Evaluation of Carboxymethyl Cellulose as an Additive for Selective Protein Removal from Wine
Abstract
1. Introduction
2. Materials and Methods
2.1. Formulation Pre-Trials in Model System and Wine
2.2. Application Study in White and Rose Wines
2.3. Data Analysis
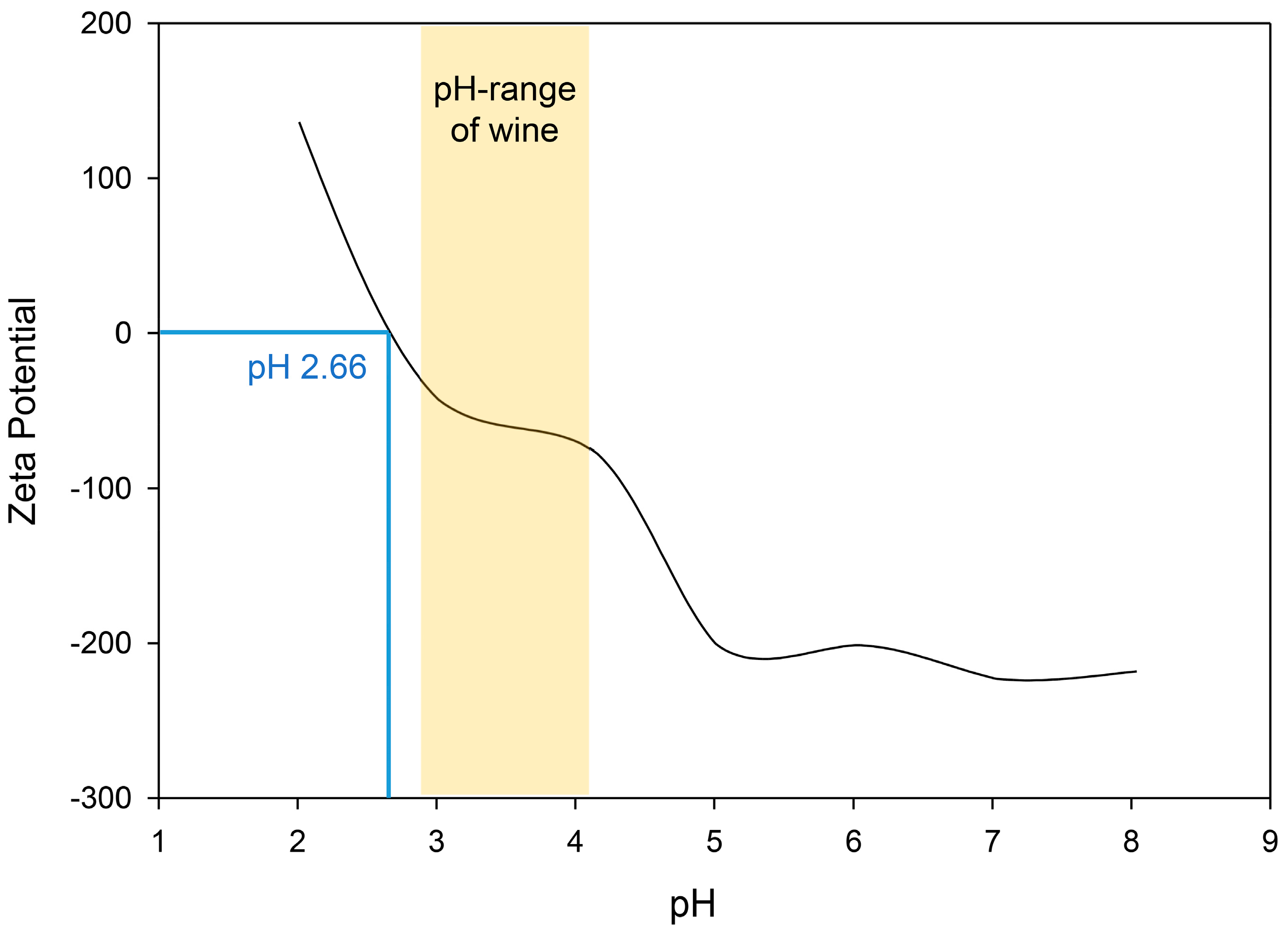
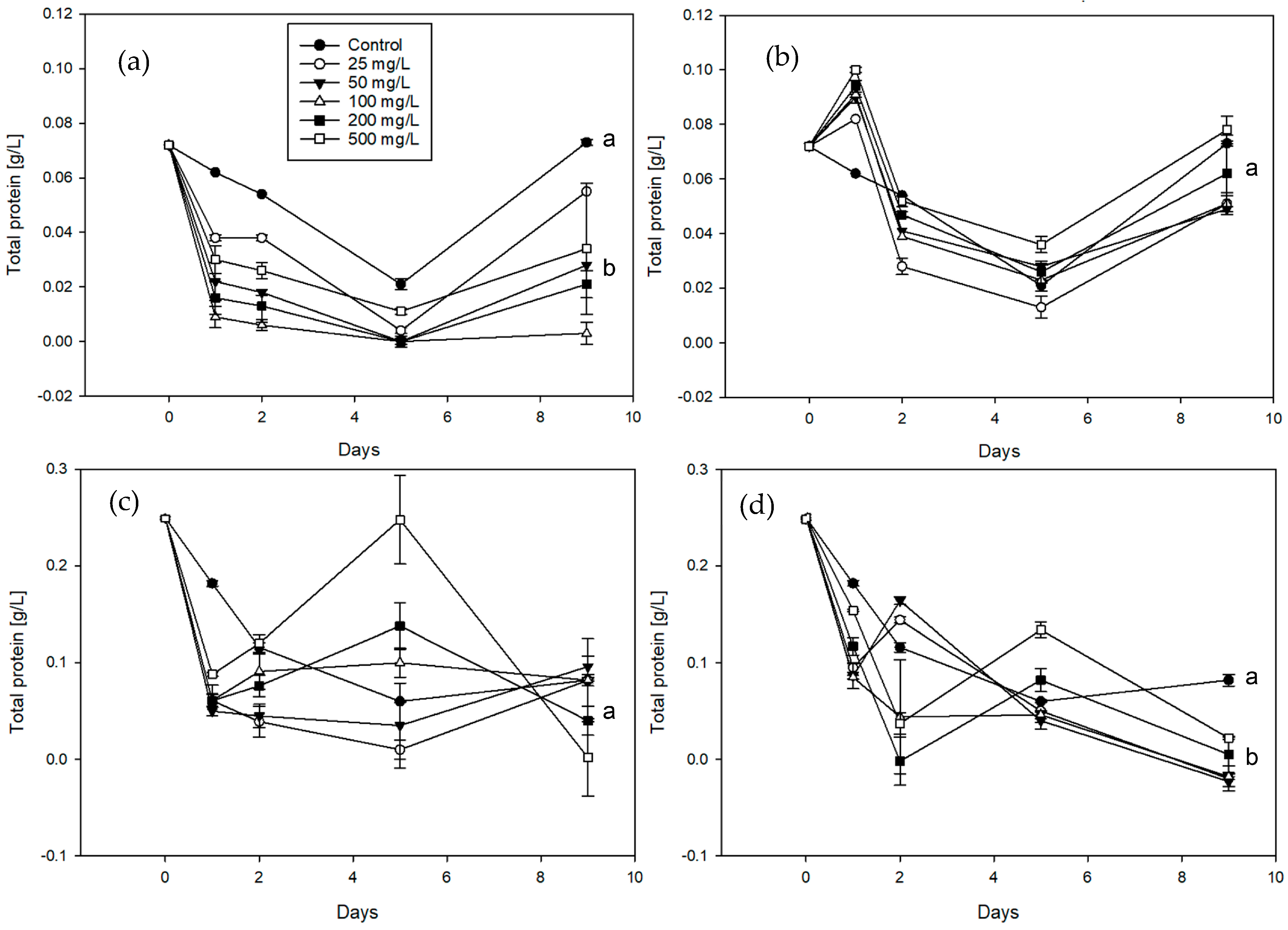
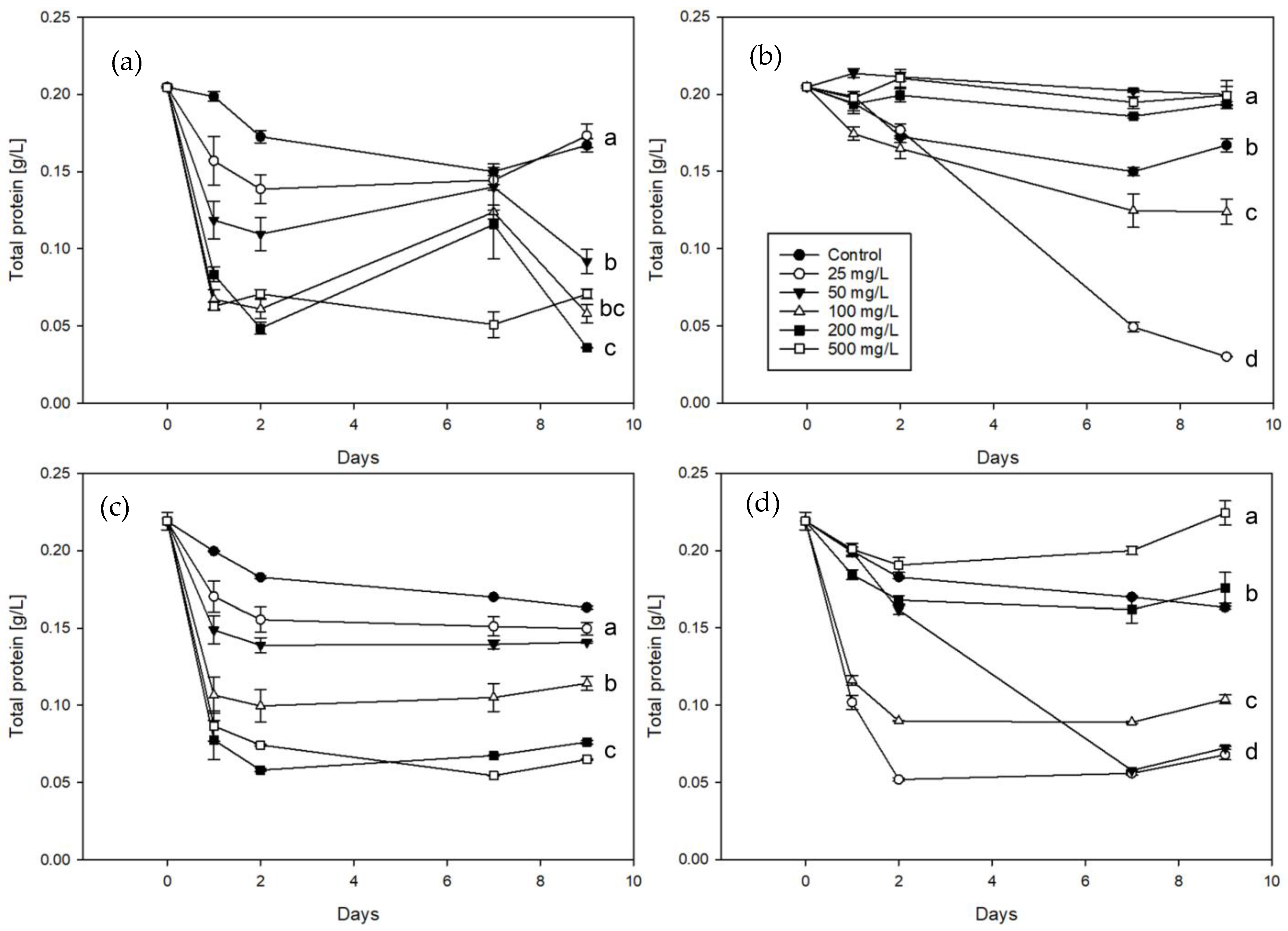
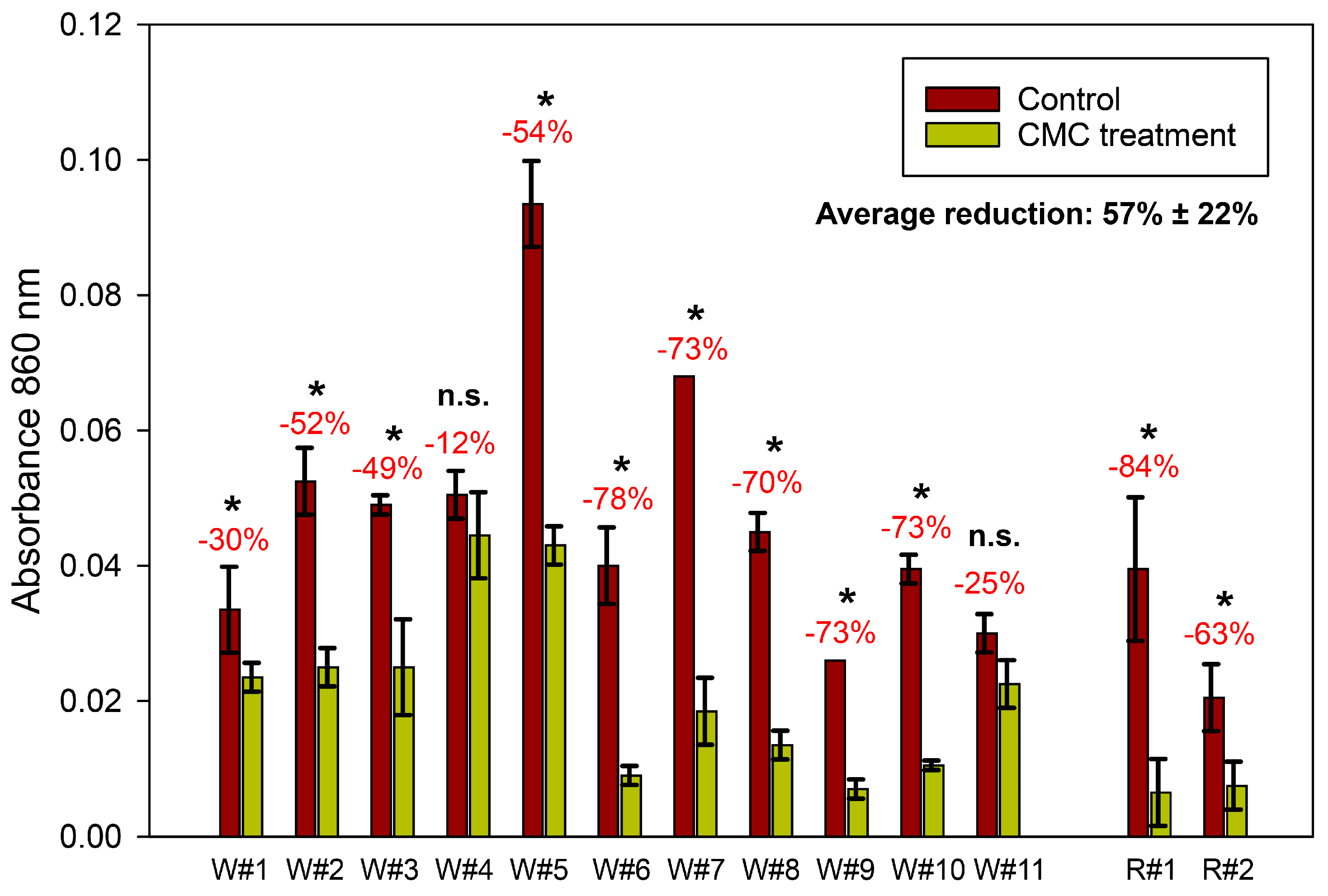
3. Results
3.1. Application Pre-Trials
3.2. Application in Commercial Wines
4. Discussion
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sommer, S.; Wegmann-Herr, P.; Fischer, U. Correlating the Demand for Bentonite in Wine with Ambient Data and Weather Anomalies. J. Wine Res. 2015, 26, 29–39. [Google Scholar] [CrossRef]
- Pasquier, G.; Feilhes, C.; Dufourcq, T.; Geffroy, O. Potential Contribution of Climate Change to the Protein Haze of White Wines from the French Southwest Region. Foods 2021, 10, 1355. [Google Scholar] [CrossRef] [PubMed]
- Norton, E.L.; Sacks, G.L.; Talbert, J.N. Nonlinear Behavior of Protein and Tannin in Wine Produced by Cofermentation of an Interspecific Hybrid (Vitis spp.) and vinifera Cultivar. Am. J. Enol. Vitic. 2020, 71, 26–32. [Google Scholar] [CrossRef]
- Mesquita, P.R.; Picarra-Pereira, M.A.; Monteiro, S.; Loureiro, V.B.; Teixeira, A.R.; Ferreira, R.B. Effect of wine composition on protein stability. Am. J. Enol. Vitic. 2001, 52, 324–330. [Google Scholar] [CrossRef]
- Ferreira, R.B.; Piçarra-Pereira, M.A.; Monteiro, S.; Loureiro, V.l.B.; Teixeira, A.R. The wine proteins. Trends Food Sci. Technol. 2001, 12, 230–239. [Google Scholar] [CrossRef]
- Bayly, F.C.; Berg, H.W. Grape and Wine Proteins of White Wine Varietals. Am. J. Enol. Vitic. 1967, 18, 18–32. [Google Scholar] [CrossRef]
