Impact of Sulfur Dioxide and Dimethyl Dicarbonate Treatment on the Quality of White Wines: A Scientific Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Standards
2.2. Grape Varieties and Winemaking
2.3. Standard Physico–Chemical Parameters
2.4. Microbiological Protocol
2.4.1. Experimental Protocol for Yeast Strains’ Growth Conditions
2.4.2. Evaluation of Yeast Concentration
2.5. Quantification of Volatile Compounds
2.6. Chromatic Measurements
2.7. Sensory Analyses
2.8. Statistical Analyses
3. Results and Discussion
3.1. Physico–Chemical Parameters
3.2. Chromatic Measurements
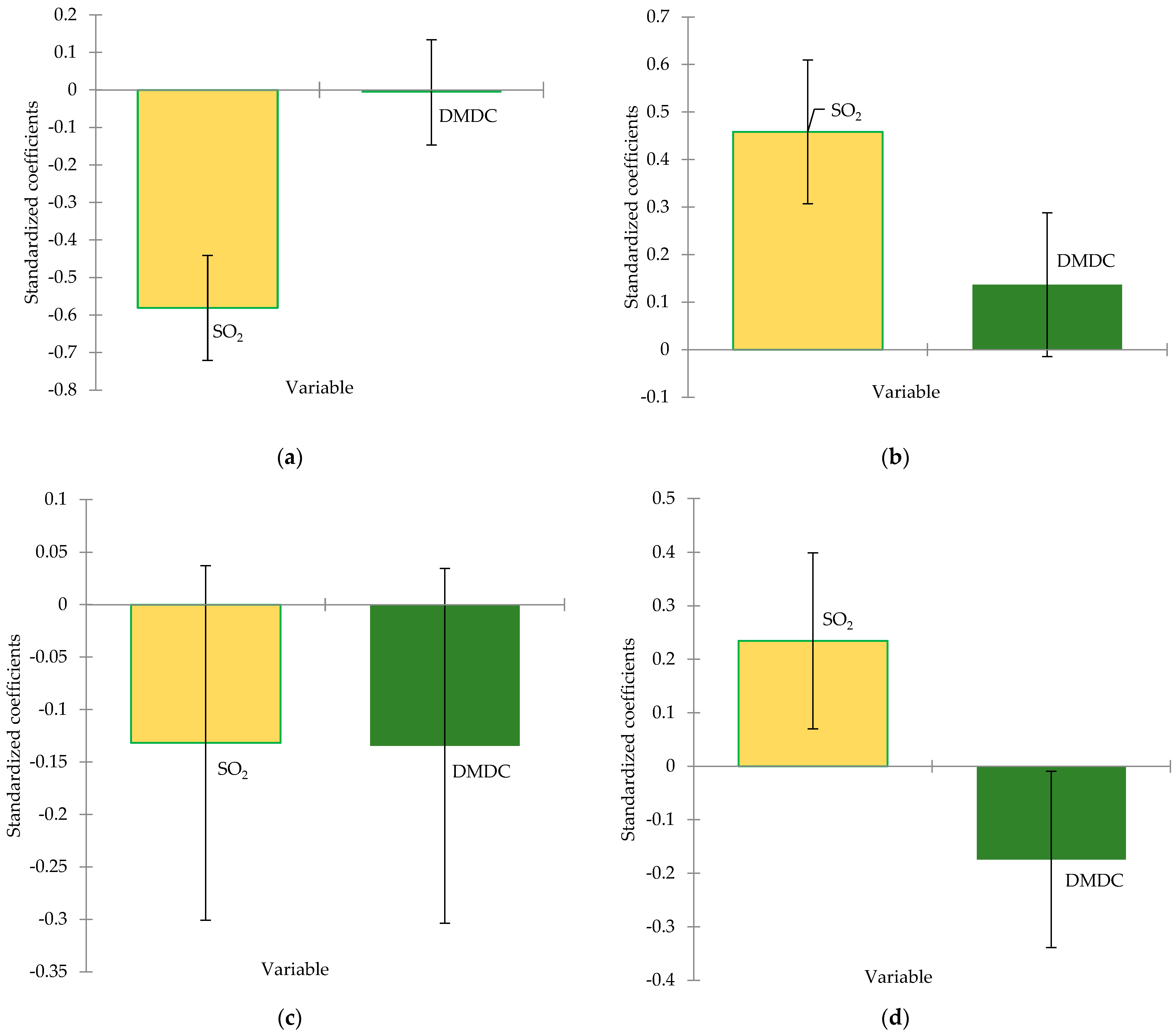
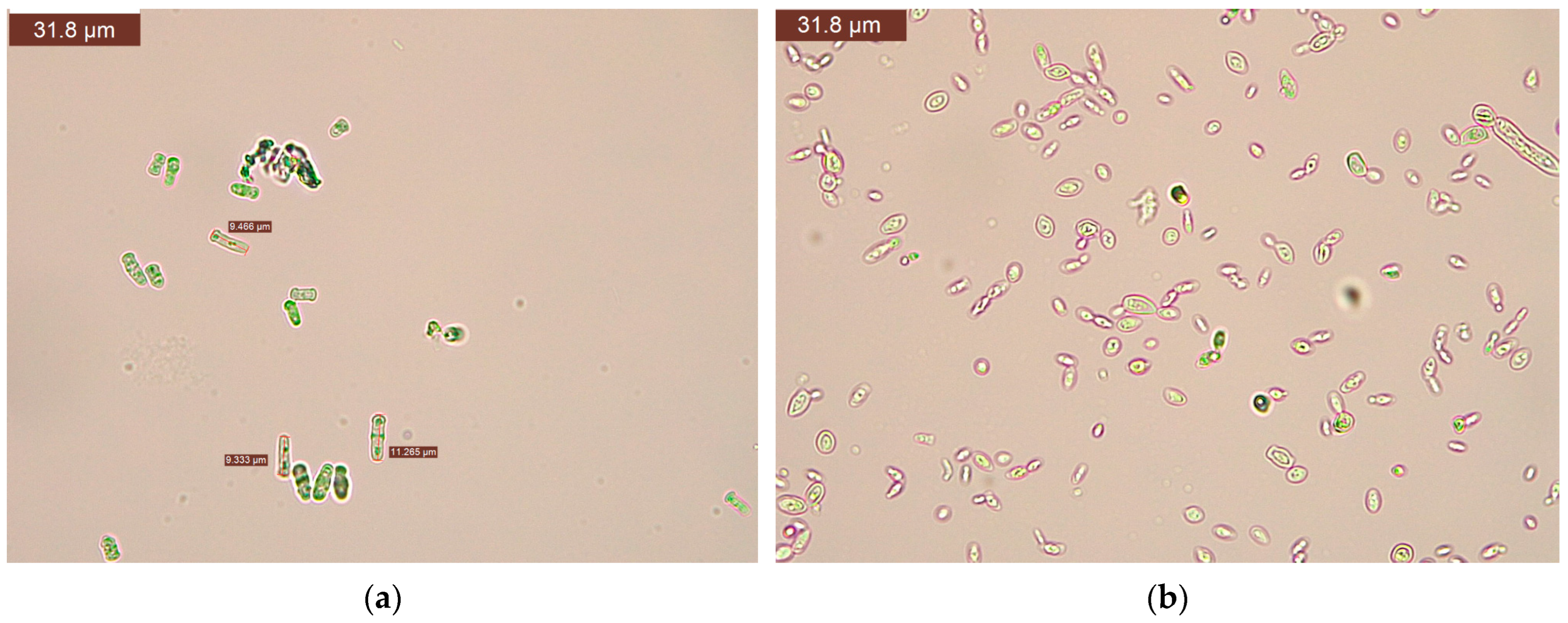
3.3. Microbiological Results
3.4. Volatile Compounds
3.5. Sensory Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morgan, S.C. The Effect of Sulfur Dioxide Addition and Alternative Fermentation Techniques on the Microbial Communities and Sensory Profiles of Wine. Ph.D. Thesis, The University of British Columbia, Vancouver, BC, Cananda, 2019. [Google Scholar]
- Cotea, V.D. Tratat De Oenologie; Ceres Publisher: București, Romania, 1985. [Google Scholar]
- Lisanti, M.T.; Gambuti, A.; Genovese, A.; Piombino, P.; Moio, L. Earthy off-flavour in wine: Evaluation of remedial treatments for geosmin contamination. Food Chem. 2014, 154, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Scutarașu, E.C.; Luchian, E.C.; Vlase, L.; Nagy, K.; Colibaba, L.C.; Trincă, L.C.; Cotea, V.V. Influence evaluation of enzyme treatments on aroma profile of white wines. Agronomy 2022, 12, 2897. [Google Scholar] [CrossRef]
- Carrascón, V.; Vallverdú-Queralt, A.; Meudec, E.; Sommerer, N.; Fernandez-Zurbano, P.; Ferreira, V. The kinetics of oxygen and SO2 consumption by red wines. What do they tell about oxidation mechanisms and about changes in wine composition? Food Chem. 2018, 241, 206214. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donèche, B.; Lonvaud, A. The microbiology of wine and vinifications. In Handbook of Enology, 2nd ed.; John Wiley & Sons Publisher: West Sussex, UK, 2006; Volume 1, pp. 214–220. [Google Scholar]
- Zironi, R.; Comuzzo, P.; Tat, L.; Scobiola, S. Sulphur dioxide management in low input winemaking. Internet J. Enol. Vitic. 2009, 12. Available online: https://www.infowine.com/wp-content/uploads/2024/04/libretto7591-01-1.pdf (accessed on 6 February 2025).
