Enhanced Fermentation Process for Production of High Docosahexaenoic Acid Content by Schizochytrium sp. GCD2032
Abstract
1. Introduction
2. Materials and Methods
2.1. Strain and Medium
2.2. Experimental Methods
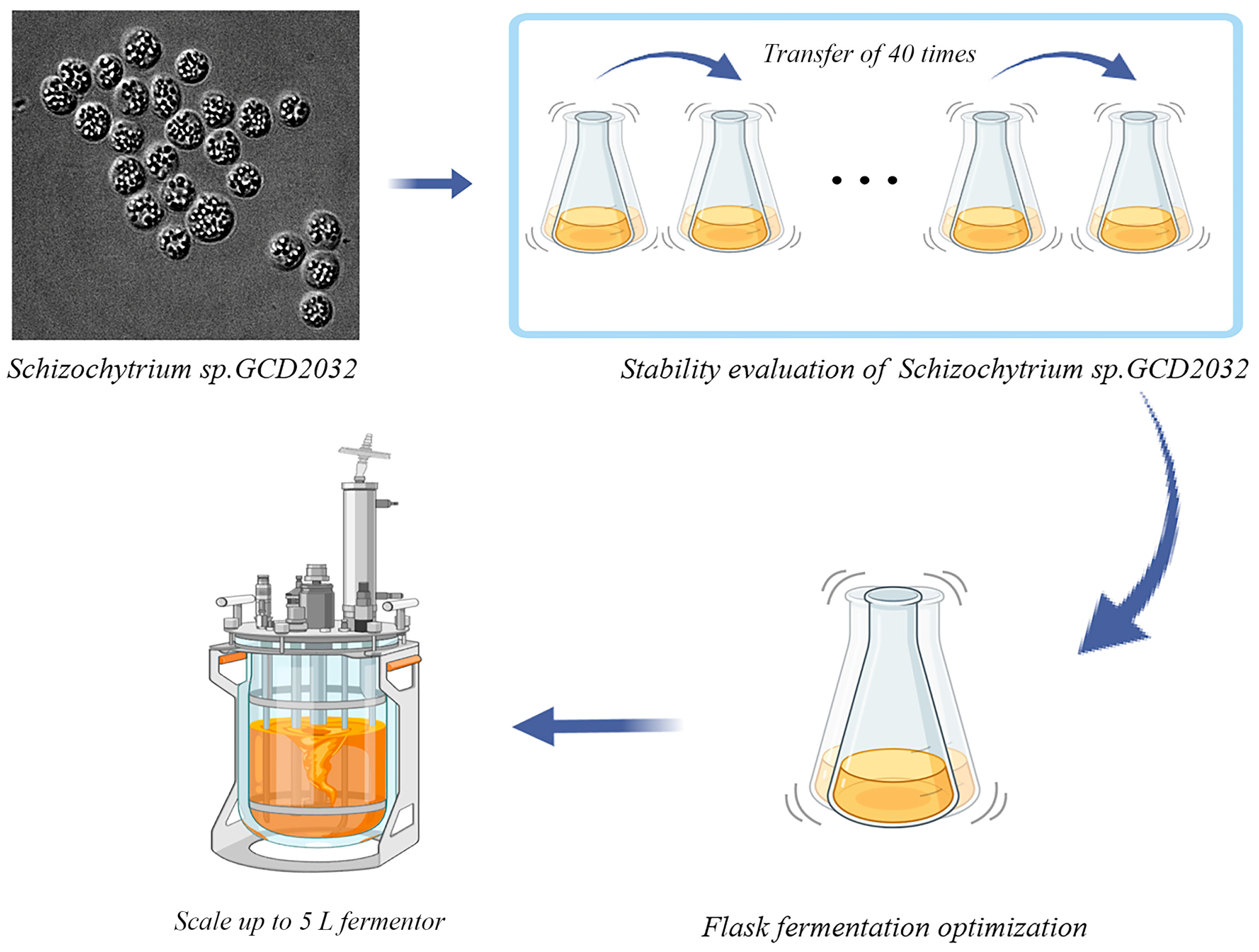
2.2.1. Preparation of Seeds
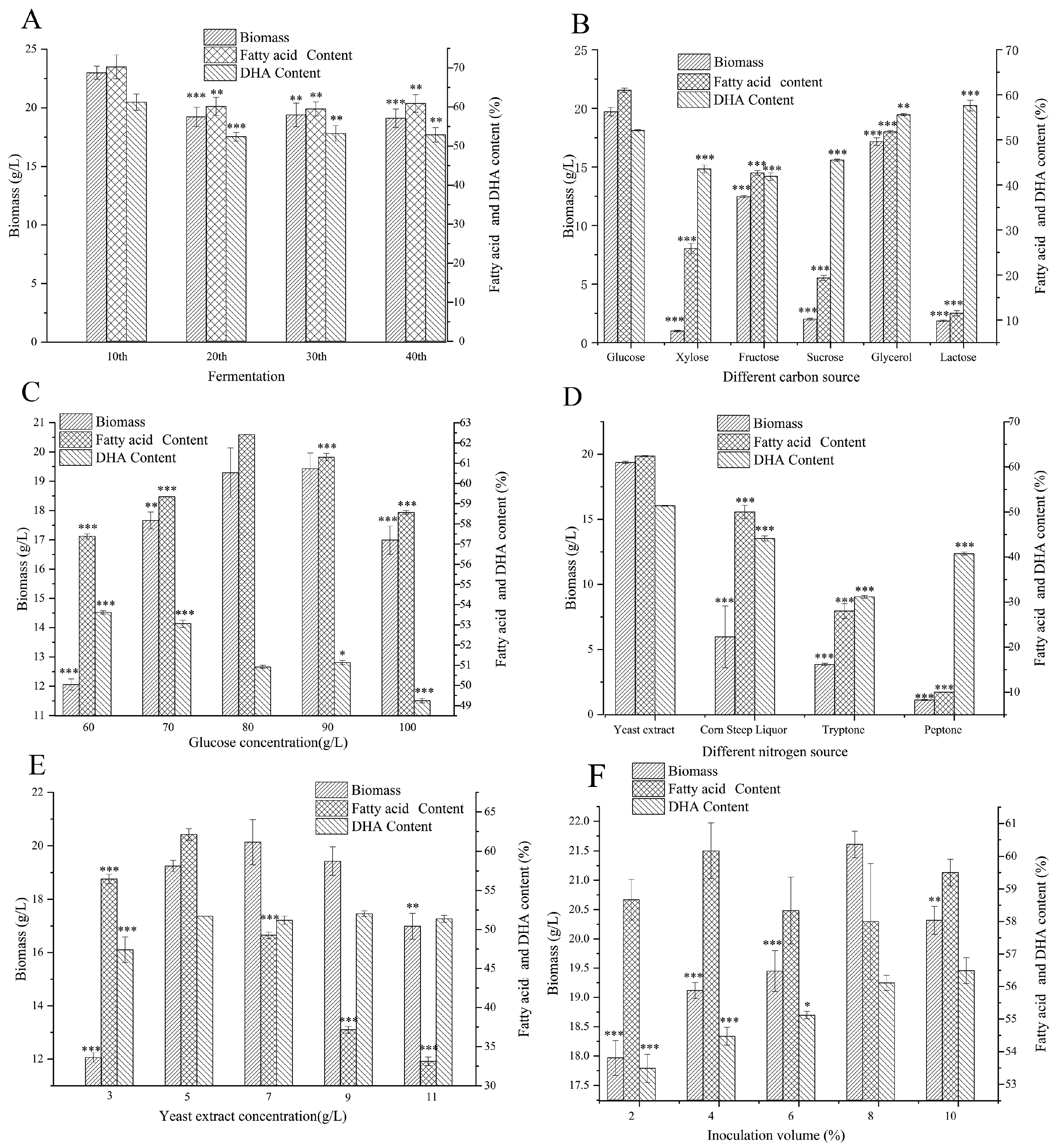
2.2.2. Strain Stability Evaluation
2.2.3. Single-Factor Fermentation Optimization of Schizochytrium sp. GCD2032
Carbon Source
Glucose Concentration
Nitrogen Source
Yeast Extract Concentration
Inoculation Volume
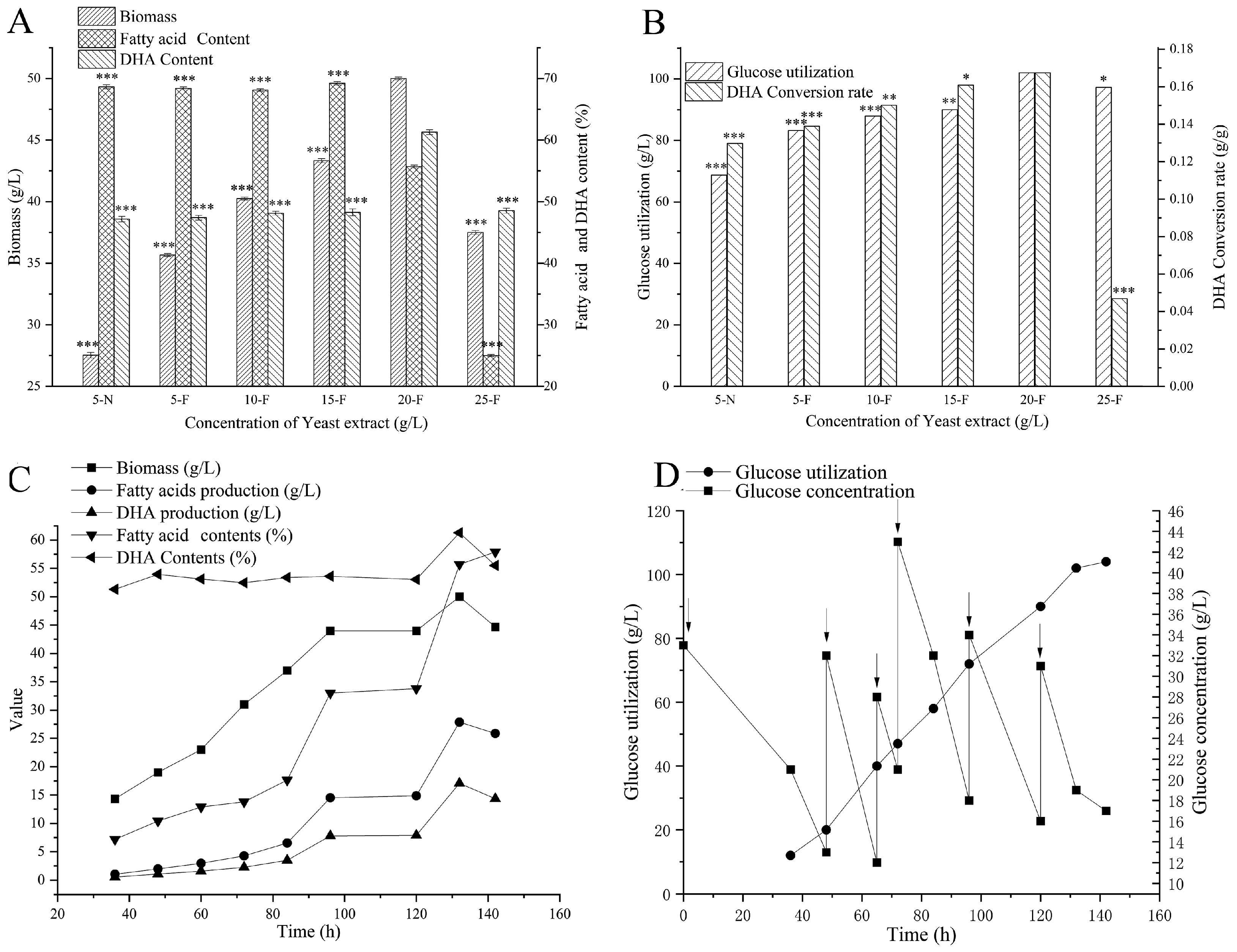
Effects of Different Concentrations of Yeast Extract in a 5 L Stirred Tank Fermenter
2.3. Analytical Methods
2.3.1. Glucose Concentration and Glucose Consumption Rate Determination
2.3.2. Determination of the Biomass
2.3.3. Fatty Acid Extraction and Quantification
2.3.4. Fatty Acid Composition and DHA Content Analysis
2.3.5. DHA Conversion Rate Analysis
2.4. Data Statistics and Analysis
3. Results
3.1. Stability Assessment of Schizochytrium sp. GCD2032 Fermentation
3.2. Shake Flask Fermentation Optimization of Schizochytrium sp. GCD2032
3.3. Scale up in 5 L Fermenters
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, Y.; Zhang, Y.; Su, G. Recent advances in treatment of retinitis pigmentosa. Curr. Stem. Cell Res. Ther. 2015, 10, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Allaire, J.; Couture, P.; Leclerc, M.; Charest, A.; Marin, J.; Lépine, M.C.; Talbot, D.; Tchernof, A.; Lamarche, B. A randomized, crossover, head-to-head comparison of eicosapentaenoic acid and docosahexaenoic acid supplementation to reduce inflammation markers in men and women: The Comparing EPA to DHA (ComparED) Study. Am. J. Clin. Nutr. 2016, 104, 280–287. [Google Scholar] [CrossRef]
