Diet Formulated with Rice Bran Fermented by Rhizopus oryzae and Saccharomyces cerevisiae: Impacts on Zootechnical Performance and Intestinal Gene Expression in Zebrafish (Danio rerio)
Abstract
1. Introduction
2. Materials and Methods
2.1. Source of Inputs
2.2. Solid-State Fermentation (SSF) of Rice Bran by Rhizopus oryzae
2.3. Fermentation of Rice Bran by Saccharomyces cerevisiae
2.4. Proximate Composition of the Brans
2.5. Experimental Diet Formulation
2.6. Quantification of Fatty Acids (FA) in the Diets
2.7. Experimental Design
2.8. Biometry
2.9. Total RNA Extraction and Complementary DNA (cDNA) Synthesis
2.10. Quantitative PCR (qPCR)
2.11. Statistical Analysis
3. Results
3.1. Proximate Composition of Rice Bran
3.2. Fatty Acids
3.3. Zootechnical Performance
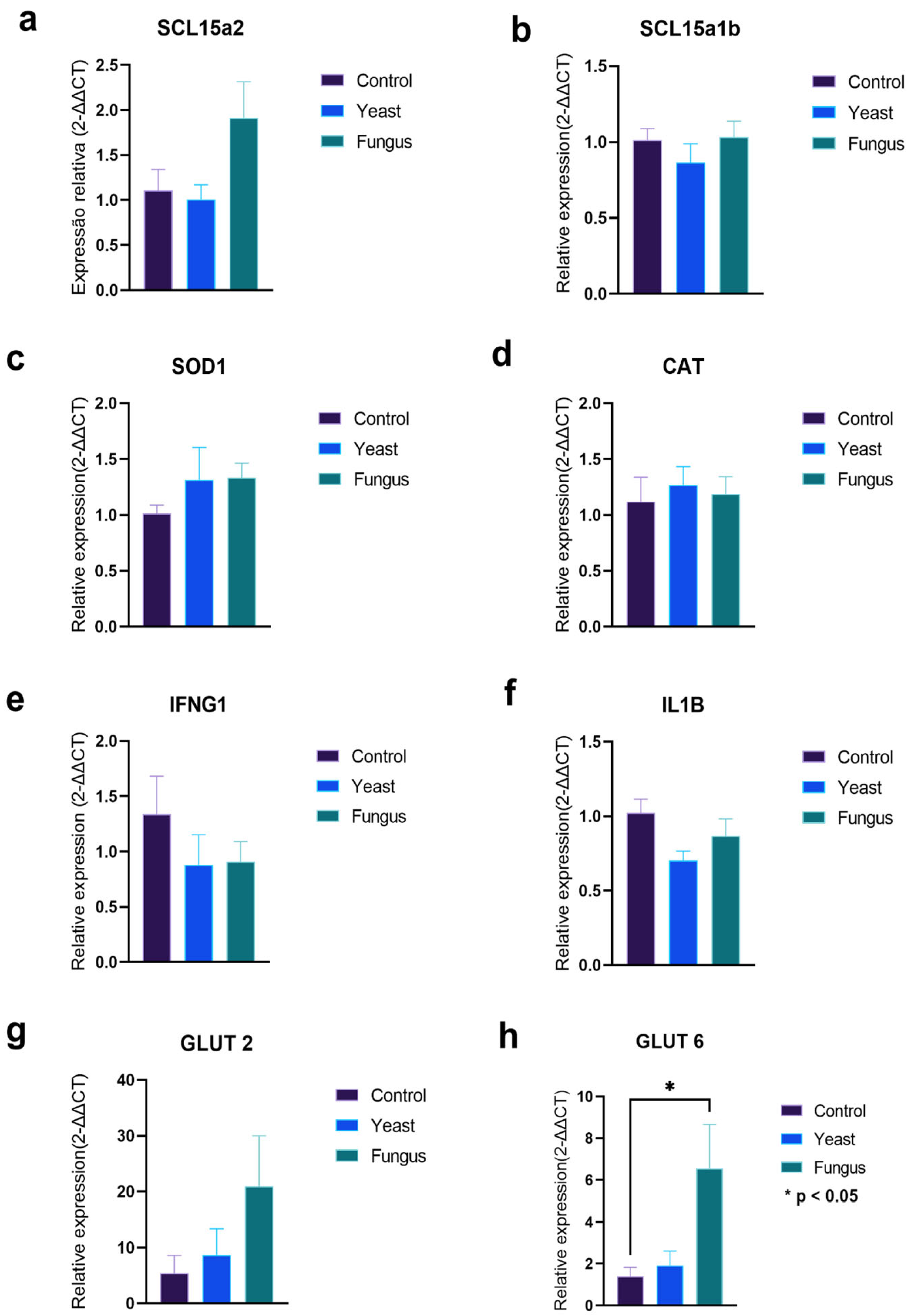
3.4. Gene Expression
4. Discussion
4.1. Bioconversion of Macromolecules in Rice Bran
4.2. Characterization of the Dietary Lipid Profile
4.3. Zootechnical Performance
4.4. Gene Expression Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2024; FAO: Rome, Italy, 2024; Available online: https://www.fao.org/publications (accessed on 11 June 2025).
