Recent Advances in Biosurfactant Production in Solid-State Fermentation
Abstract
1. Introduction
2. Classification of Biosurfactants
3. Solid State Fermentation (SSF)
4. Industrial and Environmental Applications
5. Production of Biosurfactants by SSF
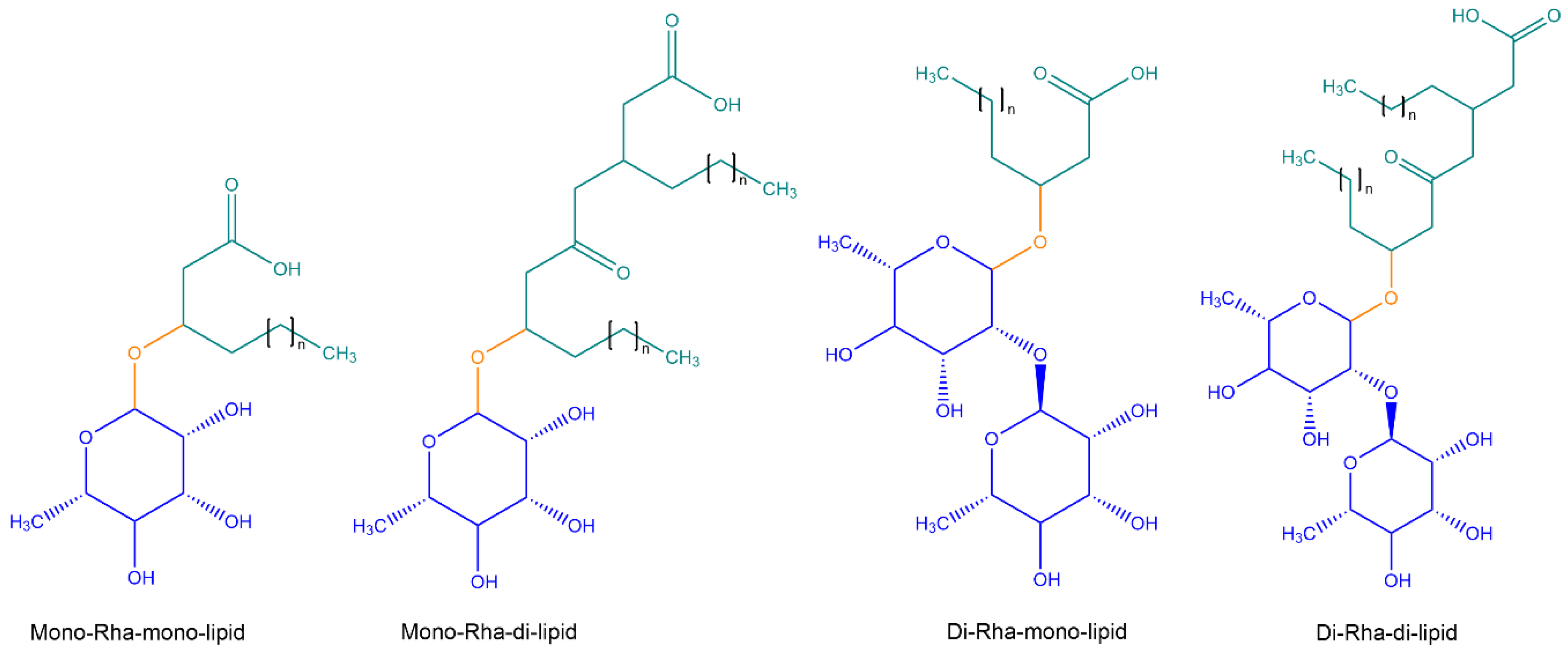
5.1. Rhamnolipids
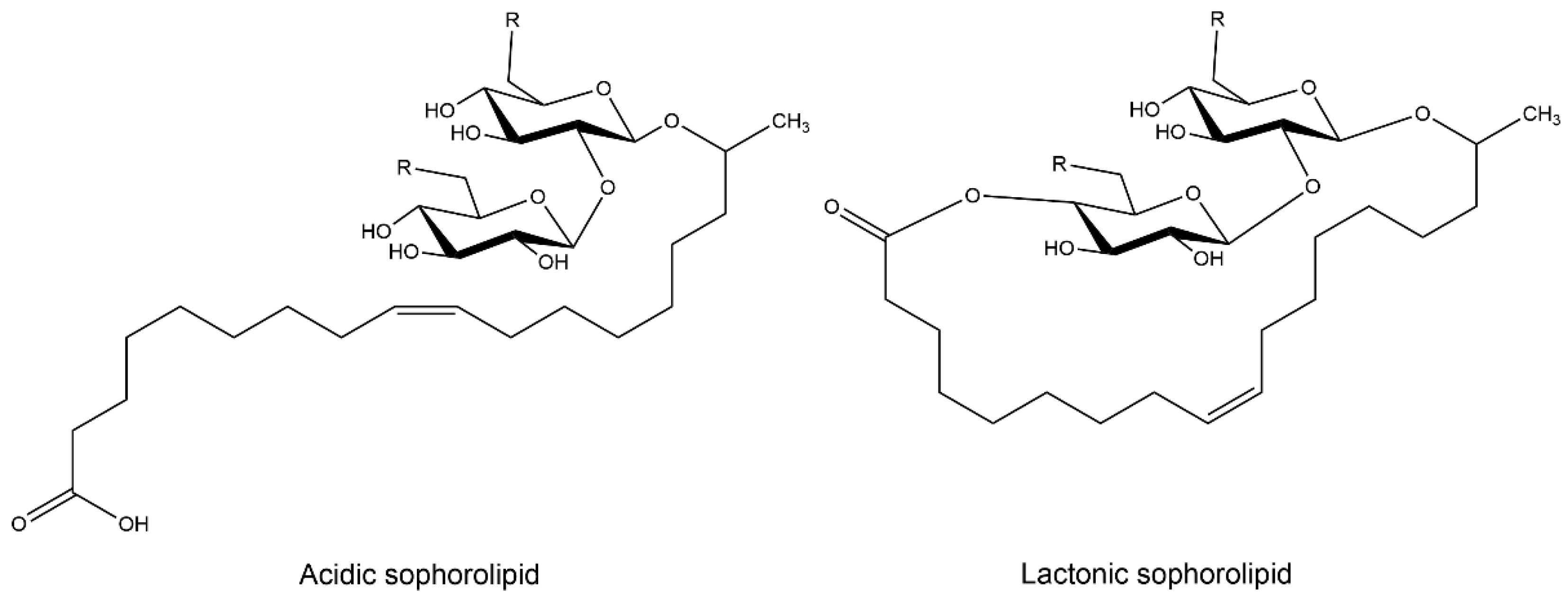
5.2. Sophorolipids
5.3. Mannosyl-Erythritol Lipids (MELs)

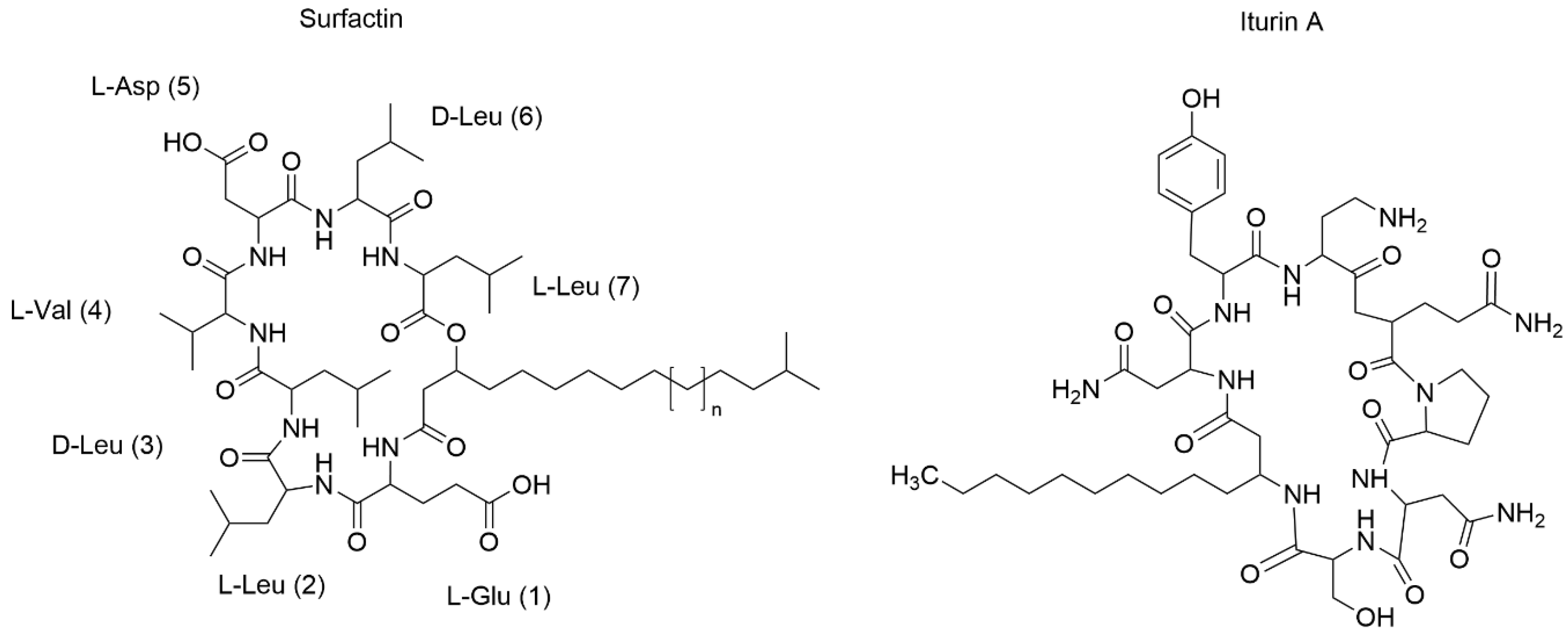
5.4. Lipopeptides
6. Techniques Used to Optimize Solid-State Fermentation
7. Challenges for the Production of Biosurfactants in SSF
7.1. Monitoring and Controlling Process Parameters
7.2. Heat Transfer
7.3. Scale up of SSF
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Artificial intelligence |
| CMC | Critical micellar concentration |
| LP | Lipopeptide |
| MELs | Mannosyl-erythritol lipids |
| RL | Rhamnolipid |
| RTDs | Resistance Temperature Detectors |
| SmF | Submerged fermentation |
| SSF | Solid State Fermentation |
| YPS | Product-substrate yield |
| WOC | Winterization oil cake |
References
- Desai, J.D.; Banat, I.M. Microbial production of surfactants and their commercial potential. Microbiol. Mol. Biol. Rev. 1997, 61, 47–64. [Google Scholar] [CrossRef]
- Banat, I.M.; Makkar, R.S.; Cameotra, S.S. Potential commercial applications of microbial surfactants. Appl. Microbiol. Biotechnol. 2000, 53, 495–508. [Google Scholar] [CrossRef]
- Van Hamme, J.D.; Singh, A.; Ward, O.P. Physiological aspects: Part 1 in a series of papers devoted to surfactants in microbiology and biotechnology. Biotechnol. Adv. 2006, 24, 604–620. [Google Scholar] [CrossRef] [PubMed]
- Nitschke, M.; Silva, S.S.E. Recent food applications of microbial surfactants. Crit. Rev. Food Sci. Nutr. 2018, 58, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Roelants, S.L.K.W.; Soetaert, W. Industrial Perspectives for (Microbial) Biosurfactants. In Biosurfactants for the Biobased Economy; Hausmann, R., Henkel, M., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 1–15. [Google Scholar]
- Varjani, S.J.; Upasani, V.N. A new look on factors affecting microbial degradation of petroleum hydrocarbon pollutants. Int. Biodeterior. Biodegrad. 2017, 120, 71–83. [Google Scholar] [CrossRef]
- Jiménez-Peñalver, P.; Rodríguez, A.; Daverey, A.; Font, X.; Gea, T. Use of wastes for sophorolipids production as a transition to circular economy: State of the art and perspectives. Rev. Environ. Sci. Bio/Technol. 2019, 18, 413–435. [Google Scholar] [CrossRef]
- Pichardo Sánchez, M.A.; Gutiérrez Olvera, Y.M.; Rodríguez Durán, N.A.; Rodríguez Durán, L.V. Producción de biosurfactantes microbianos a partir de subproductos agrícolas. In Bioproductos a Partir de Residuos Agroindustriales; Bustos Vázquez, M.G., Ramírez, D.T., Eds.; Fontamara: Mexico City, Mexico, 2025; pp. 173–192. [Google Scholar]
- Sundaram, T.; Govindarajan, R.K.; Vinayagam, S.; Krishnan, V.; Nagarajan, S.; Gnanasekaran, G.R.; Baek, K.-H.; Rajamani Sekar, S.K. Advancements in biosurfactant production using agro-industrial waste for industrial and environmental applications. Front. Microbiol. 2024, 15, 1357302. [Google Scholar] [CrossRef]
- Eras-Muñoz, E.; Gea, T.; Font, X. Carbon and nitrogen optimization in solid-state fermentation for sustainable sophorolipid production using industrial waste. Front. Bioeng. Biotechnol. 2024, 11, 1252733. [Google Scholar] [CrossRef]
- Taherzadeh, M.J.; Ferreira, J.A.; Pandey, A. Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2023; pp. xvii–xviii. [Google Scholar]
