1. Introduction
In recent years, interest in fermented foods has grown significantly due to their association with health benefits, especially because they are sources of probiotic microorganisms able to modulate the intestinal microbiota and promote the balance of the gastrointestinal tract [
1]. According to the World Health Organization (WHO), probiotics are living microorganisms that, when administered in appropriate quantities, confer beneficial effects on the host [
2]. Several studies have linked the consumption of probiotics to improved digestion, modulation of the immune response, reduction in cholesterol levels, and antioxidant effects [
3].
Among fermented foods, kefir stands out due to its complex and diverse microbial composition. Kefir grains are microbial structures formed by a matrix of lactic acid bacteria, yeasts, and exopolysaccharides, constituting a symbiotic colony. These grains appear as small granules or gelatin-like structures, in which the microorganisms are encapsulated within a polysaccharide matrix, such as kefiran, an exopolysaccharide produced by certain
Lactobacillus species. Grain formation begins with the auto-aggregation of
Lactobacillus kefiranofaciens and
Saccharomyces spp., forming small granules, to which the biofilm-forming
Lentilactobacillus kefiri later attaches, co-aggregating with other microorganisms and milk components to form mature grains [
4].
Traditionally, kefir is obtained by fermenting milk or sugary solutions using grains composed of a symbiotic community in which 65–90% consists of bacteria from the family
Lactobacillaceae, including members of the genera
Lactococcus,
Streptococcus,
Leuconostoc, and
Acetobacter. The predominant bacterial species in kefir grains include
Lacticaseibacillus paracasei,
Lactobacillus acidophilus,
Lactobacillus delbrueckii ssp.
bulgaricus,
Lactiplantibacillus plantarum,
Lactobacillus helveticus, Leuconostoc mesenteroides,
Lactococcus lactis,
Lentilactobacillus kefiri,
Lentilactobacillus parakefiri, and
Lactobacillus kefiranofaciens. Yeasts identified in kefir include members of the genera
Candida, Debaryomyces, Kazachstania, Kluyveromyces, Pichia, Saccharomyces, and Torulaspora, among others [
4,
5]. Fermentation results from the combined action of lactic acid bacteria, yeasts, and acetic acid bacteria, converting lactose and other milk components into acids, ethanol, CO
2, and bioactive compounds, which determine the beverage’s flavor, aroma, texture, and functional properties. The process, influenced by temperature (20–25 °C) and time (12–48 h), involves microbial succession that shapes bioactive compounds and enhances health benefits. After fermentation, the grains are removed, and the product is typically refrigerated to stabilize its microbiological and sensory profile [
4,
5,
6].
Recently, attention has been given to adapting kefir grains to non-dairy substrates, providing an alternative for consumers with milk restrictions, such as lactose intolerance, milk protein allergies, or vegetarian and vegan diets [
6,
7,
8]. Substrates successfully used include fruit and vegetable juices (e.g., apple, grape, carrot), which are rich in nutrients and sugars essential for probiotic growth, as well as cereals and plant-based milks (e.g., soy, almond, oat), which contain compounds with prebiotic effects and help protect probiotic cells from adverse gastrointestinal conditions [
6]. This adaptation typically involves gradual acclimatization over successive fermentation cycles, maintaining microbial viability and functional properties while enabling efficient metabolism of available sugars and nutrients [
6].
In this context, particular attention is given to the licuri (
Syagrus coronata), a palm native to the Brazilian semiarid region. It is traditionally consumed locally, and its fruits are eaten raw or processed into oil, flour, and other food products, playing an important role in the regional diet and culture. Like other Brazilian fruits, licuri is relatively unknown internationally and is commercially available mainly at a local or regional level. Its fruits are rich in lipids, predominantly medium-chain fatty acids such as lauric and oleic acids, as well as proteins and minerals including calcium, magnesium, and potassium. In addition, licuri contains bioactive compounds such as phenolic acids, flavonoids, and carotenoids, which contribute to its antioxidant and antimicrobial properties [
9,
10,
11]. Despite its high nutritional value and functional potential, the use of water-based licuri extract as a substrate for probiotic fermentations is still poorly explored in the scientific literature [
12].
Therefore, the present study aimed to develop and characterize a non-dairy fermented drink using the aqueous extract of licuri as substrate, fermented by grains of milk kefir and water kefir. The microbiological and physicochemical characteristics of the drink, its stability during storage, the viability of probiotic microorganisms against simulated conditions of the gastrointestinal tract (GIT), as well as the microbiological safety of the isolates obtained, were evaluated. This investigation sought to contribute to the valorization of regional food resources and to the development of innovative functional products with probiotic potential.
2. Materials and Methods
2.1. Materials
The materials used were as follows: sodium chloride (NaCl) (Qhemis, Sao Paulo, Brazil), pH meter buffer solutions 4, 7 and 10 (Exôdo cientifica, Sao Paulo, Brazil), sodium hydroxide NAoH (Neon, Sao Paulo, Brazil), Phenophthalein PA-ACS (Fmaia, Sao Paulo, Brazil), crystal violet (Laborclin, Pinhais, Brazil), Lugol’s iodine (NewProv), acetone alcohol (Qhemis, Sao Paulo, Brazil), safranin (Exôdo cientifica, Brazil), hydrogen peroxide (H2O2) (Exôdo cientifica, Brazil), and oxidase strips (Hardy Diagnostics, CA, USA). Culture media were as follows: peptone water (Merck, Darmstadt, Germany), Dichloran Rose Bengal Chloramphenicol Base (DRBC) agar (NutriSelect® Plus, Darmstadt, Germany), De Man–Rogosa–Sharpe MRS (GranuCult® prime, Darmstadt, Germany), trypticase soy agar (TSA) (Acumedia®, Lansing, USA), MRS broth (GranuCult® prime, Darmstadt, Germany), yeast extract (Merck, Germany), Tryptone (Kasvi, São José dos Pinhais, Brazil), sheep blood (NovaLab, Sao Paulo, Brazil), agar (Sigma-Aldrich, St. Louis, USA), Baird-Parker agar (NutriSelect® Plus, Darmstadt, Germany), and egg yolk emulsion enriched with potassium tellurite (Biolog, Hayward, USA). Enzymes were as follows: α-amylase (Sigma Aldrich, St. Louis, USA), pepsin (Sigma Aldrich), pancreatin (Sigma Aldrich), and bile salts (Sigma Aldrich). Control microorganisms were as follows: Listeria monocytogenes ATCC 7644, S. aureus ATCC 6538, Escherichia coli ATCC 94863, S. aureus ATCC 6538. Brown sugar (União, Sao Paulo, Brazil), UHT whole milk (Piracanjuba, Bela Vista de Goiás, Brazil), and water and milk kefir grains donated by the Food Microbiology Studies Laboratory (Faculty of Pharmacy, UFBA, Salvador, Brazil).