- Branco, P.; Coutinho, R.; Malfeito-Ferreira, M.; Prista, C.; Albergaria, H. Wine Spoilage Control: Impact of Saccharomycin on Brettanomyces bruxellensis and its conjugated effect with sulfur dioxide. Microorganisms 2021, 9, 2528. [Google Scholar] [CrossRef]
- Christofi, S.; Katsaros, G.; Mallouchos, A.; Cotea, V.; Kallithraka, S. Reducing SO2 content in wine by combining high pressure and glutathione addition. OENO One 2021, 1, 235–252. [Google Scholar] [CrossRef]
- Oliveira, C.M.; Ferreira, A.C.S.; De Freitas, V.; Silva, A.M.S. Oxidation mechanisms occurring in wines-Review. Food Res. Int. 2011, 44, 1115–1126. [Google Scholar] [CrossRef]
- Guerrero, R.F.; Cantos-Villar, E. Demonstrating the efficiency of sulphur dioxide replacements in wine: A parameter review. Trends Food Sci. Technol. 2015, 42, 27–43. [Google Scholar] [CrossRef]
- Ough, C.S.; Were, L. Sulfur dioxide and sulfites. In Antimicrobials in Food, 3rd ed.; Davidson, P.M., Sofos, J.N., Branen, A.L., Eds.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2005; pp. 143–168. [Google Scholar]
- Payan, C.; Gancel, A.L.; Jourdes, M.; Christmann, M.; Teissedre, P.L. Wine acidification methods: A review. OENO One 2023, 57, 113–126. [Google Scholar] [CrossRef]
- Threlfall, R.T.; Morris, J.R. Using dimethyl dicarbonate to minimize sulfur dioxide for prevention of fermentation from excessive yeast contamination in juice and semi-sweet wine. J. Food Sci. 2002, 67, 2758–2762. [Google Scholar] [CrossRef]
- Yíldírím, H.K.; Darící, B. Alternative methods of sulfur dioxide used in wine production. J. Microbiol. Biotechnol. Food Sci. 2020, 9, 675–687. [Google Scholar] [CrossRef]
- Arriagada-Carrazana, J.P.; Saez-Navarrete, C.; Bordeu, E. Membrane filtration effects on aromatic and phenolic quality of Cabernet Sauvignon wines. J. Food Eng. 2005, 68, 363–368. [Google Scholar] [CrossRef]
- Santos, M.C.; Nunes, C.; Saraiva, J.A.; Coimbra, M.A. Chemical and physical methodologies for the replacement/reduction of sulfur dioxide use during winemaking: Review of their potentialities and limitations. Eur. Food Res. Technol. 2012, 234, 1–12. [Google Scholar] [CrossRef]
- Ough, C.S. Dimethyl dicarbonate as a wine sterilant. Am. J. Enol. Vitic. 1975, 26, 130–133. [Google Scholar] [CrossRef]
- Chen, K.; Han, S.; Zhang, B.; Li, M.; Sheng, W. Development of lysozyme-combined antibacterial system to reduce sulfur dioxide and to stabilize Italian Riesling ice wine during aging process. Food Sci. Nutr. 2015, 3, 453–465. [Google Scholar] [CrossRef]
- Ancín-Azpilicueta, C.; Jiménez-Moreno, N.; Moler, J.A.; Nietorojo, R.; Urmeneta, H. Effects of reduced levels of sulfite in wine production using mixtures with lysozyme and dimethyl dicarbonate on levels of volatile and biogenic amines. Food Addit. Contam. 2016, 33, 1518–1526. [Google Scholar] [CrossRef]
- Escott, C.; Loira, I.; Morata, A.; Bañuelos, M.A.; Suárez-Lepe, J.A. Wine spoilage yeasts: Control strategy. In Yeast—Industrial Applications; Morata, A., Loira, I., Eds.; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Bruhn, C.; Feng, Y. Exploring consumer response to labeling a processing aid that enhances food safety. Food Prot. Trends 2021, 41, 305–313. [Google Scholar] [CrossRef]
- Stafford, P.A.; Ough, C.S. Formation of methanol and ethyl methyl carbonate by dimethyl dicarbonate in wine and model solutions. Am. J. Enol. Vitic. 1976, 27, 7–11. [Google Scholar] [CrossRef]
- Stockley, C.; Paschke-Kratzin, A.; Teissedre, P.L.; Restani, P.; Garcia Tejedor, N.; Quini, C. SO2 and Wine: A Review; OIV Collective Expertise Document; OIV: Dijon, France, 2021. [Google Scholar]
- International Organization of Wine and Vine. International Code of Oenological Practices; International Organization of Wine and Vine: Paris, France, 2020; Available online: https://www.oiv.int/standards/international-code-of-oenological-practices (accessed on 20 May 2024).
- Lanxess Emerging Chemistry. Velcorin®. Available online: https://lanxess.com/en/products-and-brands/products/v/velcorin- (accessed on 1 June 2024).
- Commission Regulation (EC) No 606/2009 of 10 July 2009 Laying Down Certain Detailed Rules for Implementing Council Regulation (EC) 479/2008 as Regards the Categories of Grapevine Products, Oenological Practices and the Applicable Restrictions. Available online: https://www.legislation.gov.uk/eur/2009/606/contents (accessed on 2 June 2024).
