Abstract
Bioethanol manifests an extraordinary potential to overcome the severe energy crises and reliance on fossil fuels, yet it supports the sustainable and cost-effective production of fuels for automobile engines and contributes to the reduction of greenhouse gas (GHG) emissions and other global climate-related challenges. The present study examines the potential of Mixed Lignocellulosic Biomass (MLB) as a sustainable feedstock for the consistent year-round production of bioethanol. The primary MLB sources considered in this research to underscore the significance of this heterogeneous strategy include sweet sorghum bagasse (SSB), sugarcane bagasse (SCB), and date palm trunk (DPT). Each of the three feedstocks, i.e., SSB, SCB, and DPT, were individually subjected to alkaline pretreatment, a step aimed at breaking down structural barriers and facilitating greater release of fermentable sugars during fermentation. Likewise, the alkaline-pretreated biomasses were subjected to simultaneous saccharification and fermentation (SSF) for 96 h, both individually as well as in various combined proportions. Individually, pretreated sweet sorghum bagasse (SSB) fibers produced the highest ethanol concentration, of 30.79 ± 0.44 g/L; an ethanol yield of 0.40 ± 0.62 g/g; an ethanol productivity of 0.42 ± 0.87 g/L/h; and a theoretical ethanol yield of 79.81% at 72 h. In contrast, the combination of MLB (50% of pretreated SSB and 50% of DPT fibers) produced a significantly higher ethanol concentration of 31.47 ± 0.57 g/L and an ethanol productivity of 0.653 ± 0.24 g/L/h in much less time, i.e., 48 h of SSF fermentation. The empirical data confirms that MLB offers a sustainable paradigm for ethanol biosynthesis by curtailing fermentation time and optimizing economic and operational efficacy.
1. Introduction
The growing global demand for eco-friendly energy alternatives, driven by factors such as population growth, industrial expansion, and shifting geopolitical dynamics, is accelerating the immediate transition to a sustainable, bio-based economy. This shift is not only critical for mitigating pressing environmental crises such as intensifying greenhouse gas emissions (GHG), plastic pollution, and the depletion of crude oil resources, but also for reshaping industries toward a more resilient and sustainable future [1,2]. At the same time, the rapid expansion of biofuel production is fostering the development of a diversified and sustainable economy, with the potential to generate long-term benefits across social, economic, and environmental dimensions.
Second-generation (2G) lignocellulosic biomass (LCB) is an abundant biomass source, with a global yield of 13 billion tons per year, making it an exceptionally significant source for biofuels and chemical production [3]. Despite various kinds of LCB, the maximum production of bioethanol relies on its availability and abundance within a specific area. LCB yields high-value-added products, including biofuels, biogas, bio-polymers, and construction materials [4], consisting of interwoven cellulose (30–60%), hemicellulose (20–40%), and lignin (15–25%) polymers to create a robust, dense, and resilient structure [5].
The successful conversion of LCB to bioethanol requires consistency, viability, and reasonably priced access to a biorefinery. The mixed lignocellulosic biomass (MLB) approach is introduced to address potential challenges and enhance the feasibility and profitability of biomass conversion. Data on the use of MLB for bioethanol production is limited owing to its ongoing underdeveloped research applications, though mixed feedstocks have previously been explored for the generation of biohydrogen, biogas, microbial enzymes, energy, refrigeration, and heating applications. Nevertheless, analyses conducted over the past five years clearly highlight the growing significance of this approach to overcoming feedstock availability and biorefinery logistical challenges [6,7].
Pretreatment techniques are essential for breaking down lignocellulosic biomass (LCB), separating recalcitrant lignin from cellulose and hemicellulose, and enabling efficient conversion of sugars into ethanol for eco-friendly biofuel production [8]. Due to the dissimilarity of LCB feedstocks in their composition and structure, no single pretreatment method is broadly applicable. Various techniques, including mechanical, chemical, physicochemical, and biological methods, aim to improve polymer accessibility and breakdown [9]. Following pretreatment, enzymatic hydrolysis depolymerizes cellulose and hemicellulose into fermentable sugars through enzyme-mediated degradation, although acids or alkalis can also be employed as alternatives [10]. Enzymes for this procedure can be obtained either from microorganisms or commercially (i.e., Cellic® CTec2 and Cellic® HTec2) [11]. For processes like saccharification and fermentation, either simultaneous saccharification and fermentation (SSF) or separate hydrolysis and fermentation (SHF) is followed to convert the glucose into the desired products [12,13].
Prior studies on biomass primarily focused on single-source feedstocks overlook the advantages of combining multiple substrates to enhance biofuel production, which is now crucial to reassessing the technology [14]. Mixed lignocellulosic biomass (MLB) refers to the use of multiple feedstocks, in either equal or varying proportions, which may have similar or different characteristics and often require separate treatments. Compared to the use of a single biomass, MLB offers greater advantages for ethanol production [15].
The primary intention of utilizing the mixed lignocellulosic biomass approach is ensuring continuous availability of feedstock during production while facilitating easier harvesting, efficient collection, convenient transportation, and effective storage [16]. Moreover, such approaches facilitate the uninterrupted and continuous operation of biorefineries, enhancing their reliability by reducing upstream logistical challenges and associated costs, including the seasonal dependency on a single feedstock [15,17,18]. Consequently, the use of MLB can expand production capacity and strengthen the economic resilience of bio-refineries at a large scale [19].
The advantage of using mixed lignocellulosic biomasses as substrates for bioethanol production creates an adequate balance of the ratio of carbon and nitrogen, which are compulsory to add in the fermentation process as micro and macronutrients [10]. Furthermore, the combination of MLB produces fewer inhibitors in the hydrolysate and increases ethanol production [20]. In addition to increasing enzymatic hydrolysis, such combinations may also reduce overall ethanol fermentation time [21]. A similar observation was reported by Ref. [22], who managed to produce a 34% higher ethanol production within the initial 24 h of fermentation using waste copier paper and wheat straw.
