Co-Digestion and Mono-Digestion of Sewage Sludge and Steam-Pretreated Winter Wheat Straw in Continuous Stirred-Tank Reactors—Nutrient Composition and Process Performance
Abstract
1. Introduction
- To investigate the process performance of PWS mono-digestion;
- To investigate the theoretical potential of balancing the nutrient composition in the co-digestion of SS and PWS based on the nutrient element/carbon ratio;
- To investigate the process performance and potential synergistic effects, in addition to the nutrient complementation, of co-digestion in relation to the mono-digestion of PWS and SS.
2. Materials and Methods
2.1. Materials
2.1.1. Substrates
2.1.2. Inocula
2.1.3. Continuous Stirred-Tank Reactors
2.2. Methods
2.2.1. Substrate Treatment
2.2.2. Fibre Composition Analysis and Enzymatic Hydrolysis
2.2.3. Estimation of the Microbial Nutrient Demands
2.2.4. Biochemical Methane Potential Tests
2.2.5. Start-Up and Operation of AD in CSTRs
2.2.6. Nutrient Supplements
2.2.7. Determination of SCFA over 24 h
2.3. Analytical Methods
2.3.1. Gas Volume Determination and Leakage Detection
2.3.2. pH, Alkalinity, and SCFAs
2.3.3. Nutrient Elements and Ions
2.4. Statistical Analysis
3. Results and Discussion
3.1. Substrate Characteristics
3.2. Nutrient Composition and Microbial Nutrient Demand
3.3. Process Variables in the Liquid of CSTR Experiments
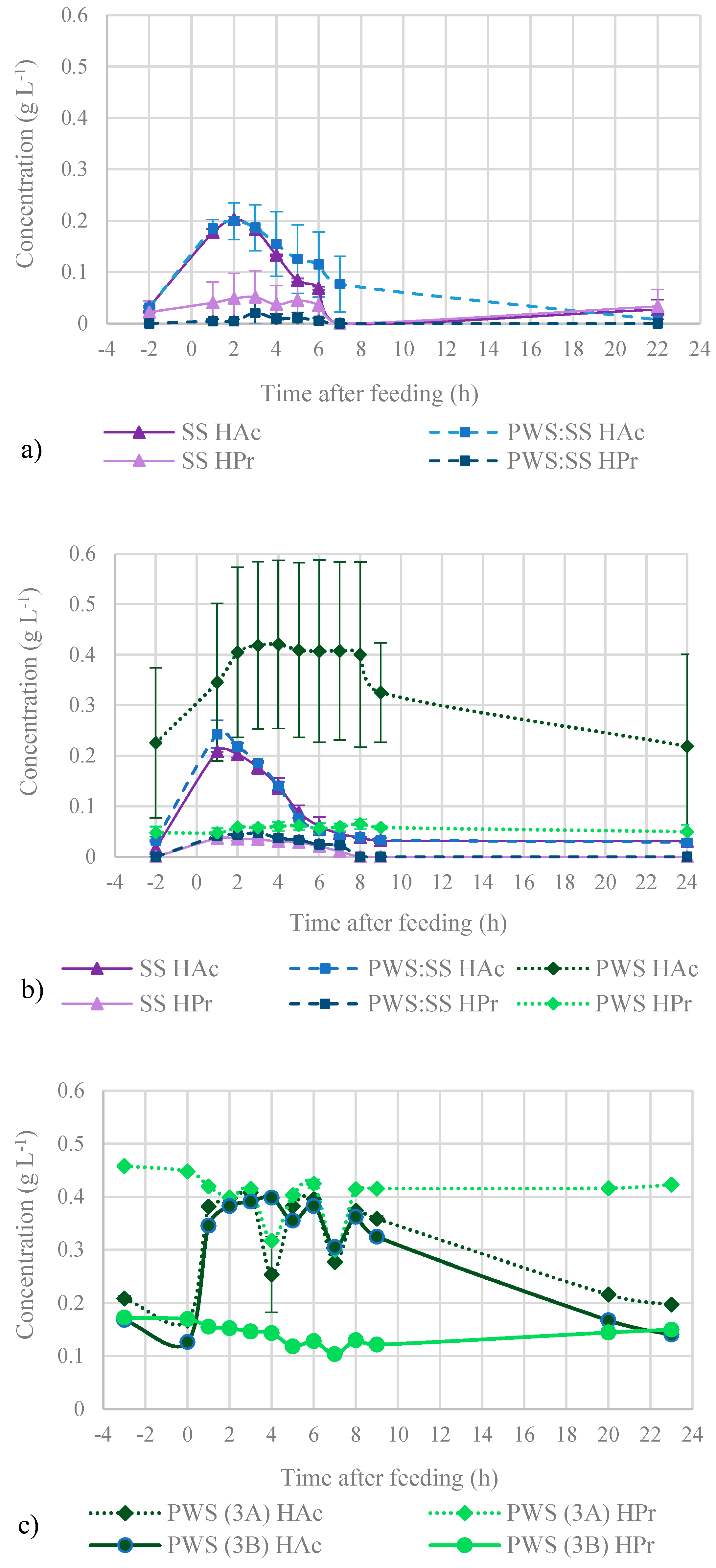
3.3.1. Short-Chain Fatty Acids and Inhibitors in SS Mono-Digestion and Co-Digestion
3.3.2. Short-Chain Fatty Acids and Inhibitors in PWS Mono-Digestion
3.3.3. Ammonia and pH
3.4. Methane Production in CSTR Experiments and Methane Yield in BMP Tests
3.4.1. Methane Production and VS Conversion in CSTR Experiments
3.4.2. Methane Yields in Biochemical Methane Potential Tests
3.4.3. Residual Methane Yield after 22 Days’ HRT
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European-Commission. Implementing the Repower EU Action Plan: Investment Needs, Hydrogen Accelerator and Achieving the Bio-Methane Targets; European-Commission: Brussels, Belgium, 2022. [Google Scholar]
- Croce, S.; Wei, Q.; D’Imporzano, G.; Dong, R.J.; Adani, F. Anaerobic digestion of straw and corn stover: The effect of biological process optimization and pre-treatment on total bio-methane yield and energy performance. Biotechnol. Adv. 2016, 34, 1289–1304. [Google Scholar] [CrossRef] [PubMed]
- Bondesson, P.M.; Galbe, M. Process design of SSCF for ethanol production from steam-pretreated, acetic-acid-impregnated wheat straw. Biotechnol. Biofuels 2016, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Byrne, E.; Kovacs, K.; van Niel, E.W.J.; Willquist, K.; Svensson, S.E.; Kreuger, E. Reduced use of phosphorus and water in sequential dark fermentation and anaerobic digestion of wheat straw and the application of ensiled steam-pretreated lucerne as a macronutrient provider in anaerobic digestion. Biotechnol. Biofuels 2018, 11, 1–16. [Google Scholar] [CrossRef]
