Abstract
Dark fermentation of food waste for biohydrogen production can simultaneously achieve waste resource utilization and clean energy production. However, the widespread application of this technology remains constrained by challenges such as low substrate hydrolysis efficiency and suboptimal metabolic performance of functional microorganisms. This study evaluated the synergistic enhancement of biohydrogen production from food waste through dark fermentation by integrating thermal–alkaline (TA) pretreatment with varying concentrations (50, 100, 150, and 200 mg/L) of nickel–cobalt oxide nanoparticles (NiCo2O4 NPs), and the underlying mechanisms involved were systematically elucidated. The results demonstrated that individual TA pretreatment (pH 11, 70 °C, 1 h) and TA coupled with NiCo2O4 NPs (100 mg/L) significantly (p < 0.01) enhanced the cumulative biohydrogen yields of the food waste dark fermentation by 20.89% and 35.76%, respectively. Mechanism research revealed that TA pretreatment effectively facilitated the dissolution and hydrolysis of macro-molecular organics such as polysaccharides and proteins, thereby enhancing the bio-accessibility of fermentation substrates. The introduction of NiCo2O4 NPs further intensified the microbial biohydrogen-producing metabolism by augmenting enzymatic activity and enriching functional bacteria. NiCo2O4 NPs significantly (p < 0.001) enhanced the overall activity of hydrogenase by 95.10% compared to the control group (CG) by providing the cofactor of hydrogenase and accelerating electron transfer. Additionally, this synergistic strategy significantly (p < 0.01) increased the activities of hydrolases (e.g., protease and α-glucosidase), as well as key enzymes in acetate-type and butyrate-type fermentation pathways (e.g., acetate kinase and butyrate kinase), and enriched the biohydrogen-producing microbial community centered on Clostridium_sensu_stricto_1. This study systematically elucidated the synergistic strategy of TA pretreatment and NiCo2O4 NPs, which achieved dual-pathway reinforcement from substrate degradability to microbial metabolic activity. The findings are expected to provide theoretical support for developing efficient biohydrogen production technology from perishable organic solid waste.
1. Introduction
Given the continuous growth of global energy demand and the increasingly severe environmental issues arising from the consumption of fossil fuels [1], the development of clean and sustainable alternative energy sources has emerged as a prominent focus in contemporary academic research [2]. Hydrogen energy, characterized by its high energy density and pollution-free combustion products, has been regarded as one of the most promising future energy carriers [3]. Among numerous hydrogen production technologies, biohydrogen production technology [4], especially dark fermentation for biohydrogen production utilizing organic solid waste [5], can simultaneously achieve waste resource utilization and clean energy production [6], demonstrating significant application prospects and economic value.
With the in-depth implementation of the national strategy of refuse classification, the amount of food waste separation is increasing, and the demand for its treatment and disposal is also rising continuously [7]. Food waste, a principal constituent of urban perishable organic solid waste, consists of complex organic matter including carbohydrates, proteins, and lipids, making it an ideal substrate for dark fermentation to produce biohydrogen [8]. However, the complex structure formed by particulate organics in food waste limited its biohydrogen generation efficiency from dark fermentation. Pretreatment is generally regarded as a feasible method to disintegrate the complex structure and improve the biohydrogen production from dark fermentation of food waste [9, 10]. For instance, Jia et al. found that hydrothermal pretreatment markedly disintegrate particulate organics efficiently and enhanced protein hydrolysis, thereby increasing the hydrogen production rate in dark fermentation of food waste [11]. Dinesh et al. summarized the strategies for enhanced biohydrogen production from food waste and proposed that OH- from alkaline pretreatment can attack the complex molecules of particulate organics to form monomers and thereby increases the biohydrogen production rate [12]. The thermal–alkaline pretreatment integrated the structure disruption effect of heat treatment and the swelling and degradation effect of alkaline treatment on particulate organics [11, 12]. That has been proven to effectively enhance the solubilization of complex carbohydrates in food waste [13], providing more abundant substrates for hydrogen-producing microorganisms and thereby significantly enhancing the biohydrogen production performance.
Although pretreatment methods can optimize substrate conditions, the ultimate efficiency of the dark fermentation biohydrogen production process remains highly dependent on the metabolic activity of hydrogen-producing microorganisms [14]. The biohydrogen production process constitutes a series of intricate biochemical reactions catalyzed by multiple enzyme systems like hydrolases and hydrogenases [15]. The metabolic activities of these enzymes directly influence the hydrogen production performance. In recent years, incorporating trace metal elements as additives into fermentation systems to stimulate microbial growth [16], enhance the activity of key enzymes, and optimize the electron transport chain has emerged as an effective bioaugmentation strategy [17]. Among them, nickel (Ni) is an indispensable trace element in the hydrogen-producing metabolism [18], which serves as a vital constituent of the active center of [Ni-Fe] hydrogenase and plays a crucial role in the assembly and catalytic activity of hydrogenase [19]. Ni-based nanoparticles (NPs), especially Ni-based bimetallic NPs, have garnered significant attention in the realm of enzyme catalysis, due to the synergy of the two functional metal ions and their unique nanostructure [20]. When implemented in the dark fermentation system [21], they were anticipated to act as a continuous electron donor or activator, substantially enhancing the activity of hydrogenase and subsequently facilitating the synthesis of hydrogen [22]. However, current applications of Ni-based bimetallic NPs in dark fermentation are mostly concentrated on wastewater or simulated wastewater systems with relatively simple compositions [20, 23]. The effect and mechanism of their application in the dark fermentation of complex organic solid waste, such as food waste, remain unknown. More importantly, whether the synergistic effect between these NPs and pretreatment technologies can break through the dual bottlenecks of the accessibility of organic solid waste substrates and the metabolic efficiency of microorganisms also urgently requires in-depth exploration.
Against the backdrop described above, this study aimed to systematically explore the synergistic enhancement effects and the underlying mechanism of thermal–alkaline pretreatment coupled with nickel–cobalt oxide NPs (NiCo2O4 NPs) on hydrogen production via dark fermentation of food waste. The research content encompassed the following aspects: (1) evaluating the enhancement effects of thermal–alkaline pretreatment alone and in combination with different concentrations of NiCo2O4 NPs on hydrogen yield; (2) unveiling the influence patterns of pretreatment and additives on the processes of organic matter dissolution, hydrolysis, and acidification; (3) analyzing the impact of NiCo2O4 NPs on the activities of key enzymes; and (4) deciphering the succession patterns of the fermentation microbial community structure and dominant microorganisms under this synergistic strategy. The anticipated outcomes of this study were expected to provide crucial theoretical basis and data support for the development of efficient biohydrogen production technologies from food waste.
2. Materials and Methods
2.1. Substrate and Inoculum
In this work, the food waste was collected from the canteen of Tongji University. After removing impurities such as bones, the waste was crushed to a particle size of less than 3 mm and stored at 4 °C for future use. The fundamental properties of the food waste were characterized as follows: pH 5.4 ± 0.2, total solids (TS) 22.9 ± 1.3%, and the ratio of volatile solids (VS) to TS 97.6 ± 0.9%, total chemical oxygen demand (TCOD) 1638.34 ± 39.22 mg/g (dry basis), protein content 28.5 ± 1.2% (dry basis), carbohydrate content 45.7 ± 2.1% (dry basis). Elemental analysis revealed the following composition: C (58.0%), H (12.1%), O (20.0%), N (3.4%), and S (0.9%). The inoculum employed in this study was sludge retrieved from a laboratory scale anaerobic digester, with pH of 6.6 ± 0.1, TS content of 2.6 ± 0.1%, and VS/TS ratio of 46.1 ± 0.3%.