This study explored the potential of three distinct lignocellulosic biomasses, sweet sorghum bagasse (SSB), sugarcane bagasse (SCB), and date palm trunk (DPT), for the production of bioethanol, assessing their performance both individually and in four defined combinatorial mixtures. The selection of these feedstocks is strategic, as they represent regionally abundant yet underutilized agricultural residues, thereby contributing to the mitigation of the food-versus-fuel dilemma and strengthening the sustainability of biofuel production pathways. Each biomass, exhibiting unique structural recalcitrance due to varying proportions of cellulose, hemicellulose, and lignin, was subjected to tailored pretreatment strategies: autoclave-assisted alkaline pretreatment for SSB, water bath-based alkaline pretreatment for SCB, and acid-assisted thermo-chemical pretreatment for DPT. The central objective of this research is to evaluate the synergistic effects of blending these complementary feedstocks on hydrolysis efficiency and fermentable sugar release. It was hypothesized that such combinatorial approaches will not only improve process efficiency and sugar yields by exploiting compositional complementarities but also minimize inhibitor formation and reduce both material and operational costs. Ultimately, this study aimed to establish a more robust, economically viable, and sustainable pathway for large-scale bioethanol production.
2. Materials and Methods
2.1. Biomass Preparation
The raw materials of SSB, SCB, and DPT were sourced from local agricultural farms located in the Quetta, Sibi, and Khairpur provinces of Balochistan and Sindh, Pakistan. All the lignocellulosic biomasses were dried under sunlight for 4–7 days to reduce their moisture content to below 10%. All the samples were then separately chopped into smaller sizes (20 × 20 × 5 mm3) using garden cutters or scissors for further processing.
2.2. Pretreatment of Each Biomass
2.2.1. Sugarcane Bagasse
The dried raw material of SCB was pretreated using the optimized pretreatment conditions of Ref. [23], which involved soaking the SCB fibers in a 3% NaOH solution at a ratio of 1:10 g/mL solid to liquid in a 1000 mL beaker and heating in a water bath at 50 °C for 4 h. Then, the combined pulp slurry was collected and squeezed to remove the hydrolysate. Subsequently, the fibers were washed with tap water until a neutral pH was achieved and dried in a hot-air oven for 24 h.
2.2.2. Date Palm Trunk
A dry sample of 150 g oil palm trunk chips was placed in a 2.5 L tank for steam explosion at 210 °C for 4 min, as per the pretreatment condition optimized by Ref. [24]. Afterwards, the combined pulp slurry was collected and squeezed to obtain the steam-exploded fibers and stored at −20 °C in the refrigerator. Thereafter, the steam-exploded fibers were treated with hot-water washing for 30 min at 80 °C with a solid:liquid ratio of 1:8 g/L for hemicellulose removal. The steam-exploded date palm trunk fibers were treated with alkaline pretreatment at 90 °C for 50 min with 15% NaOH solution [25]. After alkaline pretreatment, the combined pulp slurry was squeezed for delignification and washed util the pH was neutralized.
2.2.3. Sweet Sorghum Bagasse (SSB)
The pretreatment of SSB was carried out using the protocol described by Ref. [26]. The process involves soaking SSB in a 2% w/v NaOH solution at a solid-to-liquid ratio of 1:9 g/mL for 24 h. The resulting slurry was then transferred to a 100 mL Griffin’s glass beaker and autoclaved at 121 °C and 15 PSI for 60 min. Following autoclave, the samples were thoroughly washed with tap water until the pH was neutralized and subsequently dried at 55 °C for 24 h.
2.3. Chemical Composition Analysis
The chemical composition analysis of the raw material and pretreated fibers of the mixed lignocellulosic biomasses (SSB, DPT, SCB) was determined by following the protocol guidelines provided by the National Renewable Energy Laboratory (NREL) [27], i.e., lignin content: TAPPI T222 om-88, extractive substances: TAPPI T204 om-97, alpha-cellulose: TAPPI T203 om-88, and ash content: TAPPI T211 om-93.
2.4. Enzyme Hydrolysis of Raw and Pretreated Fibers
Enzymatic hydrolysis was conducted on both the raw materials and the pretreated fibers of SSB, DPT, and SCB separately to determine the concentration of fermentable sugars in each biomass. To achieve this, hydrolysis was performed with 10% (w/v) fibers at 50 °C and 150 rpm in a shaker incubator for 96 h. The 10% substrate on dry weight basis was placed separately in Erlenmeyer flasks (500 mL) using 270 mL sodium citrate (0.05 M, pH 4.8), and enzyme Cellic® CTec2 (15 FPU/g substrate). In addition, the enzyme activity was evaluated by using the NREL protocol LAP-006 for measurement of cellulase activity through filter paper; the cellulase activity of Cellic® CTec2 was 121.5 FPU [13].
2.5. Inoculum Preparation
The thermo-tolerant strain of the S. cerevisiae SC90 single marginal colony was cultured in YPD (yeast, peptone, dextrose) broth at 30 °C for 48 h, followed by colony growth on YPD agarose at 30 °C. In the final stage, a single colony was transferred to YPD broth and incubated in a shaker incubator at 30 °C with 150 rpm [13].
2.6. Simultaneous Saccharification and Fermentation for Ethanol Production Using Separate and Mixed Lignocellulosic Biomass
The pretreated fibers of SCB, SS, and DPT were fermented (separately) using the SSF method in 500 mL Erlenmeyer flasks with a 300 mL working volume, containing 10% dry weight of pretreated fibers, yeast–peptone (YP) media, 10% starter culture, and 15 FPU/g of Cellic® CTec2. This media was incubated in a shaker at 40 °C with 150 rpm for 96 h. Similarly, the SSF method was applied to four different combinations of MLB biomasses in 500 mL Erlenmeyer flasks with a 300 mL working volume, using 10% (w/v) fiber mixtures. These combinations included:
- SSB + SCB + DPT (3.333% each)
- SSB + SCB (50% each)
- SSB + DPT (50% each)
- SCB + DPT (50% each)
Each combination of MLB was placed in a separate YP media flask containing 10% starter culture and 15 FPU/g of Cellic® CTec2, and incubated for 96 h at 40 °C with 150 rpm. The fermentation samples were collected in triplicate at 0, 24, 48, 72, and 96 h [13].
