Sugarcane Bagasse: A Sustainable Feedstock for Biorefinery Portfolios in South Africa
Abstract
1. Introduction
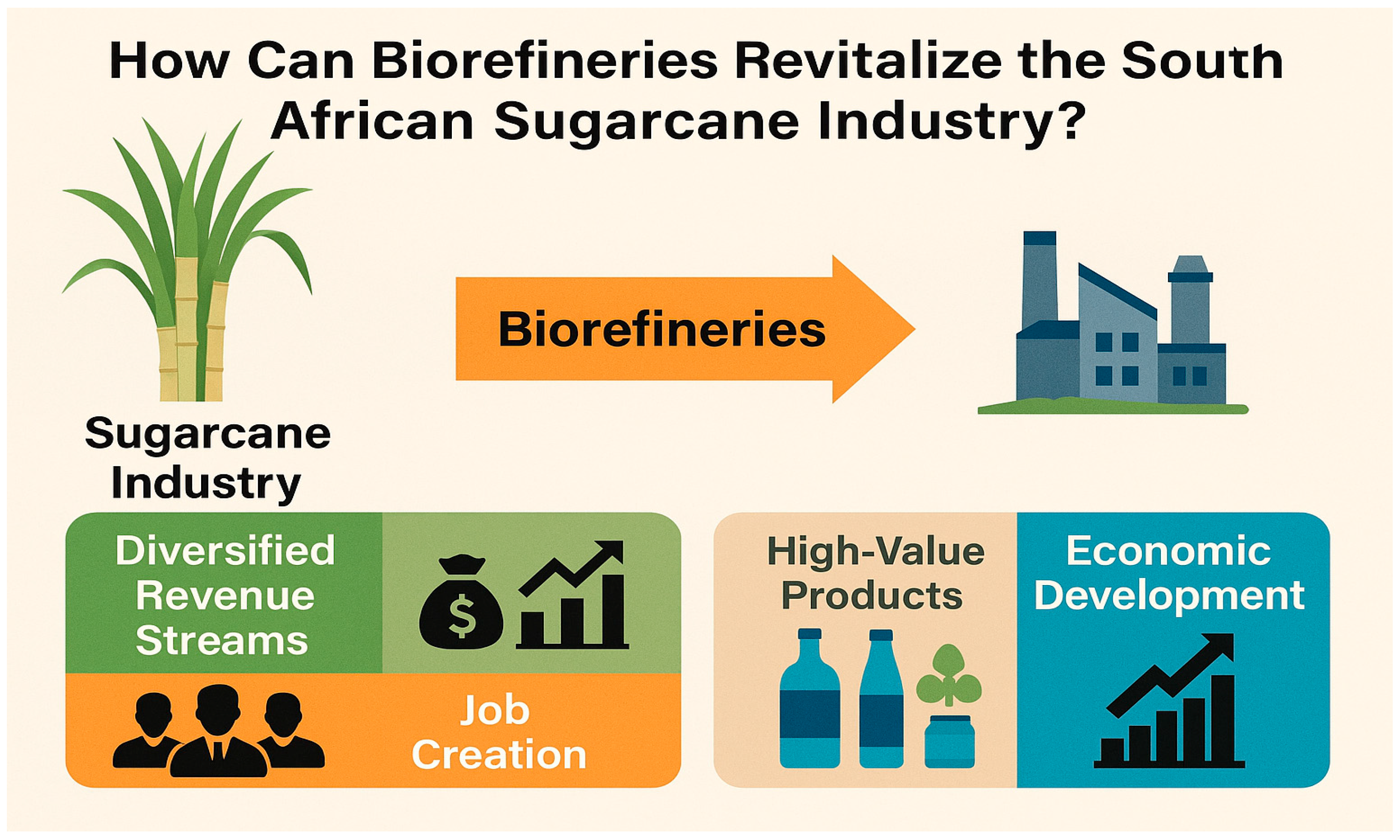
1.1. Revitalizing South Africa’s Sugarcane Industry: A Strategic Need
1.2. The Hurdles Faced by the South African Sugarcane Industry
1.3. The South African Sugarcane Master Plan 2030
2. Biorefineries Driving South Africa’s Sugarcane Revival
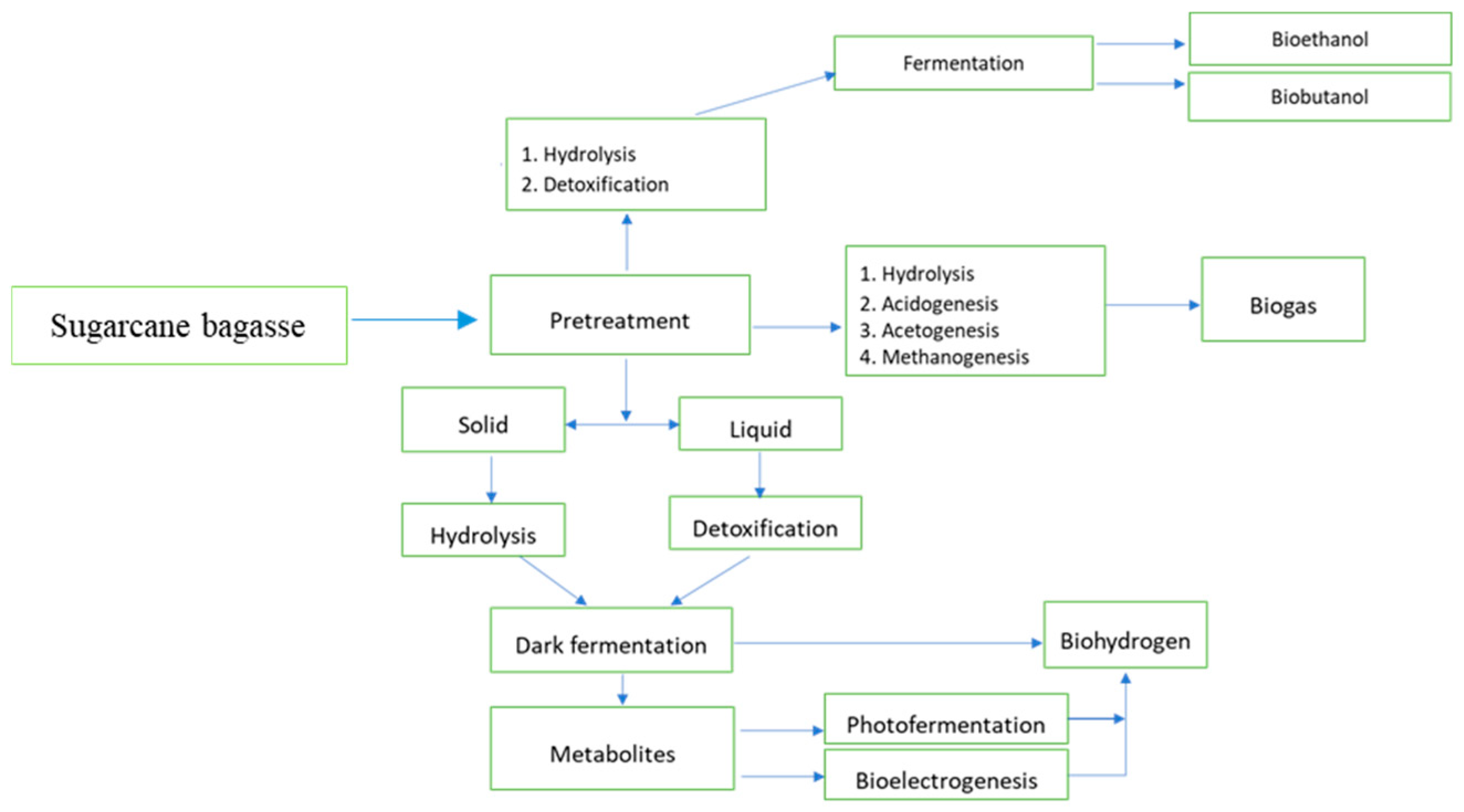
3. Extraction of Sugars from Sugarcane Bagasse
3.1. Pretreatment Strategies Used in Sugarcane Bagasse
3.1.1. Biological Pretreatment Strategies
3.1.2. Physical Pretreatment Strategies
3.1.3. Chemical Pretreatment Strategies
3.1.4. Physicochemical Pretreatment Strategies
| Pretreatment | Advantages | Drawbacks | References |
|---|---|---|---|
| Biological |
|
| [4,9,38,49,51,57] |
| Physical |
|
| [4,48,51] |
| Chemical |
|
| [4,49,51] |
| Physicochemical |
|
| [49,51] |
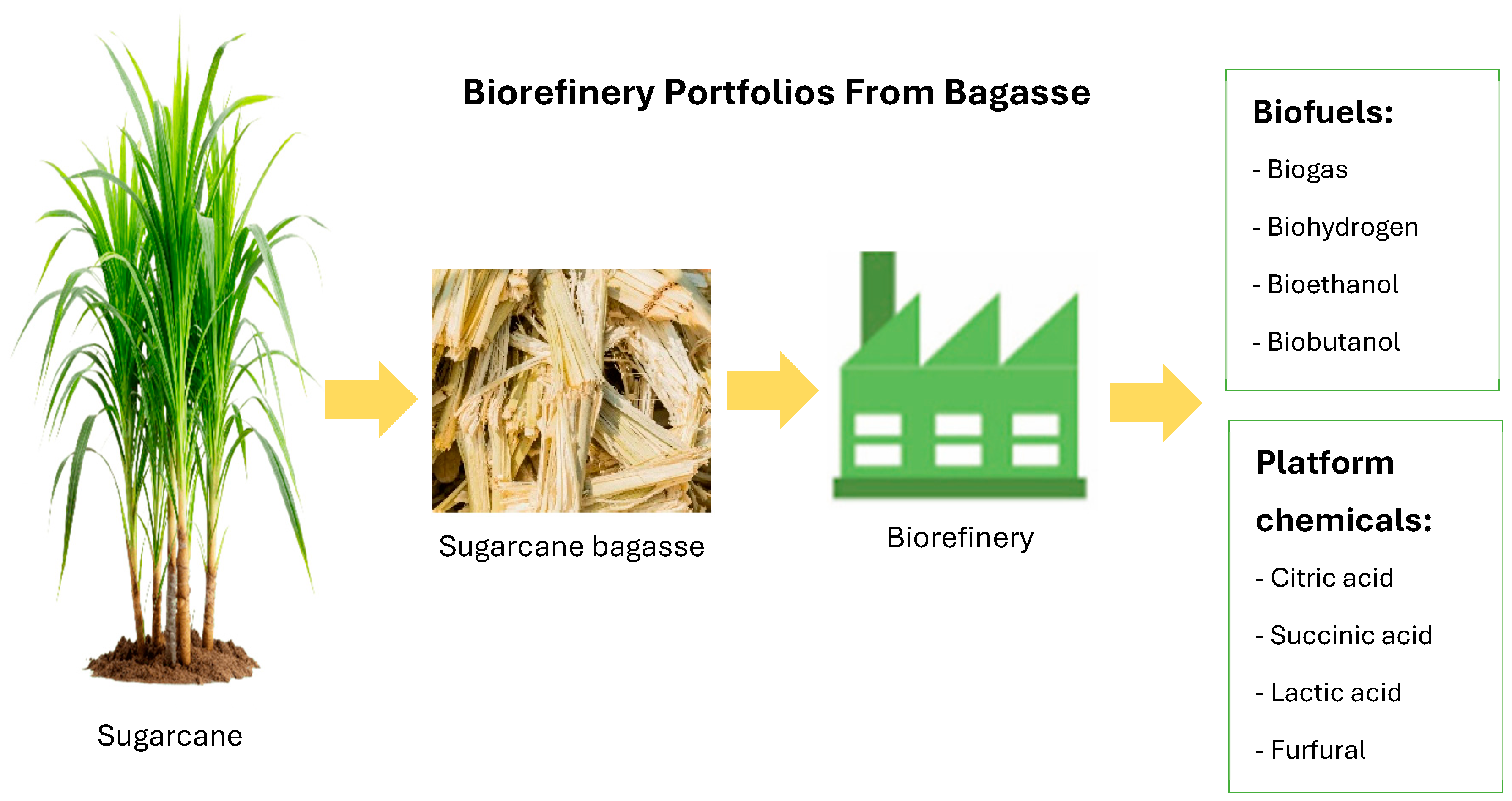
4. Biorefinery Portfolios from Sugarcane Bagasse
4.1. Biofuels
4.1.1. Biogas
4.1.2. Bioethanol
4.1.3. Biohydrogen
4.1.4. Biobutanol
4.2. Platform Chemicals
4.2.1. Lactic Acid
4.2.2. Citric Acid
4.2.3. Furfural
4.2.4. Succinic Acid
4.2.5. Xylitol
5. Biorefinery Development in South Africa
R&D and Commercialization Initiatives
6. Techno-Economic Viability of Valorizing Bagasse in South Africa
7. Conclusions and Future Outlooks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, B.; Larroche, C.; Dussap, C.G. Comprehensive assessment of 2G bioethanol production. Bioresour. Technol. 2020, 313, 123630. [Google Scholar] [CrossRef]
- Karp, S.G.; Woiciechowski, A.L.; Soccol, V.T.; Soccol, C.R. Pretreatment strategies for delignification of sugarcane bagasse: A review. Braz. Arch. Biol. Technol. 2013, 56, 679–689. [Google Scholar] [CrossRef]
- Buthelezi, A.S.; Chetty, M.; Mohammadi, A.H. Techno-Economic Assessment of Biofuels Production From Sugarcane Bagasse. Energy Sci. Eng. 2025, in press. [Google Scholar] [CrossRef]
- Fung, A.H.Y.; Rao, S.; Ngan, W.Y.; Sekoai, P.T.; Touyon, L.; Ho, T.M.; Wong, K.P.; Habimana, O. Exploring the optimization of aerobic food waste digestion efficiency through the engineering of functional biofilm Bio-carriers. Bioresour. Technol. 2021, 341, 125869. [Google Scholar] [CrossRef]
- Mkhwanazi, Z.; Isa, Y.M.; Vallabh, S.T. Production of Biocoal from Wastewater Sludge and Sugarcane Bagasse: A Review. Atmosphere 2023, 14, 184. [Google Scholar] [CrossRef]
- González, S.; Rodríguez, P.; Rigual, V.; Rivas, S. Macromolecular features of lignin, hemicellulose and cellulose fractions from biphasic organosolv fractionation of diverse biomasses: A comparative study. Int. J. Biol. Macr. 2025, 322, 146865. [Google Scholar] [CrossRef]
- Loh, Y.R.; Sujan, D.; Rahman, M.E.; Das, C.A. Sugarcane bagasse—The future composite material: A literature review. Resour. Conserv. Recycl. 2013, 75, 14–22. [Google Scholar] [CrossRef]
- Demirbas, A. Biofuels sources, biofuel policy, biofuel economy and global biofuel projections. Energy Convers. Manag. 2008, 49, 2106–2116. [Google Scholar] [CrossRef]
- Shukla, A.; Kumar, D.; Girdhar, M.; Kumar, A.; Goyal, A.; Malik, T.; Mohan, A. Strategies of pretreatment of feedstocks for optimized bioethanol production: Distinct and integrated approaches. Biotechnol. Biofuels Bioprod. 2023, 16, 44. [Google Scholar] [CrossRef]
- Geldenhuys, G.; Wattrus, M.; Fox, M.; Forbes, P.B.C. Investigations into the transition to sustainable alternative fuels in a South African underground platinum mine. Renew. Energy Focus 2023, 47, 100500. [Google Scholar] [CrossRef]