- Miao, Y.; To, M.H.; Siddiqui, M.A.; Wang, H.; Lodens, S.; Chopra, S.S.; Kaur, G.; Roelants, S.L.K.W.; Lin, C.S.K. Sustainable biosurfactant production from secondary feedstock—Recent advances, process optimization and perspectives. Front. Chem. 2024, 12, 1327113. [Google Scholar] [CrossRef]
- Kim, G.B.; Choi, S.Y.; Cho, I.J.; Ahn, D.-H.; Lee, S.Y. Metabolic engineering for sustainability and health. Trends Biotechnol. 2023, 41, 425–451. [Google Scholar] [CrossRef]
- Qamar, S.A.; Pacifico, S. Cleaner production of biosurfactants via bio-waste valorization: A comprehensive review of characteristics, challenges, and opportunities in bio-sector applications. J. Environ. Chem. Eng. 2023, 11, 111555. [Google Scholar] [CrossRef]
- Karmakar, K.; Sarkar, R.; Pal, A.; Rahaman, S.M.; Acharjee, A.; Saha, B. Recent Advances and Emerging Trends in Biosurfactants: A Concise Review. J. Solut. Chem. 2025, 54, 393–420. [Google Scholar] [CrossRef]
- Jiménez-Peñalver, P.; Gea, T.; Sánchez, A.; Font, X. Production of sophorolipids from winterization oil cake by solid-state fermentation: Optimization, monitoring and effect of mixing. Biochem. Eng. J. 2016, 115, 93–100. [Google Scholar] [CrossRef]
- Jiménez-Peñalver, P.; Castillejos, M.; Koh, A.; Gross, R.; Sánchez, A.; Font, X.; Gea, T. Production and characterization of sophorolipids from stearic acid by solid-state fermentation, a cleaner alternative to chemical surfactants. J. Clean. Prod. 2018, 172, 2735–2747. [Google Scholar] [CrossRef]
- Jiménez-Peñalver, P.; Koh, A.; Gross, R.; Gea, T.; Font, X. Biosurfactants from Waste: Structures and Interfacial Properties of Sophorolipids Produced from a Residual Oil Cake. J. Surfactants Deterg. 2020, 23, 481–486. [Google Scholar] [CrossRef]
- Costa, J.A.V.; Treichel, H.; Santos, L.O.; Martins, V.G. Solid-State Fermentation for the Production of Biosurfactants and Their Applications. In Current Developments in Biotechnology and Bioengineering; Pandey, A., Larroche, C., Soccol, C.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 357–372. [Google Scholar]
- Mitchell, D.A.; Berovič, M.; Krieger, N. Solid-State Fermentation Bioreactor Fundamentals: Introduction and Overview. In Solid-State Fermentation Bioreactors: Fundamentals of Design and Operation; Mitchell, D.A., Berovič, M., Krieger, N., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 1–12. [Google Scholar]
- Beatriz-Cuellar, H.; Martínez-Trujillo, M.A.; Membrillo-Venegas, I.d.l.L. Modelado y optimización de la fermentación en estado sólido: El poder de la inteligencia artificial en procesos biotecnológicos. Tend. En Energías Renov. Y Sustentabilidad 2024, 3, 170–174. [Google Scholar] [CrossRef]
- Martins, V.G.; Kalil, S.J.; Costa, J.A.V. In situ bioremediation using biosurfactant produced by solid state fermentation. World J. Microbiol. Biotechnol. 2009, 25, 843–851. [Google Scholar] [CrossRef]
- Eras-Muñoz, E.; Farré, A.; Sánchez, A.; Font, X.; Gea, T. Microbial biosurfactants: A review of recent environmental applications. Bioengineered 2022, 13, 12365–12391. [Google Scholar] [CrossRef]
- Banat, I.M.; Carboué, Q.; Saucedo-Castañeda, G.; de Jesús Cázares-Marinero, J. Biosurfactants: The green generation of speciality chemicals and potential production using Solid-State fermentation (SSF) technology. Bioresour. Technol. 2021, 320, 124222. [Google Scholar] [CrossRef]
- Dabaghi, S.; Ataei, S.A.; Taheri, A. Production of rhamnolipid biosurfactants in solid-state fermentation: Process optimization and characterization studies. BMC Biotechnol. 2023, 23, 2. [Google Scholar] [CrossRef]
- Dabaghi, S.; Ataei, S.A.; Taheri, A. Optimized Bioconversion of Soybean Meal Waste to Valued Biosurfactant by Pseudomonas Aeruginosa (PTCC 1074). Iran. J. Chem. Chem. Eng. -Int. Engl. Ed. 2022, 41, 3582–3590. [Google Scholar] [CrossRef]