2.2. Methods
2.2.1. Preparation of Aqueous Licuri Almond Extract (LAE)
Licuri almonds were obtained from the Production Cooperative of the Diamantina Piemonte Region (COOPES, Capim Grosso, Bahia, Brazil; 11°22′51″ S, 40°00′46″ W) and transported to the Laboratory of Studies in Food Microbiology (LEMA) of the Faculty of Pharmacy of the Federal University of Bahia (UFBA). The almonds were washed, sanitized in chlorinated water at 200 ppm for 30 min, drained, and stored at −20 °C in PVC bags until analysis.
The licuri aqueous extract (LAE) was prepared by mixing the almonds with drinking water at a 1:3 (
w/
v) ratio, followed by heating for 10 min. The mixture was homogenized for 5 min using a Mondial Easy Power 550 W blender (Mondial, Conceição do Jacuípe, Brazil), then filtered through a sieve and filter paper. Brown sugar (10%,
w/
v) was added, and the extract was manually pasteurized on a hot plate (Cientec CT 339, Cientec, Sao Paulo, Brazil) using a thermometer (Deltt DT-650, Sao Paulo, Brazil) to maintain 85 °C for 30 min, then cooled to 25 ± 2 °C [
13]. The complete procedure is illustrated in
Figure 1.
2.2.2. Activation and Fermentation with Kefir Grains
Grains of milk and water kefir previously stored at −20 °C were obtained from the Laboratory of Studies in Food Microbiology (LEMA). The reactivation was performed by successive cycles of fermentation. Milk kefir grains were inoculated in UHT whole cow’s milk (2%
w/
v) and incubated at 25 °C for 24 h, according to Januário et al. [
14]. Water kefir grains were reactivated in a 10% sucrose solution prepared with brown sugar (2%
w/
v), under the same conditions described by Paredes, Escudero-Gilete, and Vicario [
15]. After each cycle, the grains were separated with plastic sieves, rinsed with water, and submitted to a new fermentation cycle [
5].
2.2.3. Adaptation of Aqueous Licuri Almond Extract (LAE)
Reactivated grains were adapted to the LAE supplemented with 10% sucrose through three fermentation cycles of 24 or 48 h at 25 ± 2 °C. Following the adaptation phase, fermentation cycles were carried out for analysis purposes. Ultra-high temperature (UHT) cow’s milk and a 10% sucrose solution were used as control treatments. All experimental conditions are summarized in
Table 1.
After the adaptation cycles, fermented beverages were filtered and liquid fractions were collected for physicochemical and microbiological analysis, while grains were used to evaluate microbial growth and isolation. The growth percentage of kefir grains was calculated using Equation (1).
Fm = final mass; Im = initial mass.
2.2.4. Analysis of pH, Acidity, and Microbial Viability of Lactic Acid Bacteria (LAB) and Yeasts
Samples were evaluated at 0 (T0), 5 (T5), 10 (T10), 15 (T15), and 20 (T20) days of storage under refrigerated conditions (4 ± 2 °C) in sterile glass bottles sealed with screw caps. pH was determined using a properly calibrated digital potentiometer (DM-22, Digimed Instrumentação Analítica, Sao Paulo, Brazil). Titratable acidity was expressed as a percentage of lactic acid, using 0.1 N NaOH and phenolphthalein as an indicator, according to APHA [
16]. Microbial viability was assessed by surface plating in triplicate on selective culture media: De Man–Rogosa–Sharpe agar (MRS; GranuCult
® Prime, Darmstadt, Germany) for lactic acid bacteria (LAB) and Dichloran Rose Bengal Chloramphenicol agar (DRBC; NutriSelect
® Plus, Darmstadt, Germany) for yeasts were used, following the procedures recommended by APHA [
16]. Plates were incubated under specific conditions suitable for the growth of each microbial group [
16]. In addition, data obtained from physicochemical and microbiological analyses were subjected to principal component analysis (PCA) to evaluate relationships among variables [
17].
2.2.5. Evaluation of LAB and Yeasts in Simulated Gastrointestinal Conditions
In vitro resistance to the gastrointestinal tract was evaluated according to adapted protocols from Luciano et al. [
18] and Madureira et al. [
19]. Aliquots of 25 mL of the fermented licuri beverages were sequentially subjected to the following simulated digestion phases: (i) oral phase—α-amylase (100 U mL
−1, pH 6.9, 200 rpm, 2 min); (ii) gastric phase—pepsin (25 mg mL
−1, pH 2.0, 130 rpm, 90 min); (iii) duodenal phase—pancreatin (2 mg mL
−1) and bile salts (12 mg mL
−1, 45 rpm, 30 min); (iv) ileal phase—pH adjusted to 6.5, 45 rpm, 60 min. The simulations were conducted in a shaker incubator (AL-221, AmericanLab, Sao Paulo, Brazil) at 37 °C, in triplicate. Milk kefir and water kefir were used as controls.
The microbial viability of LAB and yeasts was assessed by plating 1 mL of the sample, previously homogenized in 9 mL of 0.1% peptone water, followed by serial decimal dilutions up to 10
−8 [
16]. The resistance was expressed as a survival percentage, calculated according to Equation (2).
Cf = viability count after GIT; Ci = initial viability count.
2.2.6. Safety of Isolated Strains: Hemolytic, Gelatinolytic, and Lecithinase Activities
Hemolytic Activity
Strains activated in MRS broth (37 °C/24 h, anaerobiosis) were inoculated in TSA (Kasvi, Sao Paulo, Brazil) with 7% of debrided sheep blood. After incubation (37 °C/24 h), hemolysis was classified as α (greenish halo), β (transparent halo), or γ (absence of halo). Listeria monocytogenes ATCC 7644 was the positive control.
Gelatinolytic Activity
Activated strains (37 °C/24 h) were inoculated into a medium containing 1% yeast extract, 1.5% tryptone, and 12% gelatin. After incubation (30 °C/170 h) and cooling (8 °C/30 min), gelatin liquefaction was evaluated as an indicator of gelatinolytic activity. Staphylococcus aureus ATCC 6538 and Escherichia coli ATCC 94863 were used as positive and negative controls, respectively.
Lecithinase Activity
Activated strains were grown in MRS agar (37 °C/48 h) and sown in Baird-Parker agar (Kasvi, Sao Paulo, Brazil) with egg yolk emulsion and potassium tellurite (3%). After incubation (37 °C/48 h), the presence of an opaque halo indicated lecithinase activity. Staphylococcus aureus ATCC 6538 was the positive control.
