Abstract
Spirulina is a highly nutritious microalgae commonly used as a food additive. During fermentation, different adjuncts are incorporated to act as a nutrient source for yeast and fortify or modify the sensory attributes of the final product. In this study, the effect of Spirulina on the characteristics of controlled yeast fermentation and the production of volatile organic compounds (VOCs) was analyzed. Spirulina was added to malted barley during mashing and fermented under standard conditions. An unaltered mash (negative control) and yeast extract (positive control) were also fermented. The addition of Spirulina resulted in an increased fermentation rate (~14% faster) and bigger yeast cells (~34% larger) in comparison to the negative control. There were differences in color (determined as SRM) between treatments; however, there were only minor differences in VOCs, with no statistical differences observed between chemical compound groups. No differences were observed in the pH, total number of yeast cells, or final attenuation between treatments. The primary mechanism for the observed differences is believed to be an increase in amino acids available to yeast that were contributed by the Spirulina. This shows both that Spirulina has a high potential as a fermentation adjunct and that the amino acid profile of an adjunct can significantly impact fermentation.
1. Introduction
Spirulina (Arthrospira platensis) is a multicellular, filamentous cyanobacteria that is considered a popular food supplement due to its high concentration of macro- and micronutrients, essential amino acids, protein, polyunsaturated lipids, antioxidants, minerals, and vitamins [1]. Different compounds are attributed to the vibrant coloration of Spirulina, such as carotenoids, C-phycocyanin, β-cryptoxanthin, and zeaxanthin [2,3]. These color compounds can be utilized as natural colorants to alter the color of various food products. Furthermore, Spirulina is known to be a complete protein, meaning that it contains all the essential amino acids, and is also a source of B vitamins such as thiamine and riboflavin [4]. Spirulina is currently sold as dietary supplements in pills, capsules, and powders meant to be used in water and smoothies. Spirulina has also been incorporated in food products such as bread, pasta, and chocolate biscuits [5]. However, its potential for use as a fermentation adjunct has not been well described. In this paper, the supplementation with Spirulina to a brewing fermentation was assessed with respect to the yeast performance and morphology as well as changes in chemical and organoleptic properties.
According to Habschied et al. [6], fruits, herbs, and different microorganisms like the ones found in kefir have been used to improve not only the organoleptic properties of beer but also its functional properties, serving as antioxidants and probiotics. For example, beers have been fortified with selenium to prevent deficiencies of this trace element [7]. The utilization of different gluten-free cereals and pseudo cereals has also been tested to accommodate an increasing number of people intolerant to gluten [6].
The organoleptic properties of beer (i.e., mouthfeel, color, taste, and odor) are determined by a wide range of characteristics of both the raw materials, as well as the conditions in which fermentation takes place. As an illustration, the composition of wort is considered to have a strong influence on the flavor of finished beer, as does the health of the yeast that ferments it. Wort must serve multiple purposes for the yeast: (1) it is a growing medium for new budding yeast, and (2) it serves as a fermentation medium for yeast to be able to produce ethanol, carbon dioxide, and other secondary metabolites that can ultimately influence the final sensory profile of the beer [8]. Since yeast relies on wort to obtain all the essential nutrients for their growth and multiplication, even slight modifications in the wort, like amino acid concentration, can influence yeast metabolism, impacting the final flavor profile [9,10]. Concentrations of specific amino acids (such as isoleucine, valine, phenylalanine, glycine, alanine, tyrosine, lysine, histidine, arginine, and leucine) are important to the flavor of a given beer as they are used in the biosynthesis of volatiles by yeast [11].
Several different types of nitrogenous compounds can be found in wort. However, yeast is only able to utilize specific nitrogen sources known as free amino nitrogen (FAN). FAN is a mix of amino acids, small peptides, and ammonium ions derived from the proteolysis of barley proteins during the wort-making processes [8]. These compounds are essential for yeast metabolism, affecting fermentation rate, yeast vitality, and the overall quality of the beer due to a direct impact on the volatile and semi-volatile flavor compounds [12,13]. Wort contains different amino acids that are utilized by yeasts at different rates [8].
Depending on the rate at which amino acids are utilized, they have been grouped in different categories. In this study, the amino acids were grouped based on the absorption rate as defined by Hill and Stewart [8]. Amino acids belonging to Group A are immediately absorbed and almost removed after 20 h of fermentation. Amino acids in Group B are absorbed gradually during fermentation but are not removed as fast as the ones from the previous group. The uptake of amino acids from Group C coincides with the final removal of Group A amino acids. Finally, proline is the only amino acid belonging to Group D, a group that shows a slight absorption [14].
The practice of fortifying wort to achieve the proper concentration of amino acids and increase FAN levels is a well-established technique in brewing. An improper concentration of FAN can negatively affect yeast performance and beer quality [8]. However, an excessive FAN concentration can lead to the formation of off-flavors. Therefore, it is of utmost importance to maintain an appropriate amino acid balance for optimal yeast performance and fermentation efficiency, ensuring the production of high-quality beer with a desirable flavor profile [8,13]. Typically, brewers add yeast extract to the wort due to its complete amino acid profile [15]. Adding yeast extract not only elevates the FAN content but also improves yeast growth rates, cell mass, and overall fermentation performance [16]. O’Connor et al. [17] found that while the increase in FAN did not directly correlate with higher yeast cell viability, it did lead to significant improvements in yeast growth.
Table 1 shows the amino acid profiles of Spirulina and yeast extract, grouped based on yeast absorption rates [14]. The amino acid profile is a key metric used by the brewing industry to predict yeast fermentation and beer quality [18,19]. These amino acids provide benefits during anaerobic fermentations and significantly impact VOC production throughout the fermentation process. However, it is important to note that certain amino acids can act as inhibitors, potentially affecting yeast metabolism, morphology, or the fermentation kinetics directly. Asparagine, for instance, serves as a valuable source of ammonia during fermentation. However, its impact depends on the stereoisomer present in the wort. Amino acids with a D-isomer form are known to inhibit yeast growth [20]. Asparagine can be present in wort in both L- and D- forms, with L-asparagine being a required amino acid for various metabolic processes [21]. Although S. cerevisiae strains can externally metabolize asparagine, this likely occurs primarily during nitrogen starvation [22]. Generally, it is observed during brewing operations that the addition of amino acids like lysine can reduce the fermentation time by half [10]. Similarly, when the concentration of asparagine, serine, lysine, arginine, valine, methionine, leucine, isoleucine, glycine, and tyrosine is increased by one-fold, the wort fermentability and ethanol yield also increases by 6% and 17%, respectively [23].