- Bondesson, P.-M. Evaluation of Pretreatment and Process Configurations for Combined Ethanol and Biogas Production from Lignocellulosic Biomass. Ph.D. Thesis, Lund University, Lund, Sweden, 2016. [Google Scholar]
- Peng, X.W.; Nges, I.A.; Liu, J. Improving methane production from wheat straw by digestate liquor recirculation in continuous stirred tank processes. Renew. Energy 2016, 85, 12–18. [Google Scholar] [CrossRef]
- Nges, I.A.; Wang, B.; Cui, Z.F.; Liu, J. Digestate liquor recycle in minimal nutrients-supplemented anaerobic digestion of wheat straw. Biochem. Eng. J. 2015, 94, 106–114. [Google Scholar] [CrossRef]
- Nkemka, V.N.; Murto, M. Biogas production from wheat straw in batch and UASB reactors: The roles of pretreatment and seaweed hydrolysate as a co-substrate. Bioresour. Technol. 2013, 128, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Potdukhe, R.M.; Sahu, N.; Kapley, A.; Kumar, R. Co-digestion of waste activated sludge and agricultural straw waste for enhanced biogas production. Bioresour. Technol. Rep. 2021, 15, 100769. [Google Scholar] [CrossRef]
- Weiland, P. Biogas production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2010, 85, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Pohl, M.; Heeg, K.; Mumme, J. Anaerobic digestion of wheat straw—Performance of continuous solid-state digestion. Bioresour. Technol. 2013, 146, 408–415. [Google Scholar] [CrossRef]
- Lantz, M.; Kreuger, E.; Bjornsson, L. An economic comparison of dedicated crops vs agricultural residues as feedstock for biogas of vehicle fuel quality. Aims Energy 2017, 5, 838–863. [Google Scholar] [CrossRef]
- Dawson, C.J.; Hilton, J. Fertiliser availability in a resource-limited world: Production and recycling of nitrogen and phosphorus. Food Policy 2011, 36, 14–22. [Google Scholar] [CrossRef]
- European-Commission; Directorate-General for Internal Market, Industry, Entrepreneurship, SMEs; Grohol, M.; Veeh, C. Study on the Critical Raw Materials for the EU 2023: Final Report; Publications Office of the European Union: Luxembourg, Luxembourg, 2023. [Google Scholar]
- Mata-Alvarez, J.; Mace, S.; Llabres, P. Anaerobic digestion of organic solid wastes. An overview of research achievements and perspectives. Bioresour. Technol. 2000, 74, 3–16. [Google Scholar] [CrossRef]
- Einarsson, R.; Persson, U.M. Analyzing key constraints to biogas production from crop residues and manure in the EU-A spatially explicit model. PLoS ONE 2017, 12, 23. [Google Scholar] [CrossRef]
- Kaldis, F.; Cysneiros, D.; Day, J.; Karatzas, K.A.G.; Chatzifragkou, A. Anaerobic Digestion of Steam-Exploded Wheat Straw and Co-Digestion Strategies for Enhanced Biogas Production. Appl. Sci. 2020, 10, 8284. [Google Scholar] [CrossRef]