2.2.7. Identification of the Isolate by Sequencing the 16S rRNA
After isolating several strains from water kefir grains, milk kefir grains, and adapted cultures, preliminary tests of viability and microbiological safety were conducted. Based on these technical criteria, the strain that showed the best performance in terms of viability and microbial safety was selected for DNA identification. This strain, isolated from water kefir grains adapted to licuri extract, was designated as WKL2. DNA identification was carried out by sequencing of the 16S rRNA gene (V1–V9 regions) using the Digital Microbiological Diagnostic (DMD) platform of Neoprospecta Microbiome Technologies (Florianópolis, Brazil). DNA extraction was performed with magnetic spheres, and sequencing was conducted on the MinION (Oxford Nanopore Technologies, Oxford, UK), using the V2 kit (300 cycles, single-end). The obtained sequences were processed using the company’s analysis pipeline, allowing up to 1% error. Taxonomic identification was performed by comparison with an internal database, with results available on the Neobiome platform (record no 183524.1).
2.3. Statistical Analysis
The data were evaluated using ANOVA, Tukey’s test, and Student’s
t-test (
p < 0.05). Principal component analysis (PCA) was also performed using XLSTAT® software (version 2022.1.5, Addinsoft, New York, NY, USA) [
17].
3. Results
3.1. Adaptation of Kefir Grains to Licuri Extract (WKL)
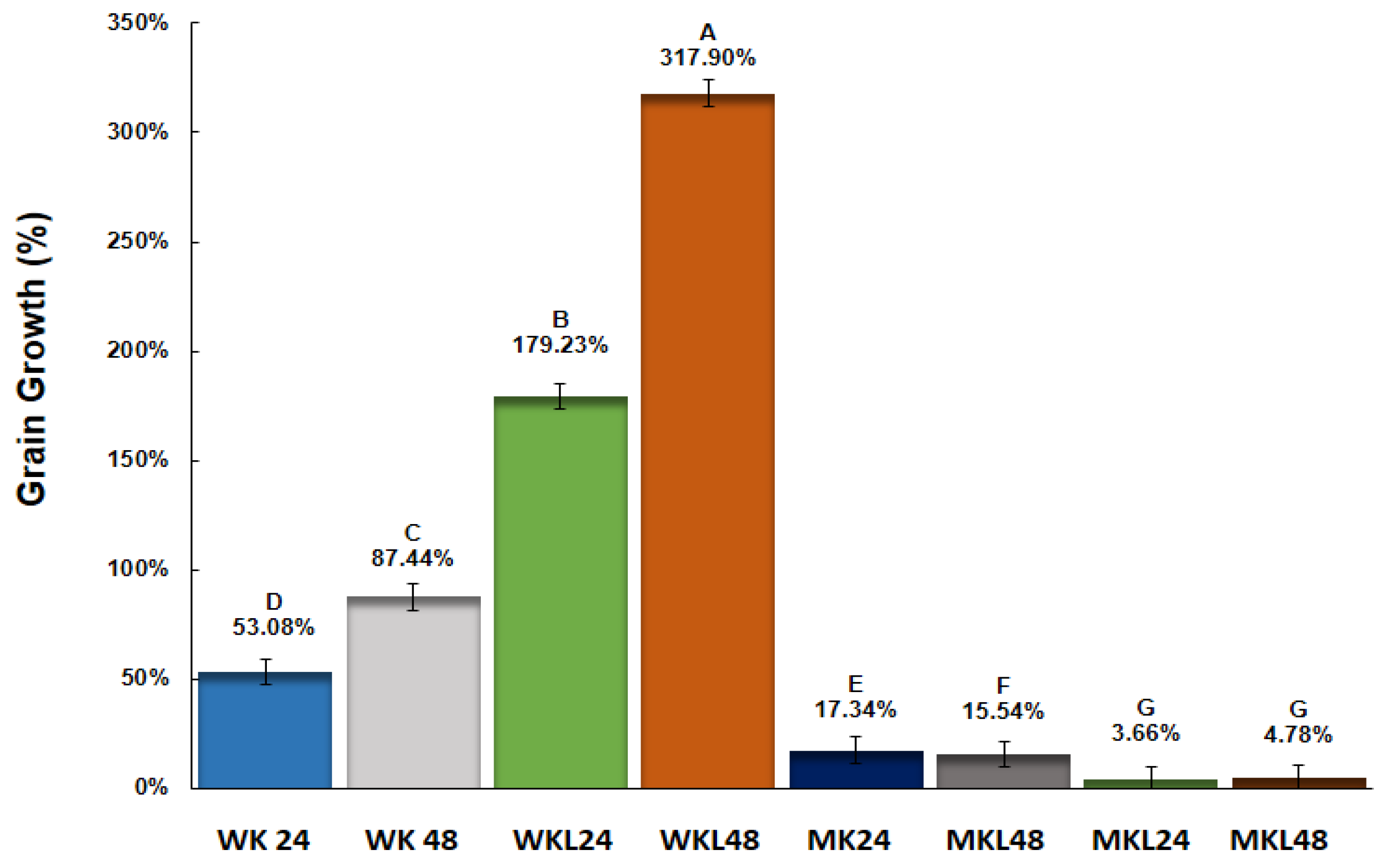
Water kefir grains adapted to the licuri aqueous extract (WKL) showed significantly higher growth rates compared to the other groups at both time points. After 48 h of incubation, the WKL 48 group exhibited the greatest biomass increase (317.90%), followed by WKL 24 (179.23%). Unadapted water kefir grains (WK) also demonstrated considerable growth, with increases of 53.08% at 24 h and 87.44% at 48 h. In contrast, unadapted milk kefir grains (MK) showed the lowest growth, with increases of 17.34% and 15.54% at 24 and 48 h, respectively (
Figure 2).
Milk kefir grains adapted to the licuri aqueous extract (MKL) showed the lowest percentage growth among all groups, with values of 3.66% after 24 h and 4.78% after 48 h. No statistically significant difference was observed between these time points (p > 0.05). Fermentation time had a positive effect on biomass increase in both adapted and unadapted water kefir grains (WKL and WK), with significantly higher growth at 48 h compared to 24 h. In contrast, prolonged fermentation did not significantly enhance the growth of milk kefir grains (MK and MKL), regardless of their adaptation status, suggesting a limited response to the LAE substrate under the evaluated conditions.
3.2. pH, Titratable Acidity, and Microbial Viability During Storage
Significant variations in pH (
Table 2) and titratable acidity (
Table 3) were observed over 20 days of refrigerated storage (4 ± 2 °C) in beverages fermented with licuri aqueous extract (LAE) and kefir grains.
The WKL 48 samples showed a decrease in pH from 3.87 (T0) to 3.69 (T20) (
Table 2), along with an increase in titratable acidity from 0.74 to 0.80 g lactic acid 100 mL
−1 (
Table 3). In the WKL 24 samples, the pH dropped from 4.43 to 3.89, while acidity increased from 0.43 to 0.46 g 100 mL
−1. The WK 24 and WK 48 samples maintained higher pH values throughout storage, with a decrease in titratable acidity over time; for WK 24, acidity declined from 0.14 g 100 mL
−1 (T0) to 0.08 g/100 mL (T20). In WK 48, acidity showed a slight increase until T15, followed by a reduction at T20.