2.7. Analytical Methods
To study the concentrations of cellobiose, glucose, and ethanol, HPLC (Agilent Technologies, Berlin, Germany) was used in conjunction with a Bio-Rad Aminex HPX-87P column (Bio-Rad®, Hercules, CA, USA), with a flow rate of around 0.6 mL per minute. For column preservation, 50 mM H2SO4 as mobile phase (50 °C) was used [28].
2.8. Statistical Analysis
The Duncan’s new multiple range test through SPSS (23 version, IBM, Armonk, NY, USA) with a significance level of 95% (p < 0.05) was followed to evaluate the statistically significant differences between ethanol concentration (g/L), ethanol yield (g/g), ethanol productivity (g/L/h), and theoretical yield (%).
3. Results
3.1. Chemical Compositional Analysis of Sweet Sorghum Bagasse, Sugarcane Bagasse, and Date Palm Trunk
The chemical compositional analysis before and after pretreatment of all three biomasses were analyzed by following different TAPPI methods. Additionally, a significant change in the content of different constitutes, such as cellulose, hemicellulose, lignin, and ash, were observed.
Prior to pretreatment, the chemical composition of SSB was 11.49% ± 0.53 extractive substances, 11.70% ± 0.31 hemicellulose, 47.11% ± 0.59% cellulose, 14.02% ± 0.21 lignin, and 7.96% ± 0.46 ash on a dry-weight basis (Table 1). However, owing to alkaline pretreatment with autoclave, the cellulose content in pretreated SSB significantly increased to 68.60% ± 0.80. Moreover, a significant decrease was observed in the content of lignin, i.e., to 55.56%; hemicellulose, i.e., to 18.11%, and ash, i.e., to 51.00%. These results were found to be similar to the findings of Ref. [26].

Table 1.
Chemical compositional analysis of different biomasses before and after pretreatment.
In SCB, the content of raw cellulose material increased significantly, from 47.11% ± 0.59 to 66.09% ± 0.29, after pretreatment. In contrast, a decrease in hemicellulose content from 31.22% ± 0.92 to 13.70%, in lignin content from 18.39% ± 0.53 to 7.31% ± 0.33, and in ash content from 2.80% ± 0.31 to 1.19% ± 0.08 was found. The results of lignin and cellulose were observed to be similar to the findings of Ref. [23].
The content of raw cellulose material in pretreated DPT increased to 73.96% ± 0.43 from 39.73% ± 0.64, which was around 46.28%. Likewise, hemicellulose content decreased from 22.86% ± 0.67% to 9.35% ± 1.01%, lignin content decreased considerably from 23.64% ± 0.29 to 6.67% ± 0.18, and, similarly, ash content dropped from 1.46% ± 0.11 to 0.80% ± 0.05. These results clearly demonstrate the effectiveness of the applied pretreatments in disrupting the recalcitrant structures and delignification, thereby ensuring maximum sugar availability to the fermentation strains during the bioethanol production process [29].
3.2. Enzyme Hydrolysis of Raw Material and Pretreated Biomasses
Enzymatic hydrolysis of both raw and pretreated SSB was performed at 50 °C/150 rpm for 96 h using an enzyme concentration of 15 FPU/g of biomass. After 96 h of enzyme hydrolysis, the raw SSB yielded a glucose concentration of 23.78 ± 2.60 g/L (Figure 1a), with no significant change observed in the texture of the biomass before and after hydrolysis. In contrast, the pretreated SSB fibers produced a substantially higher glucose concentration of 52.97 ± 0.80 g/L (Figure 1b), demonstrating the effectiveness of pretreatment in enhancing sugar release.
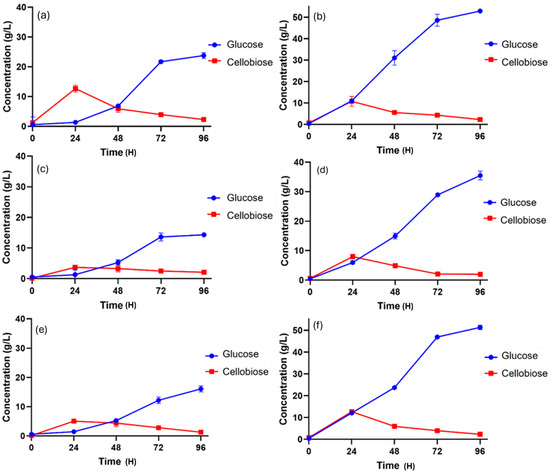
Figure 1.
Enzymatic hydrolysis of lignocellulosic biomasses with 10% fibers (w/v) at 50 °C. (a) Raw material of SSB; (b) pretreated SSB; (c) raw material of SCB; (d) pretreated SCB; (e) raw material of DPT; (f) pretreated DPT.
Figure 1c illustrates the enzymatic hydrolysis of raw SCB, which was carried out at 50 °C for 96 h using an enzyme concentration of 15 FPU/g of biomass. Under these conditions, the raw SCB yielded a maximum glucose concentration of 14.30 ± 0.54 g/L, reflecting the limited accessibility of fermentable sugars in untreated fibers. In comparison, when the pretreated SCB fibers were subjected to hydrolysis under identical experimental conditions, a markedly higher glucose concentration of 35.47 ± 1.34 g/L was obtained (Figure 1d). This significant improvement clearly demonstrates the efficiency of pretreatment in enhancing cellulose accessibility and facilitating enzymatic degradation, thereby resulting in greater sugar release.
On the other hand, Figure 1e,f presents the results of enzymatic hydrolysis for raw and alkaline-pretreated fibers of DPT. The hydrolysis was conducted at 50 °C for 96 h, with an enzyme concentration of 15 FPU/g of biomass. Under these conditions, the raw DPT yielded a maximum glucose concentration of 18.08 ± 1.11 g/L, reflecting the limited enzymatic accessibility caused by its rigid lignin-rich structure. In contrast, the alkaline-pretreated DPT fibers exhibited a substantial improvement, producing 53.30 ± 0.38 g/L of glucose after 96 h. This significant increase highlights the critical role of pretreatment in disrupting the recalcitrant lignin barrier, thus enhancing enzyme penetration and ensuring more efficient cellulose hydrolysis.