- Romano, P.; Ciani, M.; Fleet, G.H. Yeasts in the Production of Wine; Springer: New York, NY, USA, 2019; pp. 375–395. [Google Scholar]
- Loira, I.; Morata, A.; Palomero, F.; González, C.; Suárez-Lepe, J.A. Schizosaccharomyces pombe: A promising biotechnology for modulating wine composition. Fermentation 2018, 4, 70. [Google Scholar] [CrossRef]
- Kurtzman, C.P.; Fell, J.W.; Boekhout, T. The Yeasts: A Taxonomic Study; Elsevier: London, UK, 2010. [Google Scholar]
- Suárez-Lepe, J.A.; Palomero, F.; Benito, S.; Calderón, F.; Morata, A. Oenological versatility of Schizosaccharomyces spp. Eur. Food Res. Technol. 2012, 235, 375–383. [Google Scholar] [CrossRef]
- Cibrario, A.; Avramova, A.; Dimopoulou, M.; Magani, M.; MiotSertier, C.; Mas, A.; Portillo, M.C.; Ballestra, P.; Warren Albertin, W.; Masneuf-Pomarede, I.; et al. Brettanomyces bruxellensis wine isolates show high geographical dispersal and long persistence in cellars. PLoS ONE 2018, 14, e0222749. [Google Scholar] [CrossRef] [PubMed]
- Vigentini, I.; Romano, A.; Compagno, C.; Merico, A.; Molinari, F.; Tirelli, A.; Foschino, R.; Volonterio, G. Physiological and oenological traits of diferent Dekkera/Brettanomyces bruxellensis strains under wine-model conditions. FEMS Yeast Res. 2008, 8, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Crauwels, S.; Barbara, F.V.; Jaskula-Goiris, B.; Steensels, J.; Verreth, C.; Bosmans, L.; Paulussen, C.; Herrera-Malaver, B.; de Jonge, R.; De Clippeleer, J.; et al. Fermentation assays reveal diferences in sugar and (of-) favor metabolism across different Brettanomyces bruxellensis strains. FEMS Yeast Res. 2017, 17, fow105. [Google Scholar] [CrossRef]
- Joseph, C.M.L.; Gorton, L.W.; Ebeler, S.E.; Bisson, L.F. Production of volatile compounds by wine strains of Brettanomyces bruxellensis grown in the presence of different precursor substrates. Am. J. Enol. Vitic. 2013, 64, 231–240. [Google Scholar] [CrossRef]
- Romano, A.; Perello, M.C.; Revel, G.D.; Lonvaud-Funel, A. Growth and volatile compound production by Brettanomyces/Dekkera bruxellensis in red wine. J. Appl. Microbiol. 2008, 104, 1577–1585. [Google Scholar] [CrossRef]
- Galafassi, S.; Merico, A.; Pizza, F.; Hellborg, L.; Molinari, F.; Piškur, J.; Compagno, C. Dekkera/Brettanomyces yeasts for ethanol production from renewable sources under oxygen-limited and low-pH conditions. J. Ind. Microbiol. Biotechnol. 2011, 38, 1079–1088. [Google Scholar] [CrossRef]
- Vigentini, I.; Joseph, C.M.L.; Picozzi, C.; Foschino, R.; Bisson, L.F. Assessment of the Brettanomyces bruxellensis metabolome during sulphur dioxide exposure. FEMS Yeast Res. 2013, 13, 597–608. [Google Scholar] [CrossRef]
- Avramova, M.; Cibrario, A.; Peltier, E.; Coton, M.; Coton, E.; Schacherer, J.; Spano, G.; Capozzi, V.; Blaiotta, G.; Salin, F.; et al. Brettanomyces bruxellensis population survey reveals a diploid-triploid complex structured according to substrate of isolation and geographical distribution. Sci. Rep. 2018, 8, 4136. [Google Scholar] [CrossRef]
- Agnolucci, M.; Rea, F.; Sbrana, C.; Cristani, C.; Fracassetti, D.; Tirelli, A. Sulphur dioxide affects culturability and volatile phenol production by Brettanomyces/Dekkera bruxellensis. Int. J. Food Microbiol. 2010, 143, 76–80. [Google Scholar] [CrossRef]
- Campolongo, S.; Kalliopi, R.; Giordano, M.; Gerbi, V.; Cocolin, L. Prevalence and biodiversity of Brettanomyces bruxellensis in wine from Northwestern Italy. Am. J. Enol. Vitic. 2010, 61, 486–491. [Google Scholar] [CrossRef]
- Hardy, Z.; Jideani, V.A. Functional characteristics and microbiological viability of foam-mat dried Bambara groundnut (Vigna subterranea) yogurt from reconstituted Bambara groundnut milk powder. Food Sci. Nutr. 2018, 10, 5238–5248. [Google Scholar] [CrossRef] [PubMed]
- Macoviciuc, S.; Nechita, C.B.; Cioroiu, I.B.; Cotea, V.; Niculaua, M. Effect of added sulphur dioxide levels on the aroma characteristics of wines from Panciu wine region. Ştiinţa Agric. 2022, 1, 73–77. [Google Scholar] [CrossRef]
