Don’t Judge a Sausage by Its Cover: Effects of Inoculating Three Indigenous Lactic Acid Bacteria on Quality, Moisture Distribution, and Protein Structure in Fermentation
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains
2.2. Preparation of Sausages
2.3. Sensory Evaluation
2.4. Microbiological Analysis
2.5. Total Volatile Basic Nitrogen (TVB-N)
2.6. Texture Profile Analysis (TPA)
2.7. pH and aw Analysis
2.8. Low-Field Nuclear Magnetic Resonance (LF-NMR) Measurement
2.9. Raman Spectroscopic Analysis
2.10. Statistical Analysis
3. Results and Discussion
3.1. Sensory Evaluation
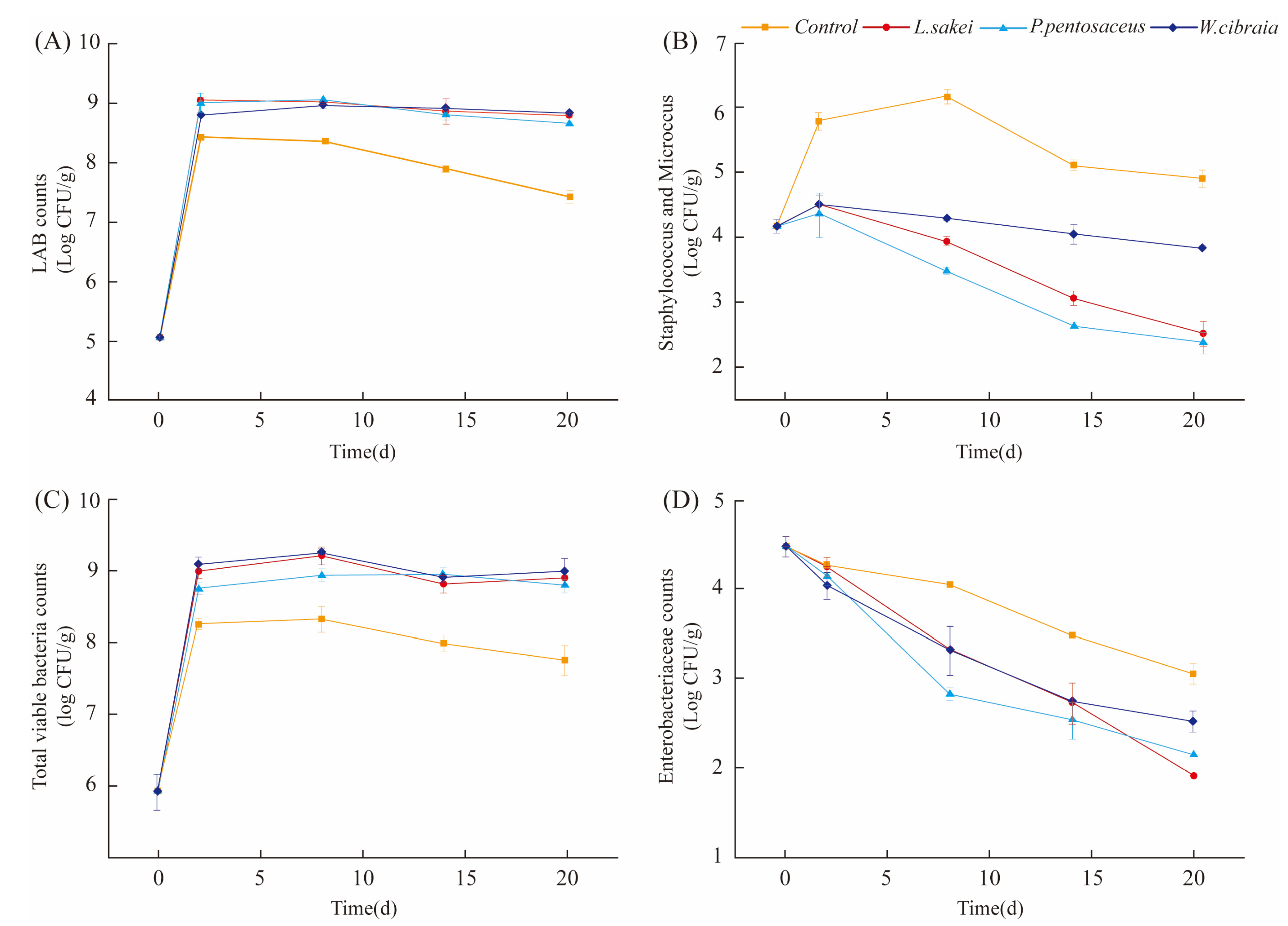
3.2. Microbiological Analysis
3.3. TVB-N Analysis
3.4. Texture Properties
3.5. pH and aw
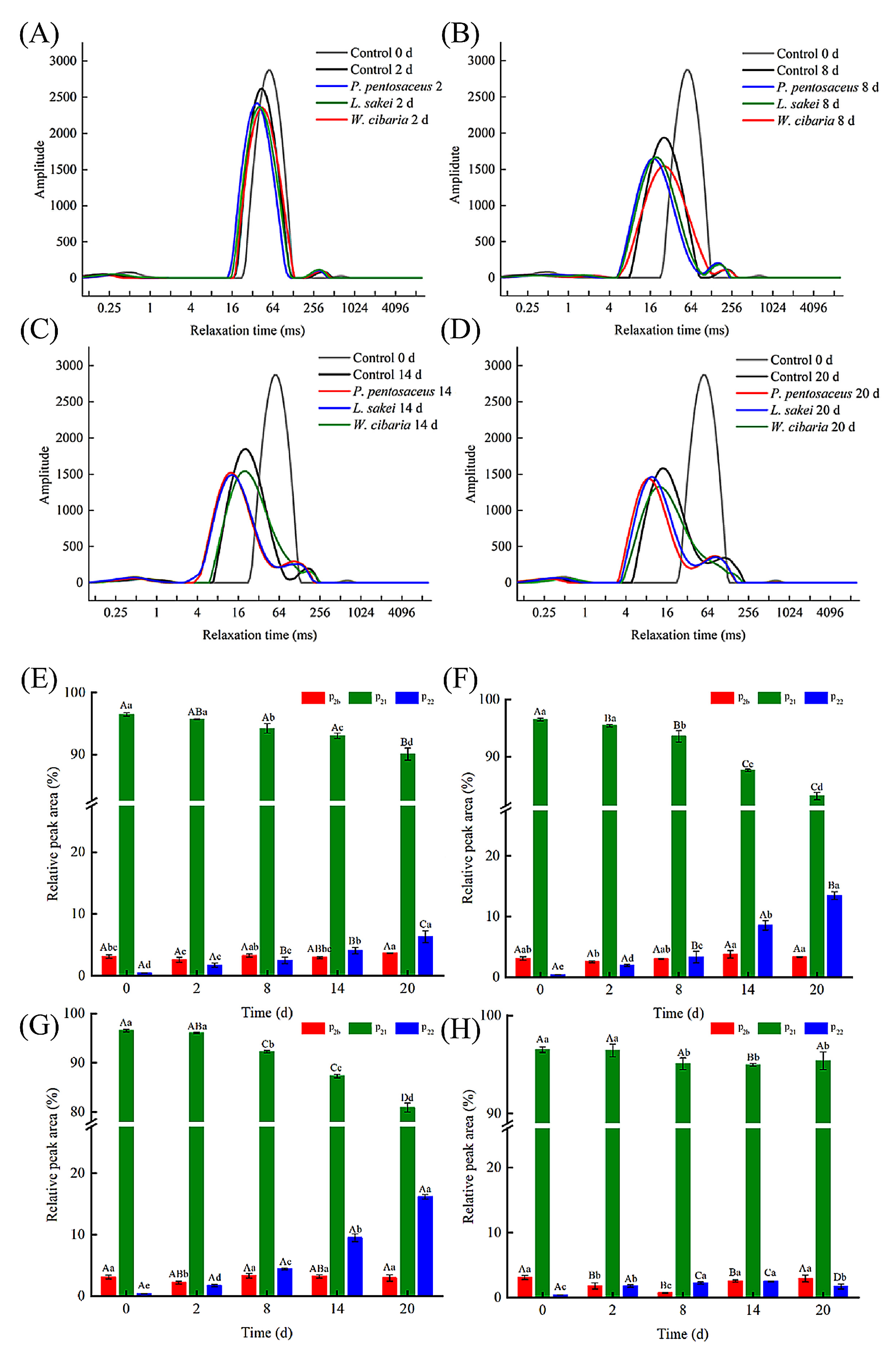
3.6. LF-NMR Analysis
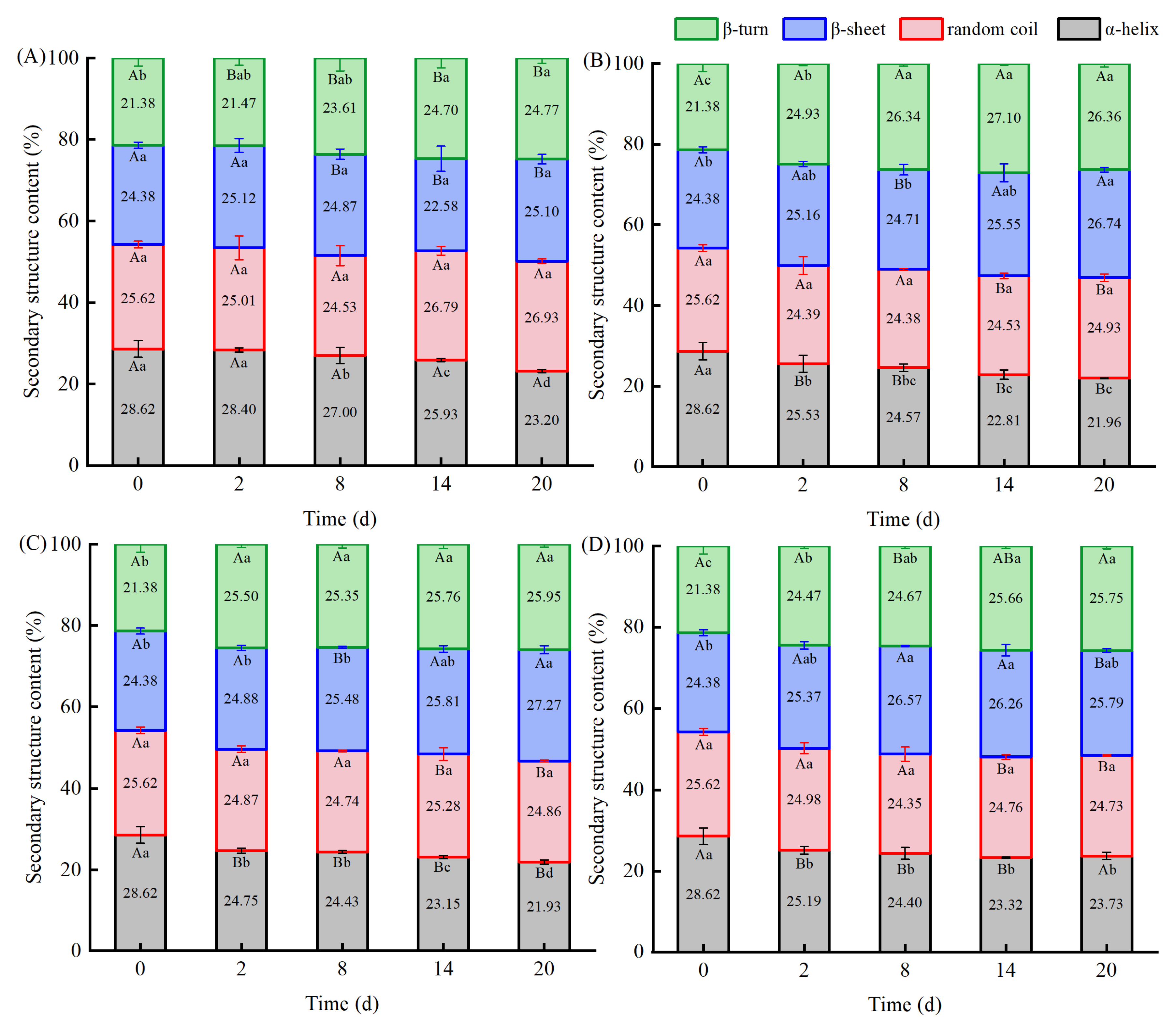
3.7. Protein Structure Based on Raman Spectroscopy Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, X.; Zhao, G.; Zheng, Y.; Wang, Y.; Bai, X.; Li, F.; Gu, Y.; Zhu, C. Effects of single fermentation of Lactobacillus sakei and compound fermentation with Staphylococcus carnosus on the metabolomics of beef sausages. Food Chem. 2025, 464, 141728. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, Y.; Li, X.-a.; Zhang, H.; Chen, Q.; Kong, B. Application of lactic acid bacteria for improving the quality of reduced-salt dry fermented sausage: Texture, color, and flavor profiles. LWT 2022, 154, 112723. [Google Scholar] [CrossRef]
- Mangia, N.P.; Trani, A.; Di Luccia, A.; Faccia, M.; Gambacorta, G.; Fancello, F.; Deiana, P. Effect of the use of autochthonous Lactobacillus curvatus, Lactobacillus plantarum and Staphylococcus xylosus strains on microbiological and biochemical properties of the Sardinian fermented sausage. Eur. Food Res. Technol. 2013, 236, 557–566. [Google Scholar] [CrossRef]
- Zhang, J.; Jin, G.; Wang, J.; Zhang, W. Effect of intensifying high-temperature ripening on lipolysis and lipid oxidation of Jinhua ham. LWT Food Sci. Technol. 2011, 44, 473–479. [Google Scholar] [CrossRef]
- Leroy, F.; Geyzen, A.; Janssens, M.; De Vuyst, L.; Scholliers, P. Meat fermentation at the crossroads of innovation and tradition: A historical outlook. Trends Food Sci. Technol. 2013, 31, 130–137. [Google Scholar] [CrossRef]
- Sun, J.; Cao, C.-C.; Feng, M.-Q.; Xu, X.-L.; Zhou, G.-H. Technological and safety characterization of coagulase-negative staphylococci with high protease activity isolated from Traditional Chinese fermented sausages. LWT 2019, 114, 108371. [Google Scholar] [CrossRef]
- Li, H.; Zhu, Q.; Chen, X.; Zhou, J.; Wu, J. Isolation and characterization of coagulase negative staphylococci with high proteolytic activity from dry fermented sausages as a potential starter culture. Food Res. Int. 2022, 162, 111957. [Google Scholar] [CrossRef]