3.3. Simultaneous Saccharification and Fermentation of Sweet Sorghum Bagasse, Sugarcane Bagasse, and Date Palm Trunk Separately
3.3.1. Simultaneous Saccharification and Fermentation of Pretreated Sweet Sorghum Bagasse for Ethanol Production
During SSB-based SSF, ethanol production was negligible at 0 h but steadily increased over time due to the hydrolysis of cellulose by Cellic® CTec2, reaching its maximum concentration of 30.79 ± 0.44 g/L at 72 h (Figure 2a). Following this peak, ethanol levels began to decline. In contrast, glucose concentration reached its highest value of 14.82 ± 0.47 g/L at 24 h, coinciding with the peak cellobiose concentration of 0.50 ± 0.02 g/L. The accumulation of glucose at this stage resulted from the rapid enzymatic hydrolysis of cellobiose, demonstrating the efficiency of the SSF process in converting intermediates into fermentable sugars for ethanol production. The findings demonstrate that the SSF process is an efficient approach for converting sugarcane bagasse into bioethanol, achieved through the combined effect of enzymatic hydrolysis and fermentation.
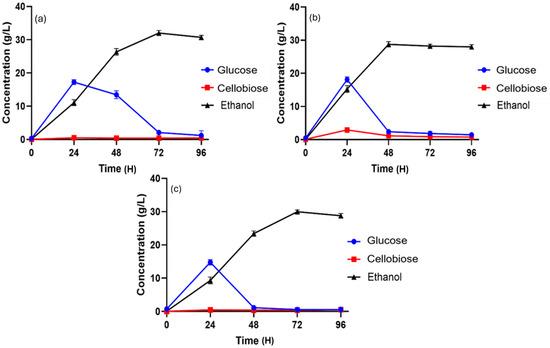
Figure 2.
Ethanol production during batch SSF at 10% (w/v) substrate loading using different lignocellulosic biomasses. (a) Pretreated SSB; (b) pretreated SCB; (c) pretreated DPT.
3.3.2. Simultaneous Saccharification and Fermentation of Pretreated Sugarcane Bagasse for Ethanol Production
In the SSF of sugarcane bagasse, ethanol concentration was initially negligible but gradually increased, attaining a maximum value of 28.20 ± 0.49 g/L at 48 h (Figure 2b), after which it began to decline. Glucose concentration showed a temporary rise during the early phase, corresponding with the highest cellobiose level observed at 24 h. This glucose accumulation resulted from the enzymatic breakdown of cellobiose by Cellic® CTec2, followed by its consumption as S. cerevisiae converted it into ethanol. Overall, the results confirm the effectiveness of the SSF process in transforming sugarcane bagasse into bioethanol through the synergistic action of enzymatic hydrolysis and fermentation.
3.3.3. Simultaneous Saccharification and Fermentation of Pretreated Date Palm Trunk for Ethanol Production
Ethanol production during the SSF of DPT was carried out at 40 °C. Glucose concentration initially increased, reaching its peak of 16.71 ± 0.48 g/L at 18 h. The buildup of glucose was attributed to the enzymatic hydrolysis of cellobiose mediated by Cellic® CTec2. Following this, glucose levels began to decline as it was progressively converted into ethanol. Ethanol production steadily increased throughout the process, achieving a maximum concentration of 29.98 ± 0.31 g/L at 72 h (Figure 2c).
3.4. Simultaneous Saccharification and Fermentation of Mixed Lignocellulosic Biomass
The SSF of the mixed feedstock showed outstanding results compared to individual results of single feedstocks. Rapid increases in ethanol production were observed when a mixed feedstock approach was carried out.
3.4.1. Simultaneous Saccharification and Fermentation of Mixed Lignocellulosic Biomass (Pretreated Sweet Sorghum Bagasse + Sugarcane Bagasse + Date Palm Trunk) for Ethanol Production
When the pretreated combination of 33.34% of SSB fibers, 33.33% of SCB, and 33.33% of DPT (completes the 10% ration) was fermented, the maximum ethanol production was obtained at 72 h, which was 27.87 ± 0.11 g/L (Figure 3a). However, higher glucose production was observed in the initial 24 h, and then a sudden decline was observed. This result shows that the mixed biomasses facilitated each other during fermentation and provided max ethanol productivity.
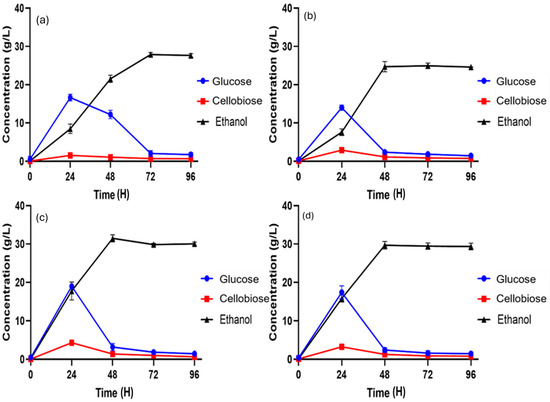
Figure 3.
Ethanol production during batch SSF at 10% (w/v) substrate loading using different percentages of mixed lignocellulosic biomasses. (a) Pretreated SCB (33.34%), S.S (33.33%), and DPT (33.33%); (b) pretreated SCB (50%) and SSB (50%); (c) pretreated SSB (50%) and DPT (50%); (d) pretreated SCB (50%) and DPT (50%).
3.4.2. Simultaneous Saccharification and Fermentation of Mixed Lignocellulosic Biomass (Pretreated Sugarcane Bagasse Fibers and Sweet Sorghum Bagasse Fibers) for Ethanol Production
In the 50% SCB fiber and 50% SSB fiber combination, ethanol production was monitored over a 96 h fermentation period. At 24 h, a sharp increase in cellobiose breakdown was observed, leading to a higher glucose concentration of 14.01 ± 0.31 g/L. By 72 h, ethanol production reached its peak at 24.96 ± 0.70 g/L, after which a gradual decline was recorded (Figure 3b). However, this combination produced one of the lowest ethanol yields compared to the other biomass mixtures.