- Aristizábal, M.V.; Gómez, P.Á.; Cardona, A.C.A. Biorefineries based on coffee cut-stems and sugarcane bagasse: Furan-based compounds and alkanes as interesting products. Bioresour. Technol. 2015, 196, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Demirbaş, A. Energy balance, energy sources, energy policy, future developments and energy investments in Turkey. Energy Convers. Manag. 2001, 42, 1239–1258. [Google Scholar] [CrossRef]
- Farzad, S.; Mandegari, M.A.; Guo, M.; Haigh, K.F.; Shah, N.; Görgens, J.F. Multi-product biorefineries from lignocelluloses: A pathway to revitalisation of the sugar industry? Biotechnol. Biofuels 2017, 10, 87. [Google Scholar] [CrossRef]
- Sindhu, R.; Gnansounou, E.; Binod, P.; Pandey, A. Bioconversion of sugarcane crop residue for value added products—An overview. Renew. Energy 2016, 98, 203–215. [Google Scholar] [CrossRef]
- Furtado, L.A.; Ribeiro, S.G.; Pradelle, F.; Reis Parise, J.A. Modeling and techno-economic analysis of a hybrid sugarcane plant fed by vinasse biogas and bagasse surplus for electricity generation. J. Clean. Prod. 2023, 413, 137511. [Google Scholar] [CrossRef]
- Mankar, A.R.; Pandey, A.; Modak, A.; Pant, K.K. Pretreatment of lignocellulosic biomass: A review on recent advances. Bioresour. Technol. 2021, 334, 125235. [Google Scholar] [CrossRef]
- Armah, E.K.; Chetty, M.; Deenadayalu, N. Biogas production from sugarcane bagasse with South African industrial wastewater and novel kinetic study using response surface methodology. Sci. Afr. 2020, 10, e00556. [Google Scholar] [CrossRef]
- Nieder-Heitmann, M.; Haigh, K.; Görgens, J.F. Process design and economic evaluation of integrated, multi-product biorefineries for the co-production of bio-energy, succinic acid, and polyhydroxybutyrate (PHB) from sugarcane bagasse and trash lignocelluloses. Biofuels Bioprod. Biorefining 2019, 13, 599–617. [Google Scholar] [CrossRef]
- Sekoai, P.T.; Chunilall, V.; Msele, K.; Buthelezi, L.; Johakimu, J.; Andrew, J.; Zungu, M.; Moloantoa, K.; Maningi, N.; Habimana, O.; et al. Biowaste biorefineries in South Africa: Current status, opportunities, and research and development needs. Renew. Sustain. Energy Rev. 2023, 188, 113870. [Google Scholar] [CrossRef]
- Baiyegunhi, L.; Arnold, C.A. Economics of sugarcane production on large scale farms in the Eshowe/Entumeni areas of KwaZulu-Natal, South Africa. Afr. J. Agric. Res. 2011, 6, 4960–4967. [Google Scholar]
- Mandree, P.; Thopil, G.A.; Ramchuran, S. Potential Opportunities to Convert Waste to Bio-Based Chemicals at an Industrial Scale in South Africa. Fermentation 2023, 9, 908. [Google Scholar] [CrossRef]
- Zulu, N.S.; Sibanda, M.; Tlali, B.S. Factors Affecting Sugarcane Production by Small-Scale Growers in Ndwedwe Local Municipality, South Africa. Agriculture 2019, 9, 170. [Google Scholar] [CrossRef]
- Dlamini, P.J. Drought stress tolerance mechanisms and breeding effort in sugarcane: A review of progress and constraints in South Africa. Plant Stress 2021, 2, 100027. [Google Scholar] [CrossRef]
- Metiso, H.; Tsvakirai, C.Z. Factors affecting small-scale sugarcane production in Nkomazi local municipality in Mpumalanga province, South Africa. S. Afr. J. Agri. Ext. 2019, 47, 1–8. [Google Scholar] [CrossRef][Green Version]
- Özüdoğru, H.M.R.; Nieder-Heitmann, M.; Haigh, K.F.; Görgens, J.F. Techno-economic analysis of product biorefineries utilizing sugarcane lignocelluloses: Xylitol, citric acid and glutamic acid scenarios annexed to sugar mills with electricity co-production. Ind. Crops Prod. 2019, 133, 259–268. [Google Scholar] [CrossRef]
- Del Carmen Cordova Molina, C.; Ortiz Enriquez, O.; Catalina Alfaro-De la Torre, M.; Reyes Agüero, J.A.; Cardona Benavides, A. Temporal hydrogeochemical evolution of surface water and groundwater in a karst system discharging into a continental-type Ramsar site in the Huasteca Potosina, Mexico. Environ. Sci. Pollut. Res. 2025, 32, 3912–3950. [Google Scholar] [CrossRef]
