Abstract
This study presents an integrated valorization strategy for oat husks through microwave-assisted pretreatment using a deep eutectic solvent (DES) composed of choline chloride and glycerol (1:2). The process was designed to enhance the release of fermentable sugars, enable xylooligosaccharide (XOS) production, and support inulinase production by Aspergillus niger A42 via submerged fermentation of the hydrolysate and solid-state fermentation of the residual biomass. Response surface methodology (RSM) was applied to evaluate the effects of microwave power, treatment time, and liquid-to-solid ratio (LSR) on fermentable sugar content (FSC) and total phenolic compounds (TPCs). Following pretreatment, the biomass was hydrolyzed using 1.99% sulfuric acid for 1 min. Optimal pretreatment conditions (350 W, 30 s, LSR 4 w/w) yielded an FSC of 51.14 g/L. Additionally, 230.78 mg/L xylohexaose and 6.47 mg/L xylotetraose were detected. Submerged fermentation of the liquid fraction with A. niger A42 resulted in inulinase and invertase activities of 60.45 U/mL and 21.83 U/mL, respectively. Solid-state fermentation of the pretreated solids produced 37.03 U/mL inulinase and 17.64 U/mL invertase. The integration of microwave-assisted DES pretreatment, dilute acid hydrolysis, and fungal fermentation established a robust strategy for the sequential production of XOS, fermentable sugars, and inulinase from oat husks, supporting their comprehensive utilization within a sustainable biorefinery framework.
1. Introduction
Population growth, industrialization, and urbanization have intensified waste generation, with agricultural activities being a major contributor. While agricultural waste is not inherently harmful, improper disposal methods can lead to environmental pollution. Bioconversion has emerged as a sustainable approach to obtain value-added products, reducing waste and dependence on non-renewable resources [1]. Utilizing lignocellulosic biomass to generate valuable bioproducts enhances sustainability [2].
Lignocellulosic materials are recognized as inexpensive, widely available, and renewable resources [3]. Lignocellulosic materials, comprising approximately 50% of Earth’s biomass, primarily consist of cellulose (30–50%), hemicellulose (20–30%), and lignin (15–30%) [4,5]. The cross-linking of lignin with carbohydrates, particularly hemicellulose, through benzyl ester, benzyl ether, and phenyl glycoside functional groups, as well as strong covalent and hydrogen bonding, contributes to the recalcitrance of biomass [6]. The inherent resistance of biomass and the complex molecular interactions within its structure present significant challenges for its efficient utilization in biorefineries. To overcome these obstacles, selecting an appropriate pretreatment technology is essential. Selecting an appropriate pretreatment strategy and designing an efficient biorefinery process are key factors in maximizing biomass conversion efficiency. Various pretreatment approaches have been developed, including chemical, physical, physicochemical, and biological methods, each with inherent limitations. Chemical pretreatments often pose environmental concerns, while physical methods tend to be energy-intensive and have low efficiency. Physicochemical techniques require complex processes and high-cost equipment, whereas biological treatments are typically slow, costly, and inefficient. Recently, the focus has shifted toward the use of green solvents [7].
Deep eutectic solvents (DESs) are synthesized by combining a hydrogen bond donor and acceptor, eliminating the need for complex solvents or purification steps [2]. Due to their non-toxic, biodegradable, and recyclable nature, DES enhance the deconstruction of biomass and enable the conversion of cellulose and hemicellulose into valuable products. Recent studies have explored the integration of DES with microwave pretreatment of biomass to enhance production of fermentable sugars [8]. Microwave irradiation induces micro-explosions within the material, facilitating the breakdown of recalcitrant structures. Aligned with green chemistry principles, the adoption of microwave as a thermal process for lignocellulosic biomass pretreatment has gained widespread interest in biorefinery applications [9].
Lignocellulosic biomass has attracted increasing interest in recent years as a sustainable raw material for oligosaccharide production. Prebiotic oligosaccharides are resistant to enzymatic digestion in the human gastrointestinal tract. These compounds remain intact as they pass through the digestion system. They are selectively utilized by probiotic bacteria in the colon [10]. Xylooligosaccharides (XOSs) are oligomers composed of β-d-xylopyranosyl units connected by β(1→4)-xylosidic bonds. These sugars occur naturally in sources like bamboo shoots, fruits, vegetables, milk, and honey. Industrial production of XOS typically involves the hydrolysis of xylan from lignocellulosic biomass. In recent years, XOS has gained considerable interest in the market due to its potent prebiotic properties in both humans and animals, even at minimal doses (1.4 g per day in adults) [11]. XOS has been classified as prebiotic oligosaccharides by the International Association of Probiotics and Prebiotics (IAAPP). XOSs serve as substrates that support the growth of the commensal microbiota in the lower gastrointestinal tract of animals, demonstrating prebiotic activity [12].
Inulinase is an enzyme that hydrolyzes the β-(2,1) linkages of inulin. Inulinases are classified into exo- and endoinulinases. Exoinulinase cleaves terminal fructose units from inulin, producing fructose as the primary product, while endoinulinase hydrolyzes internal linkages, generating fructooligosaccharides [13]. Inulinase is widely utilized in industries for hydrolyzing inulin into fructose and fructooligosaccharides (FOSs). In the food sector, it supports the production of high-fructose syrups and prebiotics, known for their bifidogenic effect. Inulinase facilitates the degradation of inulin-containing raw materials, yielding fermentable sugars that are applicable in various industrial processes [14]. Microorganisms such as Aspergillus, Penicillium, Kluyveromyces, and Bacillus are efficient producers of inulinase under solid-state or submerged fermentation conditions [15].
Oat (Avena sativa) is among the most widely cultivated cereals globally. During its processing, a significant amount of husk is generated as a byproduct [16]. Given that the husk accounts for nearly half of the total grain weight, its effective valorization is essential to prevent inefficient waste management [17].