- Elsayed, M.; Andres, Y.; Blel, W.; Gad, A.; Ahmed, A. Effect of VS organic loads and buckwheat husk on methane production by anaerobic co-digestion of primary sludge and wheat straw. Energy Conv. Manag. 2016, 117, 538–547. [Google Scholar] [CrossRef]
- Zhao, Z.S.; Li, Y.; Quan, X.; Zhang, Y.B. Improving the co-digestion performance of waste activated sludge and wheat straw through ratio optimization and ferroferric oxide supplementation. Bioresour. Technol. 2018, 267, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Olsson, J.; Forkman, T.; Gentili, F.G.; Zambrano, J.; Schwede, S.; Thorin, E.; Nehrenheim, E. Anaerobic co-digestion of sludge and microalgae grown in municipal wastewater—A feasibility study. Water Sci. Technol. 2018, 77, 682–694. [Google Scholar] [CrossRef]
- Koch, K.; Hefner, S.D.; Weinrich, S.; Astals, S.; Holliger, C. Power and Limitations of Biochemical Methane Potential (BMP) Tests. Front. Energy Res. 2020, 8, 63. [Google Scholar] [CrossRef]
- Holtzapple, M.T.; Humphrey, A.E.; Taylor, J.D. Energy-requirements for the size-reduction of poplar and aspen wood. Biotechnol. Bioeng. 1989, 33, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Shafiei, M.; Kabir, M.M.; Zilouei, H.; Horvath, I.S.; Karimi, K. Techno-economical study of biogas production improved by steam explosion pretreatment. Bioresour. Technol. 2013, 148, 53–60. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhou, T.T.; Wang, Y.B.; Cao, X.H.; Wu, S.Q.; Zhao, M.L.; Wang, H.Y.; Xu, M.; Zheng, B.D.; Zheng, J.G.; et al. Pretreatment of wheat straw leads to structural changes and improved enzymatic hydrolysis. Sci. Rep. 2018, 8, 1321. [Google Scholar] [CrossRef]
- Fernando-Foncillas, C.; Estevez, M.M.; Uellendahl, H.; Varrone, C. Co-Management of Sewage Sludge and Other Organic Wastes: A Scandinavian Case Study. Energies 2021, 14, 3411. [Google Scholar] [CrossRef]
- Jenicek, P.; Bartacek, J.; Kutil, J.; Zabranska, J.; Dohanyos, M. Potentials and limits of anaerobic digestion of sewage sludge: Energy self-sufficient municipal wastewater treatment plant? Water Sci. Technol. 2012, 66, 1277–1281. [Google Scholar] [CrossRef] [PubMed]
- Parkin, G.F.; Owen, W.F. Fundamentals of anaerobic digestion of wastewater sludges. J. Environ. Eng. 1986, 112, 867–920. [Google Scholar] [CrossRef]
- Bolzonella, D.; Pavan, P.; Battistoni, P.; Cecchi, F. Mesophilic anaerobic digestion of waste activated sludge: Influence of the solid retention time in the wastewater treatment process. Process Biochem. 2005, 40, 1453–1460. [Google Scholar] [CrossRef]
- The Department of Soil and Environment, SLU Field Research—Plant Nutrition, Electronic Database on Long-Term Cultivation Trials, Uppsala, Sweden. Available online: https://www.slu.se/en/departments/soil-environment/research/soil-nutrient-cycling/slu-field-research-plant-nutrition (accessed on 23 May 2023).
