Development of Fermented Milks with Lacticaseibacillus casei B5 and Lactiplantibacillus plantarum B7 Isolated from Minas Artisanal Cheese
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material Acquisition
2.2. Activation and Fermentation Curve of LAB Strains
2.3. Development of Fermented Milk Samples
2.4. Physicochemical Analyses
2.5. Microbiological Analyses
2.6. Sensory Analysis
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gusmão, A.C.M.; Neves, L.F.; Matos Junior, F.E.; Cardoso, T.S.; Menegali, I.; Pereira, S.; Costa, T.O.; Pinto, M.S. Probiotic Potential of Fermented Milk Containing Lactobacillus rhamnosus and Lactobacillus plantarum Isolated from Artisanal Cheeses from Northern Minas Gerais. Rev. Inst. Laticínios Cândido Tostes 2023, 78, 924. [Google Scholar] [CrossRef]
- Cancella, M.J.; Santos, T.A.; Miguel, E.M.; Moreira, G.D.M.M.; Sobral, D.; de Almeida, F.A. Lactic Acid Bacteria as Probiotics, Biopreservatives, and Bacteriocin Producers: A Review. Rev. Inst. Laticínios Cândido Tostes 2024, 79, 37–47. [Google Scholar] [CrossRef]
- Kegele, C.S.; Valladares, L.S.; Silva, J.F.M.; Alonso, B.V.; Ribeiro, J.B. Technological Potential of Lactic Acid Bacteria as Biopreservants: Literature Review. 2023. Available online: https://agronfoodacademy.com/potencial-tecnologico-de-bacterias-acido-laticas-como-bioconservantes-revisao-de-literatura/ (accessed on 22 September 2025).
- Freire, T.T.; Silva, A.L.T.; Ferreira, B.K.O.; Santos, T.M. Lactic Acid Bacteria: Characteristics and Importance—Review. Res. Soc. Dev. 2021, 10, 19964. [Google Scholar] [CrossRef]
- Mello, T.C.S. In Vitro Probiotic Properties of Lactic Acid Bacteria Isolated from Artisanal Minas Cheese from Campo das Vertentes, Minas Gerais. Master’s Thesis, Federal University of Minas Gerais, Belo Horizonte, Brazil, 2022. [Google Scholar]
- Brazil. Law No. 13,860 of 18 July 2019. Provides for the Production and Commercialization of Artisanal Cheeses and Other Provisions. Off. Gaz. Union 2019, 138, 1. [Google Scholar]
- Embrapa. Business Guide for Artisanal Cheeses: Brazilian Artisanal Cheeses; Embrapa: Brasília, Brazil, 2021. [Google Scholar]
- Brazil. Ministry of Agriculture, Livestock and Food Supply. Technical Regulation on Hygienic-Sanitary Conditions and Good Manufacturing Practices for Food Processing/Manufacturing Establishments (Ordinance No. 368 of 4 September 1997). Off. Gaz. Union 1997, 1, 1. [Google Scholar]
- Brazil. Ministry of Agriculture, Livestock and Supply. Normative Instruction No. 53 of 16 October 2018. Technical Regulation of Identity and Quality of Milk Powder. Off. Gaz. Union 2018, 1, 11–12. [Google Scholar]
- Codex Alimentarius Comission. General Standard for Milk and Milk Products, 2nd ed.; FAO: Rome, Italy; OMS: Rome, Italy, 2011; ISSN 0259-2916. [Google Scholar]
- Oliveira, M.C.P. Protective Effect of Lactobacillus plantarum (B7) and L. rhamnosus (D1) from Artisanal Minas Cheese Against Experimental Escherichia coli EHEC and EIEC Infection and the Development of Fermented Buffalo Milk. Ph.D. Thesis, Federal University of Minas Gerais, Belo Horizonte, Brazil, 2016. [Google Scholar]
- Senz, M.; Keil, C.; Schmacht, M.; Palinski, S.; Cämmerer, B.; Hageböck, M. Influence of Media Heat Sterilization Process on Growth Performance of Representative Strains of the Genus Lactobacillus. Fermentation 2019, 5, 20. [Google Scholar] [CrossRef]
- Mendes, D.P.G. Physicochemical, Microbiological Characteristics and Sensory Acceptance of Fermented Milks by Lactic Acid Bacteria Isolated from Coalho Cheese from Pernambuco. Master’s Thesis, Federal University of Minas Gerais, Belo Horizonte, Brazil, 2011. [Google Scholar]
- NBR 12894; Sensory Analysis of Foods and Beverages—Classification. ABNT—Brazilian Association of Technical Standards: Rio de Janeiro, Brazil, 1994.
