Valorization of Byproducts from the Sugarcane Industry Through Production of 1,3-Propanediol by Lentilactobacillus diolivorans
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganism and Culture Media
2.2. Acid and Organosolv Pretreatment of Sugarcane Bagasse
2.3. Growth and Fermentation
2.4. Analytical Methods and Statistical Analysis
3. Results and Discussion
3.1. Effects of Sugar Source on L. diolivorans Growth and Fermentation Performance
3.2. Evaluation of Fermentation with Co-Consumption of Carbohydrates
3.3. Characterization of the Sugarcane Industry Byproducts
3.4. Production of 1,3-PDO Using Waste from the Sugarcane Industry
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 1,3-PDO | 1,3-Propanediol |
| 1G | First-generation |
| 2G | Second-generation |
| 3-HPA | 3-hydroxypropionaldehyde |
| ABC | ATP-Binding Cassette |
| DAD | Diode Array Detector |
| EMP | Embden–Meyerhof–Parnas pathway |
| HPLC | High-Performance Liquid Chromatography |
| LAB | Lactic Acid Bacteria |
| MRS | Man, Rogosa and Sharpe medium |
| PLO | Pretreatment Liquor Organosolv |
| PPP | Pentose Phosphate Pathway |
| PTS | Phosphotransferase System |
| RID | Refractive Index Detector |
| SBH | Sugarcane bagasse acid hydrolyzed |
References
- Freitas, J.V.; Bilatto, S.; Squinca, P.; Pinto, A.S.S.; Brondi, M.G.; Bondancia, T.J.; Batista, G.; Klaic, R.; Farinas, C.S. Sugarcane biorefineries: Potential opportunities towards shifting from wastes to products. Ind. Crop. Prod. 2021, 172, 114057. [Google Scholar] [CrossRef]
- Basso, L.C.; Basso, T.O.; Rocha, S.N. Ethanol production in Brazil: The industrial process and Its impact on yeast fermentation. In Biofuel Production-Recent Developments and Prospects; Bernardes, M.A.S., Ed.; Intech: Rijeka, Croatia, 2011; pp. 85–100. [Google Scholar] [CrossRef]
- Vaidya, A.A.; Murton, K.D.; Smith, D.A.; Dedual, G. A review on organosolv pretreatment of softwood with a focus on enzymatic hydrolysis of cellulose. Biomass Conv. Bioref. 2022, 12, 5427–5442. [Google Scholar] [CrossRef]