Table 1.
Amino acid profiles of spirulina (Arthrospira platensis) and yeast extract in milligrams per 100 g of protein, organized by absorption rate groups for yeast.
The addition of Spirulina to the alcoholic fermentation process significantly impacts the formation of VOCs and plays a crucial role in determining the sensory quality of fermented products [27]. A study on Spirulina food supplements identified 128 VOCs, including major volatiles like heptadecane, ketones, aldehydes, and oct-1-en-3-ol [28]. Beisler and Sandmann [29] replaced 5% (w/w) of the malt with Spirulina powder. They found that while the alcoholic fermentation was slightly influenced by the cyanobacterial biomass, the resulting beer maintained a typical beer-like characteristic. Sensory evaluations indicated a complex alteration in the sensory properties. This includes a dominant algal taste that disturbs the character of pale ale beers but also a deep blue color of the beer if Spirulina was included during the wort cooling phase. However, when Spirulina was added at the beginning of the mashing, they observed a complete loss of the blue color. Similarly, when adjuncts, such as fruits, are added in the initial stages of brewing, there is little effect on the final product [30]. For example, when pineapple juice was added at the beginning of fermentation, it led to beers with only a weak pineapple aroma [31].
The objective of this study was to analyze the effect of Spirulina on the rate of controlled yeast fermentations, yeast performance, and morphology, as well as its impact on the production of volatile organic compounds. It was hypothesized that the additional amino acids would result in a faster fermentation, but this addition would also affect the volatile characteristics of the final product.
2. Materials and Methods
2.1. Test Materials
The malted barley used for these experiments was Copeland pale B05-071 from Skagit Valley Malting (Burlington, WA, USA). Spirulina (Arthrospira platensis) powder was purchased from Carlyle Nutritionals LLC (Melville, NY, USA), and yeast extract was bought from Becton Dickinson and Company (Franklin Lakes, NJ, USA). The yeast used for all the experiments was Diamond lager yeast (Saccharomyces pastorianus) from Lallemand (DISTILAMAX SR—Montreal, QC, Canada).
2.2. Mashing
To begin, 450 g of malted barley was ground using a FOSS Cyclotec 1093 (Hillerød, Denmark). After grinding, the barley was equally divided into nine 500 mL glass beakers. Then, 2.5 g of Spirulina (5% w/w) was added to three beakers, and 2.5 g of yeast extract was added to three other beakers. The three remaining were left as control. The mashing regime was performed as described by the European Brewing Convention-ANALYTICA (EBC-ANALYTICA) Malt 4.5.1 method (EBC). Briefly, it consisted of adding water to each beaker and heating them in an immersion bath at 45 °C for 30 min. Subsequently, the temperature was increased by 1 °C per minute for 25 min until it reached 70 °C. At this point, additional water was added to each beaker, and they were further heated at 70 °C for one hour. After mashing, the wort was filtered using P8 filter paper from Fisherbrand (Thermo Fisher Scientific Inc., Waltham, MA, USA). Density was measured using a DMA 35 Density Meter (Anton Paar, Graz, Austria) and adjusted to ~12 °Plato using distilled water and glucose, and the wort was frozen until the fermentation assay was done.
2.3. Yeast Rehydration, Propagation, and Pitching
Briefly, 3.07 g of Diamond lager yeast was added to three glass beakers with 50 mL of previously prepared and autoclaved YEPD (yeast extract peptone dextrose) broth. The yeast culture was incubated for 48 h at 30 °C as described in the American Society of Brewing Chemists (ASBC) Yeast-14 method [32]. After the initial 24 h of incubation, the slurry was centrifuged, and the yeast pellets were resuspended in sterile, distilled water. The process was repeated for three washes. After the final centrifugation, the yeast pellets were resuspended in a single tube, pitched at 1.5 × 107 cells/mL into two 250 mL flasks each containing 100 mL of YEPD broth, and incubated for 24 h. Before pitching, the yeast cell count was determined following the ASBC Yeast-4 method [33].
Subsequently, wort was separated into the three separate treatments, and yeast was pitched at identical concentrations of 1.5 × 107 cells/mL in each vessel, as stated in the ASBC Yeast-14 method [32]. Finally, 15 mL of each wort was aseptically transferred to 30 sterile 15 mL glass test tubes per treatment.
2.4. Fermentation
For each wort, small scale standardized fermentations were conducted as described by the official ASBC Yeast-14 method, which has been shown to reflect industrial scale [34]. After pitching, the fermentation tubes were stoppered with a sterile sponge bung and were placed in a water bath at 21 °C until sampling. Sampling times were modified from the ASBC Yeast-14 method [32], and samples were taken at 0, 1.5, 6.5, 20.5, 22.5, 25.5, 28.5, 31.5, 44.5, 47.5, 50.5, 53.5, 69.5, and 100 h time points. Three fermentation tubes treatment were destructively sampled at each time, and the analyses done were density, pH, total cells, and cells in suspension. Total cells and cells in suspension were measured using an automated cell counter (TC20 automated cell counter, Bio-Rad, Hercules, CA, USA).
2.5. Fermentation Measurements of Samples
Density was determined on filtered samples using a DMA 35 Density Meter (Anton Paar, Graz, Austria) following the ASBC Beer-4 method (Instrumental) [35].
pH was measured using an Accumet® AB15 pH meter (Thermo Fisher scientific, Waltham, MA, USA). The instrument was calibrated using a three-point curve prior to use.
The color of samples was determined using a UV-1900 Shimadzu spectrophotometer (Shimadzu Corporation, Kyoto, Japan). The color was measured in filtered samples at the beginning and end of fermentation. The official ASBC Beer-10 method was used to analyze color based on the wavelength and the color determination calculation observed using Equation (1).
