Bioremediation of Polycyclic Aromatic Hydrocarbons (PAHs) in Aqueous Environments: A Review of Biofiltration, Biosorption, and Biodegradation Strategies Using Living Fungal Mycelium
Abstract
1. Introduction
1.1. Biological Strategies for Water Remediation of PAHs: Biofiltration, Biosorption, and Biodegradation
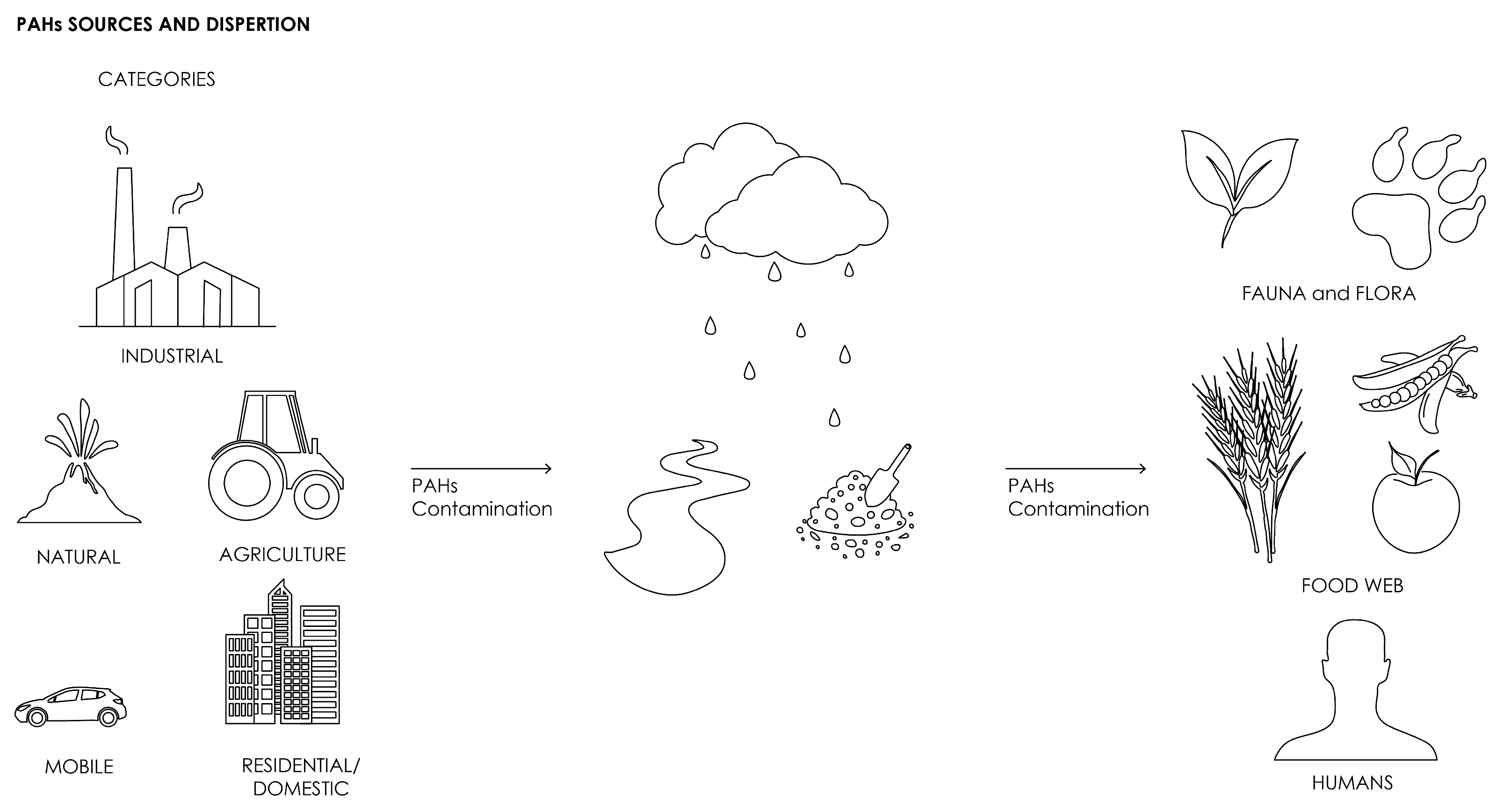
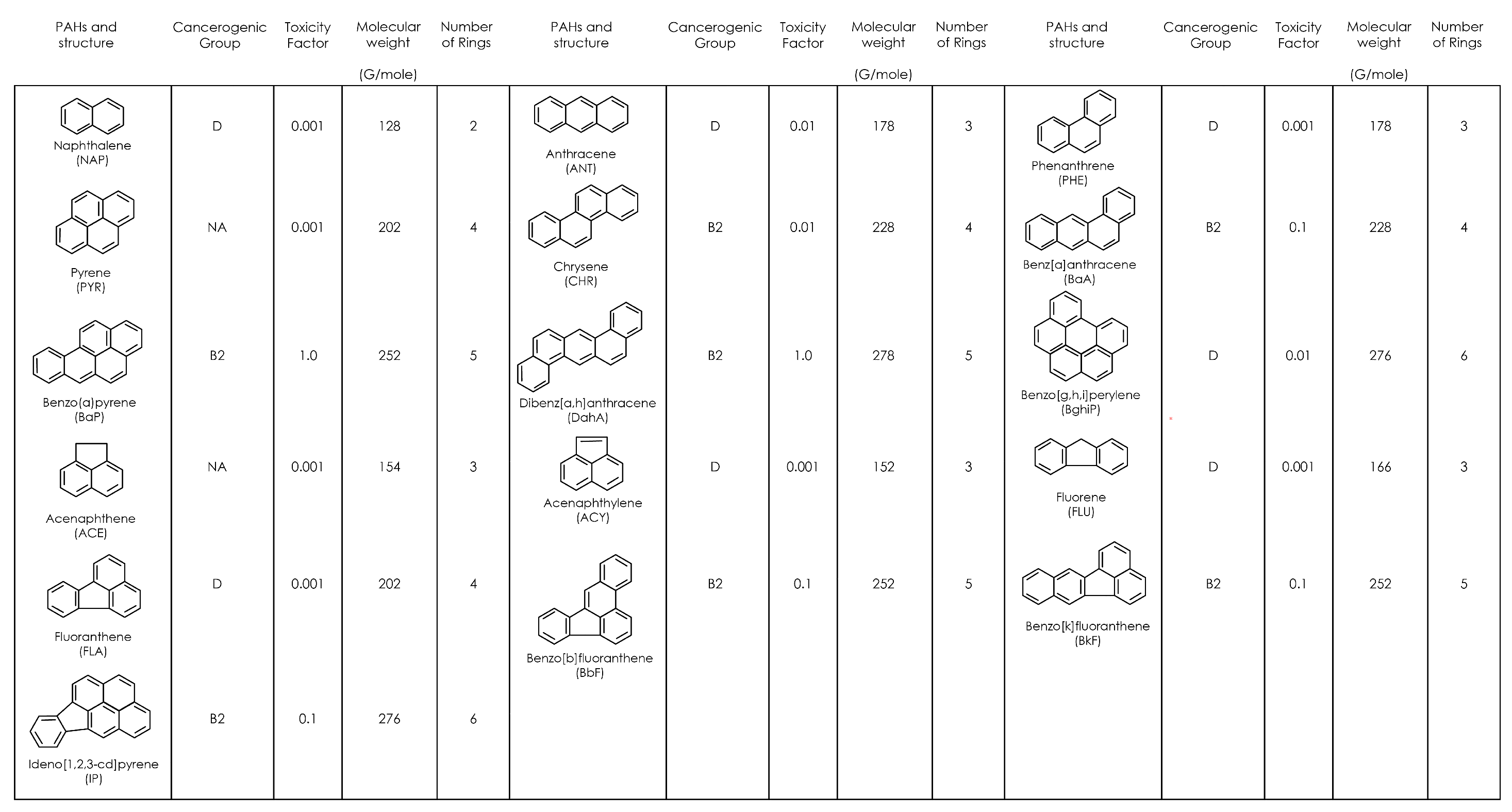
1.2. PAHs
2. Biofiltration and Other Methods
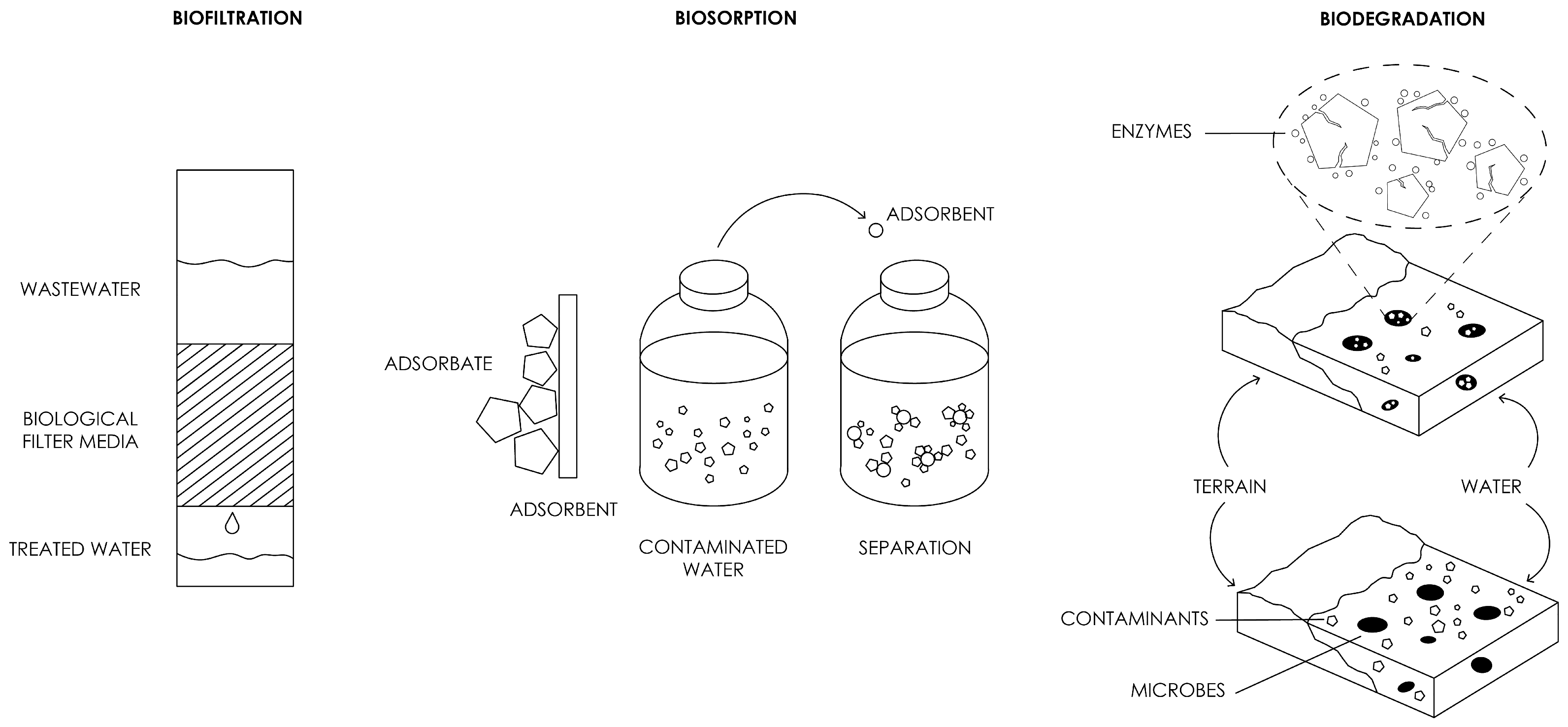
2.1. Overview of Biofiltration
2.2. Biofiltration of PAHs
2.3. Membrane Filtration of PAHs Through Fungi
3. Biosorption and Other Methods
3.1. Overview of Biosorption
3.2. Biosorption of PAHs
3.3. Fungal Biosorption of PAHs
4. Biodegradation and Other Methods
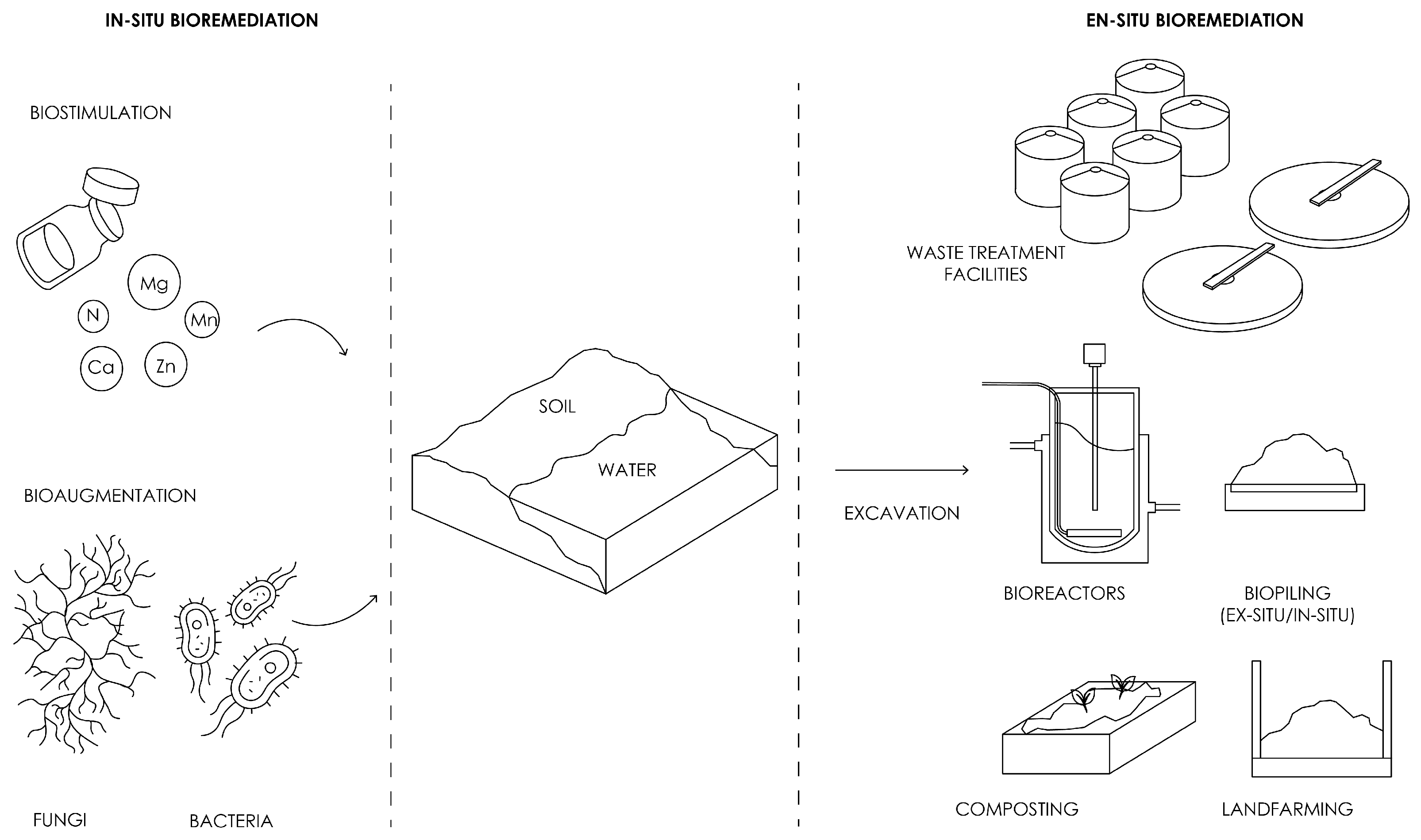
4.1. Overview of Biodegradation
| Pollutant | Organism | Enzyme/Process | Effectiveness | References |
|---|---|---|---|---|
| Benzo[a]pyrene, Pyrene, Chrysene, Fluoranthene, Naphthalene, Phenanthrene, Anthracene | Mycobacterium gilvum MI, Novosphingobium pentaromativorans sp. nov | Ring-hydroxylating dioxygenases (RHDs), Dehydrogenases, Monooxygenases | 88.2–99.9 % after 8 days | Sohn et al., 2004 [103] |
| Phenanthrene, Pyrene, Benzo[a]pyrene, Benzo[b]fluoranthene | Mycobacterium gilvum MI, Mycobacterium sp. ZL7, Rhodococcus rhodochrous Q3 | Ring-hydroxylating dioxygenases (RHDs), Dehydrogenases, Monooxygenases | consortium H6 (Q3:ZL7:MI = 1:2:2) 59% within 8 days | Zhou et al., 2023 [104] |
| Pyrene | Pseudomonas aeruginosa strain ASU-B6 | not specified | 92% after 15 days | Mawad et al., 2024 [105] |
| Hexavalent chromium (Cr(VI)) | Geobacter sulfurreducens | Extracellular protein-mediated reduction; intracellular accumulation | 99% of 100 mg/L Cr(VI) using a cell density of 5.8 × cells/mL. 99% of 200 mg/L Cr(VI) using 11.4 × cells/mL. | Elmeihy et al., 2021 [106] |
| Cadmium () | Pseudomonas aeruginosa | Bioaccumulation and Biosorption | Up to 94.7% | Chellaiah, 2018 [107] |
| Hexavalent chromium (Cr(VI)) | Priestia megaterium strain BM.1 | Bioreduction and adsorption on hydrochar | 97% removal of Cr(VI) (initially 60 mg/L) | Wu et al., 2025 [108] |
| Pentachlorophenol (PCP) | Sphingomonas chlorophenolica | Pentachlorophenol hydroxylase, Tetrachlorohydroquinone dehalogenase, 2,6-Dichlorohydroquinone dioxygenase | Not specified | Copley, 2000 [109] |
| Perchloroethylene, Trichloroethylene, Dichloroethene, Vinyl chloride | Dehalococcoides spp | Reductive dehalogenases (RDases) | Not specified | Vainberg et al., 2009 [110] |
| Anthracene, Acenaphthene, Fluoranthene, Fluorene | Aspergillus niger | Reductive dehalogenases (RDases) | ter 30 days: 77.8%, 65%, 60.9%, 52.5% | Manjunatha et al., 2025 [111] |
| Phenanthrene, Pyrene | Podoscypha elegans FTG4 | Laccase, Lignin peroxidase (LiP), Manganese peroxidase (MnP) | 50.6% of PHE, 48% of PYR, for 50 mg/kg, 99% of PHE, 98.9% of PYR for 20 mg/L | Agrawal et al., 2021 [112] |
| Zinc ion (), Chromium ion (CR3+), Lead ion (Pb2+) | Phanerochaete chrysosporium, Trametes versicolor | biosorption | P. c adsorbs Cr3+ medium at concentrations of 0.5, 1 mg . T. v adsorbs Pb2+ at concentrations of 0.25, 1, 2 mg | Solis Pacheco et al., 2015 [113] |
| Pollutant | Organism | Enzyme/Process | Effectiveness | References |
|---|---|---|---|---|
| Cadmium (II), manganese (II), zinc (II) ions | Ganoderma lucidum heteropolysaccharides (GLHP) | biosorption | 98.2%, 80.6%, 82.8% | Marolt et al., 2024 [114] |
| Cadmium | Aspergillus niger | biosorption | 82.2% | Amini et al., 2009 [115] |
| Carbamates | Ascochyta sp. CBS 237.37 | not specified | 36–94.8% | Kaur and Balomajumder, 2019 [116] |
| Parathion, Terbufos, Azinphos-methyl, Phosmet, Tribufos, Trichlorfon | Bjerkandera adusta 8258, Pleurotus ostreatus 7989, Phanerochaete chrysosporium 3641 | Cytochrome P450 monooxygenases | 50–96% | Jauregui et al., 2003 [117] |
| Methyl parathion (O,O-dimethyl-O-4-nitrophenyl phosphorothioate) | Chlorella vulgaris, Scenedesmus obliquus, Nannochloropsis spp., Anabaena spp., Spirulina spp. | electrostatic attraction and complexation | 24–98% | Satpati et al., 2023 [118] |
| Naphthalene, Phenanthrene, Anthracene, Fluoranthene, Benzo[a]pyrene | Aspergillus niger | biosorption | 82.2% | Amini et al., 2009 [115] |
| Phenol | Chlorella sp., Scenedesmus sp., Spirulina sp., Chlamydomonas sp. | Phenol hydroxylase, Catechol 1,2-dioxygenase (C12O), Catechol 2,3-dioxygenase (C23O), Laccases and peroxidases, Polyphenol oxidase | Chlorella sp.: up to 90%, Scenedesmus sp.: 70–85% | Al-Dahhan et al., 2018 [119] Radziff et al., 2021 [120] |
4.2. Biodegradation Against PAHs Through Bacteria
4.3. Fungal Biodegradation Against PAHs
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mojiri, A.; Zhou, J.L.; Ohashi, A.; Ozaki, N.; Kindaichi, T. Comprehensive Review of Polycyclic Aromatic Hydrocarbons in Water Sources, Their Effects and Treatments. Sci. Total Environ. 2019, 696, 133971. [Google Scholar] [CrossRef]
- Patel, A.B.; Shaikh, S.; Jain, K.R.; Desai, C.; Madamwar, D. Polycyclic Aromatic Hydrocarbons: Sources, Toxicity, and Remediation Approaches. Front. Microbiol. 2020, 11, 562813. [Google Scholar] [CrossRef] [PubMed]