- Araújo, M.F.; Volpi, M.P.C.; Mockaitis, G.; Morais Junior, M.A.; Carvalho da Costa, A.; Rabelo, S.C. Crude glycerol organosolv pretreatment: Chain integration for the production of 2G ethanol and biogas. Fuel 2025, 379, 132984. [Google Scholar] [CrossRef]
- Vaz, F.L.; Lins, J.R.; Alencar, B.R.A.; De Abreu, I.B.S.; Vidal, E.E.; Ribeiro, E.; Sampaio, E.V.S.B.; Menezes, R.S.C.; Dutra, E.D. Chemical pretreatment of sugarcane bagasse with liquid fraction recycling. Renew. Energy 2021, 174, 666–673. [Google Scholar] [CrossRef]
- Vandenberghe, L.P.S.; Valladares-Diestra, K.K.; Bittencourt, G.A.; Torres, L.A.Z.; Vieira, S.; Karp, S.G.; Sydney, E.B.; Carvalho, J.C.; Soccol, V.T.; Soccol, C.R. Beyond sugar and ethanol: The future of sugarcane biorefineries in brazil. Renew. Sustain. Energy Rev. 2022, 167, 112721. [Google Scholar] [CrossRef]
- Ruy, A.D.S.; Alves, R.M.B.; Hewer, T.L.R.; Pontes, D.A.; Teixeira, L.S.G.; Pontes, L.A.M. Catalysts for glycerol hydrogenolysis to 1,3-propanediol: A review of chemical routes and market. Catal. Today 2021, 381, 243–253. [Google Scholar] [CrossRef]
- Guo, Y.; Dai, L.; Xin, B.; Tao, F.; Tang, H.; Shen, Y.; Xu, P. 1,3-Propanediol production by a newly isolated strain, Clostridium perfringens GYL. Bioresour. Technol. 2017, 233, 406–412. [Google Scholar] [CrossRef]
- Yang, X.; Kim, D.S.; Choi, H.S.; Kim, C.K.; Thapa, L.P.; Park, C.; Kim, S.W. Repeated batch production of 1,3-propanediol from biodiesel derived waste glycerol by Klebsiella pneumoniae. Chem. Eng. Process. 2017, 314, 660–669. [Google Scholar] [CrossRef]
- Pflügl, S.; Marx, H.; Mattanovich, D.; Sauer, M. 1,3-propanediol production from glycerol with lactobacillus diolivorans. Bioresour. Technol. 2012, 119, 133–140. [Google Scholar] [CrossRef]
- Pflügl, S.; Marx, H.; Mattanovich, D.; Sauer, M. Heading for an economic industrial upgrading of crude glycerol from biodiesel production to 1,3-propanediol by lactobacillus diolivorans. Bioresour. Technol. 2014, 152, 499–504. [Google Scholar] [CrossRef]