Beer color for 10-mm cuvette = 10 × 1.27 × A × F
Here, A is the absorbance at 430 nm, and F is the dilution factor.
2.6. Amino Acid Profile (FAN)
The amino acid profile was completed on the initial wort and samples from the end of fermentation. The amino acid profile was measured following the Agilent Technologies application note written by Henderson Jr. and Brooks [36].
2.7. Yeast Morphology (Size)
The size of yeast cells at the end of fermentation was assessed using hemocytometer images at 40× magnification, with the size of each square as a reference. Samples were prepared as described by Guadalupe-Daqui et al. [37], and dimensions were taken as the major and minor axis and then used to calculate the area via Equation (2) [38] assuming the cells are shaped as ellipsoids.
S = 4πb(sin45°(a − b) + b)
Here, a = major axis, b = minor axis, and S = surface area of yeast cell.
2.8. Volatile and Semi-Volatile Compounds
2.8.1. Extraction of the Volatile and Semi-Volatile Compounds
Extraction and concentration of the volatile and semi-volatile compounds in the experimental and control beers were performed using the solid phase microextraction (SPME) technique. A 50/30 um divinylbenzene/carboxen/polydimethylsiolxane (DVB/carboxen/PDMS) fiber (Supelco, Inc., Bellefonte, PA, USA) was exposed to the headspace above 10 mL of sample beer spiked with 25 µL 2-octanol (20 mg/L of 2-octanol in water) as the internal standard and 30% w/v of salt in 20 mL headspace vials with Teflon-lined silicone septa (Chromacol, Fisher Scientific) for 30 min at 40 °C with an agitation speed of 250 rpm. Samples were equilibrated at 40 °C for five minutes prior to exposing the fiber.
2.8.2. Separation with Gas Chromatography (GC-MS)
Volatile compounds were desorbed for five minutes in the injection port of a Shimadzu gas chromatograph (GC) 2010 Plus Series mass spectrometer detector (MSD) QP2010 SE (Shimadz Maryland, Columbia, MD, USA). The injection port was set to 250 °C, and all injections were made in splitless mode using a narrow bore, deactivated glass insert. Volatile compounds were separated using a nonpolar ZB-5MS column (ZB; 30 m × 0.25 mm id × 0.25 μm film thickness) with He as the carrier gas at a flow rate of 2.0 mL/min (linear velocity 53.8 cm/s). The GC oven temperature program was as follows: 35 °C hold for 5 min and then increased to 225 °C at a rate of 6 °C/min. Once the final temperature of 225 °C was reached, it was maintained for 10 min. The MSD was maintained at 200 °C, and the sample mass was scanned in the range of 40–800 m/z. Peaks were identified using standardized retention time (linear retention index values—LRI), pure compounds, and fragmentation spectra of standards and the Wiley 2014 mass spectral library [39].
2.8.3. Identification of Volatile Compounds
Volatile compounds were identified based upon their LRI values using nonpolar (ZB-5MS) columns (30 m × 0.25 mm i.d., 0.25 μm film; J&W, Folsom, CA, USA). The LRI values were compared to values reported in the literature. Aliphatic hydrocarbon standards were analyzed in the same manner using a DB-5 column to calculate LRI using Equation (3):
where N is the carbon number of the lowest alkane and n is the difference between the carbon number of the two n-alkanes that are bracketed between the compound; tRa, tRN, and tR(N + n) are the retention times of the unknown compound, the lower alkane, and the upper alkane, respectively [39].
2.9. Statistical Analysis and Modeling
Statistical differences between means were assessed using a one-way ANOVA and a Tukey test and were done using the Minitab 19 Statistical software (Minitab, LLC, State College, PA, USA).
The density, pH, and FAN results were modeled using the four-parameter logistic equation described in the ASBC Yeast-14 method (ASBC). The four-parameter logistic model is commonly fit to fermentation density attenuation in order to describe and compare fermentation behavior [40,41]. The equation used is provided as Equation (4). The first derivative of the sugar consumption model can be calculated as described by Guadalupe et al., [42]. The resulting curve is the rate of sugar metabolism at any point during the fermentation.
Here, P(t) is the density at time (t), expressed as °Plato; P(e) is the lower asymptote of the density curve; P(i) is the upper asymptote; B is a function of the slope (1/h); and M is the time to reach the inflection point (h).
The results of the total cell count and cells in suspension were analyzed using a “tilted Gaussian” fit model, as described in the ASBC Yeast-14 method [32], which is shown in Equation (5).
Here, the results are expressed as the Log of the cell count (cells/mL), R is the slope, t is the time (h), Ae is the absolute amplitude, µ is the midpoint, and σ is the width factor.
After modeling the results, a comparison between the means was run to determine significant differences (α = 0.05). All the analyses were done using GraphPad Prism 9 (Dotmatics Inc., Bishop’s Stortford, UK).
3. Results
3.1. Density
Fermentation of the three samples (control, Spirulina supplemented, and yeast extract supplemented) started at the same density (11.2 °Plato), which can be observed in Figure 1a. The density of the wort is an estimation of the sugar content, which decreases during the fermentation process as it is transformed into ethanol and carbon dioxide (CO2) [43,44]. While the three samples reached the same final density (0.9 °Plato), the speed in which the samples reached the final density was significantly different (p < 0.05). The M value (time to reach the inflection point) [37] was 19.7 h for the control, 15.8 h for yeast extract, and 17.3 h for Spirulina; the Pe, which is when the curve reached the lower asymptote, was 0.92 for the control, 0.82 for the yeast extract-supplemented sample, and 0.85 for the Spirulina-supplemented sample. The speed of fermentation can be altered by different factors such as temperature [45], and an appropriate nutrient concentration in the fermentation medium [15]. The difference observed in this study can likely be attributed to the changes in amino acid concentration, which will be further discussed in the sections below. As discussed in the Introduction section, our results are similar to Jacob et al. [15] who found that the addition of yeast extract increased the FAN concentration and led to a higher cell count. The rate of fermentation (sugar metabolized per hour) was determined and is presented in Figure 1d. The rate at the beginning of fermentation was not affected; however, differences in rate became apparent after ~12 h. The rate of metabolism at peak fermentation (in this case ~23 h) was most strongly impacted by the treatments in this study.