- Hitayezu, P.; Zegeye, E.W.; Ortmann, G.F. Some aspects of agricultural vulnerability to climate change in the KwaZulu-Natal Midlands, South Africa: A systematic review. J. Hum. Ecol. 2014, 48, 347–356. [Google Scholar] [CrossRef]
- Zulu, N.S.; Hlatshwayo, S.I.; Ojo, T.O.; Slotow, R.; Cele, T.; Ngidi, M.S.C. The impact of credit accessibility and information communication technology on the income of small-scale sugarcane farmers in Ndwedwe Local Municipality, KwaZulu-Natal Province, South Africa. Front. Sustain. Food Syst. 2024, 8, 1392647. [Google Scholar] [CrossRef] [PubMed]
- Ncoyini, Z.; Savage, M.J.; Strydom, S. Limited access and use of climate information by small-scale sugarcane farmers in South Africa: A case study. Clim. Serv. 2022, 26, 100285. [Google Scholar] [CrossRef]
- Rabelo, S.C.; Carrere, H.; Maciel Filho, R.; Costa, A.C. Production of bioethanol, methane and heat from sugarcane bagasse in a biorefinery concept. Bioresour. Technol. 2011, 102, 7887–7895. [Google Scholar] [CrossRef] [PubMed]
- Meena, P.K.; Patane, P.M. Biohydrogen: Advancing a sustainable transition from fossil fuels to renewable energy. Int. J. Hydrogen Energy 2025, 100, 955–970. [Google Scholar] [CrossRef]
- Alonso, D.M.; Wettstein, S.G.; Dumesic, J.A. Bimetallic catalysts for upgrading of biomass to fuels and chemicals. Chem. Soc. Rev. 2012, 41, 8075–8098. [Google Scholar] [CrossRef]
- Peng, L.; Xie, N.; Guo, L.; Wang, L.; Yu, B.; Ma, Y. Efficient Open Fermentative Production of Polymer-Grade L-Lactate from Sugarcane Bagasse Hydrolysate by Thermotolerant Bacillus sp. Strain P38. PLoS ONE 2014, 9, e107143. [Google Scholar] [CrossRef]
- Smithers, J. Review of sugarcane trash recovery systems for energy cogeneration in South Africa. Renew. Sustain. Energy Rev. 2014, 32, 915–925. [Google Scholar] [CrossRef]
- Dogbe, E.S.; Mandegari, M.; Görgens, J.F. Revitalizing the sugarcane industry by adding value to A-molasses in biorefineries. Biofuels Bioprod. Bioref. 2020, 14, 1089–1104. [Google Scholar] [CrossRef]
- Pachón, E.R.; Vaskan, P.; Raman, J.K.; Gnansounou, E. Transition of a South African sugar mill towards a biorefinery. A feasibility assessment. Appl. Energy 2018, 229, 1–17. [Google Scholar] [CrossRef]
- Niju, S.; Swathika, M. Delignification of sugarcane bagasse using pretreatment strategies for bioethanol production. Biocatal. Agric. Biotechnol. 2019, 20, 101263. [Google Scholar] [CrossRef]
- Wani, A.K.; Rahayu, F.; Fauziah, L.; Suhara, C. Advances in safe processing of sugarcane and bagasse for the generation of biofuels and bioactive compounds. J. Agric. Food Res. 2023, 12, 100549. [Google Scholar] [CrossRef]
- Lu, S.; Wang, Q.; Liang, Z.; Wang, W.; Liang, C.; Wang, Z.; Yuan, Z.; Lan, P.; Qi, W. Saccharification of sugarcane bagasse by magnetic carbon-based solid acid pretreatment and enzymatic hydrolysis. Ind. Crops Prod. 2021, 160, 113159. [Google Scholar] [CrossRef]
- Matei, J.C.; Soares, M.; Bonato, A.C.H.; de Freitas, M.P.A.; Helm, C.V.; Maroldi, W.V.; Magalhães, W.L.E.; Haminiuk, C.W.I.; Maciel, G.M. Enzymatic delignification of sugar cane bagasse and rice husks and its effect in saccharification. Renew. Energy 2020, 157, 987–997. [Google Scholar] [CrossRef]
- Martinez-Hernandez, E.; Amezcua-Allieri, M.A.; Sadhukhan, J.; Anell, J.A. Sugarcane bagasse valorization strategies for bioethanol and energy production. In Sugarcane—Technology and Research; IntechOpen: London, UK, 2018; pp. 137–144. [Google Scholar]
- Pandey, A.; Soccol, C.R.; Nigam, P.; Soccol, V.T. Biotechnological potential of agro-industrial residues. I: Sugarcane bagasse. Bioresour. Technol. 2000, 74, 69–80. [Google Scholar] [CrossRef]
- Agrawal, D.; Kumar, V. Recent progress on sugarcane-bagasse based lactic acid production: Technical advancements, potential and limitations. Ind. Crops Prod. 2023, 193, 116132. [Google Scholar] [CrossRef]
