Abstract
The semi-dry fermentation processing (SFP) of Coffea arabica is a traditional primary processing method in the coffee industry, which crucially impacts the coffee’s flavor. To further obtain useful information on microbial communities and chemical compounds during the SFP of C. arabica from Yunnan, China, the microbial community structures and the differentially changed non-volatile compounds (DCnVCs) were comprehensively analyzed. The results showed that Tatumella, Staphylococcus, Klebsiella, Brevundimonas, and Gluconobacter were the most prevalent bacteria genera, and Candida, Hannaella, Hanseniaspora, Pichia, and Lachancea were the most abundant fungal genera. Furthermore, 117 DCnVCs were found in the fermentation-finished samples compared to the raw materials. Therefore, this study can provide useful information for understanding the SFP of coffee beans, and its impact on coffee’s quality parameters.
1. Introduction
Coffee is an important commercial crop cultivated in Brazil, Vietnam, Colombia, Indonesia, India, and other countries and regions. According to the most recent report of the International Coffee Organization (ICO), world coffee exports amounted to 10.61 million bags in November 2023, and the ICO Composite Indicator Price (I-CIP) averaged at 175.73 US cents/Ib in December. Green coffee beans undergo primary processing before being roasted, and semi-dry fermentation processing (SFP) is a traditional primary processing method. This method is a hybrid of wet and dry fermentation processing, in which coffee beans without ripe fruits are dried and fermented under the sun until they reach a 13% moisture content [1,2,3]. The coffee’s flavor after SFP is sweeter, fruity, and less acidic [4]. In addition, due to the demand for specific coffee flavor profiles and processing methods from consumers, the consumption of coffee produced through SFP has been increasing in recent years [5]. The processing time mainly depends on the weather conditions. Previous studies have shown that the coffee fermentation could easily impact coffee’s flavor [6,7]. For example, SFP could improve the caramel appearance, roasted aroma, and buttery flavor of coffee beans [8].
During SFP, bacteria, yeast, and filamentous fungi are the main microbes that take part in coffee fermentation and are dynamically altered with fermentation [9]. Coffee fermentation is critical for removing mucilage through the metabolic process of oxidation of organic substances [10]. Enterobacter, Escherichia, Bacillus, Acinetobacter, Klebsiella, Lactococcus, Leuconostoc, Serratia, and Pseudomonas are the main bacterial genera; Saccharomyces, Shizosaccharomyces, Pichia, Candida, Rhodotorula, Hanseniaspore, and Kluyveromyces are the main yeast species; and filamentous fungi such as Aspergillus, Cladosporium, Penicillium, and Fusarium have also been found [1].
Notably, primary processing can impact the microbial community and chemical compounds involved in coffee fermentation. Our previous study revealed that Achromobacter, Tatumella, Weissella, Streptococcus, Trichocoleus, Cystofilobasidium, Wickerhamomyces, Hanseniaspora, Aspergillus, and Lachancea were the predominant bacteria and fungi in Coffea arabica from Yunnan, China, produced through wet fermentation processing [11]. To further confirm the properties of C. arabica from Yunnan, China, processed using the SFP method, the microbial community structures and non-volatile compounds were analyzed in this study.
2. Materials and Methods
2.1. Materials and Chemical Standards
Mature C. arabica cherries were obtained in December 2023 from Pu-er City, Yunnan, China. Then, they were processed using the SFP method, which consisted of floatation, de-pulping, and drying. Firstly, fresh coffee cherries were selected using floatation for removing unmature and postmature coffee cherries. Then, the coffee cherries were de-pulped and sun-dried under temperatures from 10 to 18 °C. During the SFP, four coffee bean samples were obtained for analysis: one at day 0 (SP1), one after 3 days (SP2), one after 6 days (SP3), and one after 9 days (SP4) (n = 3). The chemical standards included methyl alcohol, acetonitrile, and propyl alcohol, which were of ultraperformance liquid chromatography (UHPLC) grade, purchased from Fisher Co., Ltd. (Shanghai, China).
2.2. Microbial Community Structure Analysis during the SFP
The microbial community structures of coffee samples during the SFP were analyzed referencing the method of Shen et al. [11]. The hypervariable region V5–V7 of the 16S rRNA gene was amplified with forward primer 799F and reverse primer 1193R to determine bacteria. Meanwhile, the ITS1 region was amplified using the ITS1F and ITS2R primers to determine fungi.
2.3. Non-Volatile Compound Analysis during the SFP
Non-volatile compounds of the coffee samples during the SFP were analyzed using a UHPLC-MS/MS via a UHPLC-Q Exactive system from Thermo Fisher Scientific (Shanghai, China), following the method of Shen et al. [11]. In brief, 50 mg of the coffee samples was extracted using an 80% methanol solution. Then, the coffee sample extracts were analyzed with UHPLC-MS/MS. At the same time, a quality control (QC) sample was prepared by mixing equal volumes of all samples. Finally, UHPLC-MS/MS data were preprocessed using Progensis QI software 3.0 (Waters Corporation, USA). The response intensity of the samples’ mass spectrum peaks was normalized using the sum normalization method, and variables with a standard deviation (RSD) > 30% relative to the QC samples were removed, and log10 logarithmization was performed.
2.4. Statistical Analysis
Variable importance in projection (VIP) analysis ranked the overall contribution of each variable to the OPLS-DA model, and the variables with a VIP > 1.0, p < 0.05, and a fold change (FC) >1.5 or <0.67 were classified as differentially changed non-volatile compounds (DCnVCs) [12].
2.5. Accession Numbers
The raw sequencing reads of the bacterial 16S rRNA and fungal ITS1 were deposited into the NCBI Sequence Read Archive database (Accession Number: PRJNA1088924 and PRJNA1089065).
3. Results and Discussion
3.1. Microbial Community Structure at the Phylum and Genus Level during the SFP of Coffee Beans
According to the analysis using high-throughput sequencing technology, 776,410 and 829,791 sequences were identified for bacteria and fungi during coffee SFP, respectively. The coverage in all coffee samples (0 days (SP1), 3 days (SP2), 6 days (SP3), and 9 days (SP4)) was higher than 0.99, suggesting that the sequencing results accurately reflect the abundance of bacteria and fungi during the SFP [13]. Alpha diversity analysis was performed to assess the species abundance and diversity during the SFP, as shown in Figure 1.
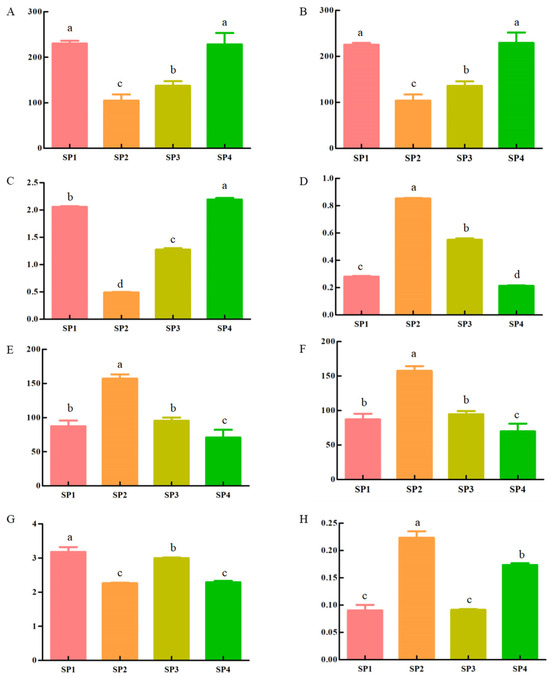
Figure 1.
