Assessing Waste Sunflower Oil as a Substrate for Citric Acid Production: The Inhibitory Effect of Triton X-100
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms
2.2. Collection of Sunflower Oils
2.3. Characterization of Sunflower Oils
2.3.1. Determination of Fatty Acid Compositions of Oils
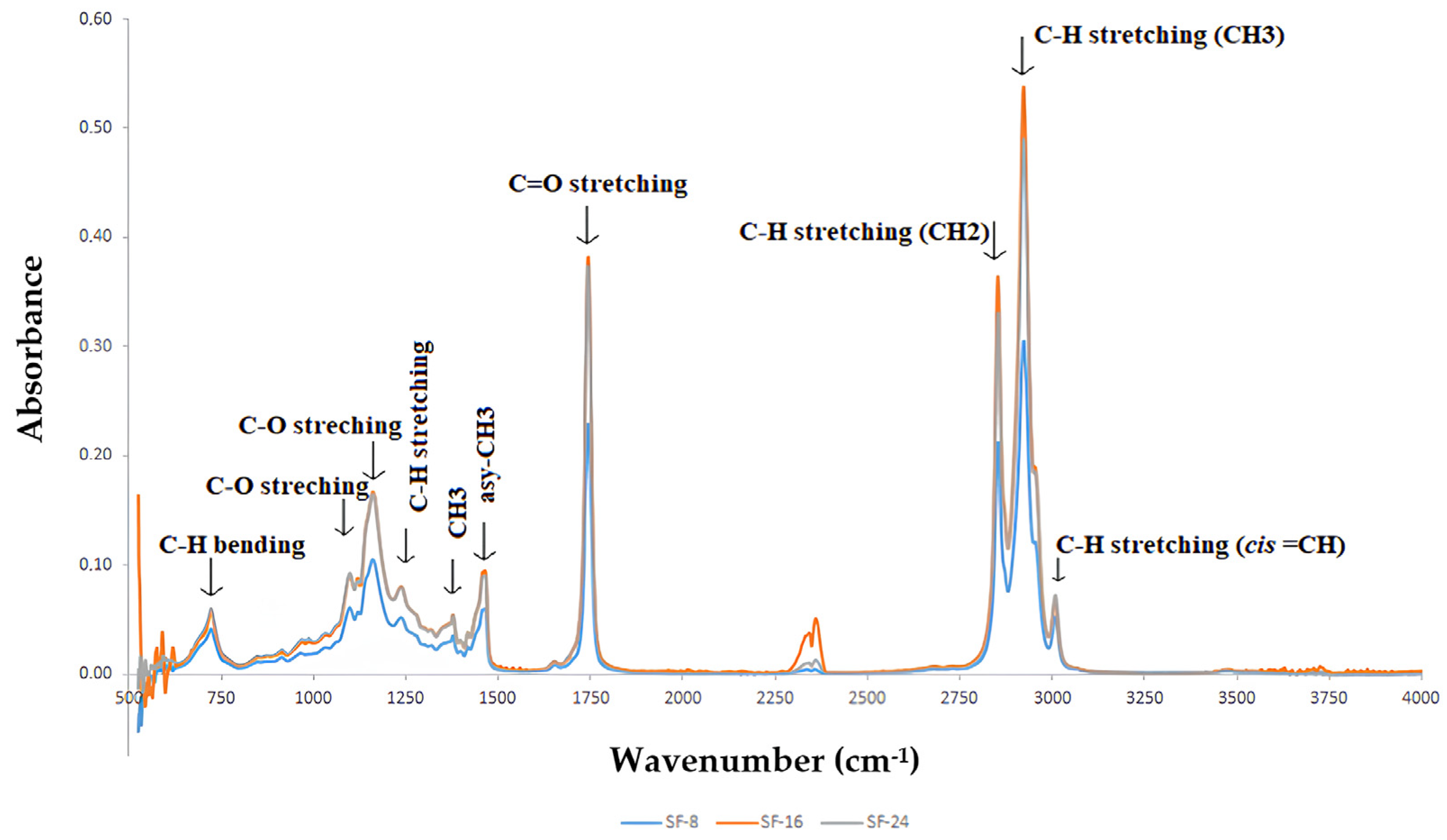
2.3.2. FTIR Analysis
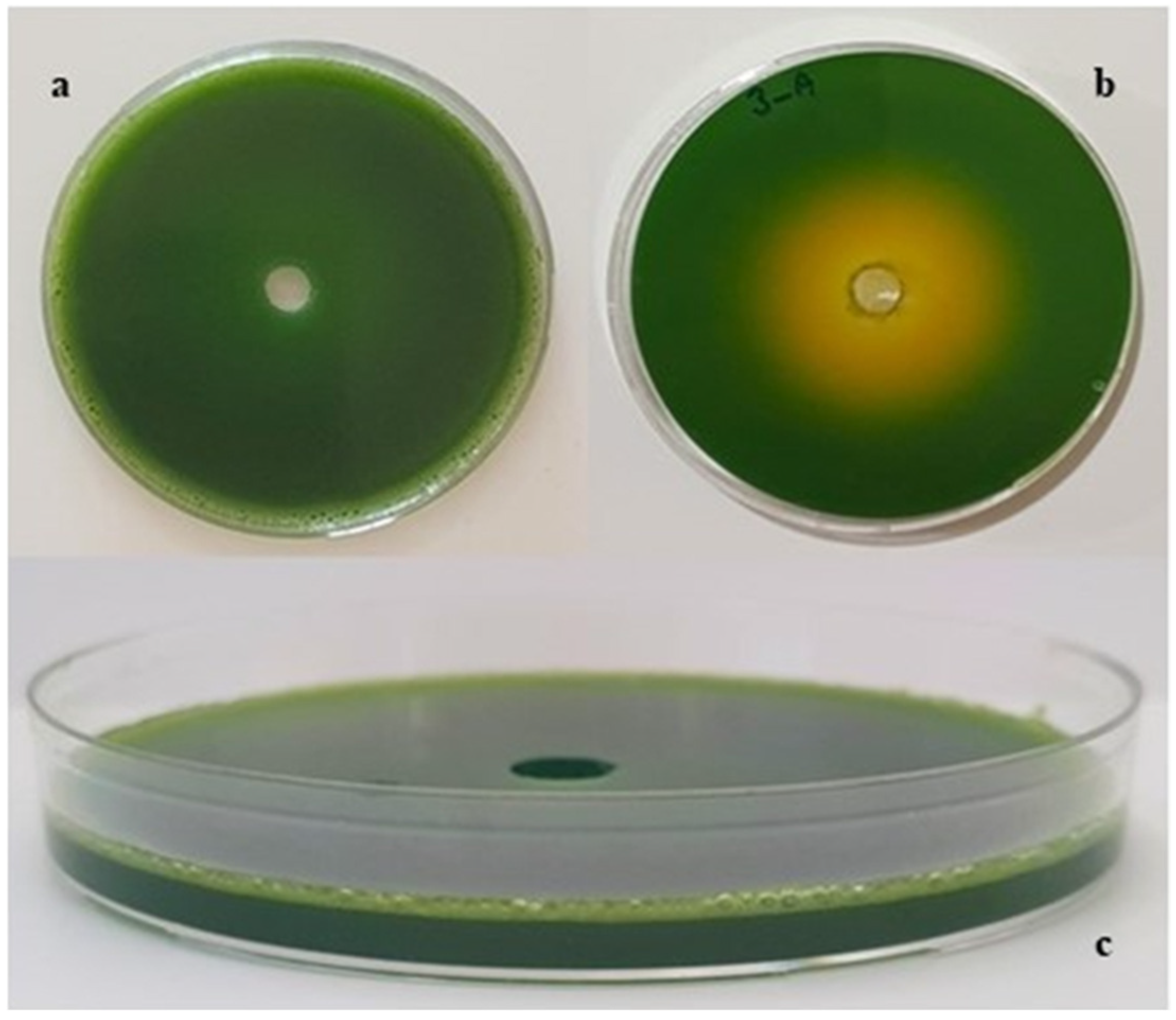
2.4. Screening of Y. lipolytica Strains for Acid Production Capacities
2.5. Fermentation Conditions
2.6. Determination of CA, ICA, and Biomass Concentrations
2.7. Process Optimization
3. Results and Discussion
3.1. Characterization of Sunflower Oil Samples
3.2. Screening of Y. lipolytica Strains for CA Production
3.3. Results of Experimental Design 1
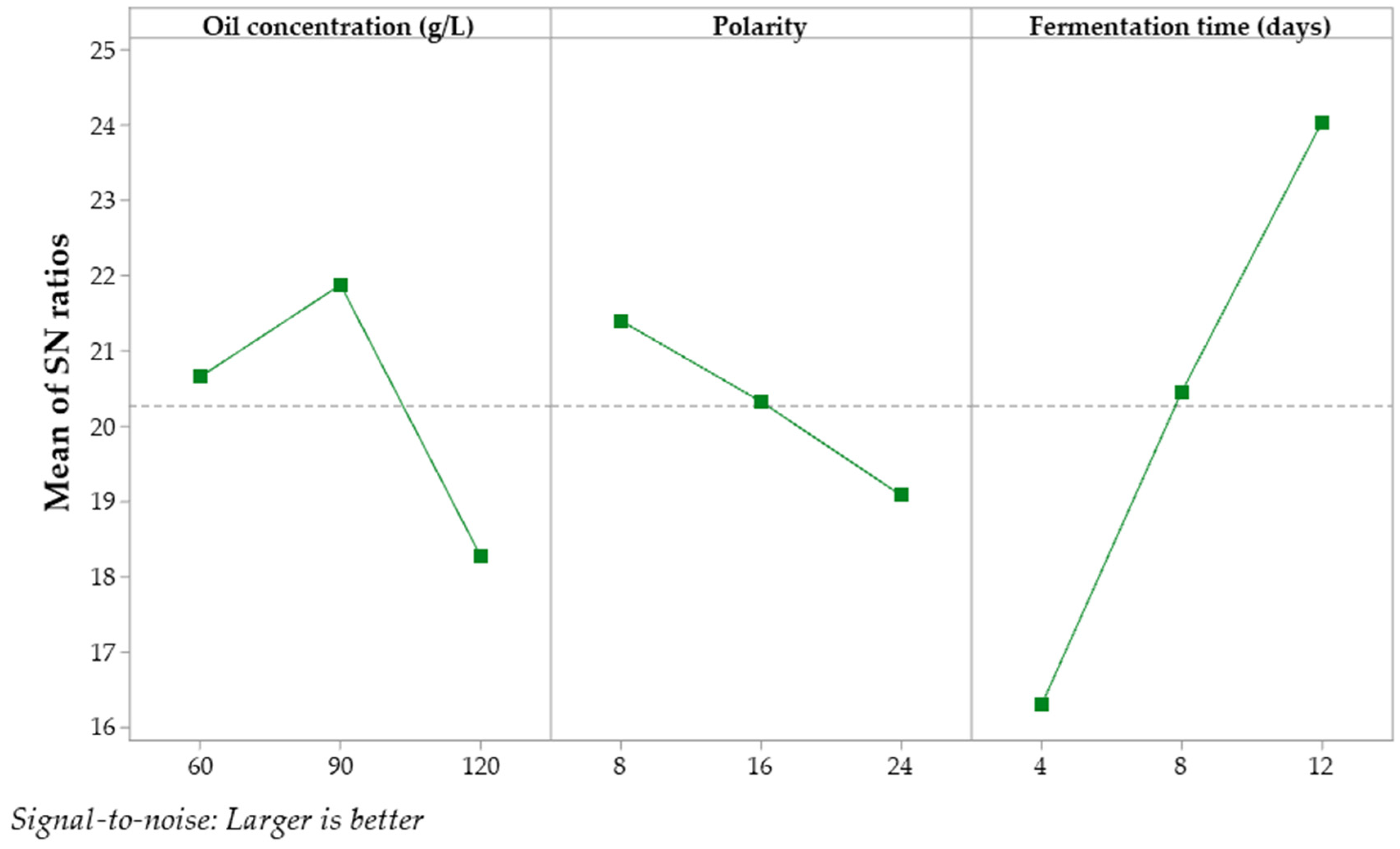
3.4. Results of Experimental Design 2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Suzuki, A.H.; Botelho, B.G.; Oliveira, L.S.; Franca, A.S. Sustainable synthesis of epoxidized waste cooking oil and its application as a plasticizer for polyvinyl chloride films. Eur. Polym. J. 2018, 99, 142–149. [Google Scholar] [CrossRef]
- Sarno, M.; Iuliano, M.; Cirillo, C. Optimized procedure for the preparation of an enzymatic nanocatalyst to produce a bio-lubricant from waste cooking oil. J. Chem. Eng. 2019, 377, 120273. [Google Scholar] [CrossRef]
- Lopes, M.; Miranda, S.M.; Belo, I. Microbial valorization of waste cooking oils for valuable compounds production–a review. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2583–2616. [Google Scholar] [CrossRef]
- Tong, Z.; Tong, Y.; Wang, D.; Shi, Y.C. Whole maize flour and isolated maize starch for production of citric acid by Aspergillus niger: A review. Starch-Stärke 2023, 75, 2000014. [Google Scholar] [CrossRef]
- Książek, E. Citric acid: Properties, microbial production, and applications in industries. Molecules 2023, 29, 22. [Google Scholar] [CrossRef] [PubMed]
- Kamzolova, S.V. A Review on citric acid production by Yarrowia lipolytica yeast: Past and present challenges and developments. Processes 2023, 1, 3435. [Google Scholar] [CrossRef]
- Kamzolova, S.V.; Lunina, J.N.; Morgunov, I.G. Biochemistry of citric acid production from rapeseed oil by Yarrowia lipolytica yeast. J. Am. Oil Chem. Soc. 2011, 88, 1965–1976. [Google Scholar] [CrossRef]
- Börekçi, B.S.; Kaban, G.; Kaya, M. Citric acid production of yeasts: An overview. EuroBiotech J. 2021, 5, 79–91. [Google Scholar] [CrossRef]
- Tan, M.J.; Chen, X.; Wang, Y.K.; Liu, G.L.; Chi, Z.M. Enhanced citric acid production by a yeast Yarrowia lipolytica over-expressing a pyruvate carboxylase gene. Bioprocess Biosyst. Eng. 2016, 39, 1289–1296. [Google Scholar] [CrossRef]
- Darvishi, F.; Nahvi, I.; Zarkesh-Esfahani, H.; Momenbeik, F. Effect of plant oils upon lipase and citric acid production in Yarrowia lipolytica yeast. J Biomed Biotechnol. 2009, 2009, 562943. [Google Scholar] [CrossRef]
- Liu, X.; Lv, J.; Xu, J.; Zhang, T.; Deng, Y.; He, J. Citric acid production in Yarrowia lipolytica SWJ-1b yeast when grown on waste cooking oil. Appl. Biochem. Biotechnol. 2015, 175, 2347–2356. [Google Scholar] [CrossRef] [PubMed]