- Cotea, V.; Focea, M.C.; Luchian, C.E.; Colibaba, L.C.; Scutarașu, E.C.; Niculaua, M.; Zamfir, C.I.; Popîrdă, A. Influence of different commercial yeasts on volatile fraction of sparkling wines. Foods 2021, 10, 247. [Google Scholar] [CrossRef] [PubMed]
- Dumitriu (Gabur), D.; Teodosiu, C.; Gabur, I.; Cotea, V.V.; Peinado, R.A.; López de Lerma, N. Alternative winemaking techniques to improve the content of phenolic and aromatic compounds in wines. Agriculture 2021, 11, 233. [Google Scholar] [CrossRef]
- ISO 3591-1977; Sensory Analysis—Apparatus—Wine-Tasting Glass. ISO: Geneva, Switzerland, 1977.
- ISO 8589-2007; Sensory Analysis—General Guidance for the Design of Test Room. ISO: Geneva, Switzerland, 2007.
- Zoecklein, B.W.; Fugelsang, K.C.; Gump, B.H.; Nury, F.S. Wine analysis and production. In Sorbic Acid, Benzoic Acid, and Dimethyldicarbonate; Chapman & Hall Publisher: New York, NY, USA, 1995; pp. 209–215. [Google Scholar]
- Samoticha, J.; Wojdyło, A.; Chmielewska, J.; Politowicz, J.; Antoni, S. The effects of enzymatic pre-treatment and type of yeast on chemical properties of white wine. LWT-Food Sci. Technol. 2017, 79, 445–453. [Google Scholar] [CrossRef]
- Țârdea, C. Chimia Și Analiza Vinului; Ion Ionescu de la Brad Publisher: Iași, Romania, 2007; pp. 574–576. [Google Scholar]
- Moreno, J.; Peinado, R. Analysis of acids. In Enological Chemistry; Academic Press: New York, NY, USA, 2012; pp. 132–135. [Google Scholar]
- Vilela-Moura, A.; Schuller, D.A.; Mendes-Faia, A.; Côrte-Real, M. Effects of acetic acid, ethanol, and SO2 on the removal of volatile acidity from acidic wines by two Saccharomyces cerevisiae commercial strains. Appl. Microbiol. Biotechnol. 2010, 87, 1317–1326. [Google Scholar] [CrossRef]
- Tian, Y.; Peng, J.; Li, X.; Wang, S. Explore the impact of free sulfur dioxide on red and white wine. In Proceedings of the 2nd International Conference on Modern Medicine and Global Health, Kuala Lumpur, Malaysia, 5 January 2024. [Google Scholar] [CrossRef]
- Kodur, S. Effects of juice pH and potassium on juice and wine quality, and regulation of potassium in grapevines through rootstocks (Vitis): A short review. Vitis 2011, 50, 1. [Google Scholar] [CrossRef]
- Jackson, R.S. Wine Science: Principles and Applications, 4th ed.; Elsevier Academic Press: Amsterdam, The Netherlands, 2014; p. 996. [Google Scholar]
- Nieto-Rojo, R.; Luquin, A.; Ancín-Azpilicueta, C. Improvement of wine aromatic quality using mixtures of lysozyme and dimethyl dicarbonate, with low SO2 concentration. Food Addit. Contam. 2015, 3, 1965–1975. [Google Scholar] [CrossRef]
- Boulton, R.B.; Singleton, V.L.; Bisson, L.F.; Cannary, J. Principles and Practices of Winemaking, 2nd ed.; Springer: New York, NY, USA, 2013. [Google Scholar]
- Gallego, R.F.; Puxeu, M.; Martín, L.; Nart, E.; Hidalgo, C.; Andorrà, I. Microbiological, physical, and chemical procedures to elaborate high-quality SO2-free wines. In Grapes and Wines—Advances in Production, Processing, Analysis and Valorization; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Delfini, C.; Gaia, P.; Schellino, R.; Strano, M.; Pagliara, A.; Ambro, S. Fermentability of grape must after inhibition with dimethyl dicarbonate (DMDC). J. Agric. Food Chem. 2002, 50, 5605–5611. [Google Scholar] [CrossRef]
- Lisanti, M.T.; Blaiotta, G.; Nioi, C.; Moio, L. Alternative Methods to SO2 for microbiological stabilization of wine. Compr. Rev. Food Sci. Food Saf. 2019, 18, 455–479. [Google Scholar] [CrossRef]
- Costa, A.; Barata, A.; Loureiro, V. Evaluation of the inhibitory effect of dimethyl dicarbonate (DMDC) against wine microorganisms. Food Microbiol. 2008, 25, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Divol, B.; Strehaiano, P.; Lonvaud-Funel, A. Effectiveness of dimethyl dicarbonate to stop alcoholic fermentation in wine. Food Microbiol. 2005, 22, 169–178. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, X.; Sun, Y.; Guan, X.; Qin, W.; Zhang, X.; Ding, Y.; Yang, W.; Zhou, J. Effects of dimethyl dicarbonate on improving the aroma of melon spirits by inhibiting spoilage microorganisms. J. Food Process. Preserv. 2022, 46, 6. [Google Scholar] [CrossRef]
