Effect of Co-Digestion Ratios and Temperature on Biomethane Production in Anaerobic Co-Digestion of Cheese Whey and Tomato Waste
Abstract
1. Introduction
2. Materials and Methods
2.1. Substrate and Inoculum
2.2. Analytical Methods
2.3. Experimental Program
2.4. Kinetic Modeling
3. Results and Discussion
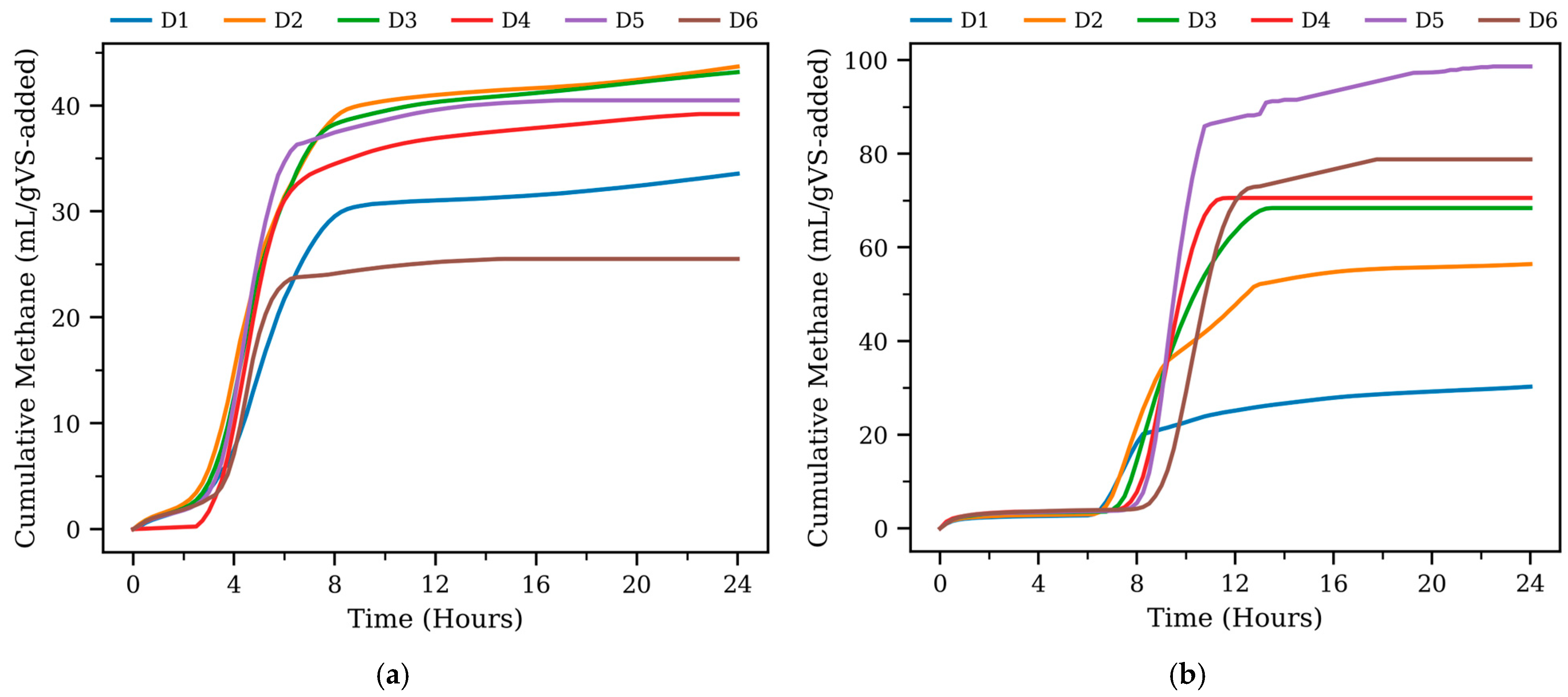
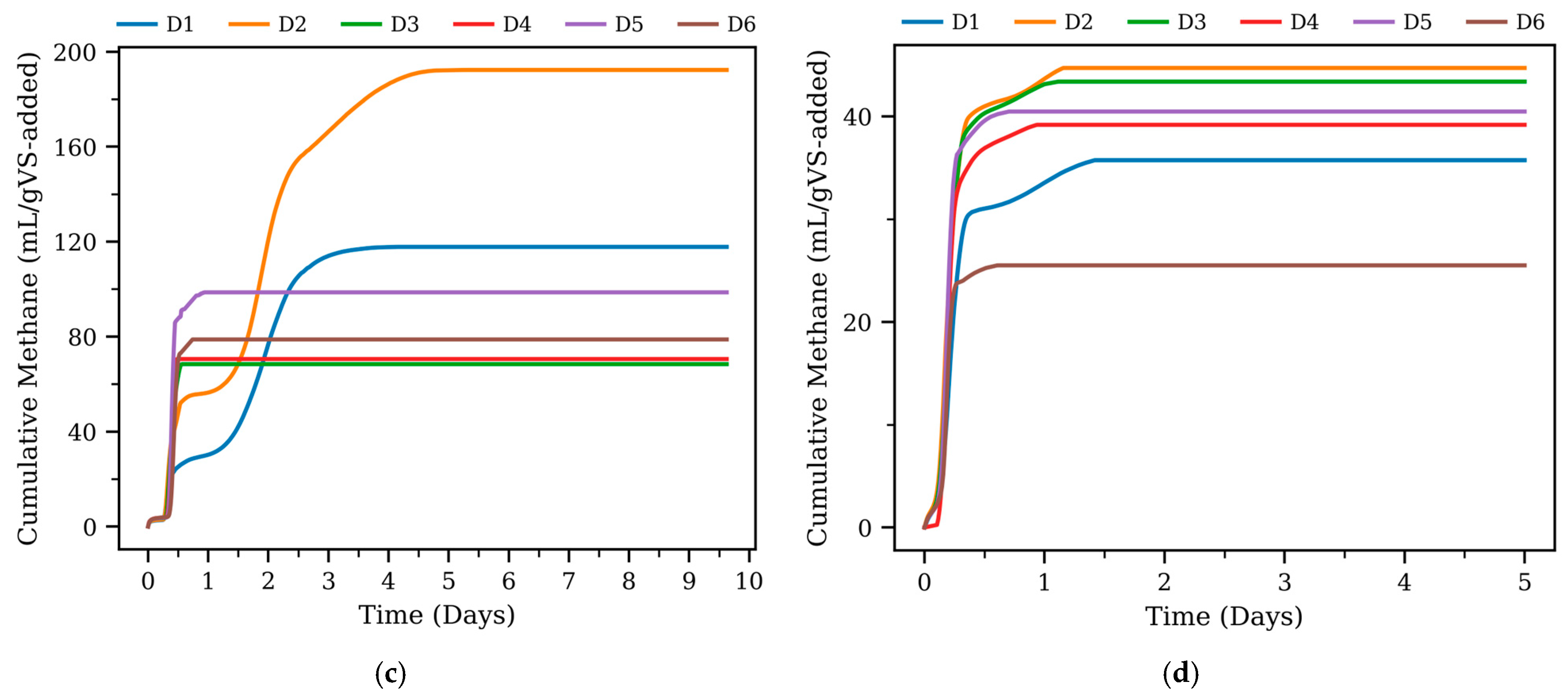
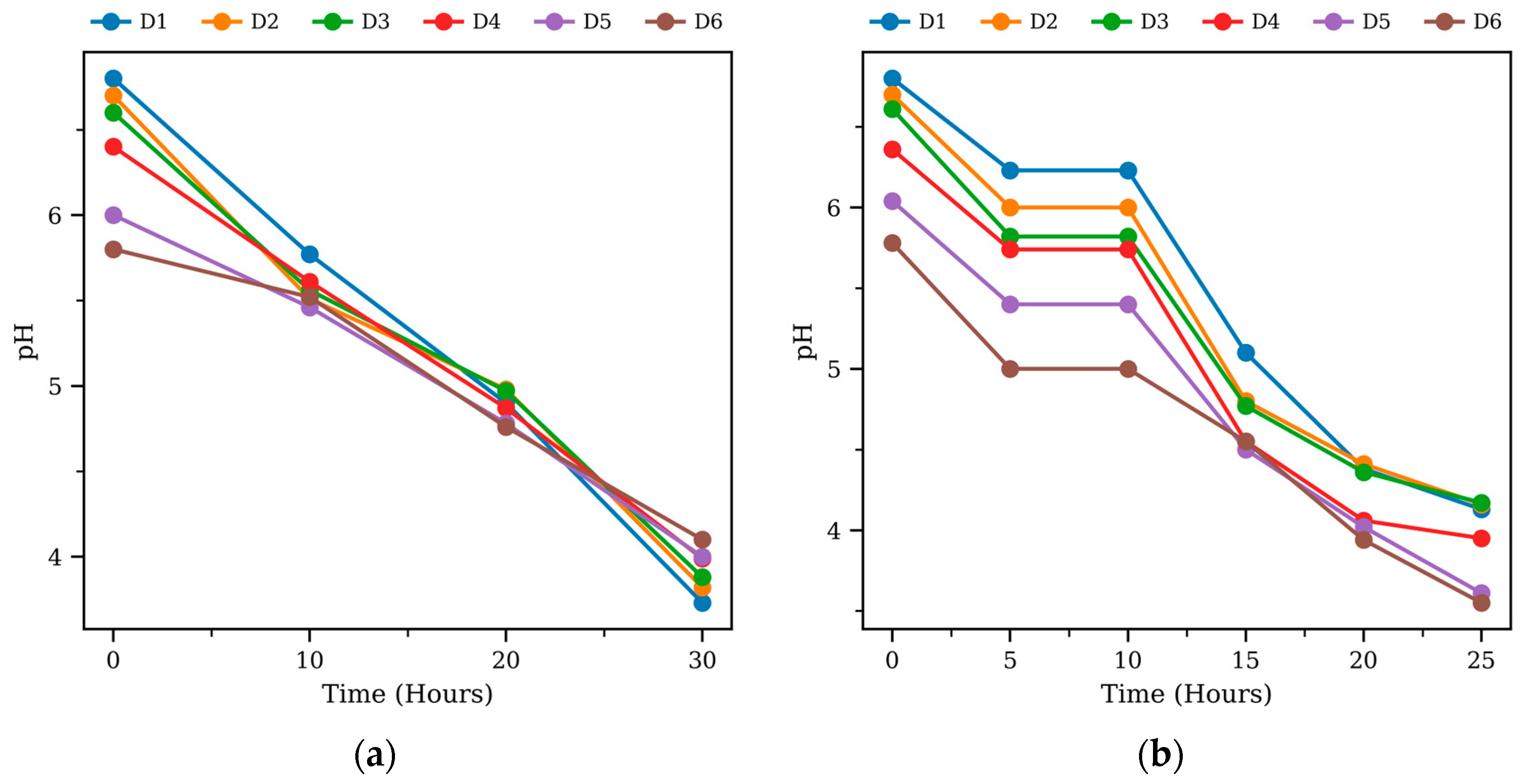
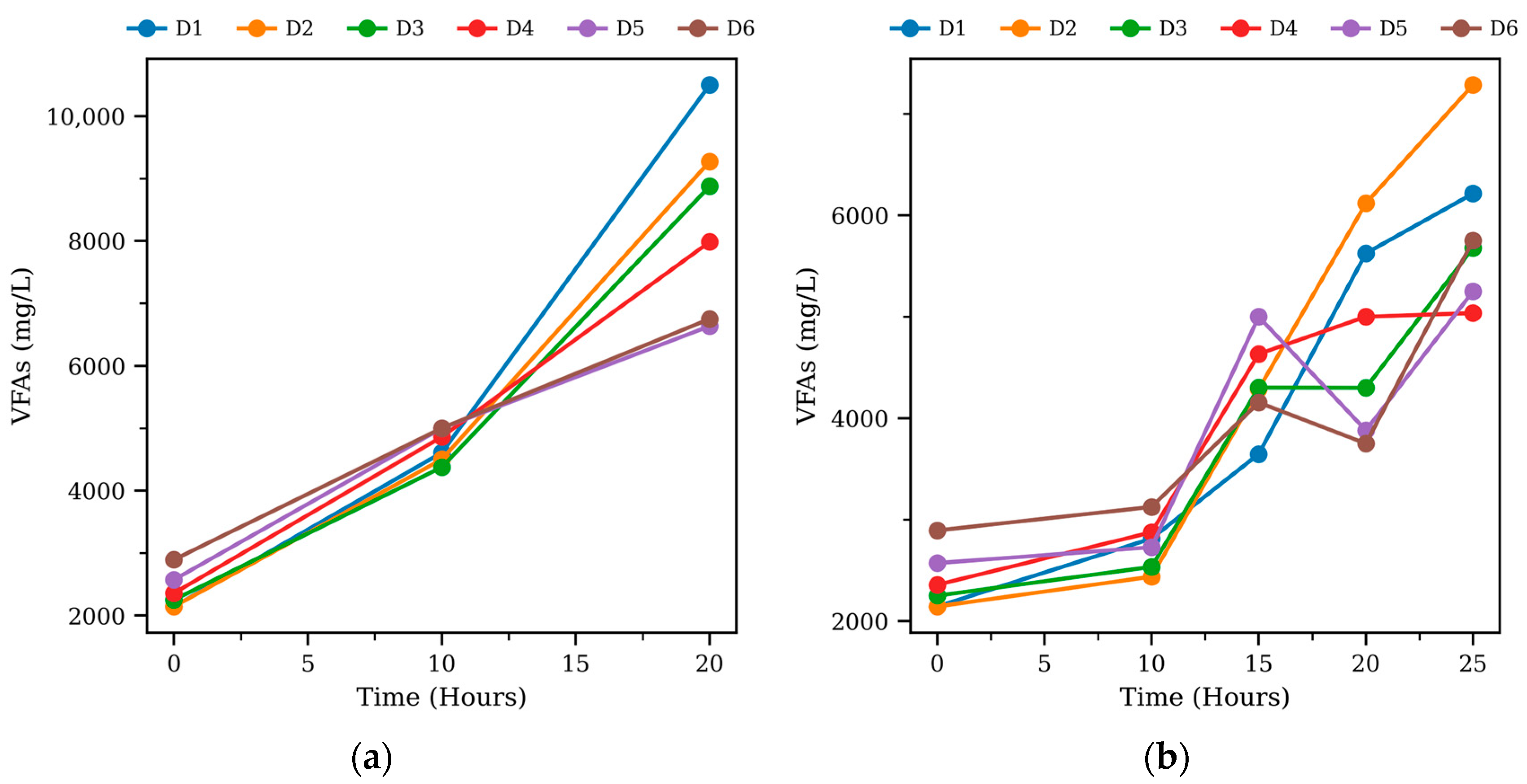
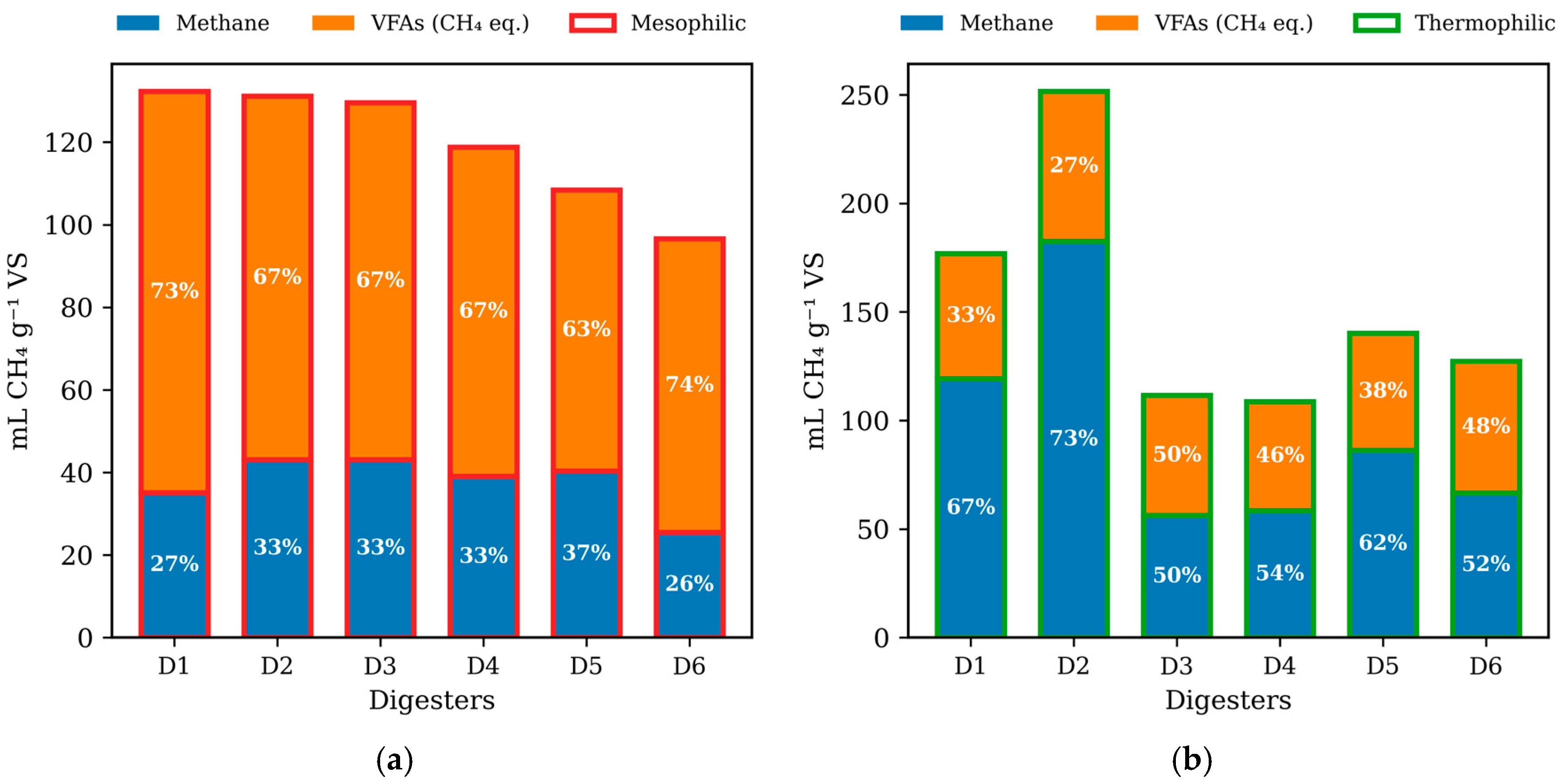
3.1. Cumulative Methane Production
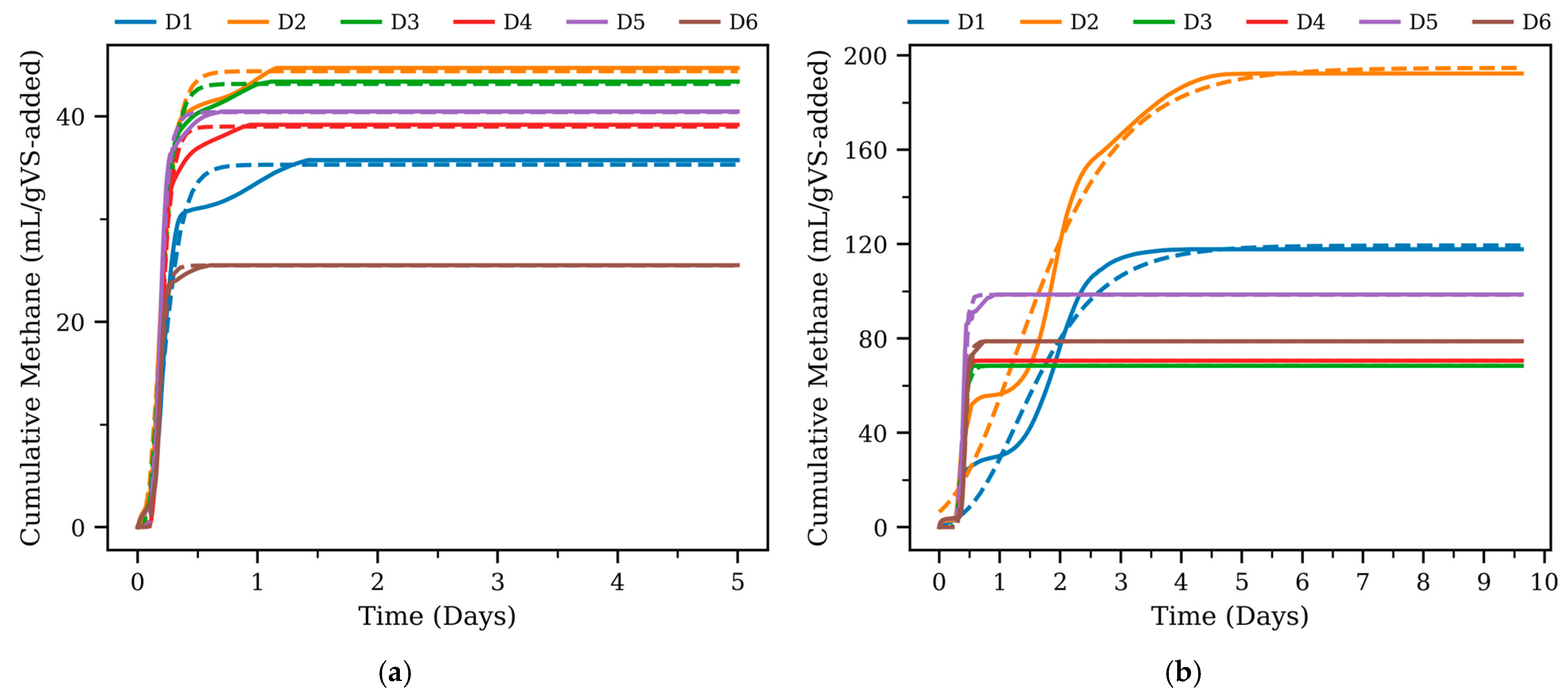
3.2. Kinetic Modeling
4. Study Limitations and Perspective
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Anaerobic Digestion |
| TW | Tomato Waste |
| CW | Cheese Whey |
| CM | Cow Manure |
| CDR | Co-Digestion Ratio |
| S/I | Substrate-to-Inoculum Ratio |
| TS | Total Solid |
| VS | Volatile Solid |
| VFA | Volatile Fatty Acid |
| TOC | Total Organic Carbon |
| TN | Total Nitrogen |
| C/N | Carbon-to-Nitrogen Ratio |
| pH | Potential of Hydrogen |
| AMPTS II | Automatic Methane Potential Test System II |
| CCU | Carbon Capturing Unit |
| Rmax | Maximum Methane Production Rate |
| Pmax | Maximum Cumulative Methane Production |
| λ | Lag Phase |
| R2 | Coefficient of Determination |
| ASTM | American Society for Testing and Materials |
| APHA | American Public Health Association |
| NDF | Neutral Detergent Fiber |
| WWTP | Wastewater Treatment Plant |
| CSTR | Continuous Stirred Tank Reactor |
| H2S | Hydrogen Sulfide |
| CH4 | Methane |
| CO2 | Carbon Dioxide |
| HRT | Hydraulic Retention Time |
| BMP | Biochemical Methane Potential |
| QRDI | Qatar Research, Development, and Innovation Council |
| ESC | Environmental Science Center |
References
- Scarlat; Dallemand, J.; Fahl, F. Biogas: Developments and perspectives in Europe. Renew. Energy 2018, 129, 457–472. [Google Scholar] [CrossRef]
- Vats, N.; Khan, A.A.; Ahmad, K. Effect of substrate ratio on biogas yield for anaerobic co-digestion of fruit vegetable waste & sugarcane bagasse. Environ. Technol. Innov. 2019, 13, 331–339. [Google Scholar] [CrossRef]