- Katre, G.; Ajmera, N.; Zinjarde, S.; RaviKumar, A. Mutants of Yarrowia lipolytica NCIM 3589 grown on waste cooking oil as a biofactory for biodiesel production. Microb. Cell Fact. 2017, 16, 176. [Google Scholar] [CrossRef] [PubMed]
- Katre, G.; Raskar, S.; Zinjarde, S.; Kumar, V.R.; Kulkarni, B.D.; RaviKumar, A. Optimization of the in situ transesterification step for biodiesel production using biomass of Yarrowia lipolytica NCIM 3589 grown on waste cooking oil. Energy 2018, 142, 944–952. [Google Scholar] [CrossRef]
- Gohain, M.; Bardhan, P.; Laskar, K.; Sarmah, S.; Mandal, M.; Bora, U.; Kalita, M.C.; Goud, V.V.; Deka, D. Rhodotorula mucilaginosa: A source of heterogeneous catalyst for biodiesel production from yeast single cell oil and waste cooking oil. Renew. Energy 2020, 160, 220–230. [Google Scholar] [CrossRef]
- Raut, G.; Jagtap, S.; Kumar, V.R.; RaviKumar, A. Enhancing lipid content of oleaginous Yarrowia lipolytica biomass grown on waste cooking oil and its conversion to biodiesel by statistical optimization. Biomass Convers. Biorefin. 2022, 1–18. [Google Scholar] [CrossRef]
- Batista, R.M.; Rufino, R.D.; Luna, J.M.; Souza, J.E.G.; Sarubbo, L.A. Effect of medium components on the production of a biosurfactant from Candida tropicalis applied to the removal of hydrophobic contaminants in soil. Water Environ. Res. 2010, 82, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Dzięgielewska, E.; Adamczak, M. Evaluation of waste products in the synthesis of surfactants by yeasts. Chem. Pap. 2013, 67, 1113–1122. [Google Scholar] [CrossRef]
- Campos, J.M.; Stamford, T.L.M.; Sarubbo, L.A. Production of a bioemulsifier with potential application in the food industry. Appl. Biochem. Biotechnol. 2014, 172, 3234–3252. [Google Scholar] [CrossRef]
- Almeida, D.G.; Silva, R.C.F.S.; Luna, J.M.; Rufino, R.D.; Santos, V.A.; Sarubbo, L.A. Response surface methodology for optimizing the production of biosurfactant by Candida tropicalis on industrial waste substrates. Front. Microbiol. 2017, 8, 157. [Google Scholar] [CrossRef]
- Csutak, O.; Corbu, V.; Stoica, I.; Vassu, T. Fatty acids effect on lipase and biosurfactant induction in Rhodotorula glutinis CMGB-RG5. In “Agriculture for Life, Life for Agriculture”, Conference Proceedings; Sciendo: Warsaw, Poland, 2018; Volume 1, pp. 515–522. [Google Scholar] [CrossRef]
- Junior, R.B.R.; Meira, H.M.; Almeida, D.G.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Sarubbo, L.A. Application of a low-cost biosurfactant in heavy metal remediation processes. Biodegradation 2018, 30, 215–233. [Google Scholar] [CrossRef]
- Souza, P.M.; Silva, N.R.A.; Souza, D.G.; Silva, T.A.L.; Freitas-Silva, M.C.; Andrade, R.F.S.; Silva, G.K.B.; Albuquerque, C.D.C.; Messias, A.S.; Campos-Takaki, G.M. Production of a biosurfactant by Cunninghamella echinulata using renewable substrates and its applications in enhanced oil spill recovery. Colloids Interfaces 2018, 2, 63–74. [Google Scholar] [CrossRef]
- Liepins, J.; Balina, K.; Soloha, R.; Berzina, I.; Lukasa, L.K.; Dace, E. Glycolipid biosurfactant production from waste cooking oils by yeast: Review of substrates, producers and products. Fermentation 2021, 7, 136. [Google Scholar] [CrossRef]