- Nges, I.A.; Escobar, F.; Fu, X.M.; Björnsson, L. Benefits of supplementing an industrial waste anaerobic digester with energy crops for increased biogas production. Waste Manag. 2012, 32, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Bondesson, P.M.; Galbe, M.; Zacchi, G. Comparison of energy potentials from combined ethanol and methane production using steam-pretreated corn stover impregnated with acetic acid. Biomass Bioenergy 2014, 67, 413–424. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; NREL/TP-510-42618; National Renewable Energy Laboratory, Midwest Research Institute: Golden, CO, USA, 2008. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Sugars, Byproducts, and Degradation Products in Liquid Fraction Process Samples; NREL/TP-510-42623; National Renewable Energy Laboratory, Midwest Research Institute: Golden, CO, USA, 2008. [Google Scholar]
- Resch, M.G.; Baker, J.O.; Decker, S.R. Low Solids Enzymatic Saccharification of Lignocellulosic Biomass; NREL/TP-5100-63351; National Renewable Energy: Golden, CO, USA, 2015. [Google Scholar]
- McCarty, P.L. Anaerobic Waste Treatment Fundamentals, Part one, Chemistry and microbiology, Public works. 1964, 95, 107–112. Available online: https://sswm.info/sites/default/files/reference_attachments/MCCARTY%201964%20Anaerobic%20Waste%20Treatment%20Fundamentals.pdf (accessed on 12 February 2024).
- Holliger, C.; Alves, M.; Andrade, D.; Angelidaki, I.; Astals, S.; Baier, U.; Bougrier, C.; Buffière, P.; Carballa, M.; de Wilde, V.; et al. Towards a standardization of biomethane potential tests. Water Sci. Technol. 2016, 74, 2515–2522. [Google Scholar] [CrossRef] [PubMed]
- Holliger, C.; Astals, S.; de Laclos, H.F.; Hafner, S.D.; Koch, K.; Weinrich, S. Towards a standardization of biomethane potential tests: A commentary. Water Sci. Technol. 2021, 83, 247–250. [Google Scholar] [CrossRef]
- Murto, M. Personal Communication; VA SYD: Malmö, Sweden, 2021. [Google Scholar]
- Switzenbaum, M.S.; Farrell, J.B.; Pincince, A.B. Relationship between the Van Kleeck and mass-balance calculation of volatile solids loss. Water Environ. Res. 2003, 75, 377–380. [Google Scholar] [CrossRef]
- Ripley, L.E.; Boyle, W.C.; Converse, J.C. Improved alkalimetric monitoring for anaerobic-digestion of high-strength wastes. J. Water Pollut. Control Fed. 1986, 58, 406–411. [Google Scholar]
- Zhang, W.; Zhang, F.; Li, Y.X.; Jiang, Y.; Zeng, R.J.X. No difference in inhibition among free acids of acetate, propionate and butyrate on hydrogenotrophic methanogen of Methanobacterium formicicum. Bioresour. Technol. 2019, 294, 122237. [Google Scholar] [CrossRef] [PubMed]
- Takashima, M.; Speece, R.E. Mineral nutrient-requirements for high-rate methane fermentation of acetate at low SRT. Res. J. Water Pollut. Control Fed. 1989, 61, 1645–1650. [Google Scholar]
- Jenkins, S.R.; Morgan, J.M.; Zhang, X. Measuring the usable carbonate alkalinity of operating anaerobic digesters. Res. J. Water Pollut. Control Fed. 1991, 63, 28–34. [Google Scholar]
- APHA. 2540 G. Total, fixed, and volatile solids in solid and semisolid samples. In Standard Methods for the Examination of Water and Wastewater, 21st ed.; Eaton, A.D., Clesceri, L.S., Rice, E.W., Greenberg, A.E., Franson, M.A., Eds.; American Public Health Association/American Water Works Association/Water Environment Federation: Baltimore, MD, USA, 2005. [Google Scholar]