- Smol, M.; Włodarczyk-Makuła, M. The Effectiveness in the Removal of PAHs from Aqueous Solutions in Physical and Chemical Processes: A Review. Polycycl. Aromat. Compd. 2017, 37, 292–313. [Google Scholar] [CrossRef]
- Badawy, M.I.; Ghaly, M.Y.; Gad-Allah, T.A. Advanced Oxidation Processes for the Removal of Organophosphorus Pesticides from Wastewater. Desalination 2006, 194, 166–175. [Google Scholar] [CrossRef]
- Li, S.; Luo, J.; Hang, X.; Zhao, S.; Wan, Y. Removal of Polycyclic Aromatic Hydrocarbons by Nanofiltration Membranes: Rejection and Fouling Mechanisms. J. Membr. Sci. 2019, 582, 264–273. [Google Scholar] [CrossRef]
- Hussain, A.; Kumari, R.; Sachan, S.G.; Sachan, A. Biological Wastewater Treatment Technology: Advancement and Drawbacks. In Microbial Ecology of Wastewater Treatment Plants; Elsevier: Amsterdam, The Netherlands, 2021; pp. 175–192. [Google Scholar] [CrossRef]
- Sutar, H.; Kumar, D. A Review on: Bioremediation. Int. J. Res. Chem. Environ. 2012, 2, 13–21. [Google Scholar]
- Kadri, T.; Rouissi, T.; Kaur Brar, S.; Cledon, M.; Sarma, S.; Verma, M. Biodegradation of Polycyclic Aromatic Hydrocarbons (PAHs) by Fungal Enzymes: A Review. J. Environ. Sci. (China) 2017, 51, 52–74. [Google Scholar] [CrossRef]
- Dinakarkumar, Y.; Ramakrishnan, G.; Gujjula, K.R.; Vasu, V.; Balamurugan, P.; Murali, G. Fungal Bioremediation: An Overview of the Mechanisms, Applications and Future Perspectives. Environ. Chem. Ecotoxicol. 2024, 6, 293–302. [Google Scholar] [CrossRef]
- Folwell, B.D.; McGenity, T.J.; Whitby, C. Biofilm and Planktonic Bacterial and Fungal Communities Transforming High-Molecular-Weight Polycyclic Aromatic Hydrocarbons. Appl. Environ. Microbiol. 2016, 82, 2288–2299. [Google Scholar] [CrossRef]
- Torres, E. Biosorption: A Review of the Latest Advances. Processes 2020, 8, 1584. [Google Scholar] [CrossRef]
- Haritash, A.K.; Kaushik, C.P. Biodegradation Aspects of Polycyclic Aromatic Hydrocarbons (PAHs): A Review. J. Hazard. Mater. 2009, 169, 1–15. [Google Scholar] [CrossRef]
- Zhang, Y.; Tao, S. Global Atmospheric Emission Inventory of Polycyclic Aromatic Hydrocarbons (PAHs) for 2004. Atmos. Environ. 2009, 43, 812–819. [Google Scholar] [CrossRef]
- Fernández, P.; Vilanova, R.M.; Martínez, C.; Appleby, P.; Grimalt, J.O. The Historical Record of Atmospheric Pyrolytic Pollution over Europe Registered in the Sedimentary PAH from Remote Mountain Lakes. Environ. Sci. Technol. 2000, 34, 1906–1913. [Google Scholar] [CrossRef]
- Marvin, C.; Tomy, G.T.; Thomas, P.J.; Holloway, A.C.; Sandau, C.D.; Idowu, I.; Xia, Z. Considerations for Prioritization of Polycyclic Aromatic Compounds as Environmental Contaminants. Environ. Sci. Technol. 2020, 54, 14787–14789. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Shafy, H.I.; Mansour, M.S.M. A Review on Polycyclic Aromatic Hydrocarbons: Source, Environmental Impact, Effect on Human Health and Remediation. Egypt. J. Pet. 2016, 25, 107–123. [Google Scholar] [CrossRef]
- Camarillo, M.; Stringfellow, W.; Jain, R. Drinking Water Security for Engineers, Planners, and Managers: Integrated Water Security Series; Butterworth-Heinemann: Oxford, UK, 2014; p. 241. [Google Scholar]
- Samer, M. Biological and Chemical Wastewater Treatment Processes. In Wastewater Treatment Engineering; IntechOpen: London, UK, 2015. [Google Scholar] [CrossRef]
- Bai, X.; Dinkla, I.J.T.; Muyzer, G. Microbial Ecology of Biofiltration Used for Producing Safe Drinking Water. Appl. Microbiol. Biotechnol. 2022, 106, 4813–4829. [Google Scholar] [CrossRef] [PubMed]
- Ejairu, U.; Aderamo, A.T.; Olisakwe, H.C.; Esiri, A.E.; Adanma, U.M.; Solomon, N.O. Eco-Friendly Wastewater Treatment Technologies (Concept): Conceptualizing Advanced, Sustainable Wastewater Treatment Designs for Industrial and Municipal Applications. Compr. Res. Rev. Eng. Technol. 2024, 2, 83–104. [Google Scholar] [CrossRef]
- Gerrity, D.; Arnold, M.; Dickenson, E.; Moser, D.; Sackett, J.D.; Wert, E.C. Microbial Community Characterization of Ozone-Biofiltration Systems in Drinking Water and Potable Reuse Applications. Water Res. 2018, 135, 207–219. [Google Scholar] [CrossRef]
- Ma, B.; LaPara, T.M.; Hozalski, R.M. Microbiome of Drinking Water Biofilters Is Influenced by Environmental Factors and Engineering Decisions but Has Little Influence on the Microbiome of the Filtrate. Environ. Sci. Technol. 2020, 54, 11526–11535. [Google Scholar] [CrossRef]
- Vignola, M.; Werner, D.; Wade, M.J.; Meynet, P.; Davenport, R.J. Medium Shapes the Microbial Community of Water Filters with Implications for Effluent Quality. Water Res. 2018, 129, 499–508. [Google Scholar] [CrossRef]
- Lu, R.; Hong, B.; Wang, Y.; Cui, X.; Liu, C.; Liu, Y.; Wu, X.; Ruan, R.; Zhang, Q. Microalgal Biofilm Cultivation on Lignocellulosic Based Bio-Carriers: Effects of Material Physical Characteristics on Microalgal Biomass Production and Composition. Chem. Eng. J. 2025, 510, 161656. [Google Scholar] [CrossRef]
- Baker, R.W. Membrane Technology and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2023. [Google Scholar]
- Hatt, B.E.; Fletcher, T.D.; Deletic, A. Treatment Performance of Gravel Filter Media: Implications for Design and Application of Stormwater Infiltration Systems. Water Res. 2007, 41, 2513–2524. [Google Scholar] [CrossRef] [PubMed]
- Lazim, S.M.; Khaleefa Ali, S.A. Treatment of Water from Irrigation Drainage by Multimedia Filtration. J. Eng. Sustain. Dev. 2020, 24, 58–71. [Google Scholar] [CrossRef]