- Nunes, P.M.B.; Martins, A.B.; Brigida, A.I.S.; Rocha-Leao, M.H.M.; Amaral, P. Intracellular lipase production by Yarrowia lipolytica using different carbon sources. Chem. Eng. Trans. 2014, 38, 421–426. [Google Scholar]
- Lopes, M.; Miranda, S.M.; Alves, J.M.; Pereira, A.S.; Belo, I. Waste cooking oils as feedstock for lipase and lipid-rich biomass production. Eur. J. Lipid Sci. Technol. 2019, 121, 1800188. [Google Scholar] [CrossRef]
- Domínguez, A.; Deive, F.J.; Angeles Sanromán, M.; Longo, M.A. Biodegradation and utilization of waste cooking oil by Yarrowia lipolytica CECT 1240. Eur. J. Lipid Sci. Technol. 2010, 112, 1200–1208. [Google Scholar] [CrossRef]
- Moftah, O.A.S.; Grbavčić, S.; Žuža, M.; Luković, N.; Bezbradica, D.; Knežević-Jugović, Z. Adding value to the oil cake as a waste from oil processing industry: Production of lipase and protease by Candida utilis in solid state fermentation. Appl. Biochem. Biotechnol. 2012, 166, 348–364. [Google Scholar] [CrossRef]
- Snopek, P.; Nowak, D.; Zieniuk, B.; Fabiszewska, A. Aeration and stirring in Yarrowia lipolytica lipase biosynthesis during batch cultures with waste fish oil as a carbon source. Fermentation 2021, 7, 88. [Google Scholar] [CrossRef]
- Fraga, J.L.; Souza, C.P.; Pereira, A.D.S.; Aguieiras, E.C.; de Silva, L.O.; Torres, A.G.; Freire, D.G.; Amaral, P.F. Palm oil wastes as feedstock for lipase production by Yarrowia lipolytica and biocatalyst application/reuse. 3 Biotech 2021, 11, 191. [Google Scholar] [CrossRef]
- Colacicco, M.; Ciliberti, C.; Agrimi, G.; Biundo, A.; Pisano, I. Towards the physiological understanding of Yarrowia lipolytica growth and lipase production using waste cooking oils. Energies 2022, 15, 5217. [Google Scholar] [CrossRef]
- El Bialy, H.; Gomaa, O.M.; Azab, K.S. Conversion of oil waste to valuable fatty acids using oleaginous yeast. World J. Microbiol. Biotechnol. 2011, 27, 2791–2798. [Google Scholar] [CrossRef]
- Katre, G.; Joshi, C.; Khot, M.; Zinjarde, S.; Ravikumar, A. Evaluation of single cell oil (SCO) from a tropical marine yeast Yarrowia lipolytica NCIM 3589 as a potential feedstock for biodiesel. AMB Express 2012, 2, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Poli, J.S.; da Silva, M.A.N.; Siqueira, E.P.; Pasa, V.M.; Rosa, C.A.; Valente, P. Microbial lipid produced by Yarrowia lipolytica QU21 using industrial waste: A potential feedstock for biodiesel production. Bioresour. Technol. 2014, 161, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Miranda, S.M.; Pereira, A.S.; Belo, I.; Lopes, M. Waste cooking oils: Low-cost substrate for co-production of lipase and microbial lipids. In WASTES—Solutions, Treatments and Opportunities II; CRC Press: Boca Raton, FL, USA, 2017; pp. 99–104. [Google Scholar]
- Niehus, X.; Casas-Godoy, L.; Rodríguez-Valadez, F.J.; Sandoval, G. Evaluation of Yarrowia lipolytica oil for biodiesel production: Land use oil yield, carbon, and energy balance. J. Lipids 2018, 2018, 6393749. [Google Scholar] [CrossRef] [PubMed]
- Tzirita, M.; Papanikolaou, S.; Chatzifragkou, A.; Quilty, B. Waste fat biodegradation and biomodification by Yarrowia lipolytica and a bacterial consortium composed of Bacillus spp. and Pseudomonas putida. Eng. Life Sci. 2018, 18, 932–942. [Google Scholar] [CrossRef] [PubMed]