- Nikoloudaki, O.; Aheto, F.; Di Cagno, R.; Gobbetti, M. Synthetic microbial communities: A gateway to understanding resistance, resilience, and functionality in spontaneously fermented food microbiomes. Food Res. Int. 2024, 192, 114780. [Google Scholar] [CrossRef]
- Xu, L.; He, J.; Duan, M.; Chang, Y.; Gu, T.; Tian, Y.; Cai, Z.; jiang, C.; Zeng, T.; Lu, L. Effects of lactic acid bacteria-derived fermented feed on the taste and quality of duck meat. Food Res. Int. 2023, 174, 113679. [Google Scholar] [CrossRef]
- Mrkonjic Fuka, M.; Tanuwidjaja, I.; Zgomba Maksimovic, A.; Zunabovic-Pichler, M.; Kublik, S.; Hulak, N.; Domig, K.J.; Schloter, M. Bacterial diversity of naturally fermented game meat sausages: Sources of new starter cultures. LWT 2020, 118, 108782. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, X.; Shan, C.; Xia, X.; Wang, Y.; Dong, M.; Zhou, J. Novel bacteriocin produced by Lactobacillus alimentarius FM-MM4 from a traditional Chinese fermented meat Nanx Wudl: Purification, identification and antimicrobial characteristics. Food Control 2017, 77, 290–297. [Google Scholar] [CrossRef]
- Wang, H.; Xu, J.; Liu, Q.; Xia, X.; Sun, F.; Kong, B. Effect of the protease from Staphylococcus carnosus on the proteolysis, quality characteristics, and flavor development of Harbin dry sausage. Meat Sci. 2022, 189, 108827. [Google Scholar] [CrossRef]
- Han, J.; Wang, Y.; Xu, Y.; Gu, Y.; Zhang, K.; Tian, J.; Jin, Y. Effects of compound fermentation of lactic acid bacteria IMAUJBP3 and IMAUJBR3 on the characteristic flavors and metabolites of mutton fermented sausages. LWT 2024, 212, 116995. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, Y.; Chen, C.; Xie, T.; Li, P. Effect of Lactobacillus plantarum and Staphylococcus xylosus on flavour development and bacterial communities in Chinese dry fermented sausages. Food Res. Int. 2020, 135, 109247. [Google Scholar] [CrossRef]
- Pavli, F.G.; Argyri, A.A.; Chorianopoulos, N.G.; Nychas, G.-J.E.; Tassou, C.C. Effect of Lactobacillus plantarum L125 strain with probiotic potential on physicochemical, microbiological and sensorial characteristics of dry-fermented sausages. LWT 2020, 118, 108810. [Google Scholar] [CrossRef]
- Pérez-Burillo, S.; Pastoriza, S.; Gironés, A.; Avellaneda, A.; Pilar Francino, M.; Rufián-Henares, J.A. Potential probiotic salami with dietary fiber modulates metabolism and gut microbiota in a human intervention study. J. Funct. Foods 2020, 66, 103790. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, P.; Zhou, Y.; Ma, F.; Chen, C. Effect of inoculating Lactobacillus pentosus R3 on N-nitrosamines and bacterial communities in dry fermented sausages. Food Control 2018, 87, 126–134. [Google Scholar] [CrossRef]
- Ge, Q.; Pei, H.; Liu, R.; Chen, L.; Gao, X.; Gu, Y.; Hou, Q.; Yin, Y.; Yu, H.; Wu, M.; et al. Effects of Lactobacillus plantarum NJAU-01 from Jinhua ham on the quality of dry-cured fermented sausage. LWT 2019, 101, 513–518. [Google Scholar] [CrossRef]