- Kawamura, S. Integrated Design and Operation of Water Treatment Facilities; John Wiley & Sons: Hoboken, NJ, USA, 2000. [Google Scholar]
- Tarikuzzaman, M. A Review on Activated Carbon: Synthesis, Properties, and Applications. Eur. J. Adv. Eng. Technol. 2023, 10, 114–123. [Google Scholar]
- Duan, J.; Gregory, J. Coagulation by Hydrolysing Metal Salts. Adv. Colloid Interface Sci. 2003, 100–102, 475–502. [Google Scholar] [CrossRef]
- Pismenskaya, N.; Nikonenko, V. Ion-Exchange Membranes and Processes. Membranes 2021, 11, 814. [Google Scholar] [CrossRef]
- Wang, L.K.; Vaccari, D.A.; Li, Y.; Shammas, N.K. Chemical Precipitation. In Physicochemical Treatment Processes; Wang, L.K., Hung, Y.T., Shammas, N.K., Eds.; Humana Press: Totowa, NJ, USA, 2005; pp. 141–197. [Google Scholar] [CrossRef]
- Magic-Knezev, A.; Wullings, B.; Van der Kooij, D. Polaromonas and Hydrogenophaga Species Are the Predominant Bacteria Cultured from Granular Activated Carbon Filters in Water Treatment. J. Appl. Microbiol. 2009, 107, 1457–1467. [Google Scholar] [CrossRef]
- Weber-Shirk, M.L.; Dick, R.I. Physical—Chemical Mechanisms in Slow Sand Filters. J. AWWA 1997, 89, 87–100. [Google Scholar] [CrossRef]
- Chaudhary, D.S.; Vigneswaran, S.; Ngo, H.H.; Shim, W.G.; Moon, H. Biofilter in Water and Wastewater Treatment. Korean J. Chem. Eng. 2003, 20, 1054–1065. [Google Scholar] [CrossRef]
- Smol, M.; Włodarczyk-Makuła, M.; Mielczarek, K.; Bohdziewicz, J.; Włóka, D. The Use of Reverse Osmosis in the Removal of PAHs from Municipal Landfill Leachate. Polycycl. Aromat. Compd. 2016, 36, 20–39. [Google Scholar] [CrossRef]
- Keyvan Hosseini, P.; Liu, L.; Keyvan Hosseini, M.; Bhattacharyya, A.; Miao, J.; Wang, F. Treatment of a Synthetic Decanted Oily Seawater in a Pilot-Scale Hollow Fiber Membrane Filtration Process: Experimental Investigation. J. Hazard. Mater. 2023, 441, 129928. [Google Scholar] [CrossRef] [PubMed]
- Smol, M.; Maria, W.M. Effectiveness in the Removal of Polycyclic Aromatic Hydrocarbons From Industrial Wastewater by Ultrafiltration Technique. Arch. Environ. Prot. 2012, 38, 49–58. [Google Scholar] [CrossRef]
- Richard, D.E.; Dwyer, D.F. Aerated Biofiltration for Simultaneous Removal of Iron and Polycyclic Aromatic Hydrocarbons from Groundwater. Water Environ. Res. 2001, 73, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, K.; Branchu, P.; Boudahmane, L.; Caupos, E.; Demare, D.; Deshayes, S.; Dubois, P.; Meffray, L.; Partibane, C.; Saad, M.; et al. Field Performance of Two Biofiltration Systems Treating Micropollutants from Road Runoff. Water Res. 2018, 145, 562–578. [Google Scholar] [CrossRef]
- Smol, M.; Włodarczyk-Makuła, M.; Mielczarek, K.; Bohdziewicz, J. Comparison of the Retention of Selected PAHs from Municipal Landfill Leachate by RO and UF Processes. Desalin. Water Treat. 2014, 52, 3889–3897. [Google Scholar] [CrossRef]
- Macedonio, F.; Ali, A.; Poerio, T.; El-Sayed, E.; Drioli, E.; Abdel-Jawad, M. Direct Contact Membrane Distillation for Treatment of Oilfield Produced Water. Sep. Purif. Technol. 2014, 126, 69–81. [Google Scholar] [CrossRef]
- Chandra, D.; Mishra, A.; Trakroo, M.D.; Chauhan, R.S.; Mishra, S.K. Impact of Mycofiltration on Water Quality. Environ. Qual. Manag. 2022, 31, 253–266. [Google Scholar] [CrossRef]
- Mehta, A.; Dubey, R.; Kumar, S. Mycofiltration: A Step Towards Sustainable Environment. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1524–1528. [Google Scholar] [CrossRef][Green Version]
- Sarwar, A.; Nayyar, B.G.; Irshad, H.; Anwar, P.; Olihk, N.; Ajmal, M. Mycofiltration of Heavy Metals (Pb, Cd, Hg) from Aqueous Solution by Living Biomass of Two Mushrooms Pleurotus Ostreatus and Agaricus Bisporus as Biosorbents. J. Water Chem. Technol. 2023, 45, 599–606. [Google Scholar] [CrossRef]
- Obayagbona, O.N.; Dunkwu-Okafor, A.; Odigie, O. Mycofiltration of Urban Derived Raw Stormwater Using Lentinus Squarrosulus. Bio-Research 2024, 22, 2336–2341. [Google Scholar] [CrossRef]
- Mnkandla, S.M.; Otomo, P.V. Effectiveness of Mycofiltration for Removal of Contaminants from Water: A Systematic Review Protocol. Environ. Evid. 2021, 10, 17. [Google Scholar] [CrossRef]
- Hai, F.I.; Yamamoto, K.; Fukushi, K. Development of a Submerged Membrane Fungi Reactor for Textile Wastewater Treatment. Desalination 2006, 192, 315–322. [Google Scholar] [CrossRef]
- Isik, Z.; Arikan, E.B.; Bouras, H.D.; Dizge, N. Bioactive Ultrafiltration Membrane Manufactured from Aspergillus carbonarius M333 Filamentous Fungi for treatment of real textile wastewater. Bioresour. Technol. Rep. 2019, 5, 212–219. [Google Scholar] [CrossRef]
- Parasnis, M.S.; Deng, E.; Yuan, M.; Lin, H.; Kordas, K.; Paltseva, A.; Frimpong Boamah, E.; Judelsohn, A.; Nalam, P.C. Heavy Metal Remediation by Dry Mycelium Membranes: Approaches to Sustainable Lead Remediation in Water. Langmuir 2024, 40, 6317–6329. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Lou, J.; Wang, H.; Yang, Y.; Wu, L.; Wu, J.; Xu, J. Biodegradation, Biosorption of Phenanthrene and Its Trans-Membrane Transport by Massilia Sp. WF1 and Phanerochaete chrysosporium. Front. Microbiol. 2016, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Legorreta-Castañeda, A.; Lucho-Constantino, C.; Beltrán-Hernández, R.; Coronel-Olivares, C.; Vázquez-Rodríguez, G. Biosorption of Water Pollutants by Fungal Pellets. Water 2020, 12, 1155. [Google Scholar] [CrossRef]