- Radha, P.; Narayanan, S.; Chaudhuri, A.; Anjum, S.; Thomas, D.L.; Pandey, R.; Ramani, K. Synthesis of single-cell oil by Yarrowia lipolytica MTCC 9520 utilizing slaughterhouse lipid waste for biodiesel production. Biomass Convers. Biorefin. 2020, 13, 1–12. [Google Scholar] [CrossRef]
- Pereira, A.S.; Lopes, M.; Miranda, S.M.; Belo, I. Bio-oil production for biodiesel industry by Yarrowia lipolytica from volatile fatty acids in two-stage batch culture. Appl. Biochem. Biotechnol. 2022, 106, 2869–2881. [Google Scholar] [CrossRef]
- Spalvins, K.; Geiba, Z.; Kusnere, Z.; Blumberga, D. Waste cooking oil as substrate for single cell protein production by yeast Yarrowia lipolytica. Rigas Teh. Univ. Zinat. Raksti. 2020, 24, 457–469. [Google Scholar] [CrossRef]
- Pang, Y.; Zhao, Y.; Li, S.; Zhao, Y.; Li, J.; Hu, Z.; Zhang, C.; Xiao, D.; Yu, A. Engineering the oleaginous yeast Yarrowia lipolytica to produce limonene from waste cooking oil. Biotechnol. Biofuels. 2019, 12, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Rong, L.; Miao, L.; Wang, S.; Wang, Y.; Liu, S.; Lu, Z.; Zhao, B.; Zhang, C.; Xiao, D.; Pushpanathan, K.; et al. Engineering Yarrowia lipolytica to produce itaconic acid from waste cooking oil. Front. Bioeng. Biotechnol. 2022, 10, 888869. [Google Scholar] [CrossRef]
- Mitrea, L.; Călinoiu, L.F.; Teleky, B.E.; Szabo, K.; Martău, A.G.; Ştefănescu, B.E.; Dulf, F.-V.; Vodnar, D.C. Waste cooking oil and crude glycerol as efficient renewable biomass for the production of platform organic chemicals through oleophilic yeast strain of Yarrowia lipolytica. Environ. Technol. Innov. 2022, 28, 102943. [Google Scholar] [CrossRef]
- Good, D.W.; Droniuk, R.; Lawford, G.R.; Fein, J.E. Isolation and characterization of a Saccharomycopsis lipolytica mutant showing increased production of citric acid from canola oil. Can. J. Microbiol. 1985, 31, 436–440. [Google Scholar] [CrossRef]
- Kamzolova, S.V.; Morgunov, I.G.; Aurich, A.; Perevoznikova, O.A.; Shishkanova, N.V.; Stottmeister, U.; Finogenova, T.V. Lipase secretion and citric acid production in Yarrowia lipolytica yeast grown on animal and vegetable fat. Food Technol. Biotech. 2005, 43, 113–122. [Google Scholar]
- Kamzolova, S.V.; Finogenova, T.V.; Morgunov, I.G. Microbiological production of citric and isocitric acids from sunflower oil. Food Technol. Biotech. 2008, 46, 51–59. [Google Scholar]
- Venter, T.; Kock, J.L.F.; Botes, P.J.; Smit, M.S.; Hugo, A.; Joseph, M. Acetate enhances citric acid production by Yarrowia lipolytica when grown on sunflower oil. Syst. Appl. Microbiol. 2004, 27, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Çelik, G.; Bahriye Uçar, F.; Akpınar, O.; Çorbacı, C. Production of citric and isocitric acid by Yarrowia lipolytica strains grown on different carbon sources. Turk. J. Biochem. 2014, 39, 285–290. [Google Scholar] [CrossRef]
- Liu, X.; Lv, J.; Xu, J.; Xia, J.; He, A.; Zhang, T.; Xu, X.; Xu, J. Effects of osmotic pressure and pH on citric acid and erythritol production from waste cooking oil by Yarrowia lipolytica. Eng. Life Sci. 2018, 18, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Liu, X.; Sun, W.; Lv, B.; Li, C. Current advances for omics-guided process optimization of microbial manufacturing. Bioresour. Bioprocess. 2023, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Taiwo, A.E.; Madzimbamuto, T.N.; Ojumu, T.V. Optimization of process variables for acetoin production in a bioreactor using Taguchi orthogonal array design. Heliyon 2020, 6, 05103. [Google Scholar] [CrossRef] [PubMed]