- Dabaghi, S.; Ataei, S.A.; Taheri, A. Performance analysis of a laboratory scale rotating drum bioreactor for production of rhamnolipid in solid-state fermentation using an agro-industrial residue. Biomass Convers. Biorefin. 2023, 13, 11513–11520. [Google Scholar] [CrossRef]
- Gong, Z.J.; He, Q.H.; Che, C.C.; Liu, J.F.; Yang, G. Optimization and scale-up of the production of rhamnolipid by Pseudomonas aeruginosa in solid-state fermentation using high-density polyurethane foam as an inert support. Bioprocess Biosyst. Eng. 2020, 43, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, S.; Hejazi, P. Modeling and validating Pseudomonas aeruginosa kinetic parameters based on simultaneous effect of bed temperature and moisture content using lignocellulosic substrate in packed-bed bioreactor. Food Bioprod. Process. 2019, 117, 51–63. [Google Scholar] [CrossRef]
- El-Housseiny, G.S.; Aboshanab, K.M.; Aboulwafa, M.M.; Hassouna, N.A. Rhamnolipid production by a gamma ray-induced Pseudomonas aeruginosa mutant under solid state fermentation. AMB Express 2019, 9, 7. [Google Scholar] [CrossRef]
- Wu, J.R.; Zhang, J.B.; Wang, P.P.; Zhu, L.; Gao, M.J.; Zheng, Z.Y.; Zhan, X.B. Production of rhamnolipids by semi-solid-state fermentation with Pseudomonas aeruginosa RG18 for heavy metal desorption. Bioprocess Biosyst. Eng. 2017, 40, 1611–1619. [Google Scholar] [CrossRef]
- Nalini, S.; Parthasarathi, R. Production and characterization of rhamnolipids produced by Serratia rubidaea SNAU02 under solid-state fermentation and its application as biocontrol agent. Bioresour. Technol. 2014, 173, 231–238. [Google Scholar] [CrossRef]
- Camilios-Neto, D.; Bugay, C.; de Santana, A.P.; Joslin, T.; de Souza, L.M.; Sassaki, G.L.; Mitchell, D.A.; Krieger, N. Production of rhamnolipids in solid-state cultivation using a mixture of sugarcane bagasse and corn bran supplemented with glycerol and soybean oil. Appl. Microbiol. Biotechnol. 2011, 89, 1395–1403. [Google Scholar] [CrossRef]
- Camilios Neto, D.; Meira, J.A.; de Araújo, J.M.; Mitchell, D.A.; Krieger, N. Optimization of the production of rhamnolipids by Pseudomonas aeruginosa UFPEDA 614 in solid-state culture. Appl. Microbiol. Biotechnol. 2008, 81, 441–448. [Google Scholar] [CrossRef]
- Rodriguez, A.; Gea, T.; Font, X. Sophorolipids Production from Oil Cake by Solid-State Fermentation. Inventory for Economic and Environmental Assessment. Front. Chem. Eng. 2021, 3, 632752. [Google Scholar] [CrossRef]
- Rashad, M.M.; Nooman, M.U.; Ali, M.M.; Al-Kashef, A.S.; Mahmoud, A.E. Production, characterization and anticancer activity of Candida bombicola sophorolipids by means of solid state fermentation of sunflower oil cake and soybean oil. Grasas Aceites 2014, 65, e017. [Google Scholar] [CrossRef]
- Bueno-Mancebo, J.; Eras-Munoz, E.; Gea, T.; Artola, A.; Barrena, R. Preliminary study on novel sustainable production of mannosylerythritol lipids by solid-state fermentation. Environ. Technol. Innov. 2025, 38, 104144. [Google Scholar] [CrossRef]
- Bouassida, M.; Mnif, I.; Hammami, I.; Triki, M.A.; Ghribi, D. Bacillus subtilis SPB1 lipopeptide biosurfactant: Antibacterial efficiency against the phytopathogenic bacteria Agrobacterium tumefaciens and compared production in submerged and solid state fermentation systems. Food Sci. Biotechnol. 2023, 32, 1595–1609. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, R.A.; Rodríguez, D.M.; Ferreira, I.N.D.; de Almeida, S.M.; Takaki, G.M.D.; de Lima, M.A.B. Novel production of biodispersant by Serratia marcescens UCP 1549 in solid-state fermentation and application for oil spill bioremediation. Environ. Technol. 2022, 43, 2956–2967. [Google Scholar] [CrossRef]