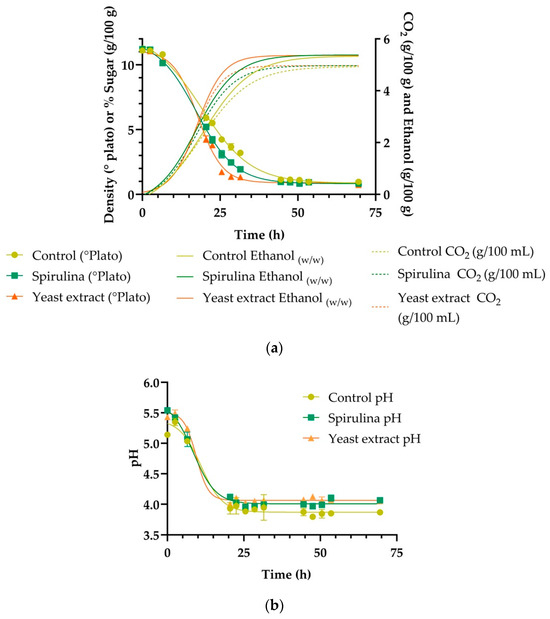
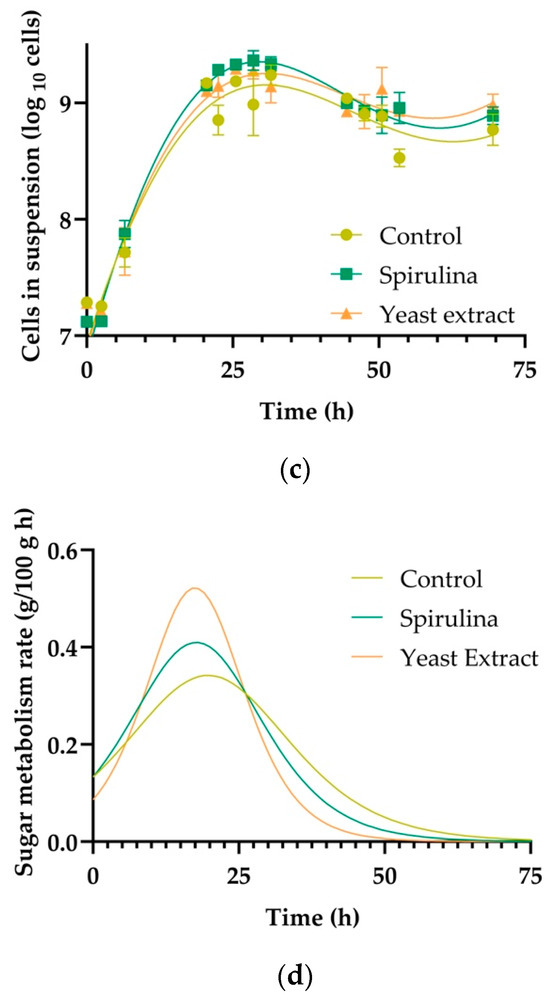
Figure 1.
(a) Fermentation density over time for each treatment: control (yellow), spirulina fortified (green), and yeast extract fortified (orange). Data points (circles, square, triangles) represent the average value of triplicate measurements, and models (continuous lines) were built using a four-parameter logistic model as described in the ASBC Yeast-14 method. (b) pH over time for each treatment: control (yellow), spirulina fortified (green), and yeast extract fortified (orange). Points (circles, square, triangles) represent the average value of triplicate measurements, and models (continuous lines) were built using a four-parameter logistic model as described in the ASBC Yeast-14 method. (c) Yeast cells in suspension during fermentation for each treatment: control (yellow), spirulina fortified (green), and yeast extract fortified (orange). Data points (circles, square, triangles) represent the average value of triplicate measurements, and models (continuous lines) were built using a tilted Gaussian fit model logistic model as described in the ASBC Yeast-14 method. (d) Rate of sugar metabolism during the fermentation determined by calculating the first derivative of the sugar consumption model (Figure 1a) as described by Guadalupe et al. [42].
3.2. pH
The changes in pH during the fermentation are depicted in Figure 1b. The statistical analysis showed that each data set had a different curve. At the beginning of the fermentation, the pH of the control was 5.14, the yeast extract supplemented sample was 5.43, and the Spirulina supplemented sample was 5.54. The final pH values were 3.74 ± 0.03, 3.94 ± 0.03, and 3.77 ± 0.04 for the control, yeast extract-supplemented sample, and Spirulina-supplemented sample, respectively. It appears as though the pH of the final beer was slightly affected by the supplementation with Spirulina; this is likely due to increased buffering capacity provided by the Spirulina powder. This effect has also been observed in fermented milk products, where the addition of Spirulina led to slower pH drop rates and was attributed to the buffering effect of the proteins, peptides, and amino acids of the microalgae [46].
3.3. Color
The color in each wort was assessed using standard methods. The findings are reported in Table 2. There was a decrease in color absorption at 430 nm in all beers when compared with the initial wort colors. This change in color can be related to oxidative degradation of polyphenols [47]. The supplementation with Spirulina, even in small concentrations, in different food products such as yogurt and cookies has been reported to cause a shift in color toward greenish hues, attributed mainly to the presence of chlorophyll and a phycobiliprotein called phycocyanin [48,49,50]. Table 2 provides the values and an image to illustrate how the vibrant green color of the Spirulina wort (center) faded post fermentation to nearly the same color as the control wort. Similarly, the color of Spirulina fermented with lactic acid bacteria decreased after 36 h [50]. These changes are likely due in part due to thermal sensitivity (degradation during the boiling step). Changes in the coloration of the samples may also be affected by the change in pH of the fermentation medium [51]. Color changes can be led by organic acid production, which can enhance oxidation of pigments present in the medium [52]. The results we observed on the effect of fermentation on the pigments provided by Spirulina including phycocyanin has been reported to be caused by a molecule that is sensitive to harsh environmental conditions, including pH [53,54].

Table 2.
Average standard research method (SRM) color results for wort and beer.