- Hsu, J.C.; Heatherbell, D. Isolation and Characterization of Soluble Proteins in Grapes, Grape Juice, and Wine. Am. J. Enol. Vitic. 1987, 38, 6–10. [Google Scholar] [CrossRef]
- Waters, E.; Wallace, W.; Williams, P.J. Heat haze characteristics of fractionated wine proteins. Am. J. Enol. Vitic. 1991, 42, 123–127. [Google Scholar] [CrossRef]
- Waters, E.; Wallace, W.; Williams, P.J. Identification of heat-unstable wine proteins and their resistance to peptidases. J. Agric. Food Chem. 1992, 40, 1514–1519. [Google Scholar] [CrossRef]
- Marangon, M.; Van Sluyter, S.C.; Neilson, K.A.; Chan, C.; Haynes, P.A.; Waters, E.J.; Falconer, R.J. Roles of Grape Thaumatin-like Protein and Chitinase in White Wine Haze Formation. J. Agric. Food Chem. 2011, 59, 733–740. [Google Scholar] [CrossRef]
- Sommer, S.; Sommer, S.J.; Gutierrez, M. Characterization of Different Bentonites and Their Properties as a Protein-Fining Agent in Wine. Beverages 2022, 8, 31. [Google Scholar] [CrossRef]
- Blade, W.H.; Boulton, R. Adsorption of protein by bentonite in a model wine solution. Am. J. Enol. Vitic. 1988, 39, 193–199. [Google Scholar] [CrossRef]
- Maxim, L.D.; Niebo, R.; McConnell, E.E. Bentonite toxicology and epidemiology—A review. Inhal. Toxicol. 2016, 28, 591–617. [Google Scholar] [CrossRef] [PubMed]
- Jaeckels, N.; Tenzer, S.; Meier, M.; Will, F.; Dietrich, H.; Decker, H.; Fronk, P. Influence of bentonite fining on protein composition in wine. LWT 2017, 75, 335–343. [Google Scholar] [CrossRef]
- Vincenzi, S.; Panighel, A.; Gazzola, D.; Flamini, R.; Curioni, A. Study of combined effect of proteins and bentonite fining on the wine aroma loss. J. Agric. Food Chem. 2015, 63, 2314–2320. [Google Scholar] [CrossRef]
- Lambri, M.; Dordoni, R.; Silva, A.; De Faveri, D.M. Effect of bentonite fining on odor-active compounds in two different white wine styles. Am. J. Enol. Vitic. 2010, 61, 225–233. [Google Scholar] [CrossRef]