- Lindlbauer, K.A.; Marx, H.; Sauer, M. Effect of carbon pulsing on the redox household of Lactobacillus diolivorans in order to enhance 1,3-propanediol production. New Biotechnol. 2017, 34, 32–39. [Google Scholar] [CrossRef]
- De Santana, J.S.; Da Silva, J.L.; Dutra, E.D.; Menezes, R.S.C.; De Souza, R.B.; Pinheiro, I.O. Production of 1,3-propanediol by lactobacillus diolivorans from agro-industrial residues and cactus cladode acid hydrolyzate. Appl. Biochem. Biotechnol. 2021, 193, 1585–1601. [Google Scholar] [CrossRef]
- Sun, C.; Ren, H.; Sun, F.; Hu, Y.; Liu, Q.; Song, G.; Abdulkhani, A.; Show, P.L. Glycerol organosolv pretreatment can unlock lignocellulosic biomass for production of fermentable sugars: Present situation and challenges. Bioresour. Technol. 2022, 344, 126264. [Google Scholar] [CrossRef]
- De Souza, R.B.; De Menezes, J.A.S.; De Souza, R.F.R.; Dutra, E.D.; De Morais, M.A., Jr. Mineral Composition of the Sugarcane Juice and Its Influence on the Ethanol Fermentation. Appl. Biochem. Biotechnol. 2015, 175, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, H.; Ji, X.; Shen, Z.; Guo, H.; Yao, S.; Wang, M.; Xiong, L.; Chen, X. Preparation, separation and purification of 5-hydroxymethylfurfural from sugarcane molasses by a self-synthesized hyper-cross-linked resin. Sep. Purif. Technol. 2023, 315, 123661. [Google Scholar] [CrossRef]
- Biebl, H.; Menzel, K.; Zeng, A.P.; Deckwer, W.D. Microbial production of 1,3-propanediol. Appl. Microbiol. Biotechnol. 1999, 52, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Doleyres, Y.; Beck, P.; Vollenweider, S.; Lacroix, C. Production of 3-hydroxypropionaldehyde using a two-step process with Lactobacillus reuteri. Appl. Microbiol. Biotechnol. 2005, 68, 467–474. [Google Scholar] [CrossRef]
- Wisselink, H.W.; Weusthuis, R.A.; Eggink, G.; Hugenholtz, J.; Grobben, G.J. Mannitol production by lactic acid bacteria: A review. Int. Dairy J. 2002, 12, 151–161. [Google Scholar] [CrossRef]