Results of the analysis of microbial alpha diversity during the SFP of C. arabica from Yunnan, China ((A–D): Ace, Chao, Shannon, and Simpson in bacteria, respectively; (E–H): Ace, Chao, Shannon, and Simpson in fungi, respectively; SP1: 0 days, SP2: 3 days, SP3: 6 days, and SP4: 9 days). Different lowercase superscripts indicate significantly different among comparisons (p < 0.05).
Ace and Chao indexes can reflect microorganism diversity, with a positive correlation between diversity and the values of the Ace and Chao indexes [13]. At the operational taxonomic unit (OUT) level, p-values of the Ace index were 0.03 and 0.05 for bacteria and fungi, respectively (Figure 1A,E). The p-values of the Chao index were 0.03 for bacteria and 0.05 for fungi. Regarding bacteria, SP4 had the highest Chao index (Figure 1B), while for fungi, the highest Chao index was observed in SP2 (Figure 1F). These results indicate that SP4 and SP2 had the highest bacterial and fungal species diversity, respectively. Shannon and Simpson indices can reflect the number and diversity of microbial species in coffee samples. According to the Shannon index, the bacterial species exhibited an initial decrease in diversity followed by an increase during the SFP (Figure 1C). The highest Shannon index was observed for SP4. For fungi, the Shannon index decreased initially and then increased cyclically, with the highest values observed in SP1 (Figure 1G). This result indicates that SP1 and SP4 had the highest species evenness for bacteria and fungi, respectively. On the other hand, the change in the Shannon index was opposite to that in the Simpson index, exhibiting a pattern of initially increasing and then decreasing regarding bacteria (Figure 1D). Based on the Shannon and Simpson indices, the diversity of bacteria decreased first and then gradually increased during the SFP. For fungi, the Shannon index was initially increased and then decreased cyclically, with the highest value observed in SP2 (Figure 1H). This means that SP2 had the highest diversity of bacteria and fungi during the SFP.
The identified bacteria during the SFP of C. arabica were classified into 18 phyla, as shown in Figure 2A. These included Proteobacteria, Firmicutes, Actinobacteriota, Acidobacteriota, Deinococcota, Bacteroidota, Gemmatimonadota, etc. Among them, Proteobacteria (comprising 42.01%–98.77% of the community abundance at the phylum level) and Firmicutes (1.15%–56.29%) were the dominant phyla. Notably, Proteobacteria was the dominant bacterial phylum with a relative percentage of over 40.00%, which initially increased from 42.01% in SP1 to 98.77% in SP2, then decreased to 58.21% in SP4. Furthermore, Firmicutes exhibited significant changes during the SFP, from a maximum of 56.29% in SP1 to a minimum of 1.15% in SP2.
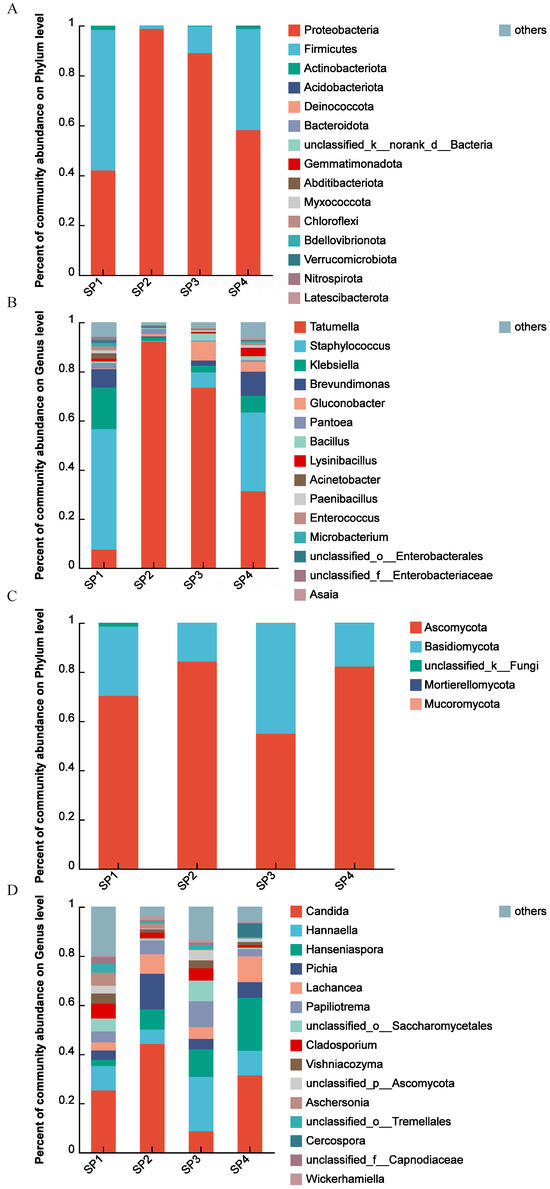
Figure 2.
Results of the analysis of microbial community structures during the SFP of C. arabica from Yunnan, China. The percentage of community abundance at the phylum level of bacteria (A); the percentage of community abundance at the genus level of bacteria (B); the percentage of community abundance at the phylum level of fungi (C); the percentage of community abundance at the genus level of fungi (D).
Furthermore, the bacteria during the SFP were classified into 31 genera, as shown in Figure 2B. They mainly included Tatumella, Staphylococcus, Klebsiella, Brevundimonas, Gluconobacter, Pantoea, Bacillus, Lysinibacillus, Acinetobacter, Paenibacillus, Enterococcus, etc. The five most abundant genera were Tatumella, Staphylococcus, Klebsiella, Brevundimonas, and Gluconobacte. At the beginning of the SFP (SP1), Staphylococcus was the dominant bacterial genus, with a relative percentage of 49.08% of community abundance at the genus level, following Klebsiella (16.93%), Brevundimonas (7.54%), and Tatumella (7.48%), respectively. Then, Staphylococcus decreased to 0.36%, whereas Tatumella increased to its highest value of 92.17% in SP2. Subsequently, Staphylococcus gradually decreased while Staphylococcus, Klebsiella, and Brevundimonas increased.
Regarding fungi, five phyla, including Ascomycota, Basidiomycota, Mortierellomycota, Mucoromycota, and an unclassified fungus, were identified during the SFP, as shown in Figure 2C. Among them, Ascomycota (comprising 54.85%–84.23% of the community abundance at the phylum level) and Basidiomycota (15.74%–45.02%) were the dominant phylum. The relative abundance of Ascomycota during the SFP was also consistently higher than 50.00%. Specifically, the relative abundance of Ascomycota first increased, reaching a maximum of 84.23% in SP2. On the other hand, the relative abundance of Basidomycota initially decreased, reaching the minimum of 15.74% in SP2, then increased.