3.4. Yeast Size
After 48 h of fermentation, yeast cells in the Spirulina-supplemented medium were significantly bigger than the ones in the control medium; however, the yeast cells in the yeast extract presented no statistical differences when compared to the control and Spirulina treatments, as shown in Table 3. Consequently, it was observed that the supplementation with Spirulina resulted in larger yeast cells. The supplementation with Spirulina increased the concentration of free amino acids, specifically those belonging to Group A (Section 3.6. Amino acid profile, Table 4), when compared to the results from the yeast extract and control samples (Section 3.5 Cell count). These differences could explain the increased size of yeast cells in the Spirulina-supplemented treatment.

Table 3.
Average yeast cell size after fermentation measured at 40× magnification.

Table 4.
Comparison of initial amino acid concentrations (mg/L) and total consumption (%) in fermentation with different supplementation sources.
3.5. Cell Count
The initial cell count (cells/mL) was 1.7 ± 0.3 × 107 cells/mL for all fermentations (control, Spirulina supplemented, and yeast extract supplemented). Over the fermentation, the number of cells in suspension increased by ~100×. The statistical analysis showed that one curve fit all the samples, meaning that there was no significant difference (p > 0.05) in the total cell count (Figure 1c).
3.6. Amino Acid Profile and Consumption
The amino acid profile of the wort from the three treatments (control, Spirulina supplemented, and yeast extract supplemented) and the change after fermentation (after 100 h) are shown in Table 4. Amino acids were grouped according to the classification by Hill and Stewart [8], with Group A including fast absorption amino acids, Group B intermediate absorption, Group C slow absorption, and Group D presenting little or no absorption by brewer’s yeast. Spirulina emerges as a richer source of amino acids than yeast extract and the control, as evidenced by both higher initial amino acid concentrations and greater total amino acid consumption throughout fermentation (Table 1), which may have contributed to observed changes in yeast morphology. This finding suggests that Spirulina’s unique composition, abundant in proteins and amino acids [29], boosts yeast activity, resulting in consistently higher yeast cell counts throughout the fermentation process, as shown in Figure 1c Notably, Group D was predominantly consumed by yeast when the fermentation process was supplemented with yeast extract. Group D, characterized by the slowest amino acid absorption rate, promotes very slow yeast growth [55,56] and serves functions such as acting as an osmoprotectant, supporting stress resistance, and maintaining cell wall integrity under stress [57]. This observation could explain the increase in yeast cells in suspension towards the end of the yeast extract supplemented fermentation compared to the Spirulina supplemented and control.
Based on the information shown in Table 1 and the results presented in Table 4, it can be hypothesized that yeast extract-derived asparagine may exist partially or entirely in a D-isomer form, negatively affecting yeast growth during fermentation. Interestingly, Spirulina lacks asparagine, potentially favoring rapid and significantly greater yeast growth when wort is supplemented with Spirulina, as shown in Figure 1c. Consequently, the slower fermentation kinetics observed with Spirulina supplementation compared to yeast extract (Figure 1a) may be attributed to the presence of glutamine and asparagine from the yeast extract addition. These findings align with the research from Coral-Medina et al. [58] who demonstrated that S. cerevisiae benefits from the presence of asparagine and glutamine fortification, resulting in faster fermentations.
3.7. Volatile Compounds
Table 5 shows the concentrations of VOCs formed post-fermentation in each treatment. It is worth noting that any initial volatile concentration from wort was minimal and did not alter the overall outcomes. The majority of VOCs formed during fermentation are by-products of yeast metabolic pathways metabolizing sugar and nitrogen compounds. While most of these VOCs are not considered toxic, some can potentially harm yeast performance and disrupt fermentation if their concentration surpasses a certain threshold. There was a significant difference between yeast extract and Spirulina supplemented fermentations regarding the acids produced. Acids like hexanoic acid, octanoic acid, nonanoic acid, and decanoic acid can hinder yeast performance at high concentrations, affecting yeast membrane integrity and fermentation efficiency [59]. Furthermore, it has been established that fermentation processes promoting yeast growth led to higher concentrations of higher alcohols [60]. For Spirulina-treated samples, it was shown that there was a slightly higher concentration of higher alcohols when compared to yeast extract and the control. This difference could be attributed to the significantly higher yeast cells in suspension for Spirulina samples. Overall, the effect of spirulina addition on VOCs was minor.

Table 5.
Volatile organic compound (VOC) concentrations (mg/L) detected after fermentation (100 h).
4. Conclusions
Spirulina is a nutrient rich microalga that has been used to fortify many different food products. As it has a high concentration of amino acids, it was hypothesized that the addition of Spirulina would improve fermentation kinetics similar to the effects of yeast extract. This study found that the addition of the Spirulina to a beer fermentation resulted in a higher fermentation rate and changes to the yeast morphology when compared to a control. Additionally, the results showed that Spirulina increased the concentration of most amino acids that were already present (except proline and cysteine). However, the effect of Spirulina was different than an equivalent amount of yeast extract that had a different amino acid profile. This strongly suggests that effects of amino acid addition are dependent upon the profile and may be able to be tailored to specific applications. After the addition of the Spirulina, the color (SRM) of the initial wort was significantly different between treatments (particularly between the red–blue wavelengths); however, the intensity degraded substantially during the mashing and fermentation steps. The VOCs present in the final product showed differences in some individual compounds (e.g., octanoic acid); however, there were no significant differences in the concentrations of compound groups when compared to the control. Similarly, there were no differences observed in the pH, total number of yeast cells, or final attenuation between treatments. Therefore, it was found that when Spirulina was added to a beer fermentation, there were positive differences in the fermentation kinetics, and few significant differences in the final product except small changes to color and the residual amino acid profile. This may facilitate Spirulina being used as either a fortification agent for beverage fermentations as a means to add bioactive components or potentially as an amino acid source for tailored fermentation to change the morphology of the microorganism and/or increase the overall rate of fermentation.
5. Future Work
This work highlights the opportunity for more research into the effect of individual amino acids on the characteristics of various food fermentations, leading to the possibility that substrates may include microorganism diets tailored to specific purposes. The potential applications of this research extend beyond the food industry, potentially impacting sectors such as pharmaceuticals, biofuels, and waste management, where microbial fermentation plays a crucial role.