2.2. Preparation and Characterization of NiCo2O4 NPs
The detailed procedure for the synthesis of NiCo2O4 NPs is as follows: (a) NiCl2·6H2O, CoCl2·6H2O, and CO(NH2)2 were dissolved in deionized water at a molar ratio of 1:2:6, and the mixture was continuously stirred to form a homogeneous reaction solution. (b) The reaction solution was then transferred into a high-pressure autoclave, then the reaction was carried out at 120 °C for 6 h. After the reaction was completed, the autoclave was allowed to cool down to room temperature naturally. The resultant product was washed thoroughly with deionized water and ethanol to remove any impurities. Subsequently, it was vacuum-dried at 60 °C for 24 h to yield the solid-phase precursor. (c) The solid-phase precursor was calcined in an air atmosphere at 300 °C for 3 h. The heating rate was set at 5 °C/min. The final product obtained after this calcination process was the nickel–cobalt oxide NPs. The morphology and chemical composition of the materials were determined using a scanning electron microscope (SEM, ZEISS, GeminiSEM 300, Oberkochen, Germany). The powder X-ray diffraction patterns of the materials were measured using an X-ray diffractometer (XRD) (Rigaku, Ultima IV, Tokyo, Japan) at a scanning speed of 2°/min within the scanning range of 10–90°.
2.3. Batch Dark Fermentation Experiments
The batch experiments were carried out in 500 mL fermentation flasks equipped with stirring rods, each with a working volume of 400 mL. The food waste and sludge were added to the fermentation flasks at a VS ratio of 6:1. A total of six groups of working conditions were set up, including the control group (CG), the thermal–alkaline pretreatment group (TA), and the thermal–alkaline pretreatment coupled with NiCo2O4 NP group (TA&NPs) with four different addition concentrations. In CG, the food waste was not subjected to any pretreatment, nor was NiCo2O4 NPs added. In TA, the food waste underwent thermal–alkaline pretreatment at 70 °C for 1 h under a pH value of 11. In TA&NPs, after the thermal–alkaline pretreatment, NiCo2O4 NPs were added to the fermentation system at concentrations of 50 mg/L, 100 mg/L, 150 mg/L, and 200 mg/L, which were labeled as TA&NPs 50, TA&NPs 100, TA&NPs 150, and TA&NPs 200, respectively. This concentration range (50–200 mg/L) has been widely reported to be effective in stimulating the enzymatic activity of anaerobic microorganisms [20, 23, 24], which facilitated the assessment of the dose-dependent effect of NiCo2O4 NPs on dark fermentation. Prior to the formal fermentation process, adjust the initial pH value to 5.5 using 2 M HCl or 2 M NaOH. Subsequently, the system was purged with nitrogen for 5 min to eliminate oxygen. Finally, each fermentation flask was connected to a pre-evacuated gas bag and then placed in an air-bath shaker at 37 °C for fermentation. All the experiments were conducted with three parallel groups to ensure the reliability of the experimental results. Gas bags were collected at 0 h, 4 h, 8 h, 12 h, 16 h, 20 h, 26 h, 32 h, 56 h, and 80 h, respectively, to determine the composition of the biogas. Meanwhile, samples were taken and preserved for the measurement of certain solubility and microbial indicators.
2.4. Determination of Biogas Components and Solubility Indicators
Gas components including N2, H2, CH4, and CO2 were analyzed using a gas chromatograph (INESA, GC112A, Shanghai, China) equipped with a thermal conductivity detector ((INESA, GC126N–TCD, Shanghai, China). Short-chain fatty acids (SCFAs) and ethanol were quantified by a gas chromatograph (Shimadzu, GC–2010 Plus, Kyoto, Japan) fitted with a gas chromatography column (Reatek, Stabilwax-DA, 30 m × 0.32 mm × 0.1 mm, Fremont, CA, USA) and a flame ionization detector (Shimadzu, FID–2010 Plus, Kyoto, Japan). The Lowry method and the anthrone method were, respectively, utilized to determine soluble proteins and soluble polysaccharides. The degree of converting particulate total organic carbon (TOC) in food waste into soluble STOC was defined as hydrolysis efficiency. A TOC analyzer (Shimadzu, TOC-L-CPH/CPN, Kyoto, Japan) was adopted to measure STOC. The molecular weight (MW) of the sample supernatant was determined by size-exclusion chromatography, using a liquid chromatography instrument (Agilent, 1260 Infinity, Santa Clara, CA, USA) equipped with a size-exclusion column (Agilent, PL aquagel-OH MIXED-M, Santa Clara, CA, USA) and a differential refractive index detector (Agilent, PL–GPC 220 RI, Santa Clara, CA, USA).
2.5. Analysis of Enzyme Activity and Microbial Community
The dark fermentation hydrogen production process involves the actions of various enzymes. Specifically, α-glucosidase, protease, hydrogenase (HYD), acetate kinase (AK), and butyrate kinase (BK) were, respectively, selected as the key enzymes involved in hydrolysis and acidification reactions. The detailed measurement procedure was as follows: a specific amount of the fermentation sample was added into an appropriate volume of PBS buffer (0.01 M, pH 7.4). After being subjected to ultrasonic disruption in an ice-water bath, the sample was centrifuged at 5000× g for 5–10 min at 4 °C. The activity of key enzymes in the supernatant extract was subsequently determined using an ELISA kit. Each sample was tested in triplicate. The mixed sample from triplicate experiments was used for microbial community test. The FastDNA® SPIN Kit (MP Biomedicals, Santa Ana, CA, USA) for Soil was employed to extract DNA from samples of each reactor. The quality of the extracted DNA was examined via 1% agarose gel electrophoresis, and the concentration and purity of the DNA were determined using the NanoDrop2000 (Thermo, NanoDrop2000, Waltham, MA, USA). Subsequently, primers 338F(5′-ACTCCTACGGGAGGCAGCAG-3′)/806R(5′-GGACTACHVGGGTWTCTAAT-3′) were utilized to perform PCR amplification on the V3-V4 variable regions of the 16S rRNA. The PCR products were sequenced using the Miseq PE300 platform of Illumina. Bioinformatic analysis was conducted using the Majorbio Cloud platform.
2.6. Analysis of Variance and Kinetic Modeling
Statistical significance was assessed by one-way ANOVA. The modified Gompertz model was employed to fit the cumulative hydrogen production curve, and its formula is presented as Equation (1):
where P(t) represents the cumulative biohydrogen production (mL), λ denotes the lag-phase time (h), Pm stands for the potential biohydrogen production of the material (mL), Rm represents the maximum biohydrogen production rate (mL/h), e is a constant with a value of 2.718281828, and t represents the fermentation time (h).
3. Results and Discussion
3.1. Characterization of the Physicochemical Properties of NiCo2O4 NPs
The synthesized NiCo2O4 NPs were characterized in terms of both physical and chemical properties. As shown in the SEM image of NiCo2O4 NPs presented in Figure 1A, the material exhibited an urchin-like spherical architecture with an approximate diameter of 4 μm, composed of numerous radially aligned nanorods growing from the center. Its high specific surface area and three-dimensional structure may provide abundant attachment sites and a protective microenvironment for hydrogen-producing microorganisms, thereby facilitating the formation of stable biofilms [25]. Furthermore, the radially oriented nanorods form rich mesoporous channels [26], which may also promote the diffusion and transport of substrate nutrients and metabolic products such as biohydrogen. The analysis of SEM-EDS (Figure 1B) and elemental mapping (Figure 1C) confirmed the presence of Co, Ni, and O in the synthesized material, with atomic percentages of 29.98%, 11.07%, and 58.95%, as well as weight percentages of 52.59%, 19.34%, and 28.07%, respectively. It was reported that NiCo2O4 NPs can release a certain amount of Ni2+ and Co2+ in the fermentation system, which possess a certain positive stimulating effect on the growth and metabolism of some biohydrogen-producing microorganisms [27]. Figure 1D presented the XRD spectra of the prepared NiCo2O4 NPs. The XRD spectra of the prepared NiCo2O4 NPs exhibited a typical spinel structure, with diffraction peaks at 2θ = 18.9°, 31.2°, 36.7°, 38.4°, 44.6°, 55.4°, 59.1°, 64.5°, 77.0°, and 82.3°, which corresponded to the characteristic peaks of the standard spectrum of Joint Committee on Powder Diffraction Standards (JCPDS) with card number 73-1702. This implied that NiCo2O4 NPs with distinctive urchin-like micromorphology, uniform element distribution, and good crystallinity have been successfully synthesized.