3.4.3. Simultaneous Saccharification and Fermentation of Mixed Lignocellulosic Biomass (Sweet Sorghum Bagasse Fibers and Date Palm Trunk Fibers) for Ethanol Production
In the 50% SSB fiber and 50% DPT fiber mixture, maximum ethanol production was achieved at 48 h, reaching 31.47 ± 0.57 g/L (Figure 3c). During the initial phase, a steady conversion of cellobiose to glucose was observed, peaking at 24 h, after which glucose was further fermented into ethanol until its maximum level was reached. These findings highlight that mixing feedstocks based on availability can reduce the sourcing radius as well as the need for extensive transportation and storage. In addition, such mixtures can be designed to minimize variations in composition, create synergies, and target specific physicochemical properties that enhance the overall conversion process. Moreover, this combination yields maximum ethanol at fewer hours of fermentation, which might be because mixed biomasses reduce sugar degradation, produce more sugar yield, and provide high sugar recovery during enzymatic saccharification than in individual ones [30].
3.4.4. Simultaneous Saccharification and Fermentation of Mixed Lignocellulosic Biomass of (Sugarcane Bagasse Fibers and Date Palm Trunk Fibers) for Ethanol Production
In the 50% DPT fiber and 50% SCB fiber mixture, higher glucose consumption was observed at 24 h, reaching 3.23 ± 0.48 g/L. The maximum conversion of glucose to ethanol occurred at 48 h, producing 29.68 ± 0.36 g/L, followed by a sudden decline in ethanol concentration (Figure 3d). This result ranked as the second highest among the tested biomass combinations, further supporting the potential of the MLB approach as a promising strategy for ethanol production because when mixtures are fermented collectively, the lag phase ultimately decreases as fewer inhibitors are produced in the mixed substrates [20].
Ultimately, these results demonstrate a clear synergistic effect, where certain combinations outperformed individual substrates. Individually, SSB achieved the highest ethanol concentration of 30.79 ± 0.44 g/L at 72 h (Figure 2a), followed closely by DPT at 29.98 ± 0.31 g/L at 72 h (Figure 2c) and SCB at 28.20 ± 0.49 g/L at 48 h (Figure 2b). Significantly, among the biomass combinations, the SSB and DPT mixture (1:1 ratio) yielded the highest ethanol production of 31.47 ± 0.57 g/L at 48 h (Figure 3c), surpassing all individual fermentations and other mixed substrates. This finding underscores the potential of strategic biomass blending to enhance hydrolysis efficiency and fermentation kinetics, ultimately leading to higher bioethanol titers and improved process economics. Additionally, improving the economics of ethanol production depends on optimizing key fermentation parameters such as ethanol concentration, ethanol yield, ethanol productivity, and theoretical efficacy. Increasing ethanol concentration from 50 g/L to above 100 g/L will significantly reduce downstream distillation costs by lowering the energy required for separation, possibly decreasing the processing costs by up to 30–40% [31]. Achieving yields close to the theoretical maximum of 0.51 g ethanol g/sugar (apparently 0.45 g/g in many processes) improves feedstock utilization and lowers raw material costs, which can conserve up to 50% of total production expenses [32]. Moreover, enhancing ethanol productivity (e.g., from <1.5 g/L/h to ≥2 g/L/h) reduces fermentation time and capital costs by enabling smaller reactors. These improvements can collectively reduce the total ethanol production costs from as high as USD 0.80/L to below USD 0.35/L, making bioethanol more competitive with fossil fuels and less dependent on subsidies [31].
3.5. Fermentation Kinetics
Fermentation kinetics refers to the rate and efficiency of the fermentation process. In this study, the parameters analyzed included maximum ethanol concentration (g/L), ethanol yield (g/g), ethanol productivity (g/L/h), and theoretical ethanol yield (%), following the protocol of NREL (2008). Ethanol concentration indicates the amount of ethanol produced per liter of fermentation broth, ethanol yield represents the ethanol obtained per gram of biomass, ethanol productivity reflects the rate of ethanol formation per hour, and theoretical yield denotes the overall efficiency of the fermentation process, expressed as a percentage (Table 2).

Table 2.
Fermentation kinetics of ethanol production using MLB fiber following SSF.
4. Discussion
Compared to mixed biomasses, the use of single biomass sources resulted in lower concentrations and yields of bioethanol, even after longer fermentation periods. Specifically, SSB and DPT produced 30.79 ± 0.44 g/L and 29.98 ± 0.31 g/L of ethanol, respectively, at 72 h of fermentation, whereas SCB produced 28.20 ± 0.49 g/L at 48 h, followed by a rapid decline. In contrast, the mixed biomass combinations yielded comparatively better results. For instance, a 50% SS + 50% DPT mixture produced 31.47 ± 0.57 g/L of ethanol, while a 50% SCB + 50% DPT mixture yielded 29.68 ± 0.36 g/L within 48 h of fermentation. However, the combination of all three biomasses (33.34% SS, 33.33% SCB, and 33.33% DPT) resulted in 27.87 ± 0.11 g/L at 72 h, and the 50% SS + 50% SCB mixture produced 24.96 ± 0.70 g/L over the same duration. In cases such as these, the mixture of different substrates creates an adequate balance of carbon–nitrogen ratio that is suitable for the fermentation process [15].
Interestingly, the combination of SS and DPT outperformed the commonly studied mixture of three biomasses and yielded higher ethanol concentrations (of 31.47 ± 0.57 g/L) than the mixed lignocellulosic feedstocks, while the combination of SS and SCB produced only 24.96 ± 0.70 g/L of ethanol within 76 h. This positive and fruitful result may be attributed to the high cellulose content and relatively lower lignin content in DPT and SS, which enhanced enzymatic accessibility and fermentation efficiency. Specifically, DPT contains approximately 73.96% cellulose and 6.67% lignin, while SS comprises about 68.60% cellulose and 7.31% lignin. The lower lignin content reduces biomass recalcitrance, facilitating saccharification and fermentation, as also reported in studies evaluating the effect of lignin content on enzymatic hydrolysis efficiency [33].