- Kim, M.; Gomec, C.Y.; Ahn, Y.; Speece, R.E. Hydrolysis and acidogenesis of particulate organic material in mesophi lic and thermophilic anaerobic digestion. Environ. Technol. 2003, 24, 1183–1190. [Google Scholar] [CrossRef]
- Pan, X.; Zhao, L.; Li, C.; Angelidaki, I.; Lv, N.; Ning, J.; Cai, G.; Zhu, G. Deep insights into the network of acetate metabolism in anaerobic digestion: Focusing on syntrophic acetate oxidation and homoacetogenesis. Water Res. 2021, 190, 116774. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, P. Basics of Anaerobic Digestion Process; Springer: Singapore, 2017. [Google Scholar] [CrossRef]
- Evangelisti, S.; Lettieri, P.; Borello, D.; Clift, R. Life cycle assessment of energy from waste via anaerobic digestion: A. Waste Manag. 2014, 34, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Piadeh, F.; Offie, I.; Behzadian, K.; Rizzuto, J.P.; Bywater, A.; Córdoba-Pachón, J.-R.; Walker, M. A critical review for the impact of anaerobic digestion on the sustainable development goals. J. Environ. Manag. 2023, 349, 119458. [Google Scholar] [CrossRef]
- Karagiannidis, A.; Perkoulidis, G. A multi-criteria ranking of different technologies for the anaerobic digestion for energy recovery of the organic fraction of municipal solid wastes. Bioresour. Technol. 2009, 100, 2355–2360. [Google Scholar] [CrossRef]
- Sevillano, C.A.; Pesantes, A.A.; Carpio, E.P.; Martínez, E.J.; Gómez, X. Anaerobic Digestion for Producing Renewable Energy—The Evolution of Th is Technology in a New Uncertain Scenario. Entropy 2021, 23, 145. [Google Scholar] [CrossRef]
- Vasco-Correa, J.; Khanal, S.; Manandhar, A.; Shah, A. Anaerobic digestion for bioenergy production: Global status, environme ntal and techno-economic implications, and government policies. Bioresour. Technol. 2018, 247, 1015–1026. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, J.; Gao, R.; Ye, F.; Zhao, G. Sustainable valorisation of tomato pomace: A comprehensive review. Trends Food Sci. Technol. 2019, 86, 172–187. [Google Scholar] [CrossRef]