Additionally, these fungi during the SFP of coffee were classified into 31 genera, as shown in Figure 2D. These included Candida, Hannaella, Hanseniaspora, Pichia, Lachancea, Papiliotrema, Cladosporium, Vishniacozyma, Aschersonia, etc. Candida, Hannaella, Hanseniaspora, Pichia, and Lachancea were the predominant genera. In the community, Candida’s percentage abundance varied from 8.65% to 44.20%, with the maximum value observed in SP2 and the minimum value in SP3. On the other hand, the minimum value for Hannaella was 5.79% in SP2, and the maximum value was 22.19% in SP3. Hanseniaspora gradually increased from SP1 with 2.50% to SP4 with 21.50%. Pichia exhibited a maximum of 14.56% in SP2 and a minimum of 3.83% in SP1.
Linear discriminant analysis (LDA) effect size (LEfSe) has been used to further assess the differences in the relative abundance of microbial community members [14]. The LEfSe results at the genus level are shown in Figure 3, unequivocally demonstrating distinct distribution patterns of predominant species during the SFP. Regarding bacteria (Figure 3A), 25 genera, such as Staphylococcus (LDA score = 5.40, p = 0.016), Klebsiella (LDA score = 4.89, p = 0.016), Acinetobacter (LDA score = 4.04, p = 0.025), Enterococcus (LDA score = 3.89, p = 0.019), Exiguobacterium (LDA score = 3.61, p = 0.018), and Stenotrohopmonas (LDA score = 3.58, p = 0.024), were significantly higher in SP1. Three genera, namely Tatumella (LDA score = 5.63, p = 0.016), Pantoea (LDA score = 3.94, p = 0.025), and Rosenbergiella (LDA score = 3.33, p = 0.016), were significantly higher in SP2. Three genera, namely Gluconobacter (LDA score = 4.57, p = 0.016), Bacillus (LDA score = 4.12, p = 0.016), and Leuconostoc (LDA score = 3.22, p = 0.032), were significantly higher in SP3. Eighteen genera, including Brevundimonas (LDA score = 4.67, p = 0.016), Lysinibacillus (LDA score = 4.20, p = 0.016), Asaia (LDA score = 3.67, p = 0.015), Neoasaia (LDA score = 3.63, p = 0.016), and Ameyamaea (LDA score = 3.60, p = 0.016), were significantly higher in SP4.
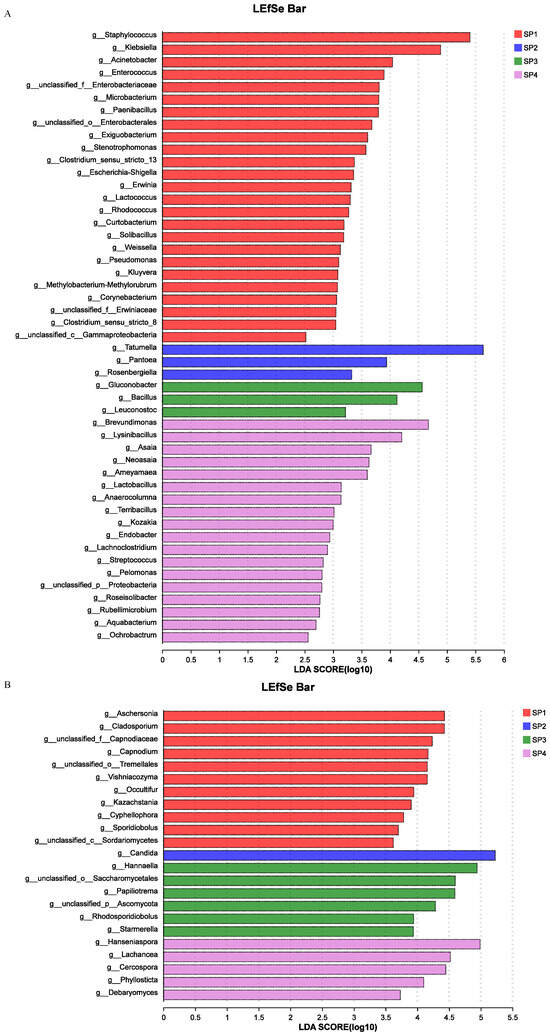
Figure 3.
LEfSe during the SFP. LDA score of bacteria (A); LDA score of fungi (B).
Regarding fungi (Figure 3B), e genera, including Aschersonia (LDA score = 4.43, p = 0.016), Cladosporium (LDA score = 4.43, p = 0.022), Vishniacozyma (LDA score = 4.16, p = 0.036), Cyphellophora (LDA score = 3.78, p = 0.041), and Sporidiobolus (LDA score = 3.70, p = 0.033), were significantly higher in SP1. One genus, Candida (LDA score = 5.23, p = 0.016), was significantly higher in SP2. Six genera, including Hannaella (LDA score = 4.94, p = 0.025), Papiliotrema (LDA score = 4.59, p = 0.022), Starmerella (LDA score = 3.94, p = 0.032), unclassified_p_Ascomycota (LDA score = 4.29, p = 0.025), and Rhodosporidiobolus (LDA score = 3.94, p = 0.038), were significantly higher in SP3. Five genera, namely Hanseniaspora (LDA score = 4.99, p = 0.019), Lachancea (LDA score = 4.52, p = 0.022), Cercospora (LDA score = 4.45, p = 0.044), Phyllosticta (LDA score = 4.10, p = 0.034), and Debaryomyces (LDA score = 3.73, p = 0.034), were significantly higher in SP4.
The coffee processing method is one of important factors influencing the microbial biodiversity in coffee fruits and grains [15]. Based on the analysis of microbial community structure during SFP, the predominant microorganisms at the genus level were Tatumella, Staphylococcus, Klebsiella, Brevundimonas, and Gluconobacter for bacteria and Candida, Hannaella, Hanseniaspora, Pichia, and Lachancea for fungi. However, Vilela et al. [16] found that Bacillus subtilis, Bacillus cereus, Escherichia coli, Enterobacter agglomerans, and Klebsiella pneumoniae were the predominant bacteria in the SFP of C. arabica originating from Brazil. At the same time, Pichia anomala, Torulaspora delbrueckii, and Rhodotorula mucilaginosa were the dominant yeast species. In coffee primary processing, the microorganism composition has been observed to vary significantly between regions. In addition, different primary processing methods also show different microorganism features. For example, species from the Bacillus, Enerobacter, Pseudomonas, Erwinia, and Proteus genera, such as Bacillus subtilis, Bacillus macerans, Bacillus megaterium, Enerobacter aerogenes, Enerobacter cloacae, Pseudomonas putrefaciens, and Pseudomonas paucimobilis, were found to be the most dominant bacteria in the dry fermentation process [1]. Leuconostoc, Streptococcus, Klebsiella, Weissela, and Lactobacillus species, such as Leuconostoc mesenteroides, Streptococcus faecalis, Klebsiella pneumonia, Weissela cibaria, and Lactobacillus plantarum, were the most dominant bacteria in the fermentation process [1]. Enterobacter, Bacillus, Acinetobacter, Klebsiella, and Lactococcus species, such as Enterobacter agglomerans, Bacillus cereus, Acinetobacter sp., Klebsiella pneumonia, and Lactococcus plantarum, were the most dominant bacteria in the SFP [1]. The microorganism community profile found in SFP falls between those observed in the dry processing and wet processing methods. Microorganisms play an important role in degrading mucilage during coffee processing [10], the ultimate determiners of quality and sensory characteristics of coffee beverages [17]. Based on the positive effect of microbiota on coffee fermentation and coffee flavor in coffee primary processes, yeasts, bacteria, and fungi can been used as starters to improve coffee flavor [18,19,20,21,22].