- Brazil. Ministry of Agriculture, Livestock and Supply. In Official Methods for Analysis of Animal Origin Products; MAPA: Brasília, Brazil, 2022. [Google Scholar]
- Brazil. Ministry of Agriculture, Livestock and Supply. Secretariat of Agricultural Defense. Department of Inspection of Animal Origin Products. Normative Instruction No. 46: Technical Regulation of Identity and Quality of Fermented Milks. Off. Gaz. Union 2007, 1, 5. [Google Scholar]
- IDF—International Dairy Federation. Yogurt: Enumeration of Characteristic Microorganisms Count Technique at 37 °C. Bull. IDF 1988, 117, 1–4. [Google Scholar]
- IDF—International Dairy Federation. Milk and Milk Products: Enumeration of Coliforms—Colony Count Technique and Most Probable Number Technique at 30 °C. Bull. IDF 1985, 73A, 1–8. [Google Scholar]
- APHA. Enumeration of Characteristic Microorganisms of Molds and Yeasts, 5 ed.; American Public Health Association: Washington, DC, USA, 2015; Chapter 21. [Google Scholar]
- Seleme, M.V.C.; Reckziegel, C.G.; Veitt, M.T.; Signor, A.; Palácio, S.M.; Gonçalves, G.C.; Barbieri, J.C.Z. Sensory Analysis of Caffeinated and Decaffeinated Coffea arabica L. of Artisanal Production and a Commercial Brand: Comparative Analysis between Coffees. Rev. Univ. Val. Rio Verde 2022, 21, 1. [Google Scholar]
- Üçok, G.; Sert, D. Growth Kinetics and Biomass Characteristics of Lactobacillus plantarum L14 Isolated from Natural Sourdough: Effect of Fermentation Time on Dough Machinability. LWT Food Sci. Technol. 2020, 129, 109516. [Google Scholar] [CrossRef]
- Lopes, D.S.; Bastos, P.A.M.B.; Rebello, L.P.G. Advances in the Application of Probiotic Lactic Acid Bacteria in Dairy Fermented Products: A Review. Vértices 2023, 25, 1. [Google Scholar] [CrossRef]
- Valente, G.L.C. In Vitro and In Vivo Probiotic Potential of Lactobacillus plantarum B7 and Lactobacillus rhamnosus D1 against Listeria monocytogenes in Experimental Infection and Goat Fermented Milk Production. Master’s Thesis, Federal University of Minas Gerais, Belo Horizonte, Brazil, 2018. [Google Scholar]