- Chojnacka, K. Biosorption and Bioaccumulation—The Prospects for Practical Applications. Environ. Int. 2010, 36, 299–307. [Google Scholar] [CrossRef]
- Aksu, Z. Application of Biosorption for the Removal of Organic Pollutants: A Review. Process Biochem. 2005, 40, 997–1026. [Google Scholar] [CrossRef]
- Wang, J.; Chen, C. Biosorption of Heavy Metals by SaccharomycesCerevisiae: A Rev. Biotechnol. Adv. 2006, 24, 427–451. [Google Scholar] [CrossRef]
- Schiewer, S.; Volesky, B. Biosorption Processes for Heavy Metal Removal. In Environmental Microbe-Metal Interactions; John Wiley & Sons, Ltd: Hoboken, NJ, USA, 2000; Chapter 14; pp. 329–362. [Google Scholar] [CrossRef]
- Naja, G.M.; Murphy, V.; Volesky, B. Biosorption, Metals. In Encyclopedia of Industrial Biotechnology; John Wiley & Sons, Ltd: Hoboken, NJ, USA, 2010; pp. 1–29. [Google Scholar] [CrossRef]
- Smoczyński, L.; Pierożyński, B.; Mikołajczyk, T. The Effect of Temperature on the Biosorption of Dyes from Aqueous Solutions. Processes 2020, 8, 636. [Google Scholar] [CrossRef]
- de Rome, L.; Gadd, G.M. Use of Pelleted and Immobilized Yeast and Fungal Biomass for Heavy Metal and Radionuclide Recovery. J. Ind. Microbiol. 1991, 7, 97–104. [Google Scholar] [CrossRef]
- Yesilada, O.; Cing Yıldırım, S.; Birhanlı, E.; Apohan, E.; Asma, D.; Boran, F. The Evaluation of Pre-Grown Mycelial Pellets in Decolorization of Textile Dyes during Repeated Batch Process. World J. Microbiol. Biotechnol. 2009, 26, 33–39. [Google Scholar] [CrossRef]
- Mogashane, T.M.; Maree, J.P.; Mokoena, L. Adsorption of Polycyclic Aromatic Hydrocarbons from Wastewater Using Iron Oxide Nanomaterials Recovered from Acid Mine Water: A Review. Minerals 2024, 14, 826. [Google Scholar] [CrossRef]
- Mohammed, A.H.; Shartooh, S.M.; Trigui, M. Biosorption and Isotherm Modeling of Heavy Metals Using Phragmites Australis. Sustainability 2025, 17, 5366. [Google Scholar] [CrossRef]
- Zhang, D.; Lu, S.; Song, X.; Zhang, J.; Huo, Z.M.; Zhao, H. Synergistic and Simultaneous Biosorption of Phenanthrene and Iodine from Aqueous Solutions by Soil Indigenous Bacterial Biomass as a Low-Cost Biosorbent. RSC Adv. 2018, 8, 39274–39283. [Google Scholar] [CrossRef] [PubMed]
- Vahabisani, A.; An, C. Use of Biomass-Derived Adsorbents for the Removal of Petroleum Pollutants from Water: A Mini-Review. Environ. Syst. Res. 2021, 10, 25. [Google Scholar] [CrossRef]
- Gupta, V.K.; Gupta, B.; Rastogi, A.; Agarwal, S.; Nayak, A. A Comparative Investigation on Adsorption Performances of Mesoporous Activated Carbon Prepared from Waste Rubber Tire and Activated Carbon for a Hazardous Azo Dye—Acid Blue 113. J. Hazard. Mater. 2011, 186, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of Methylene Blue on Low-Cost Adsorbents: A Review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef]
- Kong, H.; He, J.; Gao, Y.; Han, J.; Zhu, X. Removal of Polycyclic Aromatic Hydrocarbons from Aqueous Solution on Soybean Stalk-based Carbon. J. Environ. Qual. 2011, 40, 1737–1744. [Google Scholar] [CrossRef]
- Wießner, A.; Remmler, M.; Kuschk, P.; Stottmeister, U. The Treatment of a Deposited Lignite Pyrolysis Wastewater by Adsorption Using Activated Carbon and Activated Coke. Colloids Surf. A Physicochem. Eng. Asp. 1998, 139, 91–97. [Google Scholar] [CrossRef]
- Yuan, M.; Tong, S.; Zhao, S.; Jia, C.Q. Adsorption of Polycyclic Aromatic Hydrocarbons from Water Using Petroleum Coke-Derived Porous Carbon. J. Hazard. Mater. 2010, 181, 1115–1120. [Google Scholar] [CrossRef]
- Sarkar, M.; Das, M.; Manna, S.; Acharya, P. Removal/Reduction of Organic Pollutants from Aqueous Environment. Rocz. Ochr. Środowiska 2003, 5, 79–86. [Google Scholar]
- Lu, T.; Zhang, Q.L.; Yao, S.J. Application of Biosorption and Biodegradation Functions of Fungi in Wastewater and Sludge Treatment. In Fungal Applications in Sustainable Environmental Biotechnology; Springer: Cham, Switzerland, 2016; pp. 65–90. [Google Scholar] [CrossRef]
- Xiao, L.; Zhao, X.; Yao, J.; Lu, Q.; Feng, X.; Wu, S. Biodegradation and Adsorption of Benzo[a]Pyrene by Fungi-Bacterial Coculture. Ecotoxicol. Environ. Saf. 2024, 283, 116811. [Google Scholar] [CrossRef] [PubMed]
- Rathankumar, A.K.; Saikia, K.; Ponnusamy, S.K.; del Rayo Sánchez-Carbente, M.; Vaidyanathan, V.K. Rhamnolipid-Assisted Mycoremediation of Polycyclic Aromatic Hydrocarbons by Trametes Hirsuta Coupled with Enhanced Ligninolytic Enzyme Production. J. Air Waste Manag. Assoc. 2020, 70, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.k.; Ling Wu, L.; Fam, H. Heavy Metal Ions Affecting the Removal of Polycyclic Aromatic Hydrocarbons by Fungi with Heavy-Metal Resistance. Appl. Microbiol. Biotechnol. 2014, 98, 9817–9827. [Google Scholar] [CrossRef]
- Yu, H.; Huang, G.h.; An, C.j.; Wei, J. Combined Effects of DOM Extracted from Site Soil/Compost and Biosurfactant on the Sorption and Desorption of PAHs in a Soil–Water System. J. Hazard. Mater. 2011, 190, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Sun, Z.; Liu, P.; Li, Q.; Yang, R.; Yang, X. Competitive Adsorption of Naphthalene and Phenanthrene on Walnut Shell Based Activated Carbon and the Verification via Theoretical Calculation. RSC Adv. 2020, 10, 10703–10714. [Google Scholar] [CrossRef]