Our previous study demonstrated that choline chloride:glycerol (1:2) is highly effective in maximizing fermentable sugar content (FSC) from oat husk [18]. Similarly, in our other study, the pretreatment of wheat bran was optimized using high-pressure-assisted DES methods, which demonstrated a significant increase in fermentable sugar content (FSC) while reducing the formation of TPC, hydroxymethylfurfural (HMF) and furfural [19]. This study focused on optimizing microwave-assisted DES pretreatment using a Box–Behnken design to maximize fermentable sugar content (FSC) from oat husks. The optimization process specifically targeted FSC, which was obtained by applying dilute H2SO4 hydrolysis to the cellulose-rich solid residue generated after DES pretreatment. Under the optimized conditions, the hemicellulose-rich liquid phase separated during DES pretreatment was identified as a promising feedstock for xylooligosaccharide (XOS) production. Following DES pretreatment and hydrolysis, the resulting hydrolysate was employed in submerged fermentation with Aspergillus niger A42 for inulinase production. Additionally, the pretreated oat husk solid residue served as a substrate in solid-state fermentation, further enhancing enzyme yields. This integrated strategy highlights the sustainable potential of lignocellulosic biomass for producing high-value bioproducts and supporting circular economy principles.
2. Materials and Methods
2.1. Experimental Design
The study followed these steps (Figure 1):
- Synthesis of Chcl: Gly (1:2) for Oat husk (OH) pretreatment
- Optimization of microwave-assisted DES pretreatment using Box–Behnken design (BBD).
- Characterization of sugars and inhibitors present in hydrolysates
- Validation of production under optimum conditions
- Xylooligosaccharide analysis under optimum conditions
- Component analysis of untreated and DES-pretreated oat husk
- Submerged and solid state fermentation of inulinase using Aspergillus niger A42.
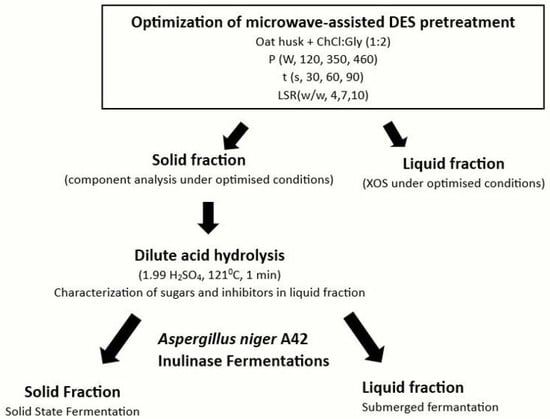
Figure 1.
Experimental flow chart.
2.2. Raw Materials
Oat husks were utilized in this study without grinding. It was stored at 4 °C.
2.3. Synthesis of DES
The method described by Abbott et al. [20] was used to synthesize DES by mixing choline chloride (ChCl) with glycerol (Gly) in a 1:2 molar ratio. The components were placed in sealed glass containers and heated to 80 °C with continuous magnetic stirring. The process continued until a clear and uniform liquid was obtained. DES was kept in an oven at 80 °C overnight [21]. The necessary chemicals were obtained from Sigma-Aldrich (St. Louis, MO, USA).
2.4. Microwave Assisted DES Pretreatment of OH
The optimization process was carried out according to the BBD using the RSM with the Design Expert 13 package (Stat-Ease Co., Mineapolis, MN, USA). Microwave power, implementation time and LSR were used as independent variables in the optimization process. The DES biomass mixture was pretreated at different microwave power settings (120, 350 and 460 W), times (30–90 s) and LSR (4–10 w/w) using a microwave oven (Beko MD 1610, Beko, Istanbul, Turkey). 3 g of OH was mixed with the corresponding DES volume according to the experimental design given in Table 1 (12 mL at LSR 4 w/w, 21 mL at LSR 7 w/w, and 30 mL at LSR 10 w/w). At the end of time, 10 mL of distilled water was added and the mixture was centrifuged at 20,000× g for 15 min. The separated liquid phase was used for xylooligosaccharide (XOS) analysis. Precipitate was rinsed twice with 250 mL of distilled water and centrifuged again under the same conditions. Solid residue dried at 60 °C for 15 h [21]. The pretreated OH was exposed to dilute acid hydrolysis using a 1.99% (v/v) H2SO4. The process was carried out at 121 °C in an autoclave for 1 min [22]. The maximum FSC was used as the response in the method. OH was hydrolyzed without DES pretreatment used as the control group.

Table 1.
Analysis results of hydrolysates obtained by microwave-assisted pretreatment of oat husk with choline chloride:glycerol (1:2) according to the Box–Behnken experimental design and dilute acid treatment in an autoclave.
2.5. Microorganism
Aspergillus niger A42 (ATCC 204447) was cultivated on malt extract agar. The incubation temperature was set at 30 °C, and the cultivation period lasted 4 days. The strain was routinely subcultured weekly and stored at 4 °C to maintain viability. It was kept in 20% glycerol at −80 °C for long-term preservation. Inulinase fermentation was conducted using submerged and solid-state fermentation methods.
2.6. Submerged Fermentation
For inulinase production, hydrolysates derived from the pretreatment stage were used as the primary substrate. The working volume in the fermentation trials was 50 mL. The fermentations were carried out in 150 mL Erlenmeyer flasks. The medium was supplemented with 1% yeast extract. The medium was adjusted to a pH of 5.0 before undergoing sterilization at 121 °C for 15 min. Spore inoculation was performed using a suspension prepared with 0.001% (v/v) Tween-80. inoculum size was 6% (v/v). The cultures were incubated under controlled conditions at 30 °C, with continuous agitation set at 200 rpm, using a CERTOMAT® IS shaking incubator (Sartorius, Göttingen, Germany). To track the progression of fermentation, 1 mL samples were collected daily and stored at 4 °C for further analysis. Before analysis, the samples were centrifuged at 6873× g for 5 min [23,24].
2.7. Solid State Fermentation
The oat husk, after acid hydrolysis, was thoroughly washed with distilled water until neutral pH was achieved and then dried. In solid-state fermentation, 5 g of pretreated biomass was used. The solid-to-liquid ratio was maintained at 4:1, yeast extract was added at 1% (w/w), and the pH of the fermentation medium was adjusted to 5.0. After sterilization at 121 °C for 15 min, the medium was inoculated with Aspergillus niger spore suspension at a volume of 7% (v/w). The fermentation process was maintained for 7 days [25]. Inulinase enzyme was extracted using 20 mL 0.1 M sodium acetate buffer (pH 4.8). The extraction process was carried out in a shaking incubator at 30 °C and 250 rpm for 1 h. After the extraction, the suspension was filtered using coarse filter paper and subsequently centrifuged at 500× g, 4 °C, for 20 min. Supernatant was used for enzyme analysis [26].