- Brodeur, G.; Yau, E.; Badal, K.; Collier, J.; Ramachandran, K.B.; Ramakrishnan, S. Chemical and Physicochemical Pretreatment of Lignocellulosic Biomass: A Review. Enzym. Res. 2011, 2011, 787532. [Google Scholar] [CrossRef]
- Alokika; Anu; Kumar, A.; Kumar, V.; Singh, B. Cellulosic and hemicellulosic fractions of sugarcane bagasse: Potential, challenges and future perspective. Int. J. Biol. Macromol. 2021, 169, 564–582. [Google Scholar] [CrossRef]
- Kapoor, K.; Tyagi, A.K.; Diwan, R.K. Effect of gamma irradiation on recovery of total reducing sugars from delignified sugarcane bagasse. Radiat. Phys. Chem. 2020, 170, 108643. [Google Scholar] [CrossRef]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Ayeni, A.O.; Daramola, M.O.; Adetayo, A.E.; Sekoai, P.T.; Nwinyi, O.C.; Ejekwu, O. Chapter 7—Valorization of Biomass to Value-Added Commodities; Daramola, M.O., Ayeni, A.O., Eds.; Springer: Cham, Switzerland, 2021; Volume 1, pp. 101–117. [Google Scholar]
- Sarker, T.C.; Azam, S.M.G.G.; Bonanomi, G. Recent Advances in Sugarcane Industry Solid By-Products Valorization. Waste Biomass Valorization 2017, 8, 241–266. [Google Scholar] [CrossRef]
- Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. Emerging technologies for the pretreatment of lignocellulosic biomass. Bioresour. Technol. 2018, 262, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Barrett, D.M.; Delwiche, M.J.; Stroeve, P. Methods for Pretreatment of Lignocellulosic Biomass for Efficient Hydrolysis and Biofuel Production. Ind. Eng. Chem. Res. 2009, 48, 3713–3729. [Google Scholar] [CrossRef]
- Silva Ortiz, P.; de Oliveira, S. Exergy analysis of pretreatment processes of bioethanol production based on sugarcane bagasse. Energy 2014, 76, 130–138. [Google Scholar] [CrossRef]
- Malhotra, M.; Suman, S.K. Laccase-mediated delignification and detoxification of lignocellulosic biomass: Removing obstacles in energy generation. Environ. Sci. Pollut. Res. 2021, 28, 58929–58944. [Google Scholar] [CrossRef]
- Salvachúa, D.; Prieto, A.; López-Abelairas, M.; Lu-Chau, T.; Martínez, Á.T.; Martínez, M.J. Fungal pretreatment: An alternative in second-generation ethanol from wheat straw. Bioresour. Technol. 2011, 102, 7500–7506. [Google Scholar] [CrossRef]
- Taherzadeh, M.J.; Karimi, K. Pretreatment of Lignocellulosic Wastes to Improve Ethanol and Biogas Production: A Review. Int. J. Mol. Sci. 2008, 9, 1621–1651. [Google Scholar] [CrossRef]
- Devi, A.; Bajar, S.; Kour, H.; Kothari, R.; Pant, D.; Singh, A. Lignocellulosic Biomass Valorization for Bioethanol Production: A Circular Bioeconomy Approach. BioEnergy Res. 2022, 15, 1820–1841. [Google Scholar] [CrossRef]
- Ren, N.; Wang, A.; Cao, G.; Xu, J.; Gao, L. Bioconversion of lignocellulosic biomass to hydrogen: Potential and challenges. Biotechnol. Adv. 2009, 27, 1051–1060. [Google Scholar] [CrossRef]
- Thite, V.S.; Nerurkar, A.S. Valorization of sugarcane bagasse by chemical pretreatment and enzyme mediated deconstruction. Sci. Rep. 2019, 9, 15904. [Google Scholar] [CrossRef] [PubMed]
- Saratale, G.D.; Saratale, R.G.; Kim, S.H.; Kumar, G. Screening and optimization of pretreatments in the preparation of sugarcane bagasse feedstock for biohydrogen production and process optimization. Int. J. Hydrogen Energy 2018, 43, 11470–11483. [Google Scholar] [CrossRef]
- Saravanan, P.; Rajeswari, S.; Divyabaskaran; López-Maldonado, E.A.; Rajeshkannan, R.; Viswanathan, S. Recent developments on sustainable biobutanol production: A novel integrative review. Environ. Sci. Pollut. Res. Int. 2024, 31, 46858–46876. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, A.; Mbohwa, C. Development of biofuels in South Africa: Challenges and opportunities. Renew. Sustain. Energy Rev. 2014, 39, 1089–1100. [Google Scholar] [CrossRef]