- Sommer, S.; Tondini, F. Sustainable Replacement Strategies for Bentonite in Wine Using Alternative Protein Fining Agents. Sustainability 2021, 13, 1860. [Google Scholar] [CrossRef]
- Sommer, S.; Dickescheid, C.; Harbertson, J.F.; Fischer, U.; Cohen, S.D. Rationale for haze formation after carboxymethyl cellulose (CMC) addition to red wine. J. Agric. Food Chem. 2016, 64, 6879–6887. [Google Scholar] [CrossRef]
- Sommer, S.; Weber, F.; Harbertson, J.F. Polyphenol-Protein-Polysaccharide Interactions in the Presence of Carboxymethyl Cellulose (CMC) in Wine-like Model Systems. J. Agric. Food Chem. 2019, 67, 7428–7434. [Google Scholar] [CrossRef]
- Claus, H.; Tenzer, S.; Sobe, M.; Schlander, M.; König, H.; Fröhlich, J. Effect of carboxymethyl cellulose on tartrate salt, protein and colour stability of red wine. Aust. J. Grape Wine Res. 2014, 20, 186–193. [Google Scholar] [CrossRef]
- Rahman, M.S.; Hasan, M.S.; Nitai, A.S.; Nam, S.; Karmakar, A.K.; Ahsan, M.S.; Shiddiky, M.J.; Ahmed, M.B. Recent developments of carboxymethyl cellulose. Polymers 2021, 13, 1345. [Google Scholar] [CrossRef] [PubMed]
- Saracino, F.; Brinco, J.; Gago, D.; Gomes da Silva, M.; Boavida Ferreira, R.; Ricardo-da-Silva, J.; Chagas, R.; Ferreira, L.M. DCMC as a Promising Alternative to Bentonite in White Wine Stabilization. Impact on Protein Stability and Wine Aromatic Fraction. Molecules 2021, 26, 6188. [Google Scholar] [CrossRef] [PubMed]
- Lankhorst, P.P.; Voogt, B.; Tuinier, R.; Lefol, B.; Pellerin, P.; Virone, C. Prevention of tartrate crystallization in wine by hydrocolloids: The mechanism studied by dynamic light scattering. J. Agric. Food Chem. 2017, 65, 8923–8929. [Google Scholar] [CrossRef]