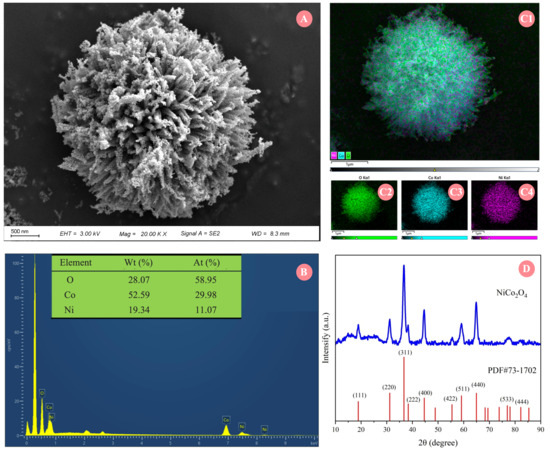
Figure 1.
(A) SEM image of NiCo2O4 NPs; (B) SEM-EDS graphic of NiCo2O4 NPs; (C1–C4) element distribution mapping of SEM; (D) XRD pattern of NiCo2O4 NPs.
3.2. Effects of TA Pretreatment Coupled with NiCo2O4 NPs on Biohydrogen Production
The effects of TA pretreatment and TA pretreatment coupled with varying concentrations of NiCo2O4 NPs on biohydrogen production from dark fermentation of food waste were evaluated separately. The time-dependent cumulative biohydrogen yields for each experimental group are illustrated in Figure 2. It was observed that the biohydrogen production in all experimental groups were nearly completed within 80 h. Throughout the dark fermentation process of food waste, the cumulative biohydrogen yield in the CG reached 56.69 mL/g VS. TA pretreatment significantly (p < 0.01) enhanced the cumulative biohydrogen yield by 20.89%, achieving a final cumulative biohydrogen production of 68.53 mL/g VS. According to the documented study, TA pretreatment can accelerate the release of organic matter from perishable organic solid waste such as food waste, thereby providing more soluble fermentation substrates for biohydrogen-producing microorganisms [28]. Furthermore, the working conditions subjected to TA pretreatment coupled with varying concentrations of NiCo2O4 NPs exhibited enhanced biohydrogen production. The cumulative biohydrogen yields reached 74.46, 76.96, 73.85, and 73.30 mL/g VS at NiCo2O4 NP concentrations of 50, 100, 150, and 200 mg/L, respectively. These values all surpassed the final biohydrogen production achieved by TA pretreatment alone. Interestingly, when the addition concentration of NiCo2O4 NPs exceeded 100 mg/L, the cumulative biohydrogen production did not show a dose-dependent increase. On one hand, NPs tend to aggregate at high dosages, thereby obstructing the binding sites between substrates and enzymes [29]. On the other hand, the excessive release of Ni2+ may also exert an inhibitory effect on microbial activity [24].
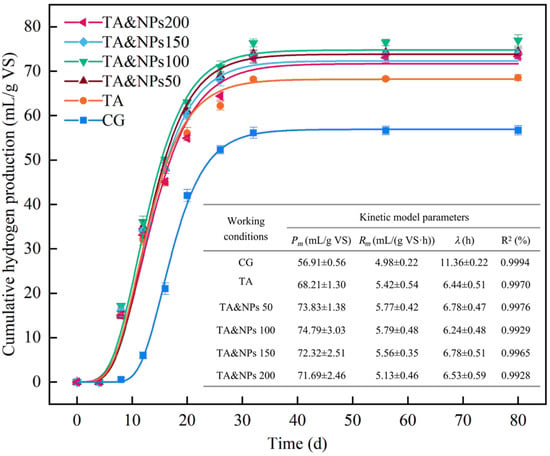
Figure 2.
Cumulative biohydrogen production with time under each working condition. The symbols denote experimental data points, while the curve represents the fitted modified Gompertz model. The table presents the kinetic model parameters for various operating conditions in dark fermentation biohydrogen production. Each symbol represents the average value of triple repetitions.
The modified Gompertz model was further utilized to conduct potential and kinetic analyses of biohydrogen production under different operating conditions, and the fitting curves and corresponding kinetic parameters were also presented in Figure 2. All of the experimental data demonstrated favorable fitting with the model, and the correlation coefficients (R2) of the kinetic model exceeded 99%, indicating that the modified Gompertz model effectively captured the trend of biohydrogen production [30]. The fitting results indicated that both individual TA pretreatment and TA coupled with NiCo2O4 NPs not only enhanced the potential biohydrogen yields and production rates but also effectively reduced the lag-phase time of food waste dark fermentation. This suggested that the combined treatment of TA and NiCo2O4 NPs not only partially alleviates the constrained reaction rate of dissolution and hydrolysis of organic matter in food waste, but also significantly improves the conversion efficiency of organic matter during the dark fermentation process [31]. Evidently, the combined treatment of TA and NiCo2O4 NPs exhibited a synergistic effect on promoting biohydrogen production via dark fermentation of food waste, with the cumulative biohydrogen yield reaching its peak at the dosage of NiCo2O4 NPs of 100 mg/L. Consequently, 100 mg/L was chosen as the optimal dosage of NiCo2O4 NPs for subsequent exploration of the underlying mechanism.
3.3. Effects of TA Pretreatment Coupled with NiCo2O4 NPs on Biochemical Process Related to Dark Fermentation for Biohydrogen Production
During the dark fermentation of food waste for biohydrogen production, the solubilization, hydrolysis, and acidification of organic matter are sequential and interrelated key steps [32, 33], which directly affect the biological accessibility of the substrate and the efficiency of organic matter conversion [34]. As shown in Figure 3A, after pretreatment with TA, the contents of soluble polysaccharides and proteins increased from 3799.04 and 599.80 mg/L in the CG to 4601.83 and 757.02 mg/L, respectively, significantly (p < 0.05) increasing by 21.13% and 26.21%. TA pretreatment facilitated the release of intracellular substances through high-temperature physical disruption, thereby supplying sufficient substrates for subsequent dark fermentation, serving as a critical preliminary step for enhancing biohydrogen production efficiency [12]. However, compared to individual TA pretreatment, the addition of NiCo2O4 NPs did not result in a statistically significant (p > 0.05) increase in the contents of soluble polysaccharides and proteins. This phenomenon may be attributed to the fact that TA pretreatment has already facilitated the release of most soluble organic matter, leading the substrate dissolution to reach a saturation state [35]. Consequently, the introduction of NiCo2O4 NPs could not further surpass this established limit.
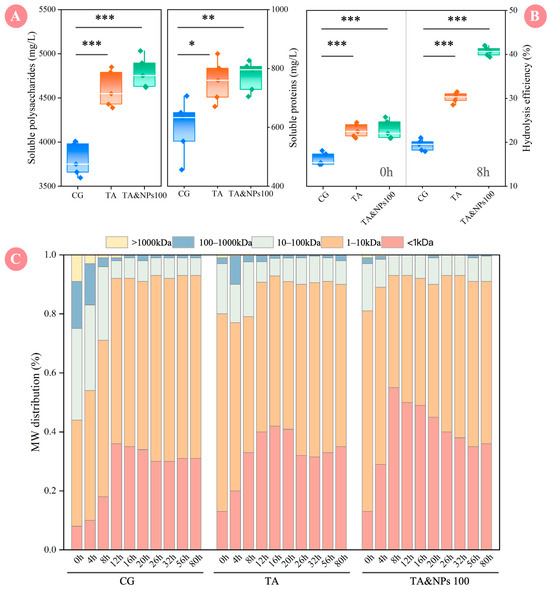
Figure 3.
(A) Concentrations of soluble polysaccharides and soluble proteins; (B) hydrolysis efficiency; (C) MW distribution profile variations throughout the dark fermentation process. Each box corresponds to five measurements and a 95% confidence interval. The asterisks denote the significant levels, * represents p < 0.05, ** represents p < 0.01, *** represents p < 0.001.