- Ali, M.Y.; Ibn Sina, A.A.I.; Khandker, S.S.; Neesa, L.; Tanvir, E.M.; Kabir, A.; Khalil, M.I.; Gan, S.H. Nutritional Composition and Bioactive Compounds in Tomatoes and Their Impact on Human Health and Disease: A Review. Foods 2020, 10, 142. [Google Scholar] [CrossRef] [PubMed]
- Trombino, S.; Cassano, R.; Procopio, D.; Di Gioia, M.L.; Barone, E. Valorization of Tomato Waste as a Source of Carotenoids. Molecules 2021, 26, 5062. [Google Scholar] [CrossRef]
- Eslami, E.; Carpentieri, S.; Pataro, G.; Ferrari, G. A Comprehensive Overview of Tomato Processing By-Product Valorization by Conventional Methods versus Emerging Technologies. Foods 2022, 12, 166. [Google Scholar] [CrossRef]
- Al-Wandawi, H.; Abdul-Rahman, M.; Al-Shaikhly, K. Tomato processing wastes as essential raw materials source. J. Agric. Food Chem. 1985, 33, 804–807. [Google Scholar] [CrossRef]
- Asunis, F.; De Gioannis, G.; Dessì, P.; Isipato, M.; Lens, P.N.; Muntoni, A.; Polettini, A.; Pomi, R.; Rossi, A.; Spiga, D. The dairy biorefinery: Integrating treatment processes for cheese whey valorisation. J. Environ. Manag. 2020, 276, 111240. [Google Scholar] [CrossRef]
- Lappa, I.K.; Papadaki, A.; Kachrimanidou, V.; Terpou, A.; Koulougliotis, D.; Eriotou, E.; Kopsahelis, N. Cheese Whey Processing: Integrated Biorefinery Concepts and Emerging Food Applications. Foods 2019, 8, 347. [Google Scholar] [CrossRef]
- Zotta, T.; Solieri, L.; Iacumin, L.; Picozzi, C.; Gullo, M. Valorization of cheese whey using microbial fermentations. Appl. Microbiol. Biotechnol. 2020, 104, 2749–2764. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, R.A.; Rodrigues, J.A.D.; Ratusznei, S.M.; Zaiat, M.; Foresti, E. Whey treatment by AnSBBR with circulation: Effects of organic loading, shock loads, and alkalinity supplementation. Appl. Biochem. Biotechnol. 2007, 143, 257–275. [Google Scholar] [CrossRef]