- Schoch, C.L.; Ciufo, S.; Domrachev, M.; Hotton, C.L.; Kanna, S.; Khovanskaya, R.; Leipe, D.; Mcveigh, R.; O’Neill, K.; Robbertse, B.; et al. NCBI Taxonomy: A comprehensive update on curation, resources and tools. Database 2020, 2020, baaa062. [Google Scholar] [CrossRef]
- Kowalcyk, M.; Mayo, B.; Fernandez, M.; Aleksandrzak-Piekarczyk, T. Updates on Metabolism in Lactic Acid Bacteria in Light of Omic Technologie. In Biotechnology of Lactic Acid Bacteria: Novel Applications, 2nd ed.; Mozzi, F., Raya, R.R., Vignolo, G.M., Eds.; Wiley-Blackwell: West Sussex, UK, 2016; p. 124. [Google Scholar] [CrossRef]
- Cheng, K.K.; Zhang, J.A.; Liu, D.H.; Sun, Y.; Liu, H.J.; Yang, M.D.; Xu, J.M. Pilot-scale production of 1,3-propanediol using Klebsiella pneumoniae. Process Biochem. 2007, 42, 740–744. [Google Scholar] [CrossRef]
- Maervoet, V.E.; De Maeseneire, S.L.; Avci, F.G.; Beauprez, J.; Soetaert, W.K.; De Mey, M. 1,3-propanediol production with Citrobacter werkmanii DSM17579: Effect of a dhaD knock-out. Microb. Cell Fact. 2014, 13, 70. [Google Scholar] [CrossRef]
- Jolly, J.; Hitzmann, B.; Ramalingam, S.; Ramachandran, K.B. Biosynthesis of 1,3-propanediol from glycerol with Lactobacillus reuteri: Effect of operating variables. J. Biosci. Bioeng. 2014, 118, 188–194. [Google Scholar] [CrossRef]
- Eiteman, M.A.; Lee, S.A.; Altman, E. A co-fermentation strategy to consume sugar mixtures effectively. J. Biol. Eng. 2008, 2, 3. [Google Scholar] [CrossRef]
- Wisedchaisri, G.; Park, M.S.; Iadanza, M.G.; Zheng, H.; Gonen, T. Proton-coupled sugar transport in the prototypical major facilitator superfamily protein XylE. Nat. Commun. 2014, 5, 4521. [Google Scholar] [CrossRef]
- Miwa, T.; Esaka, H.; Umemori, J.; Hino, T. Activity of H+-ATPase in ruminal bacteria with special reference to acid tolerance. Appl. Environ. Microbiol. 1997, 63, 2155–2158. [Google Scholar] [CrossRef] [PubMed]
- Kandler, O. Carbohydrate metabolism in lactic acid bacteria. Antonie Van Leeuwenhoek 1983, 49, 209–224. [Google Scholar] [CrossRef] [PubMed]