- Al-Dhabi, N.A.; Esmail, G.A.; Arasu, M.V. Enhanced Production of Biosurfactant from Bacillus subtilis Strain Al-Dhabi-130 under Solid-State Fermentation Using Date Molasses from Saudi Arabia for Bioremediation of Crude-Oil-Contaminated Soils. Int. J. Environ. Res. Public Health 2020, 17, 8446. [Google Scholar] [CrossRef]
- Mnif, I.; Elleuch, M.; Chaabouni, S.E.; Ghribi, D. Bacillus subtilis SPB1 biosurfactant: Production optimization and insecticidal activity against the carob moth Ectomyelois ceratoniae. Crop Prot. 2013, 50, 66–72. [Google Scholar] [CrossRef]
- Ghribi, D.; Abdelkefi-Mesrati, L.; Mnif, I.; Kammoun, R.; Ayadi, I.; Saadaoui, I.; Maktouf, S.; Chaabouni-Ellouze, S. Investigation of Antimicrobial Activity and Statistical Optimization of Bacillus subtilis SPB1 Biosurfactant Production in Solid-State Fermentation. J. Biomed. Biotechnol. 2012, 2012, 373682. [Google Scholar] [CrossRef]
- Das, K.; Mukherjee, A.K. Comparison of lipopeptide biosurfactants production by Bacillus subtilis strains in submerged and solid state fermentation systems using a cheap carbon source:: Some industrial applications of biosurfactants. Process Biochem. 2007, 42, 1191–1199. [Google Scholar] [CrossRef]
- Sun, D.F.; Liao, J.M.; Sun, L.J.; Wang, Y.L.; Liu, Y.; Deng, Q.; Zhang, N.; Xu, D.F.; Fang, Z.J.; Wang, W.J.; et al. Effect of media and fermentation conditions on surfactin and iturin homologues produced by Bacillus natto NT-6: LC-MS analysis. AMB Express 2019, 9, 120. [Google Scholar] [CrossRef]
- Valdés-Velasco, L.M.; Favela-Torres, E.; Théatre, A.; Arguelles-Arias, A.; Saucedo-Castañeda, J.G.; Jacques, P. Relationship between lipopeptide biosurfactant and primary metabolite production by Bacillus strains in solid-state and submerged fermentation. Bioresour. Technol. 2022, 345, 126556. [Google Scholar] [CrossRef]
- Lourenço, L.A.; Magina, M.D.A.; Tavares, L.B.B.; de Souza, S.; Román, M.G.; Vaz, D.A. Biosurfactant production by Trametes versicolor grown on two-phase olive mill waste in solid-state fermentation. Environ. Technol. 2018, 39, 3066–3076. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, F.G.; Wei, Z.; Ran, W.; Shen, Q.R. The usage of rice straw as a major substrate for the production of surfactin by Bacillus amyloliquefaciens XZ-173 in solid-state fermentation. J. Environ. Manag. 2013, 127, 96–102. [Google Scholar] [CrossRef]
- Ohno, A.; Ano, T.; Shoda, M. Production of a lipopeptide antibiotic, surfactin, by recombinant Bacillus-subtilis in solid-state fermentation. Biotechnol. Bioeng. 1995, 47, 209–214. [Google Scholar] [CrossRef]
- Tang, Z.M.; Cao, X.J.; Zhang, H.L. Production of iturin A by Bacillus velezensis ND and its biological control characteristics. J. Basic Microbiol. 2023, 63, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Velioglu, Z.; Ürek, R. Biosurfactant production by Pleurotus ostreatus in submerged and solid-state fermentation systems. Turk. J. Biol. 2015, 39, 160–166. [Google Scholar] [CrossRef]
- Velioglu, Z.; Urek, R.O. Optimization of cultural conditions for biosurfactant production by Pleurotus djamor in solid state fermentation. J. Biosci. Bioeng. 2015, 120, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zu, Y.; Li, X.; Meng, Q.; Long, X. Recent progress towards industrial rhamnolipids fermentation: Process optimization and foam control. Bioresour. Technol. 2020, 298, 122394. [Google Scholar] [CrossRef]
- Lavanya, M. Rhamnolipids: An insight to the overall characteristics of these extraordinary biomolecules. Green Chem. Lett. Rev. 2024, 17, 2371012. [Google Scholar] [CrossRef]