In order to quantitatively evaluate the effects of TA pretreatment and NiCo2O4 NPs on the hydrolysis process, the hydrolysis efficiency at different times was calculated based on the release amount of STOC, and the relevant results are shown in Figure 3B. At the initial stage of dark fermentation (0 h), the hydrolysis efficiencies of the CG, TA, and TA&NPs 100 groups were 16.16%, 22.68%, and 22.88%, respectively. The significant (p < 0.01) increase in hydrolysis efficiency after TA pretreatment confirmed its effectiveness in disrupting the structure of food waste and promoting dissolution. When the fermentation reached 8 h, the hydrolysis efficiency of TA&NPs 100 further increased to 40.66%, significantly (p < 0.01) higher than those of TA and CG. This implied that the introduction of NiCo2O4 NPs had a synergistic enhancement effect on the hydrolysis process, and its effect far exceeded that of TA pretreatment alone. Figure 3C further illustrated the temporal variations in the MW distribution of organic matter in the supernatant of CG, TA, and TA&NPs 100, aiming to visually elucidate the regulatory effect of TA coupled with NiCo2O4 NPs treatment on the dark fermentation hydrolysis process at the molecular level. At the initial stage of dark fermentation (0 h), compared to the CG (55.96%), the proportion of macro-molecular substances (MW > 10 kDa) in TA and TA&NPs 100 decreased to 20.04% and 19.12%, respectively. Meanwhile, a moderate increase in low-molecular-weight compounds (MW < 1 kDa) was observed in both TA and TA&NPs 100 compared to CG. This was primarily attributable to TA pretreatment, which accelerated the hydrolysis of macro-molecular organic matter into low-molecular-weight compounds by disrupting cellular structures and chemical bonds [36]. As the fermentation progressed, the MW distribution in the supernatant of each group underwent further changes. Following 8 h fermentation, the proportions of low-molecular-weight compounds (MW < 1 kDa) in the CG, TA, and TA&NPs 100 groups reached 18.03%, 33.21%, and 55.32%, respectively, demonstrating that NiCo2O4 NPs exerted a synergistic effect on the dark fermentation hydrolysis process when combined with TA pretreatment. NPs typically exhibit a high specific surface area and abundant functional groups [34]. On the one hand, NPs can enhance reaction kinetics by increasing the contact area between substrates and hydrolytic enzymes [31]. On the other hand, functional groups on the surface of NPs may catalyze the cleavage of C-O bonds in organic compounds via proton-coupled electron transfer mechanism [37], thereby accelerating the degradation of complex organic substances.
The production of biohydrogen is usually accompanied by the generation of SCFAs [6]. Therefore, the effects of TA pretreatment and TA coupled with NiCo2O4 NPs treatment on the dark fermentation acidification process were investigated. As illustrated in Figure 4, the ratios of butyric acid to acetic acid ratio (B/A > 1) across all experimental conditions in this study remained consistently elevated, indicating a predominance of butyrate-type fermentation within dark fermentation system [38]. This phenomenon was primarily attributable to the abundant carbohydrate content in food waste, which provided an optimal growth environment for butyrate-type hydrogen-producing microorganisms such as Clostridium [39]. Notably, both the TA treatment and the combined treatment of TA with NiCo2O4 NPs resulted in a progressive decrease in the butyric acid/acetic acid ratio compared to the CG, suggesting that more acetic acid was produced within the system. Previous studies have reported that 1 mol of glucose generated 4 mol of H2 when it was converted to acetic acid, while only 2 mol of H2 was released when it was converted to butyric acid [40]. The enhancement of the acetic acid pathway by TA and NiCo2O4 NPs treatment further increased the biohydrogen production within the system. Compared to the CG, the maximum SCFA production in the TA and TA&NPs 100 increased by 12.88% and 22.58%, respectively. Furthermore, the time for the production of SCFAs to reach its peak was shortened from 80 h in the CG to 32 h in both the TA pretreatment and the combined treatment of TA and NiCo2O4 NPs. This could be primarily attributed to the enhancement of the initial dissolution and hydrolysis processes achieved through both TA and NiCo2O4 NPs treatment, providing increased fermentable substrates for acidogenic microorganisms [30], thereby improving acid production efficiency. These findings indicated that TA pretreatment effectively enhanced the bio-accessibility of dark fermentation substrates, while the coupling of NiCo2O4 NPs further improved the convertibility of organic matter.
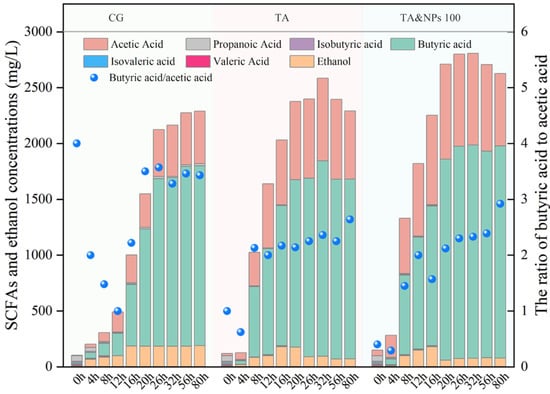
Figure 4.
Production and composition of SCFAs throughout the dark fermentation process.
3.4. Effects of TA Pretreatment Coupled with NiCo2O4 NPs on Key Enzyme Activities
The essence of biohydrogen production from food waste through dark fermentation is the decomposition of organic matter catalyzed by the multi-enzyme system of microorganisms [41], ultimately achieving the synthesis of biohydrogen. Consequently, a further investigation was conducted into the enzyme activities associated with key metabolic stages, including substrate hydrolysis, acidogenesis, and ultimate hydrogen synthesis. As shown in Figure 5, after TA pretreatment, the activities of several key enzymes increased to varying degrees, and the further coupling of NiCo2O4 NPs significantly (p < 0.05) enhanced the activities of all enzymes. Protease and α-glucosidase are key enzymes involved in the hydrolysis process [42], capable of breaking down macro-molecular organic compounds such as proteins and polysaccharides into low-molecular-weight compounds like amino acids and glucose, which are readily utilized by microorganisms. The combined treatment of TA and NiCo2O4 NPs significantly increased the activities of these two enzymes by 19.05% (p < 0.05) and 47.62% (p < 0.001), respectively. Enhancing the activities of these two enzymes could effectively increase the bioavailability of fermentation substrates [43], thereby improving the efficiency of hydrolysis as the rate-limiting step [44]. The SCFA components shown in Figure 4 suggest that the dark fermentation for hydrogen production studied in this work was dominated by butyrate-type fermentation and accompanied by acetic-type fermentation. Therefore, the activity variations in key enzymes involved in acetate and butyrate synthesis pathways including AK and BK were further analyzed. The combined treatment of TA and NiCo2O4 NPs significantly (p < 0.01) increased the activity of AK by 29.47% and extremely significantly (p < 0.001) increased the activity of BK by 41.44%, which was consistent with the elevated SCFA production observed in Figure 4. AK and BK serve as core functional enzymes in dark fermentative biohydrogen production. They not only drive the pivotal conversions in acetate-type and butyrate-type fermentation pathways for biohydrogen production, respectively, but also generate ATP via catalyzing substrate-level phosphorylation, thereby providing essential energy support for microbial biohydrogen metabolism [45]. The significant enhancement of AK and BK activities not only augmented energy supply and metabolic vitality of microbes, but also promoted more efficient channeling of carbon metabolic flux toward acetate-type and butyrate-type fermentation pathways by reinforcing the coupling between energy metabolism and biohydrogen production pathways [46]. This consequently led to a substantial increase in both biohydrogen yields and substrate conversion efficiency of the system. As a terminal biohydrogen-producing enzyme, HYD catalyzes the reduction in protons to molecular hydrogen [47]. In this study, individual TA pretreatment did not significantly (p > 0.05) enhance HYD activity, whereas the subsequent coupling with NiCo2O4 NPs resulted in a significant (p < 0.001) increase of 95.10% in HYD activity compared to CG. Based on the distinct metal composition of their active sites, HYD is primarily classified into [Fe]-HYD, [Fe-Fe]-HYD, and [Ni-Fe]-HYD [48]. Among these, [Fe-Fe]-HYD, and [Ni-Fe]-HYD are predominantly observed in dark fermentation biohydrogen production systems [19]. The catalytic center of [Ni-Fe]-HYD requires Ni2+ as essential cofactors [49]. NiCo2O4 NPs could gradually release Ni2+ and Co2+ in the aqueous phase of the fermentation system [23]. These dissolved Ni2+ could be directly absorbed by microorganisms and supplemented as essential trace elements to the active center of [Ni-Fe]-HYD, promoting its assembly and expression, thereby effectively enhancing the biosynthesis and catalytic activity of [Ni-Fe]-HYD [18]. Moreover, owing to their excellent electrical conductivity, NiCo2O4 NPs could also function as nano-electrical wires, establishing an efficient electron transfer network [50]. This characteristic could effectively increase the transfer rate of electrons from the initial electron donor, such as ferredoxin, to the active center of HYD, thereby directly enhancing the efficiency of electron utilization [51]. Notably, as an essential element in the composition of cobalamin, cobalt can also enhance the electron transfer ability of ferredoxin [20]. This is particularly significant for microorganisms that mainly produce hydrogen through [Fe-Fe]-HYD [20], as it greatly improves their catalytic efficiency. Therefore, NiCo2O4 NPs could activate two major HYD simultaneously through a dual synergistic mechanism involving chemical supplementation (e.g., providing Ni2+) and physical promotion (e.g., accelerating electron transfer), ultimately achieving a substantial enhancement in overall HYD activity.