3.2. Differentially Changed Non-Volatile Compounds Analysis during the SFP
In total, 1551 non-volatile compounds (nVCs) belonging to 15 super-classes were detected in the four coffee samples during the SFP, as shown in Figure 4. These 15 super-classes included lipids and lipid-like molecules (431 nVCs); organic acids and derivatives (238 nVCs); organoheterocyclic compounds (187 nVCs); organic oxygen compounds (184 nVCs); phenylpropanoids and polyketides (150 nVCs); benzenoids (110 nVCs); nucleosides, nucleotides, and analogs (43 nVCs); organic nitrogen compounds (19 nVCs); alkaloids and derivatives (18 nVCs); lignans, neolignans, and related compounds (6 nVCs); hydrocarbons (3 nVCs); homogeneous non-metal compounds (1 nVCs); organic 1,3-dipolar compounds (1 nVCs); organosulfur compounds (1 nVCs); and others (159 nVCs). They were further grouped into 124 classes, which mainly included carboxylic acids and derivatives (196 nVCs); organooxygen compounds (184 nVCs); fatty acyls (161 nVCs); prenol lipids (118 nVCs); benzene and substituted derivatives (74 nVCs); steroids and steroid derivatives (66 nVCs); glycerophospholipids (63 nVCs); flavonoids (58 nVCs); coumarins and derivatives (28 nVCs); cinnamic acids and derivatives (27 nVCs); indoles and derivatives (25 nVCs); phenols (23 nVCs); organonitrogen compounds (19 nVCs); imidazopyrimidines (16 nVCs); glycerolipids (15 nVCs); hydroxy acids and derivatives (14 nVCs); isoflavonoids (14 nVCs); purine nucleosides (14 nVCs); benzopyrans (12 nVCs); diazines (11 nVCs); lactones (10 nVCs); keto acids and derivatives (10 nVCs); and others.
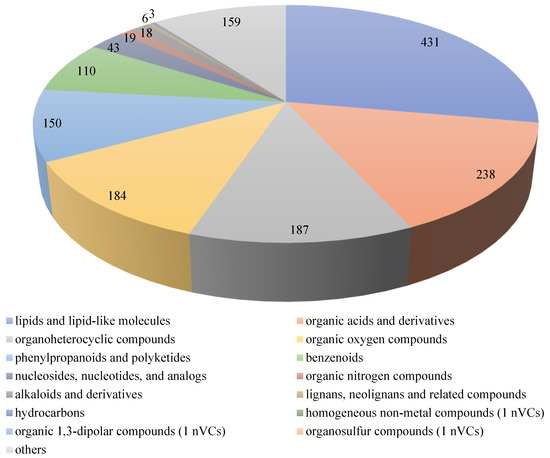
Figure 4.
Super-classes of chemical compounds during the SFP of C. arabica from Yunnan, China. The different colors represent different super-classes of chemical compounds; the different sizes represent the number of chemical compounds, with a bigger size relating to a higher number of chemical compounds.
The chemical composition of green coffee beans can directly impact the chemical constituents of coffee brews [23]. In green coffee beans, the content of organic acids (chlorogenic, quinic, citric, and malic acids) reached nearly 11%, which can form lactones through the reaction of chlorogenic acid and quinic acid during coffee bean roasting [24]. Moreover, the total contents of phenolic compounds ranged from 34.44 to 44.42 mg/g [25]. Processing methods can influence the chemical composition and physicochemical properties of coffee beans, and the coffee sensory profile [26,27]. During the SFP of C. arabica from China, fourchlorogenic acid isomers (chlorogenic acid, isochlorogenic acid, cryptochlorogenic acid, and trans-chlorogenic acid), three feruloylquinic acid isomers (feruloylquinic acid, 3-feruloylquinic acid, and 3-O-feruloylquinic acid), and seven caffeoylquinic acid isomers (1-caffeoylquinic acid, cis-5-caffeoylquinic acid, 1,3-dicaffeoylquinic acid, 1,5-dicaffeoylquinic acid, 4,5-dicaffeoylquinic acid, 3-caffeoyl-4-feruloylquinic acid, and 4-O-caffeoyl-3-O-feruloylquinic acid) were detected. Compared with the wet processing of C. arabica from China, the caffeoylquinic acid, feruloylquinic acid, and dicaffeoylquinic acid contents observed during SFP were lower [11]. At the same time, the amounts of caffeoylquinic acid and feruloylquinic acid isomers were lower than those found in wet processing [28]. While the content of dicaffeoylquinic acid isomers in the SFP was higher compared to wet processing. However, chlorogenic acid was found to have a strong highly significant negative correlation with coffee’s acidity and overall acceptability, which was not influenced by the processing method [29]. However, caffeine is a thermo-stable compound that can contribute to a coffee brew’s perceived strength, body, and bitterness [24]. The content of caffeine was found to have a strong positive highly significant correlation with the coffee’s acidity and overall acceptability [29]. The content of caffeine is not affected by the primary processing method of coffee. Trigonelline contributes to coffee’s overall aroma, astringency, and aftertaste/astringency [24,25]. The content of trigonelline in the SFP was higher than that observed during wet processing because of the lixiviation and thermal degradation with water solubility in wet processing [28]. The coffee beans produced through SFP had a highest content of lipids compared to washed coffee beans, and the content of lipids observed with the dry processing method was the lowest [29].
To gain further insights on the dynamics during the SFP of coffee, the differentially changed non-volatile compounds (DCnVCs) with variable importance in projection (VIP) >1.0, p < 0.05, and FC > 1.5 or VIP > 1.0, p < 0.05, FC < 0.67, between SP1, SP2, SP3, and SP4 were assessed and identified, as shown in Figure 5.