Milk kefir samples (MK 24) exhibited a pH decrease from 4.96 to 4.23 and an increase in acidity from 0.70 to 0.85 g 100 mL−1 during storage. MK 48 samples showed only minor variations in pH and acidity, with slight acidification until day 10, followed by a decrease. Milk kefir grains adapted to LAE (MKL 24 and MKL 48) presented stable pH values between 4.30 and 4.44, with acidity ranging from 0.32 to 0.43 g 100 mL−1 over the storage period.
3.3. Microbial Viability During Storage
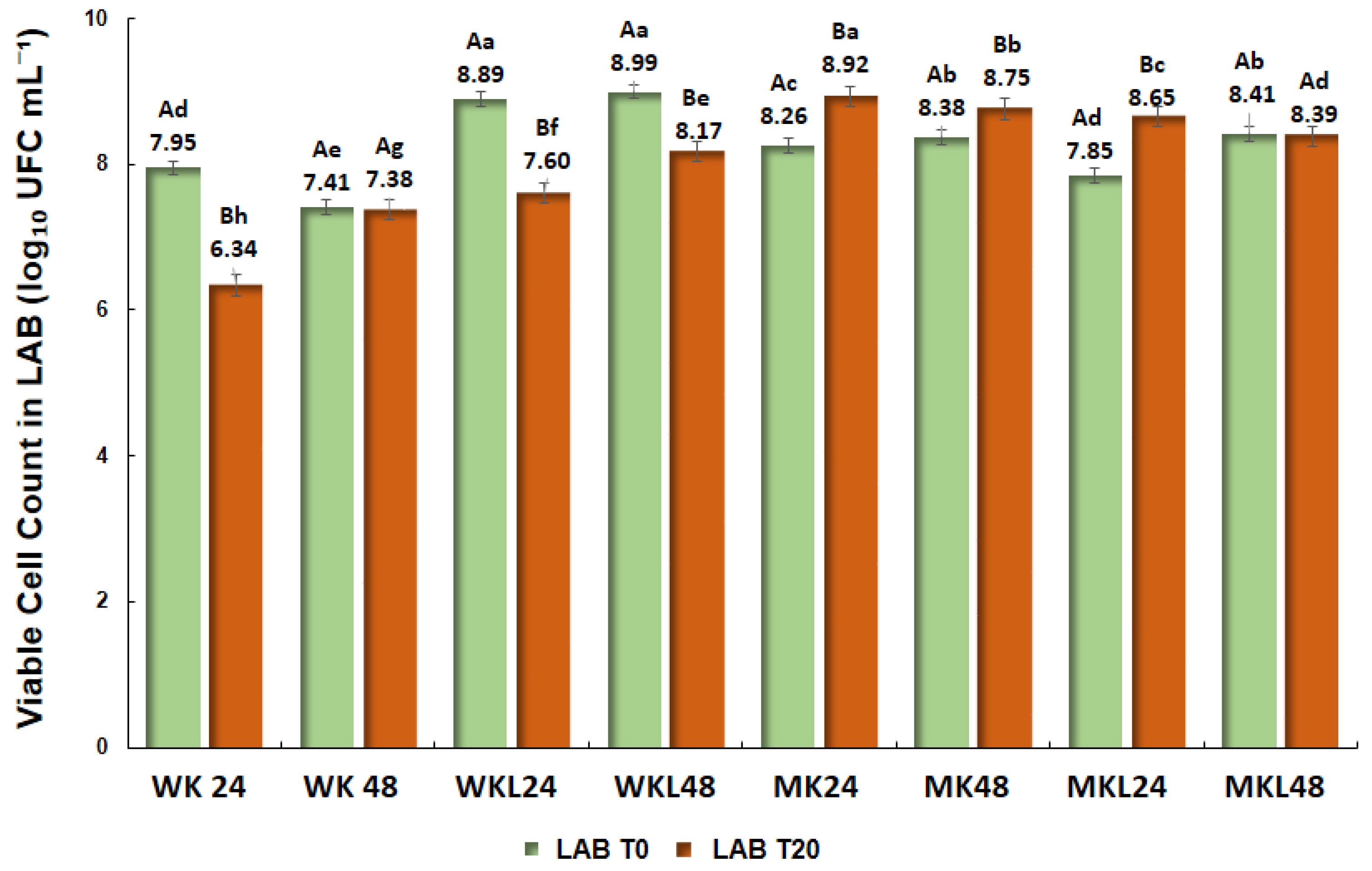
Figure 3 presents the viable cell count of lactic acid bacteria (LAB) in beverages fermented with different types of kefir, at initial times (T0) and after 20 days of refrigerated storage at 4 °C (T20). The samples fermented with water kefir not adapted to licuri extract (WK 24 and WK 48) exhibited the lowest LAB counts in both times. In WK 24, the count was significantly reduced from 7.95 to 6.34 log CFU mL
−1 (
p < 0.05), while WK 48 presented stable values, with 7.41 in T0 and 7.38 log CFU mL
−1 in T20 (
p > 0.05).
Samples produced with water kefir grains adapted to licuri (WKL 24 and WKL 48) showed the highest LAB counts, exceeding 8.6 log CFU mL−1 at both time points. In WKL 24, a slight but significant reduction was observed, from 8.89 to 8.60 log CFU mL−1 (p < 0.05), while in WKL 48, the values remained stable (8.99 at T0 and 8.81 log CFU mL−1 at T20; p > 0.05).
In samples fermented with unadapted milk kefir grains (MK), a significant increase in LAB count was observed in MK 24, from 8.26 to 8.92 log CFU mL−1 (p < 0.05). In MK 48, values were 8.38 at T0 and 8.92 log CFU mL−1 at T20, with no statistically significant difference (p > 0.05). Beverages fermented with milk kefir grains adapted to licuri (MKL 24 and MKL 48) presented intermediate counts. MKL 24 showed a significant increase from 7.85 to 8.65 log CFU mL−1 (p < 0.05), while MKL 48 remained stable, with 8.41 and 8.39 log CFU mL−1 at the evaluated time points (p > 0.05).
In all formulations, LAB counts remained above 7 log CFU mL−1 considered the minimum threshold to ensure probiotic potential.
