Integrated Pretreatment and Microbial Matching for PHA Production from Lignocellulosic Agro-Forestry Residues
Abstract
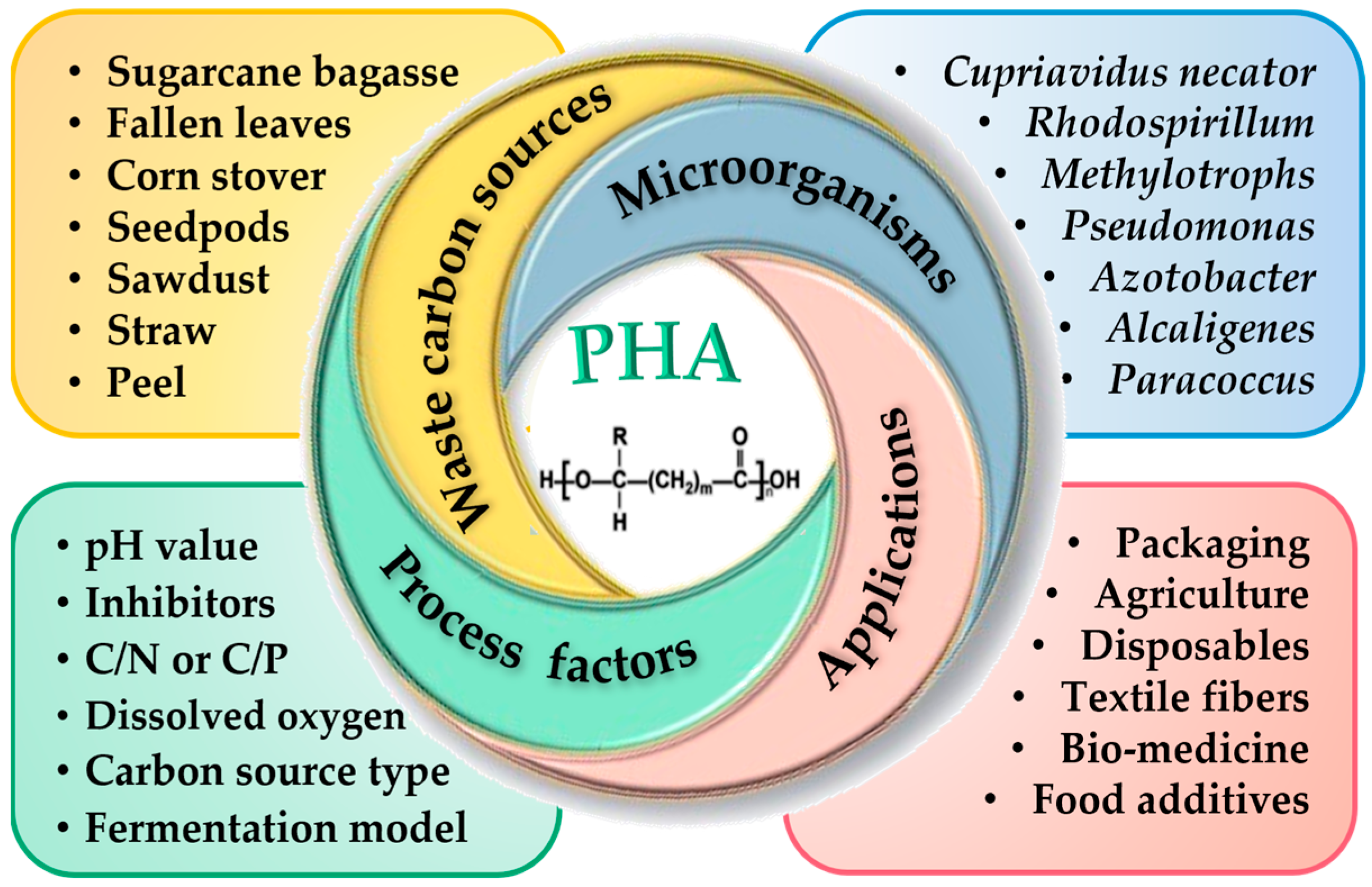
1. Introduction
2. Diversity of PHA Monomer Structures and Resulting Properties
3. Integrated Utilization of LAR-Derived Substrates for PHA Production
3.1. Pretreatment-Co-Substrate Compatibility for PHA Production
3.2. Pretreatment-Microbial Platform Matching for PHA Production
4. Functionally Oriented Microbial Platforms for PHA Production
4.1. Broad-Substrate Microorganisms: From Single Carbon Sources to Complex LAR Hydrolysate
4.2. Process-Compatible Microorganisms: Extremophiles and Resilient Consortia
4.3. Product-Customized Microorganisms: Tailoring PHA Composition and Properties
5. Integrated Co-Substrate Strategies from “Metabolic Interaction” Perspective
5.1. Carbon Flux Distribution Optimization
5.2. Microbial Community Interactions
5.3. Process Coupling Innovations
6. Kinetic Study for PHA Production, Cell Growth, Carbon Source Consumption
7. Commercial-Scale Economic Feasibility of LAR-to-PHA Conversion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LARs | Lignocellulosic agro-forestry residues |
| PHA | Polyhydroxyalkanoates |
| DEA–MW | Deacetylation–microwave |
| DES | Deep eutectic solvent |
| VFAs | Volatile fatty acids |
| Scl-PHA | Short-chain length PHA |
| Mcl-PHA | Medium-chain length PHA |
| P(3HB) | Poly(3-hydroxybutyrate) |
| P(4HB) | Poly(4-hydroxybutyrate) |
| P(3HV) | Poly(3-hydroxyvalerate) |
| P(3HP) | Poly(3-hydroxypropionate) |
| P(3HO) | Poly(3-hydroxyoctanoate) |
| P(3HD) | Poly(3-hydroxydecanoate) |
| PHBV | Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) |
| PHBH | Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) |
| P34HB | Poly(3-hydroxybutyrate-co-4-hydroxybutyrate) |
| Tm | Melting temperature |
| Tg | Glass transition temperature |
| σ | Tensile strength |
| ε | Elongation at break |
| 3HB | 3-hydroxybutyrate |
| 3HV | 3-hydroxyvalerate |
| 3HHx | 3-hydroxyhexanoate |
| 3HO | 3-hydroxyoctanoate |
| NSL | Non-sterilized lignin |
| SHF | Separate hydrolysis-fermentation |
| SSA | Succinate semialdehyde |
| 4HB | 4-hydroxybutyrate |
| GA | Glycolate |
| 2H3KBA-CoA | 2-hydroxy-3-ketobutyryl-CoA |
| 3HP | 3-hydroxypropionate |
| (R)-3HA-ACP | (R)-3-hydroxyacyl-ACP |
| 3HB-CoA | 3-hydroxybutyryl-CoA |
| 3HV-CoA | 3-hydroxyvaleryl-CoA |
| 4HB-CoA | 4-hydroxybutyryl-CoA |
| 2,3DHBA-CoA | 2,3-dihydroxybutyryl-CoA |
| 3HP-CoA | 3-hydroxypropionate-CoA |
| (R)-3HA-CoA | (R)-3-hydroxyacyl-CoA |
| ALE | Adaptive laboratory evolution |
| CDW | Cell dry weight |
| TMP | Thermomechanical-pulp |
| RMSEs | Root mean squared errors |
| MMCs | Mixed microbial cultures |
| TEA | Techno-economic analysis |
| CAPEX | Capital expenditures |
| OPEX | Operational expenditures |
| MSP | Minimum selling price |
| NAS | Nitrifier-assisted stabilization |
| AOB | Ammonia-oxidizing bacteria |
| LCA | Life cycle assessment |
References
- Sali, S.; McKay, G.; Mackey, H.R. Exploring the Influence of Light Wavelength Ranges and Nutrients Reduced Availability’s Impacts on Polyhydroxyalkanoates Accumulation in Purple Phototrophic Bacteria. Fermentation 2025, 11, 216. [Google Scholar] [CrossRef]
- Ciesielski, S.; Mozejko, J.; Pisutpaisal, N. Plant oils as promising substrates for polyhydroxyalkanoates production. J. Clean. Prod. 2015, 106, 408–421. [Google Scholar] [CrossRef]
- Wang, J.F.; Huang, J.Q.; Liu, S.J. The Production, Recovery, and Valorization of Polyhydroxybutyrate (PHB) Based on Circular Bioeconomy. Biotechnol. Adv. 2024, 72, 108340. [Google Scholar] [CrossRef]
- Li, D.N.; Wang, F.; Zheng, X.N.; Zheng, Y.Y.; Pan, X.S.; Li, J.N.; Ma, X.J.; Yin, F.; Wang, Q. Lignocellulosic Biomass as Promising Substrate for Polyhydroxyalkanoate Production: Advances and Perspectives. Biotechnol. Adv. 2025, 79, 108512. [Google Scholar] [CrossRef]
- Andler, R.; Vivod, R.; Steinbuchel, A. Synthesis of Polyhydroxyalkanoates through the Biodegradation of Poly(cis-1,4-isoprene) Rubber. J. Biosci. Bioeng. 2019, 127, 360–365. [Google Scholar] [CrossRef]
- Andler, R.; Valdes, V.; Urtuvia, V.; Andreeben, C.; Diaz-Barrera, A. Fruit Residues as a Sustainable Feedstock for the Production of Bacterial Polyhydroxyalkanoates. J. Clean. Prod. 2021, 307, 127236. [Google Scholar] [CrossRef]
- Wang, J.; Liu, S.; Huang, J.; Qu, Z. A Review on Polyhydroxyalkanoate Production from Agricultural Waste Biomass: Development, Advances, Circular Approach, and Challenges. Bioresour. Technol. 2021, 342, 126008. [Google Scholar] [CrossRef] [PubMed]
- McAdam, B.; Fournet, M.B.; McDonald, P.; Mojicevic, M. Production of Polyhydroxybutyrate (PHB) and Factors Impacting Its Chemical and Mechanical Characteristics. Polymers 2020, 12, 2908. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.B.; Li, H.C.; Li, D.Q.; You, T.T.; Nawaz, H.; Xu, F. An Efficient Approach for the Production of Polyhydroxybutyrate from Lignin by Alkali-Halophile Halomonas alkalicola M2. Ind. Crops Prod. 2023, 194, 116309. [Google Scholar] [CrossRef]