2.8. Analysis
2.8.1. Compositional Analysis of the Biomass Samples
Extractives, hemicellulose, lignin, and cellulose contents were determined using the following methods [27]. First, a dried biomass sample was extracted using a benzene/ethanol mixture (2:1 v/v) with a solid-to-liquid ratio of 1:8 (w/v) at 80 °C for 3 h. After drying at 105–110 °C until a constant weight was achieved. The extractive content was then calculated. Dried extractive free sample is boiled under reflux 150 mL of NaOH (20 g/L) for 3.5 h. After filtration, the sample was washed thoroughly to remove residual sodium ions. The residue was dried and weighed for hemicellulose content determination. Then, 1 g of the extractive-free residue was dried and treated with 30 mL of 72% H2SO4 at 8–15 °C for 24 h. 300 mL distilled water was added to mixture, followed by boiling for 1 h and subsequent filtration. After the washing and drying steps, the residue was weighed for lignin content determination.
2.8.2. Hydrolyzate Analysis
Fermentable Sugar Content and Total Phenolic Content
Fermentable sugar content was determined using the DNS method [28]. For this analysis, 0.5 mL of sample was mixed with 3.95 mL of distilled water. Then, 0.5 mL of 12 M HCl was added and the mixture was vortexed, followed by incubation in a water bath at 90 °C for 10 min. After hydrolysis, 0.1 mL of 5 N KOH was added, and 360 µL of the solution was taken. To this, 1.5 mL of DNSA reagent was added and mixed. The tubes were heated again at 90 °C for 15 min, after which 0.5 mL of 40% potassium sodium tartrate solution was added. After cooling, absorbance was measured at 575 nm with a spectrophotometer. Calibration was performed using a glucose standard curve (Equation (1)).
The total phenolic content (TPC) was calculated via the Folin–Ciocalteu method [29]. After appropriate dilutions, 0.5 mL of sample was transferred into analysis tubes, followed by the addition of 2.5 mL of 10% Folin–Ciocalteu reagent. Subsequently, 2 mL of 7.5% Na2CO3 solution was added and the mixture was vortexed. The tubes were incubated in a water bath at 50 °C for 5 min and then kept in the dark for 10 min. Absorbance was measured at 760 nm using a UV–visible spectrophotometer, with deionized water used as the blank. A gallic acid standard curve was used for quantification (Equation (2)). Both analyses were conducted using a ThermoScientific™ Evolution 201 UV-Vis spectrophotometer (Waltham, MA, USA).
FSC (g/L) = 60.401 × Abs575 + 0.5751
GA (mg GAE/L) = 97.454 × Abs760 − 3.6142
Sugars, XOS and Inhibitor Analysis
A Shimadzu HPLC (LC-20 AD) system equipped with a Carbosep Corogel-87P column was used for the analysis of xylose, arabinose, and maltose [30]. A ThermoScientific HPLC (Dionex Ultimate 3000, Thermo Fisher Scientific, Waltham, MA, USA) system was used for multiple analyses: sucrose and fructose were quantified using a Shodex KS-801 column with a refractive index detector [31]. The same method was applied for the quantification of xylooligosaccharides, including xylobiose (X2), xylotriose (X3), xylotetraose (X4), xylopentaose (X5), and xylohexaose (X6), in hydrolysates obtained under optimal pretreatment conditions, as well as in the liquid phase separated after pretreatment. Additionally, furfural and hydroxymethylfurfural (HMF) were analyzed with an ODS-2 Hypersil C18 column using a UV detector [32,33], and organic acids were determined with an ACClaim™ OA column. Acetic, benzoic, and formic acids were identified and quantified using calibration curves [34].
2.8.3. Enzyme Activity Assessment
Enzyme activity was calculated using inulin and sucrose as substrates, prepared at 2% (w/v) in 0.1 M sodium acetate buffer (pH 4.8). Samples were diluted 10–100 times based on the spectrophotometer’s detection range [35]. A reaction mixture of 100 µL diluted sample and 900 µL substrate was incubated at 60 °C. After incubation, 1.5 mL DNSA reagent was added to each tube. Control samples contained 100 µL diluted enzyme sample, 900 µL substrate, and 1.5 mL DNSA, while blank samples were prepared using 100 µL water instead of the enzyme sample. All tubes were incubated at 100 °C for 10 min to terminate enzymatic reactions. Absorbance was measured at 540 nm. The absorbance of the control sample (Abs control) was subtracted from the absorbance of the reaction mixture (Abssample) to determine the reducing sugars released by the enzyme. Enzyme activity was calculated using a fructose standard curve, following the equation below: The formula used to calculate enzyme activity is given in Equation (4). The inulinase activity (U/mL) was divided by the invertase-type activity (U/mL) to determine the I/S ratio.
where
y = 3.8983 × [Abssample − Abscontrol] − 0.0156
Enzyme activity (U/mL) = y × Df × RV/t
- y = Fructose concentration (µmol/mL)
- DF = Dilution factor
- RV = Total volume in the test tube/enzyme solution volume
- t = Reaction time (min)
3. Results and Discussion
3.1. Optimization of Microwave-Assisted DES Pretreatment of Oat Husk
OH was subjected to microwave-assisted pretreatment using ChCl: Gly (1:2). The pretreatment conditions were optimized using the BBD. Independent variables included microwave power (P), treatment time (t), and LSR, with their levels summarized in Table 1. After pretreatment, the hydrolysis was performed using 1.99% H2SO4 at 121 °C for 1 min, and the FSC was analyzed. The FSC varied between 33.22 and 45.21 g/L across different pretreatment conditions. Sugar concentrations in the hydrolysates were as follows: glucose (2.74–11.01 g/L), xylose (11.3–40.76 g/L), fructose (9.81–23.36 g/L), sucrose (0.58–1.76 g/L), and arabinose (2.29–15.37 g/L). The highest HMF content (1739.53 mg/L) was recorded in Experiment 3 (120 W, 30 s, LSR = 7), while the lowest (811.22 mg/L) was observed in Experiment 7 (350 W, 30 s, LSR = 10). The highest furfural concentration (275.94 mg/L) was found in Experiment 3 (120 W, 30 s, LSR = 7), while the lowest (119.31 mg/L) was detected in Experiment 7 (350 W, 30 s, LSR = 10). Gürler et al. [36], reported that elevated concentrations of HMF and furfural inhibited Aspergillus niger inulinase activity. They determined tolerance thresholds of 3 g/L for HMF and 0.5 g/L for furfural with respect to enzyme activities. In the present study, the measured concentrations of HMF and furfural were below these critical limits, indicating no inhibitory effect on fermentation performance.