- Burken, O.; Sommer, S. Polysaccharide Functionality in Wine-like Model Systems with Oat and Egg White Model Proteins. Fermentation 2024, 10, 573. [Google Scholar] [CrossRef]
- Kallithraka, S.; Bakker, J.; Clifford, M. Evaluation of bitterness and astringency of (+)-catechin and (-)-epicatechin in red wine and in model solution. J. Sens. Stud. 1997, 12, 25–37. [Google Scholar] [CrossRef]
- Dufrechou, M.; Poncet-Legrand, C.; Sauvage, F.-X.; Vernhet, A. Stability of White Wine Proteins: Combined Effect of pH, Ionic Strength, and Temperature on Their Aggregation. J. Agric. Food Chem. 2012, 60, 1308–1319. [Google Scholar] [CrossRef]
- Marangon, M.; Vincenzi, S.; Lucchetta, M.; Curioni, A. Heating and reduction affect the reaction with tannins of wine protein fractions differing in hydrophobicity. Anal. Chim. Acta 2010, 660, 110–118. [Google Scholar] [CrossRef]
- Watrelot, A.A.; Schulz, D.L.; Kennedy, J.A. Wine polysaccharides influence tannin-protein interactions. Food Hydrocoll. 2017, 63, 571–579. [Google Scholar] [CrossRef]
- Larsson, M.; Hill, A.; Duffy, J. Suspension stability; why particle size, zeta potential and rheology are important. Annu. Trans. Nord. Rheol. Soc. 2012, 20, 209–214. [Google Scholar]
- Burken, O.; Sommer, S. Evaluation of protein–polysaccharide interactions through ζ-potential and particle size measurements to assess their functionality in wine. J. Food Sci. 2024, 89, 6413–6424. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Lopez, C.G.; Colby, R.H.; Cabral, J.T. Electrostatic and Hydrophobic Interactions in NaCMC Aqueous Solutions: Effect of Degree of Substitution. Macromolecules 2018, 51, 3165–3175. [Google Scholar] [CrossRef]
- Harbertson, J.F.; Mireles, M.; Yu, Y. Improvement of BSA tannin precipitation assay by reformulation of resuspension buffer. Am. J. Enol. Vitic. 2015, 66, 95–99. [Google Scholar] [CrossRef]