- Andhalkar, V.V.; Montane, D.; Medina, F.; Constanti, M. Biorefinery Design with Combined Deacetylation and Microwave Pretreatment for Enhanced Production of Polyhydroxyalkanoates and Efficient Carbon Utilization from Lignocellulose. Chem. Eng. J. 2024, 486, 149754. [Google Scholar] [CrossRef]
- Li, D.N.; Ma, X.J.; Li, J.N.; Sun, B.Q. Insights into Enhanced Polyhydroxyalkanoate Production by the Synergistic Use of Waste Wood Hydrolysate and Volatile Fatty Acids by Mixed Microbial Cultures. Bioresour. Technol. 2021, 337, 125488. [Google Scholar] [CrossRef]
- Li, D.N.; Ma, X.J.; Yin, F.; Qiu, Y.J.; Yan, X. Creating Biotransformation of Volatile Fatty Acids and Octanoate as Co-Substrate to High Yield Medium-Chain-Length Polyhydroxyalkanoate. Bioresour. Technol. 2021, 331, 125031. [Google Scholar] [CrossRef]
- Sun, J.C.; Loh, K.C. One-Pot Lignin Bioconversion to Polyhydroxyalkanoates Based on Hierarchical Utilization of Heterogeneous Compounds. Bioresour. Technol. 2025, 419, 132056. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, R.Y.; Xu, T.; Liu, Z.H.; Li, B.Z.; Yuan, Y.J. Valorizing Lignin and Coprecursors into Homogeneous Polyhydroxyalkanoates by Engineered Pseudomonas putida. ACS Sustain. Chem. Eng. 2024, 12, 8402–8414. [Google Scholar] [CrossRef]
- Samrot, A.V.; Samanvitha, S.K.; Shobana, N.; Renitta, E.R.; Senthilkumar, P.; Kumar, S.S.; Abirami, S.; Dhiva, S.; Bavanilatha, M.; Prakash, P.; et al. The Synthesis, Characterization and Applications of Polyhydroxyalkanoates (PHAs) and PHA-Based Nanoparticles. Polymers 2021, 13, 3302. [Google Scholar] [CrossRef] [PubMed]
- Andreessen, C.; Steinbuchel, A. Recent Developments in Non-Biodegradable Biopolymers: Precursors, Production Processes, and Future Perspectives. Appl. Microbiol. Biotechnol. 2019, 103, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.D.; Paul, V.; Agarwal, A.; Sharma, R.; Hashempour-Baltork, F.; Rashidi, L.; Khosravi Darani, K. Production of Polyhydroxyalkanoates Using Dairy Processing Waste-A Review. Bioresour. Technol. 2021, 326, 124735. [Google Scholar] [CrossRef]
- Martinez-Herrera, R.E.; Aleman-Huerta, M.E.; Rutiaga-Quinones, O.M.; de Luna-Santillana, E.D.; Elufisan, T.O. A Comprehensive View of Bacillus cereus as a Polyhydroxyalkanoate (PHA) Producer: A Promising Alternative to Petroplastics. Process Biochem. 2023, 129, 281–292. [Google Scholar] [CrossRef]
- Panaksri, A.; Tanadchangsaeng, N. Fractionation of Medium-Chain-Length Polyhydroxyalkanoate Biosynthesized by Pilot-Scale Production for Improving Material Properties. Polym. Degrad. Stab. 2023, 213, 110368. [Google Scholar] [CrossRef]
- Huong, K.H.; Teh, C.H.; Amirul, A.A. Microbial-Based Synthesis of Highly Elastomeric Biodegradable Poly(3-Hydroxybutyrate-co-4-Hydroxybutyrate) Thermoplastic. Int. J. Biol. Macromol. 2017, 101, 983–995. [Google Scholar] [CrossRef]
- Meng, D.C.; Shi, Z.Y.; Wu, L.P.; Zhou, Q.; Wu, Q.; Chen, J.C.; Chen, G.Q. Production and Characterization of Poly(3-Hydroxypropionate-co-4-Hydroxybutyrate) with Fully Controllable Structures by Recombinant Escherichia coli Containing an Engineered Pathway. Metab. Eng. 2012, 14, 317–324. [Google Scholar] [CrossRef]
- Hu, D.; Chung, A.L.; Wu, L.P.; Zhang, X.; Wu, Q.; Chen, J.C.; Chen, G.Q. Biosynthesis and Characterization of Polyhydroxyalkanoate Block Copolymer P3HB-b-P4HB. Biomacromolecules 2011, 12, 3166–3173. [Google Scholar] [CrossRef] [PubMed]
- Govil, T.; Wang, J.; Samanta, D.; David, A.; Tripathi, A.; Rauniyar, S.; Salem, D.R.; Sani, R.K. Lignocellulosic Feedstock: A Review of a Sustainable Platform for Cleaner Production of Nature’s Plastics. J. Clean. Prod. 2020, 270, 122521. [Google Scholar] [CrossRef]
- Li, X.; Shi, Y.; Kong, W.; Wei, J.; Song, W.; Wang, S. Improving Enzymatic Hydrolysis of Lignocellulosic Biomass by Bio-Coordinated Physicochemical Pretreatment-A Review. Energy Rep. 2022, 8, 696–709. [Google Scholar] [CrossRef]
- Fu, X.S.; Qiao, J.; Xu, Z.Q.; Xu, C.; Li, X.J. Deep Eutectic Solvent Cocktail Enhanced the Pretreatment Efficiency of Lignocellulose. Ind. Crops Prod. 2024, 210, 118040. [Google Scholar] [CrossRef]
- Wang, Z.; He, X.; Yan, L.; Wang, J.; Hu, X.; Sun, Q.; Zhang, H. Enhancing Enzymatic Hydrolysis of Corn Stover by Twin-Screw Extrusion Pretreatment. Ind. Crops Prod. 2020, 143, 111960. [Google Scholar] [CrossRef]
- Liang, J.; Yu, Z.; Chen, L.; Fang, S.; Ma, X. Microwave Pretreatment Power and Duration Time Effects on the Catalytic Pyrolysis Behaviors and Kinetics of Water Hyacinth. Bioresour. Technol. 2019, 286, 121369. [Google Scholar] [CrossRef]
- Wells, J.M.; Drielak, E.; Surendra, K.C.; Khanal, S.K. Hot Water Pretreatment of Lignocellulosic Biomass: Modeling the Effects of Temperature, Enzyme and Biomass Loadings on Sugar Yield. Bioresour. Technol. 2020, 300, 122593. [Google Scholar] [CrossRef]
- Yan, X.; Li, D.N.; Ma, X.J.; Li, J. Bioconversion of Renewable Lignocellulosic Biomass into Multicomponent Substrate via Pressurized Hot Water Pretreatment for Bioplastic Polyhydroxyalkanoate Accumulation. Bioresour. Technol. 2021, 339, 125667. [Google Scholar] [CrossRef]