- Chatzifragkou, A.; Dietz, D.; Komaitis, M.; Zeng, A.P.; Papanikolaou, S. Effect of biodiesel-derived waste glycerol impurities on biomass and 1,3-propanediol production of Clostridium butyricum VPI 1718. Biotechnol. Bioeng. 2010, 107, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Jia, W.; Li, Y.; Chen, S. Performances of Lactobacillus brevis for producing lactic acid from hydrolysate of lignocellulosics. Appl. Biochem. Biotechnol. 2010, 161, 124–136. [Google Scholar] [CrossRef]
- Niel, E.W.J.; Larsson, C.U.; Lohmeier-Vogel, E.M.; Rådström, P. The potential of biodetoxification activity as a probiotic property of Lactobacillus reuteri. Int. J. Food Microbiol. 2012, 152, 206–210. [Google Scholar] [CrossRef]
- Da Silva, P.K.N.; Mendonça, A.A.; De Miranda, A.R.; Calazans, T.L.S.; De Souza, R.B.; De Morais, M.A., Jr. Nutritional requirements for Lactobacillus vini growth in sugarcane derivative substrate of ethanol fermentation. FEMS Microbiol. Lett. 2019, 366, 16. [Google Scholar] [CrossRef]
- Sharma, N.; Prasad, G.S.; Choudhury, A.R. Utilization of corn steep liquor for biosynthesis of pullulan, an important exopolysaccharide. Carbohydr. Polym. 2013, 93, 95–101. [Google Scholar] [CrossRef]
- Khan, I.; Nazir, K.; Wang, Z.P.; Liu, G.L.; Chi, Z.M. Calcium malate overproduction by Penicillium viticola 152 using the medium containing corn steep liquor. Appl. Biochem. Biotechnol. 2014, 98, 1539–1546. [Google Scholar] [CrossRef]
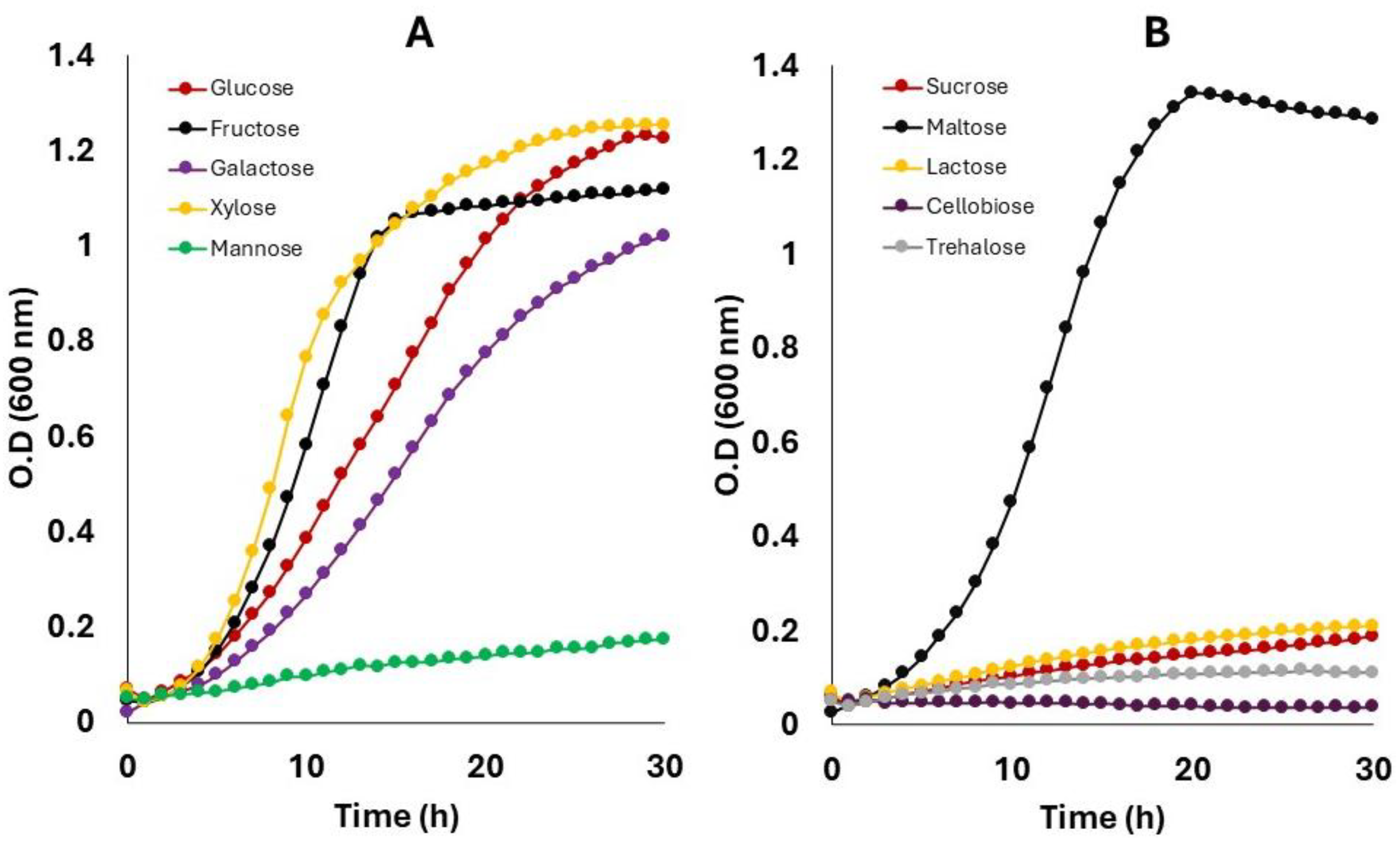
| Parameter | Glucose | Fructose | Galactose | Xylose | Maltose | Sucrose |
|---|---|---|---|---|---|---|
| Carbohydrate consumed (g/L) | 17.99 ± 0.23 b | 18.32 ± 0.93 b | 18.34 ± 0.25 b | 17.30 ± 0.82 b | 21.29 ± 0.10 a | 3.97 ± 0.11 c |
| Acetic acid (g/L) | 3.21 ± 0.51 bc | 4.28 ± 0.51 ab | 3.66 ± 0.60 ab | 4.50 ± 0.21 a | 3.34 ± 0.4 abc | 2.30 ± 0.30 c |
| Lactic acid (g/L) | 11.68 ± 0.43 b | 8.60 ± 0.23 c | 11.53 ± 0.15 b | 13.06 ± 0.20 a | 11.12 ± 0.55 b | 0.00 ± 0.00 d |
| Ethanol (g/L) | 1.26 ± 0.19 a | 0.00 ± 0.00 b | 1.15 ± 0.09 a | 0.00 ± 0.00 b | 1.01 ± 0.12 a | 0.00 ± 0.00 b |
| Mannitol (g/L) | 0.00 b | 2.86 ± 0.25 a | 0.00 b | 0.00 b | 0.00 b | 0.00 b |