- Soberón-Chávez, G.; González-Valdez, A.; Soto-Aceves, M.P.; Cocotl-Yañez, M. Rhamnolipids produced by Pseudomonas: From molecular genetics to the market. Microb. Biotechnol. 2021, 14, 136–146. [Google Scholar] [CrossRef]
- Nicolò, M.S.; Cambria, M.G.; Impallomeni, G.; Rizzo, M.G.; Pellicorio, C.; Ballistreri, A.; Guglielmino, S.P.P. Carbon source effects on the mono/dirhamnolipid ratio produced by Pseudomonas aeruginosa L05, a new human respiratory isolate. New Biotechnol. 2017, 39, 36–41. [Google Scholar] [CrossRef]
- Funston, S.J.; Tsaousi, K.; Smyth, T.J.; Twigg, M.S.; Marchant, R.; Banat, I.M. Enhanced rhamnolipid production in Burkholderia thailandensis transposon knockout strains deficient in polyhydroxyalkanoate (PHA) synthesis. Appl. Microbiol. Biotechnol. 2017, 101, 8443–8454. [Google Scholar] [CrossRef]
- Li, J.; Xia, C.; Fang, X.; Xue, H.; Song, X. Identification and characterization of a long-chain fatty acid transporter in the sophorolipid-producing strain Starmerella bombicola. Appl. Microbiol. Biotechnol. 2016, 100, 7137–7150. [Google Scholar] [CrossRef] [PubMed]
- Roelants, S.; Solaiman, D.K.Y.; Ashby, R.D.; Lodens, S.; Van Renterghem, L.; Soetaert, W. Production and applications of sophorolipids. In Biobased Surfactants, 2nd ed.; Hayes, D.G., Solaiman, D.K.Y., Ashby, R.D., Eds.; AOCS Press: Champaign, IL, USA, 2019; pp. 65–119. [Google Scholar]
- Pichardo Sánchez, M.A. Producción de Biosurfactantes en Cultivo en Medio Sólido. Master’s Thesis, Universidad Autónoma Metropolitana, Iztapalapa, Mexico, 2019. [Google Scholar]
- Hirata, Y.; Igarashi, K.; Ueda, A.; Quan, G.L. Enhanced sophorolipid production and effective conversion of waste frying oil using dual lipophilic substrates. Biosci. Biotechnol. Biochem. 2021, 85, 1763–1771. [Google Scholar] [CrossRef] [PubMed]
- Pekin, G.; Vardar-Sukan, F.; Kosaric, N. Production of sophorolipids from Candida bombicola ATCC 22214 using turkish corn oil and honey. Eng. Life Sci. 2005, 5, 357–362. [Google Scholar] [CrossRef]
- Pal, S.; Chatterjee, N.; Das, A.K.; McClements, D.J.; Dhar, P. Sophorolipids: A comprehensive review on properties and applications. Adv. Colloid Interface Sci. 2023, 313, 102856. [Google Scholar] [CrossRef]
- Wongsirichot, P.; Winterburn, J. Recent advances in sophorolipid production: From laboratory to pilot scale and beyond. J. Clean. Prod. 2025, 492, 144898. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, X.; Liu, G.; Zhao, G.; Fang, X.; Song, X. A Cumulative Effect by Multiple-Gene Knockout Strategy Leads to a Significant Increase in the Production of Sophorolipids in Starmerella Bombicola CGMCC 1576. Front. Bioeng. Biotechnol. 2022, 10, 818445. [Google Scholar] [CrossRef]
- Morita, T.; Fukuoka, T.; Imura, T.; Kitamoto, D. Mannosylerythritol lipids: Production and applications. J. Oleo Sci. 2015, 64, 133–141. [Google Scholar] [CrossRef]
- Mujumdar, S.; Bashetti, S.; Pardeshi, S.; Thombre, R.S. Industrial applications of biosurfactants. In Industrial Biotechnology; Apple Academic Press: Point Pleasant, NJ, USA, 2017; pp. 81–109. [Google Scholar]
- Paulino, B.N.; Pessôa, M.G.; Molina, G.; Kaupert Neto, A.A.; Oliveira, J.V.C.; Mano, M.C.R.; Pastore, G.M. Biotechnological production of value-added compounds by ustilaginomycetous yeasts. Appl. Microbiol. Biotechnol. 2017, 101, 7789–7809. [Google Scholar] [CrossRef] [PubMed]