- Asztalos, I.B.; Gleason, J.A.; Sever, S.; Gedik, R.; Asztalos, B.F.; Horvath, K.V.; Dansinger, M.L.; Lamon-Fava, S.; Schaefer, E.J. Effects of eicosapentaenoic acid and docosahexaenoic acid on cardiovascular disease risk factors: A randomized clinical trial. Metabolism 2016, 65, 1636–1645. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, A.M.; Ding, E.L.; Willett, W.C.; Rimm, E.B. A meta-analysis shows that docosahexaenoic acid from algal oil reduces serum triglycerides and increases HDL-cholesterol and LDL-cholesterol in Persons without coronary heart disease1–23. J. Nutr. 2012, 142, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Can Bioactive Lipids Inactivate Coronavirus (COVID-19)? Arch. Med. Res. 2020, 51, 282–286. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, Y.; Gao, H.; Li, D.; Jiang, R.; Ge, L.; Tong, C.; Xu, K. Associations among Dietary omega-3 polyunsaturated fatty acids, the Gut Microbiota, and Intestinal Immunity. Mediat. Inflamm. 2021, 2021, 8879227. [Google Scholar] [CrossRef]
- Younge, N.; Yang, Q.; Seed, P.C. Enteral high fat-polyunsaturated fatty acid blend alters the pathogen composition of the intestinal microbiome in premature infants with an enterostomy. J. Pediatr. 2017, 181, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Oh, C.E.; Kim, G.J.; Park, S.J.; Choi, S.; Park, M.J.; Lee, O.M.; Seo, J.W.; Son, H.J. Purification of high purity docosahexaenoic acid from Schizochytrium sp. SH103 using preparative-scale HPLC. Appl. Biol. Chem. 2020, 63, 56. [Google Scholar] [CrossRef]
- Chen, L.; Liu, X.; Li, C.; Li, H.; Chen, W.; Li, D. Transcriptome analyses reveal the DHA enhancement mechanism in Schizochytrium limacinum LD11 mutant. Algal Res. 2022, 67, 102868. [Google Scholar] [CrossRef]