- Porter, M.G.; Murray, R.S. The volatility of components of grass silage on oven drying and the inter-relationship between dry-matter content estimated by different analytical methods. Grass Forage Sci. 2001, 56, 405–411. [Google Scholar] [CrossRef]
- Kreuger, E.; Nges, I.A.; Bjornsson, L. Ensiling of crops for biogas production: Effects on methane yield and total solids determination. Biotechnol. Biofuels 2011, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- Capson-Tojo, G.; Moscoviz, R.; Astals, S.; Robles, A.; Steyer, J.P. Unraveling the literature chaos around free ammonia inhibition in anaerobic digestion. Renew. Sust. Energ. Rev. 2020, 117, 109487. [Google Scholar] [CrossRef]
- Miller, J.N.; Miller, J.C. Statistics and Chemometrics for Analytical Chemistry, 5th ed.; Pearson Education Ltd.: Gosport, UK, 2005. [Google Scholar]
- Hofmann, J.; Peltri, G.; Sträuber, H.; Müller, L.; Schumacher, B.; Müller, U.; Liebetrau, J. Statistical Interpretation of Semi-Continuous Anaerobic Digestion Experiments on the Laboratory Scale. Chem. Eng. Technol. 2016, 39, 643–651. [Google Scholar] [CrossRef]
- Arthur, R.; Antonczyk, S.; Off, S.; Scherer, P.A. Mesophilic and Thermophilic Anaerobic Digestion of Wheat Straw in a CSTR System with ‘Synthetic Manure’: Impact of Nickel and Tungsten on Methane Yields, Cell Count, and Microbiome. Bioengineering 2022, 9, 13. [Google Scholar] [CrossRef]
- Ghasimi, D.S.M.; Aboudi, K.; de Kreuk, M.; Zandvoort, M.H.; van Lier, J.B. Impact of lignocellulosic-waste intermediates on hydrolysis and methanogenesis under thermophilic and mesophilic conditions. Chem. Eng. J. 2016, 295, 181–191. [Google Scholar] [CrossRef]
- Tosi, V. Anaerobic co-digestion of steam pretreated wheat straw and sewage sludge. Master’s Thesis, Lund University, Lund, Sweden, 2021. [Google Scholar]
- Demirel, B.; Scherer, P. Trace element requirements of agricultural biogas digesters during biological conversion of renewable biomass to methane. Biomass Bioenergy 2011, 35, 992–998. [Google Scholar] [CrossRef]
- Lebuhn, M.; Liu, F.; Heuwinkel, H.; Gronauer, A. Biogas production from mono-digestion of maize silage-long-term process stability and requirements. Water Sci. Technol. 2008, 58, 1645–1651. [Google Scholar] [CrossRef] [PubMed]
- Munk, B.; Lebuhn, M. Process diagnosis using methanogenic Archaea in maize-fed, trace element depleted fermenters. Anaerobe 2014, 29, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Holliger, C.; de Laclos, H.F.; Hack, G. Methane Production of Full-scale anaerobic Digestion Plants calculated from substrate’s Biomethane Potentials compares Well with the One Measured On-site. Front. Energy Res. 2017, 5, 12. [Google Scholar] [CrossRef]
- Labatut, R.A.; Angenent, L.T.; Scott, N.R. Biochemical methane potential and biodegradability of complex organic substrates. Bioresour. Technol. 2011, 102, 2255–2264. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.; Bösch, P.; Friedl, A.; Amon, T. Analysis of methane potentials of steam-exploded wheat straw and estimation of energy yields of combined ethanol and methane production. J. Biotechnol. 2009, 142, 50–55. [Google Scholar] [CrossRef]
- Ferreira, L.C.; Nilsen, P.J.; Fdz-Polanco, F.; Perez-Elvira, S.I. Biomethane potential of wheat straw: Influence of particle size, water impregnation and thermal hydrolysis. Chem. Eng. J. 2014, 242, 254–259. [Google Scholar] [CrossRef]