ANOVA results for the selected model of optimization of microwave-assisted pretreatment of oat husk with choline chloride:glycerol (1:2) are presented in Table 2. The results indicated that a second-order model provided the best fit for FSC. The regression equation for FSC is given in Equation (5).

Table 2.
ANOVA results for the selected model of optimization of microwave-assisted pretreatment of oat husk with choline chloride:glycerol (1:2).
The model’s F-value of 9.07 indicates statistical significance (p < 0.05), with a 0.4% chance that the observed variability could be due to noise. The model’s performance metrics include an R2 value of 0.9218, an adjusted R2 of 0.8212, and a predicted R2 of 0.4353. The adequate precision value of 13.1691 indicates a strong signal-to-noise ratio, confirming the model’s robustness. The non-significant lack of fit (p = 0.5309) indicated that the model adequately described the experimental data without systematic deviation. Together, these metrics confirm that the model is statistically reliable and suitable for predicting FSC within the studied parameter space.
The statistical evaluation of the independent variables influencing FSC indicated that processing time was the most significant factor (F = 59.10, p = 0.0001), strongly affecting sugar release. Longer processing times facilitated the breakdown of the lignocellulosic matrix, leading to a higher yield of fermentable sugars. Microwave power also contributed to FSC production; however, its effect was not statistically significant (F = 4.28, p = 0.0774), suggesting that microwave power alone may not be a determining factor but could play a role when combined with other parameters. The interaction between microwave power and processing time was found to be statistically significant (F = 10.66, p = 0.0138), highlighting the importance of optimizing both variables simultaneously. This finding suggests that a moderate to high power level applied over an extended duration may be more effective than an excessively high power level over a short period. Additionally, the quadratic effect of microwave power was statistically significant (F = 6.64, p = 0.0366), indicating that excessively high power levels may have adverse effects, potentially causing sugar degradation. These results underscore the necessity of determining optimal microwave power and processing time conditions to maximize fermentable sugar yield efficiently.
The impact of microwave power on FSC was analyzed by comparing specific experimental conditions. In experiments 3 and 17, where the treatment time was 30 s and the liquid-to-solid ratio (LSR) was 7, an increase in microwave power enhanced FSC from 39.71 to 42.80 g/L. However, in experiments 5 and 13, which were conducted with a treatment time of 60 s and an LSR of 10, higher microwave power led to a decrease in FSC from 40.65 to 38.17 g/L. Similarly, in experiments 10 and 14, with a treatment time of 60 s and an LSR of 4, increasing microwave power reduced FSC from 40.56 to 37.69 g/L. These results indicate that while moderate microwave power may promote sugar release, excessive power levels can decrease FSC, possibly due to thermal degradation of sugars.
The influence of treatment time on FSC was also investigated. In experiments 1 and 3, performed at 120 W and an LSR of 7, extending the treatment time from 30 to 90 s lowered FSC from 39.71 to 37.99 g/L. A similar trend was observed in experiments 15 and 16, where an increase in treatment time from 30 to 90 s at 350 W and an LSR of 4 caused FSC to drop from 45.21 to 36.97 g/L. Additionally, in experiments 6 and 17, conducted at 460 W and an LSR of 7, FSC declined from 42.80 to 33.22 g/L as the treatment duration increased from 30 to 90 s. The reduction in FSC was more pronounced at higher microwave power levels, highlighting a significant interaction between microwave power and treatment time, as demonstrated in Table 1. These findings suggest that prolonged exposure to high-intensity microwave radiation may lead to sugar degradation, reducing the overall FSC yield. The effect of LSR on FSC was assessed by comparing experiments 5 and 10, experiments 2 and 16, and experiments 13 and 14. The results indicated that LSR had no statistically significant impact on FSC (p > 0.05), implying that variations in the liquid-to-solid ratio did not considerably affect fermentable sugar release under the tested conditions.
Three-dimensional response surface graphs illustrating the effects of microwave power, treatment time, and LSR on FSC are presented in Figure 2. These findings provide valuable insights into optimizing microwave-assisted DES pretreatment for enhancing fermentable sugar yields. At Graph A, the response surface demonstrates a parabolic trend, where moderate microwave power and shorter treatment times maximize FSC. At prolonged treatment times and higher microwave power, FSC decreases, suggesting thermal degradation of sugars, as also supported by ANOVA (P2: p = 0.0088). At graph B, FSC increases with rising microwave power, while the effect of LSR remains minimal, resulting in a nearly flat response surface. ANOVA results confirm that LSR alone is not statistically significant (p = 0.7266). However, the interaction between microwave power and LSR (p = 0.0439) was significant, suggesting that LSR influences FSC only when combined with microwave power. The interaction between t and LSR was statistically significant (p = 0.0133), suggesting that treatment time has a greater influence on FSC when LSR is adjusted.
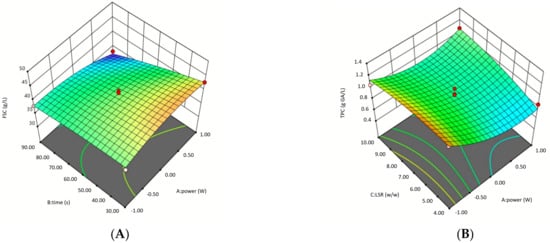
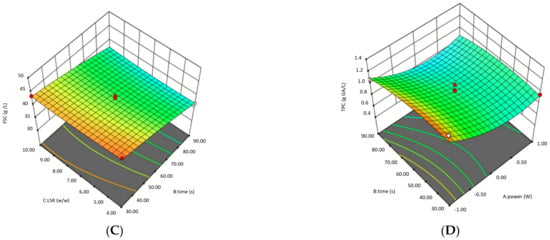
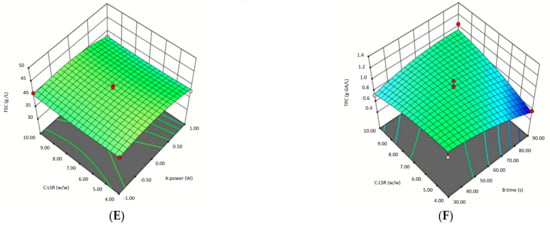
Figure 2.
Surface plots of microwave assisted DES pretreatment of oat husk by using BBD. (A) Effect of pretreatment time and microwave power on the fermentable sugar concentration, (B) Effect of liquid-to-solid ratio and microwave power on the fermentable sugar concentration, (C) Effect of liquid-to-solid ratio and pretreatment time on the fermentable sugar concentration, (D) Effect of pretreatment time and microwave power on the total phenolic substance concentration, (E) Effect of liquid-to-solid ratio and microwave power on the total phenolic substance concentration, (F) Effect of liquid-to-solid ratio and pretreatment time on the total phenolic substance concentration.