- Van Reckem, E.; Claeys, E.; Charmpi, C.; Sosa Fajardo, A.; Van der Veken, D.; Maes, D.; Weckx, S.; De Vuyst, L.; Leroy, F. High-throughput amplicon sequencing to assess the impact of processing factors on the development of microbial communities during spontaneous meat fermentation. Int. J. Food Microbiol. 2021, 354, 109322. [Google Scholar] [CrossRef]
- Bertram, H.C.; Kristensen, M.; Andersen, H.J. Functionality of myofibrillar proteins as affected by pH, ionic strength and heat treatment—A low-field NMR study. Meat Sci. 2004, 68, 249–256. [Google Scholar] [CrossRef]
- Chen, J.; Hu, Y.; Wen, R.; Liu, Q.; Chen, Q.; Kong, B. Effect of NaCl substitutes on the physical, microbial and sensory characteristics of Harbin dry sausage. Meat Sci. 2019, 156, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Nawrocka, A.; Szymańska-Chargot, M.; Miś, A.; Wilczewska, A.Z.; Markiewicz, K.H. Effect of dietary fibre polysaccharides on structure and thermal properties of gluten proteins—A study on gluten dough with application of FT-Raman spectroscopy, TGA and DSC. Food Hydrocoll. 2017, 69, 410–421. [Google Scholar] [CrossRef]
- Zhu, Y.; Guo, L.; Yang, Q. Partial replacement of nitrite with a novel probiotic Lactobacillus plantarum on nitrate, color, biogenic amines and gel properties of Chinese fermented sausages. Food Res. Int. 2020, 137, 109351. [Google Scholar] [CrossRef]
- Alrosan, M.; Tan, T.C.; Easa, A.M.; Gammoh, S.; Alu’datt, M.H.; Kubow, S.; Almajwal, A.M.; Razzak Mahmood, A.A.; Al-Qaisi, A.; Bawadi, H. Enhancing the quality of lentil proteins via combination with whey proteins based on a dual process: A novel strategy through the incorporation of complexation and fermentation. Food Sci. Biotechnol. 2025, 34, 65–78. [Google Scholar] [CrossRef]
- Han, X.; Yi, H.; Zhang, L.; Huang, W.; Zhang, Y.; Zhang, L.; Du, M. Improvement of fermented Chinese cabbage characteristics by selected starter cultures. J. Food Sci. 2014, 79, M1387–M1392. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Ruan, Y.; He, J.; Dang, Y.; Cao, J.; Sun, Y.; Pan, D.; Tian, H. Effects of microbial fermentation on the flavor of cured duck legs. Poult. Sci. 2020, 99, 4642–4652. [Google Scholar] [CrossRef]
- Ye, Y.; Ariful, I.M.; Zhu, J.; Zhou, X.; Ge, Q.; Wu, M.; Yu, H.; Liu, R. Physicochemical characteristics, texture changes, proteolysis and volatile flavor compounds of fermented sausages by mixed starters: Effects of incorporating varying proportions of pale, soft and exudative pork. LWT 2025, 216, 117349. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, T.-T.; Guo, R.-R.; Ye, Q.; Zhao, H.-L.; Huang, X.-H. The regulation of key flavor of traditional fermented food by microbial metabolism: A review. Food Chem. X 2023, 19, 100871. [Google Scholar] [CrossRef]
- Kaczmarska, K.; Taylor, M.; Piyasiri, U.; Frank, D. Flavor and Metabolite Profiles of Meat, Meat Substitutes, and Traditional Plant-Based High-Protein Food Products Available in Australia. Foods 2021, 10, 801. [Google Scholar] [CrossRef]