- Charalambous, P.; Shin, J.; Shin, S.G.; Vyrides, I. Anaerobic digestion of industrial dairy wastewater and cheese whey: Performance of internal circulation bioreactor and laboratory batch test at pH 5-6. Renew. Energy 2020, 147, 1–10. [Google Scholar] [CrossRef]
- Kataki, S.; West, H.; Clarke, M.; Baruah, D. Phosphorus recovery as struvite from farm, municipal and industrial waste: Feedstock suitability, methods and pre-treatments. Waste Manag. 2016, 49, 437–454. [Google Scholar] [CrossRef] [PubMed]
- Alvarado-Vallejo, A.; Marín-Peña, O.; Rosas-Mendoza, E.S.; Méndez-Contreras, J.M.; Alvarado-Lassman, A. The Valorization of Fruit and Vegetable Wastes Using an Anaerobic Fixed Biofilm Reactor: A Case of Discarded Tomatoes from a Traditional Market. Processes 2024, 12, 1923. [Google Scholar] [CrossRef]
- Marchetti, A.; Salvatori, G.; Astolfi, M.L.; Fabiani, M.; Fradinho, J.; Reis, M.A.M.; Gianico, A.; Bolzonella, D.; Villano, M. Evaluation of the acidogenic fermentation potential of food industry by-products. Biochem. Eng. J. 2023, 199, 109029. [Google Scholar] [CrossRef]
- Zhu, Z.; Keesman, K.J.; Yogev, U.; Gross, A. Onsite anaerobic treatment of tomato plant waste as a renewable source of energy and biofertilizer under desert conditions. Bioresour. Technol. Rep. 2022, 20, 101274. [Google Scholar] [CrossRef]
- Escalante, H.; Castro, L.; Amaya, M.P.; Jaimes, L.; Jaimes-Estévez, J. Anaerobic digestion of cheese whey: Energetic and nutritional potential for the dairy sector in developing countries. Waste Manag. 2018, 71, 711–718. [Google Scholar] [CrossRef]
- Gonzalez-Piedra, S.; Hernández-García, H.; Perez-Morales, J.M.; Acosta-Domínguez, L.; Bastidas-Oyanedel, J.-R.; Hernandez-Martinez, E. A Study on the Feasibility of Anaerobic Co-Digestion of Raw Cheese Whey with Coffee Pulp Residues. Energies 2021, 14, 3611. [Google Scholar] [CrossRef]
- Li, Y.; Xu, F.; Li, Y.; Lu, J.; Li, S.; Shah, A.; Zhang, X.; Zhang, H.; Gong, X.; Li, G. Reactor performance and energy analysis of solid state anaerobic co-digestion of dairy manure with corn stover and tomato residues. Waste Manag. 2017, 73, 130–139. [Google Scholar] [CrossRef]