- Lunardi, A.; Dantas Filho, J.V.; Ferreira, C.C.; Cavali, J.; Vais, J.O.; Dias, A.A.; Gasparotto, P.H.G. Non-Starter Lactic Acid Bacteria (NSLAB): A Challenge to the Cheese Industry. Braz. J. Dev. 2021, 7, 26383–26409. [Google Scholar] [CrossRef]
- Ali, A.H.; Abu-Jdayil, B.; Al Nabulsi, A.; Osaili, T.; Liu, S.-Q.; Kamal-Eldin, A.; Mutamed, A. Effect of Acid Whey Protein Concentrate on the Rheological Properties, Antioxidant Capacities, and Biological Activities of Bioaccessible Fractions in Fermented Camel Milk. J. Dairy Sci. 2023, 108, 1242–1260. [Google Scholar]
- Vieira, A.C.A.; Andrade, E.H.P.; Soares, C.F.; Acurcio, L.B.; Mello, T.C.S.; de Souza, M.R. Probiotic Potential of Lactic Acid Bacteria Isolated from Artisanal Minas Cheese. Rev. Inst. Laticínios Cândido Tostes 2022, 77, 32–42. [Google Scholar] [CrossRef]
- Freitas, L.S.; Costa, W.P.G.; Oliveira, D.D. Microbiological Analysis of Sweets Sold by Delivery during the COVID-19 Isolation Period. Rev. Saúde Multidiscip. 2023, 14, 33–37. [Google Scholar]
- Carneiro, C.D.S.; Cunha, F.L.; Carvalho, L.D.; Carrijo, K.D.F.; Borges, A.; Cortez, M.A.S. Fermented Milks: History, Composition, Physicochemical Characteristics, Processing Technology and Defects. PUBVET 2012, 6, 1424. [Google Scholar]
- Francisquini, J.A.; Martins, E.; Silva, P.H.F.; Schuck, P.; Perrone, Í.T.; Carvalho, A.F. Maillard Reaction: A Review. Rev. Inst. Laticínios Cândido Tostes 2017, 72, 48–57. [Google Scholar] [CrossRef]
- Silva, M.A.P.; Leão, K.M.; Santos, P.A. Technology of Fermented Dairy Manufacturing: Bibliographic Review. PUBVET 2010, 4, 814. [Google Scholar]
- Bezerril, F.F. Development of Goat Yogurt Added with Xique-Xique Jelly (Pilosocereus gounellei): Evaluation of Technological Characteristics and Functional Potential. Ph.D. Thesis, Federal University of Paraíba, João Pessoa, Brazil, 2021. [Google Scholar]
- Brazil. National Health Surveillance Agency (ANVISA). Technical Regulation on Microbiological Standards for Foods (RDC No. 12 of 2 January 2001). Off. Gaz. Union 2001, 1, 1. [Google Scholar]