- Khan, N.; Sudhakar, K.; Mamat, R. Role of Biofuels in Energy Transition, Green Economy and Carbon Neutrality. Sustainability 2021, 13, 12374. [Google Scholar] [CrossRef]
- Ebadian, M.; van Dyk, S.; McMillan, J.D.; Saddler, J. Biofuels policies that have encouraged their production and use: An international perspective. Energy Policy 2020, 147, 111906. [Google Scholar] [CrossRef]
- Veza, I.; Muhamad Said, M.F.; Latiff, Z.A. Recent advances in butanol production by acetone-butanol-ethanol (ABE) fermentation. Biomass Bioenergy 2021, 144, 105919. [Google Scholar] [CrossRef]
- Ajala, E.O.; Ighalo, J.O.; Ajala, M.A.; Adeniyi, A.G.; Ayanshola, A.M. Sugarcane bagasse: A biomass sufficiently applied for improving global energy, environment and economic sustainability. Bioresour. Bioprocess. 2021, 8, 87. [Google Scholar] [CrossRef]
- Aristizábal-Marulanda, V.; Poveda-Giraldo, J.A.; Cardona Alzate, C.A. Comparison of furfural and biogas production using pentoses as platform. Sci. Total Environ. 2020, 728, 138841. [Google Scholar] [CrossRef]
- Gupta, P.; Kurien, C.; Mittal, M. Biogas (a promising bioenergy source): A critical review on the potential of biogas as a sustainable energy source for gaseous fuelled spark ignition engines. Int. J. Hydrogen Energy 2023, 48, 7747–7769. [Google Scholar] [CrossRef]
- Agarwal, N.K.; Kumar, M.; Ghosh, P.; Kumar, S.S.; Singh, L.; Vijay, V.K.; Kumar, V. Anaerobic digestion of sugarcane bagasse for biogas production and digestate valorization. Chemosphere 2022, 295, 133893. [Google Scholar] [CrossRef] [PubMed]
- Iram, M.; Asghar, U.; Irfan, M.; Huma, Z.; Jamil, S.; Nadeem, M.; Syed, Q. Production of bioethanol from sugarcane bagasse using yeast strains: A kinetic study. Energy Sources Part A Recovery Util. Environ. Eff. 2018, 40, 364–372. [Google Scholar] [CrossRef]
- Dodo, C.M.; Mamphweli, S.; Okoh, O. Bioethanol production from lignocellulosic sugarcane leaves and tops. J. Energy South. Afr. 2017, 28, 1–11. [Google Scholar] [CrossRef]
- Mandegari, M.; Farzad, S.; Görgens, J.F. A new insight into sugarcane biorefineries with fossil fuel co-combustion: Techno-economic analysis and life cycle assessment. Energy Convers. Manag. 2018, 165, 76–91. [Google Scholar] [CrossRef]
- Sekoai, P.T.; Chunilall, V.; Sithole, B.; Habimana, O.; Ndlovu, S.; Ezeokoli, O.T.; Sharma, P.; Yoro, K.O. Elucidating the Role of Biofilm-Forming Microbial Communities in Fermentative Biohydrogen Process: An Overview. Microorganisms 2022, 10, 1924. [Google Scholar] [CrossRef]
- Han, S.-K.; Shin, H.-S. Biohydrogen production by anaerobic fermentation of food waste. Int. J. Hydrogen Energy 2004, 29, 569–577. [Google Scholar] [CrossRef]
- Mizuno, O.; Dinsdale, R.; Hawkes, F.R.; Hawkes, D.L.; Noike, T. Enhancement of hydrogen production from glucose by nitrogen gas sparging. Bioresour. Technol. 2000, 73, 59–65. [Google Scholar] [CrossRef]
- Su, H.; Liu, G.; He, M.; Tan, F. A biorefining process: Sequential, combinational lignocellulose pretreatment procedure for improving biobutanol production from sugarcane bagasse. Bioresour. Technol. 2015, 187, 149–160. [Google Scholar] [CrossRef]
- Chitaka, T.Y.; Schenck, C. Developing country imperatives in the circular bioeconomy: A review of the South African case. Environ. Dev. 2023, 45, 100812. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Tashiro, Y.; Sonomoto, K. Lactic acid production from lignocellulose-derived sugars using lactic acid bacteria: Overview and limits. J. Biotechnol. 2011, 156, 286–301. [Google Scholar] [CrossRef]
- Koutinas, A.A.; Vlysidis, A.; Pleissner, D.; Kopsahelis, N.; Lopez Garcia, I.; Kookos, I.K.; Papanikolaou, S.; Kwan, T.H.; Lin, C.S.K. Valorization of industrial waste and by-product streams via fermentation for the production of chemicals and biopolymers. Chem. Soc. Rev. 2014, 43, 2587–2627. [Google Scholar] [CrossRef]