- Rau, U.; Nguyen, L.A.; Schulz, S.; Wray, V.; Nimtz, M.; Roeper, H.; Koch, H.; Lang, S. Formation and analysis of mannosylerythritol lipids secreted by Pseudozyma aphidis. Appl. Microbiol. Biotechnol. 2005, 66, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Rau, U.; Nguyen, L.A.; Roeper, H.; Koch, H.; Lang, S. Fed-batch bioreactor production of mannosylerythritol lipids secreted by Pseudozyma aphidis. Appl. Microbiol. Biotechnol. 2005, 68, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Pilz, M.; Cavelius, P.; Qoura, F.; Awad, D.; Brück, T. Lipopeptides development in cosmetics and pharmaceutical applications: A comprehensive review. Biotechnol. Adv. 2023, 67, 108210. [Google Scholar] [CrossRef]
- Sankhyan, S.; Kumar, P.; Pandit, S.; Kumar, S.; Ranjan, N.; Ray, S. Biological machinery for the production of biosurfactant and their potential applications. Microbiol. Res. 2024, 285, 127765. [Google Scholar] [CrossRef]
- Ali, N.; Pang, Z.; Wang, F.; Xu, B.; El-Seedi, H.R. Lipopeptide Biosurfactants from Bacillus spp.: Types, Production, Biological Activities, and Applications in Food. J. Food Qual. 2022, 2022, 3930112. [Google Scholar] [CrossRef]
- Neira-Vielma, A.A.; Aguilar, C.N.; Ilyina, A.; Contreras-Esquivel, J.C.; Carneiro-da-Cunha, M.d.G.; Michelena-Álvarez, G.; Martínez-Hernández, J.L. Purification and biochemical characterization of an Aspergillus niger phytase produced by solid-state fermentation using triticale residues as substrate. Biotechnol. Rep. 2018, 17, 49–54. [Google Scholar] [CrossRef]
- Wittmann, C.L.; James, C. Industrial Biotechnology: Products and Processes; Wiley-VCH: Weinheim, Germany, 2017; Volume 4. [Google Scholar]
- Carboué, Q.; Rébufa, C.; Hamrouni, R.; Roussos, S.; Bombarda, I. Statistical approach to evaluate effect of temperature and moisture content on the production of antioxidant naphtho-gamma-pyrones and hydroxycinnamic acids by Aspergillus tubingensis in solid-state fermentation. Bioprocess Biosyst. Eng. 2020, 43, 2283–2294. [Google Scholar] [CrossRef]
- Borkertas, S.; Viskelis, J.; Viskelis, P.; Streimikyte, P.; Gasiunaite, U.; Urbonaviciene, D. Fungal Biomass Fermentation: Valorizing the Food Industry’s Waste. Fermentation 2025, 11, 351. [Google Scholar] [CrossRef]
- Kumar, V.; Yadav, S.K.; Kumar, J.; Ahluwalia, V. A critical review on current strategies and trends employed for removal of inhibitors and toxic materials generated during biomass pretreatment. Bioresour. Technol. 2020, 299, 122633. [Google Scholar] [CrossRef]
- Perwez, M.; Al Asheh, S. Valorization of agro-industrial waste through solid-state fermentation: Mini review. Biotechnol. Rep. 2025, 45, e00873. [Google Scholar] [CrossRef]
- Gassara, F.; Ajila, C.M.; Brar, S.K.; Tyagi, R.D.; Verma, M.; Valero, J. Influence of aeration and agitation modes on solid-state fermentation of apple pomace waste by Phanerochaete chrysosporium to produce ligninolytic enzymes and co-extract polyphenols. Int. J. Food Sci. Technol. 2013, 48, 2119–2126. [Google Scholar] [CrossRef]
- Pallín, M.Á.; González-Rodríguez, S.; Eibes, G.; López-Abelairas, M.; Moreira, M.T.; Lema, J.M.; Lú-Chau, T.A. Towards industrial application of fungal pretreatment in 2G biorefinery: Scale-up of solid-state fermentation of wheat straw. Biomass Convers. Biorefin. 2024, 14, 593–605. [Google Scholar] [CrossRef]
- Zhu, X.; Deng, Z.; Wang, Q.; Hao, S.; Liu, P.; He, S.; Li, X. Improvement in Palm Kernel Meal Quality by Solid-Sate Fermentation with Bacillus velezensis, Saccharomyces cerevisiae and Lactobacillus paracasei. Fermentation 2024, 10, 655. [Google Scholar] [CrossRef]
- The Food Tech. Implementación de Sistemas de Inteligencia Artificial Para Optimizar la Fermentación en la Producción de Bebidas. Available online: https://thefoodtech.com/soluciones-y-tecnologia-alimentaria/implementacion-de-sistemas-de-inteligencia-artificial-para-optimizar-la-fermentacion-en-la-produccion-de-bebidas/ (accessed on 22 August 2025).