- Chen, L.; Li, J.; Lu, M.; Guo, X.; Zhang, H.; Han, L. Integrated Chemical and Multi-Scale Structural Analyses for the Processes of Acid Pretreatment and Enzymatic Hydrolysis of Corn Stover. Carbohydr. Polym. 2016, 141, 1–9. [Google Scholar] [CrossRef]
- Huang, C.; Zhao, X.; Zheng, Y.; Lin, W.; Lai, C.; Yong, Q.; Ragauskas, A.J.; Meng, X. Revealing the Mechanism of Surfactant-Promoted Enzymatic Hydrolysis of Dilute Acid Pretreated Bamboo. Bioresour. Technol. 2022, 360, 127524. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Lee, Y.Y.; Kim, T.H. A Review on Alkaline Pretreatment Technology for Bioconversion of Lignocellulosic Biomass. Bioresour. Technol. 2016, 199, 42–48. [Google Scholar] [CrossRef]
- Yan, X.; Cheng, J.R.; Wang, Y.T.; Zhu, M.J. Enhanced Lignin Removal and Enzymolysis Efficiency of Grass Waste by Hydrogen Peroxide Synergized Dilute Alkali Pretreatment. Bioresour. Technol. 2020, 301, 122756. [Google Scholar] [CrossRef]
- Hou, X.; Wang, Z.; Sun, J.; Li, M.; Wang, S.; Chen, K.; Gao, Z. A Microwave-Assisted Aqueous Ionic Liquid Pretreatment to Enhance Enzymatic Hydrolysis of Eucalyptus and Its Mechanism. Bioresour. Technol. 2019, 272, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Lee, S.H.; Endo, T. Effect of Dimethyl Sulfoxide on Ionic Liquid 1-Ethyl-3-Methylimidazolium Acetate Pretreatment of Eucalyptus Wood for Enzymatic Hydrolysis. Bioresour. Technol. 2013, 140, 90–96. [Google Scholar] [CrossRef]
- Favaro, L.; Basaglia, M.; Casella, S. Improving Polyhydroxyalkanoate Production from Inexpensive Carbon Sources by Genetic Approaches: A Review. Biofuels Bioprod. Biorefin. 2019, 13, 208–227. [Google Scholar] [CrossRef]
- Luo, H.; Gao, L.; Xie, F.; Shi, Y.; Zhou, T.; Guo, Y.; Yang, R.; Bilal, M. A New L-Cysteine-Assisted Glycerol Organosolv Pretreatment for Improved Enzymatic Hydrolysis of Corn Stover. Bioresour. Technol. 2022, 363, 127975. [Google Scholar] [CrossRef]
- Fang, S.; Wang, W.; Tong, S.; Zhang, C.; Liu, P. Evaluation of the Effects of Isolated Lignin on Cellulose Enzymatic Hydrolysis of Corn Stover Pretreatment by NaOH Combined with Ozone. Molecules 2018, 23, 1495. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Upreti, S.; Ein-Mozaffari, F. Ozone Pretreatment of Wheat Straw for Enhanced Biohydrogen Production. Int. J. Hydrogen Energy 2013, 38, 10270–10276. [Google Scholar] [CrossRef]
- Cebreiros, F.; Risso, F.; Cagno, M.; Cabrera, M.N.; Rochon, E.; Jauregui, G.; Boix, E.; Bothig, S.; Ferrari, M.D.; Lareo, C. Enhanced Production of Butanol and Xylosaccharides from Eucalyptus grandis Wood Using Steam Explosion in a Semi-Continuous Pre-Pilot Reactor. Fuel 2021, 290, 119818. [Google Scholar] [CrossRef]
- Li, C.; Liu, G.; Nges, I.A.; Liu, J. Enhanced Biomethane Production from Miscanthus lutarioriparius Using Steam Explosion Pretreatment. Fuel 2016, 179, 267–273. [Google Scholar] [CrossRef]
- Baghbanzadeh, M.; Savage, J.; Balde, H.; Sartaj, M.; VanderZaag, A.C.; Abdehagh, N.; Strehler, B. Enhancing Hydrolysis and Biomethane Generation of Extruded Lignocellulosic Wood Waste Using Microbial Pretreatment. Renew. Energy 2021, 170, 438–448. [Google Scholar] [CrossRef]
- Tsafrakidou, P.; Bekatorou, A.; Koutinas, A.A.; Kordulis, C.; Banat, I.M.; Petsi, T.; Sotiriou, M. Acidogenic Fermentation of Wheat Straw after Chemical and Microbial Pretreatment for Biofuel Applications. Energy Convers. Manag. 2018, 160, 509–517. [Google Scholar] [CrossRef]
- Li, J.N.; Li, D.N.; Su, Y.H.; Yan, X.; Wang, F.; Yu, L.L.; Ma, X.J. Efficient and Economical Production of Polyhydroxyalkanoate from Sustainable Rubber Wood Hydrolysate and Xylose as Co-Substrate by Mixed Microbial Cultures. Bioresour. Technol. 2022, 355, 127238. [Google Scholar] [CrossRef] [PubMed]
- Lou, C.; Zhou, Y.; Yan, A.; Liu, Y. Extraction of Cellulose from Corn Stalk Taking Advantage of Pretreatment Technology with Immobilized Enzyme. RSC Adv. 2021, 12, 1208–1215. [Google Scholar] [CrossRef]
- Saratale, R.G.; Cho, S.K.; Kadam, A.A.; Ghodake, G.S.; Kumar, M.; Bharagava, R.N.; Varjani, S.; Nair, S.; Kim, D.S.; Shin, H.S.; et al. Developing Microbial Co-Culture System for Enhanced Polyhydroxyalkanoates (PHA) Production Using Acid Pretreated Lignocellulosic Biomass. Polymers 2022, 14, 1234. [Google Scholar] [CrossRef] [PubMed]
- Kucera, D.; Pernicova, I.; Kovalcik, A.; Koller, M.; Mullerova, L.; Sedlacek, P.; Mravec, F.; Nebesarova, J.; Kalina, M.; Marova, I.; et al. Characterization of the Promising Poly(3-Hydroxybutyrate) Producing Halophilic Bacterium Halomonas halophila. Bioresour. Technol. 2018, 256, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, Z.; Zhang, K.; Liu, M.; Liu, D.; Yan, X.; Si, M.; Shi, Y. Valorizing Waste Liquor from Dilute Acid Pretreatment of Lignocellulosic Biomass by Bacillus megaterium B-10. Ind. Crops Prod. 2021, 161, 113160. [Google Scholar] [CrossRef]
- Saratale, R.G.; Cho, S.K.; Ghodake, G.S.; Shin, H.S.; Saratale, G.D.; Park, Y.; Lee, H.S.; Bharagava, R.N.; Kim, D.S. Utilization of Noxious Weed Water Hyacinth Biomass as a Potential Feedstock for Biopolymers Production: A Novel Approach. Polymers 2020, 12, 1704. [Google Scholar] [CrossRef]