Author Contributions
Conceptualization, A.J.M., M.M.R.-R., and A.P.-A.; methodology, K.A.T.-W. and M.A.R.-R.; formal analysis, S.C.-P., A.J.M., S.R.M., M.G.-D., and A.P.-A.; investigation S.C.-P. and A.P.-A.; resources, A.J.M., M.A.R.-R., and M.M.R.-R.; data curation, S.C.-P. and A.P.-A.; writing—original draft preparation, A.J.M., M.G.-D., S.C.-P., S.R.M., K.A.T.-W., and A.P.-A.; writing—review and editing, A.J.M., M.G.-D., S.C.-P., S.R.M., K.A.T.-W., A.P.-A., M.A.R.-R., and M.M.R.-R.; visualization, A.J.M., M.G.-D., S.C.-P., K.A.T.-W., and A.P.-A.; supervision, A.J.M., M.M.R.-R., and K.A.T.-W.; project administration, A.J.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
Author Alexa Pérez-Alva gratefully acknowledges financial support for her Ph.D. studies from Universidad de las Américas Puebla (UDLAP) and the National Council for Science and Technology (CONACyT) of Mexico as well as the Food Analysis Laboratory, Intema S.A. de C.V. for their contribution in the performed analyses.
Conflicts of Interest
Author Melissa A. Ramírez-Rodrigues was employed by the company Intema S.A. de C.V. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Soni, R.A.; Sudhakar, K.; Rana, R.S. Spirulina—From growth to nutritional product: A review. Trends Food Sci. Technol. 2017, 69, 157–171. [Google Scholar] [CrossRef]
- Careri, M.; Furlattini, L.; Mangia, A.; Musci, M.; Anklam, E.; Theobald, A.; von Holst, C. Supercritical fluid extraction for liquid chromatographic determination of carotenoids in Spirulina Pacifica algae: A chemometric approach. J. Chromatogr. A 2001, 912, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Park, W.S.; Kim, H.J.; Li, M.; Lim, D.H.; Kim, J.; Kwak, S.S.; Kang, C.M.; Ferruzzi, M.G.; Ahn, M.J. Two Classes of Pigments, Carotenoids and C-Phycocyanin, in Spirulina Powder and Their Antioxidant Activities. Molecules 2018, 23, 2065. [Google Scholar] [CrossRef] [PubMed]
- Mathew, F.; Saral, M.A. Fucoidans: A marine antioxidant. In Marine Antioxidants: Preparations, Syntheses, and Applications; Kim, S.K., Shin, K.H., Venkatesan, J., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 355–363. [Google Scholar]
- Santos, T.D.; Freitas, B.C.B.; de Moreira, J.B.; Zanfonato, K.; Costa, J.A.V. Development of powdered food with the addition of Spirulina for food supplementation of the elderly population. Innov. Food Sci. Emerg. Technol. 2016, 37, 216–220. [Google Scholar] [CrossRef]
- Habschied, K.; Živković, A.; Krstanović, V.; Mastanjević, K. Functional Beer—A Review on Possibilities. Beverages 2020, 6, 51. [Google Scholar] [CrossRef]
- Rodrigo, S.; Santamaria, O.; Chen, Y.; McGrath, S.P.; Poblaciones, M.J. Selenium speciation in malt, wort, and beer made from selenium-biofortified two-rowed barley grain. J. Agric. Food Chem. 2014, 62, 5948–5953. [Google Scholar] [CrossRef]
- Hill, A.E.; Stewart, G.G. Free Amino Nitrogen in Brewing. Fermentation 2019, 5, 22. [Google Scholar] [CrossRef]
- Ferreira, I.M.; Guido, L.F. Impact of Wort Amino Acids on Beer Flavour: A Review. Fermentation 2018, 4, 23. [Google Scholar] [CrossRef]
- Lekkas, C.; Stewart, G.; Hill, A.; Taidi, B.; Hodgson, J. The Importance of Free Amino Nitrogen in Wort and Beer. Tech. Q. Master Brew. Assoc. Am. 2005, 42, 113–116. [Google Scholar]
- He, Y.; Dong, J.; Yin, H.; Zhao, Y.; Chen, R.; Wan, X.; Chen, P.; Hou, X.; Liu, J.; Chen, L. Wort composition and its impact on the flavour-active higher alcohol and ester formation of beer—A review. J. Inst. Brew. 2014, 120, 157–163. [Google Scholar] [CrossRef]
- Lodolo, E.J.; Kock, J.L.F.; Axcell, B.C.; Brooks, M. The yeast Saccharomyces cerevisiae—The main character in beer brewing. FEMS Yeast Res. 2008, 8, 1018–1036. [Google Scholar] [CrossRef] [PubMed]
- Walker, G.M.; Stewart, G.G. Saccharomyces cerevisiae in the Production of Fermented Beverages. Beverages 2016, 2, 30. [Google Scholar] [CrossRef]
- Jones, M.; Pierce, J.S. Absorption of amino acids from wort by yeasts. J. Inst. Brew. 1964, 70, 307–315. [Google Scholar] [CrossRef]
- Jacob, F.F.; Michel, M.; Zarnkow, M.; Hutzler, M.; Methner, F.J. The complexity of yeast extracts and its consequences on the utility in brewing: A review. Brew. Sci. 2019, 72, 50. [Google Scholar]
- D’Amore, T.; Panchal, C.J.; Russeil, I.; Stewart, G.G. Osmotic pressure effects and intracellular accumulation of ethanol in yeast during fermentation. J. Ind. Microbiol. 1988, 2, 365–372. [Google Scholar] [CrossRef]
- O’Connor-Cox, E.S.C.; Paik, J.; Ingledew, W.M. Improved ethanol yields through supplementation with excess assimilable nitrogen. J. Ind. Microbiol. 1991, 8, 45–52. [Google Scholar] [CrossRef]
- Hashimoto, T.; Maruhashi, T.; Yamaguchi, Y.; Hida, Y.; Oka, K. The effect on fermentation by-products of the amino acids in wort. In Proceedings of the World Brewing Congress, Portland, OR, USA, 28 July–1 August 2012. [Google Scholar]