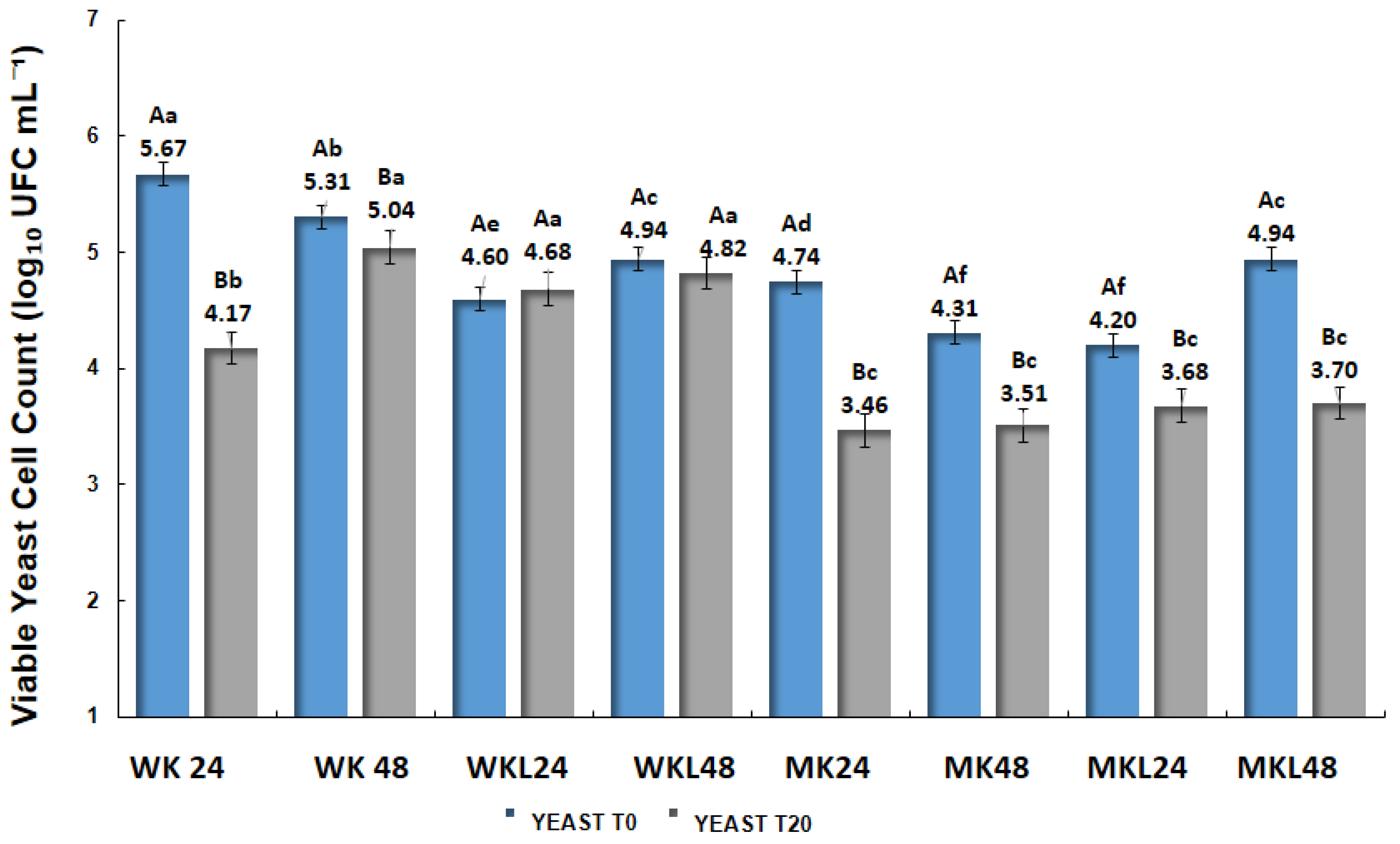
Figure 4 presents the viable yeast counts in beverages fermented with different types of kefir at T0 and T20. Overall, a significant reduction in yeast viability was observed over 20 days of storage at 4 °C (
p < 0.05), with the decrease being more pronounced in samples fermented with milk kefir. Among the beverages produced with water kefir (WK), the highest initial yeast counts were found in WK 24, decreasing from 5.67 log CFU mL
−1 at T0 to 4.17 log CFU mL
−1 at T20. WK 48 exhibited a smaller variation, from 5.31 to 5.04 log CFU mL
−1 (
p > 0.05). In formulations fermented with water kefir adapted to licuri (WKL), the counts remained relatively stable: from 4.68 to 4.60 log CFU mL
−1 in WKL 24 (
p > 0.05) and from 4.94 to 4.82 log CFU mL
−1 in WKL 48 (
p < 0.05).
Beverages fermented with milk kefir (MK) showed the lowest yeast counts, with initial values of 4.31 and 4.20 log CFU mL−1 for MK 24 and MK 48, respectively, which declined to 3.46 and 3.51 log CFU mL−1 at T20 (p < 0.05). Samples fermented with milk kefir adapted to licuri (MKL) showed better retention of viable yeast, with a slight reduction from 4.20 to 3.68 log CFU mL−1 in MKL 24 (p > 0.05) and a significant decrease from 4.94 to 3.70 log CFU mL−1 in MKL 48 (p < 0.05).