The quartic model was identified as the most suitable for predicting the total phenolic content (TPC). ANOVA results presented in Table 2. The model was found to be statistically significant (p = 0.0285, F = 8.14), indicating that the independent variables had a considerable effect on TPC. The lowest TPC value (0.57 g GA/L) was observed in Experiment 16 (350 W, 90 s, LSR = 4), while the highest TPC value (1.40 g GA/L) was recorded in Experiment 3 (120 W, 30 s, LSR = 7). Although microwave power was significant (p = 0.063, F = 6.53), its effect was not strongly significant when considered alone. Processing time was not significant (p = 0.9765), indicating that extended treatment duration had no substantial impact, whereas LSR was also not significant (p = 0.13), though it exhibited influence when interacting with other variables. Notably, the interaction between microwave power and LSR was significant (p = 0.0439), showing that their combined effect played a role in phenolic compound retention. Similarly, a strong interaction was observed between time and LSR (p = 0.0133), suggesting that these factors together affected TPC more than when considered independently. Furthermore, the quadratic effect of microwave power (P2) was highly significant (p = 0.0088), confirming that excessive microwave power may lead to phenolic degradation. These findings indicate that TPC was not significantly affected by pretreatment time or LSR alone but was influenced by their interactions with microwave power. The p-values (p < 0.05) confirmed that the interaction terms, P x (LSR), t x (LSR), and P2 are significant model terms, while other parameters were excluded from the regression equation (Equation (6)).
The R2 value of 0.9606 demonstrates the model’s high accuracy and fit with the experimental data. The adjusted R2 value of 0.8426 indicates acceptable agreement with the data. An adequate precision value of 12.1438 reflects a sufficient signal-to-noise ratio, confirming the model’s reliability for navigating the design space.
Three-dimensional (3D) response surface plots illustrate the interaction effects of various parameters and help estimate the optimal levels of each factor for maximizing TPC. These plots provide infinite combinations of two variables as responses. The 3D response surface plots showing the effects of P, t, and LSR on TPC are presented in Figure 2. The highest TPC values were observed at low microwave power and shorter treatment times. At higher microwave power and prolonged exposure, TPC declines. TPC declines significantly at high microwave power, aligning with ANOVA results where microwave power had a significant quadratic effect (p = 0.0088). Graph F illustrates that as treatment time increases, total phenolic content (TPC) decreases, forming a concave surface pattern. This trend aligns with the statistically significant interaction effect between treatment time and LSR in ANOVA (p = 0.0133), indicating that the influence of treatment time on TPC varies depending on the LSR. LSR has a minor direct effect on TPC, but its impact is more evident in interaction with treatment time.
Toxic compounds can stress fermentative microorganisms, reducing the efficient utilization of sugars and product formation [37]. OH was subjected to microwave-assisted pretreatment under optimized conditions (350 W, 30 s, and 4 w/w) using ChCl: Gly (1:2). Following hydrolysis with 1.99% H2SO4 at 121 °C for 1 min, the TPC was determined. The predicted TPC value was 0.90 g GA/L, which matched the experimentally obtained value of 0.90 g GA/L.
3.2. Impact of DES Pretreatment on Biomass Structure
The initial composition of OH before pretreatment was as follows: extractives (9.45%), hemicellulose (41.43%), lignin (21.20%), and cellulose (27.21%). After pretreatment with choline chloride:glycerol (1:2), the composition of oat husk was determined as 6.01% extractives, 29.44% hemicellulose, 16.24% lignin, and 48.31% cellulose (Table 3). Increasing temperature during pretreatment breaks hydrogen bonds between the solvent components, reducing viscosity and facilitating the diffusion of the DES system. This enhanced interaction between the biomass and the solvent promotes the removal of lignin from the biomass [38]. Hemicellulose and cellulose possess strong hydrogen bonds and high cohesive energies that hinder their solubility in DES. Therefore, DES systems are more effective in dissolving lignin compared to hemicellulose or cellulose [39]. After DES pretreatment, the extractive content and lignin ratio decreased, while the cellulose content increased. The removal of extractives, hemicellulose, and lignin from lignocellulosic materials contributed to the increase in cellulose content. Narayanan et al. [40] also observed a significant increase in cellulose content along with reduced lignin and hemicellulose after applying ternary DES to Napier grass, supporting the trend observed in this study.

Table 3.
Chemical composition of biomass and hydrolyzates.
DES and microwave assisted pretreatment has been shown to be highly effective in biomass processing. In one study, poplar treated with choline chloride:glycerol/AlCl3 and choline chloride:ethylene glycol/AlCl3 at 400 W and 110 °C for 10 min reached a saccharification yield of 96.15% [41]. In another report, DES–MW pretreatment of transgenic poplar resulted in lignin recovery yields of 45.38–53.09% with choline chloride:e thylene glycol and 46.38–54.30% with choline chloride:glycerol [42]. Further work demonstrated that DES assisted microwave pretreatment achieved delignification of 88% in miscanthus and 85% in birchwood at 110 °C, and enabled 80% lignin removal from poplar within 3 min compared to 9 h under conventional heating [38]. In line with these findings, the Deep Eutectic Solvent and Microwave-Assisted Pretreatment of oat husk in this study improved lignin removal. The hydrolysates were analyzed for their acetic acid, benzoic acid, and formic acid contents, with the results summarized in Table 3. Examination of Table 3 reveals that the benzoic acid content in the control samples was higher compared to those pretreated with ChCl: Gly, 1:2. This observation can be attributed to the presence of lignin and extractives in lignocellulosic biomass, which under acidic conditions, contribute to the formation of benzoic acid [43]. The reduction in benzoic acid formation can be attributed to the DES pretreatment, which effectively removed lignin and extractives from the lignocellulosic biomass. This removal minimized the precursors responsible for benzoic acid formation under acidic conditions. DES pretreatment facilitates delignification because the presence of chloride ions promotes the cleavage of β-O-4 aryl ether linkages, thereby increasing lignin solubility. The reduction in lignin content enhances the hydrolysis of carbohydrates and decreases their degradation into fermentation inhibitors. Consequently, DES application reduces the formation of such inhibitory compounds [44].