- Inoue, T.; Kashihara, T. The importance of indices related to nitrogen metabolism in fermentation control. Tech. Q. Master Brew. Assoc. Am. 1995, 32, 109–113. [Google Scholar]
- Rytka, J. Positive selection of general amino acid permease mutants in Saccharomyces cerevisiae. J. Bacteriol. 1975, 121, 562–570. [Google Scholar] [CrossRef]
- Dunlop, P.C.; Roon, R.J. L-Asparaginase of Saccharomyces cerevisiae: An extracellular Enzyme. J. Bacteriol. 1975, 12, 1017–1024. [Google Scholar] [CrossRef]
- Dunlop, P.C.; Roon, R.J.; Even, H.L. Utilization of D-asparagine by Saccharomyces cerevisiae. J. Bacteriol. 1976, 125, 999–1004. [Google Scholar] [CrossRef]
- Lei, H.; Feng, L.; Peng, F.; Xu, H. Amino Acid Supplementations Enhance the Stress Resistance and Fermentation Performance of Lager Yeast During High Gravity Fermentation. Appl. Biochem. Biotechnol. 2019, 187, 540–555. [Google Scholar] [CrossRef]
- Andreeva, A.; Budenkova, E.; Babich, O.; Sukhikh, S.; Ulrikh, E.; Ivanova, S.; Prosekov, A.; Dolganyuk, V. Production, Purification, and Study of the Amino Acid Composition of Microalgae Proteins. Molecules 2021, 26, 2767. [Google Scholar] [CrossRef]
- Marson, G.V.; de Castro, R.J.S.; Belleville, M.P.; Hubinger, M.D. Spent brewer’s yeast as a source of high added value molecules: A systematic review on its characteristics, processing and potential applications. World J. Microbiol. Biotechnol. 2020, 36, 95. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Yuan, H.; Liu, M.; Liu, Q.; Zhang, S.; Liu, H.; Jiang, Y.; Huang, D.; Wang, T. Yeast Extract: Characteristics, Production, Applications and Future Perspectives. J. Microbiol. Biotechnol. 2023, 33, 151–166. [Google Scholar] [CrossRef]
- Zhou, X.; Guo, T.; Lu, Y.; Hadiatullah, H.; Li, P.; Ding, K.; Zhao, G. Effects of amino acid composition of yeast extract on the microbiota and aroma quality of fermented soy sauce. Food Chem. 2022, 393, 133289. [Google Scholar] [CrossRef]
- Paraskevopoulou, A.; Kaloudis, T.; Hiskia, A.; Steinhaus, M.; Dimotikali, D.; Triantis, T.M. Volatile Profiling of Spirulina Food Supplements. Foods 2024, 13, 1257. [Google Scholar] [CrossRef] [PubMed]
- Beisler, N.; Sandmann, M. Integration of Arthrospira platensis (spirulina) into the brewing process to develop new beers with unique sensory properties. Front. Sustain. Food Syst. 2022, 6, 918772. [Google Scholar] [CrossRef]
- Moreno, S.R.; Curtis, S.J.; Sarkhosh, A.; Sarnoski, P.J.; Sims, C.A.; Dreyer, E.; Rudolph, A.B.; Thompson-Witrick, K.A.; MacIntosh, A.J. Considerations When Brewing with Fruit Juices: A Review and Case Study Using Peaches. Fermentation 2022, 8, 567. [Google Scholar] [CrossRef]
- Yang, Q.; Gong, X.; Chen, M.; Tu, J.; Zheng, X.; Yuan, Y. Comparative analysis of the aroma profile of pineapple beers brewed with juice added at different times. J. Inst. Brew. 2023, 129, 151–163. [Google Scholar] [CrossRef]
- ASBC. Yeast-14: Miniature fermentation assay. In Wort Methods; American Society of Brewing Chemists: St. Paul, MN, USA, 2011; pp. 1–3. [Google Scholar]
- ASBC. Yeast-4: Microscopic yeast cell counting. In Wort Methods; American Society of Brewing Chemists: St. Paul, MN, USA, 2004. [Google Scholar]
- MacIntosh, A.J.; Adler, J.; Eck, E.; Speers, R.A. Suitability of the Miniature Fermentability Method to Monitor Industrial Fermentations. J. Am. Soc. Brew. Chem. 2012, 70, 205–211. [Google Scholar] [CrossRef]
- ASBC. Beer-4: Alcohol (Instrumental). In Beer Methods; American Society of Brewing Chemists: St. Paul, MN, USA, 2011; pp. 1–3. [Google Scholar]
- Henderson, J.W.; Brooks, A. Improved Amino Acid Methods Using Agilent ZORBAX Eclipse Plus C18 Columns for a Variety of Agilent LC Instrumentation and Separation Goals; Agilent Technologies: Wilmington, DE, USA, 2010; pp. 1–16. [Google Scholar]
- Guadalupe-Daqui, M.; Chen, M.; Thompson-Witrick, K.A.; MacIntosh, A.J. Yeast Morphology Assessment through Automated Image Analysis during Fermentation. Fermentation 2021, 7, 44. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, J.; Liu, B.; Wang, Z.; Yuan, Y.; Yue, T. Effect of yeast cell morphology, cell wall physical structure and chemical composition on patulin adsorption. PLoS ONE 2015, 10, e0136045. [Google Scholar] [CrossRef] [PubMed]
- Thompson-Witrick, K.A.; Rouseff, R.L.; Cadawallader, K.R.; Duncan, S.E.; Eigel, W.N.; Tanko, J.M.; O’Keefe, S.F. Comparison of Two Extraction Techniques, Solid-Phase Microextraction Versus Continuous Liquid–Liquid Extraction/Solvent-Assisted Flavor Evaporation, for the Analysis of Flavor Compounds in Gueuze Lambic Beer. J. Food Sci. 2015, 80, C571–C576. [Google Scholar] [CrossRef] [PubMed]
- Reid, S.J.; Josey, M.; MacIntosh, A.J.; Maskell, D.L.; Alex Speers, R. Predicting Fermentation Rates in Ale, Lager and Whisky. Fermentation 2021, 7, 13. [Google Scholar] [CrossRef]
- Armstrong, M.; MacIntosh, A.J.; Josey, M.; Speers, R.A. Examination of premature yeast flocculation in U.K. malts. Tech. Q. Master Brew. Assoc. Am. 2018, 55, 54–60. [Google Scholar]