- Renouf, V.; Strehaiano, P.; Lonvaud-Funel, A. Effectiveness of dimethlyl dicarbonate to prevent Brettanomyces bruxellensis growth in wine. Food Contr. 2008, 19, 208–216. [Google Scholar] [CrossRef]
- Nykanen, L. Formation and occurrence of flavor compounds in wine and distilled alcoholic beverages. Am. J. Enol. Vitic. 1986, 37, 84–96. [Google Scholar] [CrossRef]
- Francois, M.O. Effect of Yeasts and Oenological Parameters on Acetaldehyde Production During Alcoholic Fermentation of South African Grape Musts. Master’s Thesis, Stellenbosch University, Stellenbosch, South Africa, 2020. [Google Scholar]
- Osborne, J.P.; Dube Morneau, A.; Mira de Ordun, R. Degradation of free and sulfur-dioxide-bound acetaldehyde by malolactic lactic acid bacteria in white wine. J. Appl. Microbiol. 2006, 101, 474–479. [Google Scholar] [CrossRef]
- Liu, S.Q.; Pilone, G.J. An overview of formation and roles of acetaldehyde in winemaking with emphasis on microbiological implications. Int. J. Food Sci. Technol. 2000, 35, 49–61. [Google Scholar] [CrossRef]
- Soldatenco, O. Bazele Științifice Și Practice Ale Utilizării Levurilor În Oenologie; Institutul Științifico-Practic de Horticultură și Tehnologii Alimentare: Chișinău, Republic of Moldova, 2021. [Google Scholar]
- Miyake, T.; Shibamoto, T. Quantitative analysis of acetaldehyde in foods and beverages. J. Agric. Food Chem. 1993, 41, 1968–1970. [Google Scholar] [CrossRef]
- Byrne, S.; Howell, G. Acetaldehyde: How to limit its formation during fermentation. Aust. Grapegrow. Winemak. 2017, 637, 68–69. [Google Scholar]
- Erhu, L.; de Orduña Heidinger, R.M. Acetaldehyde metabolism in industrial strains of Saccharomyces cerevisiae inhibited by SO2 and cooling during alcoholic fermentation. OENO One 2020, 54, 2. [Google Scholar] [CrossRef]
- Călin Buțerchi, I.; Cotea, V.V.; Colibaba, L.C.; Luchian, C.E.; Cerbu, I.M.; Zamfir, C.I.; Nechita, B.C. The dynamic of methanol and acetaldehyde in white wines treated with sulphur dioxide and dimethyl dicarbonate. Lucr. Științifice Ser. Hortic. 2021, 64, 61–68. [Google Scholar]
- Călin, I.; Luchian, C.E.; Colibaba, L.C.; Scutarașu, E.C.; Popîrdă, A.; Cimpoi, V.I.; Zamfir, C.I.; Cotea, V.V. Study concerning the influence of sulphur dioxide and dimethyl dicarbonate treatments in wine. In Scientific Papers. Series B, Horticulture; USAMV Bucharest: Bucharest, Romania, 2020; Volume LXIV. [Google Scholar]
- Cabaroglu, T. Methanol contents of Turkish varietal wines and effect of processing. Food Control 2005, 16, 177–181. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources Added to Food (ANS). Scientific Opinion on the re-evaluation of dimethyl dicarbonate (DMDC, E 242) as a food additive. EFSA J. 2015, 13, 4319. [Google Scholar] [CrossRef]
- Scientific Committee for Food. Opinion of the Scientific Committee on Food on the Use of Dimethyl Dicarbonate (DMDC) in Wines; Opinion Expressed on 11 July 2001; SCF/CS/ADD/CONS/43 Final; European Commission: Luxembourg, 2001. [Google Scholar]
- Sîrbu, A.D.; Tomoiagă, L.L.; Răcoare, H.S.; Şerbu, F.D.; Chedea, V.S. Sulfur dioxide dinamic in Sauvignon Blanc and Neuburger dry white wines. Bull. Univ. Agric. Sci. Veter-Med. Cluj-Napoca. Food Sci. Technol. 2022, 79, 21–29. [Google Scholar] [CrossRef]
- Salton, M.A.; Daudt, C.E.; Rizzon, L.A. Influence of sulfur dioxide and grape varieties at the formation of some volatile compounds and at the sensory quality of the wine distillate. Food Sci. Technol. 2000, 20, 302–308. [Google Scholar] [CrossRef]
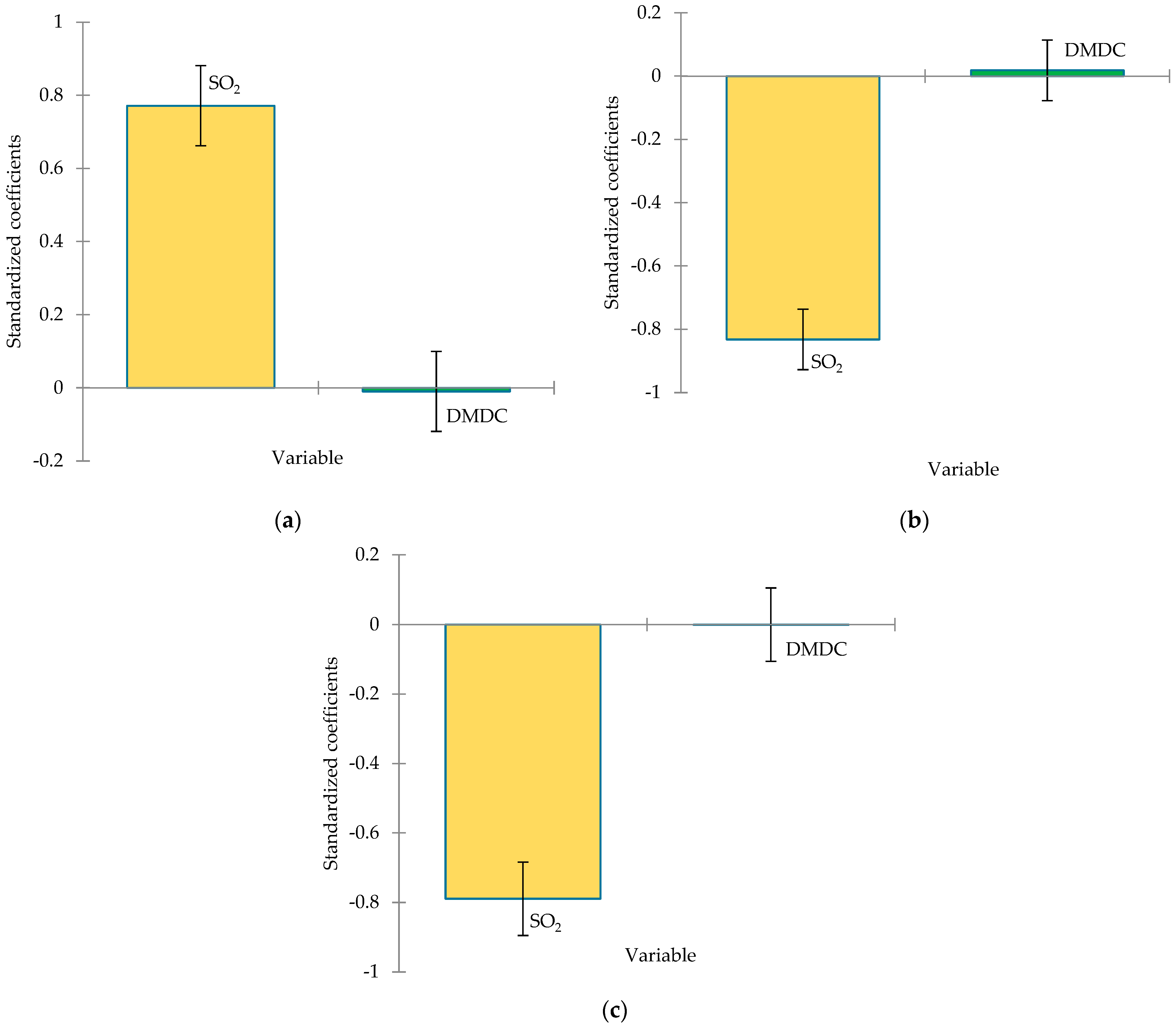

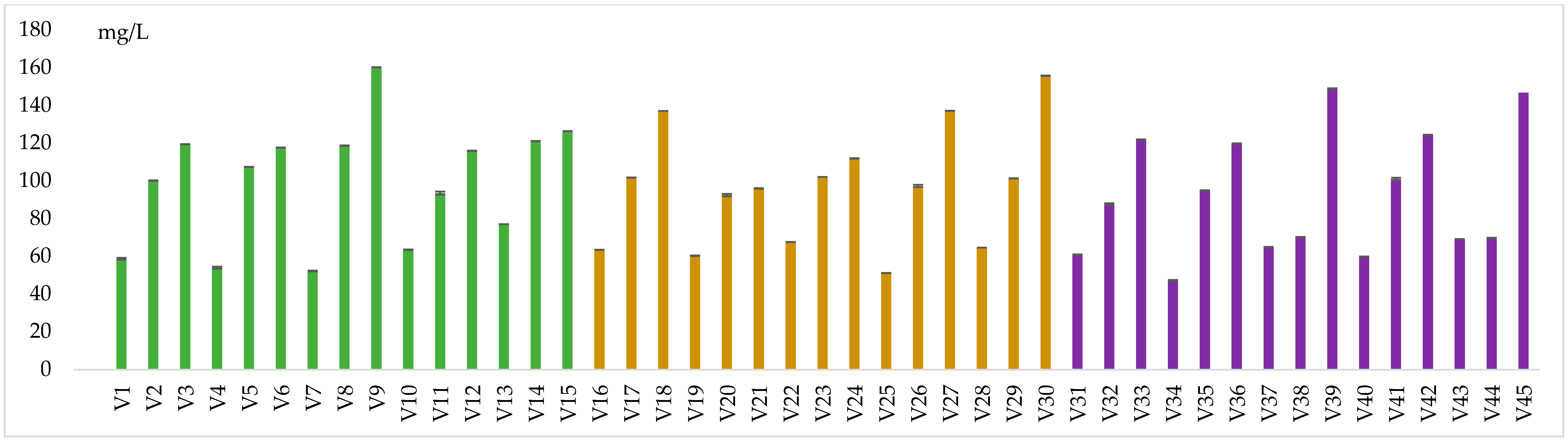
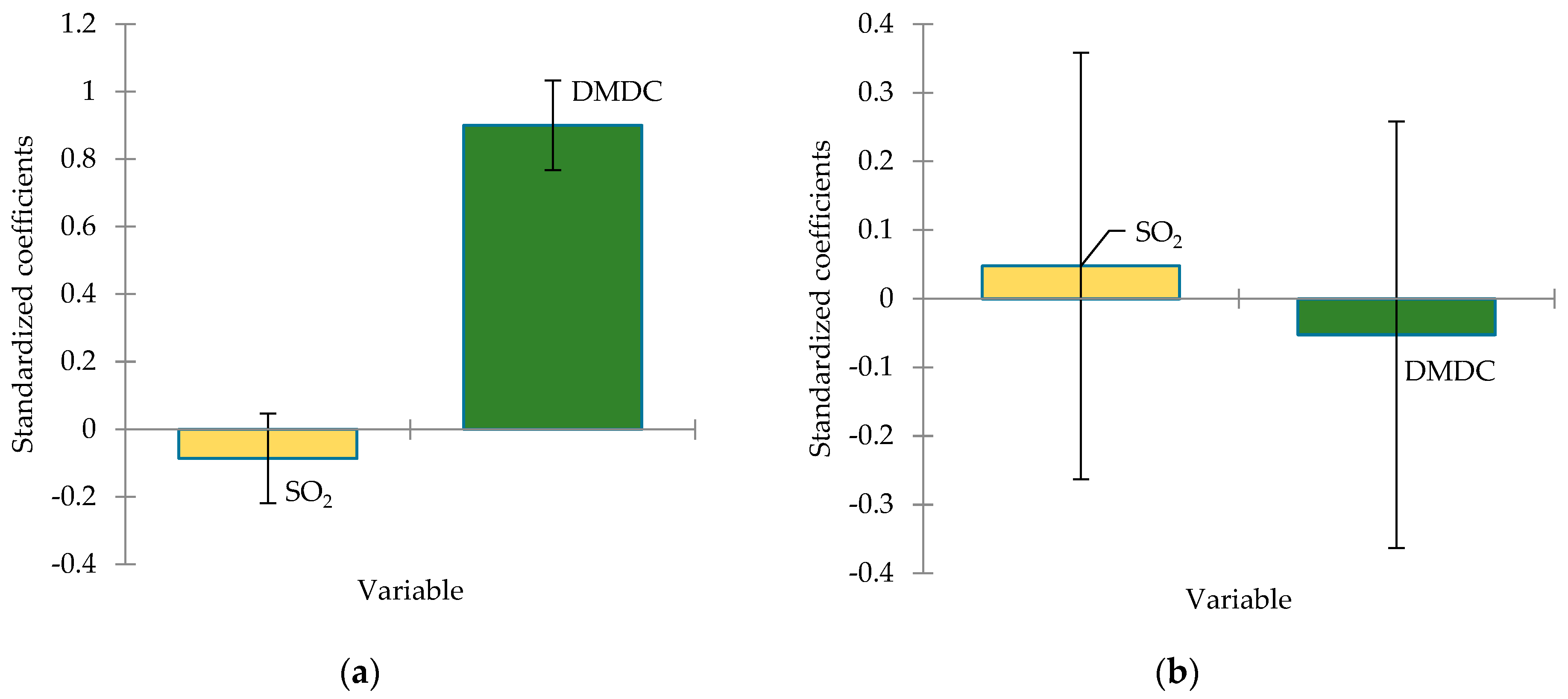
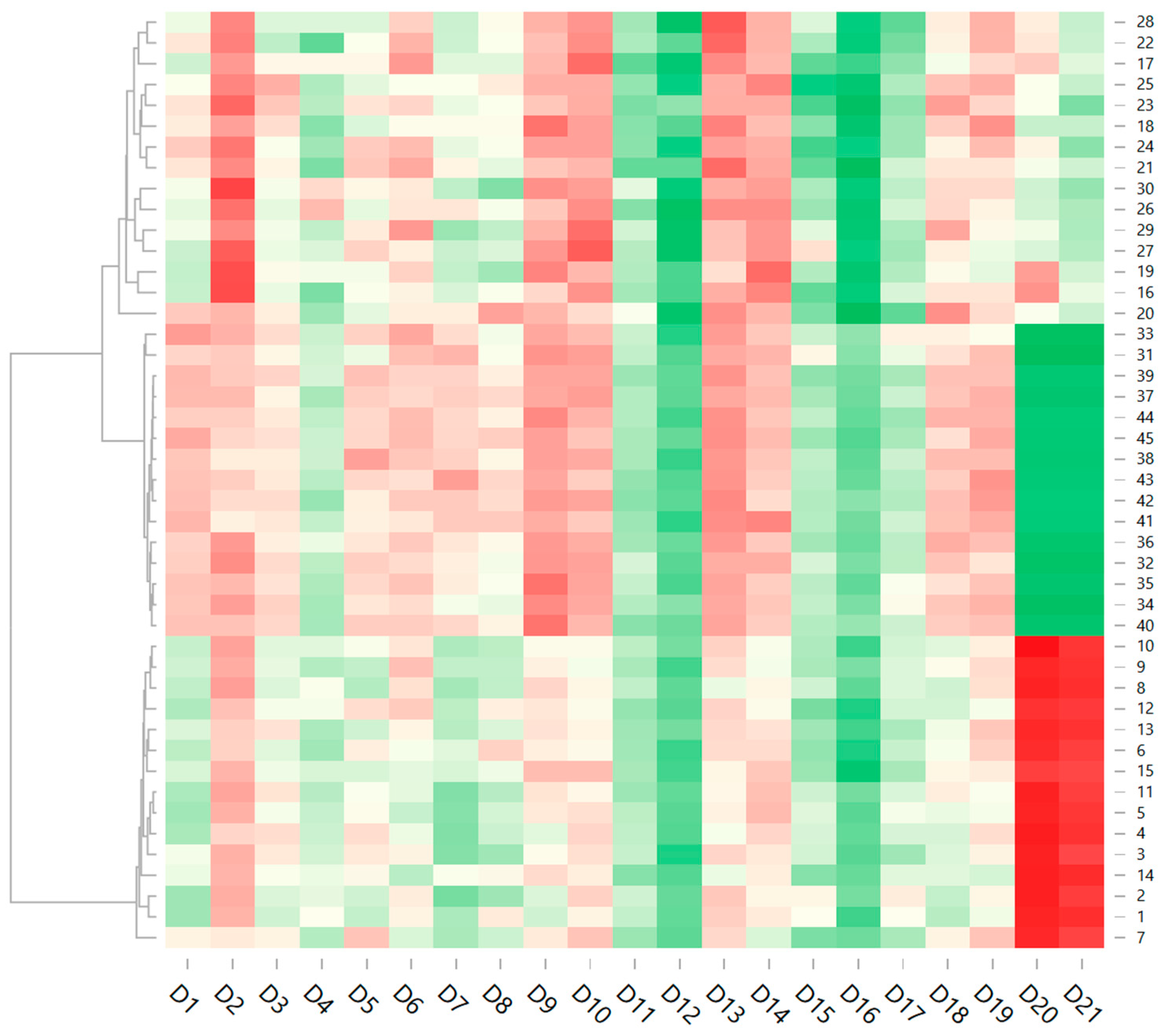
| Variant | Yeast | CFU/mL | SO2 mL | DMDC mg/L | Variant | Yeast | CFU/mL | SO2 mL | DMDC mg/L | Variant | Yeast | SO2 mL | CFU/mL | DMDC mg/L |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| V1 | Without yeast | 0 | 40 | 0 | V16 | Without yeast | 0 | 80 | 0 | V31 | Without yeast | 160 | 0 | 0 |
| V2 | 100 | V17 | 100 | V32 | 100 | |||||||||