The xylooligosaccharide content of hydrolysates obtained using optimal pretreatment conditions and dilute acid hydrolysis was determined. Additionally, xylooligosaccharide analyses were performed on the liquid phase separated during pretreatment with choline chloride:glycerol (1:2) under optimized conditions. The analysis results are presented in Table 4. X4 and X6 were detected in the analyzed samples. Since the chemical and structural variations in XOS significantly influence their functional properties, including prebiotic effects, careful evaluation of both the administered dose and the polymerization profile is essential to achieve optimal stimulation of specific probiotic strains [45]. The highest xylooligosaccharide content was obtained in the liquid phase separated during microwave-assisted pretreatment. Since hemicellulose exhibits high solubility in deep eutectic solvents, the xylooligosaccharide content in the liquid phase separated after pretreatment with choline chloride:glycerol (1:2) was higher than that in the hydrolysate obtained from the residual solid using dilute acid hydrolysis.

Table 4.
Xylooligosaccharide content in des pretreated hydrolysate and liquid phase separated during des pretreatment.
Inulinase and invertase activities in Submerged (SmF) and Solid-State Fermentation (SSF) are presented in Table 5. In submerged fermentation with 1% yeast extract (YE), inulinase activity reached 60.45 U/mL, while invertase-type activity was recorded as 21.83 U/mL, yielding an I/S ratio of 2.76. The hydrolysate used in submerged fermentation was prepared from oat biomass pretreated with ChCl: Gly (1:2) and subsequently subjected to dilute acid hydrolysis, with the solid fraction separated and retained. To enhance enzyme yield per unit of oat biomass, this residual solid fraction was then utilized in solid-state fermentation. Inulinase activity reached 37.03 U/mL under these conditions while invertase-type activity was 17.64 U/mL. As a result, the I/S ratio was determined to be 2.10. This sequential fermentation strategy aimed to maximize enzyme recovery and overall process efficiency by utilizing the residual oat substrate after hydrolysate extraction. The I/S ratio is a crucial parameter for determining the catalytic activity of inulinase. This ratio represents the activity of the enzyme preparation against inulin and sucrose [46]. An I/S value greater than 0.01 indicates the dominance of inulinase activity [47]. The values obtained in our study clearly indicate the dominance of inulinase activity. Inulinase activities reported in the literature show variation depending on the microorganism and substrate employed. For instance, A. niger produced 50 U/mL on Jerusalem artichoke extract [48], Aspergillus oryzae yielded 72.25 U/mL on wheat bran [49], and A. tamarii produced 71.97 U/mL on agricultural waste extracts of Jerusalem artichoke and chicory [50].

Table 5.
Inulinase and Invertase Activities in Submerged (SmF) and Solid-State Fermentation (SSF).
The study by Singh et al. [51] reported inulinase production from Kluyveromyces marxianus YS-1 using Asparagus racemosus root extract as the substrate. Their findings showed that in an optimized shake flask system, the maximum inulinase activity obtained was 40.5 U/mL, while in a bioreactor system, 47.3 U/mL was achieved under optimal conditions (200 rpm, 0.75 vvm aeration, 30 °C, 60 h). Inulinase production from glucose (10.40 U/mL), fructose (3.09 U/mL), xylose (0.44 U/mL), sucrose (21.87 U/mL), and inulin (22.37 U/mL) [24] was lower than the values obtained using oat husk hydrolysate in this study (60.45 U/mL in SmF, 37.03 U/mL in SSF) is demonstrates the high potential of OH hydrolysates for cost-effective and efficient inulinase production, reducing the dependency on expensive pure carbon sources.
4. Conclusions
This study successfully optimized the microwave-assisted deep eutectic solvent (DES) pretreatment of oat husks for the production of fermentable sugars, xylooligosaccharides, and inulinase. The optimized conditions (choline chloride:glycerol, 1:2; 350 W; 30 s; LSR 4 w/w) resulted in a fermentable sugar concentration of 51.14 g/L and xylooligosaccharide production (6.47 mg/L X4 and 230.78 mg/L X6). Submerged fermentation of the hydrolysate with Aspergillus niger A42 yielded 60.45 U/mL inulinase and 21.83 U/mL invertase, while solid-state fermentation of the pretreated solids produced 37.03 U/mL and 17.64 U/mL, respectively. The findings indicate that DES pretreatment enhances fermentable sugar recovery while reducing the inhibitory benzoic acid content by facilitating lignin removal. The short processing time of microwave-assisted DES pretreatment offers a significant advantage, making the method more efficient for biorefinery applications. Moreover, the characterization of the sugar profile and inhibitor content in the hydrolysates provides a useful dataset that supports the evaluation of hydrolysate quality for fermentation applications and may serve as a reference for future microbial and enzymatic studies. Future work will focus on scaling up the microwave-assisted DES pretreatment process, conducting detailed techno-economic and life cycle assessment analyses, and exploring the application of hydrolysates with different microorganisms and enzymatic systems to broaden the valorization potential of oat husk.
Author Contributions
H.G.H.Y.: Writing-original draft, Visualization, Investigation; I.Y.: Conceptualization, Investigation, Resources; I.T.: Writing-review&editing, Resources, Supervision, Conceptualization, Methodology. All authors have read and agreed to the published version of the manuscript.
Funding
Akdeniz University, FBA-2024-6630.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Correction Statement
This article has been republished with a minor correction to the Data Availability Statement. This change does not affect the scientific content of the article.