3.4. Resistance to Simulated Gastrointestinal Conditions
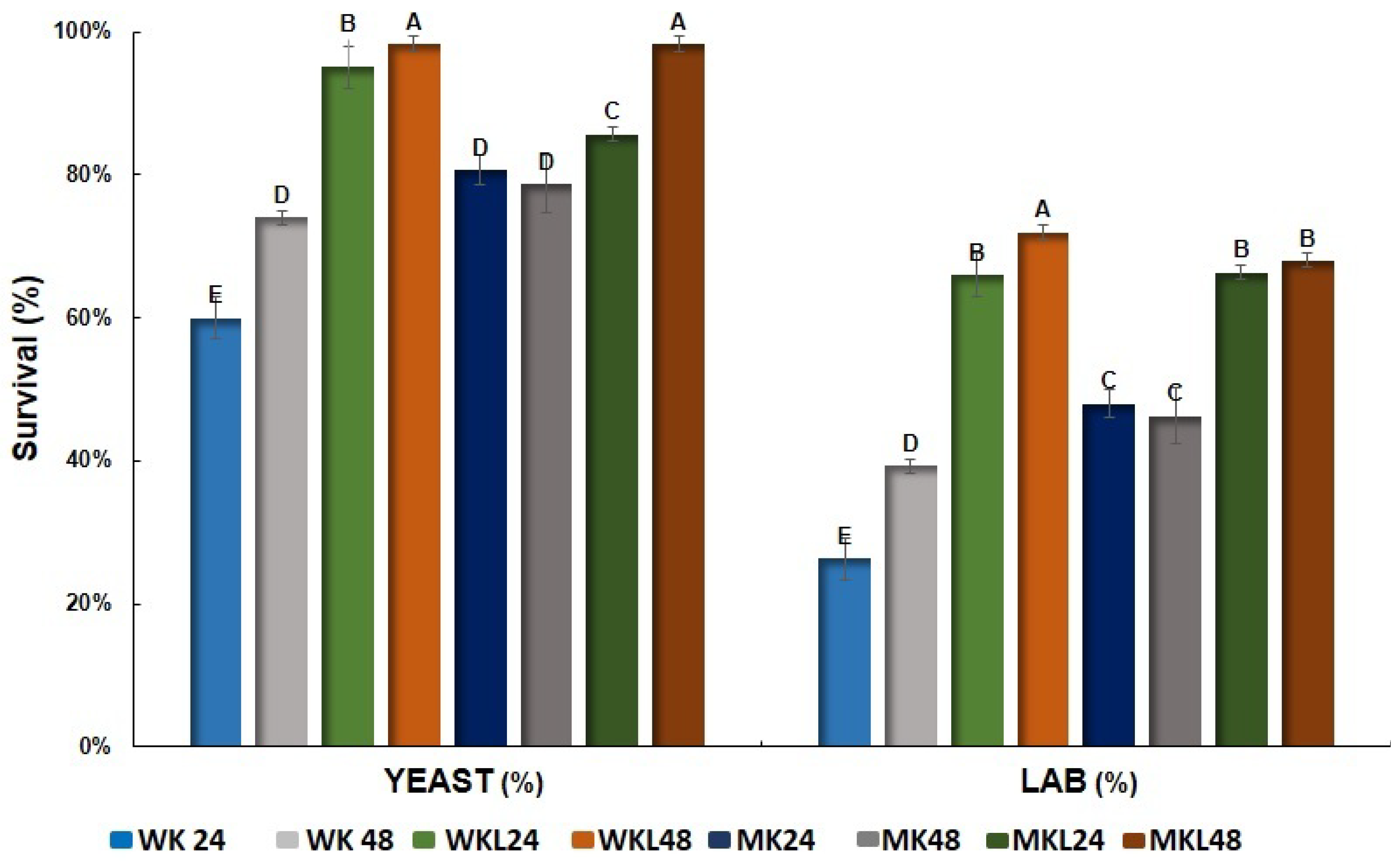
Figure 5 presents the survival percentages of yeasts and lactic acid bacteria (LAB) after exposure to simulated gastrointestinal tract (GIT) conditions in beverages fermented with water kefir, milk kefir, and their combinations with licuri extract, fermented for 24 and 48 h. For a more detailed view, the microbial counts (log CFU mL
−1) before and after GIT simulation are presented in
Table 4.
In general, samples fermented for 48 h exhibited significantly higher survival rates for both microbial groups compared to those fermented for 24 h. Among the two groups, yeasts demonstrated greater resistance to simulated GIT conditions than LAB. The WKL 48 sample (water kefir with licuri extract, fermented for 48 h) showed the highest survival, reaching approximately 85% for LAB and 99% for yeasts (
p < 0.05), consistent with the counts observed in
Table 4. The MKL 48 sample (milk kefir with licuri extract, fermented for 48 h) also achieved high survival, with around 70% for LAB and 95% for yeasts.
Conversely, WK24 and MKL24 presented the lowest survival rates, particularly WK24, which exhibited only ~25% LAB survival and ~60% yeast survival. These values were significantly lower than those of the other treatments (
p < 0.05), consistent with the lower log CFU mL
−1 values reported in
Table 4. This demonstrates the correlation between absolute microbial counts and percentage survival, providing a more complete understanding of microbial viability under simulated GIT conditions.
3.5. Microbiological Safety of Isolates
The aim of this analysis was to ensure the microbiological safety of the isolates obtained from kefir grains used in the fermentation. The isolated LAB strains (n = 15) were obtained from kefir grains after fermentation under different conditions (substrate type and fermentation time). Briefly, the grains were macerated in peptone water, and 1 mL aliquots were plated on MRS agar and incubated under anaerobic conditions for 48 h. Individual colonies were then picked and purified to obtain distinct isolates. These isolates were selected to represent the microbial diversity present in the kefir grains, rather than in the fermented beverages. None of the isolated LAB strains showed β-hemolytic activity. All were classified as non-pathogenic, displaying either no hemolysis (γ-hemolysis) or weak α-hemolysis (non-harmful). Positive controls (Listeria monocytogenes and Staphylococcus aureus) confirmed the efficacy of the assays.
3.6. Identification of the WKL 2 Strain
The purpose of this step was to identify at least one representative isolate from the microbial consortium. The WKL 2 strain, isolated from adapted water kefir grains, was identified as Lacticaseibacillus paracasei by high-performance sequencing of the 16S rRNA gene, covering regions V1 to V9. The analysis revealed high similarity with sequences deposited in reference databases, confirming the identity of the strain.
3.7. Principal Component Analysis (PCA) of Physical–Chemical, Microbiological, and Functional Parameters
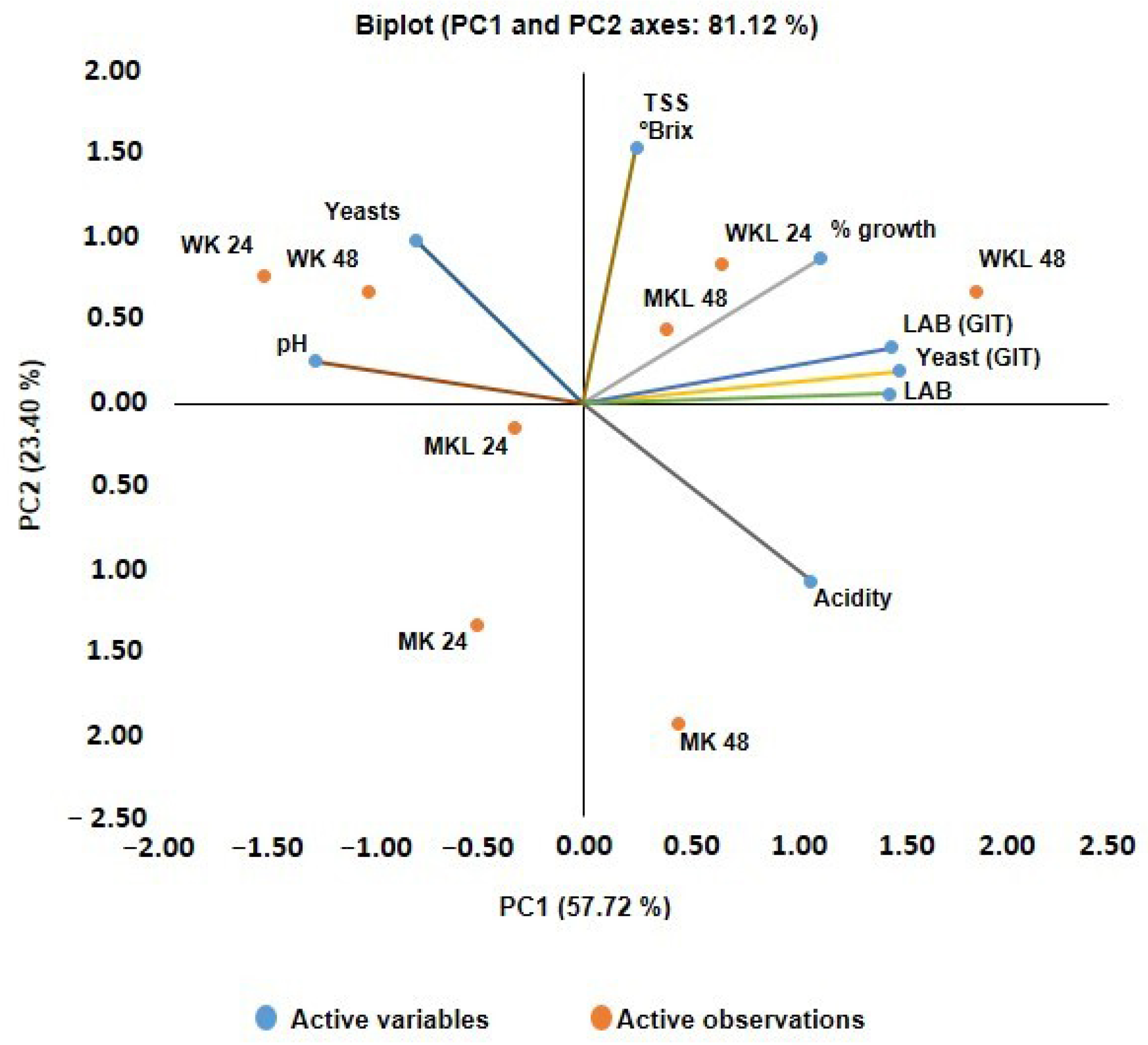
The principal component analysis (PCA), presented in
Figure 6, highlights the influence of the fermentation matrix and fermentation time on the physicochemical, microbiological, and functional parameters of the beverages.