| Glycerol consumed (g/L) | 8.36 ± 0.28 a | 9.01 ± 0.45 a | 8.64 ± 0.13 a | 3.58 ± 0.31 b | 8.95 ± 0.54 a | 2.40 ± 0.24 c |
| 1,3-Propanediol (g/L) | 6.22 ± 0.18 b | 7.05 ± 0.17 a | 7.07 ± 0.18 a | 1.51 ± 0.10 c | 7.15 ± 0.14 a | 1.30 ± 0.14 c |
| Y, 1,3-PDO | 0.75 ± 0.03 a | 0.78 ± 0.06 a | 0.82 ± 0.02 a | 0.42 ± 0.05 b | 0.80 ± 0.05 a | 0.54 ± 0.05 b |
| E (%) | 90.20 ± 3.9 a | 95.00 ± 6.85 a | 99.01 ± 2.69 a | 51.24 ± 5.85 b | 96.96 ± 5.90 a | 65.73 ± 6.39 b |
| Parameter | SG Mix | SX Mix | GX Mix | SGX Mix |
|---|---|---|---|---|
| Sucrose consumed (g/L) | 4.58 ± 0.50 c | 9.32 ± 0.21 a | - | 5.87 ± 0.25 b |
| Glucose consumed (g/L) | 10.90 ± 0.04 a | - | 10.89 ± 0.08 a | 7.62 ± 0.05 b |
| Xylose consumed (g/L) | - | 11.27 ± 0.08 a | 10.27 ± 0.83 b | 7.32 ± 0.06 c |
| Carbohydrates consumed (g/L) | 15.48 ± 0.54 c | 20.58 ± 0.16 b | 21.16 ± 0.76 a | 20.82 ± 0.29 ab |
| Acetic acid (g/L) | 3.78 ± 0.28 c | 6.27 ± 0.03 a | 5.55 ± 0.06 b | 5.27 ± 0.04 b |
| Lactic acid (g/L) | 9.96 ± 0.15 d | 14.70 ± 0.60 b | 16.28 ± 0.47 a | 13.19 ± 0.31 c |
| Yp/s Lactic acid | 0.95 ± 0.06 a | 0.71 ± 0.02 bc | 0.77 ± 0.03 b | 0.63 ± 0.02 c |
| Ethanol (g/L) | 0.00 ± 0.00 c | 0.00 ± 0.00 c | 1.82 ± 0.13 a | 1.34 ± 0.09 b |
| Glycerol consumed (g/L) | 10.52 ± 0.58 a | 7.99 ± 0.51 b | 5.27 ± 0.22 c | 8.20 ± 0.32 b |
| 1.3-Propanediol (g/L) | 7.63 ± 0.61 a | 6.18 ± 0.17 b | 4.31 ± 0.06 c | 6.59 ± 0.04 ab |
| Yp/s 1.3-PDO | 0.73 ± 0.02 b | 0.77 ± 0.04 ab | 0.82 ± 0.03 a | 0.80 ± 0.04 a |
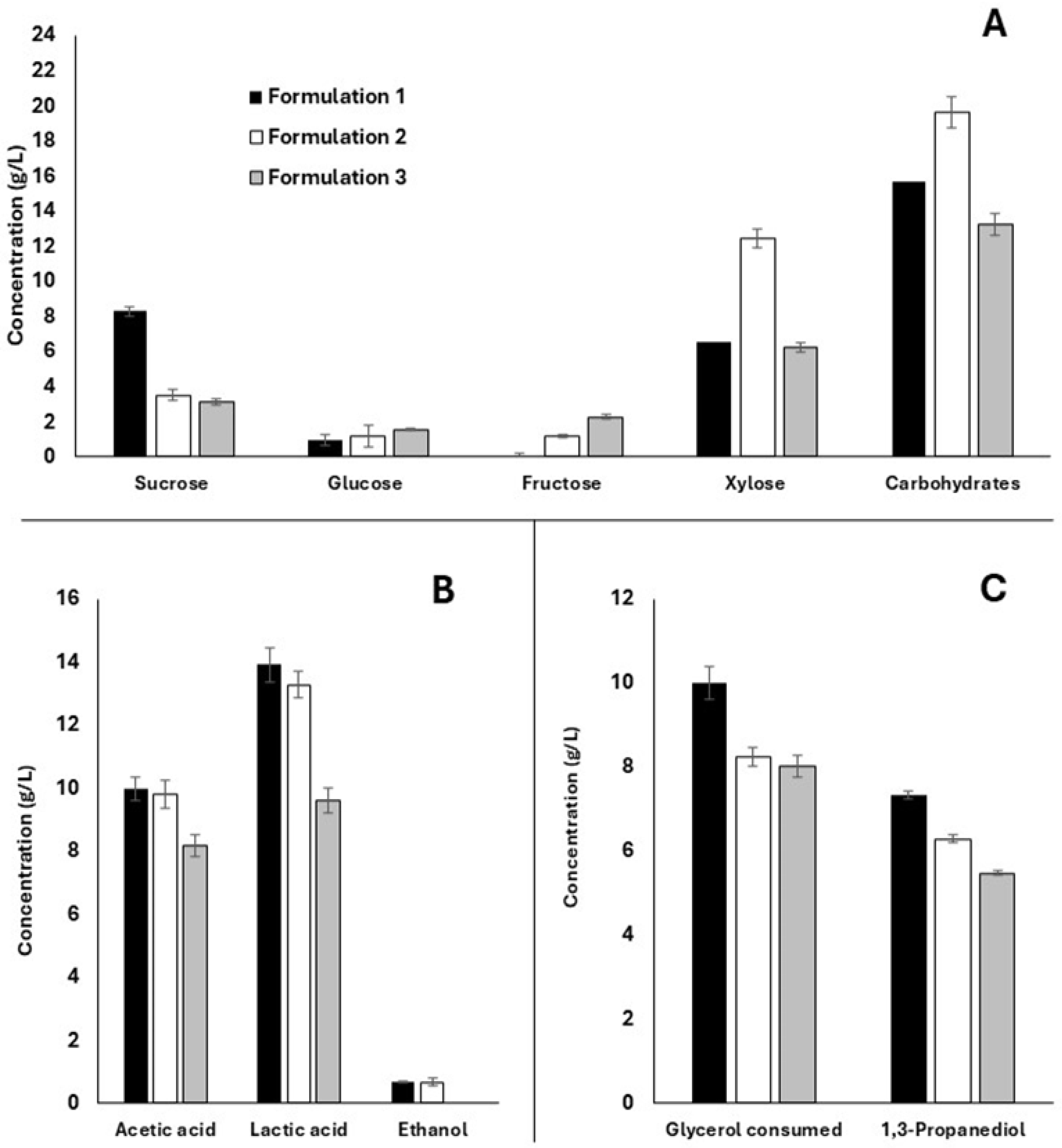
| Parameter | Molasses | SBH |
|---|---|---|
| Sucrose (g/L) | 255.48 ± 0.11 | 0.0 |
| Glucose (g/L) | 23.48 ± 1.36 | 3.60 ± 0.38 |
| Fructose (g/L) | 31.55 ± 1.21 | 0.0 |
| Xylose (g/L) | 0.00 | 14.77 ± 1.42 |
| Acetic acid (g/L) | 0.00 | 2.60 ± 0.09 |
| pH | 6.0 | 4.8 |
| Density | 1.35 | N.D. |
| Ashes (%) | 6.03 | N.D. |
| Parameter | Pure Glycerol | Organosolv Hydrolysate |
|---|---|---|
| Glucose consumed (g/L) | 21.38 ± 0.23 a | 20.51 ± 0.24 b |