- Heng, K.S.; Hatti-Kaul, R.; Adam, F.; Fukui, T.; Sudesh, K. Conversion of Rice Husks to Polyhydroxyalkanoates (PHA) via a Three-Step Process: Optimized Alkaline Pretreatment, Enzymatic Hydrolysis, and Biosynthesis by Burkholderia cepacia USM (JCM 15050). J. Chem. Technol. Biotechnol. 2017, 92, 100–108. [Google Scholar] [CrossRef]
- Annamalai, N.; Sivakumar, N. Production of Polyhydroxybutyrate from Wheat Bran Hydrolysate Using Ralstonia eutropha through Microbial Fermentation. J. Biotechnol. 2016, 237, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Sun, W.D.; Wang, H.W.; Geng, A.L. Polyhydroxybutyrate Production from Oil Palm Empty Fruit Bunch Using Bacillus megaterium R11. Bioresour. Technol. 2013, 147, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Thuoc, D.V.; Chung, N.T.; Hatti-Kaul, R. Polyhydroxyalkanoate Production from Rice Straw Hydrolysate Obtained by Alkaline Pretreatment and Enzymatic Hydrolysis Using Bacillus Strains Isolated from Decomposing Straw. Bioresour. Bioprocess. 2021, 8, 98. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Wang, L.; Li, T.T.; Shen, Y.B.; Luo, J.B. Acid Soaking Followed by Steam Flash-Explosion Pretreatment to Enhance Saccharification of Rice Husk for Poly(3-Hydroxybutyrate) Production. Int. J. Biol. Macromol. 2020, 160, 446–455. [Google Scholar] [CrossRef]
- Mohan, G.; Johnson, R.L.; Yu, J. Conversion of pine sawdust into polyhydroxyalkanoate bioplastics. ACS Sustain. Chem. Eng. 2021, 9, 8383–8392. [Google Scholar] [CrossRef]
- Yin, F.; Li, D.N.; Ma, X.J.; Zhang, C. Pretreatment of lignocellulosic feedstock to produce fermentable sugars for poly(3-hydroxybutyrate-co-3-hydroxyvalerate) production using activated sludge. Bioresour. Technol. 2019, 290, 121773. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Gurav, R.; Cho, D.H.; Kim, B.; Jung, H.J.; Kim, S.H.; Choi, T.R.; Kim, H.J.; Yang, Y.H. Algal biochar mediated detoxification of plant biomass hydrolysate: Mechanism study and valorization into polyhydroxyalkanoates. Bioresour. Technol. 2023, 370, 128571. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Garcia, Y.; Grieve, J.; Meza-Contreras, J.C.; Clifton-Garcia, B.; Silva-Guzman, J.A. Tequila agave bagasse hydrolysate for the production of polyhydroxybutyrate by Burkholderia sacchari. Bioengineering 2019, 6, 115. [Google Scholar] [CrossRef]
- Guo, H.; Zhao, Y.; Chang, J.S.; Lee, D.J. Inhibitor formation and detoxification during lignocellulose biorefinery: A review. Bioresour. Technol. 2022, 361, 127666. [Google Scholar] [CrossRef]
- Xu, Z.X.; Xu, M.L.; Cai, C.G.; Chen, S.T.; Jin, M.J. Microbial Polyhydroxyalkanoate Production from Lignin by Pseudomonas putida NX-1. Bioresour. Technol. 2021, 319, 124210. [Google Scholar] [CrossRef]
- Hrabalova, V.; Opial, T.A.; Musilova, J.; Sedlar, K.; Obruca, S. Biotransformation of Ferulic Acid into Vanillyl Alcohol and Vanillic Acid Employing Thermophilic Bacterium Caldimonas thermodepolymerans. Enzyme Microb. Technol. 2024, 179, 110475. [Google Scholar] [CrossRef]
- Xu, Q.J.; Liu, H.; Wang, L.; Liu, Z.H.; Li, B.Z.; Yuan, Y.J. Identifying Ligninolytic Bacteria for Lignin Valorization to Bioplastics. Bioresour. Technol. 2022, 358, 127383. [Google Scholar] [CrossRef]
- Arreola-Vargas, J.; Meng, X.Z.; Wang, Y.Y.; Ragauskas, A.J.; Yuan, J.S. Enhanced Medium Chain Length-Polyhydroxyalkanoate Production by Co-Fermentation of Lignin and Holocellulose Hydrolysates. Green Chem. 2021, 23, 8226–8237. [Google Scholar] [CrossRef]
- Salvachua, D.; Rydzak, T.; Auwae, R.; de Capite, A.; Black, B.A.; Bouvier, J.T.; Cleveland, N.S.; Elmore, J.R.; Huenemann, J.D.; Katahira, R.; et al. Metabolic Engineering of Pseudomonas putida for Increased Polyhydroxyalkanoate Production from Lignin. Microb. Biotechnol. 2020, 13, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Lee, H.J.; Kim, S.H.; Suh, M.J.; Cho, J.Y.; Ham, S.; Song, H.S.; Bhatia, S.K.; Gurav, R.; Jeon, J.M.; et al. Engineering of Shewanella marisflavi BBL25 for Biomass-Based Polyhydroxybutyrate Production and Evaluation of Its Performance in Electricity Production. Int. J. Biol. Macromol. 2021, 183, 1669–1675. [Google Scholar] [CrossRef]
- Li, M.; Wilkins, M. Fed-Batch Cultivation and Adding Supplements to Increase Yields of Polyhydroxybutyrate Production by Cupriavidus necator from Corn Stover Alkaline Pretreatment Liquor. Bioresour. Technol. 2020, 299, 122676. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Ray, S.; Kalia, V.C. Production of Co-Polymers of Polyhydroxyalkanoates by Regulating the Hydrolysis of Biowastes. Bioresour. Technol. 2016, 200, 100483. [Google Scholar] [CrossRef]
- Simo-Cabrera, L.; Garcia-Chumillas, S.; Hagagy, N.; Saddiq, A.; Tag, H.; Selim, S.; AbdElgawad, H.; Arribas Aguero, A.; Monzo Sanchez, F.; Canovas, V.; et al. Haloarchaea as Cell Factories to Produce Bioplastics. Mar. Drugs 2021, 19, 159. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Mitra, R.; Zhang, S.J.; Zuo, Z.; Lin, L.; Zhao, D.; Xiang, H.; Han, J. Unusual Phosphoenolpyruvate (PEP) Synthetase-Like Protein Crucial to Enhancement of Polyhydroxyalkanoate Accumulation in Haloferax mediterranei Revealed by Dissection of PEP-Pyruvate Interconversion Mechanism. Appl. Environ. Microbiol. 2019, 85, e00984. [Google Scholar] [CrossRef]