- Pereira, M.T.; Santana, E.H.W.; Santos, J.S. Importance of Lactic Acid and Non-Starter Bacteria (NSLAB) in Dairy Derivatives Production Technology. Ensaios 2020, 24, 348–352. [Google Scholar] [CrossRef]
- Dan, T.; Chen, H.; Li, T.; Tian, J.; Ren, W.; Zhang, H.; Sun, T. Influence of Lactobacillus plantarum P-8 on Fermented Milk Flavor and Storage Stability. Front. Microbiol. 2019, 9, 3133. [Google Scholar] [CrossRef] [PubMed]
| TRAT1B5 | TRAT2B5 | TRAT3B5 | TRAT1B7 | TRAT2B7 | TRAT3B7 | |
|---|---|---|---|---|---|---|
| DAY 1 | 4.15 ± 0.01 A,a | 3.95 ± 0.02 A,c | 3.95 ± 0.01 A,c | 3.76 ± 0.02 A,d | 4.02 ± 0.02 A,b | 3.98 ± 0.00 A,bc |
| DAY 15 | 4.02 ± 0.01 B,a | 3.87 ± 0.01 B,c | 3.93 ± 0.01 A,b | 3.76± 0.01 A,d | 3.88 ± 0.01 B,c | 3.92 ± 0.01 B,b |
| DAY 30 | 3.84 ± 0.01 C,a | 3.65 ± 0.00 C,d | 3.82 ± 0.00 B,a | 3.57 ± 0.01 B,e | 3.71 ± 0.01 C,c | 3.79 ± 0.00 D,b |
| DAY 45 | 3.57 ± 0.02 D,de | 3.64 ± 0.06 C,c | 3.82 ± 0.02 B,a | 3.55 ± 0.03 B,e | 3.70 ± 0.03 C,bc | 3.83 ± 0.03 C,a |
| TRAT1B5 | TRAT2B5 | TRAT3B5 | TRAT1B7 | TRAT2B7 | TRAT3B7 | |
|---|---|---|---|---|---|---|
| DAY 1 | 1.17 ± 0.01 AB,e | 1.52 ± 0.02 C,b | 1.27 ± 0.00 C,d | 1.46 ± 0.02 A,c | 1.60 ± 0.02 A,a | 1.19 ± 0.02 A,e |
| DAY 15 | 1.33 ± 0.17 A,abc | 1.63 ± 0.15 BC,a | 1.45 ± 0.29 ABC,ab | 1.32 ± 0.08 B,abc | 1.52 ± 0.04 B,ab | 1.07 ± 0.04 C,b |
| DAY 30 | 1.21 ± 0.03 AB,e | 1.81 ± 0.02 AB,a | 1.73 ± 0.02 A,b | 1.34 ± 0.00 B,d | 1.66 ± 0.03 A,c | 1.09 ± 0.01 B,f |
| DAY 45 | 1.14 ± 0.00 B,e | 1.78 ± 0.07 B,a | 1.69 ± 0.02 AB,b | 1.35 ± 0.01 B,d | 1.64 ± 0.03 A,c | 1.11 ± 0.02 B,e |
| TRAT1B5 | TRAT2B5 | TRAT3B5 | TRAT1B7 | TRAT2B7 | TRAT3B7 | |
|---|---|---|---|---|---|---|
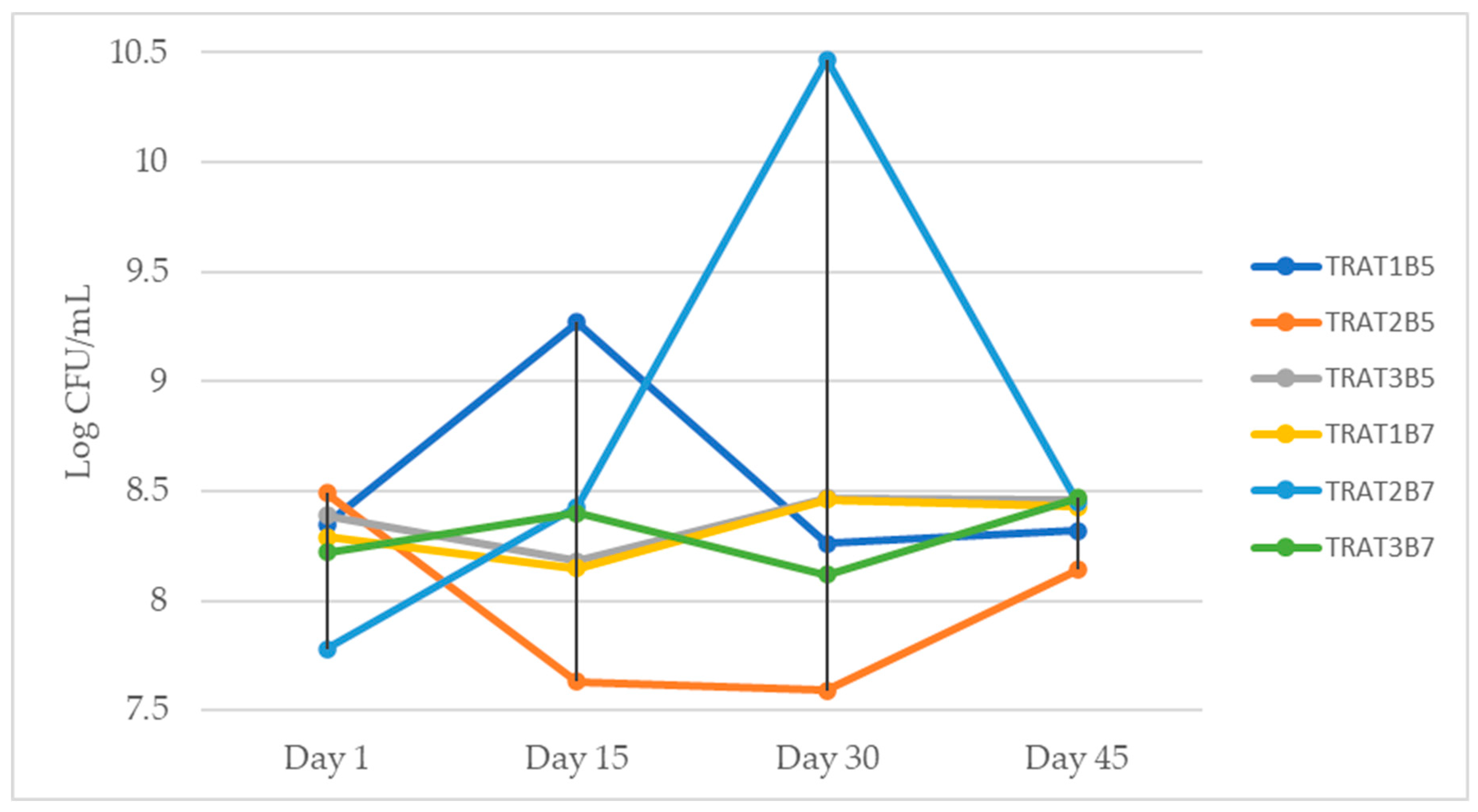
| DAY 1 | 8.35 ± 0.70 A,a | 8.49 ± 0.12 A,a | 8.39 ± 0.28 A,a | 8.29 ± 0.04 A,a | 7.78 ±0.01 A,a | 8.22 ± 0.19 A,a |
| DAY 15 | 9.27 ± 0.05 A,a | 7.63 ± 0.28 B,c | 8.18 ± 0.08 B,b | 8.15 ± 0.02 B,b | 8.43 ± 0.01 B,b | 8.40 ±0.05 A,b |
| DAY 30 | 8.26 ±0.03 A,ab | 7.59 ± 0.19 B,c | 8.47 ± 0.14 A,a | 8.46 ± 0.01 C,a | 10.47 ± 0.01 C,a | 8.12 ± 0.0 A,b |