- Hesham, A.E.L.; Mostafa, Y.S.; AlSharqi, L.E.O. Optimization of citric acid production by immobilized cells of novel yeast isolates. Mycobiology 2020, 48, 122–132. [Google Scholar] [CrossRef]
- Kamzolova, S.V.; Morgunov, I.G. Metabolic peculiarities of the citric acid overproduction from glucose in yeasts Yarrowia lipolytica. Bioresour. Technol. 2017, 243, 433–440. [Google Scholar] [CrossRef]
- Alavi-Borazjani, S.A.; da Cruz Tarelho, L.A.; Capela, M.I. Parametric optimization of the dark fermentation process for enhanced biohydrogen production from the organic fraction of municipal solid waste using Taguchi method. Int. J. Hydrogen Energy 2021, 46, 21372–21382. [Google Scholar] [CrossRef]
- Chenthamarakshan, A.; Parambayil, N.; Miziriya, N.; Soumya, P.S.; Lakshmi, M.K.; Ramgopal, A.; Dileep, A.; Nambisan, P. Optimization of laccase production from Marasmiellus palmivorus LA1 by Taguchi method of design of experiments. BMC Biotechnol. 2017, 17, 12. [Google Scholar] [CrossRef] [PubMed]
- Kamil, F.H.; Salmiaton, A.; Hafriz, R.S.R.M.; Hussien, I.R.; Omar, R. Characterization and application of molten slag as catalyst in pyrolysis of waste cooking oil. Bull. Chem. React. Eng. Catal. 2020, 15, 119–127. [Google Scholar] [CrossRef]
- Poiana, M.A.; Alexa, E.; Munteanu, M.F.; Gligor, R.; Moigradean, D.; Mateescu, C. Use of ATR-FTIR spectroscopy to detect the changes in extra virgin olive oil by adulteration with soybean oil and high temperature heat treatment. Open Chem. 2015, 13, 489–498. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Ristivojevic, P.; Gegechkori, V.; Litvinova, T.M.; Morton, D.W. Essential Oil Quality and Purity Evaluation via FT-IR Spectroscopy and Pattern Recognition Techniques. Appl. Sci. 2020, 10, 7294. [Google Scholar] [CrossRef]
- Vlachos, N.; Skopelitis, Y.; Psaroudaki, M.; Konstantinidou, V.; Chatzilazarou, A.; Tegou, E. Applications of Fourier transform-infrared spectroscopy to edible oils. Anal. Chim. Acta. 2006, 573, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Mousa, M.A.; Wang, Y.; Antora, S.A.; Al-Qurashi, A.D.; Ibrahim, O.H.; He, H.J.; Liu, S.; Kamruzzaman, M. An overview of recent advances and applications of FT-IR spectroscopy for quality, authenticity, and adulteration detection in edible oils. Crit. Rev. Food Sci. Nutr. 2022, 62, 8009–8027. [Google Scholar] [CrossRef]
- Matwijczuk, A.; Zając, G.; Karcz, D.; Chruściel, E.; Matwijczuk, A.; Kachel-Jakubowska, M.; Łapczyńska-Kordon, B.; Gagoś, M. Spectroscopic studies of the quality of WCO (Waste Cooking Oil) fatty acid methyl esters. In BIO Web of Conferences; EDP Sciences: Paris, France, 2018; Volume 10, p. 02019. [Google Scholar]
- Jović, O.; Smolić, T.; Jurišić, Z.; Meić, Z.; Hrenar, T. Chemometric analysis of croatian extra virgin olive oils from central Dalmatia region. Croat. Chem. Acta 2013, 86, 335–344. [Google Scholar] [CrossRef]