- Gu, H.; Luo, X.; Wang, H.; Wu, L.; Wu, J.; Xu, J. The Characteristics of Phenanthrene Biosorption by Chemically Modified Biomass of Phanerochaete Chrysosporium. Environ. Sci. Pollut. Res. 2015, 22, 11850–11861. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, S.Y.; Lv, M.J.; Xie, S.G. Biosorption Characteristics of Ectomycorrhizal Fungal Mycelium for Anthracene. Biomed. Environ. Sci. 2010, 23, 378–383. [Google Scholar] [CrossRef]
- Bullen, J.; Saleesongsom, S.; Weiss, D.J. A Revised Pseudo-Second Order Kinetic Model for Adsorption, Sensitive to Changes in Sorbate and Sorbent Concentrations. Langmuir 2021, 37, 3189–3201. [Google Scholar] [CrossRef]
- Gadd, G.M. Heavy Metal Accumulation by Bacteria and Other Microorganisms. Experientia 1990, 46, 834–840. [Google Scholar] [CrossRef]
- Gadd, G.M. Interactions of Fungi with Toxic Metals. In The Genus Aspergillus: From Taxonomy and Genetics to Industrial Application; Powell, K.A., Renwick, A., Peberdy, J.F., Eds.; Springer: Boston, MA, USA, 1994; pp. 361–374. [Google Scholar] [CrossRef]
- Kapoor, A.; Viraraghavan, T.; Cullimore, D.R. Removal of Heavy Metals Using the Fungus Aspergillus Niger. Bioresour. Technol. 1999, 70, 95–104. [Google Scholar] [CrossRef]
- Chen, B.; Wang, Y.; Hu, D. Biosorption and Biodegradation of Polycyclic Aromatic Hydrocarbons in Aqueous Solutions by a Consortium of White-Rot Fungi. J. Hazard. Mater. 2010, 179, 845–851. [Google Scholar] [CrossRef]
- Raghukumar, C.; Shailaja, M.; Singh, S.K. Removal of Polycyclic Aromatic Hydrocarbons from Aqueous Media by the Marine Fungus NIOCC # 312: Involvement of Lignin-Degrading Enzymes and Exopolysaccharides. Indian J. Mar. Sci. 2006, 35, 373–379. [Google Scholar]
- Hamby, D. Site Remediation Techniques Supporting Environmental Restoration Activities—A Review. Sci. Total Environ. 1996, 191, 203–224. [Google Scholar] [CrossRef]
- Kuppusamy, S.; Palanisami, T.; Megharaj, M.; Venkateswarlu, K.; Naidu, R. Ex-Situ Remediation Technologies for Environmental Pollutants: A Critical Perspective. In Reviews of Environmental Contamination and Toxicology Volume 236; De Voogt, P., Ed.; Springer International Publishing: Cham, Switzerland, 2016; Volume 236, pp. 117–192. [Google Scholar] [CrossRef]
- Reddy, K.; Cameselle, C. Electrochemical Remediation Technologies for Polluted Soils, Sediments and Groundwater; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Lee, S.H.; Kim, S.O.; Lee, S.W.; Kim, M.S.; Park, H. Application of Soil Washing and Thermal Desorption for Sustainable Remediation and Reuse of Remediated Soil. Sustainability 2021, 13, 12523. [Google Scholar] [CrossRef]
- Vidonish, J.; Zygourakis, K.; Masiello, C.; Sabadell, G.; Alvarez, P. Thermal Treatment of Hydrocarbon-Impacted Soils: A Review of Technology Innovation for Sustainable Remediation. Engineering 2016, 2, 426–437. [Google Scholar] [CrossRef]
- Song, W.; Vidonish, J.E.; Kamath, R.; Yu, P.; Chu, C.; Moorthy, B.; Gao, B.; Zygourakis, K.; Alvarez, P.J.J. Pilot-Scale Pyrolytic Remediation of Crude-Oil-Contaminated Soil in a Continuously-Fed Reactor: Treatment Intensity Trade-Offs. Environ. Sci. Technol. 2019, 53, 2045–2053. [Google Scholar] [CrossRef]
- Han, C.; Zhu, X.; Xiong, G.; Gao, J.; Wu, J.; Wang, D.; Wu, J. Quantitative Study of Situ Chem. Oxid. Remediat. Coupled Therm. Desorption. Water Res. 2023, 239, 120035. [Google Scholar] [CrossRef]
- Waris, A.A.; Athar, T.; Nisar, M. Recent Advances in Chemical Methods for Remediation of Heavy Metals Contaminated Soils: A Review. Emergent Life Sci. Res. 2018, 4, 45–50. [Google Scholar] [CrossRef]
- Oro, C.E.D.; Saorin Puton, B.M.; Venquiaruto, L.D.; Dallago, R.M.; Tres, M.V. Effective Microbial Strategies to Remediate Contaminated Agricultural Soils and Conserve Functions. Agronomy 2024, 14, 2637. [Google Scholar] [CrossRef]
- Tyagi, M.; da Fonseca, M.M.R.; de Carvalho, C.C. Bioaugmentation and Biostimulation Strategies to Improve the Effectiveness of Bioremediation Processes. Biodegradation 2011, 22, 231–241. [Google Scholar] [CrossRef]
- Kakde, P.; Sharma, J. Microbial Bioremediation of Petroleum Contaminated Soil: Structural Complexity, Degradation Dynamics and Advanced Remediation Techniques. J. Pure Appl. Microbiol. 2024, 18, 2244. [Google Scholar] [CrossRef]
- Agrawal, N.; Verma, P.; Shahi, S. Degradation of Polycyclic Aromatic Hydrocarbons (Phenanthrene and Pyrene) by the Ligninolytic Fungi Ganoderma Lucidum Isolated from the Hardwood Stump. Bioresour. Bioprocess. 2018, 5, 11. [Google Scholar] [CrossRef]
- Brooijmans, R.J.W.; Pastink, M.I.; Siezen, R.J. Hydrocarbon-degrading Bacteria: The Oil-spill Clean-up Crew. Microb. Biotechnol. 2009, 2, 587. [Google Scholar] [CrossRef]
- Gupte, A.; Tripathi, A.; Patel, H.; Rudakiya, D.; Gupte, S. Bioremediation of Polycyclic Aromatic Hydrocarbon (PAHs): A Perspective. Open Biotechnol. J. 2016, 10, 363–378. [Google Scholar] [CrossRef]
- Marquez-Rocha, F.; Hernández-Rodríguez, V.; Vazquez-Duhalt, R. Biodegradation of Soil-Adsorbed Polycyclic Aromatic Hydrocarbons by the White Rot Fungus Pleurotus Ostreatus. Biotechnol. Lett. 2000, 22, 469–472. [Google Scholar] [CrossRef]