- Chen, L.; Tong, S.; Liu, W.; Zhang, Y.; Khalid, H.; Long, L.; Li, Y.; Li, D.; Yan, B.; Chen, G. Electroporation-induced mutation and transcriptome analysis for high DHA production in Schizochytrium limacinum GCD2032. Algal Res. 2023, 76, 103297. [Google Scholar] [CrossRef]
- Jiang, Y.; Fan, K.W.; Wong, R.T.; Chen, F. Fatty acid composition and squalene content of the marine microalga Schizochytrium mangrovei. J. Agric. Food. Chem. 2004, 52, 1196–1200. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Chen, L.; Liu, J.; Gao, F.; He, R.; Chen, W.; Guo, W.; Chen, S.; Li, D. Effects of butanol on high value product production in Schizochytrium limacinum B4D1. Enzyme Microb. Technol. 2017, 102, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Min, Q.; Xu, J.; Zhang, K.; Chen, S.; Wang, H.; Li, D. Effect of malate on docosahexaenoic acid production from Schizochytrium sp. B4D1 Electron. J. Biotechnol. 2016, 19, 56–60. [Google Scholar]
- Wang, Z.; Wang, S.; Feng, Y.; Wan, W.; Zhang, H.; Bai, X.; Cui, Q.; Song, X. Obtaining high-purity docosahexaenoic acid oil in Thraustochytrid aurantiochytrium through a combined metabolic engineering strategy. J. Agric. Food Chem. 2021, 69, 10215–10222. [Google Scholar] [CrossRef] [PubMed]
- Castro-Cosio, P.; Monreal-Escalante, E.; Romero-Geraldo, R.; Angulo, C. Natural and recombinant bioactive compounds from Schizochytrium sp.: Recent advances and future prospects. Algal Res. 2023, 75, 103273. [Google Scholar] [CrossRef]
- Półbrat, T.; Konkol, D.; Korczyński, M. Optimization of docosahexaenoic acid production by Schizochytrium sp.—A review. Biocatal. Agric. Biotechnol. 2021, 35, 102076. [Google Scholar]
- Ding, J.; Fu, Z.; Zhu, Y.; He, J.; Ma, L.; Bu, D. Enhancing docosahexaenoic acid production of Schizochytrium sp. by optimizing fermentation using central composite design. BMC Biotechnol. 2022, 22, 39. [Google Scholar] [CrossRef]