- Theuretzbacher, F.; Lizasoain, J.; Lefever, C.; Saylor, M.K.; Enguidanos, R.; Weran, N.; Gronauer, A.; Bauer, A. Steam explosion pretreatment of wheat straw to improve methane yields: Investigation of the degradation kinetics of structural compounds during anaerobic digestion. Bioresour. Technol. 2015, 179, 299–305. [Google Scholar] [CrossRef]
- Sapci, Z.; Morken, J.; Linjordet, R. An Investigation of the Enhancement of Biogas Yields from Lignocellulosic Material using Two Pretreatment Methods: Microwave Irradiation and Steam Explosion. Bioresources 2013, 8, 1976–1985. [Google Scholar] [CrossRef]
- Ambye-Jensen, M.; Thomsen, S.T.; Kádár, Z.; Meyer, A.S. Ensiling of wheat straw decreases the required temperature in hydrothermal pretreatment. Biotechnol. Biofuels 2013, 6, 1–9. [Google Scholar] [CrossRef]
- Monavari, S.; Galbe, M.; Zacchi, G. Influence of impregnation with lactic acid on sugar yields from steam pretreatment of sugarcane bagasse and spruce, for bioethanol production. Biomass Bioenergy 2011, 35, 3115–3122. [Google Scholar] [CrossRef]
- Hamnér, K.; Weih, M.; Eriksson, J.; Kirchmann, H. Influence of nitrogen supply on macro- and micronutrient accumulation during growth of winter wheat. Field Crops Res. 2017, 213, 118–129. [Google Scholar] [CrossRef]





| SS | PWS:SS | PWS:SS | PWS | |
|---|---|---|---|---|
| Reactors | 1A, 1B | 2A, 2B | 2A, 2B | 3A, 3B |
| OLR (gVS L−1 d−1) | 2.1 ± 0.1 | 2.1 ± 0.1 | 2.8 ± 0.1 | 2.1 ± 0.1 |
| SS | 100% | 65% | 88.1% | - |
| PWS | - | 8.78% | 11.9% | 25.1% |
| Macronutrient supplements | - | - | - | 38.04% |
| Micronutrient supplements | - | - | - | 0 (23.67%) 2 |
| Water 1 | - | 26.21% | - | 36.86% (13.19%) 2 |
| Operational Day | Comments |
|---|---|
| −12 | Mono-digestion of SS in all six reactors. |
| 0 | Reactors 2A, 2B, 3A, and 3B were shifted from SS mono-digestion to co-digestion. Start of operational period 1. |
| 25 | Determination of SCFAs prior to feeding, hourly for 7 h after feeding, and at 22 and 24 h after feeding. |
| 33 | Reactors 3A and 3B were shifted from co-digestion to PWS mono-digestion. |
| 70 | The IA/PA threshold quotient was increased from 0.3 to 0.40. |
| 92 | Determination of SCFAs prior to feeding, hourly for 9 h after feeding, and at 24 h after feeding. |
| 81–93 | Reference period 1 for reactors 1A, 1B, 2A, and 2B. The gas production of reactor 2B was excluded from SMP calculations and the determination of residual methane determination due to gas leakage. |
| 94–102 | The OLR was temporarily increased from 2.1 to 2.8 gVS L−1 d−1 for reactors 2A and 2B. |
| 98 | The IA/PA threshold quotient was increased from 0.4 to 0.45. |
| 104–108 | Reduced feeding to reactor 3A (only fed day 107) due to high IA/PA quotient. |
| 110–114 | Reference period 1 for reactors 3A and 3B. The gas production for reactor 3A was excluded due to reduced feeding at the end of operational period 1. |
| 122 | End of operational period 1. |
| 122–124 | There was a break in feeding for 50 days between operational periods 1 and 2. Residual methane production was measured. Reactors 2B and 3B were excluded due to gas leakages. For reactor 3B, the leakage occurred 5 days after operational period 1. |
| 124 | Start of operational period 2. |
| 123–136 | Start-up period with reduced feeding to reactors 1A, 1B, 2A, and 2B. |
| 123–139 | Start-up period with reduced feeding to reactors 1A, 1B, 2A, and 2B. |
| 123–160 | Start-up period with reduced feeding to reactor 3A. |
| 123–170 | Start-up period with reduced feeding to reactor 3B. |