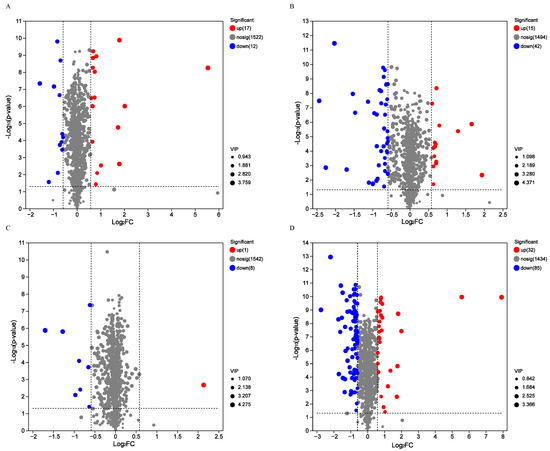
Figure 5.
In total, we found 29 DCnVCs between SP2 and SP1 (A), including 17 up-regulated DCnVCs and 12 down-regulated DCnVCs; 57 DCnVCs between SP3 and SP2 (B), including 15 up-regulated DCnVCs and 42 down-regulated DCnVCs; 9 DCCs between SP4 and SP3 (C), including 1 up-regulated DCnVC and 8 down-regulated DCnVCs; and 117 DCnVCs between SP4 and SP1 (D), including 32 up-regulated DCnVCs and 85 down-regulated DCnVCs.
In total, 29 DCnVCs were detected in the SP2 vs. SP1 comparison (Figure 5A). These included 17 up-regulated DCnVCs and 12 down-regulated DCnVCs. The up-regulated DCnVCs included phenylpropanoids and polyketides (four DCnVCs: 7-ethoxycoumarin, luteolin 7-glucuronide, subaphylline, and pelargonidin 3-(6″-malonylglucoside)), lipids and lipid-like molecules (three DCnVCs: 3alpha-O-trans-feruloyl-2alpha-hydroxy-12-ursen-28-oic acid, (2′E,4′Z,8E)-colneleic acid, and 3-methylthiopropionic acid), organic oxygen compounds (three DCnVCs: (2S,3S,4S,5R)-3,4,5-trihydroxy-6-sulfooxyoxane-2-carboxylic acid, 4-O-alpha-D-Galactopyranuronosyl-D-galacturonic acid, and L-Xylonate), organic acids and derivatives (two DCnVCs: homocarnosine and deferoxamine), organoheterocyclic compounds (one DCnVC: stercobilin), benzenoids (one DCnVC: N-(6-aminopyridin-2-yl)-4′-cyanobiphenyl-4-sulfonamide), nucleosides, nucleotides, and analogs (one DCnVC: xanthylic acid), and others (two DCnVCs: SM(d14:0/2:0) and Ser Gly His). Among them, the most up-regulated DCnVCs with an FC over 3.0 were 7-ethoxycoumarin, 3alpha-O-trans-feruloyl-2alpha-hydroxy-12-ursen-28-oic acid, N-(6-aminopyridin-2-yl)-4′-cyanobiphenyl-4-sulfonamide, xanthylic acid, and luteolin 7-glucuronide. Meanwhile, organic acids and derivatives (three DCnVCs: arginosuccinate, advantame, and N-feruloylglycyl-L-phenylalanine), phenylpropanoids and polyketides (three DCnVCs: Isofraxidin, (E)-4-(1,2,3,6-Tetrahydro-2,6-dioxo-1,3-dipropyl-9H-purin-8-yl)cinnamic acid, and glyceollin II), lipids and lipid-like molecules (two DCnVCs: 4alpha-carboxy-4beta-methyl-5alpha-cholesta-8,24-dien-3beta-ol and macrophorin D), organic oxygen compounds (two DCnVCs: 3-(2-propenoic acid)-o-benzoquinone and isopentenyladenine-9-N-glucoside), benzenoids (one DCnVC: 2-(((3,5-Dichlorophenyl)carbamoyl)oxy)-2-methyl-3-butenoic acid), and organoheterocyclic compounds (one DCnVC: licorice glycoside A) were the down-regulated DCnVCs. Notably, licorice glycoside A, macrophorin D, and glyceollin II were the most down-regulated DCnVCs, with an FC lower than 0.5.
Similarly, 57 DCnVCs were identified when comparing SP3 and SP2 (Figure 5B), including 15 up-regulated DCnVCs and 42 down-regulated DCnVCs. Among the up-regulated DCnVCs were organic acids and derivatives (seven DCnCVs, e.g., zofenopril, thiomorpholine 3-carboxylate, N-eicosapentaenoyl aspartic acid, 2-deoxy-2,3-dehydro-n-acetyl-neuraminic acid, N-carbamoylputrescine, etc.), phenylpropanoids and polyketides (three DCnVCs: fukinolic acid, trans-p-feruloyl-beta-D-glucopyranoside, and 6-hydroxyluteolin 6-xyloside), lipids and lipid-like molecules (two DCnVCs: lobetyolin, and sarmentosin), benzenoids (one DCnVC: aminosalicylic acid), organoheterocyclic compounds (one DCnVC: 3-indolebutyric acid), alkaloids and derivatives (one DCnVC: 7Z,14Z-eicosadienoic acid). Among them, zofenopril, thiomorpholine 3-carboxylate, and N-eicosapentaenoyl aspartic acid were the most up-regulated DCnVCs with an FC higher than 2.0. On the other hand, down-regulated DCnVCs included phenylpropanoids and polyketides (nine DCnVCs, e.g., sanguisorbic acid dilactone, sakuranetin, 3,7-dimethylquercetin, alpha-solanine, 6′-malonyltrifolirhizin, etc.), organic acids and derivatives (seven DCnVCs, 5-phosphonooxy-L-lysine, deferoxamine, cysteinyl-alanine, Na-p-hydroxycoumaroyltryptophan, majoroside F6, etc.), lipids and lipid-like molecules (seven DCnVCs, e.g., (3b,5b,22a,25R)-furostane-22-methoxy-3,26-diol 3-[glucosyl-(1->2)-glucoside] 26-glucoside, 8-hydroxyhesperetin 7-[6-acetylglucosyl-(1->2)-glucoside], PA(i-21:0/8:0), medicoside G, PC(DiMe(9,3)/DiMe(9,3)), etc.), alkaloids and derivatives (five DCnVCs: desacetoxyvindoline, lycorine, delimotecan, 4-desacetylvinblastine hydrazide, and 10-hydroxycamptothecin), organoheterocyclic compounds (four DCnVCs: nipradilol, citrusinine I, stercobilin, and ivabradine),organic oxygen compounds (three DCnVCs: acetyl CoA, amygdalin, and thermophillin), benzenoids (two DCnVCs: 3,5-dinitrobenzoic acid, and p-hydroxyfelbamate), and others (five DCnVCs: PI(20:4(6Z,8E,10E,14Z)-2OH(5S,12R)/20:0), PS(6 keto-PGF1alpha/18:3(9Z,12Z,15Z)), Cer(d17:1/22:6(4Z,7Z,10Z,13E,15E,19Z)-OH(17)), PI(18:1(11Z)/PGJ2), and PS(six keto-PGF1alpha/20:5(5Z,8Z,11Z,14Z,17Z)). Among them, amygdalin, cysteinyl-Alanine, Na-p-hydroxycoumaroyltryptophan, p-hydroxyfelbamate, delimotecan, kaempferol-3-O-glucoside, 6′-malonyltrifolirhizin, 2″,6″-diacetylorientin, ivabradine, and PI(20:4(6Z,8E,10E,14Z)-2OH(5S,12R)/20:0) were the most down-regulated DCnVCs with a FC lower than 0.5.