- OECD-FAO Agricultural Outlook 2025–2034, Chapter 7: Fish and Other Aquatic Products; OECD Publishing: Paris, France; FAO: Rome, Italy, 2025. [CrossRef]
- Al Khawli, F.; Martí-Quijal, F.J.; Ferrer, E.; Ruiz, M.J.; Berrada, H.; Gavahian, M.; Barba, F.J.; de la Fuente, B. Aquaculture and Its By-Products as a Source of Nutrients and Bioactive Compounds. Adv. Food Nutr. Res. 2020, 92, 1–33. [Google Scholar] [CrossRef]
- Lenz, G.; Ayres, T.S.M.; Tesser, M.B.; Christ-Ribeiro, A. Rice bran as an agro-industrial by-product in aquaculture: Types, properties, and applications. Trop. Anim. Health Prod. 2025, 57, 402. [Google Scholar] [CrossRef]
- He, Y.; Guo, X.; Tan, B.; Dong, X.; Yang, Q.; Liu, H.; Zhang, S.; Chi, S. Replacing fish meal with fermented rice protein in diets for hybrid groupers (Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus ♂): Effects on growth, digestive and absorption capacities, inflammatory-related gene expression, and intestinal microbiota. Aquac. Rep. 2021, 19, 100603. [Google Scholar] [CrossRef]
- AMIS—Agricultural Market Information System. AMIS Market Monitor, No. 129, June 2025; AMIS Secretariat: Rome, Italy, 2025; Available online: https://www.amis-outlook.org (accessed on 11 June 2025).
- Christ-Ribeiro, A. Fermentation process in the availability of nutrients in rice bran. Res. Rev. J. Microbiol. Biotechnol. 2017, 6, 45–52. [Google Scholar]
- Kahlon, T.S. Chapter 14. Rice bran: Production, composition, functionality and food applications, physiological benefits. In Fiber Ingredients: Food Applications and Health Benefits; Cho, S.S., Samuel, P., Eds.; CRC Press: Boca Raton, FL, USA, 2009; pp. 305–321. [Google Scholar]
- Manlapig, J.; Matsui, H. Production and utilization of fermented rice bran as animal feed. Anim. Sci. J. 2025, 96, e70037. [Google Scholar] [CrossRef]
- Bhatnagar, A.S.; Prabhakar, D.S.; Prasanth Kumar, P.K.; Raja Rajan, R.G.; Gopala Krishna, A.G. Processing of commercial rice bran for the production of fat and nutraceutical rich rice brokens, rice germ and pure bran. LWT-Food Sci. Technol. 2014, 58, 306–311. [Google Scholar] [CrossRef]
- IAFFD—International Aquaculture Feed Formulation Database. Available online: https://www.iaffd.com/ (accessed on 16 June 2025).