| 199–200 | Remediation of leakages by improved sealing. |
| 201–208 | Reference period 2 for reactors 1A, 1B, 2A, and 2B. |
| 208 | End of operational period 2 for reactors 1A, 1B, 2A, and 2B. Residual methane yields from these four reactors were excluded due to a higher TS content of the SS used on days 209–221 than the SS used on days 1–208. |
| 219–227 | Reference period 2 for reactors 3A and 3B. |
| 224 | Determination of SCFAs in reactors 3A and 3B prior to and hourly for 9 h after feeding, and at 20 and 24 h after feeding. |
| 227 | End of operational period 2 for reactors 3A and 3B. Residual methane production was measured for reactors 3A and 3B for 58 days. |
| Compound | Operational Period 1 | Operational Period 2 | ||
|---|---|---|---|---|
| Stock Solution Concentration (g L−1) | Content of Element in Feed (mg kg−1) 1 | Stock Solution Concentration (g L−1) | Content of Element in Feed (mg kg−1) 1 | |
| Macronutrient solutions | ||||
| (NH)2CO | 47.880 2 | 1723 (N) | 40.296 2 | 1874 (N) |
| Na2HPO4 | 2.458 2 | 40.8 (P) | 41.982 2 | 705 (P) |
| (NH4)2SO4 | 1.51 2 | 27.8 (S) | 27.53 2 | 508 (S) |
| CaCl2 | 1.25 2 | 34.4 (Ca) | 19.91 2 | 547 (Ca) |
| MgCl2 | 0.428 2 | 8.31 (Mg) | 3.578 3 | 34.7 (Mg) |
| KH2PO4 | 0 | 0.938 3 | 10.3 (K) | |
| Micronutrient solutions 4 | ||||
| FeCl2·4H2O | 4.64 | 103 (Fe) | 14.10 | 312 (Fe) |
| CuCl2·2H2O | 3.16 × 10−2 | 9.28 × 10−2 (Cu) | 1.02 × 10−1 | 3.00 (Cu) |
| ZnCl2 | 2.39 × 10−4 | 9.06 × 10−3 (Zn) | 7.92 × 10−2 | 3.00 (Zn) |
| MnCl2·4H2O | 1.35 × 10−4 | 2.96 × 10−3 (Mn) | 5.08 × 10−2 | 1.11 (Mn) |
| NiCl2·6H2O | 1.09 × 10−5 | 2.10 × 10−4 (Ni) | 3.69 × 10−3 | 7.19 × 10−2 (Ni) |
| NaSeO3·5H2O | 6.76 × 10−8 | 1.61 × 10−6 (Se) | 2.99 × 10−4 | 7.07 × 10−3 (Se) |
| (NH4)6Mo7O24·4H2O | 2.90 × 10−8 | 1.15 × 10−6 (Mo) | 2.89 × 10−4 | 1.24 × 10−2 (Mo) |
| CoCl2·6H2O | 5.31 × 10−7 | 9.93 × 10−6 (Co) | 1.57 × 10−3 | 3.07 × 10−2 (Co) |
| Na2O4W·2H2O | 1.58 × 10−7 | 7.07 × 10−6 (W) | 5.40 × 10−4 | 2.37 × 10−2 (W) |
| Na2HPO4 NaSeO3·5H2O Na2O4W·2H2O | See above. | 60.6 (Na) | See above. | 1046 (Na) |
| SS Batch 1 | SS Batch 2 | Primary Sludge | WAS | |
|---|---|---|---|---|
| TS, % of WW | 5.44 (0.1) | 4.75 (0.48) | 6.25 (0.1) | 4.44 (0.03) |
| VS, % of TS | 81.6 (1.6) | 79.3 (0.2) | 82.8 (0.1) | 79.6 (0.2) |
| n TS and VS measurements | 33 | 12 | 4 | 4 |
| TAN (mg kg−1) | 274 | ND | 120 | 752 |
| NO3-N (mg kg−1) | 1.1 | ND | 0.7 | 0.8 |
| Element | WS1 | Average of WS1–WS13 1 | SD and CV, WS1–WS13 1 | PWS | Primary Sludge 2 | WAS 2 | SS 2 | AD Inoculum |
|---|---|---|---|---|---|---|---|---|
| Total C | 435 | 430 | 20.5 (5%) | 472 | 437 | 405 | 430 | 33 |
| Total N 3 | 6.3 | 4.5 | 1.8 (41%) | 6.1 | 49.6 | 63.7 | 53.8 | 41.6 |
| Ca | 2892 | 2185 | 617 (28%) | 1591 | 15,464 | 15,798 | 15,972 | 22,368 |
| Cu | 3.11 | 2.47 | 0.65 (26%) | 3.42 | 335 | 301 | 328 | 438 |
| Cl | 750 | 467 | 270 (57%) | 148 | 307 | 335 | 321 | 780 |
| Fe | 28.3 | 31.0 | 22.5 (76%) | 39.8 | 30,693 | 39,141 | 33,151 | 52,293 |
| K | 10,661 | 8121 | 2210 (27%) | 2286 | 1618 | 4082 | 2291 | 3948 |
| Mg | 846 | 699 | 152 (22%) | 244 | 1865 | 2650 | 2094 | 2979 |
| Mn | 25.4 | 15.4 | 11.2 (73%) | 10.0 | 131 | 121 | 128 | 178 |
| Na | 127 | 80.0 | 36.1 (43%) | 49.7 | 2581 | 3865 | 3019 | 4777 |
| P | 490 | 412 | 110 (7%) | 235 | 16,818 | 25,726 | 19,085 | 27,725 |
| S | 1494 | 832 | 290 (27%) | 607 | 16,665 | 9571 | 14,659 | 18,356 |
| Si | 722 | 627 | 117 (19%) | ND | ND | ND | ND | ND |