- Yokochi, T.; Honda, D.; Higashihara, T.; Nakahara, T. Optimization of docosahexaenoic acid production by Schizochytrium limacinum SR21. Appl. Microbiol. Biotechnol. 1998, 49, 72–76. [Google Scholar] [CrossRef]
- Jia, Y.L.; Zhang, Y.; Xu, L.W.; Zhang, Z.X.; Xu, Y.S.; Ma, W.; Gu, Y.; Sun, X.M. Enhanced fatty acid storage combined with the multi-factor optimization of fermentation for high-level production of docosahexaenoic acid in Schizochytrium sp. Bioresour. Technol. 2024, 398, 130532. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.L.; Yang, L.H.; He, S.J.; Du, Y.H.; Guo, D.S. Development of a green fermentation strategy with resource cycle for the docosahexaenoic acid production by Schizochytrium sp. Bioresour. Technol. 2023, 385, 129434. [Google Scholar] [CrossRef]
- Zhu, X.; Meng, C.; Du, H.; Chen, L.; Sun, F.; Chen, W.; Wei, Z.; Ren, J.; Gao, Z.; Li, D. Enhancement of astaxanthin production in Schizochytrium limacinum B4D1 under ethanol induction. Algal Res. 2022, 61, 102537. [Google Scholar] [CrossRef]
- Guo, D.S.; Ji, X.J.; Ren, L.J.; Yin, F.W.; Sun, X.M.; Huang, H.; Zhen, G. Development of a multi-stage continuous fermentation strategy for docosahexaenoic acid production by Schizochytrium sp. Bioresour. Technol. 2018, 269, 32–39. [Google Scholar] [CrossRef]
- Keskin, A.; Ünlü, A.E.; Takaç, S. Utilization of olive mill wastewater for selective production of lipids and carotenoids by Rhodotorula glutinis. Appl. Microbiol. Biotechnol. 2023, 107, 4973–4985. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Li, Y.; Mbifile, M.D.; Li, C.; Yang, H.; Wang, W. Improvement of docosahexaenoic acid fermentation from Schizochytrium sp. AB-610 by staged pH control based on cell morphological changes. Eng. Life Sci. 2017, 17, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.J.; Huang, H.; Xiao, A.H.; Lian, M.; Jin, L.J.; Ji, X.J. Enhanced docosahexaenoic acid production by reinforcing acetyl-CoA and NADPH supply in Schizochytrium sp. HX-308. Bioprocess. Biosyst. Eng. 2009, 32, 837–843. [Google Scholar] [CrossRef]
- Tene, S.T.; Zokou, R.; Albendea, P.; Bemmo, L.G.D.; Purcaro, G.; Womeni, H.M. Effect of derivatization method (KOH and BF3) on fatty acid profile data of boiled Tetracarpidium conophorum, and egusi pudding oils. Data Brief. 2024, 54, 110362. [Google Scholar] [CrossRef] [PubMed]
- Martzy, R.; Mello-de-Sousa, T.M.; Mach, R.L.; Yaver, D.; Mach-Aigner, A.R. The phenomenon of degeneration of industrial Trichoderma reesei strains. Biotechnol. Biofuels 2021, 14, 193. [Google Scholar] [CrossRef] [PubMed]
- Danner, C.; Mach, R.L.; Mach-Aigner, A.R. The phenomenon of strain degeneration in biotechnologically relevant fungi. Appl. Microbiol. Biotechnol. 2023, 107, 4745–4758. [Google Scholar] [CrossRef]
- Heggeset, T.M.B.; Ertesvåg, H.; Liu, B.; Ellingsen, T.E.; Vadstein, O.; Aasen, I.M. Lipid and DHA-production in Aurantiochytrium sp.—Responses to nitrogen starvation and oxygen limitation revealed by analyses of production kinetics and global transcriptomes. Sci. Rep. 2019, 9, 19470. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Han, X.; Chen, Z.; Yan, Y.; Chen, Z. Selectively superior production of docosahexaenoic acid in Schizochytrium sp. through engineering the fatty acid biosynthetic pathways. Biotechnol. Biofuels 2024, 17, 75. [Google Scholar] [CrossRef]
- Zhao, G.; Chen, M.; Liu, J.; Wang, S.; Fu, D.; Zhang, C. Concentration-dependent dual roles of proanthocyanidins on oxidative stress and docosahexaenoic acid production in Schizochytrium sp. ATCC 20888. Bioresour. Technol. 2024, 398, 130537. [Google Scholar] [CrossRef] [PubMed]