- Matsubara, M.; Lynch, J.M.; De Leij, F.A.A.M. A Simple Screening Procedure for Selecting Fungi with Potential for Use in the Bioremediation of Contaminated Land. Enzym. Microb. Technol. 2006, 39, 1365–1372. [Google Scholar] [CrossRef]
- Singh, H. Mycoremediation: Fungal Bioremediation, 1st ed.; Wiley: Hoboken, NJ, USA, 2006. [Google Scholar] [CrossRef]
- Aydin, S.; Karaçay, H.; Gökçe, S.; Shahi, A.; Ince, B.; Ince, O. Aerobic and Anaerobic Fungal Metabolism and Omics Insights for Increasing Polycyclic Aromatic Hydrocarbons Biodegradation. Fungal Biol. Rev. 2016, 31, 61–72. [Google Scholar] [CrossRef]
- Sohn, J.; Kwon, K.K.; Kang, J.H.; Jung, H.B.; Kim, S.J. Novosphingobium Pentaromativorans Sp Nov., a High-Molecular-Mass Polycyclic Aromatic Hydrocarbon-Degrading Bacterium Isolated from Estuarine Sediment. Int. J. Syst. Evol. Microbiol. 2004, 54, 1483–1487. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Gao, X.; Wang, S.; Zhang, Y.; Coulon, F.; Cai, C. Enhanced Bioremediation of Aged Polycyclic Aromatic Hydrocarbons in Soil Using Immobilized Microbial Consortia Combined with Strengthening Remediation Strategies. Int. J. Environ. Res. Public Health 2023, 20, 1766. [Google Scholar] [CrossRef]
- Mawad, A.M.M.; Aldaby, E.S.E.; Madany, M.M.Y.; Dawood, M.F.A. The Application of PAHs-Degrading PseudomonasAeruginosa Mitigate Phytotoxic Impact Pyrene Barley (Hordeumvulgare L.) Broad Bean (Viciafaba L.) Plants. Plant Physiol. Biochem. 2024, 215, 108959. [Google Scholar] [CrossRef]
- Elmeihy, R.; Shi, X.C.; Tremblay, P.L.; Zhang, T. Fast Removal of Toxic Hexavalent Chromium from an Aqueous Solution by High-Density Geobacter Sulfurreducens. Chemosphere 2021, 263, 128281. [Google Scholar] [CrossRef]
- Chellaiah, E.R. Cadmium (Heavy Metals) Bioremediation by Pseudomonas Aeruginosa: A Minireview. Appl. Water Sci. 2018, 8, 154. [Google Scholar] [CrossRef]
- Wu, M.; Ouyang, X.; Li, Y.; Zhang, J.; Liu, J.; Yin, H. Mechanisms in Hexavalent Chromium Removal from Aquatic Environment by the Modified Hydrochar-Loaded Bacterium Priestia Megaterium Strain BM.1. Sustainability 2025, 17, 5172. [Google Scholar] [CrossRef]
- Copley, S. Evolution of a Metabolic Pathway for Degradation of a Toxic Xenobiotic: The Patchwork Approach. Trends Biochem. Sci. 2000, 25, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Vainberg, S.; Condee, C.W.; Steffan, R.J. Large-Scale Production of Bacterial Consortia for Remediation of Chlorinated Solvent-Contaminated Groundwater. J. Ind. Microbiol. Biotechnol. 2009, 36, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Manjunatha, B.K.; Vamshee, V.K.; Poojitha, N.G.; Kiran, R.J.; Mythili, S.T.; Divakara, R.; Sreenivasa, R.A.; Vidya, S.M. Bioremediation of Polycyclic Aromatic Hydrocarbons (Acenaphthene, Anthracene, Fluoranthene, Fluorene) by Aspergillus Niger HQ170509.1 Fungus. Int. J. Environ. Sci. Technol. 2025, 22, 12567–12582. [Google Scholar] [CrossRef]
- Agrawal, N.; Barapatre, A.; Shahi, M.P.; Shahi, S.K. Biodegradation Pathway of Polycyclic Aromatic Hydrocarbons by Ligninolytic Fungus Podoscypha Elegans Strain FTG4 and Phytotoxicity Evaluation of Their Metabolites. Environ. Process. 2021, 8, 1307–1335. [Google Scholar] [CrossRef]
- Solís Pacheco, J.; Santana, M.; Aguilar Uscanga, M.G.; Cavazos Garduño, A.; Serrano Niño, J.; Gómez, H.; Aguilar Uscanga, B.; Solís Pacheco, J.; Santana, M.; Aguilar Uscanga, M.G.; et al. Ability of Phanerochaete Chrysosporium and Trametes Versicolor to Remove Zn2+, CR3+, Pb2+ Metal Ions. Terra Latinoam. 2015, 33, 189–198. [Google Scholar]
- Marolt, G.; Kralj, T.; Slapničar, M.; Gregori, A. Potential of Ganoderma Lucidum Heteropolysaccharides for Heavy Metals Removal from Water Solutions; Slovenski Kemijski Dnevi: Portorož, Slovenia, 2024. [Google Scholar]
- Amini, M.; Younesi, H.; Bahramifar, N. Statistical Modeling and Optimization of the Cadmium Biosorption Process in an Aqueous Solution Using Aspergillusniger. Colloids Surf. A Physicochem. Eng. Asp. 2009, 337, 67–73. [Google Scholar] [CrossRef]
- kaur, P.; Balomajumder, C. Simultaneous Biodegradation of Mixture of Carbamates by Newly Isolated Ascochyta Sp. CBS 237.37. Ecotoxicol. Environ. Saf. 2019, 169, 590–599. [Google Scholar] [CrossRef]
- Jauregui, J.; Valderrama, B.; Albores, A.; Vazquez-Duhalt, R. Microsomal Transformation of Organophosphorus Pesticides by White Rot Fungi. Biodegradation 2003, 14, 397–406. [Google Scholar] [CrossRef]
- Satpati, G.G.; Gupta, S.; Biswas, R.K.; Choudhury, A.K.; Kim, J.W.; Davoodbasha, M. Microalgae Mediated Bioremediation of Polycyclic Aromatic Hydrocarbons: Strategies, Advancement and Regulations. Chemosphere 2023, 344, 140337. [Google Scholar] [CrossRef]
- Al-Dahhan, M.; Al-Ani, F.; Obeid, A. Biodegradation of Phenolic Components in Wastewater by Micro Algae: A Review. MATEC Web Conf. 2018, 162, 05009. [Google Scholar] [CrossRef]
- Radziff, S.B.M.; Ahmad, S.A.; Shaharuddin, N.A.; Merican, F.; Kok, Y.Y.; Zulkharnain, A.; Gomez-Fuentes, C.; Wong, C.Y. Potential Application of Algae in Biodegradation of Phenol: A Review and Bibliometric Study. Plants 2021, 10, 2677. [Google Scholar] [CrossRef] [PubMed]
- Cébron, A.; Norini, M.P.; Beguiristain, T.; Leyval, C. Real-Time PCR Quantification of PAH-ring Hydroxylating Dioxygenase (PAH-RHDα) Genes from Gram Positive and Gram Negative Bacteria in Soil and Sediment Samples. J. Microbiol. Methods 2008, 73, 148–159. [Google Scholar] [CrossRef]