The first two principal components explained 81.12% of the total variance, with Principal Component 1 (PC1) strongly associated with titratable acidity, grain biomass increase, LAB counts, and resistance to simulated gastrointestinal conditions (GIT). In turn, Principal Component 2 (PC2) primarily discriminated yeast viability and pH values.
Samples fermented with licuri aqueous extract, particularly WKL 48, showed a positive correlation with desirable functional attributes, such as higher LAB growth and greater GIT resistance, highlighting the potential of licuri as a functional fermentation matrix. In contrast, control samples with water kefir (WK) exhibited higher yeast viability and higher pH values, suggesting a distinct fermentative profile with reduced organic acid production.
These findings reinforce that both the type of substrate and fermentation time significantly influence the composition and functionality of fermented beverages. The licuri extract emerges as a promising substrate for the development of symbiotic foods, since it provides phenolic compounds and other bioactive molecules with potential prebiotic effects while supporting the growth and viability of probiotic microorganisms, thus combining both probiotic and prebiotic benefits [
9,
10,
11,
12].
4. Discussion
The results demonstrated that the prior adaptation of water kefir grains to licuri aqueous extract (LAE) was a determining factor in promoting greater grain biomass increase, enhancing fermentative activity, and maintaining microbial viability throughout refrigerated storage. The WKL 48 group exhibited a biomass increase of 317.90%, significantly higher than all other experimental groups, including milk kefir grains adapted to the same extract (MKL), which showed growth below 5%. This marked contrast reinforces the hypothesis that water kefir grains possess greater adaptability, efficiently utilizing the nutrients present in LAE.
As expected, milk kefir grains exhibited limited performance, likely due to physiological stress resulting from the substitution of their native dairy matrix—rich in lactose and specific proteins—with a plant-based medium of distinct composition. This adaptive limitation has also been reported by Atalar [
20] and Lynch et al. [
21]. The inclusion of milk kefir in this study, however, served as a control for comparison, confirming its restricted applicability in non-dairy fermentations and reinforcing the superior adaptability of water kefir grains to plant-based substrates.
Furthermore, the observed grain growth may have been favored by the mineral composition of LAE. Previous studies have indicated that the presence of ions, particularly calcium, contributes to pH stability during fermentation by acting as a buffering agent and preventing abrupt pH shifts that could compromise microbial viability [
22]. This stabilization of the microenvironment is essential for grain structural development and supports the proliferation of adapted microbial consortia.
In addition, the microbial profile of kefir grains can undergo significant shifts depending on the cultivation substrate. Hsieh et al. [
23] demonstrated that different media—such as water with brown sugar, cow’s milk, and goat’s milk—promote distinct selections of dominant microorganisms, influencing the microbial community structure over time. In this context, it is plausible to assume that adaptation to LAE induced a positive selection of strains better suited to the plant-based matrix, gradually replacing species originally dominant in dairy environments. This selective process may account for the functional superiority observed in the WKL 48 group compared to those containing milk kefir grains.
Another relevant finding was the positive impact of extended fermentation time (48 h), which enhanced both the fermentative performance and growth of water kefir grains, whether adapted to LAE or not. This effect, however, was not observed in milk kefir grains, suggesting that the response to fermentation duration is intrinsically linked to the compatibility between the microbial consortium and the substrate. Thus, prolonged fermentation appears to benefit only metabolically active and well-adapted consortia, as seen in the water kefir and LAE treatments.
Regarding fermentation behavior during storage, the samples produced with adapted water kefir grains (WKL 24 and WKL 48) exhibited greater acidification and a more pronounced pH reduction, reinforcing the conclusion that prior adaptation favored the persistence of microbial activity even under refrigeration. The higher titratable acidity observed in these samples (up to 0.80 g 100 mL−1) was consistent with elevated counts of viable lactic acid bacteria (LAB), which remained above 8.6 log CFU mL−1 at the end of the 20-day storage period. These values exceed the minimum threshold generally recommended for probiotic efficacy, which is typically 6 log CFU mL−1, as reported in the literature.
In contrast, samples with unadapted grains (WK 24 and WK 48) also showed reductions in pH and increases in titratable acidity, but these changes were accompanied by lower microbial viability over time. This suggests a limited fermentative activity, possibly due to the lack of prior adaptation of the grains to the plant-based substrate, thereby compromising the technological and probiotic potential of the product. These findings highlight the importance of the adaptation process as a critical step for ensuring the stability and metabolic performance of starter cultures in unconventional matrices.
According to Normative Instruction No. 46 of 23 October 2007 (IN 46/2007), issued by the Ministry of Agriculture, Livestock and Supply (MAPA), which establishes the identity and quality standards for fermented milks in Brazil, the titratable acidity of these products during storage shall not vary by more than 1 g of lactic acid per 100 g of product [
24]. In the present study, all samples remained within this limit, demonstrating that despite variations in physical and chemical parameters, the fermented beverages met the legal quality criteria, including those produced with kefir grains not adapted to licuri extract.