However, only nine DCnVCs were detected when comparing SP4 and SP3 (Figure 5C), including one up-regulated DCnVC (cysteinyl-alanine) and eight down-regulated DCnVCs. These down-regulated DCnVCs included phenylpropanoids and polyketides (four DCnVCs: (+/−)-catechin, rhamnocitrin, biochanin A 7-(6-malonylglucoside), and limocitrin 3-rhamnoside), organic acids and derivatives (two DCnVCs: gluten exorphin B4 and thiomorpholine 3-carboxylate), one benzenoid (14-methoxymetopon), and one lipid/lipid-like molecule (3-methylthiopropionic acid). Notably, thiomorpholine 3-carboxylate, limocitrin 3-rhamnoside, and gluten exorphin B4 were the most significantly down-regulated DCnVCs, with an FC lower than 0.5.
Overall, 117 DCnVCs were detected when comparing SP4 and SP1 (Figure 5D), including 32 up-regulated DCnVCs and 85 down-regulated DCnVCs. The up-regulated DCnVCs included organic acids and derivatives (seven DCnVCs, e.g., phenylacetylglutamine, homocarnosine, N-eicosapentaenoyl aspartic acid, L-beta-aspartyl-L-threonine, aspartame, etc.), lipids and lipid-like molecules (seven DCnVCs, e.g., (2′E,4′Z,8E)-colneleic acid, sarmentosin, 3-hexaprenyl-4-hydroxybenzoic acid, abscisic alcohol, etc.), phenylpropanoids and polyketides (six DCnVCs, e.g., 7-ethoxycoumarin, luteolin 7-glucuronide, fukinolic acid, 6-hydroxyluteolin 6-xyloside, prenyl cis-caffeate, etc.), organic oxygen compounds (three DCnVCs: tuliposide A, 4-O-alpha-D-Galactopyranuronosyl-D-galacturonic acid, and (2S,3S,4S,5R)-3,4,5-trihydroxy-6-sulfooxyoxane-2-carboxylic acid), benzenoids (two DCnVCs: N-(6-aminopyridin-2-yl)-4′-cyanobiphenyl-4-sulfonamide and aminosalicylic acid), nucleosides, nucleotides, and analogs (two DCnVCs: 3′-C-ethynylcytidine and xanthylic acid), one organoheterocyclic compound (5-hydroxymethyluracil), and others (four DCnVCs: SM(d14:0/2:0), stearoyl serotonin, palmitoyl serotonin, and Ile Arg). Among them, the most significantly up-regulated DCnVCs with an FC greater than 3.0 were zofenopril, 7-ethoxycoumarin, 3alpha-O-trans-feruloyl-2alpha-hydroxy-12-ursen-28-oic acid, N-(6-aminopyridin-2-yl)-4′-cyanobiphenyl-4-sulfonamide, luteolin 7-glucuronide, and xanthylic acid. On the other hand, the down-regulated DCnVCs included lipids and lipid-like molecules (22 DCnVCs, e.g., deltonin, vinaginsenoside R6, 8-hydroxyhesperetin 7-[6-acetylglucosyl-(1->2)-glucoside], spinasaponin A, PGP(i-13:0/a-25:0), etc.), phenylpropanoids and polyketides (19 DCnVCs, e.g., 6″-O-malonylglycitin, sakuranetin, sanguisorbic acid dilactone, rhamnocitrin, 6′-malonyltrifolirhizin, etc.), organic acids and derivatives (12 DCnVCs, e.g., laninamivir, Na-p-hydroxycoumaroyltryptophan, 2-S-glutathionyl acetate, 5-phosphonooxy-L-lysine, N-feruloylglycyl-L-phenylalanine, etc.), organic oxygen compounds (8 DCnVCs, e.g., chlorogenoquinone, clarithromycin, amygdalin, acetyl CoA, 3-(2-propenoic acid)-o-benzoquinoneetc, etc.), organoheterocyclic compounds (5 DCnVCs: licorice glycoside A, toralactone, nipradilol, citrusinine I, and ivabradine), alkaloids and derivatives (5 DCnVCs: camptothecin sodium, 4-desacetylvinblastine hydrazide, lycorine, 10-hydroxycamptothecin, and delimotecan), benzenoids (4 DCnVCs: 14-methoxymetopon, 7-amino-4-hydroxy-2-naphthalenesulfonic acid, 3,5-dinitrobenzoic acid, and p-hydroxyfelbamate), nucleosides, nucleotides, and analogs (1 DCnVC: 3′,5′-Cyclic GMP), and others (9 DCnVCs, e.g., PGP(18:3(9,11,15)-OH(13)/i-24:0), PI(16:1(9Z)/6 keto-PGF1alpha), PI(22:4(10Z,13Z,16Z,19Z)/PGJ2), PI(PGF2alpha/16:0), PI(20:4(6Z,8E,10E,14Z)-2OH(5S,12R)/20:0), etc.). Among them, amygdalin, Na-p-hydroxycoumaroyltryptophan, and kaempferol-3-O-glucoside were the most down-regulated DCnVCs, with FC values < 0.20.
A Venn diagram was drawn to assess the numbers of DCnVCs that were either unique or shared during the SFP, as shown in Figure 6. Based on the Venn diagram analysis, 9, 10, 1, and 52 DCnVCs were unique to SP2 vs. SP1, SP3 vs. SP2, SP4 vs. SP3, and SP4 vs. SP1, respectively. Additionally, compared to the DCnVCs in SP4 vs. SP3, more DCnVCs were identified in SP2 vs. SP1 and SP3 vs. SP2. The number of DCnVCs decreased with the processing time during SFP. In addition, two shared DCnVCs were identified in SP2 vs. SP1 and S3 vs. SP2. However, no DCnVCs were identified in all four comparison groups.
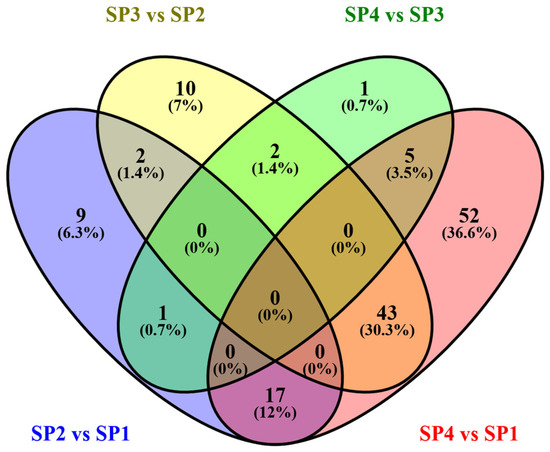
Figure 6.