- Pastore, S.C.G.; Gaiotto, J.R.; Ribeiro, F.A.S.; Nunes, A.J.P. Boas práticas de fabricação e formulação de rações para peixes. In NutriAqua: Nutrição e Alimentação de Espécies de Interesse Para a Aquicultura Brasileira; Fracalossi, D.M., Cyrino, J.E.P., Eds.; Sociedade Brasileira de Aquicultura e Biologia Aquática: Florianópolis, Brazil, 2016; Volume 1, Chapter 16; pp. 295–346. [Google Scholar]
- Feddern, V.; Furlong, E.B.; Soares, L.A.S. Effects of fermentation on the physicochemical and nutritional properties of rice bran. Ciência E Tecnol. Aliment. 2007, 27, 800–804. [Google Scholar] [CrossRef]
- Magalhães, A.P.L.; Teixeira, A.Z.A. Análises organolépticas e proteica da fermentação do farelo de arroz com a levedura Saccharomyces cerevisiae. Rev. Ibero-Am. Humanidades Ciências E Educ. 2020, 6, 9. [Google Scholar] [CrossRef]
- Londoño-Hernandez, L.; Ruiz, H.A.; Ramírez, C.; Ascacio, J.A.; Rodríguez-Herrera, R.; Aguilar, C.N. Fungal detoxification of coffee pulp by solid-state fermentation. Biocatal. Agric. Biotechnol. 2020, 23, 101467. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; Sant’Anna, E.S. Effect of sodium chloride on protein production (Saccharomyces cerevisiae) in semi-solid fermentation. Ciência E Tecnol. Aliment. 2001, 21, 57–62. [Google Scholar] [CrossRef]
- Aquarone, E.; Borzani, W.; Schmidell Netto, W.; Lima, U.D.A. Biotecnologia Industrial—Biotecnologia na Produção de Alimentos; Edgard Blücher: São Paulo, Brazil, 2001; p. 523. [Google Scholar]
- Liu, N.; Wang, Y.; An, X.; Qi, J. Study on the enhancement of antioxidant properties of rice bran using mixed-bacteria solid-state fermentation. Fermentation 2022, 8, 212. [Google Scholar] [CrossRef]
- Coronado, M.; Solis, C.J.; Hernandez, P.P.; Feijóo, C.G. Dietary modulation of intestinal inflamemation in zebrafish: Insights into the cellular and molecular mechanisms. Front. Immunol. 2019, 10, 610. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, F.; Guo, M.; Qin, M.; Wang, J.; Yu, H.; Xu, J.; Liu, Y.; Tong, T. Growth performance, antioxidant and immunity capacity were significantly affected by feeding fermented soybean meal in juvenile coho salmon (Oncorhynchus kisutch). Animals 2023, 13, 945. [Google Scholar] [CrossRef]
- Christ-Ribeiro, A.; Graça, C.S.; Kupski, L.; Badiale-Furlong, E.; Souza-Soares, L.A. Cytotoxicity, antifungal and antimycotoxin effects of phenolic compounds from fermented rice bran and Spirulina sp. Process Biochem. Process Biochem. 2019, 80, 190–196. [Google Scholar] [CrossRef]
- Christ-Ribeiro, A.; da Silva Graça, C.; Massarolo, K.C.; Jaeschke, D.P.; de Souza Soares, L.A. Production of high-value-added biomass by Saccharomyces cerevisiae using lignocellulosic substrate. Fermentation 2025, 11, 257. [Google Scholar] [CrossRef]
- AOAC—Association of Official Analytical Chemists. Official Methods of Analysis, 18th ed.; AOAC: Gaithersburg, MD, USA, 2007. [Google Scholar]
- Silva, D.J.; Queiroz, A.C. Análise de Alimentos: Métodos Químicos e Biológicos, 3rd ed.; Universidade Federal de Viçosa: Viçosa, MG, Brazil, 2006; p. 235. [Google Scholar]