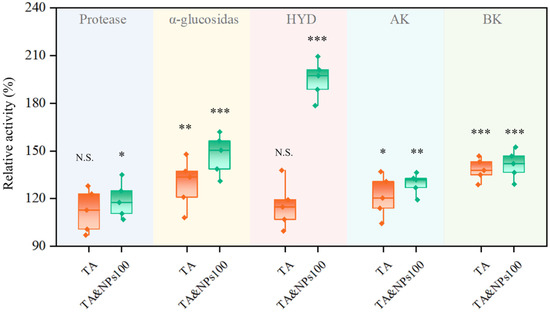
Figure 5.
The relative activities of key enzymes involved in hydrolysis, acidification, and biohydrogen production. Each box corresponds to five measurements and a 95% confidence interval. The asterisks denote the significant levels, * represents p < 0.05, ** represents p < 0.01, *** represents p < 0.001, N.S. represents no significant.
3.5. Effects of TA Pretreatment Coupled with NiCo2O4 NPs on Microbial Community
The succession patterns of microbial community structures during dark fermentation hydrogen production under TA and NiCo2O4 NP treatment was further explored. As shown in Figure 6A, at the initial phase of dark fermentation (0 h), 10 bacterial phyla with abundance ratio greater than 1% were detected across the CG, TA, and TA&NPs 100, and minimal structural distribution differences were observed among them. During this phase, microorganisms were generally in the acclimatization period and have yet developed distinct metabolic functional differentiation. As fermentation progressed to 12 h, Firmicutes gradually emerged as the dominant phylum in both TA and TA&NPs 100 groups, with relative abundances reaching 60.00% in each group, distinctly higher than the 17.00% observed in CG. Firmicutes typically serve as the predominant microbial phylum in dark fermentation biohydrogen production systems utilizing food waste [52], governing both butyrate-type and acetate-type biohydrogen-producing metabolic pathways [53]. The marked increase in its abundance indicated the successful establishment of the ecological niche of biohydrogen-producing microorganisms, laying the community structure foundation for the high biohydrogen production performance of the dark fermentation system. This observation was also consistent with the significant elevation in cumulative biohydrogen yield depicted in Figure 2. Further comparative analysis was conducted to examine the successional rules of microbial communities at the genus level in the three groups as time advanced. As illustrated in Figure 6B, with the progression of dark fermentation process, the genera related to biohydrogen production including Clostridium_sensu_stricto_1, Thermoanaerobacterium, Butyrivibrio, and Ethanoligenens gradually proliferated and became the dominant in all groups. Among them, Clostridium_sensu_stricto_1 was the dominant genus with the highest relative abundance, exhibiting a progressive increase over the fermentation period. At 32 h of fermentation, its relative abundances reached 49.65% in CG, 68.04% in TA, and 69.35% in TA&NPs 100, respectively. As a core biohydrogen-producing bacterium, Clostridium_sensu_stricto_1 typically achieved efficient biohydrogen production through the ferredoxin-hydrogenase pathway [54], a process that relied on the highly efficient involvement of HYD. On the one hand, Clostridium_sensu_stricto_1 possessed the gene encoding HYD, and its enrichment provided a microbial basis for the synthesis of HYD [55]. On the other hand, highly active HYD could also provide a suitable ecological niche for the enrichment of Clostridium_sensu_stricto_1 by modulating the redox balance within the fermentation system [56]. The principal biohydrogen-producing pathway of Thermoanaerobacterium and Butyrivibrio is butyrate-type fermentation [57, 58], and their relative abundances were enriched to varying degrees under the combined treatment of TA and NiCo2O4 NPs. Ethanoligenens, an ethanol-type biohydrogen-producing bacterium [59], did not exhibit enrichment in either the TA or TA&NPs 100, which aligned with the ethanol concentration profiles observed in Figure 4. Furthermore, the combined treatment of TA and NiCo2O4 NPs also enriched functional microorganisms associated with hydrolysis (e.g., Bacteroides) and acidification (e.g., Acetobacterium), which further elucidated the enhancement in hydrolysis rates and SCFA production. The proliferation of these microorganisms equipped with hydrolytic and acidogenic functions established a coherent functional loop with the enhanced activities of key enzymes involved in the hydrolysis (e.g., protease and α-glucosidase) and acidification (e.g., AK and BK) process as previously described, thereby elucidating the ecological mechanism underlying the comprehensive improvement in substrate hydrolysis efficiency, acidification capacity, and ultimate biohydrogen production performance within the dark fermentation system. These findings indicated that the synergistic effect of TA and NiCo2O4 NPs effectively enhanced biohydrogen production efficiency in dark fermentation of food waste by enriching a functional microbial community centered on Clostridium_sensu_stricto_1 and supported by hydrolytic and acidogenic microbes.
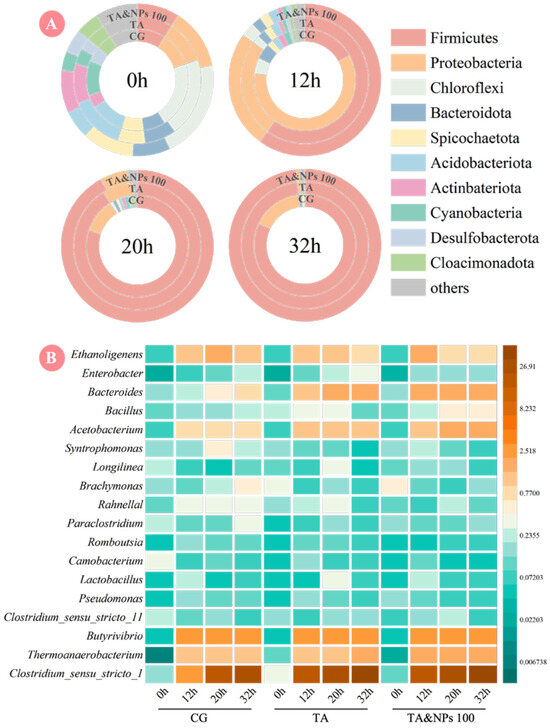
Figure 6.
(A) Variations in microbial community distribution at phylum level during dark fermentation; (B) heatmap of microbial succession at genus level.