However, the combination of all three biomasses (SS, SC, and DPT) in equal proportions produced a lower ethanol concentration of 27.87 ± 0.11 g/L at 72 h compared to other binary mixtures. This could be the result of inhibitory interactions among the biomasses or the lack of synergistic effects in a ternary mixture. Moreover, while alkaline pretreatment was used to improve biomass digestibility, its efficacy can vary depending on the specific biomass composition. Previous research has shown that alkaline pretreatment is effective at delignification and preserving sugars, but its impact can differ across biomasses, affecting overall yields [34]. Additionally, acidic pretreatment, while effective in breaking down biomass, can degrade sugars into inhibitory compounds like furfural, HMF, acetic acid, and phenolic acids through dehydration reaction under high temperature. These harsh conditions lead to the formation of furan derivatives, which hinders enzyme hydrolysis, microbial growth, and sugar consumption in downstream fermentation processes [35,36].
These findings are consistent with previous research highlighting the potential of mixed biomass for enhanced bioethanol yields. For instance, Ref. [37] demonstrated that a 1:1:1 mixture of rice straw (RS), Napier grass (NG), and SCB produced higher bioethanol concentrations compared to individual biomasses. Their NaOH-pretreated mixed sample yielded a maximum bioethanol concentration of 0.82% (v/v), with reduced sugar and ethanol yields of 0.43 g/g and 0.12 g/g, respectively. This suggests that combining complementary biomasses can enhance enzymatic hydrolysis and fermentation by leveraging their compositional attributes [37]. Similarly, the versatility of this research can be justified through the literature itself; when coffee husks were mixed with cassava stems and coconut coir in equal proportions, the end yield of ethanol was 9.5 mg/mL [38], which was greater than the individual result of the husks of 8.5 mg/mL. In addition, Ref. [22] found that when different parts of an individual “sugarcane,” i.e., bagasse, straw, and hoops, were integrated, it showed 55% higher enzymatic conversion and 25% higher ethanol production.
Moreover, the significance of using mixed feedstocks for ethanol production is well supported in the literature. Studies have shown that combining multiple feedstocks produces fewer inhibitors in the hydrolysate and enhances ethanol production. For example, it was reported that the proportion of inhibitors such as acetic acid, HMF, and formic acid was lower in a mixture of waste copier paper and wheat straw compared to wheat straw alone. As a result, the mixed feedstock achieved 34% higher ethanol production within the first 24 h, compared to only 3% from wheat straw alone. In addition, the mixture exhibited a detoxifying effect comparable to that of pure CaCO3 on filter paper [20].
Additional nutritional supplements are not required during the fermentation process when the mixed feedstock is used, e.g., clover-regress was supplemented with “nitrogen content” when processed individually to meet 80% of the theoretical yield for ethanol production. However, when the same biomass was mixed with lower fraction of “straw,” “no nitrogen” was added and the production of ethanol was similar [17]. Parallel to this, the hydrolysates of wet oxidation-pretreated clover-regress were sufficient for microorganisms (S. cerevisiae and Mucor indicus) during simultaneous saccharification and co-fermentation (SSCF) to perform their metabolic activities, so the additional urea supplementation did not have any substantial effect on ethanol yield or the fermentation process [18]. Therefore, the mixture of substrates creates an adequate/balanced ratio of carbon–nitrogen for fermentation, and extra supplementation is required for processing. This point could be elaborated more by highlighting an example from lignin biorefinery paper: One way or the other during a chemical reaction, the different biomasses compensate one another, i.e., individually, the filtration of Euphorbia is crucial due to the higher presence of proteins (10.8) in its composition, which results in lower permeate flow, while hemp shive shows good filtration properties, and mixing the two biomasses increases the permeate flows, as “H” can act as a filtration adjuvant and decrease membrane fouling [39].
Furthermore, the mixed feedstock approach is economical when 70% of woody and 30% of agricultural biomasses are mixed to overcome storage and logistic issues. Mixed feedstock has shown many benefits over utilizing a single biomass for higher ethanol yields, maximum bestowal in building, and maintaining sustainable industries to combat global issues like climate change and global warming [16].
The findings of this study emphasize the need for careful selection of biomass combinations and optimization of pretreatment methods to achieve higher ethanol yields. Future studies should explore the mechanistic basis of the observed synergistic effects in specific biomass mixtures, such as SS and DPT. Investigating alternative pretreatment methods, including hybrid alkaline–acid or enzymatic approaches, may further enhance ethanol yields. Assessing the economic feasibility and scalability of mixed biomass use for industrial bioethanol production will also be crucial in moving toward commercial applications [40].
5. Conclusions
Overall, the main focus of this research pinpoints reducing constraints such as the availability of an individual feedstock throughout the year, the management of finances for the storage of that single biomass, its transportation, and non-continuous production of ethanol when switching from one biomass to another, which can result in closure of the industry. These problems can be primarily handled by mixing the various biomasses found to be the best solutions. By integrating biomasses, similar and even higher ethanol production was obtained compared to a single feedstock, which can be justified by quoting the production of ethanol from this research as well, which was comparatively better than that of the individual feedstock, signifying the importance of utilizing MLB for efficient and consistent ethanol production. Moreover, the mixed lignocellulosic approach brings economic stability for the reaction process by reducing the production cost together with lessening the extra supplementation demand. To conclude, this interesting area of biotechnology, known as “biofuels,” can make a huge difference in combating the ongoing crises. Moreover, they release a half portion of carbon dioxide and are considered a carbon-neutral alternative to conventional fossil-based fuels.
Author Contributions
Investigation and write-up of the original draft, M.A. (Malaika Amjad); investigation, editing, and reviewing of the manuscript, M.A. (Muhammad Abbas); investigation and writing—original draft, A.L.; validation of data, supervision, resources, I.N.S.; supervision, resources, conceptualization, formal analysis, data curation, A.K.T. All authors have read and agreed to the published version of the manuscript.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study is included in this published article. Further details can be provided upon request to the corresponding author.
Acknowledgments
This research was supported by the Department of Biotechnology, Faculty of Life Sciences and Informatics, BUITEMS, Quetta, Balochistan, Pakistan. The authors also acknowledge the support of Pramuk Parakulsuksatid, Department of Biotechnology, Faculty of Agro-Industry, Kasetsart University Chatuchuk, Bangkok 10900, Thailand.