- Guillén, M.D.; Cabo, N. Usefulness of the frequencies of some Fourier transform infrared spectroscopic bands for evaluating the composition of edible oil mixtures. Lipid/Fett 1999, 101, 71–76. [Google Scholar] [CrossRef]
- Mores, S.; de Souza Vandenberghe, L.P.; Júnior, A.I.M.; de Carvalho, J.C.; de Mello, A.F.M.; Pandey, A.; Soccol, C.R. Citric acid bioproduction and downstream processing: Status, opportunities, and challenges. Bioresour. Technol. 2021, 320, 124426. [Google Scholar] [CrossRef]
- Holz, M.; Förster, A.; Mauersberger, S.; Barth, G. Aconitase overexpression changes the product ratio of citric acid production by Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 2009, 81, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Hapeta, P.; Rakicka-Pustułka, M.; Juszczyk, P.; Robak, M.; Rymowicz, W.; Lazar, Z. Overexpression of citrate synthase increases isocitric acid biosynthesis in the yeast Yarrowia lipolytica. Sustainability 2020, 12, 7364. [Google Scholar] [CrossRef]
- Förster, A.; Jacobs, K.; Juretzek, T.; Mauersberger, S.; Barth, G. Overexpression of the ICL1 gene changes the product ratio of citric acid production by Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 2007, 77, 861–869. [Google Scholar] [CrossRef]
- Yuzbasheva, E.Y.; Scarcia, P.; Yuzbashev, T.V.; Messina, E.; Kosikhina, I.M.; Palmieri, L.; Shutov, A.V.; Taratynova, M.O.; Amaro, R.L.; Palmieri, F.; et al. Engineering Yarrowia lipolytica for the selective and high-level production of isocitric acid through manipulation of mitochondrial dicarboxylate–tricarboxylate carriers. Metab. Eng. 2021, 65, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Finogenova, T.; Kamzolova, S.; Dedyukhina, E.; Shishkanova, N.; Il’Chenko, A.; Morgunov, I.; Chernyavskaya, O.; Sokolov, A. Biosynthesis of citric and isocitric acids from ethanol by mutant Yarrowia lipolytica N 1 under continuous cultivation. Appl. Microbiol. Biotechnol. 2002, 59, 493–500. [Google Scholar] [CrossRef]
- Kamzolova, S.V.; Samoilenko, V.A.; Lunina, J.N.; Morgunov, I.G. Effects of medium components on isocitric acid production by Yarrowia lipolytica yeast. Fermentation 2020, 6, 112. [Google Scholar] [CrossRef]
- Cavallo, E.; Charreau, H.; Cerrutti, P.; Foresti, M.L. Yarrowia lipolytica: A model yeast for citric acid production. FEMS Yeast Res. 2017, 17, fox084. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yu, X.; Gao, S.; Dong, X.; Xia, J.; Xu, J.; He, A.; Hu, L.; Yan, Y.; Wang, Z. Enhancing the erythritol production by Yarrowia lipolytica from waste oil using loofah sponge as oil-in-water dispersant. Biochem. Eng. J. 2019, 151, 107302. [Google Scholar] [CrossRef]
- Rakicka, M.; Rywinska, A.; Cybulski, K.; Rymowicz, W. Enhanced production of erythritol and mannitol by Yarrowia lipolytica in media containing surfactants. Braz. J. Microbiol. 2016, 47, 417–423. [Google Scholar] [CrossRef]
- Mirbagheri, M.; Nahvi, I.; Emtiazi, G.; Darvishi, F. Enhanced production of citric acid in Yarrowia lipolytica by Triton X-100. Appl. Biochem. Biotechnol. 2011, 165, 1068–1074. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, F.; Li, X.; Mao, G.; Xie, H.; Song, A.; Santos, J.C.; Zhang, Z. Tailored production of citric acid and mannitol by Yarrowia lipolytica from corn stover pretreated by glycerol-assisted instant catapult steam explosion. Ind. Crops Prod. 2022, 189, 115820. [Google Scholar] [CrossRef]