- Coral Mendoza, B.A.; Córdova Rey, M.B.; Jiménez Delgado, R.R. Evaluación de la calidad de bebidas fermentadas mediante la aplicación de un flujograma establecido por Inteligencia Artificial. Código Científico Rev. Investig. 2025, 6, 1460–1482. [Google Scholar] [CrossRef]
- Secpho. Inteligencia Artificial y Machine Learning Aplicada a la Identificación de Microorganismos de la Fermentación en Bebidas. Available online: https://www.secpho.org/noticias/inteligencia-artificial-y-machine-learning-aplicada-a-la-identificacion-de-microorganismos-de-la-fermentacion-en-bebidas (accessed on 9 October 2025).
- Jimenez-Peralta, F.; Pizango-Linares, G.E. Sistema inteligente basado en deep learning para la optimización de la fermentación del cacao. Rev. Amaz. Digit. 2023, 2, e277. [Google Scholar] [CrossRef]
- Bezerra, C.O.; Carneiro, L.L.; Carvalho, E.A.; das Chagas, T.P.; de Carvalho, L.R.; Uetanabaro, A.P.T.; da Silva, G.P.; da Silva, E.G.P.; da Costa, A.M. Artificial Intelligence as a Combinatorial Optimization Strategy for Cellulase Production by Trichoderma stromaticum AM7 Using Peach-Palm Waste Under Solid-State Fermentation. BioEnergy Res. 2021, 14, 1161–1170. [Google Scholar] [CrossRef]
- Galeano-Arias, L.F.; Aguirre, S.G.; Castrillón-Gómez, O.D. Análisis de calidad del vino por medio de técnicas de inteligencia artificial. Inf. Tecnol. 2021, 32, 17–26. [Google Scholar] [CrossRef]
- Rivera Demanuel, D.R.; Huamani Huancara, C.; Charca Ccama, Y.A. Sistema automático para calificación de vino mediante Redes Neuronales. Innovación Y Softw. 2022, 3, 30–46. [Google Scholar] [CrossRef]
- Palencia Argel, M.P.B.A.; Diana, M.; Roncancio Lucas, O.D. Propuesta de Modelo Predictivo para Determinar Preferencias y Factores que Influyen en el Consumo de Cerveza Artesanal en Mujeres. Bachelor’s thesis, Universidad Ean, Bogotá, Colombia, 2024. [Google Scholar]
- Rodera Martínez, P. La Inteligencia Artificial como aliada en el proceso creativo: Un estudio de caso con celulosa bacteriana como biomaterial para el diseño. Cuad. Del Cent. De Estud. De Diseño Y Comun. 2025, 256, 93–110. [Google Scholar] [CrossRef]
- Kabir, M.F.; Ovi, A.Q.; Ju, L.-K. Real-time pH and temperature monitoring in solid-state fermentation reveals culture physiology and optimizes enzyme harvesting for tailored applications. Microb. Cell Factories 2025, 24, 188. [Google Scholar] [CrossRef]
- Jiang, H.; Liu, G.; Mei, C.; Yu, S.; Xiao, X.; Ding, Y. Rapid determination of pH in solid-state fermentation of wheat straw by FT-NIR spectroscopy and efficient wavelengths selection. Anal. Bioanal. Chem. 2012, 404, 603–611. [Google Scholar] [CrossRef]
- Fernández, M.; Pérez-Correa, J.R. Instrumentation for Monitoring SSF Bioreactors. In Solid-State Fermentation Bioreactors: Fundamentals of Design and Operation; Mitchell, D.A., Berovič, M., Krieger, N., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 363–374. [Google Scholar]
- Jin, G.; Zhao, Y.; Xin, S.; Li, T.; Xu, Y. Solid-State Fermentation engineering of traditional chinese fermented food. Foods 2024, 13, 3003. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Duran, L.V.; Torres-Mancera, M.T.; Trujillo-Roldán, M.A.; Valdez-Cruz, N.A.; Favela-Torres, E.; Saucedo-Castañeda, G. Standard Instruments for Bioprocess Analysis and Control. In Current Developments in Biotechnology and Bioengineering; Larroche, C., Sanromán, M.Á., Du, G., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 593–626. [Google Scholar]
- Méndez-González, F.; Loera, O.; Saucedo-Castañeda, G.; Favela-Torres, E. Forced aeration promotes high production and productivity of infective conidia from Metarhizium robertsii in solid-state fermentation. Biochem. Eng. J. 2020, 156, 107492. [Google Scholar] [CrossRef]
- Pitol, L.O.; Finkler, A.T.J.; Dias, G.S.; Machado, A.S.; Zanin, G.M.; Mitchell, D.A.; Krieger, N. Optimization studies to develop a low-cost medium for production of the lipases of Rhizopus microsporus by solid-state fermentation and scale-up of the process to a pilot packed-bed bioreactor. Process Biochem. 2017, 62, 37–47. [Google Scholar] [CrossRef]
- Lopez-Ramirez, N.; Volke-Sepulveda, T.; Gaime-Perraud, I.; Saucedo-Castañeda, G.; Favela-Torres, E. Effect of stirring on growth and cellulolytic enzymes production by Trichoderma harzianum in a novel bench-scale solid-state fermentation bioreactor. Bioresour. Technol. 2018, 265, 291–298. [Google Scholar] [CrossRef]
- Kumar, V.; Ahluwalia, V.; Saran, S.; Kumar, J.; Patel, A.K.; Singhania, R.R. Recent developments on solid-state fermentation for production of microbial secondary metabolites: Challenges and solutions. Bioresour. Technol. 2021, 323, 124566. [Google Scholar] [CrossRef]
| Group | Sub-Group | Class |