- Ip, C.H.; Higuchi, H.; Wu, C.Y.; Okuda, T.; Katsuya, S.; Ogawa, J.; Ando, A. Production of docosahexaenoic acid by a novel isolated Aurantiochytrium sp. 6-2 using fermented defatted soybean as a nitrogen source for sustainable fish feed development. Biosci. Biotechnol. Biochem. 2024, 88, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.S.; Patel, A.K.; Singhania, R.R.; Vadrale, A.P.; Chen, C.W.; Giri, B.S.; Chang, J.S.; Dong, C.D. Fine-tuning of key parameters to enhance biomass and nutritional polyunsaturated fatty acids production from Thraustochytrium sp. Bioresour. Technol. 2024, 394, 130252. [Google Scholar] [CrossRef]
- Li, J.; Zheng, Y.; Yang, W.Q.; Wei, Z.Y.; Xu, Y.S.; Zhang, Z.X.; Ma, W.; Sun, X.M. Enhancing the accumulation of lipid and docosahexaenoic acid in Schizochytrium sp. by co-overexpression of phosphopantetheinyl transferase and ω-3 fatty acid desaturase. Biotechnol. J. 2023, 18, 2300314. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; Rani, R.; Gupta, R.; Mathur, A.; Ramakumar, S.S.V. Simultaneous production of high-value lipids in Schizochytrium sp. by synergism of chemical modulators. Appl. Microbiol. Biot. 2023, 107, 6135–6149. [Google Scholar] [CrossRef] [PubMed]
| Strains | Working Volume/Reactor Volume | Biomass | Fatty Acid Content | DHA Content |
|---|---|---|---|---|
| A recombinant strain of OPKSABC-PPT originated from Schizochytrium sp. ATCC20888 [30] | - | - | - | 40.60% |
| Schizochytrium sp. HX-308 (CCTCC M209059) overexpressing the diacylglycerol acyltransferase (ScDGAT2C) gene [19] | 100 mL/250 mL | - | - | 50.10% |
| Schizochytrium sp. ATCC 20888 with 5 mg/L proanthocyanidins [31] | 50 mL/250 mL | 36.7 g/L | 55.31% | 48.30% |