- Kongraksawech, T.; Vazquez-Landaverde, P.; Huerta-Ruelas, J.; Torres, J.A. Ionic Strength and pH Effects on Optical Thermographs for Bovine Serum Albumin (BSA). Cienc. Y Tecnol. Aliment. 2007, 5, 259–264. [Google Scholar] [CrossRef][Green Version]
- Saha, D.; Bhattacharya, S. Hydrocolloids as thickening and gelling agents in food: A critical review. J. Food Sci. Technol. 2010, 47, 587–597. [Google Scholar] [CrossRef]
- Keller, J.D. Sodium carboxymethylcellulose (CMC). In Food Hydrocolloids; CRC Press: Boca Raton, FL, USA, 2020; pp. 43–109. [Google Scholar]
- Mehrabi, A.; Jalise, S.Z.; Hivechi, A.; Habibi, S.; Kebria, M.M.; Haramshahi, M.A.; Latifi, N.; Karimi, A.; Milan, P.B. Evaluation of inherent properties of the carboxymethyl cellulose (CMC) for potential application in tissue engineering focusing on bone regeneration. Polym. Adv. Technol. 2024, 35, e6258. [Google Scholar] [CrossRef]
- Bosso, A.; Salmaso, D.; De Faveri, E.; Guaita, M.; Franceschi, D. The use of carboxymethylcellulose for the tartaric stabilization of white wines, in comparison with other oenological additives. Vitis 2010, 49, 95–99. [Google Scholar]
- Guise, R.; Filipe-Ribeiro, L.; Nascimento, D.; Bessa, O.; Nunes, F.; Cosme, F. Comparison between different types of carboxylmethylcellulose and other oenological additives used for white wine tartaric stabilization. Food Chem. 2014, 156, 250–257. [Google Scholar] [CrossRef]
- Bosso, A.; Panero, L.; Petrozziello, M.; Sollazzo, M.; Asproudi, A.; Motta, S.; Guaita, M. Use of polyaspartate as inhibitor of tartaric precipitations in wines. Food Chem. 2015, 185, 1–6. [Google Scholar] [CrossRef]
- Cui, W.; Wang, X.; Han, S.; Guo, W.; Meng, N.; Li, J.; Sun, B.; Zhang, X. Research progress of tartaric acid stabilization on wine characteristics. Food Chem. X 2024, 23, 101728. [Google Scholar] [CrossRef]