- Ping, L.; Yuan, X.; Zhang, M.; Chai, Y.; Shan, S. Improvement of extracellular lipase production by a newly isolated Yarrowia lipolytica mutant and its application in the biosynthesis of L-ascorbyl palmitate. Int. J. Biol. Macromol. 2018, 106, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, E.; Nobile, M.; Cerrutti, P.; Foresti, M.L. Exploring the production of citric acid with Yarrowia lipolytica using corn wet milling products as alternative low-cost fermentation media. Biochem. Eng. J. 2020, 155, 107463. [Google Scholar] [CrossRef]
- Panadare, D.C. Applications of waste cooking oil other than biodiesel: A review. Iran. J. Chem. Eng. 2015, 12, 55–76. [Google Scholar]

| Factors | Level 1 | Level 2 | Level 3 |
|---|---|---|---|
| Sunflower oil polarity | 8 | 16 | 24 |
| Oil concentration (g/L) | 60 | 90 | 120 |
| Fermentation time (days) | 4 | 8 | 12 |
| * Triton X-100 concentration (g/L) | 0 | 1 | 2 |
| Run | Polarity | Oil (g/L) | Time (Days) | Triton X-100 (g/L) * |
|---|---|---|---|---|
| 1 | 8 | 60 | 4 | 0 |
| 2 | 8 | 90 | 8 | 1 |
| 3 | 8 | 120 | 12 | 2 |
| 4 | 16 | 60 | 8 | 2 |
| 5 | 16 | 90 | 12 | 0 |
| 6 | 16 | 120 | 4 | 1 |
| 7 | 24 | 60 | 12 | 1 |
| 8 | 24 | 90 | 4 | 2 |
| 9 | 24 | 120 | 8 | 0 |
| Wavenumber (cm−1) | Assignment | Functional Group | Fatty Acid Containing |
|---|---|---|---|
| 720 | C-H bending [55] | methylene (CH2) groups and alkanes | long-chain fatty acids (e.g., palmitic acid and stearic acid) |
| 1100–1200 | C-O stretching [56] | esters | triglycerides formed from fatty acids (e.g., oleic acid, linoleic acid, palmitic acid, and stearic acid) |
| 1375–1385 | CH3 bending (symmetric) [57] | methyl (CH3) groups in alkanes | fatty acids with methyl groups (e.g., palmitic acid and stearic acid) |
| 1465 | CH2 bending [58] | alkanes | long-chain fatty acids (e.g., palmitic acid and stearic acid) |
| 1740 | C=O stretching [59] | esters | triglycerides and fatty acids (e.g., oleic acid, linoleic acid, palmitic acid, and stearic acid) |
| 2850 | C-H stretching (CH2) [60] | methylene (CH2) groups and alkanes | long-chain fatty acids (e.g., palmitic acid and stearic acid) |
| 2925 | C-H stretching (CH3) [58,61] | methyl (CH3) groups in alkanes | fatty acids with methyl groups (e.g., palmitic acid and stearic acid) |
| 3008 | C-H stretching (cis=CH) [62] | aromatic rings and alkanes (unsaturated) | unsaturated fatty acids (e.g., oleic acid and linoleic acid) |
| Microorganisms | Diameter (cm) | |
|---|---|---|
| 24 h | 48 h | |
| Y. lipolytica NRRLY-1094 | 0.60 ± 0.07 | 1.00 ± 0.14 |
| Y. lipolytica NRRL YB-423 | 0.05 ± 0.07 | 0.50 ± 0.00 |
| Y. lipolytica IFP29 | 0.75 ± 0.07 | 2.00 ± 0.00 |
| Y. lipolytica NRRL YB 423-12 | 0.40 ± 0.14 | 1.25 ± 0.07 |
| Run | Polarity | Oil (g/L) | Time (Days) | Triton X-100 (g/L) | Biomass (g/L) | CA (g/L) | ICA (g/L) | Final pH |
|---|---|---|---|---|---|---|---|---|
| 1 | 8 | 60 | 4 | 0 | 3.30 ± 0.42 | 4.36 ± 0.24 | 3.76 ± 0.38 | 2.74 ± 0.14 |
| 2 | 8 | 90 | 8 | 1 | 2.83 ± 0.18 | 4.53 ± 1.44 | 1.64 ± 0.26 | 3.60 ± 0.23 |
| 3 | 8 | 120 | 12 | 2 | 2.34 ± 0.25 | - | - | 5.15 ± 0.28 |