- Shi, Z.; Wu, L.; Fan, X.; Sun, Y.; Zhang, H.; Tian, H.; Zhou, F.; Wang, Z.; Pan, X.; Pan, D. Impact of inoculating Staphylococcus xylosus CICC21445 and Staphylococcus vitulinus CICC10850 on lipid metabolism and flavor development in dry-cured duck. Food Biosci. 2024, 61, 104864. [Google Scholar] [CrossRef]
- Zheng, S.-S.; Hu, Y.-Y.; Tan, L.-J.; Yang, L.; Wang, C.-Y.; Xu, B.-C. Innovative insights into dry fermented sausages flavor: Unraveling the impact of varied lactobacillus genera-driven fermentation. Food Biosci. 2024, 60, 104331. [Google Scholar] [CrossRef]
- Wang, H.; Sui, Y.; Liu, J.; Kong, B.; Li, H.; Qin, L.; Chen, Q. Analysis and comparison of the quality and flavour of traditional and conventional dry sausages collected from northeast China. Food Chem. X 2023, 20, 100979. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Yue, J.; Wang, Y.; Shao, J.; Li, Z.; Li, M. The regulation of volatile flavor compounds in fermented meat products mediated by microorganisms: A review. Food Biosci. 2024, 62, 105180. [Google Scholar] [CrossRef]
- Chung, C.; Baier, S.; McClements, D.J.; Decker, E.A. Stabilization of myoglobin from different species (produced by cellular agriculture) using food-grade natural and synthetic antioxidants. Food Res. Int. 2024, 178, 113965. [Google Scholar] [CrossRef] [PubMed]
- Patinho, I.; Cavalcante, C.L.; Saldaña, E.; Gagaoua, M.; Behrens, J.H.; Contreras-Castillo, C.J. Assessment of beef sensory attributes and physicochemical characteristics: A comparative study of intermediate versus normal ultimate pH striploin cuts. Food Res. Int. 2024, 175, 113778. [Google Scholar] [CrossRef]
- Zhang, Y.; Qin, Y.; Wang, Y.; Huang, Y.; Li, P.; Li, P. Lactobacillus plantarum LPL-1, a bacteriocin producing strain, changed the bacterial community composition and improved the safety of low-salt fermented sausages. LWT 2020, 128, 109385. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, P.; Xie, Y.; Wang, X. Co-fermentation with Lactobacillus curvatus LAB26 and Pediococcus pentosaceus SWU73571 for improving quality and safety of sour meat. Meat Sci. 2020, 170, 108240. [Google Scholar] [CrossRef]
- Juárez-Castelán, C.; García-Cano, I.; Escobar-Zepeda, A.; Azaola-Espinosa, A.; Álvarez-Cisneros, Y.; Ponce-Alquicira, E. Evaluation of the bacterial diversity of Spanish-type chorizo during the ripening process using high-throughput sequencing and physicochemical characterization. Meat Sci. 2019, 150, 7–13. [Google Scholar] [CrossRef]
- Kang, J.; Zhou, X.; Zhang, W.; Pei, F.; Ge, J. Transcriptomic analysis of bacteriocin synthesis and stress response in Lactobacillus paracasei HD1.7 under acetic acid stress. LWT 2022, 154, 112897. [Google Scholar] [CrossRef]