3.6. Overall Understanding and Implication
This study systematically elucidated the synergistic enhancement mechanism of TA pretreatment coupled with NiCo2O4 NPs on biohydrogen production from dark fermentation of food waste. The findings demonstrated that this synergistic strategy not only effectively improved the bio-accessibility of substrates through TA pretreatment, but also profoundly optimized the biohydrogen-producing metabolic pathways of microorganisms at the levels of both enzyme activities and microbial community via the incorporation of NiCo2O4 NPs. On the one hand, Ni2+ released from NiCo2O4 NPs served as indispensable cofactors for [Ni-Fe]-HYD, directly facilitating the assembly and expression of key biohydrogen-producing enzymes. On the other hand, the distinctive nanostructure and favorable electrical conductivity of NiCo2O4 NPs functioned as efficient nano-electrical conduits, efficaciously enhancing the electron transfer rate to the HYD active centers. This synergistic effect was further manifested in the enhanced activities of critical enzymes and the selective enrichment of functional microbial communities dominated by core biohydrogen-producing genera.
It should be noted that, as an exploration mechanism, this study employed a short-term batch experimental system. Long-term continuous-flow experiments are necessary for evaluating the application potential of this synergistic strategy, including its stability, safety, and economy. The following key issues need to be addressed in future research.
- (1)
- Nanomaterials often exhibit a concentration-dependent double-edged biological effect characterized by stimulation at low doses and inhibition at high concentrations [60]. The sustained release of Ni2+ and Co2+ from high-concentration NiCo2O4 NPs may exceed the tolerance threshold of microorganisms, thereby inducing metal ion toxicity that disrupted enzymatic functions and triggered oxidative stress [17]. Furthermore, a substantial number of nanomaterials were prone to undergo uncontrolled aggregation and adsorption on cell surfaces, which might not only impede nutrient transport but also cause physical damage to the cell membrane [24]. The combined physical–chemical toxicity can inhibit microbial growth and metabolism, thereby impeding the sustained progression of biohydrogen production. This also constituted the primary reason why no additional biohydrogen production was observed when the concentration of NiCo2O4 NPs exceeded 100 mg/L in this study. Consequently, in future studies, real-time tracking of the stability (e.g., zeta potential, aggregation) of its physical and chemical properties during the fermentation process, as well as the kinetics of metal ion release, will be crucial for precisely regulating the NP addition strategy and ensuring the safety and efficiency of its application.
- (2)
- During long-term operation, NiCo2O4 NPs may lose their activity or even be lost due to physical erosion, chemical corrosion or biological encapsulation. A feasible solution is to utilize the inherent ferromagnetic property of NiCo2O4 NPs, and achieve recovery and reuse by applying an external magnetic field [61, 62], which can significantly reduce long-term operational costs. Therefore, future research should focus on the changes in activity and recovery efficiency of NiCo2O4 NPs after multiple uses in continuous-flow reactors.
- (3)
- Ultimately, a comprehensive life-cycle and techno-economic assessments in long-term continuous-flow reactors are necessary for the transformation of this technology from concept to practice. This encompasses a full-process analysis spanning from the synthesis of nanomaterials, energy consumption during system operation, biohydrogen production revenue, to end-of-life waste disposal, in order to determine its net environmental benefits and economic competitiveness.
4. Conclusions
In summary, the synergistic combination of TA pretreatment and 100 mg/L NiCo2O4 NPs significantly boosted biohydrogen production from food waste by 35.76% (p < 0.01). Mechanism studies indicated that this improvement derived from a dual reinforcement mechanism: TA pretreatment promoted substrate solubilization and hydrolysis, while NiCo2O4 NPs further elevated the activities of key metabolic enzymes, particularly HYD, which showed a 95.10% enhancement (p < 0.01). This strategy successfully created a functional community dominated by the core biohydrogen-producing bacterium Clostridium_sensu_stricto_1, providing a microbial basis for efficient biohydrogen production.
Author Contributions
Conceptualization, Y.-T.Z. and Y.X.; methodology, X.A.; software, Y.-T.Z.; validation, Y.-T.Z., X.A. and J.H.; formal analysis, Y.-T.Z.; investigation, X.A.; resources, X.D.; data curation, X.A.; writing—original draft preparation, Y.-T.Z.; writing—review and editing, Y.X.; visualization, Y.-T.Z.; supervision, X.D.; project administration, Y.X.; funding acquisition, X.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. And we have applied the full waiver to this manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Dataset available on request from the authors.
Acknowledgments
This work was supported by the Natural Science Foundation of China (52470157 and 52131002), the Social Development Program of Science and Technology Committee Foundations of Shanghai (24YF2742100), and the China Postdoctoral Science Foundation (2024M752428).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Don’t wait for COP: The end of the fossil-fuel age must start now. Nature 2023, 613, 216. [CrossRef]
- Sagar, A.D.; Mathur, A.; Birol, F.; Mulugetta, Y.; Ogunbiyi, D.; Sokona, Y.; Steiner, A. Mission Energy Access for a just and sustainable future for all. Nat. Energy 2023, 8, 1171–1173. [Google Scholar] [CrossRef]
- Liu, H.F.; Ampah, J.D.; Afrane, S.; Adun, H.; Jin, C.; Yao, M.F. Potential benefits and trade-offs associated with hydrogen transition under diverse carbon dioxide removal strategies. Sci. Bull. 2024, 69, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.X.; Zhou, Q.; Yu, D.H. The future of hydrogen energy: Bio-hydrogen production technology. Int. J. Hydrogen Energy 2022, 47, 33677–33698. [Google Scholar] [CrossRef]
- Dahiya, S.; Chatterjee, S.; Sarkar, O.; Mohan, S.V. Renewable hydrogen production by dark-fermentation: Current status, challenges and perspectives. Bioresour. Technol. 2021, 321, 124354. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Wei, W.; Wang, C.; Ni, B.J. Microbial and physicochemical responses of anaerobic hydrogen-producing granular sludge to polyethylene micro(nano)plastics. Water Res. 2022, 221, 118745. [Google Scholar] [CrossRef]
- Zan, F.X.; Iqbal, A.; Lu, X.J.; Wu, X.H.; Chen, G.H. “Food waste-wastewater-energy/resource” nexus: Integrating food waste management with wastewater treatment towards urban sustainability. Water Res. 2022, 211, 118089. [Google Scholar] [CrossRef]
- Mohanakrishna, G.; Sneha, N.P.; Rafi, S.M.; Sarkar, O. Dark fermentative hydrogen production: Potential of food waste as future energy needs. Sci. Total Environ. 2023, 888, 163801. [Google Scholar] [CrossRef]