| 4 | 16 | 60 | 8 | 2 | 2.11 ± 0.31 | - | - | 4.78 ± 0.27 |
| 5 | 16 | 90 | 12 | 0 | 3.10 ± 0.17 | 32.17 ± 2.04 | 29.44 ± 1.80 | 4.65 ± 0.41 |
| 6 | 16 | 120 | 4 | 1 | 2.52 ± 0.20 | - | - | 3.42 ± 0.50 |
| 7 | 24 | 60 | 12 | 1 | 2.22 ± 0.61 | - | - | 5.50 ± 0.21 |
| 8 | 24 | 90 | 4 | 2 | 1.51 ± 0.10 | - | - | 4.48 ± 0.06 |
| 9 | 24 | 120 | 8 | 0 | 3.00 ± 0.41 | 11.40 ± 1.27 | 7.27 ± 1.10 | 3.68 ± 0.64 |
| Run | Oil (g/L) | Polarity | Time (Days) | Biomass (g/L) | Final pH | CA (g/L) | S/N Ratio | ICA (g/L) | S/N Ratio |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 60 | 8 | 4 | 3.14 ± 0.27 | 4.48 ± 0.07 | 8.18 ± 1.22 | 18.26 | 6.32 ± 1.15 | 16.01 |
| 2 | 60 | 16 | 8 | 2.19 ± 0.21 | 4.03 ± 0.08 | 10.97 ± 0.49 | 20.80 | 7.66 ± 1.10 | 17.68 |
| 3 | 60 | 24 | 12 | 1.64 ± 0.35 | 4.01 ± 0.01 | 13.98 ± 0.18 | 22.91 | 10.03 ± 1.54 | 20.03 |
| 4 | 90 | 8 | 8 | 3.76 ± 0.61 | 4.01 ± 0.16 | 13.92 ± 0.47 | 22.87 | 10.59 ± 0.93 | 20.50 |
| 5 | 90 | 16 | 12 | 2.97 ± 0.27 | 4.36 ± 0.06 | 20.31 ± 2.76 | 26.15 | 13.63 ± 1.46 | 22.69 |
| 6 | 90 | 24 | 4 | 1.79 ± 0.10 | 4.95 ± 0.03 | 6.77 ± 0.66 | 16.61 | 5.76 ± 0.71 | 15.21 |
| 7 | 120 | 8 | 12 | 3.89 ± 0.47 | 3.88 ± 0.11 | 14.25 ± 1.09 | 23.08 | 10.65 ± 0.89 | 20.55 |
| 8 | 120 | 16 | 4 | 3.86 ± 0.28 | 5.00 ± 0.02 | 5.02 ± 0.20 | 14.01 | 4.99 ± 0.34 | 13.96 |
| 9 | 120 | 24 | 8 | 3.64 ± 0.51 | 4.51 ± 0.01 | 7.68 ± 0.69 | 17.70 | 6.44 ± 1.20 | 16.18 |
| Source | DF | Seq SS | Contribution | Adj SS | Adj MS | F | P |
|---|---|---|---|---|---|---|---|
| Oil concentration (g/L) | 2 | 20.27 | 16.94% | 20.27 | 10.14 | 21.46 | 0.05 |
| Polarity | 2 | 8.12 | 6.79% | 8.12 | 4.06 | 8.60 | 0.10 |
| Fermentation time (days) | 2 | 90.34 | 75.49% | 90.34 | 45.17 | 95.63 | 0.01 |
| Error | 2 | 0.95 | 0.79% | 0.94 | 0.47 | ||
| Total | 8 | 119.68 | 100% |
| Source | DF | Seq SS | Contribution | Adj SS | Adj MS | F | P |
|---|---|---|---|---|---|---|---|
| Oil concentration (g/L) | 2 | 10.05 | 14.32% | 10.05 | 5.03 | 27.17 | 0.04 |
| Polarity | 2 | 5.32 | 7.57% | 5.32 | 2.66 | 14.37 | 0.07 |
| Fermentation time (days) | 2 | 54.47 | 77.58% | 54.47 | 27.24 | 147.22 | 0.01 |
| Error | 2 | 0.37 | 0.53% | 0.37 | 0.19 | ||
| Total | 8 | 70.22 | 100% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sayın, B.; Bozkurt, A.G.; Kaban, G. Assessing Waste Sunflower Oil as a Substrate for Citric Acid Production: The Inhibitory Effect of Triton X-100. Fermentation 2024, 10, 374. https://doi.org/10.3390/fermentation10070374
Sayın B, Bozkurt AG, Kaban G. Assessing Waste Sunflower Oil as a Substrate for Citric Acid Production: The Inhibitory Effect of Triton X-100. Fermentation. 2024; 10(7):374. https://doi.org/10.3390/fermentation10070374
Chicago/Turabian StyleSayın, Bilge, Akif Göktuğ Bozkurt, and Güzin Kaban. 2024. "Assessing Waste Sunflower Oil as a Substrate for Citric Acid Production: The Inhibitory Effect of Triton X-100" Fermentation 10, no. 7: 374. https://doi.org/10.3390/fermentation10070374
APA StyleSayın, B., Bozkurt, A. G., & Kaban, G. (2024). Assessing Waste Sunflower Oil as a Substrate for Citric Acid Production: The Inhibitory Effect of Triton X-100. Fermentation, 10(7), 374. https://doi.org/10.3390/fermentation10070374