- Pantazaki, A.A.; Papaneophytou, C.P.; Lambropoulou, D.A. Simultaneous Polyhydroxyalkanoates and Rhamnolipids Production by Thermus thermophilus HB8. AMB Express 2011, 1, 17. [Google Scholar] [CrossRef]
- Musilova, J.; Kourilova, X.; Pernicova, I.; Bezdicek, M.; Lengerova, M.; Obruca, S.; Sedlar, K. Novel Thermophilic Polyhydroxyalkanoates Producing Strain Aneurinibacillus thermoaerophilus CCM 8960. Appl. Microbiol. Biotechnol. 2022, 106, 4669–4681. [Google Scholar] [CrossRef] [PubMed]
- Sheu, D.S.; Chen, W.M.; Yang, J.Y.; Chang, R.C. Thermophilic Bacterium Caldimonas taiwanensis Produces Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate) from Starch and Valerate as Carbon Sources. Enzyme Microb. Technol. 2009, 44, 289–294. [Google Scholar] [CrossRef]
- Wang, S.Q.; Liu, Y.J.; Guo, H.F.; Meng, Y.; Xiong, W.N.; Liu, R.H.; Yang, C. Establishment of Low-Cost Production Platforms of Polyhydroxyalkanoate Bioplastics from Halomonas cupida J9. Biotechnol. Bioeng. 2024, 121, 2106–2120. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.; Kleerebezem, R.; van Loosdrecht, M.C. Model-Based Data Evaluation of Polyhydroxybutyrate Producing Mixed Microbial Cultures in Aerobic Sequencing Batch and Fed-Batch Reactors. Biotechnol. Bioeng. 2009, 104, 50–67. [Google Scholar] [CrossRef]
- Villano, M.; Valentino, F.; Barbetta, A.; Martino, L.; Scandola, M.; Majone, M. Polyhydroxyalkanoates Production with Mixed Microbial Cultures: From Culture Selection to Polymer Recovery in a High-Rate Continuous Process. New Biotechnol. 2014, 31, 289–296. [Google Scholar] [CrossRef]
- Lin, L.; Chen, J.Y.; Mitra, R.; Gao, Q.X.; Cheng, F.Y.; Xu, T.; Zuo, Z.Q.; Xiang, H.; Han, J. Optimising PHBV Biopolymer Production in Haloarchaea via CRISPRi-Mediated Redirection of Carbon Flux. Commun. Biol. 2021, 4, 1007. [Google Scholar] [CrossRef]
- Holmes, E.C.; Bleem, A.C.; Johnson, C.W.; Beckham, G.T. Adaptive Laboratory Evolution and Metabolic Engineering of Cupriavidus necator for Improved Catabolism of Volatile Fatty Acids. Metab. Eng. 2024, 86, 262–273. [Google Scholar] [CrossRef]
- Yin, F.; Li, D.N.; Ma, X.J.; Li, J.N.; Qiu, Y.J. Poly(3-Hydroxybutyrate-3-Hydroxyvalerate) Production from Pretreated Waste Lignocellulosic Hydrolysates and Acetate Co-Substrate. Bioresour. Technol. 2020, 316, 123911. [Google Scholar] [CrossRef]
- Yuan, Y.P.; Wang, H.; He, H.T.; Zhang, Z.N.; Yang, F.; Chen, Y.L.; Wu, F.Q.; Wu, Q.; Chen, G.Q. Polyhydroxyalkanoate Production by Engineered Halomonas Grown in Lignocellulose Hydrolysate. Bioresour. Technol. 2025, 425, 132313. [Google Scholar] [CrossRef]
- Singh, S.; Sithole, B.; Lekha, P.; Permaul, K.; Govinden, R. Optimization of Cultivation Medium and Cyclic Fed-Batch Fermentation Strategy for Enhanced Polyhydroxyalkanoate Production by Bacillus thuringiensis Using a Glucose-Rich Hydrolyzate. Bioresour. Bioprocess. 2021, 8, 11. [Google Scholar] [CrossRef]
- Pinto-Ibieta, F.; Cea, M.; Cabrera, F.; Abanto, M.; Felissia, F.E.; Area, M.C.; Ciudad, G. Strategy for Biological Co-Production of Levulinic Acid and Polyhydroxyalkanoates by Using Mixed Microbial Cultures Fed with Synthetic Hemicellulose Hydrolysate. Bioresour. Technol. 2020, 309, 123323. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Singh, M.; Mehariya, S.; Patel, S.K.S.; Lee, J.K.; Kalia, V.C. Ecobiotechnological Approach for Exploiting the Abilities of Bacillus to Produce Co-Polymer of Polyhydroxyalkanoate. Indian J. Microbiol. 2014, 54, 151–157. [Google Scholar] [CrossRef]
- Diietrich, K.; Dumont, M.J.; Orsat, V.; Del-Rio, L.F. Consumption of Sugars and Inhibitors of Softwood Hemicellulose Hydrolysates as Carbon Sources for Polyhydroxybutyrate (PHB) Production with Paraburkholderia sacchari IPT 101. Cellulose 2019, 26, 7939–7952. [Google Scholar] [CrossRef]
- Alcantara, J.M.G.; Iannacci, F.; Morbidelli, M.; Sponchioni, M. Soft Sensor Based on Raman Spectroscopy for the In-Line Monitoring of Metabolites and Polymer Quality in the Biomanufacturing of Polyhydroxyalkanoates. J. Biotechnol. 2023, 377, 23–33. [Google Scholar] [CrossRef]
- Wang, J.; Guo, H.; Huang, J.; Jiang, S.; Hou, S.; Chen, X.; Lv, H.; Bi, X.; Hou, M.; Lin, H.; et al. L-Lactic Acid Production from Fructose by Chitosan Film-Coated Sodium Alginate-Polyvinyl Alcohol Immobilized Lactobacillus pentosus Cells and Its Kinetic Analysis. Bioresour. Bioprocess. 2021, 8, 27. [Google Scholar] [CrossRef]
- Prasad, D.M.R.; Pendyala, R.; Senthilkumar, R.; Bin Azri, M.H. Microbial Production of Poly(3-Hydroxybutyrate) (PHB) from Rubber Seed Oil Using Cupriavidus necator H16. IOP Conf. Ser. Earth Environ. Sci. 2019, 398, 012008. [Google Scholar] [CrossRef]
- Wang, J.; Yue, Z.B.; Sheng, G.P.; Yu, H.Q. Kinetic Analysis on the Production of Polyhydroxyalkanoates from Volatile Fatty Acids by Cupriavidus necator with a Consideration of Substrate Inhibition, Cell Growth, Maintenance, and Product Formation. Biochem. Eng. J. 2010, 49, 422–428. [Google Scholar] [CrossRef]