Conflicts of Interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Chandel, A.K.; Garlapati, V.K.; Jeevan Kumar, S.P.; Hans, M.; Singh, A.K.; Kumar, S. The role of renewable chemicals and biofuels in building a bioeconomy. Biofuels Bioprod. Biorefin. 2020, 14, 830–844. [Google Scholar] [CrossRef]
- Scarlat, N.; Motola, V.; Dallemand, J.F.; Monforti-Ferrario, F.; Mofor, L. Evaluation of energy potential of Municipal Solid Waste from African urban areas. Renew. Sustain. Energy Rev. 2015, 50, 1269–1286. [Google Scholar] [CrossRef]
- Joshi, M.; Manjare, S. Chemical approaches for the biomass valorisation: A comprehensive review of pretreatment strategies. Environ. Sci. Pollut. Res. 2024, 31, 48928–48954. [Google Scholar] [CrossRef]
- Devi, A.; Bajar, S.; Kour, H.; Kothari, R.; Pant, D.; Singh, A. Lignocellulosic biomass valorization for bioethanol production: A circular bioeconomy approach. Bioenergy Res. 2022, 15, 1820–1841. [Google Scholar] [CrossRef] [PubMed]
- Dahadha, S.; Amin, Z.; Bazyar Lakeh, A.A.; Elbeshbishy, E. Evaluation of different pretreatment processes of lignocellulosic biomass for enhanced biomethane production. Energy Fuels 2017, 31, 10335–10347. [Google Scholar] [CrossRef]
- Singhvi, M.; Zinjarde, S.; Kim, B.-S. Sustainable Strategies for the Conversion of Lignocellulosic Materials into Biohydrogen: Challenges and Solutions toward Carbon Neutrality. Energies 2022, 15, 8987. [Google Scholar] [CrossRef]
- Imamoglu, E.; Sukan, F.V. The effects of single and combined cellulosic agrowaste substrates on bioethanol production. Fuel 2014, 134, 477–484. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, N.; Saini, S.; Singh, C.; Barnwal, P.; Panghal, A. Ethanol from lignocellulosic biomass: Pretreatment challenges and opportunities in sustainable waste management and energy Production. Biofuels Bioprod. Biorefin. 2025. [Google Scholar] [CrossRef]
- Chen, J.; Ma, X.; Liang, M.; Guo, Z.; Cai, Y.; Zhu, C.; Wang, Z.; Wang, S.; Xu, J.; Ying, H. Physical–Chemical–Biological Pretreatment for Biomass Degradation and Industrial Applications: A Review. Waste 2024, 2, 451–473. [Google Scholar] [CrossRef]
- Broda, M.; Yelle, D.J.; Serwańska, K. Bioethanol Production from Lignocellulosic Biomass—Challenges and Solutions. Molecules 2022, 27, 8717. [Google Scholar] [CrossRef]
- Borisova, A.S.; Pihlajaniemi, V.; Kont, R.; Niemelä, K.; Koitto, T.; Mikkelson, A.; Väljamäe, P.; Kruus, K.; Marjamaa, K. The effect of soluble phenolic compounds from hydrothermally pretreated wheat straw on Trichoderma reesei cellulases and commercial enzyme cocktails. Biomass Convers. Biorefin. 2024, 14, 971–984. [Google Scholar] [CrossRef]
- Sultan, I.N.; Khienpanya, N.; Tareen, A.K.; Laemsak, N.; Sirisansaneeyakul, S.; Vanichsriratana, W.; Parakulsuksatid, P. Kinetic study of ethanol production from different sizes of two-step pretreated oil palm trunk by fed-batch simultaneous saccharification and fermentation. Agric. Nat. Resour. 2022, 56, 287–298. [Google Scholar]
- Tareen, A.K.; Sultan, I.N.; Songprom, K.; Laemsak, N.; Sirisansaneeyakul, S.; Vanichsriratana, W.; Parakulsuksatid, P. Two-step pretreatment of oil palm trunk for ethanol production by thermotolerent Saccharomyces cerevisiae SC90. Bioresour. Technol. 2021, 320, 124298. [Google Scholar] [CrossRef] [PubMed]
- Singla, D.; Taggar, M.S. Mixed Lignocellulosic Feedstocks: An Effective Approach for Enhanced Biofuel Production. In Agroindustrial Waste for Green Fuel Application; Springer: Berlin/Heidelberg, Germany, 2023; pp. 249–279. [Google Scholar]
- Oke, M.A.; Annuar, M.S.M.; Simarani, K. Mixed Feedstock Approach to Lignocellulosic Ethanol Production—Prospects and Limitations. BioEnergy Res. 2016, 9, 1189–1203. [Google Scholar] [CrossRef]
- Sultana, A.; Kumar, A. Optimal configuration and combination of multiple lignocellulosic biomass feedstocks delivery to a biorefinery. Bioresour. Technol. 2011, 102, 9947–9956. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, M.H.; Haugaard-Nielsen, H. Sustainable bioethanol production combining biorefinery principles using combined raw materials from wheat undersown with clover-grass. J. Ind. Microbiol. Biotechnol. 2008, 35, 303–311. [Google Scholar] [CrossRef][Green Version]
- Martín, C.; Thomsen, M.H.; Hauggaard-Nielsen, H.; BelindaThomsen, A. Wet oxidation pretreatment, enzymatic hydrolysis and simultaneous saccharification and fermentation of clover–ryegrass mixtures. Bioresour. Technol. 2008, 99, 8777–8782. [Google Scholar] [CrossRef]
- Rentizelas, A.A.; Tolis, A.J.; Tatsiopoulos, I.P. Logistics issues of biomass: The storage problem and the multi-biomass supply chain. Renew. Sustain. Energy Rev. 2009, 13, 887–894. [Google Scholar] [CrossRef]
- Elliston, A.; Wilson, D.R.; Wellner, N.; Collins, S.R.A.; Roberts, I.N.; Waldron, K.W. Effect of steam explosion on waste copier paper alone and in a mixed lignocellulosic substrate on saccharification and fermentation. Bioresour. Technol. 2015, 187, 136–143. [Google Scholar] [CrossRef][Green Version]