- Arbulu, S.; Kjos, M. Revisiting the Multifaceted Roles of Bacteriocins: The Multifaceted Roles of Bacteriocins. Microb. Ecol. 2024, 87, 41. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, C.; Ning, J.; Wang, S.; Nie, Q.; Wang, W.; Zhang, J.; Ji, L. Effect of fermentation by Pediococcus pentosaceus and Staphylococcus carnosus on the metabolite profile of sausages. Food Res. Int. 2022, 162, 112096. [Google Scholar] [CrossRef] [PubMed]
- Zhong, A.; Chen, W.; Duan, Y.; Li, K.; Tang, X.; Tian, X.; Wu, Z.; Li, Z.; Wang, Y.; Wang, C. The potential correlation between microbial communities and flavors in traditional fermented sour meat. LWT 2021, 149, 111873. [Google Scholar] [CrossRef]
- Bekhit, A.E.-D.A.; Holman, B.W.B.; Giteru, S.G.; Hopkins, D.L. Total volatile basic nitrogen (TVB-N) and its role in meat spoilage: A review. Trends Food Sci. Technol. 2021, 109, 280–302. [Google Scholar] [CrossRef]
- Li, Y.; Tang, X.; Shen, Z.; Dong, J. Prediction of total volatile basic nitrogen (TVB-N) content of chilled beef for freshness evaluation by using viscoelasticity based on airflow and laser technique. Food Chem. 2019, 287, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Bekhit, A.E.A.; Giteru, S.G.; Holman, B.W.B.; Hopkins, D.L. Total volatile basic nitrogen and trimethylamine in muscle foods: Potential formation pathways and effects on human health. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3620–3666. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Long, Y.; Peng, Y.; Li, Y.; Chao, K.; Tang, X. A feasibility study of rapid nondestructive detection of total volatile basic nitrogen (TVB-N) content in beef based on airflow and laser ranging technique. Meat Sci. 2018, 145, 367–374. [Google Scholar] [CrossRef]
- Li, Y.; Cao, Z.; Yu, Z.; Zhu, Y.; Zhao, K. Effect of inoculating mixed starter cultures of Lactobacillus and Staphylococcus on bacterial communities and volatile flavor in fermented sausages. Food Sci. Hum. Wellness 2023, 12, 200–211. [Google Scholar] [CrossRef]
- Liu, M.; Luo, H.; Xiao, Q.; Chen, C.; Xu, B.; Li, P. Effect of Latilactobacillus sakei and Staphylococcus xylosus on the textural characteristics of dry fermented sausages. Food Biosci. 2024, 59, 103972. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, Y.; Huang, D.; Bayinbate, B.; Zheng, S.; Xu, B. The effects of fermented sausage quality driven by reduction of NaCl: Investigation into the microbial community and flavor profiles. Food Res. Int. 2025, 204, 115867. [Google Scholar] [CrossRef]
- Gensollen, G.; Pourcher, A.-M.; Duedal, A.-L.; Picard, S.; Le Roux, S.; Peu, P. Impact of pH in the first-stage of a two-stage anaerobic digestion on metabolic pathways and methane production. Bioresour. Technol. Rep. 2022, 20, 101256. [Google Scholar] [CrossRef]
- Demeyer, D.I.; Vandekerckhove, P.; Moermans, R. Compounds determining pH in dry sausage. Meat Sci. 1979, 3, 161–167. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Gómez, M.; Fonseca, S. Effect of commercial starter cultures on physicochemical characteristics, microbial counts and free fatty acid composition of dry-cured foal sausage. Food Control 2014, 46, 382–389. [Google Scholar] [CrossRef]