- Tamis, J.; Marang, L.; Jiang, Y.; van Loosdrecht, M.C.; Kleerebezem, R. Modeling PHA-Producing Microbial Enrichment Cultures—Towards a Generalized Model with Predictive Power. New Biotechnol. 2014, 31, 324–334. [Google Scholar] [CrossRef]
- Sakthiselvan, P.; Madhumathi, R. Kinetic Evaluation on Cell Growth and Biosynthesis of Polyhydroxybutyrate (PHB) by Bacillus safensis EBT1 from Sugarcane Bagasse. Eng. Agric. Environ. Food 2018, 11, 145–152. [Google Scholar] [CrossRef]
- Segura, P.C.; Wattiez, R.; Vande Wouwer, A.; Leroy, B.; Dewasme, L. Dynamic Modeling of Rhodospirillum rubrum PHA Production Triggered by Redox Stress during VFA Photoheterotrophic Assimilations. J. Biotechnol. 2022, 360, 45–54. [Google Scholar] [CrossRef]
- Wang, X.; Carvalho, G.; Reis, M.A.M.; Oehmen, A. Metabolic Modeling of the Substrate Competition among Multiple VFAs for PHA Production by Mixed Microbial Cultures. J. Biotechnol. 2018, 280, 62–69. [Google Scholar] [CrossRef]
- Mozumder, M.S.I.; Amin, M.S.A.; Shishir, M.F.R. Unified Model to Predict and Enhance the Mixed Culture Polyhydroxyalkanoates (PHA) Production. Bioresour. Technol. Rep. 2020, 11, 100537. [Google Scholar] [CrossRef]
- Borchers, A.; Pieler, T. Programming Pluripotent Precursor Cells Derived from Xenopus Embryos to Generate Specific Tissues and Organs. Genes 2010, 1, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, S.; Ravi, Y.K.; Gunasekaran, M.; Chattopadhyay, I.; Palani, S.; Kumar, V.; Kumar, G.; Jeyakumar, R.B. Development of an Integrated Biorefinery System for Bioconversion of Lignocellulosic Biomass to Polyhydroxyalkanoates and Biohydrogen. ACS Sustain. Chem. Eng. 2023, 11, 4606–4622. [Google Scholar] [CrossRef]
- Calvo, M.V.; Colombo, B.; Corno, L.; Eisele, G.; Cosentino, C.; Papa, G.; Scaglia, B.; Pilu, R.; Simmons, B.; Adani, F. Bioconversion of Giant Cane for Integrated Production of Biohydrogen, Carboxylic Acids, and Polyhydroxyalkanoates (PHAs) in a Multistage Biorefinery Approach. ACS Sustain. Chem. Eng. 2018, 6, 15361–15373. [Google Scholar] [CrossRef]
- Silva, A.R.G.D.; Errico, M.; Rong, B.G. Techno-Economic Analysis of Organosolv Pretreatment Process from Lignocellulosic Biomass. Clean Technol. Environ. Policy 2018, 20, 1401–1412. [Google Scholar] [CrossRef]
- Peng, J.J.; Xu, H.F.; Wang, W.X.; Kong, Y.; Su, Z.N.; Li, B. Techno-Economic Analysis of Bioethanol Preparation Process via Deep Eutectic Solvent Pretreatment. Ind. Crops Prod. 2021, 172, 114036. [Google Scholar] [CrossRef]
- Ullah, A.; Zhang, Y.X.; Liu, C.; Qiao, Q.; Shao, Q.; Shi, J. Process Intensification Strategies for Green Solvent Mediated Biomass Pretreatment. Bioresour. Technol. 2022, 369, 128394. [Google Scholar] [CrossRef]
- Vasco-Correa, J.; Shah, A. Techno-Economic Bottlenecks of the Fungal Pretreatment of Lignocellulosic Biomass. Fermentation 2019, 5, 30. [Google Scholar] [CrossRef]
- Manikandan, N.A.; Pakshirajan, K.; Pugazhenthi, G. Techno-Economic Assessment of a Sustainable and Cost-Effective Bioprocess for Large-Scale Production of Polyhydroxybutyrate. Chemosphere 2021, 284, 131371. [Google Scholar] [CrossRef]
- Ventura, J.R.S.; Ventura, R.L.G. Techno-Economic Feasibility of Polyhydroxybutyrate (PHB) Production from Corn Stover. Philipp. Agric. Sci. 2024, 107, 55–69. [Google Scholar] [CrossRef]
- Rajendran, N.; Han, J. Techno-Economic Analysis of Food Waste Valorization for Integrated Production of Polyhydroxyalkanoates and Biofuels. Bioresour. Technol. 2022, 348, 126796. [Google Scholar] [CrossRef] [PubMed]
- Derippe, G.; Philip, L.; Lemechko, P.; Eyheraguibel, B.; Meistertzheim, A.L.; Pujo-Pay, M.; Conan, P.; Barbe, V.; Bruzaud, S.; Ghiglione, J.F. Marine Biodegradation of Tailor-Made Polyhydroxyalkanoates (PHA) Influenced by the Chemical Structure and Associated Bacterial Communities. J. Hazard. Mater. 2024, 462, 132782. [Google Scholar] [CrossRef]
- Zhou, W.; Colpa, D.I.; Permentier, H.; Offringa, R.A.; Rohrbach, L.; Euverink, G.J.W.; Krooneman, J. Insight into Polyhydroxyalkanoate (PHA) Production from Xylose and Extracellular PHA Degradation by a Thermophilic Schlegelella thermodepolymerans. Resour. Conserv. Recycl. 2023, 194, 107006. [Google Scholar] [CrossRef]
- Sepehri, A.; Sarrafzadeh, M.H. Effect of Nitrifiers Community on Fouling Mitigation and Nitrification Efficiency in a Membrane Bioreactor. Chem. Eng. Process. 2018, 128, 10–18. [Google Scholar] [CrossRef]
- Sepehri, A.; Sarrafzadeh, M.H.; Avateffazeli, M. Interaction between Chlorella vulgaris and Nitrifying-Enriched Activated Sludge in the Treatment of Wastewater with Low C/N Ratio. J. Clean. Prod. 2020, 247, 119164. [Google Scholar] [CrossRef]
- Sepehri, A.; Sarrafzadeh, M.H. Activity Enhancement of Ammonia-Oxidizing Bacteria and Nitrite-Oxidizing Bacteria in Activated Sludge Process: Metabolite Reduction and CO2 Mitigation Intensification Process. Appl. Water Sci. 2019, 9, 131. [Google Scholar] [CrossRef]
- Kawaguchi, H.; Takada, K.; Elkasaby, T.; Pangestu, R.; Toyoshima, M.; Kahar, P.; Ogino, C.; Kaneko, T.; Kondo, A. Recent Advances in Lignocellulosic Biomass White Biotechnology for Bioplastics. Bioresour. Technol. 2022, 344, 126165. [Google Scholar] [CrossRef]