- Lasanta, C.; Gómez, J. Tartrate stabilization of wines. Trends Food Sci. Technol. 2012, 28, 52–59. [Google Scholar] [CrossRef]
- Pittari, E.; Catarino, S.; Andrade, M.C.; Ricardo-da-Silva, J.M. Preliminary results on tartaric stabilization of red wine by adding different carboxymethylcelluloses. Ciência E Técnica Vitivinícola 2018, 33, 47–57. [Google Scholar] [CrossRef]
- Lali, A.; Balan, S.; John, R.; D’Souza, F. Carboxymethyl cellulose as a new heterobifunctional ligand carrier for affinity precipitation of proteins. Bioseparation 1998, 7, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.; Zadow, J.G. Recovery of whey proteins from precipitated complexes of carboxymethyl cellulose and protein. J. Dairy Res. 1978, 45, 77–83. [Google Scholar] [CrossRef]
- Benchabane, A.; Bekkour, K. Rheological properties of carboxymethyl cellulose (CMC) solutions. Colloid Polym. Sci. 2008, 286, 1173–1180. [Google Scholar] [CrossRef]
- Lopez, C.G.; Richtering, W. Oscillatory rheology of carboxymethyl cellulose gels: Influence of concentration and pH. Carbohydr. Polym. 2021, 267, 118117. [Google Scholar] [CrossRef]
- Burchard, W. Solubility and Solution Structure of Cellulose Derivatives. Cellulose 2003, 10, 213–225. [Google Scholar] [CrossRef]
- Jaeckels, N.; Meier, M.; Dietrich, H.; Will, F.; Decker, H.; Fronk, P. Influence of polysaccharides on wine protein aggregation. Food Chem. 2016, 200, 38–45. [Google Scholar] [CrossRef]
- Jones-Moore, H.R.; Jelley, R.E.; Marangon, M.; Fedrizzi, B. The interactions of wine polysaccharides with aroma compounds, tannins, and proteins, and their importance to winemaking. Food Hydrocoll. 2022, 123, 107150. [Google Scholar] [CrossRef]