Venn diagram of the number of DCnVCs during SFP. Overall, 9, 10, 1, and 52 DCnVCs were unique to SP2 vs. SP1, SP3 vs. SP2, SP4 vs. SP3, and SP4 vs. SP1, respectively. In SP3 vs SP2, twocommon DCnVCs were found in SP2 vs. SP1, two common DCnVCs were found in SP4 vs. SP3, and 43 common DCnVCs were found in SP4 vs. SP1.
The primary processing methods used for coffee can influence the chemical compound compositions in green coffee beans and roasted coffee beans, and coffee flavor [30,31]. In recent years, some novel processing methods for coffee have been demonstrated to improve coffee’s quality. When compared with the three traditional primary processing methods (dry fermentation processing, SFP, and wet fermentation processing), anaerobic fermentation could increase the variety of nVCs by producing extracellular enzymes, catalyzing phenolic acid’s transformation to other compounds [25]. Highly fruity, floral, and sweet aromas are the characteristics provided by dry processing. Moreover, traditional dry fermentation processing shows the strongest aftertaste, astringency, and umami. SFP enhanced the caramel, roasted aroma, and buttery flavor of coffee [8]. High contents of amino acids and derivatives have been found in dry fermentation processing and increased contents of lipids and phenolic acids have been found in SFP.
In addition, microbial communities and chemical compounds interact during the primary processing of coffee. The Metschnikowia and Apiotrichum fungi genera were extremely strongly positively correlated with Leuconostoc. For example, during wet processing, L-quinate was found to be strongly positively correlated with Leuconostoc, Metschnikowia, and Apiotrichum [11]. Therefore, using specific microorganisms as a starter in the primary processing of coffee can improve coffee flavor. For example, caramel and fruity flavors can be produced during SFP when C. arabica is inoculated with Saccharomyces cerevisiae, Candida parapsilosis, and Pichia guilliermondii [2]. Moreover, specific microorganisms in primary coffee processing are beneficial to the microorganisms’ abundance during coffee processing [18]. Therefore, further research is needed on the controlled fermentation of coffee in the future.
4. Conclusions
The market price of coffee, as an important agricultural economic product, is usually determined by the coffee bean quality and the beverage quality. However, primary processing is a crucial factor in coffee’s quality, influencing the genetics, environment, handling, and storage of coffee. In this study, the microbial communities and non-volatile compounds of C. arabica beans from Yunnan, China, during SFP were analyzed and compared. Tatumella, Staphylococcus, Klebsiella, Brevundimonas, and Gluconobacter were the predominant bacterial genera, and Candida, Hannaella, Hanseniaspora, Pichia, and Lachancea were the predominant fungal genera during SFP. Furthermore, 1551 non-volatile compounds from 15 super-classes were identified. Among them, 29 DCnVCs were detected in SP2 vs. SP1, among which 17 were up-related and 12 were down-related. Moreover, 57 DCnVCs were detected in SP3 vs. SP2, among which 15 were up-related and 42 were down-related. Nine DCnVCs were detected in SP4 vs. SP3, among which one was up-related and eight were down-related. Overall, 117 DCnVCs were detected in SP4 vs. SP1, of which 32 were up-related and 85 were down-related. In addition, zofenopril, 7-ethoxycoumarin, 3alpha-O-trans-feruloyl-2alpha-hydroxy-12-ursen-28-oic acid, N-(6-aminopyridin-2-yl)-4′-cyanobiphenyl-4-sulfonamide, luteolin 7-glucuronide, xanthylic acid, amygdalin, Na-p-hydroxycoumaroyltryptophan, and kaempferol-3-O-glucoside were the most differentially accumulated DCnVCs, with FC values > 3.0 or FC values < 0.20.
Therefore, this study provides valuable insights that can be used to improve our understanding of SFP and its impact on coffee’s quality.
Author Contributions
Conceptualization, X.S. and K.L.; methodology, X.S., Q.W. and W.Y.; software, W.Y.; formal analysis, X.S., Q.W., K.L. and W.J.; resources, B.P. and Y.G.; data curation, X.S., B.P. and Y.G.; writing—original draft preparation, X.S. and Q.W.; writing—review and editing, K.L. and W.J.; funding acquisition, K.L. and W.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Project of Yunnan Province Agricultural Basic Research Joint Foundation (No. 202101BD070001-046), the Reserve Talent Project of Young and Middle-aged Academic and Technical Leaders Yunnan Province (No. 202405AC350064) and the Science and Technology Innovation Team Project of Yibin Vocational and Technical College (No. ybzy21cxtd-03); the Scientific Research Project of Yibin Vocational and Technical College (No. ZRZD24-12).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The experimental data provided in this work are available in articles.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Elhalis, H.; Cox, J.; Zhao, J. Coffee fermentation: Expedition from traditional to controlled process and perspectives for industrialization. Appl. Food Res. 2023, 3, 100253. [Google Scholar] [CrossRef]
- Evangelista, S.R.; Miguel, M.G.D.C.P.; Cordeiro, C.D.S.; Silva, C.F.; Pinheiro, A.C.M.; Schwan, R.F. Inoculation of stater cultures in a semi-dry coffee (Coffea arabica) fermentation process. Food Microbiol. 2014, 44, 87–95. [Google Scholar] [CrossRef]
- Huch, M.; Franz, C.M.A.P. Coffee: Fermentation and Microbiota. In Advances in Fermented Foods and Beverages; Woodhead Publishing: Sawston, UK, 2015; pp. 501–513. [Google Scholar]
- Aswathi, K.N.; Murthy, P.S. Pulped natural/honey coffee process: An innovative approach. Food Humanit. 2024, 2, 100287. [Google Scholar] [CrossRef]
- Aswsthi, K.N.; Shirke, A.; Praveen, A.; Chaudhari, S.R.; Murthy, P.S. Pulped natural/honey robusta coffee fermentation metabolites, physicochemical and sensory profiles. Food Chem. 2023, 429, 136897. [Google Scholar] [CrossRef]
- Shen, X.J.; Zi, C.T.; Yang, Y.J.; Wang, Q.; Zhang, Z.L.; Shao, J.W.; Zhao, P.C.; Liu, K.Y.; Li, X.Y.; Fan, J.P. Effects of different primary processing methods on the flavor of Coffea arabica beans by metabolomics. Fermentation 2023, 9, 717. [Google Scholar] [CrossRef]
- Lee, L.W.; Cheong, M.W.; Curran, P.; Yu, B.; Liu, S.Q. Coffee fermentation and flavor- an intricate and delicate relationship. Food Chem. 2015, 185, 182–191. [Google Scholar] [CrossRef]