- Bligh, E.C.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Christie, W.W. Lipid Analysis: Isolation, Separation, Identification and Structural Analysis of Lipids; Pergamon Press: Oxford, UK, 1982. [Google Scholar]
- Schneider, A.C.R.; Maurer, R.L.; Matte, U.; Porawski, M.; Schaefer, P.G.; dos Santos, J.L.; da Silveira, T.R. Implementação de um novo modelo de experimentação animal—Zebrafish. Clin. Biomed. Res. 2009, 29, 100–103. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Oliveira, M.D.S.; Feddern, V.; Kupski, L.; Cipolatti, E.P.; Badiale-Furlong, E.; de Souza-Soares, L.A. Physico-chemical characterization of fermented rice bran biomass. CyTA-J. Food 2010, 8, 229–236. [Google Scholar] [CrossRef]
- Zhang, Q.; Guo, M.; Li, F.; Qin, M.; Yang, Q.; Yu, H.; Xu, J.; Liu, Y.; Tong, T. Evaluation of fermented soybean meal to replace a portion fish meal on growth performance, antioxidant capacity, immunity, and mTOR signaling pathway of coho salmon (Oncorhynchus kisutch). Aquac. Nutr. 2023, 2023, 2558173. [Google Scholar] [CrossRef]
- Albuquerque, L.F.G. Zootechnical and Economic Performance of Penaeus vannamei Culture Using the Aquamimicry System. Ph.D. Thesis, Federal University of Ceará, Fortaleza, Brazil, 2019. [Google Scholar]
- Helal, S.E.; Abdelhady, H.M.; Abou-Taleb, K.A.; Hassan, M.G.; Amer, M.M. Lipase from Rhizopus oryzae R1: In-depth characterization, immobilization, and evaluation in biodiesel production. J. Genet. Eng. Biotechnol. 2021, 19, 1. [Google Scholar] [CrossRef]
- Silveira, C.M.; Badiale-Furlong, E. Characterization of nitrogenated compounds in solid-state fermented bran. Ciênc. Tecnol. Aliment. 2007, 27, 805–811. [Google Scholar] [CrossRef]
- Parrish, C.C. Essential Fatty Acids in Aquatic Food Webs. In Lipids in Aquatic Ecosystems; Arts, M.T., Brett, M.T., Kainz, M., Eds.; Springer: New York, NY, USA, 2009; pp. 309–326. [Google Scholar] [CrossRef]
- Kupski, L.; Cipolatti, E.; Rocha, M.; Oliveira, M.S.; Souza-Soares, L.A.; Badiale-Furlong, E. Solid-state fermentation for the enrichment and extraction of proteins and antioxidant compounds in rice bran by Rhizopus oryzae. Braz. Arch. Biol. Technol. 2012, 55, 937–942. [Google Scholar] [CrossRef]
- Shi, Q.; Xiong, X.; Wen, Z.-Y.; Qin, C.; Li, R.; Zhang, Z.; Gong, Q.; Wu, X. Cu/Zn Superoxide Dismutase and Catalase of Yangtze Sturgeon, Acipenser dabryanus: Molecular Cloning, Tissue Distribution and Response to Fasting and Refeeding. Fishes 2022, 7, 35. [Google Scholar] [CrossRef]
- Mansoori, A.; Allaf Noveirian, H.; Hoseinifar, S.H.; Sajjadi, M.; Ashouri, G.; Imperatore, R.; Paolucci, M. Polyphenol-rich extracts enhance growth, immune function, and antioxidant defense in juvenile rainbow trout (Oncorhynchus mykiss). Front. Nutr. 2024, 11, 1487209. [Google Scholar] [CrossRef] [PubMed]
- Ng’onga, L.; Amoah, K.; Chen, H.; Huang, Y.; Wang, B.; Shija, V.M.; Mpwaga, A.Y.; Fachri, M.; Cai, J.; Adjei-Boateng, D. The metabolism and antioxidant properties of probiotics and prebiotics in fish: A review. Front. Mar. Sci. 2025, 12, 1622474. [Google Scholar] [CrossRef]