| DAY 45 | 8.32 ±0.35 A,a | 8.14 ± 0.14 AB,c | 8.46 ± 0.01 A,b | 8.43 ± 0.21 C,b | 8.45 ± 0.02 B,b | 8.47 ± 0.0 A,b |
| Preference Ranking | ||||||
|---|---|---|---|---|---|---|
| TRAT3B7 | TRAT2B7 | TRAT2B5 | TRAT3B5 | TRAT1B7 | TRAT1B5 | |
| TRAT3B7 | 397 | 283 | 256 | 245 | 222 | 181 |
| TRAT2B7 | - | 114 | 141 | 152 | 175 | 216 |
| TRAT2B5 | - | - | 27 | 38 | 61 | 102 |
| TRAT3B5 | - | - | - | 11 | 34 | 75 |
| TRAT1B7 | - | - | - | - | 23 | 64 |
| TRAT1B5 | - | - | - | - | - | 41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Queiroz, C.S.d.; Rekowsky, B.S.d.S.; Salgado, M.J.G.; Lopes, M.F.B.d.H.; Souza, M.R.d.; Costa, M.P.d.; Silva, J.G.d. Development of Fermented Milks with Lacticaseibacillus casei B5 and Lactiplantibacillus plantarum B7 Isolated from Minas Artisanal Cheese. Fermentation 2025, 11, 560. https://doi.org/10.3390/fermentation11100560
Queiroz CSd, Rekowsky BSdS, Salgado MJG, Lopes MFBdH, Souza MRd, Costa MPd, Silva JGd. Development of Fermented Milks with Lacticaseibacillus casei B5 and Lactiplantibacillus plantarum B7 Isolated from Minas Artisanal Cheese. Fermentation. 2025; 11(10):560. https://doi.org/10.3390/fermentation11100560
Chicago/Turabian StyleQueiroz, Camila Selles de, Bruna Samara dos Santos Rekowsky, Madian Johel Galo Salgado, Maria Fernanda Barreto da Hora Lopes, Marcelo Resende de Souza, Marion Pereira da Costa, and José Givanildo da Silva. 2025. "Development of Fermented Milks with Lacticaseibacillus casei B5 and Lactiplantibacillus plantarum B7 Isolated from Minas Artisanal Cheese" Fermentation 11, no. 10: 560. https://doi.org/10.3390/fermentation11100560
APA StyleQueiroz, C. S. d., Rekowsky, B. S. d. S., Salgado, M. J. G., Lopes, M. F. B. d. H., Souza, M. R. d., Costa, M. P. d., & Silva, J. G. d. (2025). Development of Fermented Milks with Lacticaseibacillus casei B5 and Lactiplantibacillus plantarum B7 Isolated from Minas Artisanal Cheese. Fermentation, 11(10), 560. https://doi.org/10.3390/fermentation11100560