- He, Q.; Peng, H.; Sheng, M.; Hu, S.; Qiu, J.; Gu, J. Humidity Control Strategies for Solid-State Fermentation: Capillary Water Supply by Water-Retention Materials and Negative-Pressure Auto-controlled Irrigation. Front. Bioeng. Biotechnol. 2019, 7, 263. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Ding, Z.; Mei, J.; Xie, J. Effect of cellobiose on the myofibrillar protein denaturation induced by pH changes during freeze-thaw cycles. Food Chem. 2022, 373, 131511. [Google Scholar] [CrossRef]
- Tan, M.; Ye, J.; Xie, J. Freezing-induced myofibrillar protein denaturation: Role of pH change and freezing rate. LWT 2021, 152, 112381. [Google Scholar] [CrossRef]
- Zhu, C.; Yin, F.; Tian, W.; Zhu, Y.; Zhao, L.; Zhao, G. Application of a pressure-transform tumbling assisted curing technique for improving the tenderness of restructured pork chops. LWT 2019, 111, 125–132. [Google Scholar] [CrossRef]
- Shao, J.-H.; Deng, Y.-M.; Jia, N.; Li, R.-R.; Cao, J.-X.; Liu, D.-Y.; Li, J.-R. Low-field NMR determination of water distribution in meat batters with NaCl and polyphosphate addition. Food Chem. 2016, 200, 308–314. [Google Scholar] [CrossRef]
- Møller, S.M.; Grossi, A.; Christensen, M.; Orlien, V.; Søltoft-Jensen, J.; Straadt, I.K.; Thybo, A.K.; Bertram, H.C. Water properties and structure of pork sausages as affected by high-pressure processing and addition of carrot fibre. Meat Sci. 2011, 87, 387–393. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, W.; Li, T.; Zheng, H.; Khan, M.A.; Xu, X.; Sun, J.; Zhou, G. Effect of protein structure on water and fat distribution during meat gelling. Food Chem. 2016, 204, 239–245. [Google Scholar] [CrossRef]
- Kang, Z.-L.; Chen, F.-S.; Ma, H.-J. Effect of pre-emulsified soy oil with soy protein isolate in frankfurters: A physical-chemical and Raman spectroscopy study. LWT 2016, 74, 465–471. [Google Scholar] [CrossRef]
- Dumetz, A.C.; Chockla, A.M.; Kaler, E.W.; Lenhoff, A.M. Effects of pH on protein–protein interactions and implications for protein phase behavior. Biochim. Et. Biophys. Acta (BBA) Proteins Proteom. 2008, 1784, 600–610. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, M.; Chen, H.; Zhang, Q.; Li, F.; Gu, Y.; Wang, K.; Zhao, G. Effect of NaCl on the structure and digestive properties of heat-treated myofibrillar proteins. Food Chem. 2025, 463, 141521. [Google Scholar] [CrossRef]