- Karthikeyan, O.P.; Trably, E.; Mehariya, S.; Bernet, N.; Wong, J.W.C.; Carrere, H. Pretreatment of food waste for methane and hydrogen recovery: A review. Bioresour. Technol. 2018, 249, 1025–1039. [Google Scholar] [CrossRef]
- Gbiete, D.; Narra, S.; Kongnine, D.M.; Narra, M.M.; Nelles, M. Insights into Biohydrogen Production Through Dark Fermentation of Food Waste: Substrate Properties, Inocula, and Pretreatment Strategies. Energies 2024, 17, 6350. [Google Scholar] [CrossRef]
- Jia, X.; Li, M.X.; Zhu, J.L.; Jiang, Y.H.; Wang, Y.; Wang, Y.J. Enhancement split-phase hydrogen production from food waste during dark fermentation: Protein substances degradation and transformation during hydrothermal pre-treatments. Int. J. Hydrogen Energy 2019, 44, 17334–17345. [Google Scholar] [CrossRef]
- Dinesh, G.K.; Chauhan, R.; Chakma, S. Influence and strategies for enhanced biohydrogen production from food waste. Renew. Sust. Energ. Rev. 2018, 92, 807–822. [Google Scholar] [CrossRef]
- Yun, Y.M.; Lee, M.K.; Im, S.W.; Marone, A.; Trably, E.; Shin, S.R.; Kin, M.G.; Cho, S.K.; Kim, D.H. Biohydrogen production from food waste: Current status, limitations, and future perspectives. Bioresour. Technol. 2018, 248, 79–87. [Google Scholar] [CrossRef]
- Yang, G.; Wang, J.L. Changes in microbial community structure during dark fermentative hydrogen production. Int. J. Hydrogen Energy 2019, 44, 25542–25550. [Google Scholar] [CrossRef]
- Yang, G.; Wang, J.L. Various additives for improving dark fermentative hydrogen production: A review. Renew. Sustain. Energy Rev. 2018, 95, 130–146. [Google Scholar] [CrossRef]
- Yang, Y.J.; Bu, J.; Tiong, Y.W.; Xu, S.; Zhang, J.X.; He, Y.L.; Zhu, M.J.; Tong, Y.W. Enhanced thermophilic dark fermentation of hydrogen production from food waste by Fe-modified biochar. Environ. Res. 2024, 244, 117946. [Google Scholar] [CrossRef]
- Arun, J.; Sasipraba, T.; Copinath, K.P.; Priyadharsini, P.; Nachiappan, S.; Nachiappan, N.; Dawn, S.S.; Chi, N.T.L.; Pugazhendhi, A. Influence of biomass and nanoadditives in dark fermentation for enriched bio-hydrogen production: A detailed mechanistic review on pathway and commercialization challenges. Fuel 2022, 327, 125112. [Google Scholar] [CrossRef]
- Salazar-Batres, K.J.; Moreno-Andrade, I. Effect of nickel concentration on biohydrogen production: Organic solid waste vs. glucose. Int. J. Hydrogen Energy 2022, 47, 30097–30106. [Google Scholar] [CrossRef]
- Trchounian, K.; Sawers, R.G.; Trchounian, A. Improving biohydrogen productivity by microbial dark- and photo-fermentations: Novel data and future approaches. Renew. Sustain. Energy Rev. 2017, 80, 1201–1216. [Google Scholar] [CrossRef]
- Zhang, J.S.; Zhao, W.Q.; Yang, J.W.; Li, Z.M.; Zhang, J.C.; Zang, L.H. Comparison of mesophilic and thermophilic dark fermentation with nickel ferrite nanoparticles supplementation for biohydrogen production. Bioresour. Technol. 2021, 329, 124853. [Google Scholar] [CrossRef]
- Mishra, P.; Akaniro, I.R.; Zhang, R.L.; Wang, P.X.; Geng, Y.Q.; Li, D.Y.; Xu, Q.X.; Wong, J.W.C.; Zhao, J. Unlocking the Potential of Ni/Fe2O3 Bimetallic Nanoparticles for Fermentative Biohydrogen Production. ACS EST Eng. 2024, 4, 2424–2434. [Google Scholar] [CrossRef]
- Marousek, J.; Gavurova, B.; Marouskova, A. Machine learning enables more efficient (nano)catalyst management, enhancing the competitiveness of (bio)hydrogen production from sewage sludge. Renew. Energy 2026, 256, 124025. [Google Scholar] [CrossRef]
- Li, Z.M.; Wang, J.M.; Tian, K.X.; Zhou, C.; Pei, Y.; Zhang, J.S.; Zang, L.H. Nickel-Cobalt Oxide Nanoparticle-Induced Biohydrogen Production. ACS Omega 2022, 7, 41594–41605. [Google Scholar] [CrossRef]
- He, C.S.; Ding, R.R.; Wang, Y.R.; Li, Q.; Wang, Y.X.; Mu, Y. Insights into short- and long-term effects of loading nickel nanoparticles on anaerobic digestion with flocculent sludge. Environ. Sci. Nano 2019, 6, 2820–2831. [Google Scholar] [CrossRef]
- Liu, J.; Chen, T.T.; Jian, P.M.; Wang, L.X.; Yan, X.D. Hollow urchin-like NiO/NiCo2O4 heterostructures as highly efficient catalysts for selective oxidation of styrene. J. Colloid Interface Sci. 2018, 526, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Xu, D.; Zhang, D.F.; Zhang, G.Z.; Zhang, L. Superior performance of 3 D Co-Ni bimetallic oxides for catalytic degradation of organic dye: Investigation on the effect of catalyst morphology and catalytic mechanism. Appl. Catal. B-Environ. 2016, 186, 193–203. [Google Scholar] [CrossRef]
- Srivastava, N.; Srivastava, M.; Malhotra, B.D.; Gupta, V.K.; Ramteke, P.W.; Silva, R.N.; Shunkla, P.; Dubey, K.K.; Mishra, P.K. Nanoengineered cellulosic biohydrogen production via dark fermentation: A novel approach. Biotechnol. Adv. 2019, 37, 107384. [Google Scholar] [CrossRef]
- Ouafa, A.; Kerroum, D.; Antonio, P.; Lamis, R.; Chaima, B.; Rania, Z.Z.; Mossaab, B.L. Coffee grounds and fruit and vegetable waste co-digestion in dark Fermentation: Evaluation of mixing ratio and hybrid pretreatments impact on bio-hydrogen production. Biomass Bioenergy 2025, 199, 107896. [Google Scholar] [CrossRef]
- Ji, J.; Shen, L. Screening of enhanced biohydrogen production from anaerobic fermentation amended with nano-sized barium ferrite supported by aluminum oxide. Int. J. Hydrogen Energy 2024, 69, 961–973. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, J.W.; Li, C. Synergistic increase of bio-hydrogen production from sludge anaerobic fermentation by thermal hydrolysis coupled with calcium hypochlorite pretreatment. J. Clean. Prod. 2025, 522, 146304. [Google Scholar] [CrossRef]
- Palanivel, P.D.; Jovita, A.; Babu, K.; Mohamed, S.N. Harnessing dark fermentation: Mechanisms, metabolic pathways, and nanoparticle innovations for biohydrogen enhancement. Int. J. Hydrogen Energy 2025, 109, 383–396. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Wei, W.; Huang, Q.S.; Wang, C.; Wang, Y.; Ni, B.J. Insights into the microbial response of anaerobic granular sludge during long-term exposure to polyethylene terephthalate microplastics. Water Res. 2020, 179, 115898. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Wei, W.; Sun, J.; Xu, Q.X.; Ni, B.J. Long-Term Effects of Polyvinyl Chloride Microplastics on Anaerobic Granular Sludge for Recovering Methane from Wastewater. Environ. Sci. Technol. 2020, 54, 9662–9671. [Google Scholar] [CrossRef] [PubMed]
- Brindhadevi, K.; Pugazhendhi, A. Enhancing biohydrogen production through microbial fermentation with the addition of nanometal ions. Renew. Sustain. Energy Rev. 2025, 215, 115552. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, W.; Yang, D.H.; Wu, L.; Chen, X.M.; Dai, X.H.; Ni, B.J. Unraveling temperature effects on caproate and caprylate production from waste activated sludge. Bioresour. Technol. 2025, 417, 131844. [Google Scholar] [CrossRef]
- Chen, H.; Yi, H.; Li, H.C.; Guo, X.S.; Xiao, B.Y. Effects of thermal and thermal-alkaline pretreatments on continuous anaerobic sludge digestion: Performance, energy balance and, enhancement mechanism. Renew. Energy 2020, 147, 2409–2416. [Google Scholar] [CrossRef]