| V3 | 200 | V18 | 200 | V33 | 200 | |||||||||
| V4 | Brettanomyces bruxellensis | 30 | 0 | V19 | Brettanomyces bruxellensis | 30 | 0 | V34 | Brettanomyces bruxellensis | 30 | 0 | |||
| V5 | 100 | V20 | 100 | V35 | 100 | |||||||||
| V6 | 200 | V21 | 200 | V36 | 200 | |||||||||
| V7 | 100 | 0 | V22 | 100 | 0 | V37 | 100 | 0 | ||||||
| V8 | 100 | V23 | 100 | V38 | 100 | |||||||||
| V9 | 200 | V24 | 200 | V39 | 200 | |||||||||
| V10 | Schizzossacharomyces pombe | 30 | 0 | V25 | Schizossacharomyces pombe | 30 | 0 | V40 | Schizossacharomyces pombe | 30 | 0 | |||
| V11 | 100 | V26 | 100 | V41 | 100 | |||||||||
| V12 | 200 | V27 | 200 | V42 | 200 | |||||||||
| V13 | 100 | 0 | V28 | 100 | 0 | V43 | 100 | 0 | ||||||
| V14 | 100 | V29 | 100 | V44 | 100 | |||||||||
| V15 | 200 | V30 | 200 | V45 | 200 |
| Variants | Alc. Strength % vol. alc. | Tot. Acidity g/L ac. Tartaric | Vol. Acidity g/L ac. Acetic | Res. Sugars g/L | Density | pH | Free SO2 mg/L | Tot. SO2 mg/L |
|---|---|---|---|---|---|---|---|---|
| 40 mg/L SO2 With/Without DMDC | ||||||||
| V1 | 14.20 ± 0.23 ab | 5.80 ± 0.04 a | 0.30 ± 0.13 a | 20.70 ± 0.19 ghi | 0.9971 ± 0.24 a | 3.42 ± 0.07 a | 10 ± 0.24 b | 40 ± 0.05 b |
| V2 | 14.30 ± 0.04 abc | 5.80 ± 0.16 a | 0.27 ± 0.23 a | 21.10 ± 0.13 ijk | 0.9969 ± 0.05 a | 3.40 ± 0.08 a | 10 ± 0.15 b | 40 ± 0.12 b |
| V3 | 14.30 ± 0.19 abc | 5.82 ± 0.13 a | 0.21 ± 0.19 a | 21.60 ± 0.07 lmno | 0.9972 ± 0.04 a | 3.26 ± 0.16 a | 11 ± 0.18 b | 40 ± 0.15 b |
| V4 | 14.60 ± 0.07 abcde | 6.20 ± 0.16 a | 0.19 ± 0.24 a | 22.00 ± 0.23 o | 0.9975 ± 0.13 a | 3.36 ± 0.04 a | 10 ± 0.11 b | 40 ± 0.18 b |
| V5 | 14.70 ± 0.03 bcdef | 5.90 ± 0.07 a | 0.21 ± 0.19 a | 21.00 ± 0.23 jk | 0.9972 ± 0.13 a | 3.41 ± 0.16 a | 10 ± 0.11 b | 40 ± 0.05 b |
| V6 | 14.80 ± 0.18 cdef | 6.10 ± 0.24 a | 0.18 ± 0.04 a | 20.50 ± 0.19 fgh | 0.9970 ± 0.23 a | 3.23 ± 0.13 a | 11 ± 0.15 b | 40 ± 0.19 b |
| V7 | 14.50 ± 0.24 abcd | 5.90 ± 0.04 a | 0.28 ± 0.07 a | 21.80 ± 0.13 no | 0.9970 ± 0.16 a | 3.41 ± 0.23 a | 10 ± 0.03 b | 39 ± 0.03 a |
| V8 | 14.30 ± 0.05 abc | 5.90 ± 0.04 a | 0.26 ± 0.24 a | 19.90 ± 0.16 cde | 0.9972 ± 0.05 a | 3.41 ± 0.01 a | 10 ± 0.03 a | 39 ± 0.11 a |
| V9 | 14.50 ± 0.12 abcd | 5.90 ± 0.04 a | 0.19 ± 0.23 a | 21.30 ± 0.24 klm | 0.9970 ± 0.13 a | 3.24 ± 0.16 a | 11 ± 0.11 b | 40 ± 0.05 b |
| V10 | 15.00 ± 0.23 def | 6.00 ± 0.13 a | 0.21 ± 0.19 a | 21.70 ± 0.01 mno | 0.9967 ± 0.16 a | 3.38 ± 0.24 a | 10 ± 0.19 b | 39 ± 0.13 a |
| V11 | 14.80 ± 0.08 cdef | 6.12 ± 0.16 a | 0.20 ± 0.13 a | 21.20 ± 0.19 jkl | 0.9972 ± 0.24 a | 3.25 ± 0.04 a | 11 ± 0.04 b | 40 ± 0.00 b |
| V12 | 14.80 ± 0.29 cdef | 6.43 ± 0.23 a | 0.16 ± 0.13 a | 19.20 ± 0.24 b | 0.9972 ± 0.08 a | 3.26 ± 0.04 a | 11 ± 0.22 b | 40 ± 0.05 b |
| V13 | 14.70 ± 0.16 bcdef | 5.90 ± 0.19 a | 0.28 ± 0.13 a | 20.30 ± 0.04 efg | 0.9967 ± 0.16 a | 3.37 ± 0.24 a | 10 ± 0.03 b | 39 ± 0.19 a |
| V14 | 14.90 ± 0.04 def | 6.12 ± 0.16 a | 0.18 ± 0.13 a | 21.70 ± 0.19 mno | 0.9970 ± 0.23 a | 3.21 ± 0.04 a | 11 ± 0.05 b | 39 ± 0.15 a |
| V15 | 14.80 ± 0.26 cdef | 6.30 ± 0.16 a | 0.18 ± 0.13 a | 21.10 ± 0.24 ijk | 0.9972 ± 0.04 a | 3.23 ± 0.23 a | 11 ± 0.11 b | 40 ± 0.19 b |
| p-value (V1–V15) | <0.001 * | 0.681 | 0.681 | <0.001 * | 1.000 | 0.550 | 0.122 | 1.000 |
| 80 mg/L SO2 With/Without DMDC | ||||||||
| V16 | 15.20 ± 0.02 f | 6.28 ± 0.09 a | 0.17 ± 0.01 a | 20.70 ± 0.19 ghi | 0.9969 ± 0.08 a | 3.25 ± 0.13 a | 31 ± 0.24 c | 79 ± 0.13 c |
| V17 | 15.20 ± 0.13 f | 6.12 ± 0.01 a | 0.18 ± 0.08 a | 21.30 ± 0.02 klm | 0.9968 ± 0.03 a | 3.21 ± 0.08 a | 31 ± 0.05 c | 79 ± 0.05 c |
| V18 | 15.10 ± 0.01 ef | 6.43 ± 0.02 a | 0.20 ± 0.01 a | 18.50 ± 0.19 a | 0.9971 ± 0.11 a | 3.26 ± 0.04 a | 31 ± 0.07 c | 79 ± 0.24 c |
| V19 | 15.10 ± 0.08 ef | 6.12 ± 0.19 a | 0.18 ± 0.07 a | 21.00 ± 0.02 ijk | 0.9971 ± 0.07 a | 3.21 ± 0.15 a | 31 ± 0.11 c | 79 ± 0.11 c |
| V20 | 15.10 ± 0.11 ef | 6.43 ± 0.08 a | 0.16 ± 0.07 a | 19.60 ± 0.01 bc | 0.9971 ± 0.02 a | 3.27 ± 0.19 a | 31 ± 0.03 c | 79 ± 0.08 c |
| V21 | 15.10 ± 0.21 ef | 6.12 ± 0.19 a | 0.20 ± 0.13 a | 21.40 ± 0.01 klmn | 0.9967 ± 0.03 a | 3.21 ± 0.08 a | 31 ± 0.19 c | 79 ± 0.02 c |