References
- Kuthiala, T.; Thakur, K.; Sharma, D.; Singh, G.; Khatri, M.; Arya, S.K. The eco-friendly approach of cocktail enzyme in agricultural waste treatment: A comprehensive review. Int. J. Biol. Macromol. 2022, 209, 1956–1974. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Tian, X. Development of different pretreatments and related technologies for efficient biomass conversion of lignocellulose. Int. J. Biol. Macromol. 2022, 202, 256–268. [Google Scholar] [CrossRef]
- Wischral, D.; Arias, J.M.; Modesto, L.F.; de França Passos, D.; Pereira, N., Jr. Lactic acid production from sugarcane bagasse hydrolysates by Lactobacillus pentosus: Integrating xylose and glucose fermentation. Biotechnol. Prog. 2019, 35, e2718. [Google Scholar] [CrossRef] [PubMed]
- Haldar, D.; Purkait, M.K. A review on the environment-friendly emerging techniques for pretreatment of lignocellulosic biomass: Mechanistic insight and advancements. Chemosphere 2020, 264, 128523. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lee, D.-J. Lignocellulosic biomass pretreatment by deep eutectic solvents on lignin extraction and saccharification enhancement: A review. Bioresour. Technol. 2021, 339, 125587. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, W.; Xia, Q.; Guo, B.; Wang, Q.; Liu, S.; Liu, Y.; Li, J.; Yu, H. Efficient cleavage of lignin–carbohydrate complexes and ultrafast extraction of lignin oligomers from wood biomass by microwave-assisted treatment with deep eutectic solvent. ChemSusChem 2017, 10, 1692–1700. [Google Scholar] [CrossRef]
- Li, Y.; Sun, H.; Mu, T.; Garcia-Vaquero, M. Sustainable closed-loop biorefinery of γ-valerolactone from lignocellulosic biomass: Pretreatments of multiple biomass and synthesis of γ-valerolactone from multiple biomass-derived feedstocks. Biomass Bioenergy 2025, 193, 107594. [Google Scholar] [CrossRef]
- Tan, J.; Li, Y.; Tan, X.; Wu, H.; Li, H.; Yang, S. Advances in pretreatment of straw biomass for sugar production. Front. Chem. 2021, 9, 696030. [Google Scholar] [CrossRef]
- Mankar, A.R.; Pandey, A.; Modak, A.; Pant, K.K. Pretreatment of lignocellulosic biomass: A review on recent advances. Bioresour. Technol. 2021, 334, 125235. [Google Scholar] [CrossRef]
- Cho, E.J.; Trinh, L.T.P.; Song, Y.; Lee, Y.G.; Bae, H.-J. Bioconversion of biomass waste into high value chemicals. Bioresour. Technol. 2020, 298, 122386. [Google Scholar] [CrossRef]
- Santibáñez, L.; Henríquez, C.; Corro-Tejeda, R.; Bernal, S.; Armijo, B.; Salazar, O. Xylooligosaccharides from lignocellulosic biomass: A comprehensive review. Carbohydr. Polym. 2021, 251, 117118. [Google Scholar] [CrossRef]
- Kaur, G.; Kaur, P.; Kaur, J.; Singla, D.; Taggar, M.S. Xylanase, xylooligosaccharide and xylitol production from lignocellulosic biomass: Exploring biovalorization of xylan from a sustainable biorefinery perspective. Ind. Crops Prod. 2024, 215, 118610. [Google Scholar] [CrossRef]
- Singh, R.S.; Chauhan, K.; Kennedy, J.F. A panorama of bacterial inulinases: Production, purification, characterization and industrial applications. Int. J. Biol. Macromol. 2017, 96, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.S.; Singh, T.; Hassan, M.; Kennedy, J.F. Updates on inulinases: Structural aspects and biotechnological applications. Int. J. Biol. Macromol. 2020, 164, 193–210. [Google Scholar] [CrossRef] [PubMed]
- Canatar, M.; Tufan, H.N.G.; Ünsal, S.B.E.; Koc, C.Y.; Ozcan, A.; Kucuk, G.; Basmak, S.; Yatmaz, E.; Germec, M.; Yavuz, I.; et al. Inulinase and fructooligosaccharide production from carob using Aspergillus niger A42 (ATCC 204447) under solid-state fermentation conditions. Int. J. Biol. Macromol. 2023, 245, 125520. [Google Scholar] [CrossRef]
- Ruviaro, A.S.; Santana, H.A.; dos Santos Lima, G.T.; Barraza, M.T.; Silvestro, L.; Gleize, P.J.P.; Pelisser, F. Valorization of oat husk ash in metakaolin-based geopolymer pastes. Constr. Build. Mater. 2023, 367, 130341. [Google Scholar] [CrossRef]
- Neitzel, N.; Eder, M.; Hosseinpourpia, R.; Walther, T.; Adamopoulos, S. Chemical composition, particle geometry, and micro-mechanical strength of barley husks, oat husks, and wheat bran as alternative raw materials for particleboards. Mater. Today Commun. 2023, 36, 106602. [Google Scholar] [CrossRef]
- Hosta Yavuz, H.G.; Ibrahim Isci, A.; Turhan, I. Harnessing deep eutectic solvent for enhanced inulinase production from agricultural via submerged fermentation with Aspergillus niger. Int. J. Biol. Macromol. 2025, 295, 139592. [Google Scholar] [CrossRef]
- Hosta Yavuz, H.G.; Yavuz, I.; Yakan, A.I.; Turhan, I. Conversion of wheat bran into fermentable sugars using deep eutectic solvent pretreatment in a high-pressure reactor. Biomass Convers. Biorefin. 2023, 14, 24515–24525. [Google Scholar] [CrossRef]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep eutectic solvents formed between choline chloride and carboxylic acids: Versatile alternatives to ionic liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef]
- Isci, A.; Erdem, G.M.; Bagder Elmaci, S.; Sakiyan, O.; Lamp, A.; Kaltschmitt, M. Effect of microwave-assisted deep eutectic solvent pretreatment on lignocellulosic structure and bioconversion of wheat straw. Cellulose 2020, 27, 8949–8962. [Google Scholar] [CrossRef]
- Germec, M.; Ozcan, A.; Turhan, I. Bioconversion of wheat bran into high value-added products and modelling of fermentations. Ind. Crops Prod. 2019, 139, 111565. [Google Scholar] [CrossRef]
- Ilgın, M.; Germec, M.; Turhan, I. Statistical and kinetic modeling of Aspergillus niger inulinase fermentation from carob extract and its partial concentration. Ind. Crops Prod. 2020, 156, 112866. [Google Scholar] [CrossRef]
- Germec, M.; Turhan, I. Evaluation of carbon sources for the production of inulinase by Aspergillus niger A42 and its characterization. Bioproc. Biosyst. Eng. 2019, 42, 1993–2005. [Google Scholar] [CrossRef]