- Zhu, C.; Zheng, Y.; Zhang, G.; Yu, X.; Zhang, Q.; Zhao, G.; Li, F. Enhacing emulsification of meat broth system mixed with myofibrillar proteins and type I collagen: The role of NaCl and heat. Food Chem. X 2024, 24, 101945. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, A.; Hong, P.; Zhou, C.; Li, X.; Xie, M. pH-induced interface protein structure changes to adjust the stability of tilapia protein isolate emulsion prepared by high-pressure homogenization. Food Chem. X 2024, 24, 101841. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, J.; Zhang, R.; Zhou, L.; Ni, L.; Zhang, W. Study on the effects of pre-slaughter transport stress on water holding capacity of pork: Insights from oxidation, structure, function, and degradation properties of protein. Food Chem. X 2024, 24, 101913. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, S.-M.; Xie, B.-J.; Xiong, S.-B. Contribution of protein conformation and intermolecular bonds to fish and pork gelation properties. Food Hydrocoll. 2011, 25, 898–906. [Google Scholar] [CrossRef]
- Herrero, A.M.; Cambero, M.I.; Ordóñez, J.A.; de la Hoz, L.; Carmona, P. Raman spectroscopy study of the structural effect of microbial transglutaminase on meat systems and its relationship with textural characteristics. Food Chem. 2008, 109, 25–32. [Google Scholar] [CrossRef]
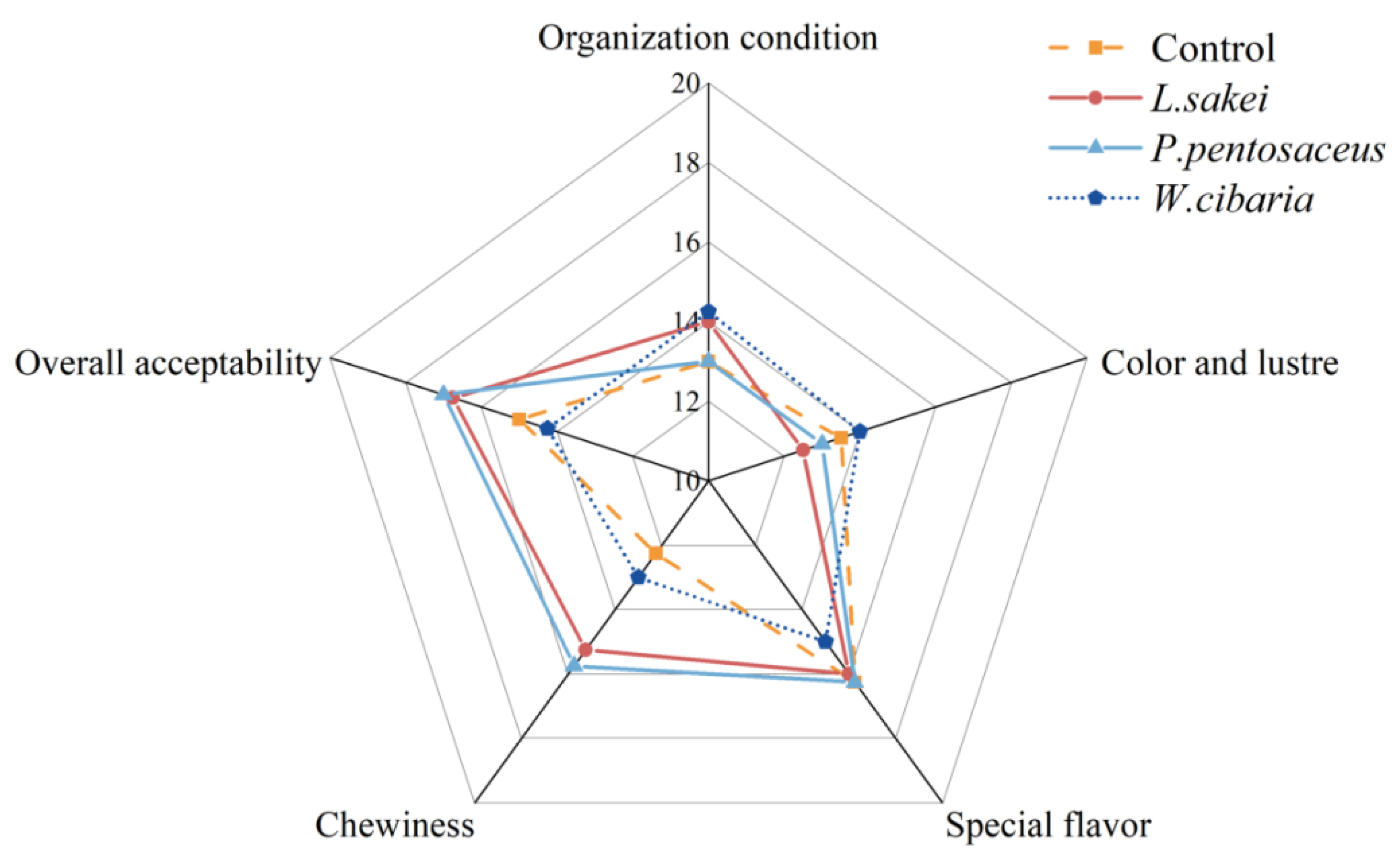

| Evaluation Criteria (Weight) | Standard | Score |
|---|---|---|
| Organization condition (20%) | The meat is firm in texture, with a smooth and even surface, and distinct separation between lean meat and fat particles. | 15~20 |
| The meat is relatively firm in texture, with a relatively smooth and even surface. | 10~15 | |
| The meat has a somewhat loose texture, allowing for slicing, but lacks smoothness and evenness on the surface. | 5~10 | |
| The meat is not densely compacted, making it impossible to form slices. | 1~5 | |
| Color and lustre (20%) | The lean meat presents a deep red hue, while the fat appears pristine white with a lustrous sheen. | 15~20 |
| The lean meat is slightly red, appearing slightly dull, while the fat is whiter. | 10~15 | |
| The lean meat is a dark red, while the fat has a slight yellowish hue. | 5~10 | |
| The lean meat darkens or takes on a greenish hue, while the fat becomes yellowish, with indistinct boundaries. | 1~5 | |
| Special flavor (20%) | The unique flavor is rich and free from any off-notes. | 15~20 |
| The fermented aroma is pronounced, with no off-notes present. | 10~15 | |
| The fermented aroma is subtle, with slight off-notes present. | 5~10 | |
| There are no distinctive flavors, but there are off-notes present. | 1~5 | |
| Chewiness (20%) | Firm and resilient with good chewiness. | 15~20 |
| There is a certain level of firmness and resilience, with good chewability. | 10~15 | |
| Too hard or somewhat loose, with average chewability. | 5~10 | |
| Difficult to chew. | 1~5 | |
| Overall acceptability (20%) | Strong appetite. | 15~20 |
| Intense appetite. | 10~15 | |
| Has appetite. | 5~10 | |
| Essentially no appetite. | 1~5 |
| Time | Treatment | Hardness | Cohesiveness | Spring | Cohesion | Chewiness |
|---|---|---|---|---|---|---|
| 2 d | Control | 31.40 ± 2.60 b | 0.20 ± 0.00 a | 1.33 ± 0.09 b | 7.47 ± 0.76 b | 10.07 ± 1.50 b |