| V22 | 15.10 ± 0.22 ef | 6.12 ± 0.02 a | 0.18 ± 0.08 a | 21.60 ± 0.07 lmno | 0.9973 ± 0.01 a | 3.21 ± 0.08 a | 31 ± 0.13 c | 79 ± 0.15 c |
| V23 | 15.00 ± 0.26 def | 6.12 ± 0.02 a | 0.17 ± 0.03 a | 21.20 ± 0.08 jkl | 0.9970 ± 0.15 a | 3.20 ± 0.07 a | 31 ± 0.05 c | 79 ± 0.12 c |
| V24 | 15.00 ± 0.06 def | 6.28 ± 0.13 a | 0.18 ± 0.01 a | 21.90 ± 0.15 o | 0.9975 ± 0.02 a | 3.21 ± 0.03 a | 31 ± 0.09 c | 79 ± 0.03 c |
| V25 | 15.00 ± 0.11 def | 6.43 ± 0.08 a | 0.13 ± 0.02 a | 20.10 ± 0.04 def | 0.9971 ± 0.13 a | 3.27 ± 0.07 a | 31 ± 0.00 c | 79 ± 0.03 c |
| V26 | 15.00 ± 0.17 def | 6.12 ± 0.09 a | 0.17 ± 0.01 a | 20.80 ± 0.08 hij | 0.9968 ± 0.04 a | 3.22 ± 0.13 a | 31 ± 0.05 c | 79 ± 0.14 c |
| V27 | 15.00 ± 0.27 def | 6.12 ± 0.13 a | 0.13 ± 0.09 a | 19.70 ± 0.03 cd | 0.9965 ± 0.13 a | 3.22 ± 0.01 a | 31 ± 0.03 c | 79 ± 0.05 c |
| V28 | 14.90 ± 0.12 def | 6.12 ± 0.13 a | 0.18 ± 0.02 a | 21.30 ± 0.08 klm | 0.9972 ± 0.07 a | 3.22 ± 0.09 a | 31 ± 0.19 c | 79 ± 0.01 c |
| V29 | 14.90 ± 0.14 def | 6.28 ± 0.08 a | 0.17 ± 0.01 a | 20.70 ± 0.13 ghi | 0.9966 ± 0.04 a | 3.22 ± 0.09 a | 31 ± 0.15 c | 79 ± 0.02 c |
| V30 | 14.80 ± 0.21 cdef | 6.12 ± 0.13 a | 0.19 ± 0.01 a | 21.40 ± 0.19 klmn | 0.9968 ± 0.08 a | 3.21 ± 0.07 a | 31 ± 0.11 c | 79 ± 0.09 c |
| p-value (V16–V30) | 0.972 | 0.129 | 0.129 | <0.001 * | 1.000 | 0.999 | 1.000 | 0.972 |
| 160 mg/L SO2 With/Without DMDC | ||||||||
| V31 | 14.20 ± 0.13 ab | 6.20 ± 0.01 a | 0.27 ± 0.19 a | 21.30 ± 0.02 klm | 0.9953 ± 0.03 a | 3.19 ± 0.08 a | 95 ± 0.09 d | 159 ± 0.28 d |
| V32 | 14.30 ± 0.02 abc | 6.28 ± 0.01 a | 0.27 ± 0.08 a | 21.20 ± 0.09 jkl | 0.9950 ± 0.04 a | 3.19 ± 0.07 a | 95 ± 0.23 d | 159 ± 0.16 d |
| V33 | 14.20 ± 0.19 ab | 6.20 ± 0.19 a | 0.26 ± 0.03 a | 21.30 ± 0.08 klm | 0.9953 ± 0.13 a | 3.18 ± 0.15 a | 95 ± 0.01 d | 159 ± 0.24 d |
| V34 | 14.20 ± 0.07 ab | 6.40 ± 0.13 a | 0.25 ± 0.01 a | 21.30 ± 0.08 klm | 0.9953 ± 0.01 a | 3.18 ± 0.19 a | 95 ± 0.00 d | 159 ± 0.02 d |
| V35 | 14.20 ± 0.01 ab | 6.28 ± 0.09 a | 0.24 ± 0.08 a | 21.30 ± 0.09 klm | 0.9953 ± 0.07 a | 3.18 ± 0.13 a | 95 ± 0.28 d | 159 ± 0.28 d |
| V36 | 14.20 ± 0.03 ab | 6.43 ± 0.08 a | 0.24 ± 0.02 a | 21.30 ± 0.15 klm | 0.9953 ± 0.09 a | 3.22 ± 0.19 a | 95 ± 0.23 d | 159 ± 0.07 d |
| V37 | 14.10 ± 0.07 a | 6.30 ± 0.13 a | 0.24 ± 0.04 a | 21.40 ± 0.02 klmn | 0.9954 ± 0.09 a | 3.19 ± 0.08 a | 95 ± 0.09 d | 159 ± 0.16 d |
| V38 | 14.20 ± 0.21 ab | 6.20 ± 0.08 a | 0.18 ± 0.04 a | 21.30 ± 0.07 klm | 0.9953 ± 0.13 a | 3.18 ± 0.09 a | 95 ± 0.03 d | 159 ± 0.01 d |
| V39 | 14.20 ± 0.15 ab | 6.28 ± 0.13 a | 0.17 ± 0.09 a | 21.30 ± 0.15 klm | 0.9953 ± 0.08 a | 3.18 ± 0.07 a | 95 ± 0.24 d | 159 ± 0.28 d |
| V40 | 14.20 ± 0.02 ab | 6.28 ± 0.13 a | 0.17 ± 0.08 a | 21.30 ± 0.07 klm | 0.9953 ± 0.03 a | 3.20 ± 0.09 a | 95 ± 0.02 d | 159 ± 0.05 d |
| V41 | 14.10 ± 0.13 a | 6.28 ± 0.15 a | 0.16 ± 0.13 a | 21.40 ± 0.11 klmn | 0.9954 ± 0.19 a | 3.17 ± 0.08 a | 95 ± 0.07 d | 159 ± 0.09 d |
| V42 | 14.10 ± 0.21 a | 6.28 ± 0.07 a | 0.16 ± 0.13 a | 21.40 ± 0.15 klmn | 0.9954 ± 0.08 a | 3.17 ± 0.19 a | 95 ± 0.02 d | 159 ± 0.24 d |
| V43 | 14.10 ± 0.15 a | 6.12 ± 0.08 a | 0.16 ± 0.07 a | 21.40 ± 0.02 klmn | 0.9954 ± 0.04 a | 3.18 ± 0.13 a | 95 ± 0.03 d | 159 ± 0.33 d |
| V44 | 14.10 ± 0.13 a | 6.12 ± 0.04 a | 0.16 ± 0.13 a | 21.40 ± 0.07 klmn | 0.9954 ± 0.15 a | 3.18 ± 0.02 a | 95 ± 0.07 d | 159 ± 0.11 d |
| V45 | 14.10 ± 0.03 a | 6.12 ± 0.19 a | 0.16 ± 0.11 a | 21.40 ± 0.09 klmn | 0.9954 ± 0.01 a | 3.18 ± 0.19 a | 95 ± 0.16 d | 159 ± 0.00 d |
| p-value (V31–V45) | 0.722 | <0.001 * | 0.055 | 0.268 | 1.000 | 1.000 | 1.000 | 0.987 |
| p-value (V1–V45) | <0.001 * | <0.001 * | 0.006 * | <0.001 * | 1.000 | 0.367 | <0.001 * | 0.722 |
| Samples | Luminosity L 0–100 | Colorimetric Coordinates | |
|---|---|---|---|
| a Red (+)/Green (−) | b Yellow (+)/Blue (−) | ||
| 40 mg SO2 | |||
| V1 | 55.3 ± 0.03 a | 8.86 ± 0.19 a | 31.29 ± 0.01 zd |
| V2 | 58.5 ± 0.11 a | 8.52 ± 0.07 a | 31.81 ± 0.05 zf |
| V3 | 54.7 ± 0.13 a | 9.15 ± 0.19 a | 32.61 ± 0.09 zm |
| V4 | 63.5 ± 0.09 a | 8.44 ± 0.18 a | 32.40 ± 0.11 zj |