- Manolescu, A.R.; Witkowska, K.; Kinnaird, A.; Cessford, T.; Cheeseman, C. Facilitated hexose transporters: New perspectives on form and function. Physiology 2007, 22, 234–240. [Google Scholar] [CrossRef]
- Lennartsson, P.R.; Taherzadeh, M.J.; Edebo, L. Rhizopus. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Academic Press: London, UK, 2014; pp. 284–290. [Google Scholar]
- Massarolo, J. Cultivo de Rhizopus Oryzae em Farelo de Arroz Integral e Seus Impactos na Fração Lipídica. Master’s Thesis, Universidade Federal do Rio Grande, Rio Grande, Brasil, 2016; p. 92. [Google Scholar]
- Nagarajan, M.; Rajasekaran, B.; Venkatachalam, K. Metabólitos microbianos em produtos alimentícios fermentados e seus benefícios potenciais. Int. Food Res. J. 2022, 29, 466–486. [Google Scholar] [CrossRef]
- Yao, W.; Gong, Y.; Li, L.; Hu, X.; You, L. Os efeitos das fibras alimentares do farelo de arroz e do farelo de trigo na microbiota intestinal: Uma visão geral. Food Chem. X 2022, 13, 100252. [Google Scholar] [CrossRef]
- Siddik, M.A.B.; Julien, B.B.; Islam, S.M.M.; Francis, D.S. Fermentation in Aquatic Feed Processing: Achieving Sustainability in Global Aquaculture Feed Production. Rev. Aquac. 2024, 16, 1244–1265. [Google Scholar] [CrossRef]
- Eide, L.H.; Morales-Lange, B.; Kuiper, R.V.; Dale, O.B.; Rocha, S.D.C.; Djordjevic, B.; Øverland, M. Fermented Sunflower Meal in Diets for Atlantic Salmon under Commercial Farming Conditions Promotes Intestinal Lactic Acid Bacteria and Controls Distal Intestine Inflammation. Aquaculture 2024, 595, 741517. [Google Scholar] [CrossRef]
- Li, C.; Zhang, B.; Liu, C.; Zhou, H.; Wang, X.; Mai, K.; He, G. Effects of Dietary Raw or Enterococcus faecium Fermented Soybean Meal on Growth, Antioxidant Status, Intestinal Microbiota, Morphology, and Inflammatory Responses in Turbot (Scophthalmus maximus L.). Fish Shellfish Immunol. 2020, 100, 261–271. [Google Scholar] [CrossRef]
- Catalán, N.; Villasante, A.; Wacyk, J.; Ramírez, C.; Romero, J. Fermented Soybean Meal Increases Lactic Acid Bacteria in the Intestinal Microbiota of Atlantic Salmon (Salmo salar). Probiotics Antimicrob. Proteins 2018, 10, 566–576. [Google Scholar] [CrossRef]
- Wang, A.; Meng, D.; Hao, Q.; Xia, R.; Zhang, Q.; Ran, C.; Yang, Y.; Li, D.; Liu, W.; Zhang, Z.; et al. Effect of Supplementation of Solid-State Fermentation Product of Bacillus subtilis HGcc-1 to High-Fat Diet on Growth, Hepatic Lipid Metabolism, Epidermal Mucus, Gut and Liver Health and Gut Microbiota of Zebrafish. Aquaculture 2022, 560, 738542. [Google Scholar] [CrossRef]
- Kasubuchi, M.; Hasegawa, S.; Hiramatsu, T.; Ichimura, A.; Kimura, I. Dietary Gut Microbial Metabolites, Short-Chain Fatty Acids, and Host Metabolic Regulation. Nutrients 2015, 7, 2839–2849. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, X.-F.; He, S.; Chen, X.; Wang, J.; Li, J.; Zhu, Q.; Zhang, Z.; Li, L.; Alam, M.S. Effects of Diet-Modulated Microbiota on the Health of Chinese Perch (Siniperca chuatsi) under a Carbohydrate-Rich Diet. Front. Microbiol. 2020, 11, 575102. [Google Scholar] [CrossRef]