- Weber, F. Noncovalent polyphenol–macromolecule interactions and their effects on the sensory properties of foods. J. Agric. Food Chem. 2021, 70, 72–78. [Google Scholar] [CrossRef]
- Gonçalves, F.; Heyraud, A.; de Pinho, M.N.; Rinaudo, M. Characterization of white wine mannoproteins. J. Agric. Food Chem. 2002, 50, 6097–6101. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.; Ricardo-da-Silva, J.M.; Lucas, C.; Laureano, O. Effect of commercial mannoproteins on wine colour and tannins stability. Food Chem. 2012, 131, 907–914. [Google Scholar] [CrossRef]
- Wang, S.; Ma, Z.; Zhao, P.; Du, G.; Sun, X.; Wang, X. The role of Arabic gum on astringency by modulating the polyphenol fraction-protein reaction in model wine. Food Chem. 2023, 417, 135927. [Google Scholar] [CrossRef] [PubMed]
- Ridge, M.; Sommer, S.; Dycus, D.A. Addressing Enzymatic Clarification Challenges of Muscat Grape Juice. Fermentation 2021, 7, 198. [Google Scholar] [CrossRef]
- Gago, D.; Chagas, R.; Ferreira, L.M. The Effect of Dicarboxymethyl Cellulose on the Prevention of Protein Haze Formation on White Wine. Beverages 2021, 7, 57. [Google Scholar] [CrossRef]
| Matrix | Protein | CMC Formulation | CMC Addition |
|---|---|---|---|
| Model wine | Bovine serum albumin | liquid | 0, 25, 50, 100, 200, 500 ppm |
| solid | 0, 25, 50, 100, 200, 500 ppm | ||
| Egg white protein | liquid | 0, 25, 50, 100, 200, 500 ppm | |
| solid | 0, 25, 50, 100, 200, 500 ppm | ||
| White wine | Egg white protein | liquid | 0, 25, 50, 100, 200, 500 ppm |
| solid | 0, 25, 50, 100, 200, 500 ppm | ||
| Rose wine | Egg white protein | liquid | 0, 25, 50, 100, 200, 500 ppm |
| solid | 0, 25, 50, 100, 200, 500 ppm |
| ID | Wine Description | Alcohol [%] | Titratable Acidity [g/L] | pH | Tartaric Acid [g/L] | Malic Acid [g/L] | Lactic Acid [g/L] | Glycerol [g/L] |