- Mujtaba, M.; Fraceto, L.F.; Fazeli, M.; Mukherjee, S.; Savassa, S.M.; de Medeiros, G.A.; Pereira, A.D.S.; Mancini, S.D.; Lipponen, J.; Vilaplana, F. Lignocellulosic Biomass from Agricultural Waste to the Circular Economy: A Review with Focus on Biofuels, Biocomposites and Bioplastics. J. Clean. Prod. 2023, 402, 136815. [Google Scholar] [CrossRef]



| Review (First Author, Year) | Scope | Pretreatments Coverage | Microbial Coverage | TEA Depth |
|---|---|---|---|---|
| Ciesielski et al., 2015 [2] | Review of plant oils (including waste oils) as PHA feedstocks, covering PHA structures, metabolic pathways, microbial species, fermentation strategies, material properties, and economic aspects | Focused on oil-based feedstocks; no systematic discussion of lignocellulosic pretreatments; only brief mention that oil by-products may require simple processing | Various pure cultures (Cupriavidus, Pseudomonas, Bacillus, etc.); mentions mixed microbial cultures (MMC) and some engineered strains | Brief qualitative discussion of cost components (feedstock, downstream recovery); no quantitative TEA modeling |
| Li et al., 2025 [4] | Systematic review of full lignocellulosic fractions (cellulose, hemicellulose, lignin) to PHA, covering feedstock-metabolism-engineering-TEA-life cycle assessment (LCA) | Comprehensive coverage of physical, chemical (acid/alkali/organic solvent/ionic liquid/DES), physicochemical (steam explosion/hydrothermal), and biological pretreatments; tables comparing pros/cons, sugar/inhibitor yields, and PHA outputs | Wide range of pure cultures (Cupriavidus, Ralstonia, Halomonas, Pseudomonas, etc.), MMC, and engineered strains; includes feedstock-strain-product mapping | Dedicated TEA section citing multiple quantitative TEAs (CAPEX, OPEX, MSP, sensitivity analysis) and integrated with LCA (GHG, energy use, downstream processing) |
| Andler et al., 2021 [6] | Review of fruit residues as sustainable feedstock for PHA production, including biochemical pathways, pretreatments, production yields, and biotechnological aspects | Focus on fruit residues; covers physical, chemical (acid, alkali, ionic liquids), enzymatic, and biological pretreatments; tables summarizing pretreatment methods and effects | Various pure cultures (Cupriavidus, Bacillus, Halomonas, Pseudomonas, Pandoraea, etc.), some co-cultures; includes both PHB and mcl-PHA producers | Brief mention of techno-economic and environmental analysis in the context of banana residues; no dedicated or quantitative TEA section |
| Wang et al., 2021 [7] | Review of agricultural wastes (including lignocellulose, lipids, molasses, whey, etc.) for PHA production, covering fermentation parameter optimization, kinetic modeling, and circular utilization | Detailed discussion of acid/alkali/physical/enzymatic/ultrasound/microwave pretreatments; tables summarizing different feedstocks and pretreatment effects | Multiple pure cultures (Bacillus, Paracoccus, Burkholderia, Halomonas, etc.), some inhibitor-tolerant strains, engineered strains; includes MMC | No dedicated TEA section; only mentions cost factors in conclusions; no quantitative analysis |
| This study | Comprehensive review of LAR-to-PHA covering feedstock properties, advanced pretreatment (including green solvents and non-sterile strategies), co-substrate blending, microbial platform selection (pure, mixed, engineered), kinetic modeling, and integrated TEA; provides practitioner frameworks and decision tools | High coverage: systematic comparison of conventional (acid/alkali/steam), green (DES, ionic liquid, MW-assisted), and non-sterile pretreatments; detailed discussion of inhibitor management and process integration | Broad: pure cultures (Cupriavidus, Bacillus, Halomonas, Pseudomonas, etc.), MMC, extremophiles, and engineered strains; explicit mapping of substrate-platform-product relationships; focus on process-compatible and product-customized platforms | Medium-high: dedicated TEA section with regional scenario analysis, MSP/payback sensitivity, coproduct valorization |
| Type | Pretreatment Method | Advantage | Disadvantage | Criticism | Ref. |
|---|---|---|---|---|---|
| Physical Pretreatment | Mechanical pretreatment | Effective reduction of particle size and cellulose crystallinity | High energy consumption | High operational costs, potential clogging in downstream processes | [25,26] |
| Microwave pretreatment | High thermal efficiency and low energy consumption | High cost | Uneven energy distribution, inconsistent treatment effectiveness | [27] | |
| Liquid hot water pretreatment | Green, economical, and easy to operate | Residual lignin reduces enzymatic digestion effectiveness | Incomplete lignin removal requiring post-treatment steps | [28,29] | |
| Ultrasonic pretreatment | Low energy consumption and cost | Unfavorable to enzymatic hydrolysis | Negative effects on enzyme efficiency, cavitation can produce inhibitors | [24] | |