- Story, S.; Kline, E.; Hughes, T.A.; Riley, M.; Hayasaka, S. Degradation of Aromatic Hydrocarbons by Sphingomonas Paucimobilis Strain EPA505. Arch. Environ. Contam. Toxicol. 2004, 47, 168–176. [Google Scholar] [CrossRef]
- Aitken, M.D.; Stringfellow, W.T.; Nagel, R.D.; Kazunga, C.; Chen, S.H. Characteristics of Phenanthrene-Degrading Bacteria Isolated from Soils Contaminated with Polycyclic Aromatic Hydrocarbons. Can. J. Microbiol. 1998, 44, 743–752. [Google Scholar] [CrossRef]
- Tarekegn, M.; Zewdu, F.; Ishetu, A. Microbes Used as a Tool for Bioremediation of Heavy Metal from the Environment. Cogent Food Agric. 2020, 6, 1783174. [Google Scholar] [CrossRef]
- Cerniglia, C.E. Biodegradation of Polycyclic Aromatic Hydrocarbons. Biodegradation 1992, 3, 351–368. [Google Scholar] [CrossRef]
- Bala, S.; Garg, D.; Thirumalesh, B.V.; Sharma, M.; Sridhar, K.; Inbaraj, B.S.; Tripathi, M. Recent Strategies for Bioremediation of Emerging Pollutants: A Review for a Green and Sustainable Environment. Toxics 2022, 10, 484. [Google Scholar] [CrossRef] [PubMed]
- Robichaud, K.; Stewart, K.; Labrecque, M.; Hijri, M.; Cherewyk, J.; Amyot, M. An Ecological Microsystem to Treat Waste Oil Contaminated Soil: Using Phytoremediation Assisted by Fungi and Local Compost, on a Mixed-Contaminant Site, in a Cold Climate. Sci. Total Environ. 2019, 672, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Stamets, P. Delivery Systems for Mycotechnologies, Mycofiltration and Mycoremediation. WO WO200206 5836A2, 2002.
- Hacıoğlu, B.; Dupaul, G.; Paladino, G.; Edman, M.; Hedenström, E. Unlocking the Biodegradative Potential of Native White-Rot Fungi: A Comparative Study of Fiberbank Organic Pollutant Mycoremediation. Bioengineered 2024, 15, 2396642. [Google Scholar] [CrossRef]
- Juwarkar, A.A.; Singh, S.K.; Mudhoo, A. A Comprehensive Overview of Elements in Bioremediation. Rev. Environ. Sci. Bio/Technol. 2010, 9, 215–288. [Google Scholar] [CrossRef]
- Abatenh, E.; Gizaw, B.; Tsegaye, Z.; Wassie, M.; Abatenh, E.; Gizaw, B.; Tsegaye, Z.; Wassie, M. The Role of Microorganisms in Bioremediation—A Review. Open J. Environ. Biol. 2017, 2, 38–46. [Google Scholar] [CrossRef]
- Malik, S.; Bora, J.; Nag, S.; Sinha, S.; Mondal, S.; Rustagi, S.; Hazra, R.; Kumar, H.; Rajput, V.D.; Minkina, T.; et al. Fungal-Based Remediation in the Treatment of Anthropogenic Activities and Pharmaceutical-Pollutant-Contaminated Wastewater. Water 2023, 15, 2262. [Google Scholar] [CrossRef]
- Al-Hawash, A.; Alkooranee, J.; Zhang, X.; Ma, F. Fungal Degradation of Polycyclic Aromatic Hydrocarbons. Int. J. Pure Appl. Biosci. 2018, 6, 8–24. [Google Scholar] [CrossRef]
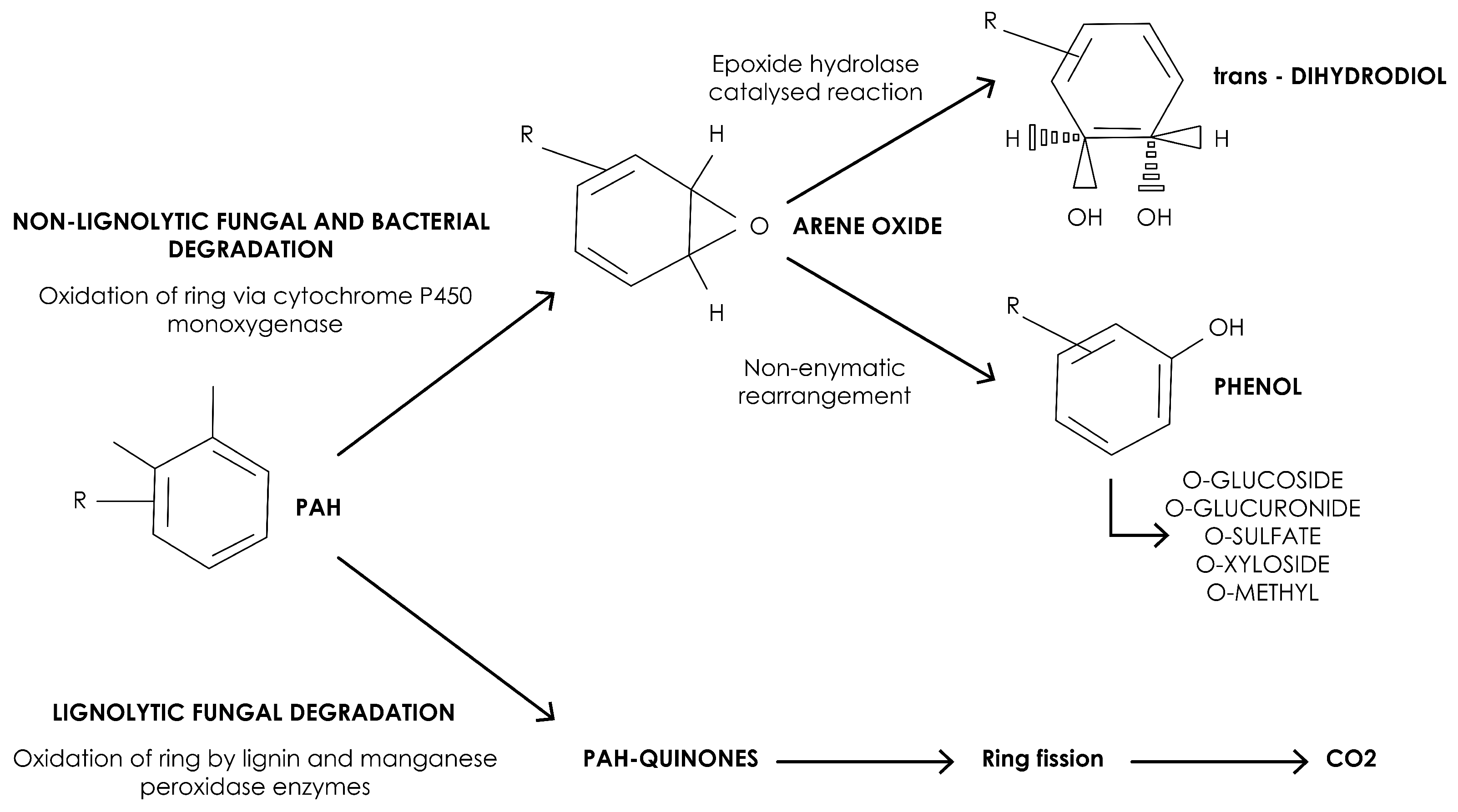
- Sutherland, J.; Rafii, F.; Khan, A.; Cerniglia, C. Mechanisms of Polycyclic Aromatic Hydrocarbon Degradation, Microbial Transformation and Degradation of Toxic Organic Chemical. , Environ Health Perspect 1995, 15, 269. [Google Scholar]
- Cerniglia, C.E.; Sutherland, J.B. Bioremediation of Polycyclic Aromatic Hydrocarbons by Ligninolytic and Non-Ligninolytic Fungi. In Fungi in Bioremediation, 1st ed.; Gadd, G.M., Ed.; Cambridge University Press: Cambridge, UK, 2001; pp. 136–187. [Google Scholar] [CrossRef]
- Özer, A.; Ay Sal, F.; Belduz, A.; Kirci, H.; Canakci, S. Use of Feruloyl Esterase as Laccase-Mediator System in Paper Bleaching. Appl. Biochem. Biotechnol. 2020, 190, 721–731. [Google Scholar] [CrossRef]
- Pothiraj, C.; Kanmani, P.; Balaji, P. Bioconversion of Lignocellulose Materials. Mycobiology 2006, 34, 159–165. [Google Scholar] [CrossRef]
- Hammel, K.E. Mechanisms for Polycyclic Aromatic Hydrocarbon Degradation by Ligninolytic Fungi. Environ. Health Perspect. 1995, 103, 41–43. [Google Scholar] [PubMed]
- Deshmukh, R.; Khardenavis, A.A.; Purohit, H.J. Diverse Metabolic Capacities of Fungi for Bioremediation. Indian J. Microbiol. 2016, 56, 247–264. [Google Scholar] [CrossRef] [PubMed]
- Latif, W.; Ciniglia, C.; Iovinella, M.; Shafiq, M.; Papa, S. Role of White Rot Fungi in Industrial Wastewater Treatment: A Review. Appl. Sci. 2023, 13, 8318. [Google Scholar] [CrossRef]
- Pozdnyakova, N.N. Involvement of the Ligninolytic System of White-Rot and Litter-Decomposing Fungi in the Degradation of Polycyclic Aromatic Hydrocarbons. Biotechnol. Res. Int. 2012, 2012, 243217. [Google Scholar] [CrossRef]
- Bezalel, L.; Hadar, Y.; Cerniglia, C. Mineralization of Polycyclic Aromatic Hydrocarbons by the White Rot Fungus Pleurotus Ostreatus. Appl. Environ. Microbiol. 1996, 62, 292–295. [Google Scholar] [CrossRef]
- Zain ul Arifeen, M.; Ma, Y.; Wu, T.; Chu, C.; Liu, X.; Jiang, J.; Li, D.; Xue, Y.R.; Liu, C.H. Anaerobic Biodegradation of Polycyclic Aromatic Hydrocarbons (PAHs) by Fungi Isolated from Anaerobic Coal-Associated Sediments at 2.5 km below the Seafloor. Chemosphere 2022, 303, 135062. [Google Scholar] [CrossRef]

| Physical Filtration | Energy Efficiency | Methods | Limitations | References |
|---|---|---|---|---|
| Membrane filtration | Energy intensive in reverse osmosis (RO); moderate for ultrafiltration/microfiltration | Semi-permeable membranes to separate contaminants | High operational costs due to membrane fouling and frequent replacement | Baker, 2023 [25] |