- Gil, A.; Siles, J.Á.; Serrano, A.; Martín, M.Á. Mixture optimization of anaerobic co-digestion of tomato and cucumber waste. Environ. Technol. 2015, 36, 2628–2636. [Google Scholar] [CrossRef] [PubMed]
- Hallaji, S.M.; Kuroshkarim, M.; Moussavi, S.P. Enhancing methane production using anaerobic co-digestion of waste activated sludge with combined fruit waste and cheese whey. BMC Biotechnol. 2019, 19, 19. [Google Scholar] [CrossRef]
- David, A.; Govil, T.; Tripathi, A.K.; McGeary, J.; Farrar, K.; Sani, R.K. Thermophilic Anaerobic Digestion: Enhanced and Sustainable Methane Production from Co-Digestion of Food and Lignocellulosic Wastes. Energies 2018, 11, 2058. [Google Scholar] [CrossRef]
- Kulcu, R. Composting of Greenhouse Tomato Plant Residues, Wheat Straw, and Separated Dairy Manure, and the Effect of Free Air Space on the Process. Pol. J. Environ. Stud. 2014, 23, 1341–1346. [Google Scholar]
- Şevik, F.; Tosun, I.; Ekinci, K. The effect of FAS and C/N ratios on co-composting of sewage sludge, dairy manure and tomato stalks. Waste Manag. 2018, 80, 450–456. [Google Scholar] [CrossRef]
- Oladejo, O.S.; Dahunsi, S.O.; Adesulu-Dahunsi, A.T.; Ojo, S.O.; Lawal, A.I.; Idowu, E.O.; Olanipekun, A.A.; Ibikunle, R.A.; Osueke, C.O.; Ajayi, O.E.; et al. Energy generation from anaerobic co-digestion of food waste, cow dung and piggery dung. Bioresour. Technol. 2020, 313, 123694. [Google Scholar] [CrossRef]
- Chen, J.L.; Ortiz, R.; Steele, T.W.; Stuckey, D.C. Toxicants inhibiting anaerobic digestion: A review. Biotechnol. Adv. 2014, 32, 1523–1534. [Google Scholar] [CrossRef]
- APHA. 2005. Available online: https://www.standardmethods.org/doi/book/10.2105/smww.2882 (accessed on 15 January 2025).
- Method 9060A–Total Organic Carbon, EPA. 2004. Available online: https://www.epa.gov/hw-sw846/sw-846-test-method-9060a-total-organic-carbon (accessed on 15 January 2025).