Supporting these findings, Dantas [
25] evaluated probiotic drinks prepared from coconut milk and jambolão pulp and observed an initial drop in pH followed by a gradual increase during 21 days of storage. The pH values ranged from 5.83 to 4.45 in lactose-fermented milk beverages and from 3.67 to 3.58 in plant-based formulations. Additionally, no significant differences in titratable acidity were detected between the different drink types, with values remaining between 0.62 and 0.87 g of lactic acid per 100 g over the evaluation period. These results reinforce the hypothesis that the physicochemical stability of fermented beverages depends both on the substrate type and on the prior adaptation of the microbiota, factors that directly influence viability and probiotic functionality during storage [
25].
The adaptation of the grains to licuri extract was successful, particularly for water kefir, which may be related to the composition of the matrix and the synergy with the resident yeasts [
22,
26]. The nutritional composition of LAE proved sufficient to sustain microbial growth and preserve viability over 20 days of refrigerated storage, reinforcing the potential of this matrix as a functional vehicle for probiotic cultures.
In formulations containing milk kefir grains, adaptation was less efficient in terms of growth. However, the viability of lactic acid bacteria (LAB) remained high (above 8 log CFU mL−1), indicating that part of the microbiota associated with this matrix shows resilience to the new fermentation environment. Nevertheless, the limited grain expansion and instability in physicochemical parameters (pH and titratable acidity) during storage suggest that the use of milk kefir in LAE is less effective from a fermentative and technological perspective compared to adapted water kefir.
Previous studies corroborate this observation. Łopusiewicz et al. [
27] reported significant growth of LAB and yeast in beverages fermented from flaxseed oil cake, even without supplementation, with counts maintained during storage between 7 and 8 log CFU mL
−1 for LAB and between 5 and 6 log CFU mL
−1 for yeasts. Similarly, Pinto et al. [
28] demonstrated that beverages fermented with yam extract and kefir grains maintained populations of 7.01 log CFU mL
−1 of LAB and 6.32 log CFU mL
−1 of yeast after 28 days of storage. Consistent results were also observed in other plant products fermented with kefir, such as soy aqueous extract, apple vinegar-based substrates, and hazelnut [
20,
29,
30], reinforcing the feasibility of using plant matrices for the development of functional fermented beverages.
According to IN 46/2007 [
24], fermented products must maintain minimum counts of ≥6 log CFU g
−1 or mL
−1 for lactic acid bacteria and yeasts throughout their entire shelf life. In the present study, all formulations met the minimum viability requirements for LAB. However, samples ML24, ML48, MKL24, and MKL48 exhibited yeast counts below the required threshold, which may compromise their classification as fermented beverages according to regulatory standards. This finding highlights the importance of microbiota adaptation to the substrate to ensure the expected functional composition in fermented products.
The resistance of microbial cultures to simulated gastrointestinal tract conditions was consistent with internationally accepted criteria for probiotic claims [
31]. The detection of
Lacticaseibacillus paracasei in a native and safe matrix such as the licuri aqueous extract (LAE) reinforces the potential of this raw material for functional beverage formulations and represents a promising avenue for in vivo studies and commercial applications.
As highlighted by Del Piano et al. [
31], the main obstacle faced by probiotic microorganisms during passage through the gastrointestinal tract (GIT) is the gastric environment, characterized by an extremely acidic pH and the presence of proteolytic enzymes such as pepsin. Following this stage, the surviving microorganisms encounter the conditions of the small intestine, where pancreatin and bile salts present additional challenges to their viability, typically resulting in an average reduction of 35 to 40% in the microbial population. Therefore, for microorganisms in fermented foods to be considered probiotics, it is recommended that they withstand simulated GIT conditions and maintain viable counts equal to or greater than 6 log CFU mL
−1, the minimum level necessary to confer health benefits to the host [
32].
In the present study, only samples MKL24, MKL48, WKL24, and WKL48 met this criterion, exhibiting counts of 6.10, 6.60, 7.00, and 7.68 log CFU mL
−1, respectively, after exposure to simulated GIT conditions. Rodrigues [
33] evaluated the resistance of a bacterial H7 isolate in a milk matrix and observed a reduction of approximately 2 log cycles after GIT simulation, resulting in a final concentration of 5.99 ± 0.04 log CFU mL
−1. Despite this decrease, the isolate was considered promising from a probiotic perspective, as it remained near the recommended minimum value.
Similar findings were reported by Ferreira et al. [
34], who assessed the viability of
Pichia kluyveri (CCMA 0615) and
Debaryomyces hansenii (CCMA 1761) under simulated GIT conditions. Both yeasts maintained nearly unchanged cell concentrations, with survival rates exceeding 90% in a plant matrix, demonstrating remarkable physiological resistance. Bernat et al. [
13] also observed positive results using vegetable hazelnut milk, in which approximately 60–65% of the bacteria survived passage through the GIT, resulting in counts close to 5 log CFU mL
−1.
These results indicate that, although the conditions in the gastrointestinal tract (GIT) pose a significant challenge to the survival of probiotic microorganisms, certain formulations based on plant matrices, such as the licuri aqueous extract (LAE), can effectively protect the probiotic microbiota. This protective effect may be attributed to the presence of phenolic compounds, natural sugars, and other bioactive molecules in LAE, which can act as prebiotic substrates, enhance cell resistance to acidic and bile conditions, and contribute to maintaining microbial viability during GIT transit [
18]. The improved survival observed in MKL24, MKL48, WKL24, and WKL48 may be explained by several factors: the protective effects of fibers, phenolic compounds, and natural sugars present in the licuri extract, the buffering capacity of the substrate, and the prior adaptation of water kefir grains to the plant medium, which likely enhanced microbial resistance. The use of licuri extract, a regional plant resource, shows great promise for the development of non-dairy fermented beverages with functional and probiotic properties. Here, “functional beverage” refers to a drink that provides additional health benefits beyond basic nutrition, such as supporting gut health or modulating the immune system, particularly when fermented with water kefir grains previously adapted to the substrate, which appeared to support higher microbial viability and stability during storage and simulated GIT conditions [
4,
15].
It should be noted that sensory evaluation of the final products was not performed in this study, which represents a limitation. Future studies should include sensory analyses to fully assess the technological potential and consumer acceptance of these beverages
Moreover, lactic acid bacteria (LAB) strains isolated from kefir grains exhibited microbiological safety, showing no pathogenic traits such as β-hemolysis, gelatinolytic activity, or lecithinase production. All strains were classified as non-pathogenic, presenting only α-hemolysis halos. These results reflect intrinsic characteristics of the kefir-derived strains rather than changes induced by the fermentation process. The absence of virulence factors further supports the safety of these strains as functional cultures for application in fermented foods. Although LAB are generally recognized as safe (GRAS), thorough evaluation of potential virulence factors remains essential, particularly for new or previously uncharacterized strains [
33,
35].