- Yang, X.; Lai, Z.; Lai, C.; Zhu, M.; Li, S.; Wang, J.; Wang, X. Efficient production of l-lactic acid by an engineered Thermoanaerobacterium aotearoensewith broad substrate specificity. Biotechnol. Biofuels 2013, 6, 124. [Google Scholar] [CrossRef] [PubMed]
- Weissgram, M.; Herwig, C.; Weber, H.K. Biotechnological Generation of Value Added Products from Spent Pulping Liquors: Assessing the Potential of Extremophiles. J. Bioprocess. Biotech. 2015, 5, 1–14. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, J.; Su, X.; Bao, J.; Wang, K.; Mao, Z. Citric acid production by recycling its wastewater treated with anaerobic digestion and nanofiltration. Process Biochem. 2017, 58, 245–251. [Google Scholar] [CrossRef]
- Hou, W.; Bao, J. Simultaneous saccharification and aerobic fermentation of high titer cellulosic citric acid by filamentous fungus Aspergillus niger. Bioresour. Technol. 2018, 253, 72–78. [Google Scholar] [CrossRef]
- Anastassiadis, S.; Rehm, H.-J. Citric acid production from glucose by yeast Candida oleophila ATCC 20177 under batch, continuous and repeated batch cultivation. Electron. J. Biotechnol. 2006, 9, 26–39. [Google Scholar] [CrossRef]
- De Jong, W.; Marcotullio, G. Overview of biorefineries based on co-production of furfural, existing concepts and novel developments. Int. J. Chem. React. Eng. 2010, 8. [Google Scholar] [CrossRef]
- Jaswal, A.; Singh, P.P.; Mondal, T. Furfural—A versatile, biomass-derived platform chemical for the production of renewable chemicals. Green Chem. 2022, 24, 510–551. [Google Scholar] [CrossRef]
- Cai, C.M.; Zhang, T.; Kumar, R.; Wyman, C.E. Integrated furfural production as a renewable fuel and chemical platform from lignocellulosic biomass. J. Chem. Technol. Biotechnol. 2014, 89, 2–10. [Google Scholar] [CrossRef]
- Mamman, A.S.; Lee, J.-M.; Kim, Y.-C.; Hwang, I.T.; Park, N.-J.; Hwang, Y.K.; Chang, J.-S.; Hwang, J.-S. Furfural: Hemicellulose/xylosederived biochemical. Biofuels Bioprod. Biorefin. 2008, 2, 438–454. [Google Scholar] [CrossRef]
- Takkellapati, S.; Li, T.; Gonzalez, M.A. An overview of biorefinery-derived platform chemicals from a cellulose and hemicellulose biorefinery. Clean Technol. Environ. Policy 2018, 20, 1615–1630. [Google Scholar] [CrossRef] [PubMed]
- Reshmy, R.; Athiyaman Balakumaran, P.; Divakar, K.; Philip, E.; Madhavan, A.; Pugazhendhi, A.; Sirohi, R.; Binod, P.; Kumar Awasthi, M.; Sindhu, R. Microbial valorization of lignin: Prospects and challenges. Bioresour. Technol. 2022, 344, 126240. [Google Scholar] [CrossRef]
- Borges, E.R.; Pereira, N. Succinic acid production from sugarcane bagasse hemicellulose hydrolysate by Actinobacillus succinogenes. J. Ind. Microbiol. Biotechnol. 2011, 38, 1001–1011. [Google Scholar] [CrossRef]
- Zeikus, J.G.; Jain, M.K.; Elankovan, P. Biotechnology of succinic acid production and markets for derived industrial products. Appl. Microbiol. Biotechnol. 1999, 51, 545–552. [Google Scholar] [CrossRef]
- Dessie, W.; Luo, X.; Duns, G.J.; Wang, M.; Qin, Z. Towards the development of efficient, economic and environmentally friendly downstream processing for bio-based succinic acid. Environ. Technol. Innov. 2023, 32, 103243. [Google Scholar] [CrossRef]
- Tang, Y.; Ju, X.; Chen, X.; Li, L. Advances in the biological production of sugar alcohols from biomass-derived xylose. World J. Microbiol. Biotechnol. 2025, 41, 110. [Google Scholar] [CrossRef] [PubMed]
- Ndaba, B.; Rama, H.; Bingwa, N.; Roopnarain, A. Unravelling the role of nanoparticles during bioethanol production: A review on pretreatment, hydrolysis and fermentation. Fuel 2025, 396, 135336. [Google Scholar] [CrossRef]