| L. sakei | 53.65 ± 4.98 a | 0.30 ± 0.00 a | 2.37 ± 0.17 a | 15.20 ± 1.31 a | 36.13 ± 4.74 a | |
| P. pentosaceus | 56.33 ± 3.03 a | 0.30 ± 0.00 a | 2.38 ± 0.11 a | 15.67 ± 0.85 a | 37.33 ± 2.00 a | |
| W. cibaria | 27.03 ± 3.78 c | 0.20 ± 0.00 b | 1.47 ± 0.41 b | 5.24 ± 1.05 c | 7.70 ± 1.55 c | |
| 8 d | Control | 78.00 ± 2.45 b | 0.30 ± 0.00 a | 1.89 ± 0.05 b | 26.37 ± 1.01 c | 52.33 ± 2.61 b |
| L. sakei | 109.27 ± 2.96 a | 0.30 ± 0.00 a | 2.10 ± 0.10 a | 32.17 ± 0.40 a | 67.60 ± 2.42 a | |
| P. pentosaceus | 110.77 ± 3.99 a | 0.27 ± 0.06 a | 2.37 ± 0.07 a | 27.73 ± 0.87 b | 62.57 ± 2.97 a | |
| W. cibaria | 31.30 ± 0.56 c | 0.20 ± 0.00 b | 1.27 ± 0.03 c | 6.87 ± 0.25 d | 8.70 ± 0.56 c | |
| 14 d | Control | 95.70 ± 2.42 b | 0.30 ± 0.00 a | 2.72 ± 0.08 a | 26.80 ± 1.64 b | 72.73 ± 4.44 b |
| L. sakei | 152.98 ± 14.43 a | 0.30 ± 0.00 a | 2.84 ± 0.13 a | 40.30 ± 3.91 a | 114.30 ± 9.16 a | |
| P. pentosaceus | 151.65 ± 5.06 a | 0.30 ± 0.00 a | 2.80 ± 0.11 a | 38.78 ± 1.50 a | 108.50 ± 4.86 a | |
| W. cibaria | 46.75 ± 2.16 c | 0.20 ± 0.00 b | 1.25 ± 0.08 b | 8.18 ± 0.36 c | 10.20 ± 0.81 c | |
| 20 d | Control | 150.00 ± 24.85 b | 0.20 ± 0.00 a | 2.42 ± 0.35 a | 31.43 ± 8.32 b | 73.93 ± 13.11 b |
| L. sakei | 208.48 ± 17.80 a | 0.23 ± 0.05 a | 2.53 ± 0.17 a | 50.30 ± 3.02 a | 126.95 ± 3.82 a | |
| P. pentosaceus | 222.90 ± 12.10 a | 0.20 ± 0.00 a | 2.69 ± 0.22 a | 49.50 ± 4.55 a | 132.85 ± 13.69 a | |
| W. cibaria | 61.43 ± 3.24 c | 0.13 ± 0.05 b | 1.38 ± 0.04 b | 8.88 ± 0.10 c | 12.23 ± 0.33 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Zhao, G.; Zhao, S.; Li, X.; Cui, W.; Xu, L.; Zhu, C.; Tong, L. Don’t Judge a Sausage by Its Cover: Effects of Inoculating Three Indigenous Lactic Acid Bacteria on Quality, Moisture Distribution, and Protein Structure in Fermentation. Fermentation 2025, 11, 134. https://doi.org/10.3390/fermentation11030134
Zheng Y, Zhao G, Zhao S, Li X, Cui W, Xu L, Zhu C, Tong L. Don’t Judge a Sausage by Its Cover: Effects of Inoculating Three Indigenous Lactic Acid Bacteria on Quality, Moisture Distribution, and Protein Structure in Fermentation. Fermentation. 2025; 11(3):134. https://doi.org/10.3390/fermentation11030134
Chicago/Turabian StyleZheng, Yangyi, Gaiming Zhao, Shichang Zhao, Xuan Li, Wenming Cui, Long Xu, Chaozhi Zhu, and Lin Tong. 2025. "Don’t Judge a Sausage by Its Cover: Effects of Inoculating Three Indigenous Lactic Acid Bacteria on Quality, Moisture Distribution, and Protein Structure in Fermentation" Fermentation 11, no. 3: 134. https://doi.org/10.3390/fermentation11030134
APA StyleZheng, Y., Zhao, G., Zhao, S., Li, X., Cui, W., Xu, L., Zhu, C., & Tong, L. (2025). Don’t Judge a Sausage by Its Cover: Effects of Inoculating Three Indigenous Lactic Acid Bacteria on Quality, Moisture Distribution, and Protein Structure in Fermentation. Fermentation, 11(3), 134. https://doi.org/10.3390/fermentation11030134