| V5 | 60.9 ± 0.20 a | 8.74 ± 0.90 a | 32.46 ± 0.01 zl |
| V6 | 64.2 ± 0.05 a | 8.38 ± 0.19 a | 31.98 ± 0.03 zh |
| V7 | 56.4 ± 0.16 a | 9.27 ± 0.09 a | 32.41 ± 0.11 zk |
| V8 | 54.8 ± 0.07 a | 10.09 ± 0.20 a | 33.34 ± 0.01 zo |
| V9 | 55.5 ± 0.18 a | 9.28 ± 0.03 a | 31.86 ± 0.07 zg |
| V10 | 59.8 ± 0.19 a | 9.00 ± 0.11 a | 32.82 ± 0.05 zn |
| V11 | 67.4 ± 0.01 a | 7.95 ± 0.90 a | 32.15 ± 0.18 zi |
| V12 | 60.2 ± 0.20 a | 9.33 ± 0.19 a | 30.65 ± 0.01 zb |
| V13 | 65.9 ± 0.22 a | 7.47 ± 0.03 a | 31.13 ± 0.07 zc |
| V14 | 61.9 ± 0.11 a | 8.91 ± 0.20 a | 31.38 ± 0.18 ze |
| V15 | 60.2 ± 0.03 a | 8.93 ± 0.19 a | 32.15 ± 0.09 zi |
| p-value (V1–V15) | 0.000 * | 0.000 * | 0.000 * |
| 80 mg SO2 | |||
| V16 | 96.6 ± 0.25 b | 1.17 ± 0.03 a | 7.39 ± 0.18 w |
| V17 | 96.8 ± 0.09 b | 1.08 ± 0.05 a | 7.31 ± 0.20 u |
| V18 | 96.7 ± 0.11 b | 1.12 ± 0.19 a | 7.29 ± 0.07 s |
| V19 | 96.5 ± 0.07 b | 1.29 ± 0.01 a | 7.48 ± 0.09 za |
| V20 | 96.7 ± 0.03 b | 1.09 ± 0.05 a | 7.37 ± 0.18 v |
| V21 | 96.7 ± 0.22 b | 1.13 ± 0.09 a | 7.29 ± 0.20 s |
| V22 | 97.1 ± 0.16 b | 0.65 ± 0.11 a | 7.12 ± 0.07 n |
| V23 | 96.9 ± 0.19 b | 0.88 ± 0.20 a | 7.20 ± 0.01 p |
| V24 | 96.8 ± 0.05 b | 0.99 ± 0.03 a | 7.25 ± 0.18 q |
| V25 | 96.9 ± 0.01 b | 0.96 ± 0.07 a | 7.17 ± 0.11 o |
| V26 | 96.7 ± 0.18 b | 1.15 ± 0.09 a | 7.41 ± 0.19 y |
| V27 | 96.7 ± 0.11 b | 1.15 ± 0.05 a | 7.40 ± 0.16 x |
| V28 | 96.4 ± 0.09 b | 1.00 ± 0.03 a | 7.26 ± 0.18 r |
| V29 | 96.8 ± 0.20 b | 0.97 ± 0.11 a | 7.30 ± 0.01 t |
| V30 | 96.7 ± 0.03 b | 0.98 ± 0.07 a | 7.37 ± 0.16 v |
| p-value (V16–V30) | 0.000 * | 0.000 * | 0.000 * |
| 160 mg SO2 | |||
| V31 | 98.2 ± 0.11 b | −0.25 ± 0.20 a | 5.67 ± 0.01 g |
| V32 | 98.2 ± 0.19 b | −0.25 ± 0.18 a | 5.64 ± 0.16 f |
| V33 | 98.2 ± 0.01 b | −0.24 ± 0.09 a | 5.67 ± 0.03 g |
| V34 | 98.2 ± 0.20 b | −0.36 ± 0.11 a | 5.79 ± 0.13 h |
| V35 | 98.2 ± 0.07 b | −0.34 ± 0.19 a | 5.80 ± 0.01 i |
| V36 | 98.1 ± 0.09 b | −0.28 ± 0.18 a | 5.85 ± 0.22 j |
| V37 | 98.4 ± 0.20 b | −0.40 ± 0.09 a | 5.52 ± 0.03 b |
| V38 | 98.4 ± 0.19 b | −0.38 ± 0.05 a | 5.56 ± 0.01 d |
| V39 | 98.3 ± 0.03 b | −0.22 ± 0.11 a | 5.62 ± 0.16 e |
| V40 | 98.4 ± 0.20 b | −0.31 ± 0.07 a | 5.54 ± 0.09 c |
| V41 | 98.2 ± 0.01 b | −0.29 ± 0.05 a | 5.61 ± 0.01 a |
| V42 | 98.3 ± 0.09 b | −0.31 ± 0.03 a | 5.51 ± 0.18 k |
| V43 | 98.2 ± 0.18 b | −0.22 ± 0.07 a | 5.92 ± 0.01 m |
| V44 | 98.1 ± 0.19 b | −0.17 ± 0.09 a | 5.97 ± 0.20 l |
| V45 | 98.1 ± 0.11 b | −0.16 ± 0.16 a | 5.96 ± 0.05 l |
| p-value (V31–V45) | 0.000 * | 0.000 * | 0.000 * |
| p-value (V1–V45) | 0.000 * | 0.000 * | 0.000 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buțerchi, I.; Colibaba, L.C.; Luchian, C.E.; Lipșa, F.D.; Ulea, E.; Zamfir, C.I.; Scutarașu, E.C.; Nechita, C.B.; Irimia, L.M.; Cotea, V.V. Impact of Sulfur Dioxide and Dimethyl Dicarbonate Treatment on the Quality of White Wines: A Scientific Evaluation. Fermentation 2025, 11, 86. https://doi.org/10.3390/fermentation11020086
Buțerchi I, Colibaba LC, Luchian CE, Lipșa FD, Ulea E, Zamfir CI, Scutarașu EC, Nechita CB, Irimia LM, Cotea VV. Impact of Sulfur Dioxide and Dimethyl Dicarbonate Treatment on the Quality of White Wines: A Scientific Evaluation. Fermentation. 2025; 11(2):86. https://doi.org/10.3390/fermentation11020086
Chicago/Turabian StyleBuțerchi, Ioana, Lucia Cintia Colibaba, Camelia Elena Luchian, Florin Daniel Lipșa, Eugen Ulea, Cătălin Ioan Zamfir, Elena Cristina Scutarașu, Constantin Bogdan Nechita, Liviu Mihai Irimia, and Valeriu V. Cotea. 2025. "Impact of Sulfur Dioxide and Dimethyl Dicarbonate Treatment on the Quality of White Wines: A Scientific Evaluation" Fermentation 11, no. 2: 86. https://doi.org/10.3390/fermentation11020086
APA StyleBuțerchi, I., Colibaba, L. C., Luchian, C. E., Lipșa, F. D., Ulea, E., Zamfir, C. I., Scutarașu, E. C., Nechita, C. B., Irimia, L. M., & Cotea, V. V. (2025). Impact of Sulfur Dioxide and Dimethyl Dicarbonate Treatment on the Quality of White Wines: A Scientific Evaluation. Fermentation, 11(2), 86. https://doi.org/10.3390/fermentation11020086