- Vacca, F.; Barca, A.; Gomes, A.; Mazzei, A.; Piccinni, B.; Cinquetti, R.; Del Vecchio, G.; Romano, A.; Rønnestad, I.; Bossi, E.; et al. The peptide transporter 1a of zebrafish Danio rerio, an emerging model in nutrigenomics and nutrition research: Molecular characterization, functional properties and expression analysis. Genes Nutr. 2019, 14, 33. [Google Scholar] [CrossRef]
- Con, P.; Nitzan, T.; Cnaani, A. Salinity-dependent shift in the localization of three peptide transporters along the intestine of the Mozambique tilapia (Oreochromis mossambicus). Front. Physiol. 2017, 8, 1039. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Han, P.; Pan, L.; Lu, W.; Xiong, J.-W.; Zhang, M.; Zhang, W.; Li, L.; Wen, Z. Il-1β and reactive oxygen species differentially regulate neutrophil directional migration and basal random motility in a zebrafish injury–induced inflammation model. J. Immunol. 2014, 192, 5998–6008. [Google Scholar] [CrossRef] [PubMed]
| Ingredients (%) | Treatments | ||
|---|---|---|---|
| Control | Yeast | Fungus | |
| Fish meal | 32 | 32 | 32 |
| Soybean meal | 15 | 15 | 15 |
| cornflour | 12 | 12 | 12 |
| Rice bran | 25 | 0 | 0 |
| Rice bran fermented by yeast | 0 | 25 | 0 |
| Rice bran fermented by fungus | 0 | 0 | 25 |
| Casein | 10 | 10 | 10 |
| Gelatin | 2 | 2 | 2 |
| Premix mineral and vitamin | 2 | 2 | 2 |
| Cellulose | 2 | 2 | 2 |
| TOTAL | 100 | 100 | 100 |
| Total polyphenol content (mg/g) | 3.55 ± 0.02 | 3.72 ± 0.01 | 4.56 ± 0.05 |
| Crude protein (%) | 42.15 ± 0.27 | 44.16 ± 0.83 | 47.13 ± 0.67 |
| Lipids (%) | 10.4 ± 0.78 | 11.22 ± 0.42 | 10.03 ± 0.59 |
| Humidity (%) | 1.03 ± 0.05 | 4.31 ± 0.14 | 2.02 ± 0.02 |
| Ash (%) | 13.49 ± 0.10 | 13.35 ± 0.24 | 13.84 ± 0.25 |
| Gene | Sequence (5′–3′) | Efficiency (%) | GenBank Accession |
|---|---|---|---|
| slc15a2 | F: cacagccggagaagtcatgt | 98 | NM_001039828.1 |
| R: gaacggatttcatgctcgcc | |||
| slc15a1b | F: gcatctacgcaaagcagagc | 98 | NM_198064.1 |
| R: atgagggcaaccaccatgag | |||
| ifng1 | F: acgcttgcaaaggattgggttgg | 99 | NM001020793 |
| R: acacagcctggcaagtgcagg | |||
| il-1b | F: ccacgtatgcgtcgcccagt | 100 | NM212844 |
| R: gggcaacaggccaggtacagg | |||
| sod1 | F: caccgtctatttcaatcaagagg | 114 | BC055516 |
| R: agaatgttggcctgacaaagtta | |||
| cat | F: aacaacacccccatcttctttat | 103 | BC051626 |
| R: atgtgtgtctgggtaggagaaaa | |||
| glut2 | F: ttaacaggcacgctcgctct | 98 | NM001042721 |
| R: ttcatgctctgtgccatttcc | |||
| glut6 | F: tttggcctgattttgccgtg | 98 | XM684000 |
| R: gtggtaacgtggagaggtcg | |||
| ef1α | F: caaaattggaggtattggaactgtac | 99 | NM131263 |
| R: tcaacagacttgacctcagtggtt | |||
| rpl13a | F: tctggaggactgtaagaggtatgc | 99 | NM212784 |
| R: agacgcacaatcttgagagcag |
| Component | Control | Yeast | Fungus |
|---|---|---|---|
| Ash % | 9.48 ± 0.13 b | 8.68 ± 0.09 a | 9.65 ± 0.10 b |
| Crude protein % | 15.58 ± 0.15 c | 16.18 ± 0.62 bc | 21.26 ± 0.52 a |
| Lipids % | 17.91 ± 0.79 b | 17.20 ± 1.73 ab | 15.46 ± 0.64 a |
| Crude fiber % | 6.81 ± 0.43 c | 10.03 ± 0.99 b | 16.43 ± 0.68 a |