- Roopnarain, A.; Adeleke, R. Current status, hurdles and future prospects of biogas digestion technology in Africa. Ren. Sustain. Energy Rev. 2017, 67, 1162–1179. [Google Scholar] [CrossRef]
- Baloyi, R.B.; Gbadeyan, O.J.; Sithole, B.; Chunilall, V. Recent advances in recycling technologies for waste textile fabrics: A review. Text. Res. J. 2024, 94, 508–529. [Google Scholar] [CrossRef]
- O’Brien, F.; Ramchuran, S.; Chunilall, V. Assessing the succinic acid production capabilities of Corynebacterium glutamicum using industrial feedstocks. J. Chem. Technol. Biotechnol. 2025, 100, 1238–1243. [Google Scholar] [CrossRef]
- Brobbery, M.S.; Louw, J.; Görgens, J.F. A techno-economic and life cycle assessment of multiproduct sugarcane biorefinery: Lactic acid as a platform chemical. Biomass Bioenergy 2025, 193, 107605. [Google Scholar]
- van Dyk, J.; Görgens, J.F.; van Rensburg, E. Valorisation of Industrial Waste for Second-Generation Ethanol Production: Towards the Scale-Up of High-Solids Paper Sludge Fermentation. Waste Biomass Valorization 2025, in press. [Google Scholar] [CrossRef]
- Tshemese, Z.; Deenadayalu, N.; Linganiso, L.Z.; Chetty, M. An Overview of Biogas Production from Anaerobic Digestion and the Possibility of Using Sugarcane Wastewater and Municipal Solid Waste in a South African Context. Appl. Syst. Innov. 2023, 6, 13. [Google Scholar] [CrossRef]
- Bishop, B.D.C.; Sanusi, I.A.; Kana, G.E.B. Enhanced substrate suitability of autoclave-assisted acid pre-treated waste sugarcane molasses: Pre-treatment optimization, sequential nano-based detoxification strategies, and bioproduct production. Biomass Convers. Biorefinery 2025, 15, 12175–12188. [Google Scholar] [CrossRef]
- Ngerem, E.C.; Sanusi, I.A.; Kana, G.E.B.; Olaniran, A.O. Optimization of co-valorisation techniques for dairy and paper pulp wastewater in the cultivation of Chlorococcum sp. with a focus on mixture design, microwave-assisted pretreatment, and bioethanol production. Heliyon 2025, 11, e42531. [Google Scholar] [CrossRef]
- Rashama, C.; Ijoma, G.N.; Matambo, T.S. The effects of phytochemicals on methanogenesis: Insights from ruminant digestion and implications for industrial biogas digesters management. Phytochem. Rev. 2021, 20, 1245–1271. [Google Scholar] [CrossRef]
- Marx, S.; Laubscher, A.N.E.; Bunt, J.R.; Venter, R.J.; Uwaoma, R.C.; Strydom, C.A. Evaluation of sugar cane bagasse hydrothermal liquefaction products for co-gasification with coal as green coal pellet production. Bioresour. Technol. Rep. 2023, 22, 101503. [Google Scholar] [CrossRef]
- van Coller, R.H.; Marx, S. Techno-economic baseline study for a waste-based biorefinery in South Africa. Bioresour. Technol. Repot. 2022, 20, 101280. [Google Scholar] [CrossRef]
- Ansari, F.A.; Hassan, H.; Mahmud, U.; Rawat, I.; Bux, F. Cultivation of Arthrospira platensis in non-conventional water resources: A comparative analysis of biomass productivity and chemical constituents. Biomass Bioenergy 2025, 202, 108163. [Google Scholar] [CrossRef]
- Ramanna, L.; Ansari, F.A.; Rawat, I.; Bux, F. Microalgae-driven carbon sequestration and bio-fertiliser: Steps towards a sustainable future. Chem. Eng. J. 2025, 519, 164892. [Google Scholar] [CrossRef]
- Boonzaier, J.J.; Hart, R.S.; Hoff, J.W.; du Plessis, H.W.; den Haan, R. Characterization of Zalaria obscura Y1223 hydrolases: Implications for lignocellulose conversion. AMB Express 2025, 15, 109. [Google Scholar] [CrossRef]
- Fortuin, J.; Hoffmeester, L.J.; Minnaar, L.S.; den Haan, R. Advancing cellulose utilization and engineering consolidated bioprocessing yeasts: Current state and perspectives. Appl. Microbiol. Biotechnol. 2025, 109, 43. [Google Scholar] [CrossRef]
- Watson, E. Available online: https://agfundernews.com/mycosure-seeks-to-put-south-africa-on-the-alt-protein-map-with-biomass-fermentation-platform (accessed on 29 July 2025).