|---|---|---|---|---|---|---|---|---|
| W#1 | 2021 White Blend | 12.9 | 6.6 | 3.43 | 2.5 | 3.3 | <0.2 | 4.5 |
| W#2 | 2022 Chardonel | 12.1 | 5.8 | 3.71 | 1.9 | 3.2 | 0.2 | 6.2 |
| W#3 | 2020 Chardonel | 12.8 | 5.9 | 3.54 | 1.5 | 0.4 | 2.4 | 5.6 |
| W#4 | 2024 White Blend | 14.8 | 6.3 | 3.53 | 2.8 | 1.8 | <0.2 | 8.3 |
| W#5 | 2022 Traminette | 10.2 | 6.4 | 3.98 | 1.6 | 0.3 | 5.2 | 5.7 |
| W#6 | 2023 Vidal Blanc | 13.5 | 5.9 | 3.39 | 1.9 | 2.2 | <0.2 | 7.7 |
| W#7 | 2021 White Blend | 12.1 | 6.5 | 3.41 | 1.6 | 3.0 | 0.2 | 7.7 |
| W#8 | 2023 White Blend | 11.0 | 8.4 | 3.24 | 3.4 | 2.8 | 0.8 | 7.4 |
| W#9 | 2023 Vignoles | 12.0 | 9.8 | 3.62 | 1.7 | 6.4 | <0.2 | 6.3 |
| W#10 | 2022 Vignoles | 11.7 | 8.8 | 3.47 | 1.7 | 5.5 | <0.2 | 6.2 |
| W#11 | 2023 Vidal Blanc | 11.1 | 6.7 | 3.25 | 2.9 | 2.6 | <0.2 | 4.2 |
| R#1 | 2023 Rose Blend | 10.9 | 6.3 | 3.26 | 3.3 | 0.5 | 1.1 | 7.3 |
| R#2 | 2024 Concord Rose | 11.2 | 6.6 | 3.29 | 3.5 | 1.7 | <0.2 | 4.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sommer, S. Evaluation of Carboxymethyl Cellulose as an Additive for Selective Protein Removal from Wine. Fermentation 2025, 11, 273. https://doi.org/10.3390/fermentation11050273
Sommer S. Evaluation of Carboxymethyl Cellulose as an Additive for Selective Protein Removal from Wine. Fermentation. 2025; 11(5):273. https://doi.org/10.3390/fermentation11050273
Chicago/Turabian StyleSommer, Stephan. 2025. "Evaluation of Carboxymethyl Cellulose as an Additive for Selective Protein Removal from Wine" Fermentation 11, no. 5: 273. https://doi.org/10.3390/fermentation11050273
APA StyleSommer, S. (2025). Evaluation of Carboxymethyl Cellulose as an Additive for Selective Protein Removal from Wine. Fermentation, 11(5), 273. https://doi.org/10.3390/fermentation11050273