| Total polyphenol content (mg/g) | 4.47 ± 0.01 c | 3.17 ± 0.05 b | 9.24 ± 0.02 a |
| Fatty Acids | Control | Yeast | Fungus |
|---|---|---|---|
| 8:00 | 0.08 ± 0.06 ac | 0.06 ± 0.01 ab | 0.09 ± 0.01 c |
| 10:0 | 0.02 ± 0.00 | 0.01 ± 0.01 | 0.02 ± 0.00 |
| 11:0 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 12:0 | 0.07 ± 0.01 | 0.07 ± 0.01 | 0.07 ± 0.01 |
| 13:0 | 0.05 ± 0.00 | 0.05 ± 0.00 | 0.06 ± 0.00 |
| 14:0 | 1.33 ± 0.02 a | 1.27 ± 0.07 a | 1.40 ± 0.01 b |
| 14:1n-5 | 0.09 ± 0.00 | 0.09 ± 0.00 | 0.10 ± 0.00 |
| 15:0 | 0.25 ± 0.01 a | 0.24 ± 0.02 a | 0.27 ± 0.01 b |
| 15:1n-5 | 0.06 ± 0.00 | 0.06 ± 0.01 | 0.07 ± 0.00 |
| 16:0 | 14.12 ± 0.02 a | 12.36 ± 0.59 b | 11.62 ± 0.06 c |
| 16:1n-9 | 0.23 ± 0.00 a | 0.22 ± 0.03 a | 0.28 ± 0.01 b |
| 16:1n-7 | 2.83 ± 0.02 a | 2.80 ± 0.13 a | 3.05 ± 0.01 b |
| 16:1n-5 | 0.05 ± 0.00 | 0.05 ± 0.00 | 0.05 ± 0.00 |
| 16:2 | 0.13 ± 0.00 | 0.13 ± 0.01 | 0.15 ± 0.00 |
| 16:2n-4 | 0.03 ± 0.00 | 0.02 ±0.02 | 0.05 ± 0.00 |
| 17:0 | 0.30 ± 0.01 a | 0.29 ± 0.01 a | 0.32 ±0.02 b |
| 17:1n-7 | 0.17 ± 0.00 | 0.17 ± 0.00 | 0.20 ± 0.02 |
| 16:3n-4 | 0.15 ± 0.00 | 0.15 ± 0.00 | 0.16 ± 0.01 |
| 16:4 | 0.01 ± 0.01 | 0.03 ± 0.02 | 0.02 ± 0.00 |
| 18:0 | 2.75 ± 0.01 a | 2.62 ± 0.13 b | 2.79 ± 0.02 a |
| 18:1n-11 | 0.04 ± 0.01 | 0.03 ± 0.00 | 0.06 ± 0.00 |
| 18:1n-9 | 18.66 ± 0.12 a | 16.38 ± 0.76 b | 15.41 ± 0.13 c |
| 18:1n-7 | 1.39 ± 0.02 a | 1.31 ± 0.06 b | 1.44 ± 0.00 c |
| 18:1n-5 | 0.06 ± 0.00 | 0.05 ± 0.01 | 0.06 ± 0.00 |
| 18:2n-6 | 12.55 ± 0.07 a | 10.25 ± 0.44 b | 8.57 ± 0.05 c |
| 18:2n-4 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 18:3n-6 | 0.07 ± 0.00 | 0.09 ± 0.01 | 0.33 ± 0.02 |
| 18:3n-4 | 0.06 ± 0.01 | 0.07 ± 0.01 | 0.07 ± 0.01 |
| 18:3n-3 | 0.94 ± 0.01 a | 0.82 ± 0.07 b | 0.69 ± 0.01c |
| 18:4n-3 | 0.17 ± 0.00 | 0.17 ± 0.00 | 0.19 ± 0.00 |
| 18:4n1 | 0.01 ± 0.01 | 0.01 ± 0.00 | 0.00 ± 0.00 |
| 20:0 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 20:1n-11 | 0.26 ± 0.01 a | 0.25 ± 0.00 a | 0.28 ± 0.00 b |
| 20:1n-9 | 0.62 ± 0.02 a | 0.58 ± 0.01 b | 0.65 ± 0.00 c |
| 20:1n-7 | 0.12 ± 0.01 | 0.12 ± 0.01 | 0.14 ± 0.00 |
| 20:2n-9 | 0.16 ± 0.00 | 0.15 ± 0.01 | 0.14 ± 0.00 |
| 21:0 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 20:3n-6 | 0.10 ± 0.00 | 0.10 ± 0.02 | 0.10 ± 0.02 |
| 20:4n-6 (ARA) | 0.55 ± 0.01 a | 0.56 ± 0.02 a | 0.61 ± 0.01 b |
| 20:3n-3 | 0.07 ± 0.01 | 0.07 ± 0.01 | 0.06 ± 0.00 |
| 20:4n-3 | 0.12 ± 0.01 a | 0.11 ± 0.01 a | 0.13 ± 0.01 b |
| 22:0 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 20:5n-3 (EPA) | 1.82 ± 0.01 a | 1.76 ± 0.10 a | 1.95 ± 0.05 b |
| 22:1n-11 | 0.12 ± 0.00 | 0.12 ± 0.00 | 0.13 ± 0.00 |
| 22:1n-9 | 0.09 ± 0.00 | 0.08 ± 0.00 | 0.09 ± 0.00 |
| 22:1n-7 | 0.00 ± 0.01 | 0.07 ± 0.01 | 0.06 ± 0.00 |
| 22:2n-6 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 21:5n-3 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 23:0 | 0.05 ± 0.01 | 0.05 ± 0.01 | 0.03 ± 0.00 |
| 22:4n-6 | 0.18 ± 0.00 | 0.17 ± 0.01 | 0.19 ± 0.01 |
| 22:5 n-6 | 0.20 ± 0.01 | 0.19 ± 0.01 | 0.21 ± 0.01 |