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Gallipoli, A.; Braguglia, C.M.; Gianico, A.; Montecchio, D.; Pagliaccia, P. Kitchen waste valorization through a mild-temperature pretreatment to enhance biogas production and fermentability: Kinetics study in mesophilic and thermophilic regimen. J. Environ. Sci. 2020, 89, 167–179. [Google Scholar] [CrossRef]
- Raposo, F.; Banks, C.; Siegert, I.; Heaven, S.; Borja, R. Influence of inoculum to substrate ratio on the biochemical methane potential of maize in batch tests. Process Biochem. 2006, 41, 1444–1450. [Google Scholar] [CrossRef]
- Atasoy, M.; Eyice, O.; Cetecioglu, Z. A Comprehensive Study of Volatile Fatty Acids Production from Batch Reactor to Anaerobic Sequencing Batch Reactor Using Cheese Processing Wastewater. Bioresour. Technol. 2020, 311, 123529. [Google Scholar] [CrossRef]
- Astals, S.; Koch, K.; Weinrich, S.; Hafner, S.D.; Tait, S.; Peces, M. Impact of Storage Conditions on the Methanogenic Activity of Anaerobic Digestion Inocula. Water 2020, 12, 1321. [Google Scholar] [CrossRef]
- Syaichurrozi, B.I.; Sumardiono, S. Kinetic Model of Biogas Yield Production from Vinasse at Various Initial pH: Comparison between Modified Gompertz Model and First Order Kinetic Model. Res. J. Appl. Sci. Eng. Technol. 2014, 7, 2798–2805. [Google Scholar] [CrossRef]
- Cioabla, A.E.; Dumitrel, G.A.; Ionel, I. Evaluation by Kinetic Models of Anaerobe Digestion Performances for Various Substrates and Co-substrates. Rev. De Chim. 2017, 68, 2614–2617. [Google Scholar] [CrossRef]
- Yong-sheng, W. Application of Modified Gompertz Model to Study on Anaerobic Digestion of Organic Fraction of Municipal Solid Waste. Environ. Sci. 2011, 32, 8. [Google Scholar]
- Lower, L.; Qiu, Y.; Sartor, R.C.; Sagues, W.J.; Cheng, J.J. Kinetic Modeling of Thermophilic Anaerobic Digestion of Lemnaceae for Biogas Production. BioEnergy Res. 2025, 18, 23. [Google Scholar] [CrossRef]
- Pramanik, S.K.; Suja, F.B.; Porhemmat, M.; Pramanik, B.K. Performance and Kinetic Model of a Single-Stage Anaerobic Digestion System Operated at Different Successive Operating Stages for the Treatment of Food Waste. Processes 2019, 7, 600. [Google Scholar] [CrossRef]
- Filer, J.; Ding, H.H.; Chang, S. Biochemical Methane Potential (BMP) Assay Method for Anaerobic Digestion Research. Water 2019, 11, 921. [Google Scholar] [CrossRef]
- Mohammadianroshanfekr, M.; Pazoki, M.; Pejman, M.B.; Ghasemzadeh, R.; Pazoki, A. Kinetic modeling and optimization of biogas production from food waste and cow manure co-digestion. Results Eng. 2024, 24, 103477. [Google Scholar] [CrossRef]
- Tian, Y.; Yang, K.; Zheng, L.; Han, X.; Xu, Y.; Li, Y.; Li, S.; Xu, X.; Zhang, H.; Zhao, L. Modelling Biogas Production Kinetics of Various Heavy Metals Exposed Anaerobic Fermentation Process Using Sigmoidal Growth Functions. Waste Biomass Valorization 2020, 11, 4837–4848. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Q.; Wang, X.; Zhou, X.; Zhu, J. Effect of pH on volatile fatty acid production from anaerobic digestion of potato peel waste. Bioresour. Technol. 2020, 316, 123851. [Google Scholar] [CrossRef]
- Marchetti, A.; Palhas, M.; Villano, M.; Reis, M.A.; Fradinho, J. Unlocking the potential of food industry by-products: Sustainable volatile fatty acids production via mixed culture acidogenic fermentation of reground pasta. J. Clean. Prod. 2025, 526, 146633. [Google Scholar] [CrossRef]
- Koch, K.; Hafner, S.D.; Weinrich, S.; Astals, S. Identification of Critical Problems in Biochemical Methane Potential (BMP) Tests From Methane Production Curves. Front. Environ. Sci. 2019, 7, 178. [Google Scholar] [CrossRef]
- Xing, B.-S.; Cao, S.; Han, Y.; Wen, J.; Zhang, K.; Wang, X.C. Stable and high-rate anaerobic co-digestion of food waste and cow manure: Optimisation of start-up conditions. Bioresour. Technol. 2020, 307, 123195. [Google Scholar] [CrossRef]
- Girotto, F.; Lavagnolo, M.C.; Acar, G.; Piazza, L. Bio-methane production from tomato pomace: Preliminary evaluation of process intensification through ultrasound pre-treatment. J. Mater. Cycles Waste Manag. 2020, 23, 416–422. [Google Scholar] [CrossRef]
- Yangin-Gomec, C.; Agnihotri, S.; Ylitervo, P.; Horváth, I.S. Assessment of Microbial Diversity during Thermophilic Anaerobic Co-Digestion for an Effective Valorization of Food Waste and Wheat Straw. Energies 2023, 16, 55. [Google Scholar] [CrossRef]
- Papirio, S.; Matassa, S.; Pirozzi, F.; Esposito, G. Anaerobic Co-Digestion of Cheese Whey and Industrial Hemp Residues Opens New Perspectives for the Valorization of Agri-Food Waste. Energies 2020, 13, 2820. [Google Scholar] [CrossRef]
- Peng, X.; Zhang, S.; Li, L.; Zhao, X.; Ma, Y.; Shi, D. Long-term high-solids anaerobic digestion of food waste: Effects of ammonia on process performance and microbial community. Bioresour. Technol. 2018, 262, 148–158. [Google Scholar] [CrossRef]