| Aurantiochytrium sp. 6–2 [32] | 1 L/2 L | - | - | 28.80% |
| Thraustochytrium sp. [33] | 100 mL/250 mL | 9.88 g/L | 66.50% | 24.80% |
| Schizochytrium sp. co-overexpressed PPTase and ω-3 FAD. [34] | -/5 L | - | - | 55.70% |
| Schizochytrium sp. with seawater and fermentation wastewater as fermentation broth [20] | 30 L/50 L | 195.8 g/L | 38% | 62.40% |
| The synergistic effect of chemical regulators is applied to Schizochytrium sp. [35] | 1.5 L/2 L | 2.04 ± 1.12 g/L/d | 49.02% | 38.60% |
| Schizochytrium sp. GCD2032 | 3.5 L/5 L | 50 g/L | 55.71% | 61.29% (this study) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, L.; Ren, X.; Zhang, F.; Shi, A.; Zhai, Y.; Chen, W.; Duan, Y.; Shi, P.; Chen, L.; Li, D. Enhanced Fermentation Process for Production of High Docosahexaenoic Acid Content by Schizochytrium sp. GCD2032. Fermentation 2024, 10, 460. https://doi.org/10.3390/fermentation10090460
Long L, Ren X, Zhang F, Shi A, Zhai Y, Chen W, Duan Y, Shi P, Chen L, Li D. Enhanced Fermentation Process for Production of High Docosahexaenoic Acid Content by Schizochytrium sp. GCD2032. Fermentation. 2024; 10(9):460. https://doi.org/10.3390/fermentation10090460
Chicago/Turabian StyleLong, Liucheng, Xiaoqing Ren, Feiyu Zhang, Aijia Shi, Yida Zhai, Wuxi Chen, Yu Duan, Pengbao Shi, Limei Chen, and Demao Li. 2024. "Enhanced Fermentation Process for Production of High Docosahexaenoic Acid Content by Schizochytrium sp. GCD2032" Fermentation 10, no. 9: 460. https://doi.org/10.3390/fermentation10090460
APA StyleLong, L., Ren, X., Zhang, F., Shi, A., Zhai, Y., Chen, W., Duan, Y., Shi, P., Chen, L., & Li, D. (2024). Enhanced Fermentation Process for Production of High Docosahexaenoic Acid Content by Schizochytrium sp. GCD2032. Fermentation, 10(9), 460. https://doi.org/10.3390/fermentation10090460