| B | 8.31 | 6.54 | 1.55 (26%) | 18.02 | BDL | BDL | BDL | BDL |
| Cr | 6.46 | 0.548 | 0.144 (23%) | 0.532 | 4.52 | 6.22 | 8.14 | 10.47 |
| Co | 0.077 | 0.020 | 0.019 (43%) | 0.038 | 2.19 | 3.49 | 4.14 | 4.99 |
| Ni | 3.90 | 0.560 | 0.226 (40%) | 0.272 | 5.12 | 6.95 | 10.3 | 11.5 |
| Zn | 8.17 | 3.31 | 2.07 (46%) | 6.01 | 278 | 268 | 289 | 472 |
| Se | 0.071 | 0.058 | 0.014 (24%) | BDL | 1.24 | 2.52 | 2.65 | 2.61 |
| Mo | 0.383 | 0.642 | 0.318 (50%) | 0.243 | 1.99 | 3.72 | 3.96 | 5.66 |
| W | BDL | BDL | ND | BDL | 0.957 | 2.70 | 2.36 | 5.14 |
| WS1 | Average of 4 WS-Samples | SD | SS | Primary Sludge | WAS | |
|---|---|---|---|---|---|---|
| Glucan | 38.6 | 37.4 | 2.3 | 13.3 | 14.7 | BC |
| Xylan | 21.2 | 20.9 | 1.6 | BC | BC | BC |
| Galactan | BC | BC | BC | BC | BC | BC |
| Arabinan | 3.4 | BC | BC | BC | BC | BC |
| Mannan | BC | BC | BC | BC | BC | BC |
| AIL | 16.0 | 14.5 | 0.8 | 13.5 | 9.9 | 8.0 |
| ASL | 5.0 | 5.9 | 0.8 | 3.0 | 3.0 | 3.4 |
| Lignin ash | 0.2 | 0.6 | 1.0 | 2.8 | 2.7 | 1.9 |
| Water extractives | 13.0 | 13.5 | 1.8 | 23.0 | 23.9 | 30.1 |
| Ethanol extractives | 2.1 | 2.4 | 0.3 | 17.0 | 18.1 | 17.4 |
| Sum | 99.31 | 95.19 | 8.56 | 72.71 | 72.23 | 60.66 |
| Fibre (% of WW) | Monomers (% of WW) | Oligomers (% of WW) | Sum (% of WW) | Sum (% of TS) | |
|---|---|---|---|---|---|
| Glucan/glucose | 9.16 | 0.11 | 0.31 | 9.58 | 46.1 |
| Xylan/xylose | 0.99 | 0.68 | 3.09 | 4.76 | 22.9 |
| Galactan/galactose | 0.04 | 0.07 | 0.11 | 0.5 | |
| Arabinan/arabinose | 0.25 | 0.23 | 0.48 | 2.3 | |
| Mannan/mannose | |||||
| Formic acid | 0.14 | 0.14 | 0.6 | ||
| Acetic acid | 0.62 | 0.62 | 3.0 | ||
| Lactic acid | 0.39 | 0.39 | 1.9 | ||
| Glycerol | 0.02 | 0.02 | 0.1 | ||
| HMF | 0.02 | 0.02 | 0.1 | ||
| Furfural | 0.20 | 0.20 | 0.9 | ||
| Ammonia-N | 0.016 | 0.003 | |||
| AIL | 3.96 | 3.96 | 19.0 | ||
| ASL | 0.40 | 0.40 | 1.9 | ||
| Lignin ash | 0.45 | 0.45 | 2.2 | ||
| Sum | 21.12 | 101.6 | |||
| WIS | 14.35 (0.16) 1 | ||||
| TS un-corrected | 20.07 (0.65) 1 | ||||
| TS corrected | 20.79 (0.68) 1 | ||||
| VS corrected | 19.89 (0.55) 1 | 95.69 (0.10) 1 | |||
| Total ash | 0.90 (0.03) 1 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kreuger, E.; Tosi, V.; Lindblad, M.; Davidsson, Å. Co-Digestion and Mono-Digestion of Sewage Sludge and Steam-Pretreated Winter Wheat Straw in Continuous Stirred-Tank Reactors—Nutrient Composition and Process Performance. Fermentation 2024, 10, 414. https://doi.org/10.3390/fermentation10080414
Kreuger E, Tosi V, Lindblad M, Davidsson Å. Co-Digestion and Mono-Digestion of Sewage Sludge and Steam-Pretreated Winter Wheat Straw in Continuous Stirred-Tank Reactors—Nutrient Composition and Process Performance. Fermentation. 2024; 10(8):414. https://doi.org/10.3390/fermentation10080414
Chicago/Turabian StyleKreuger, Emma, Virginia Tosi, Maja Lindblad, and Åsa Davidsson. 2024. "Co-Digestion and Mono-Digestion of Sewage Sludge and Steam-Pretreated Winter Wheat Straw in Continuous Stirred-Tank Reactors—Nutrient Composition and Process Performance" Fermentation 10, no. 8: 414. https://doi.org/10.3390/fermentation10080414
APA StyleKreuger, E., Tosi, V., Lindblad, M., & Davidsson, Å. (2024). Co-Digestion and Mono-Digestion of Sewage Sludge and Steam-Pretreated Winter Wheat Straw in Continuous Stirred-Tank Reactors—Nutrient Composition and Process Performance. Fermentation, 10(8), 414. https://doi.org/10.3390/fermentation10080414