| Gravel and multimedia filtration | Energy efficient | Phisical entrapment of through the porous bed, | Ineffective towards ions or molecules dissolved in water, the lifespan is dictated by physical clogging | Hatt et al., 2007 [26] M. Lazim, 2020 [27] |
| Slow and rapid sand filtration | Energy efficient | Water passes through layers of gravel, sand, and anthracite | Limited ability to remove very fine particles and dissolved substances | Kawamura, 2000 [28] |
| Activated carbon filtration | Energy efficient in use, energy intensive in production | Adsorbs contaminants onto the porous surface of activated carbon | High cost, limited lifespan; requires frequent regeneration or replacement | Tarikuzzaman, 2023 [29] |
| Chemical Filtration | Energy Efficiency | Methods | Limitations | References |
|---|---|---|---|---|
| Coagulation and flocculation followed by filtration | Moderately energy-intensive | Coagulants like alum or ferric chloride neutralize particle charges, forming larger aggregates (flocs) that are filtered out | Chemical costs and sludge disposal issues; requires precise dosage | Gregory & Duan, 2003 [30] |
| Ion exchange filtration | System related. In dilute streams there is a higher energy consumption per unit ion removed | Ion-exchange membrane fundamentals | Concentration polarisation, low current efficiency with complex ions, scaling/fouling, membrane heterogeneity | Pismenskaya & Nikonenko, 2021 [31] |
| Chemical precipitation | Moderately energy-intensive | Contaminants are converted into insoluble precipitates through chemical reactions and then filtered or settled out | High sludge production; requires proper chemical handling | Wang, 2005 [32] |
| Oxidation | Moderately/high energy-intensive | Advanced oxidation processes (AOPs) using engineered nanomaterials (ENMs) as catalysts. Risk of forming halogenated or toxic oxidation products | Might require large surface area | Pismenskaya & Nikonenko, 2021 [31] |
| Fungus/Matrix | Media | Contaminant | Effectiveness | Relevance to PAH Filtration | References |
|---|---|---|---|---|---|
| Ganoderma sp. dried fungal membrane | Cross-flow filtration | Pb2+ | 85–90 % | structural analogue | Parasnis et al., 2024; [50] |
| Mixed mycelium | Mycofiltration bed (e.g., wood chips) | Bacteria, pathogens | >95 | functional model | Chandra et al., 2022; [43] Mehta et al., 2017; [44] |
| Coriolus versicolor fungal membrane | Synthetic waste water | 97% TOC and 99% color removal | Textile dye | structural analogue/potential for PAH capture | Hai et al., 2006; [48] |
| Aspergillus carbonarius fungal membrane | Synthetic waste water | Textile dye | 91% decolorisation and 73.2% COD removal | structural analogue/potential for PAH capture | Isik et al., 2019; [49] |
| Treatment | Approximate Cost (£25/Tonne Soil) |
|---|---|
| Biological | 5–170 |
| Chemical | 12–600 |
| Physical | 20–170 |
| Solidification/stabilisation | 17–171 |
| Thermal | 30–750 |
| Aspect | Fungal Membrane Filtration | Fungal Biosorption | Fungal Biodegradation |
|---|---|---|---|
| Pollutant range | Potential to filter microbes and organic particles | Binds metals, dyes, hydrocarbons, complex organics | Degrades complex pollutants |
| Mechanism type | Functional groups on fungal cell walls | Physical entrapment of through the membrane | Extracellular enzymes |
| Scalability & maintenance | Potential for in locus degradation therefore reduce fouling, improving durability | Depends on production process of membrane. Potential to reuse biomass, low cost | Scalable for liquid and solid phases |
| Environmental tolerance | Potentially more resilient | Can tolerate harsh conditions | Can tolerate harsh conditions |
| Substrate growth and cost | Potentially can grow on low-cost substrates and self-regenerating membranes | Low-cost biomass from industrial waste | Can grow on agro-industrial waste |
| Synergy potential | Potentially can be integrated in a consortia with bacteria | Compatible with integrated remediation | High synergy with bacteria and plants |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colmo, C.; Tegelaar, M.; Ayres, P. Bioremediation of Polycyclic Aromatic Hydrocarbons (PAHs) in Aqueous Environments: A Review of Biofiltration, Biosorption, and Biodegradation Strategies Using Living Fungal Mycelium. Fermentation 2025, 11, 573. https://doi.org/10.3390/fermentation11100573
Colmo C, Tegelaar M, Ayres P. Bioremediation of Polycyclic Aromatic Hydrocarbons (PAHs) in Aqueous Environments: A Review of Biofiltration, Biosorption, and Biodegradation Strategies Using Living Fungal Mycelium. Fermentation. 2025; 11(10):573. https://doi.org/10.3390/fermentation11100573
Chicago/Turabian StyleColmo, Claudia, Martin Tegelaar, and Phil Ayres. 2025. "Bioremediation of Polycyclic Aromatic Hydrocarbons (PAHs) in Aqueous Environments: A Review of Biofiltration, Biosorption, and Biodegradation Strategies Using Living Fungal Mycelium" Fermentation 11, no. 10: 573. https://doi.org/10.3390/fermentation11100573
APA StyleColmo, C., Tegelaar, M., & Ayres, P. (2025). Bioremediation of Polycyclic Aromatic Hydrocarbons (PAHs) in Aqueous Environments: A Review of Biofiltration, Biosorption, and Biodegradation Strategies Using Living Fungal Mycelium. Fermentation, 11(10), 573. https://doi.org/10.3390/fermentation11100573