- Basmak, S.; Turhan, I. Production of β-mannanase, inulinase, and oligosaccharides from coffee wastes and extracts. Int. J. Biol. Macromol. 2024, 261, 129798. [Google Scholar] [CrossRef]
- Chen, H.-Q.; Chen, X.-M.; Chen, T.-X.; Xu, X.-M.; Jin, Z.-Y. Extraction optimization of inulinase obtained by solid state fermentation of Aspergillus ficuum JNSP5-06. Carbohydr. Polym. 2011, 85, 446–451. [Google Scholar] [CrossRef]
- Li, S.; Xu, S.; Liu, S.; Yang, C.; Lu, Q. Fast pyrolysis of biomass in free-fall reactor for hydrogen-rich gas. Fuel Process. Technol. 2004, 85, 1201–1211. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Demirel, F.; Germec, M.; Coban, H.B.; Turhan, I. Optimization of dilute acid pretreatment of barley husk and oat husk and determination of their chemical composition. Cellulose 2018, 25, 6377–6393. [Google Scholar] [CrossRef]
- Kuhn, R.C.; Maugeri Filho, F.; Silva, V.; Palacio, L.; Hernández, A.; Prádanos, P. Mass transfer and transport during purification of fructooligosaccharides by nanofiltration. J. Membr. Sci. 2010, 365, 356–365. [Google Scholar] [CrossRef]
- Germec, M.; Kartal, F.K.; Bilgic, M.; Ilgin, M.; Ilhan, E.; Güldali, H.; Isci, A.; Turhan, I. Ethanol production from rice hull using Pichia stipitis and optimization of acid pretreatment and detoxification processes. Biotechnol. Prog. 2016, 32, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Germec, M.; Tarhan, K.; Yatmaz, E.; Tetik, N.; Karhan, M.; Demirci, A.; Turhan, I. Ultrasound-assisted dilute acid hydrolysis of tea processing waste for production of fermentable sugar. Biotechnol. Prog. 2016, 32, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Hijazi, A.; Pisano, I.; Illek, P.; Leahy, J.J. A rapid HPLC method for the simultaneous determination of organic acids and furans: Food applications. Beverages 2022, 8, 6. [Google Scholar] [CrossRef]
- Dinarvand, M.; BAriff, A.; Moeini, H.; Masomian, M.; Mousavi, S.S.; Nahavandi, R.; Mustafa, S. Effect of extrinsic and intrinsic parameters on inulinase production by Aspergillus niger ATCC 20611. Electron. J. Biotechnol. 2012, 15, 5. [Google Scholar] [CrossRef]
- Gürler, H.N.; Çoban, H.B.; Turhan, I. Investigation of the inhibitory effects of furfural and hydroxymethylfurfural on the production of Aspergillus niger inulinase and modeling of the process. Biomass Convers. Biorefinery 2023, 13, 4291–4303. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Roberto, I.C. Alternatives for detoxification of diluted-acid lignocellulosic hydrolyzates for use in fermentative processes: A review. Bioresour Technol. 2004, 93, 1–10. [Google Scholar] [CrossRef]
- Kohli, K.; Katuwal, S.; Biswas, A.; Sharma, B.K. Effective delignification of lignocellulosic biomass by microwave assisted deep eutectic solvents. Bioresour. Technol. 2020, 303, 122897. [Google Scholar] [CrossRef]
- Park, C.W.; Gwon, J.; Han, S.Y.; Park, J.S.; Bandi, R.; Dadigala, R.; Kim, J.K.; Kwon, G.J.; Lee, S.H. Effect of deep eutectic solvent pretreatment on defibrillation efficiency and characteristics of lignocellulose nanofibril. Wood Sci. Technol. 2023, 57, 197–209. [Google Scholar] [CrossRef]
- Narayanan, K.; Venkatachalam, P.; Panakkal, E.J.; Tantayotai, P.; Tandhanskul, A.; Selvasembian, R.; Chuetor, S.; Sriariyanun, M. Exploring ternary deep eutectic solvent pretreatment in a one-pot process with Napier grass for bioethanol production. BioEnergy Res. 2024, 17, 2213–2225. [Google Scholar] [CrossRef]
- Xu, L.H.; Ma, C.Y.; Zhang, C.; Liu, J.; Peng, X.P.; Yao, S.Q.; Min, D.Y.; Yuan, T.Q.; Wen, J.L. Ultrafast fractionation of wild-type and CSE down-regulated poplars by microwave-assisted deep eutectic solvents (DES) for cellulose bioconversion enhancement and lignin nanoparticles fabrication. Ind. Crops Prod. 2022, 176, 114275. [Google Scholar] [CrossRef]
- Ma, C.Y.; Xu, L.H.; Sun, Q.; Sun, S.N.; Cao, X.F.; Wen, J.L.; Yuan, T.Q. Ultrafast alkaline deep eutectic solvent pretreatment for enhancing enzymatic saccharification and lignin fractionation from industrial xylose residue. Bioresour. Technol. 2022, 352, 127065. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef]
- Moreira, B.P.; Draszewski, C.P.; Celante, D.; Brondani, L.; Lachos-Perez, D.; Mayer, F.D.; Abaide, E.R.; Castilhos, F. Defatted rice bran pretreated with deep eutectic solvents and sequential use as feedstock for subcritical water hydrolysis. Bioresour. Technol. 2022, 351, 127063. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wang, H.; Sun, Z.; Ni, Z.; Li, C. Catabolism mechanism and growth-promoting effect of xylooligosaccharides in Lactiplantibacillus plantarum strain B20. Fermentation 2025, 11, 280. [Google Scholar] [CrossRef]
- Jain, S.C.; Jain, P.; Kango, N. Production of inulinase from Kluyveromyces marxianus using Dahlia tuber extract. Braz. J. Microbiol. 2012, 43, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Urrutia, C.; Volke-Sepulveda, T.; Figueroa-Martinez, F.; Favela-Torres, E. Solid-state fermentation enhances inulinase and invertase production by Aspergillus brasiliensis. Process Biochem. 2021, 108, 169–175. [Google Scholar] [CrossRef]
- Öngen-Baysal, G.; Sukan, Ş.S.; Vassilev, N. Production and properties of inulinase from Aspergillus niger. Biotechnol. Lett. 1994, 16, 275–280. [Google Scholar] [CrossRef]
- Ali, S.; Shahzadi, H. Nutritional optimizations for improved exo-inulinase production from Aspergillus oryzae for high fructose syrup preparations. Int. J. Curr. Microbiol. App. Sci. 2015, 4, 618–631. [Google Scholar]
- Saber, W.; El-Naggar, N. Optimization of fermentation conditions for the biosynthesis of inulinase by the new source; Aspergillus tamarii and hydrolysis of some inulin containing agro-wastes. Biotechnology 2009, 8, 425–433. [Google Scholar] [CrossRef]
- Singh, R.S.; Dhaliwal, R.; Puri, M. Production of inulinase from Kluyveromyces marxianus YS-1 using root extract of Asparagus racemosus. Process Biochem. 2006, 41, 1703–1707. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).