| Chemical Pretreatment | Acid pretreatment | Improved cellulose accessibility | High corrosiveness | Formation of toxic by-products, requires neutralization | [30,31] |
| Alkali pretreatment | Effective for delignification | Difficult to treat wastewater | Wastewater disposal challenges, limited efficacy on high-lignin feedstocks | [32,33] | |
| Ionic liquid pretreatment | Environmentally friendly and thermally stable | High cost | Difficulty in recovery | [34,35] | |
| Organic solvent pretreatment | Efficient for hemicellulose dissolution and lignin removal | Solvent flammability and operational hazards | Expensive solvents, complex solvent recovery | [36,37] | |
| Ozone pretreatment | Effective lignin removal | High operational cost | Formation of unwanted by-products | [38,39] | |
| Physico- Chemical Pretreatment | Steam pretreatment | Effective hemicellulose dissolution, cost-effective | High temperature and pressure | High energy consumption, sugar degradation, equipment corrosion risks | [40,41] |
| Ammonia fiber expansion pretreatment | Increased cellulose accessibility | Inefficient for biomass with high lignin content | Ammonia recovery inefficiency and process scalability challenges | [24] | |
| Supercritical fluids pretreatment | Environmentally green | High capital and maintenance costs | Specialized equipment required, limited applicability to industrial-scale operations | [24] | |
| Wet oxidation pretreatment | Efficient lignin removal | Control of process parameters and high energy cost | Formation of inorganic salts complicating downstream processing | [24,36] | |
| Biological Pretreatment | Microbial pretreatment | Lignin removal with low energy consumption | Slow degradation rate, environmental sensitivity | Reduced carbon yield, slower degradation process | [42,43] |
| Enzyme pretreatment | High substrate specificity with minimal by-product formation | High enzyme costs and susceptibility to inhibition | Optimization challenges for mixed-substrate systems | [44,45] |
| Substrate | Pretreatment Strategy | Pretreatment Result | PHA Production, g/L | PHA Content, wt% | Ref. |
|---|---|---|---|---|---|
| Sugarcane Bagasse | 1% H2SO4 at 121 °C for 40 min | Sugar yield: 569.0 mg/g | 6.38 | 70.0 | [46] |
| Corn Stover | 1 M HCl at 110 °C for 40 min | Sugar concentration: 18.7 g/L | 0.82 | 38.7 | [47] |
| Pine Sawdust | 1 M HCl at 110 °C for 40 min | Sugar concentration: 8.93 g/L | 1.00 | 46.9 | [47] |
| Rice Straw | 0.5% H2SO4 at 121 °C for 40 min | Sugar concentration: 19.6 g/L | 1.50 | 32.6 | [48] |
| Wheat Biomass | 2% NaOH at 100 °C for 3 h | Sugar yield: 418.0 mg/g; Hydrolysis yield: 64.3% | 5.67 | 67.5 | [49] |
| Rice Husks | 1.0 mol/L KOH at 121 °C and 0.1 MPa for 15 min | Sugar concentration: 20.0 g/L | 3.90 | 50.0 | [50] |
| Wheat Bran | 1% NaOH at 121 °C for 30 min | Sugar concentration: 62.9 g/L | 2.69 | 43.8 | [51] |
| Oil Palm Fruit | 10 g/L NaOH at 121 °C for 60 min | Sugar concentration: 78.1 g/L | 2.72 | 43.1 | [52] |
| Rice Straw | 20% NH3 at 80 °C for 10 h and enzymatic at 50 °C for 40 h | Maximum delignification: 63%; Glucan conversion: 92.0% | 2.96 | 59.3 | [53] |
| Rice Husks | 0.4% H2SO4 at 100 °C for 30 min and steam explosion of 1.8 MPa for 5 min | Sugar yield: 266.5 mg/g | 5.00 | - | [54] |
| Key Model (Equation) | Key Kinetic Parameter | Definition of Parameters | Operational Context | Ref. |
|---|---|---|---|---|
| x: Biomass (g/L); xmax: Max biomass (g/L); μmax: Max growth rate (h−1); α: Growth-associated coefficient; β: Non-growth-associated coefficient | C. necator, rubber seed oil, 30 °C, pH not controlled, batch | [86] | ||
| S: Substrate (g/L); KS: Half-saturation constant; A: Cell activity (unitless); m: Maintenance coefficient (h−1) | C. necator, mixed VFAs, 30 °C, pH 7.0, batch | [87] | ||
| - | qPHA: PHA prod. Rate; YPHA,S: PHA yield/substrate; CX: Active biomass (g/L) | Mixed VFAs, 30 °C, aerobic sequencing batch reactor (SBR), feast–famine operation | [88] | |
| μ: Specific growth rate (h−1); Ks: Half-saturation constant; m: Maintenance coefficient (h−1) | Bacillus safensis, sugarcane bagasse, 30 °C, pH 7.0, batch | [89] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Liu, S.; Wang, Q.; Ma, X.; Li, J. Integrated Pretreatment and Microbial Matching for PHA Production from Lignocellulosic Agro-Forestry Residues. Fermentation 2025, 11, 563. https://doi.org/10.3390/fermentation11100563
Li D, Liu S, Wang Q, Ma X, Li J. Integrated Pretreatment and Microbial Matching for PHA Production from Lignocellulosic Agro-Forestry Residues. Fermentation. 2025; 11(10):563. https://doi.org/10.3390/fermentation11100563
Chicago/Turabian StyleLi, Dongna, Shanshan Liu, Qiang Wang, Xiaojun Ma, and Jianing Li. 2025. "Integrated Pretreatment and Microbial Matching for PHA Production from Lignocellulosic Agro-Forestry Residues" Fermentation 11, no. 10: 563. https://doi.org/10.3390/fermentation11100563
APA StyleLi, D., Liu, S., Wang, Q., Ma, X., & Li, J. (2025). Integrated Pretreatment and Microbial Matching for PHA Production from Lignocellulosic Agro-Forestry Residues. Fermentation, 11(10), 563. https://doi.org/10.3390/fermentation11100563