- Guadalupe-Daqui, M.; Goodrich-Schneider, R.M.; Sarnoski, P.J.; Carriglio, J.C.; Sims, C.A.; Pearson, B.J.; MacIntosh, A.J. The effect of CO2 concentration on yeast fermentation: Rates, metabolic products, and yeast stress indicators. J. Ind. Microb. Biotechnol. 2023, 50, kuad001. [Google Scholar] [CrossRef]
- Hoche, S.; Hussein, M.A.; Becker, T. Critical process parameter of alcoholic yeast fermentation: Speed of sound and density in the temperature range 5–30 °C. Int. J. Food Sci. Technol. 2014, 49, 2441–2448. [Google Scholar] [CrossRef]
- Pires, E.J.; Teixeira, J.A.; Brányik, T.; Vicente, A.A. Yeast: The soul of beer’s aroma—A review of flavour-active esters and higher alcohols produced by the brewing yeast. Appl. Microbiol. Biotechnol. 2014, 98, 1937–1949. [Google Scholar] [CrossRef] [PubMed]
- Kucharczyk, K.; Tuszyński, T. The effect of temperature on fermentation and beer volatiles at an industrial scale. J. Inst. Brew. 2018, 124, 230–235. [Google Scholar] [CrossRef]
- Beheshtipour, H.; Mortazavian, A.M.; Haratian, P.; Khosravi-Darani, K. Effects of Chlorella vulgaris and Arthrospira platensis addition on viability of probiotic bacteria in yogurt and its biochemical properties. Eur. Food Res. Technol. 2012, 235, 719–728. [Google Scholar] [CrossRef]
- Salanță, L.C.; Coldea, T.E.; Ignat, M.V.; Pop, C.R.; Tofană, M.; Mudura, E.; Borșa, A.; Pasqualone, A.; Zhao, H. Non-Alcoholic and Craft Beer Production and Challenges. Processes 2020, 8, 1382. [Google Scholar] [CrossRef]
- Barkallah, M.; Dammak, M.; Louati, I.; Hentati, F.; Hadrich, B.; Mechichi, T.; Ayadi, M.A.; Fendri, I.; Attia, H.; Abdelkafi, S. Effect of Spirulina platensis fortification on physicochemical, textural, antioxidant and sensory properties of yogurt during fermentation and storage. LWT 2017, 84, 323–330. [Google Scholar] [CrossRef]
- Batista, A.P.; Niccolai, A.; Fradinho, P.; Fragoso, S.; Bursic, I.; Rodolfi, L.; Biondi, N.; Tredici, M.R.; Sousa, I.; Raymundo, A. Microalgae biomass as an alternative ingredient in cookies: Sensory, physical and chemical properties, antioxidant activity and in vitro digestibility. Algal Res. 2017, 26, 161–171. [Google Scholar] [CrossRef]
- de Marco Castro, E.; Shannon, E.; Abu-Ghannam, N. Effect of Fermentation on Enhancing the Nutraceutical Properties of Arthrospira platensis (Spirulina). Fermentation 2019, 5, 28. [Google Scholar] [CrossRef]
- Tolpeznikaite, E.; Bartkevics, V.; Skrastina, A.; Pavlenko, R.; Mockus, E.; Zokaityte, E.; Starkute, V.; Klupsaite, D.; Ruibys, R.; Rocha, J.M.; et al. Changes in Spirulina’s Physical and Chemical Properties during Submerged and Solid-State Lacto-Fermentation. Toxins 2023, 15, 75. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, Y.K.; Lele, V.; Sakiene, V.; Zavistanaviciute, P.; Zokaityte, E.; Klupsaite, D.; Bartkevics, V.; Guiné, R.P.F.; Bartkiene, E. Plant-based proteinaceous snacks: Effect of fermentation and ultrasonication on end-product characteristics. Food Sci. Nutr. 2020, 8, 4746–4756. [Google Scholar] [CrossRef] [PubMed]
- Chaiklahan, R.; Chirasuwan, N.; Bunnag, B. Stability of phycocyanin extracted from Spirulina sp.: Influence of temperature, pH and preservatives. Process Biochem. 2012, 47, 659–664. [Google Scholar] [CrossRef]
- İlter, I.; Akyıl, S.; Demirel, Z.; Koç, M.; Conk-Dalay, M.; Kaymak-Ertekin, F. Optimization of phycocyanin extraction from Spirulina platensis using different techniques. J. Food Compos. Anal. 2018, 70, 78–88. [Google Scholar] [CrossRef]
- Gobbi, M.; Comitini, F.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Lachancea thermotolerans and Saccharomyces cerevisiae in simultaneous and sequential co-fermentation: A strategy to enhance acidity and improve the overall quality of wine. Food Microbiol. 2013, 33, 271–281. [Google Scholar] [CrossRef]
- Henschke, P.A.; Jiranek, V. Yeasts-metabolism of nitrogen compounds. In Wine Microbiology and Biotechnology; Fleet, G.H., Ed.; Taylor & Francis: London, UK, 2002; pp. 77–164. [Google Scholar]
- Takagi, H. Proline as a stress protectant in yeast: Physiological functions, metabolic regulations, and biotechnological applications. Appl. Microbiol. Biotechnol. 2008, 81, 211–223. [Google Scholar] [CrossRef]
- Coral-Medina, A.; Morrissey, J.P.; Camarasa, C. The growth and metabolome of Saccharomyces uvarum in wine fermentations are strongly influenced by the route of nitrogen assimilation. J. Ind. Microbiol. Biotechnol. 2022, 49, kuac025. [Google Scholar]
- Viegas, C.A.; Rosa, M.F.; Sá-Correia, I.; Novais, J.M. Inhibition of Yeast Growth by Octanoic and Decanoic Acids Produced during Ethanolic Fermentation. Appl. Environ. Microbiol. 1989, 55, 21–28. [Google Scholar] [PubMed]
- Dragone, G.; Mussatto, S.I.; Almeida e Silva, J.B. Influence of temperature on continuous high gravity brewing with yeasts immobilized on spent grains. Eur. Food Res. Technol. 2008, 228, 257–264. [Google Scholar] [CrossRef][Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).