- Peng, X.; Zhang, S.; Li, L.; Zhao, X.; Ma, Y.; Shi, D. The effects of C/N (10-25) on the relationship of substrates, metabolites, and microorganisms in “inhibited steady-state” of anaerobic digestion. Water Res. 2020, 188, 116466. [Google Scholar] [CrossRef]
- Sarada, R.; Nand, K. Start-up of anaerobic digestion of tomato-processing wastes for methane generation. Biol. Wastes 1989, 30, 231–237. [Google Scholar] [CrossRef]
- Saghouri, M.; Mansoori, Y.; Rohani, A.; Khodaparast, M.H.H.; Sheikhdavoodi, M.J. Modelling and evaluation of anaerobic digestion process of tomato processing wastes for biogas generation. J. Mater. Cycles Waste Manag. 2018, 20, 561–567. [Google Scholar] [CrossRef]
- Saev, M.; Koumanova, B.; Simeonov, I.V. Anaerobic co-digestion of wasted tomatoes and cattle dung for biogas production. J. Univ. Chem. Technol. Metall. 2009, 44, 55–60. [Google Scholar]
- Dreschke, G.; Probst, M.; Walter, A.; Pümpel, T.; Walde, J.; Insam, H. Lactic acid and methane: Improved exploitation of biowaste potential. Bioresour. Technol. 2015, 176, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Labatut, R.A.; Angenent, L.T.; Scott, N.R. Biochemical methane potential and biodegradability of complex organic substrates. Bioresour. Technol. 2011, 102, 2255–2264. [Google Scholar] [CrossRef]
- Abdallah, M.; Greige, S.; Beyenal, H.; Harb, M.; Wazne, M. Investigating microbial dynamics and potential advantages of anaerobic co-digestion of cheese whey and poultry slaughterhouse wastewaters. Sci. Rep. 2022, 12, 10529. [Google Scholar] [CrossRef]
- Almeida, P.V.; Rodrigues, R.P.; Teixeira, L.M.; Santos, A.F.; Martins, R.C.; Quina, M.J. Bioenergy Production through Mono and Co-Digestion of Tomato Residues. Energies 2021, 14, 5563. [Google Scholar] [CrossRef]
- Belhadj, S.; Joute, Y.; El Bari, H.; Serrano, A.; Gil, A.; Siles, J.Á.; Chica, A.F.; Martín, M.Á. Evaluation of the anaerobic co-digestion of sewage sludge and tomato waste at mesophilic temperature. Appl. Biochem. Biotechnol. 2014, 172, 3862–3874. [Google Scholar] [CrossRef]
- Kovačić, Đ.; Kralik, D.; Jovičić, D.; Spajić, R. An Assessment of Anaerobic Thermophilic Co-Digestion of Dairy Cattle Manure and Separated Tomato Greenhouse Waste in Lab-Scale Reactors. Acta Technol. Agric. 2019, 22, 38–42. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Yao, L.; Fu, L.; Liu, Z. The Biogas Production Potential and Community Structure Characteristics of the Co-Digestion of Dairy Manure and Tomato Residues. Agronomy 2024, 14, 881. [Google Scholar] [CrossRef]
- Ruiz-Aguilar, G.M.L.; Martínez-Martínez, J.H.; Costilla-Salazar, R.; Camarena-Martínez, S. Using Central Composite Design to Improve Methane Production from Anaerobic Digestion of Tomato Plant Waste. Energies 2023, 16, 5412. [Google Scholar] [CrossRef]
- Zhang, H.; An, D.; Cao, Y.; Tian, Y.; He, J. Modeling the Methane Production Kinetics of Anaerobic Co-Digestion of Agricultural Wastes Using Sigmoidal Functions. Energies 2021, 14, 258. [Google Scholar] [CrossRef]
- de la Cueva, S.C.; Balagurusamy, N.; Pérez-Vega, S.B.; Pérez-Reyes, I.; Vázquez-Castillo, J.A.; de la Serna, F.Z.D.; Salmerón-Ochoa, I. Effects of different nitrogen sources on methane production, free ammonium and hydrogen sulfide in anaerobic digestion of cheese whey with cow manure. Rev. Mex. De Ing. Química 2021, 20, Bio2566. [Google Scholar] [CrossRef]
- Casallas-Ojeda, M.; Perez-Esteban, N.; Cabeza, I.; Cobo, M.; Olaya-Rincon, M.; Caicedo-Concha, D.M.; Astals, S. Understanding the acidification risk of cheese whey anaerobic digestion under psychrophilic and mesophilic conditions. Heliyon 2024, 10, e26476. [Google Scholar] [CrossRef]
- La Mantia, M.C.; Calì, M.; Rossi, E.; Scalella, R.; Signorini, A.; Santangelo, E.; Chiariotti, A. Metagenomic Analysis during Co-Digestion Buffalo Sludge and Tomato Pomace Post Thermal Stress: A Case Study. J. Buffalo Sci. 2024, 13, 104–115. [Google Scholar] [CrossRef]
- Dareioti, M.A.; Vavouraki, A.I.; Tsigkou, K.; Kornaros, M. Assessment of Single- vs. Two-Stage Process for the Anaerobic Digestion of Liquid Cow Manure and Cheese Whey. Energies 2021, 14, 5423. [Google Scholar] [CrossRef]
- Ergüder, T.; Tezel, U.; Güven, E.; Demirer, G. Anaerobic biotransformation and methane generation potential of cheese whey in batch and UASB reactors. Waste Manag. 2001, 21, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Lahbab, A.; Djaafri, M.; Kalloum, S.; Benatiallah, A.; Atelge, M.; Atabani, A. Co-digestion of vegetable peel with cow dung without external inoculum for biogas production: Experimental and a new modelling test in a batch mode. Fuel 2021, 306, 121627. [Google Scholar] [CrossRef]
- Yan, Y.; Du, Z.; Zhang, L.; Feng, L.; Sun, D.; Dang, Y.; Holmes, D.E.; Smith, J.A. Identification of parameters needed for optimal anaerobic co-digestion of chicken manure and corn stover. RSC Adv. 2019, 9, 29609–29618. [Google Scholar] [CrossRef] [PubMed]
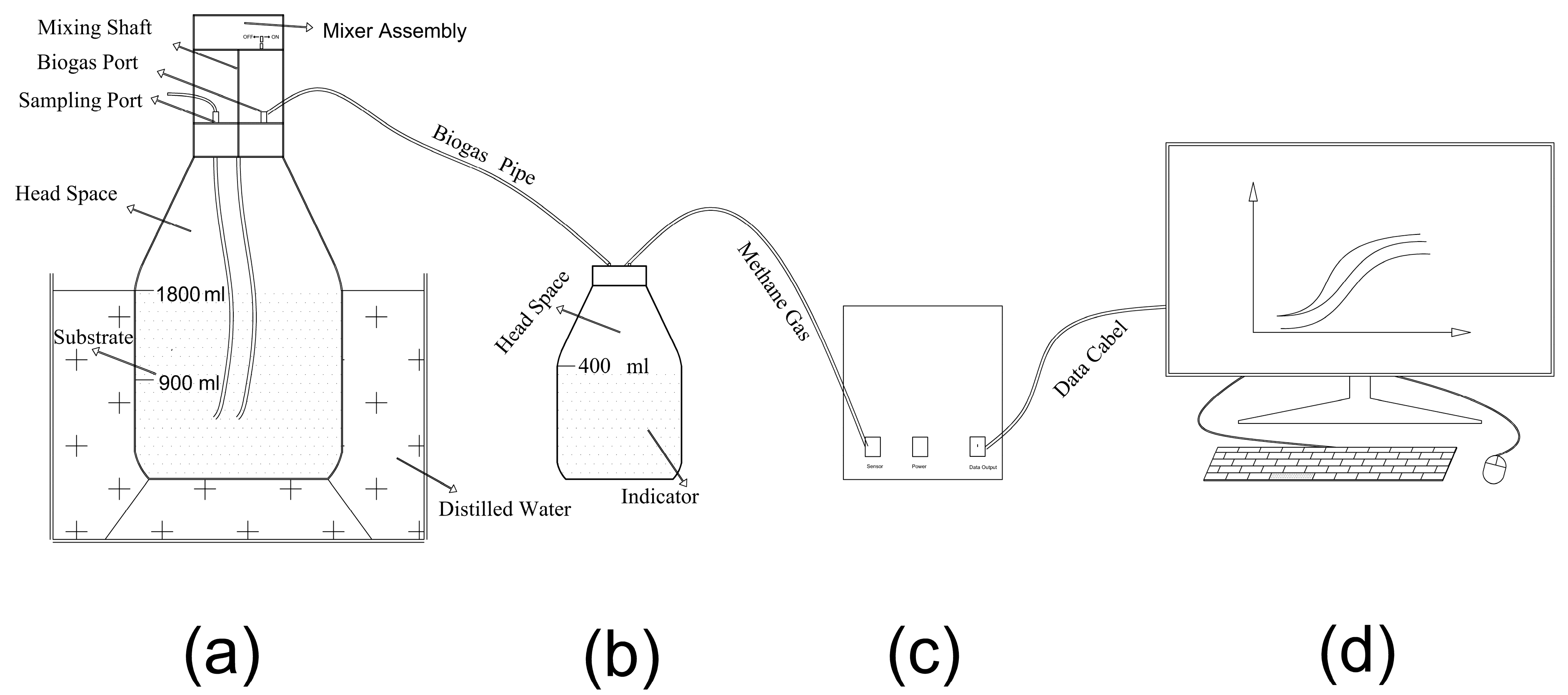
| Parameter | CW | TW | Inoculum |
|---|---|---|---|
| pH | 5.07 | 4.27 | 8.5 |
| TS (%) | 5.1 | 4.65 | 4.8 |