- Zhai, H.; Dong, W.; Tang, Y.; Hu, R.; Yu, X.; Chen, X. Characterization of the volatile flavour compounds in Yunnan arabica coffee prepared by different primary processing methods using HS-SPME/GC-MS and HS-GC-IMS. LWT 2024, 192, 115717. [Google Scholar] [CrossRef]
- Ribeiro, L.S.; Miguel, M.G.D.C.P.; Evangelista, S.R.; Martins, P.M.M.; Mullem, J.V.; Belizario, M.H.; Schwan, R.F. Behavior of yeast inoculated during semi-dry coffee fermentation and the effect on chemical and sensorial properties of the final beverage. Food Res. Int. 2017, 92, 26–32. [Google Scholar] [CrossRef]
- Haile, M.; Kang, W.H. The role of mocrobes in coffee fermentation and their impact on coffee quality. J. Food Qual. 2019, 12, 4836709. [Google Scholar]
- Shen, X.J.; Wang, B.J.; Zi, C.T.; Huang, L.L.; Wang, Q.; Zhou, C.C.; Wen, W.; Liu, K.Y.; Yuan, W.J.; Li, X.Y. Interaction and metabolic function of microbiota during the washed processing of Coffea arabica. Molecules 2023, 28, 6092. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Liu, X.; Wang, T.; Wang, Q.; Feng, L.; Su, R.; Zhang, M.; Xu, B.; Chen, F.; Li, P. Optimization of main ingredient ratio, metabolomics analysis, and antioxidant activity analysis of lycopene-enriched compound fruit wine. Fermentation 2023, 9, 591. [Google Scholar] [CrossRef]
- Liu, K.; Han, L.; Wang, Q.; Yang, L.; Liu, X.; Jiang, B.; Zeng, X.; Liu, Y.; Li, M.; Jiao, W.; et al. Interaction and metabolic function of microbiota during Tibetan tea fermentation through bioaugmentation with Aspergillus niger. Fermentation 2023, 9, 690. [Google Scholar] [CrossRef]
- Liu, K.Y.; Wang, L.Y.; An, J.S.; Luo, H.; Wang, X.H.; Ma, Y.; LV, C.Y.; Zhao, M. Research on the fermentation of Pu-erh tea through inoculation with Aspergillus amstelodami. J. Light Ind. 2022, 37, 1–9. [Google Scholar]
- Batista, L.R.; Chalfoun, S.M.; Silva, C.F.; Cirillo, M.; Varga, E.A.; Prado, G.; Schwan, R.F. Ocratoxin A in coffee beans (Coffea arabica L.) processed by dry and wet methods. Food Control 2009, 20, 784–790. [Google Scholar] [CrossRef]
- Vilela, D.M.; Pereira, G.V.D.; Silva, C.F.; Batista, L.R.; Schwan, R.F. Molecular ecology and polyphasic characterization of the microbiota associated with semi-dry processed coffee (Coffea arabica L.). Food Microliol. 2010, 27, 1128–1135. [Google Scholar] [CrossRef]
- Gomes, W.D.S.; Pereira, L.L.; Luz, J.M.R.D.; Silva, M.D.C.S.D.; Veloso, T.G.R.; Partelli, F.L. Exploring the microbiome of coffee plants: Implication for coffee quality and production. Food Res. Int. 2024, 179, 113972. [Google Scholar] [CrossRef]
- Martinez, S.J.; Bressani, A.P.P.; Miguel, M.G.D.P.; Dias, D.R.; Schwan, R.F. Different inoculation methods for semi-dry processed coffee using yeasts as stater cultures. Food Res. Int. 2017, 102, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Bressani, A.P.P.; Batista, N.N.; Ferreira, G.; Martinez, S.J.; Simao, J.B.P.; Dias, D.R.; Schwan, R.F. Characterization of bioactive, chemical, and sensory compounds from fermented coffees with different yeast species. Food Res. Int. 2021, 150, 110755. [Google Scholar] [CrossRef]
- Pereira, G.V.D.M.; Sampaio, V.D.M.; Wiele, N.; Vale, A.D.S.; Neto, D.P.D.C.; Souza, A.D.F.D.D.; Santos, D.V.N.D.; Ruiz, I.R.; Rogez, H.; Soccol, C.R. How yeast has transformed the coffee market by creating new flavors and aromas through modern post-harvest fermentation systems. Trends Food Sci. Technol. 2024, 151, 104641. [Google Scholar] [CrossRef]
- Cassimiro, D.M.D.J.; Batista, N.N.; Fonseca, H.C.; Naves, J.A.O.; Dias, D.R.; Schwan, R.F. Coinoculation of lactic acid bacteria and yeast increases the quality of wet fermentation arabia coffee. Int. J. Food Microbiol. 2022, 369, 109627. [Google Scholar] [CrossRef]
- Iamanaka, B.T.; Teixeira, A.A.; Teixeira, A.R.R.; Vicente, E.; Frisvad, J.C.; Taniwaki, M.H.; Bragagnolo, N. Potrntial of volatile compounds produced by fungi to influence sensory quality of coffee beverage. Food Res. Int. 2014, 64, 166–170. [Google Scholar] [CrossRef]
- Wang, X.; Meng, Q.Q.; Peng, X.R.; Hu, G.L.; Qiu, M.H. Identification of new diterpene esters from green Arabica coffee beans, and their platelet aggregation accelerating activities. Food Chem. 2018, 263, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Sunarharum, W.B.; Williams, D.J.; Smyth, H.E. Complexity of coffee flavor: A compositional and sensory perspective. Food Res. Int. 2014, 62, 315–325. [Google Scholar] [CrossRef]
- Zhai, H.; Dong, W.J.; Fu, X.; Li, G.; Hu, F. Integration of widely targeted metabolomics and the e-tongue reveals the chemical variation and taste quality of Yunnan arabica coffee prepared using different primary processing method. Food Chem. X 2024, 15, 101286. [Google Scholar] [CrossRef]
- Cortes-Macias, E.T.; Lopez, C.F.; Gentile, P.; Giron-Hernandez, J.; Lopez, A.F. Impact of post-harvest treatments on physicochemical and sensory characteristics of coffee beans in Huila, Colombia. Postharvest Biol. Technol. 2022, 187, 111852. [Google Scholar] [CrossRef]
- Santanatoglia, A.; Caprioli, G.; Ricciutelli, M.; Vittori, S.; Angeloni, S. Quantification of two derivatives of malic acid first-time discovered in coffee: Influence of postharvest processing method. Food Chem. 2024, 460, 140644. [Google Scholar] [CrossRef]
- Duarte, G.S.; Pereira, A.A.; Farah, A. Chlorogenic acids and other relevant compounds in Brazilian coffees processed by semi-dry and wet post-harvesting method. Food Chem. 2010, 118, 851–855. [Google Scholar] [CrossRef]
- Banti, M.; Atlaw, T. Effect of processing methods on physicochemical and cup quality of coffee at Jimma, Ethiopia. Heliyon 2024, 10, e30480. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Park, S.J.; Yu, J.S.; Lee, D.Y. Interactive effect of post-harvest processing method, roasting degree, and brewing mentod on coffee metabolite profiles. Food Chem. 2022, 397, 133749. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Hu, G.; Zhang, Z.; Al-Romaima, A.; Bai, X.; Li, J.; Zhou, L.; Li, Z.; Qiu, M. Comparative studies of fermented coffee fruits post-treatments on chemical and sensory properties of roasted beans in Yunnan, China. Food Chem. 2023, 423, 136332. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).