- Siddiqui, Y.; Meon, S.; Ismail, R.; Rahmani, M. Bio-potential of compost tea from agro-waste to suppress Choanephora cucurbitarum L. the causal pathogen of wet rot of okra. Biol. Control 2009, 49, 38–44. [Google Scholar] [CrossRef]
- Pereira, S.C.; Maehara, L.; Machado, C.M.M.; Farinas, C.S. 2G ethanol from the whole sugarcane lignocellulosic biomass. Biotechnol. Biofuels 2015, 8, 44. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, W.; Tan, X.; Chen, X.; Guo, Y.; Yu, Q.; Yuan, Z.; Zhuang, X. Low-temperature sodium hydroxide pretreatment for ethanol production from sugarcane bagasse without washing process. Bioresour. Technol. 2019, 291, 121844. [Google Scholar] [CrossRef]
- Tareen, A.K.; Punsuvon, V.; Sultan, I.N.; Khan, M.W.; Parakulsuksatid, P. Cellulase addition and pre-hydrolysis effect of high solid fed-batch simultaneous saccharification and ethanol fermentation from a combined pretreated oil palm trunk. ACS Omega 2021, 6, 26119–26129. [Google Scholar] [CrossRef] [PubMed]
- Tareen, A.K.; Punsuvon, V.; Parakulsuksatid, P. Investigation of alkaline hydrogen peroxide pretreatment to enhance enzymatic hydrolysis and phenolic compounds of oil palm trunk. 3 Biotech 2020, 10, 179. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Sun, C.; Qiu, J.; Li, X.; Liu, R.; Zhang, L. Pretreatment of sweet sorghum bagasse by alkaline hydrogen peroxide for enhancing ethanol production. Korean J. Chem. Eng. 2016, 33, 873–879. [Google Scholar] [CrossRef]
- Selig, M.; Weiss, N.; Ji, Y. Enzymatic Saccharification of Lignocellulosic Biomass; Laboratory Analytical Procedure (LAP); National renewable energy laboratory technical report, NREL/TP510-42629; National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2008.
- Xue, S.; Nirmal, U.; Bowman, J.M.; David, C.; Leonardo, D.C.S.; Bruce, E.D.; Venkatesh, B. Sugar loss and enzyme inhibition due to oligosaccharide accumulation during high solids loading enzymatic hydrolysis. Biotechnol. Biofuels 2015, 8, 195. [Google Scholar] [CrossRef]
- Mankar, A.R.; Pandey, A.; Modak, A.; Pant, K.K. Pretreatment of lignocellulosic biomass: A review on recent advances. Bioresour. Technol. 2021, 334, 125235. [Google Scholar] [CrossRef]
- Vera, R.M.; Bura, R.; Gustafson, R. Synergistic effects of mixing hybrid poplar and wheat straw biomass for bioconversion processes. Biotechnol. Biofuels 2015, 8, 226. [Google Scholar] [CrossRef]
- Lee, S.C.; Oh, H.W.; Woo, H.C.; Kim, Y.H. Energy-efficient bioethanol recovery process using deep eutectic solvent as entrainer. Biomass Convers. Biorefin. 2023, 13, 15815–15826. [Google Scholar] [CrossRef]
- Shukla, A.; Kumar, D.; Girdhar, M.; Kumar, A.; Goyal, A.; Malik, T.; Mohan, A. Strategies of pretreatment of feedstocks for optimized bioethanol production: Distinct and integrated approaches. Biotechnol. Biofuels Bioprod. 2023, 16, 44. [Google Scholar] [CrossRef]
- Sun, R.; Song, X.L.; Sun, R.; Jiang, J.X. Effect of lignin content on enzymatic hydrolysis of furfural residues. Bioresources 2011, 6, 317–328. [Google Scholar] [CrossRef]
- Beluhan, S.; Mihajlovski, K.; Šantek, B.; Ivančić Šantek, M. The production of bioethanol from lignocellulosic biomass: Pretreatment methods, fermentation, and downstream processing. Energies 2023, 16, 7003. [Google Scholar] [CrossRef]
- Guo, H.; Zhao, Y.; Chang, J.-S.; Lee, D.-J. Inhibitor formation and detoxification during lignocellulose biorefinery: A review. Bioresour. Technol. 2022, 361, 127666. [Google Scholar] [CrossRef] [PubMed]
- Mujtaba, M.; Fernandes Fraceto, L.; Fazeli, M.; Mukherjee, S.; Savassa, S.M.; Araujo de Medeiros, G.; do Espírito Santo Pereira, A.; Mancini, S.D.; Lipponen, J.; Vilaplana, F. Lignocellulosic Biomass from Agricultural Waste to the Circular Economy: A Review with Focus on Biofuels, Biocomposites and Bioplastics. J. Clean. Prod. 2023, 402, 136815. [Google Scholar] [CrossRef]
- Cheenkachorn, K.; Mensah, R.Q.; Dharmalingam, B.; Gundupalli, M.P.; Rattanaporn, K.; Tantayotai, P.; Show, P.L.; Sriariyanun, M. The Versatility of Mixed Lignocellulose Feedstocks for Bioethanol Production: An Experimental Study and Empirical Prediction. BioEnergy Res. 2023, 17, 1004–1014. [Google Scholar] [CrossRef]
- Nguyen, Q.A.; Yang, J.; Bae, H.-J. Bioethanol production from individual and mixed agricultural biomass residues. Ind. Crops Prod. 2017, 95, 718–725. [Google Scholar] [CrossRef]
- Berchem, T.; Schmetz, Q.; Lepage, T.; Richel, A. Single and mixed feedstocks biorefining: Comparison of primary metabolites recovery and lignin recombination during an alkaline process. Front. Chem. 2020, 8, 479. [Google Scholar] [CrossRef]
- Arora, S.; Sarao, L.K.; Singh, A. Bioenergy from Cellulose of Woody Biomass. In Agroindustrial Waste for Green Fuel Application; Srivastava, N., Verma, B., Mishra, P.K., Eds.; Springer Nature: Singapore, 2023; pp. 89–120. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).