|---|---|---|
| Low Molecular weight | Glycolipids | Rhamnolipids |
| Sophorolipids | ||
| Trehalolipids | ||
| Mannosylerythritol lipids | ||
| Lipopeptides | Bacillus-related LP | |
| Pseudomonas-related LP | ||
| Other bacteria-related LP | ||
| Fungi-related LP | ||
| Fatty acids, neutral lipids, and phospholipids | Fatty acids | |
| Neutral lipids | ||
| Phospholipids | ||
| High molecular weight | Polymeric biosurfactants | Emulsan |
| Biodispersan | ||
| Alasan | ||
| Liposan | ||
| Particulate biosurfactants | Vesicles and fimbriae | |
| Whole cells |
| Biosurfactant Type | Microorganism | Solid Support | Yield (g/kg Solid Support) | Reference |
|---|---|---|---|---|
| Rhamnolipids | Pseudomonas aeruginosa | Soybean meal | 19.68 | [25] |
| Rhamnolipids | Pseudomonas aeruginosa | Soybean meal | 17.05 | [26] |
| Rhamnolipids | Pseudomonas aeruginosa | Soybean meal | 12.6 | [27] |
| Rhamnolipids | Pseudomonas aeruginosa | Polyurethane foam | 39.8 * | [28] |
| Rhamnolipids | Pseudomonas aeruginosa | Corn bran and corn germ | 19.5 | [29] |
| Rhamnolipids | Pseudomonas aeruginosa | Sugarcane bagasse and sunflower seed meal | 46.85 * | [30] |
| Rhamnolipids | Pseudomonas aeruginosa | Rapeseed meal and wheat bran | 18.7 * | [31] |
| Rhamnolipids | Serratia rubidaea | Mahua oil cake | N.D. | [32] |
| Rhamnolipids | Pseudomonas aeruginosa | Sugarcane bagasse and corn bran | 45 * | [33] |
| Rhamnolipids | Pseudomonas aeruginosa | Sugarcane bagasse and sunflower seed meal | 172 | [34] |
| Sophorolipids | Starmerella bombicola | Wheat bran and winterization oil cake | 190 | [35] |
| Sophorolipids | Starmerella bombicola | Winterization oil cake | 141 | [10] |
| Sophorolipids | Starmerella bombicola | Sunflower oil cake | N.D. | [18] |
| Sophorolipids | Starmerella bombicola | Polyurethane foam | 211 | [17] |
| Sophorolipids | Starmerella bombicola | Winterization oil cake and wheat straw | 179 | [16] |
| Sophorolipids | Starmerella bombicola | Sunflower oil cake | 495 | [36] |
| Mannosyl-erythritol lipids | Moesziomyces bullatus | Wheat bran and winterization oil cake | 98.0 | [37] |
| Mannosyl-erythritol lipids | Ustilago maydis | Wheat bran and winterization oil cake | 12.2 | [37] |
| Lipopeptides | Bacillus subtilis | Aleppo pine waste | 27.59 | [38] |
| Lipopeptides | Serratia marcescens | Wheat bran | 52 | [39] |
| Lipopeptides | Bacillus subtilis | Date molasses | N.D. | [40] |
| Lipopeptides | Bacillus subtilis | Tuna fish flour and potato waste flour | 116 | [41] |
| Lipopeptides | Bacillus subtillis | Millet | 20.8 | [42] |
| Lipopeptides | Bacillus subtilis | Potato peels | 67 | [43] |
| Lipopeptides (surfactin and iturin) | Bacillus natto | Wheat bran and bean pulp | 4.40 | [44] |
| Lipopeptides (surfactin and fengycin) | Bacillus subtillis | Polyurethane foam | 3.1 * | [45] |
| Lipopeptides | Trametes versicolor | Two-phase olive mill waste | 3.7 | [46] |
| Surfactin | Bacillus amyloliquefaciens | Rice straw and soybean flour | 15.03 | [47] |
| Surfactin | Bacillus subtilis | Okara | 2 | [48] |
| Iturin | Bacillus velezensis | Soybean meal powder | 12.46 | [49] |
| Carbohydrate–peptide–lipid complex | Pleurotus ostreatus | Sunflower seed shell | 4.69 * | [50] |
| Protein-polysaccharide-lipid complex | Pleurotus djamor | Sunflower seed shell | 10.2 | [51] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bustos-Vázquez, M.G.; Rodríguez-Durán, L.V.; Pichardo-Sánchez, M.A.; Rodríguez-Durán, N.R.; Rodríguez-Durán, N.A.; Trujillo-Ramírez, D.; Torres-de los Santos, R. Recent Advances in Biosurfactant Production in Solid-State Fermentation. Fermentation 2025, 11, 592. https://doi.org/10.3390/fermentation11100592
Bustos-Vázquez MG, Rodríguez-Durán LV, Pichardo-Sánchez MA, Rodríguez-Durán NR, Rodríguez-Durán NA, Trujillo-Ramírez D, Torres-de los Santos R. Recent Advances in Biosurfactant Production in Solid-State Fermentation. Fermentation. 2025; 11(10):592. https://doi.org/10.3390/fermentation11100592
Chicago/Turabian StyleBustos-Vázquez, Ma. Guadalupe, Luis V. Rodríguez-Durán, María Alejandra Pichardo-Sánchez, Nubia R. Rodríguez-Durán, Nadia A. Rodríguez-Durán, Daniel Trujillo-Ramírez, and Rodolfo Torres-de los Santos. 2025. "Recent Advances in Biosurfactant Production in Solid-State Fermentation" Fermentation 11, no. 10: 592. https://doi.org/10.3390/fermentation11100592
APA StyleBustos-Vázquez, M. G., Rodríguez-Durán, L. V., Pichardo-Sánchez, M. A., Rodríguez-Durán, N. R., Rodríguez-Durán, N. A., Trujillo-Ramírez, D., & Torres-de los Santos, R. (2025). Recent Advances in Biosurfactant Production in Solid-State Fermentation. Fermentation, 11(10), 592. https://doi.org/10.3390/fermentation11100592