- Surroop, D.; Bundhoo, Z.M.A.; Raghoo, P. Waste to energy through biogas to improve energy security and to transform Africa’s energy landscape. Curr. Opin. Green Sustain. Chem. 2019, 18, 79–83. [Google Scholar] [CrossRef]
- Ramlucken, U.; Ramchuran, S.O.; Moonsamy, G.; van Rensburg, C.J.; Thantsha, M.S.; Lalloo, R. Production and stability of a multi-strain Bacillus based probiotic product for commercial use in poultry. Biotechnol. Rep. 2021, 29, e00575. [Google Scholar] [CrossRef]
- Molefe, M.; Nkazi, D.; Mukaya, H.E. Method Selection for Biojet and Biogasoline Fuel Production from Castor Oil: A Review. Energy Fuels 2019, 33, 5918–5932. [Google Scholar] [CrossRef]
- Harrisberg, K. Biochar Boom? South Africa Bets on Super Charcoal for Green Jobs. Available online: https://www.timeslive.co.za/sunday-times/business/business/2025-07-08-biochar-boom-south-africa-bets-on-super-charcoal-for-green-jobs/ (accessed on 29 July 2025).
- Fajimi, L.I.; Chrisostomou, J.; Oboirien, B.O. A techno-economic analysis (TEA) of a combined process of torrefaction and gasification of lignocellulose biomass (bagasse) for methanol and electricity production. Biomass Convers. Biorefinery 2024, 14, 12501–12516. [Google Scholar] [CrossRef]
- Petersen, A.M.; Aneke, M.C.; Görgens, J.F. Techno-economic comparison of ethanol and electricity coproduction schemes from sugarcane residues at existing sugar mills in Southern Africa. Biotechnol. Biofuels 2014, 7, 105. [Google Scholar] [CrossRef]
- Nieder-Heitmann, M.; Haigh, K.F.; Görgens, J.F. Life cycle assessment and multi-criteria analysis of sugarcane biorefinery scenarios: Finding a sustainable solution for the South African sugar industry. J. Clean. Prod. 2019, 239, 118039. [Google Scholar] [CrossRef]
- Louw, J.; Dogbe, E.G.; Yang, B.; Görgens, J.F. Prioritisation of biomass-derived products for biorefineries based on economic feasibility: A review on the comparability of techno-economic assessment results. Renew. Sustain. Energy Rev. 2023, 188, 113840. [Google Scholar] [CrossRef]

| University/Company Name | Sector | Products | Publications |
|---|---|---|---|
| Agricultural Research Council | Science council | Biofuels | [94,95] |
| Biorefinery Industry Development Facility—Council for Scientific and Industrial Research | Science council | Biofuels and platform chemicals | [96,97] |
| Stellenbosch University | Academia | Platform chemicals—lactic acid, succinic acid, furfural, citric acid | [98,99] |
| Cape Peninsula University of Technology | Academia | Biofuels | [100] |
| University of KwaZulu-Natal | Academia | Biofuels and platform chemicals | [101,102] |
| University of South Africa | Academia | Biofuels | [103] |
| North-West University | Academia | Biofuels, biochar, hydrochar | [104,105] |
| Durban University of Technology | Academia | Biobased products | [106,107] |
| University of the Western Cape | Academia | Biobased products | [108,109] |
| Ziziba | SMME | Protein | N/A |
| MycoSure | SMME | Protein | [110] |
| Bio2Watt | Company | Biogas | [111] |
| OptimusBío | Company | Fertilizers, skincare products, platform chemicals | [112] |
| Selokong Sa Dimelana | SMME | Castor oil, biodiesel | [113] |
| Adsorb Technologies | SMME | Biochar, activated carbon | [114] |
| Energy Phambili | Company | Biochar, activated carbon, bio-oil, wood vinegar | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nhleko, L.; Sekoai, P.T. Sugarcane Bagasse: A Sustainable Feedstock for Biorefinery Portfolios in South Africa. Fermentation 2025, 11, 489. https://doi.org/10.3390/fermentation11090489
Nhleko L, Sekoai PT. Sugarcane Bagasse: A Sustainable Feedstock for Biorefinery Portfolios in South Africa. Fermentation. 2025; 11(9):489. https://doi.org/10.3390/fermentation11090489
Chicago/Turabian StyleNhleko, Lindile, and Patrick T. Sekoai. 2025. "Sugarcane Bagasse: A Sustainable Feedstock for Biorefinery Portfolios in South Africa" Fermentation 11, no. 9: 489. https://doi.org/10.3390/fermentation11090489
APA StyleNhleko, L., & Sekoai, P. T. (2025). Sugarcane Bagasse: A Sustainable Feedstock for Biorefinery Portfolios in South Africa. Fermentation, 11(9), 489. https://doi.org/10.3390/fermentation11090489