| Acetic acid (g/L) | 6.04 ± 0.03 a | 5.63 ± 0.09 b |
| Lactic acid (g/L) | 13.90 ± 0.13 a | 13.68 ± 0.43 a |
| Ethanol (g/L) | 2.78 ± 0.12 a | 2.84 ± 0.13 a |
| Glycerol consumed (g/L) | 10.49 ± 0.42 a | 9.27 ± 0.35 b |
| 1,3-Propanediol (g/L) | 8.14 ± 0.06 a | 7.19 ± 0.05 b |
| Yp/s 1,3-PDO | 0.78 ± 0.03 a | 0.78 ± 0.02 a |
| Efficiency (%) | 94.04 ± 3.25 a | 93.99 ± 2.78 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, R.K.; da Silva, S.B.; da Paixão, G.A.; Júnior, F.G.F.; Araújo, M.F.; Rabelo, S.C.; Padilha, C.E.d.A.; Dutra, E.D.; da Costa, A.C.; de Morais Júnior, M.A.; et al. Valorization of Byproducts from the Sugarcane Industry Through Production of 1,3-Propanediol by Lentilactobacillus diolivorans. Fermentation 2025, 11, 554. https://doi.org/10.3390/fermentation11100554
Silva RK, da Silva SB, da Paixão GA, Júnior FGF, Araújo MF, Rabelo SC, Padilha CEdA, Dutra ED, da Costa AC, de Morais Júnior MA, et al. Valorization of Byproducts from the Sugarcane Industry Through Production of 1,3-Propanediol by Lentilactobacillus diolivorans. Fermentation. 2025; 11(10):554. https://doi.org/10.3390/fermentation11100554
Chicago/Turabian StyleSilva, Rayssa Karla, Sophia Bezerra da Silva, Giselle Alves da Paixão, Fábio Gabriel Ferreira Júnior, Michelle Fernandes Araújo, Sarita Cândida Rabelo, Carlos Eduardo de Araújo Padilha, Emmanuel Damilano Dutra, Aline Carvalho da Costa, Marcos Antônio de Morais Júnior, and et al. 2025. "Valorization of Byproducts from the Sugarcane Industry Through Production of 1,3-Propanediol by Lentilactobacillus diolivorans" Fermentation 11, no. 10: 554. https://doi.org/10.3390/fermentation11100554
APA StyleSilva, R. K., da Silva, S. B., da Paixão, G. A., Júnior, F. G. F., Araújo, M. F., Rabelo, S. C., Padilha, C. E. d. A., Dutra, E. D., da Costa, A. C., de Morais Júnior, M. A., & de Souza, R. B. (2025). Valorization of Byproducts from the Sugarcane Industry Through Production of 1,3-Propanediol by Lentilactobacillus diolivorans. Fermentation, 11(10), 554. https://doi.org/10.3390/fermentation11100554