- He, Y.Q.; Zeng, X.; Lu, Z.R.; Mo, S.H.; An, Q.Z.; Liu, Q.H.; Yang, Y.L.; Lan, W.; Wang, S.Y.; Zou, Y.Q. Aqueous Electrocatalytic Hydrogenation Depolymerization of Lignin β-O-4 Linkage via Selective Caryl-O(C) Bond Cleavage: The Regulation of Adsorption. J. Am. Chem. Soc. 2024, 146, 32022–32031. [Google Scholar] [CrossRef]
- Li, W.M.; He, L.; Cheng, C.; Cao, G.L.; Ren, N.Q. Effects of biochar on ethanol-type and butyrate-type fermentative hydrogen productions. Bioresour. Technol. 2020, 306, 123088. [Google Scholar] [CrossRef]
- Detman, A.; Laubitz, D.; Chojnacka, A.; Kiela, P.R.; Salamon, A.; Barberan, A.; Chen, Y.J.; Yang, F.; Blaszczyk, M.K.; Sikora, A. Dynamics of dark fermentation microbial communities in the light of lactate and butyrate production. Microbiome 2021, 9, 158. [Google Scholar] [CrossRef]
- Ghimire, A.; Frunzo, L.; Pirozzi, F.; Trably, E.; Escudie, R.; Lens, P.N.L.; Esposito, G. A review on dark fermentative biohydrogen production from organic biomass: Process parameters and use of by-products. Appl. Energy 2015, 144, 73–95. [Google Scholar] [CrossRef]
- Chen, D.; Kuang, Y.; Wang, H.Y.; Liang, J.J.; Zhao, J.W. Insights into the mechanism of naproxen inhibiting biohydrogen production from sludge dark fermentation. Process Saf. Environ. 2022, 167, 390–397. [Google Scholar] [CrossRef]
- Gao, P.T.; Guo, L.; Sun, J.; Wang, Y.; She, Z.L.; Gao, M.C.; Zhao, Y.G. Accelerating waste sludge hydrolysis with alkyl polyglucose pretreatment coupled with biological process of thermophilic bacteria: Hydrolytic enzyme activity and organic matters transformation. J. Environ. Manag. 2019, 247, 161–168. [Google Scholar] [CrossRef]
- Zhou, W.Y.; Fang, Q.; Ding, W.X.; Xiao, Z.L.; Luo, F.; Ren, P.F.; Wang, G.H. Enhancement of waste activated sludge hydrolysis and decomposition by combined free nitrous acid and sodium citrate pretreatment. J. Water Process Eng. 2023, 54, 103945. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Wei, W.; Ni, B.J. Revealing the mechanism of zinc oxide nanoparticles facilitating hydrogen production in alkaline anaerobic fermentation of waste activated sludge. J. Clean. Prod. 2021, 328, 129580. [Google Scholar] [CrossRef]
- Wang, D.B.; Duan, Y.Y.; Yang, Q.; Liu, Y.W.; Ni, B.J.; Wang, Q.L.; Zeng, G.M.; Li, X.M.; Yuan, Z.G. Free ammonia enhances dark fermentative hydrogen production from waste activated sludge. Water Res. 2018, 133, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.N.; Zhu, M.J.; Zhao, L. Enhanced thermophilic hydrogen production from co-substrate of pretreated waste activated sludge and food waste: Analysis from microbial growth and metabolism. Int. J. Hydrogen Energy 2025, 97, 431–443. [Google Scholar] [CrossRef]
- Ji, H.S.; Wan, L.; Gao, Y.X.; Du, P.; Li, W.J.; Luo, H.; Ning, J.R.; Zhao, Y.Y.; Wang, H.W.; Zhang, L.X.; et al. Hydrogenase as the basis for green hydrogen production and utilization. J. Energy Chem. 2023, 85, 348–362. [Google Scholar] [CrossRef]
- Shobana, S.; Saratale, G.D.; Pugazhendhi, A.; Arvindnarayan, S.; Periyasamy, S.; Kumar, G.; Kim, S.H. Fermentative hydrogen production from mixed and pure microalgae biomass: Key challenges and possible opportunities. Int. J. Hydrogen Energy 2017, 42, 26440–26453. [Google Scholar] [CrossRef]
- Elreedy, A.; Ibrahim, E.; Hassan, N.; El-Dissouky, A.; Fujii, M.; Yoshimura, C.; Tawfik, A. Nickel-graphene nanocomposite as a novel supplement for enhancement of biohydrogen production from industrial wastewater containing mono-ethylene glycol. Energy Convers. Manag. 2017, 140, 133–144. [Google Scholar] [CrossRef]
- Chen, Y.W.; Zhu, K.; Qin, W.L.; Jiang, Z.W.; Hu, Z.F.; Sillanpa, M.; Yan, K. Enhanced electron transfer using NiCo2O4@C hollow nanocages with an electron-shuttle effect for efficient tetracycline degradation. Chem. Eng. J. 2024, 488, 150786. [Google Scholar] [CrossRef]
- Yang, G.; Wang, J.L. Improving mechanisms of biohydrogen production from grass using zero-valent iron nanoparticles. Bioresour. Technol. 2018, 266, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Silva-Martínez, R.D.; Aguilar-Juárez, O.; Díaz-Jiménez, L.; Valdez-Guzmán, B.E.; Aranda-Jaramillo, B.; Carlos-Hernández, S. Biological Hydrogen Production Through Dark Fermentation with High-Solids Content: An Alternative to Enhance Organic Residues Degradation in Co-Digestion with Sewage Sludge. Fermentation 2025, 11, 398. [Google Scholar] [CrossRef]
- Zhang, L.G.; Dong, J.; Guan, S.Y.; Cui, Y.Q.; Du, C.Z.; Ban, Q.Y. Anthraquinone-2,6-disulfonate as exogenous electron shuttles to improve the biohydrogen production from co-fermentation of waste activated sludge and corn stover. J. Environ. Chem. Eng. 2025, 13, 118887. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, S.; Dong, Z.; Cao, S.X.; Yuan, A.K.; Sha, H.; Chen, N. Enhancing dark fermentative hydrogen production from wheat straw through synergistic effects of active electric fields and enzymatic hydrolysis pretreatment. Bioresour. Technol. 2024, 406, 130993. [Google Scholar] [CrossRef]
- Yang, G.; Wang, J. Biohydrogen production by co-fermentation of antibiotic fermentation residue and fallen leaves: Insights into the microbial community and functional genes. Bioresour. Technol. 2021, 337, 125380. [Google Scholar] [CrossRef]
- Chen, L.; Xu, D.; Liang, J.S.; Zhang, Y.J.; Fang, W.; Zhang, P.Y.; Zhang, G.M. New insight into effects of waste scrap iron on sludge anaerobic digestion: Performances, microbial community, and potential metabolic functions. J. Water Process Eng. 2023, 55, 104230. [Google Scholar] [CrossRef]
- Khamtib, S.; Reungsang, A. Biohydrogen production from xylose by Thermoanaerobacterium thermosaccharolyticum KKU19 isolated from hot spring sediment. Int. J. Hydrogen Energy 2012, 37, 12219–12228. [Google Scholar] [CrossRef]
- Li, S.L.; Lin, J.S.; Wang, Y.H.; Lee, Z.K.; Kuo, S.C.; Tseng, I.C.; Cheng, S.S. Strategy of controlling the volumetric loading rate to promote hydrogen-production performance in a mesophilic-kitchen-waste fermentor and the microbial ecology analyses. Bioresour. Technol. 2011, 102, 8682–8687. [Google Scholar] [CrossRef]
- Li, Z.; Gu, J.Y.; Ding, J.; Ren, N.Q.; Xing, D.F. Molecular mechanism of ethanol-H2 co-production fermentation in anaerobic acidogenesis: Challenges and perspectives. Biotechnol. Adv. 2021, 46, 107679. [Google Scholar] [CrossRef]
- Mu, H.; Zheng, X.; Chen, Y.G.; Chen, H.; Liu, K. Response of Anaerobic Granular Sludge to a Shock Load of Zinc Oxide Nanoparticles during Biological Wastewater Treatment. Environ. Sci. Technol. 2012, 46, 5997–6003. [Google Scholar] [CrossRef]
- Geng, H.; Xu, Y.; Liu, R.; Xu, J.; Li, X.; Yang, D.H.; Dai, X.H. Magnetic porous microspheres altering interfacial thermodynamics of sewage sludge to drive metabolic cooperation for efficient methanogenesis. Water Res. 2024, 261, 122022. [Google Scholar] [CrossRef]
- Geng, H.; Xu, Y.; Liu, R.; Yang, D.H.; Dai, X.H. Magnetic porous microspheres enhancing the anaerobic digestion of sewage sludge: Synergistic free and attached methanogenic consortia. Water Res. 2024, 254, 121393. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).