| 24:0 | 0.25 ± 0.01 a | 0.24 ± 0.00 b | 0.30 ± 0.01 c |
| 22:5n-3 (DPA) | 0.57 ± 0.01 a | 0.56 ± 0.02 a | 0.63 ± 0.01 b |
| 24:1n-9 | 0.11 ± 0.00 a | 0.12 ± 0.00 a | 2.63 ± 0.10 b |
| 22:6n-3 (DHA) | 2.33 ± 0.03 a | 2.26 ± 0.13 a | 2.62 ± 0.10 b |
| SAFA | 19.22 ± 0.09 a | 17.21 ± 0.84 b | 16.90 ± 0.06 b |
| MUFA | 24.99 ± 0.04 a | 22.50 ± 1.03 b | 22.22 ± 0.09 b |
| PUFA | 20.23 ± 0.01 a | 17.65 ± 0.88 b | 16.92 ± 0.11 b |
| n-9 | 19.77 ± 0.08 a | 17.45 ± 0.81 b | 16.64 ± 0.11 c |
| n-6 | 13.81 ± 0.05 a | 11.50 ± 0.49 b | 10.19 ± 0.07 c |
| n-3 | 5.95 ± 0.05 a | 5.68 ± 0.33a | 6.21 ± 0.19 b |
| n-3 HUFA | 4.84 ± 0.06 a | 4.70 ± 0.26 a | 5.33 ± 0.17 b |
| n-6/n-3 | 2.32 ± 0.03 a | 2.03 ± 0.03 b | 1.64 ± 0.06 c |
| DHA/EPA | 1.28 ± 0.01 a | 1.28 ± 0.00 a | 1.34 ± 0.02 c |
| Control | Yeast | Fungus | |
|---|---|---|---|
| IW (g) | 0.09 ± 0.02 | 0.09 ± 0.01 | 0.09 ± 0.008 |
| FW (g) | 0.17 ± 0.037 b | 0.18 ± 0.02 ab | 0.22 ± 0.02 a |
| WG (g) | 0.075 ± 0.01 b | 0.091 ± 0.02 b | 0.127 ± 0.02 a |
| SGR (%) | 1.30 ± 0.16 b | 1.40 ± 0.20 b | 1.78 ± 0.27 a |
| S (%) | 100 | 100 | 100 |
| FCR (g/g) | 1.09 ± 0.10 b | 0.82 ± 0.18 a | 0.71 ± 0.09 a |
| CF | 1.84 ± 0.11 a | 1.64 ± 0.08 b | 1.82 ± 0.05 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lenz, G.; Martins, R.M.; Riet, J.; Azevedo, R.d.S.; Cardoso, A.; Nornberg, B.F.d.S.; Bessonart, M.; Magnone, L.; Marins, L.F.F.; Anni, I.S.A.; et al. Diet Formulated with Rice Bran Fermented by Rhizopus oryzae and Saccharomyces cerevisiae: Impacts on Zootechnical Performance and Intestinal Gene Expression in Zebrafish (Danio rerio). Fermentation 2025, 11, 567. https://doi.org/10.3390/fermentation11100567
Lenz G, Martins RM, Riet J, Azevedo RdS, Cardoso A, Nornberg BFdS, Bessonart M, Magnone L, Marins LFF, Anni ISA, et al. Diet Formulated with Rice Bran Fermented by Rhizopus oryzae and Saccharomyces cerevisiae: Impacts on Zootechnical Performance and Intestinal Gene Expression in Zebrafish (Danio rerio). Fermentation. 2025; 11(10):567. https://doi.org/10.3390/fermentation11100567
Chicago/Turabian StyleLenz, Gabriela, Rejane Macedo Martins, Jade Riet, Raíza dos Santos Azevedo, Arthur Cardoso, Bruna Félix da Silva Nornberg, Martín Bessonart, Larisa Magnone, Luis Fernando Fernandes Marins, Iuri Salim Abou Anni, and et al. 2025. "Diet Formulated with Rice Bran Fermented by Rhizopus oryzae and Saccharomyces cerevisiae: Impacts on Zootechnical Performance and Intestinal Gene Expression in Zebrafish (Danio rerio)" Fermentation 11, no. 10: 567. https://doi.org/10.3390/fermentation11100567
APA StyleLenz, G., Martins, R. M., Riet, J., Azevedo, R. d. S., Cardoso, A., Nornberg, B. F. d. S., Bessonart, M., Magnone, L., Marins, L. F. F., Anni, I. S. A., Gonçalves, T. P., Christ-Ribeiro, A., & Tesser, M. B. (2025). Diet Formulated with Rice Bran Fermented by Rhizopus oryzae and Saccharomyces cerevisiae: Impacts on Zootechnical Performance and Intestinal Gene Expression in Zebrafish (Danio rerio). Fermentation, 11(10), 567. https://doi.org/10.3390/fermentation11100567