| TS (g/L) | 50.7 | 42.25 | 47 |
| VS (g/L) | 45 | 36.9 | 40 |
| VS (% of TS) | 88 | 87 | 85 |
| C/N | 30 | 14.1 | 13 |
| TC (%) * | 2.4 | 36.8 | 36.2 |
| TN (%) * | 0.08 | 2.6 | 2.9 |
| Cellulose + Hemicellulose + Lignin + Ash | <1% | 14.5% | - |
| Digester | CDRs (gVS/gVS) | CW (mL) | TW (mL) | CM (mL) | CW (VSg/L) | TW (VS g/L) | S/I | pH |
|---|---|---|---|---|---|---|---|---|
| D1 | Control | 900 | 0 | 900 | 37.80 | 0.00 | 1 | 6.8 |
| D2 | 4.6 | 720 | 180 | 900 | 30.24 | 6.64 | 1 | 6.7 |
| D3 | 1.7 | 540 | 360 | 900 | 22.68 | 13.28 | 1 | 6.61 |
| D4 | 0.8 | 360 | 540 | 900 | 15.12 | 19.93 | 1 | 6.36 |
| D5 | 0.3 | 180 | 720 | 900 | 7.56 | 26.57 | 1 | 6.04 |
| D6 | Control | 0 | 900 | 900 | 0.00 | 33.21 | 1 | 5.78 |
| Blank | Blank | 0 | 0 | 900 | 0 | 0 | - | 8.5 |
| Digestion Type | Substrate(s) | Inoculum | Key Influencing Factors | Methane Yield (mL CH4/g VS-Added) | References |
|---|---|---|---|---|---|
| Co-digestion | Tomato residues + cow manure | Anaerobic digester sludge (municipal WWTP) | Mesophilic (35 °C), Batch | 12–75 | [65] |
| Mono-digestion | Tomato residues | Anaerobic digester sludge (municipal WWTP) | Mesophilic (35 °C), Batch | 324 | [65] |
| Co-digestion | Tomato waste + sewage sludge | Anaerobic digester sludge (municipal WWTP) | Mesophilic (35 °C), Batch | 159 | [66] |
| Co-digestion | Tomato residues + cow manure | Fresh cow manure | Thermophilic (55 °C), Batch | ~200 | [67] |
| Co-digestion | Tomato residues | Anaerobic digestate from dairy manure digester | Mesophilic (35 °C), Batch | 30 | [68] |
| Mono-digestion | Tomato plant waste (stems, leaves, etc.) | Anaerobic digestate from dairy manure digester | Mesophilic (40 °C), Batch | 210.8 | [69] |
| Co-digestion | Tomato leaves + cow manure | Fresh cow manure | Mesophilic (35 °C), Batch | 30–35 | [70] |
| Mono-digestion | Cheese whey | Fresh cow manure | Mesophilic (35 °C), Batch | 7–400 | [71] |
| Mono-digestion | Cheese whey (dairy effluent) | Anaerobic digester sludge (municipal WWTP) | Psychrophilic (20 °C) vs. Mesophilic (35 °C), Batch | 389–436 | [72] |
| Co-digestion | Raw cheese whey + coffee pulp | Anaerobic sludge pre-acclimated to cheese whey | Mesophilic (35 °C), Batch | 71 | [26] |
| Co-digestion | Tomato pomace + buffalo manure | Fresh buffalo manure | Mesophilic (35 °C), Thermophilic (55 °C), Batch | 50–300 | [36,73] |
| Co-digestion | Cheese whey + liquid cow manure | Lab CSTRs seeded with acclimated anaerobic consortia | Mesophilic (35 °C), Two-phase AD (acidogenic + methanogenic) | 290 | [74] |
| Mono-digestion | Cheese whey + supplementation | Digestate from farm dairy manure digester | Mesophilic (35 °C), Batch | 424 | [75] |
| Co-digestion | Food waste + cow manure | Fresh cow manure | Mesophilic (35 °C), Batch | 130–170 | [76] |
| Co-digestion | chicken manure + corn stover | Mesophilic digester effluent | Mesophilic (35 °C), Batch | 160–230 | [77] |
| Co-digestion | Food waste + cow/pig manure | Fresh cow/pig manure | Mesophilic (35 °C), Batch | 14–30 | [33] |
| Co-digestion | Tomato waste + cheese whey | Fresh cow manure | Mesophilic (35 °C), Thermophilic (55 °C), Batch | 25–182.5 | This Study |
| Type | Digester | Pmax (mL/gVS-Added) | Rmax (mL/h) | Lag Phase (h) | R2 |
|---|---|---|---|---|---|
| Thermophilic | D1 | 119.3 | 75.8 | 12 | 0.97 |
| D2 | 182.5 | 97.3 | 6 | 0.98 | |
| D3 | 56.29 | 591.1 | 7 | 0.99 | |
| D4 | 58.44 | 1030.2 | 8 | 0.99 | |
| D5 | 86.33 | 1161.0 | 8 | 0.99 | |
| D6 | 66.63 | 852.9 | 9 | 0.99 | |
| Mesophilic | D1 | 35.11 | 173.5 | 2 | 0.97 |
| D2 | 43.16 | 268.5 | 2 | 0.99 | |
| D3 | 43.15 | 296.4 | 2 | 0.99 | |
| D4 | 39.1 | 352.4 | 3 | 0.99 | |
| D5 | 40.41 | 429.4 | 3 | 0.99 | |
| D6 | 25.47 | 334.2 | 3 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, I.; Ayari, M.A.; Talhami, M.; Das, P.; Al-Ejji, M.; Benzarti, S.; Hawari, A.H. Effect of Co-Digestion Ratios and Temperature on Biomethane Production in Anaerobic Co-Digestion of Cheese Whey and Tomato Waste. Fermentation 2025, 11, 659. https://doi.org/10.3390/fermentation11120659
Ullah I, Ayari MA, Talhami M, Das P, Al-Ejji M, Benzarti S, Hawari AH. Effect of Co-Digestion Ratios and Temperature on Biomethane Production in Anaerobic Co-Digestion of Cheese Whey and Tomato Waste. Fermentation. 2025; 11(12):659. https://doi.org/10.3390/fermentation11120659
Chicago/Turabian StyleUllah, Irfan, Mohamed Arselene Ayari, Mohammed Talhami, Probir Das, Maryam Al-Ejji, Saoussen Benzarti, and Alaa H. Hawari. 2025. "Effect of Co-Digestion Ratios and Temperature on Biomethane Production in Anaerobic Co-Digestion of Cheese Whey and Tomato Waste" Fermentation 11, no. 12: 659. https://doi.org/10.3390/fermentation11120659
APA StyleUllah, I., Ayari, M. A., Talhami, M., Das, P., Al-Ejji, M., Benzarti, S., & Hawari, A. H. (2025). Effect of Co-Digestion Ratios and Temperature on Biomethane Production in Anaerobic Co-Digestion of Cheese Whey and Tomato Waste. Fermentation, 11(12), 659. https://doi.org/10.3390/fermentation11120659





