Physicochemical and Biochemical Changes in Cocoa during the Fermentation Step
Abstract
1. Introduction
2. Materials and Methods
2.1. Cocoa Material
2.2. Assembly of the Fermentation Process
2.3. Sample Preparation
2.4. Physicochemical Parameter Determination
2.4.1. pH Determination
2.4.2. Total Acidity Determination
2.4.3. Moisture Determination
2.4.4. Fermentation Index (FI)
2.5. Biochemical Determinations
2.5.1. Total Phenolic Content (TPC)
2.5.2. Total Flavonoids Content (TFC)
2.5.3. Methylxanthine Contents (Theobromine (TC) and Caffeine (CC))
2.5.4. Antioxidant Activity Determination
2.5.5. Cocoa FTIR-ATR Analysis
2.6. Statistical Analyses
3. Results
3.1. pH Changes
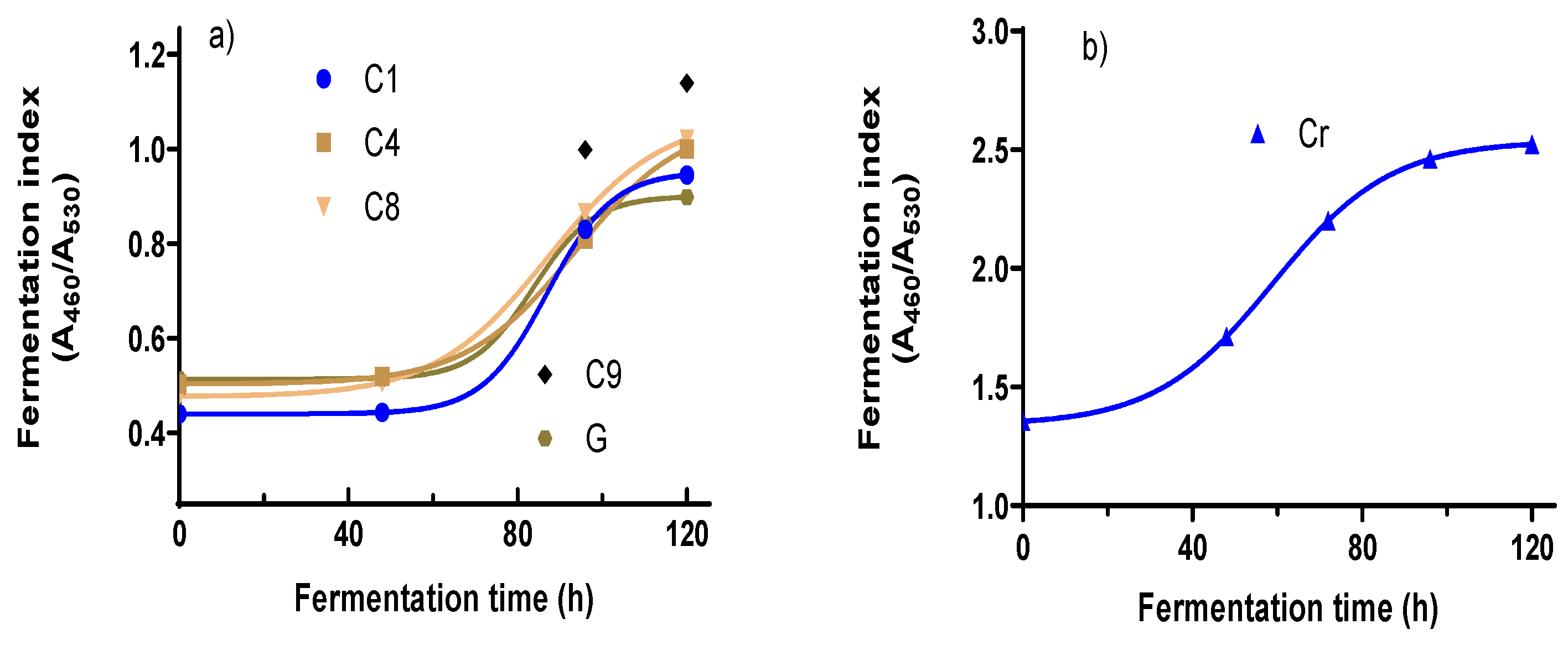
3.2. Fermentation Index (FI)
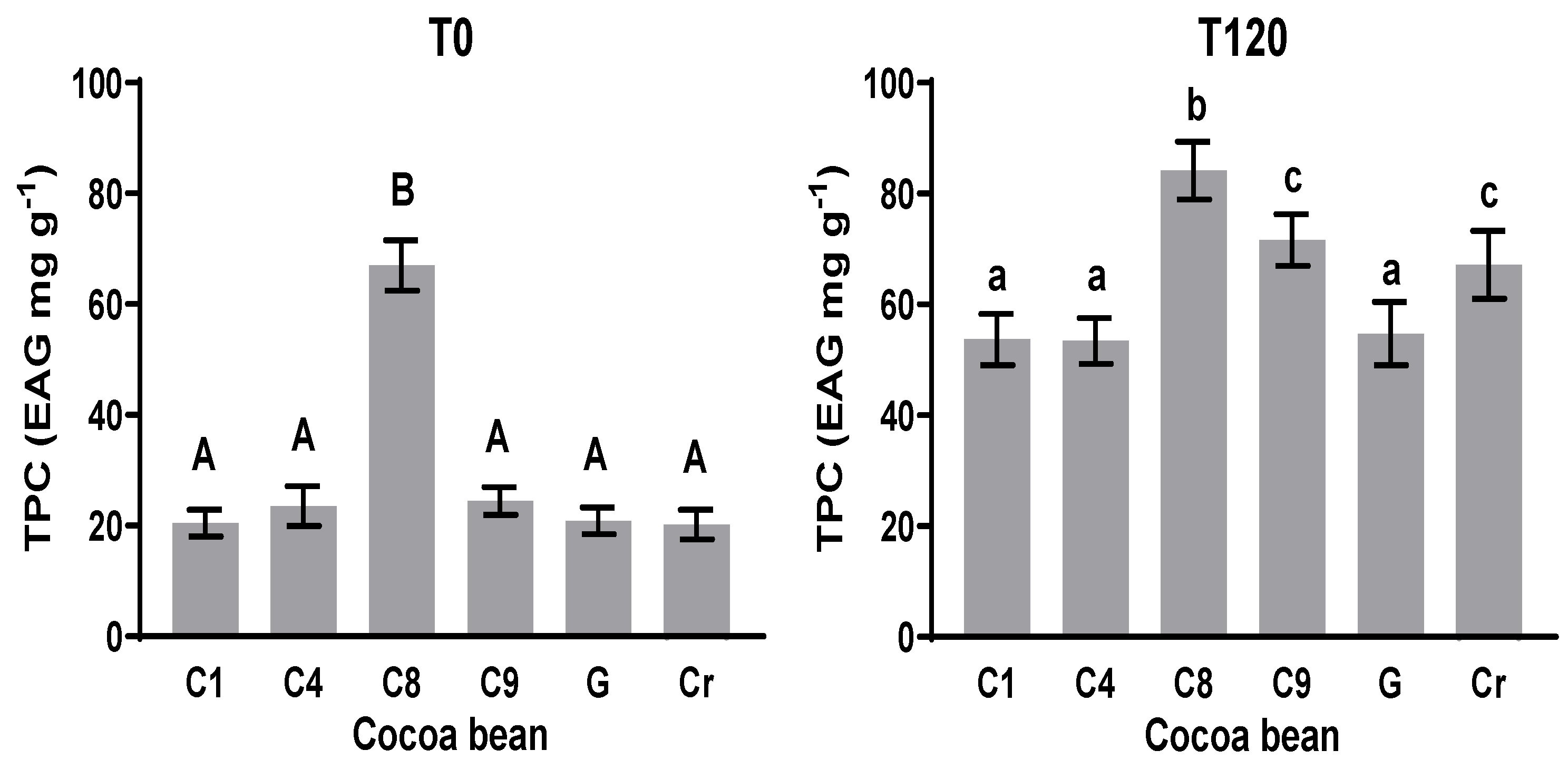
3.3. Total Polyphenol Content (TPC)
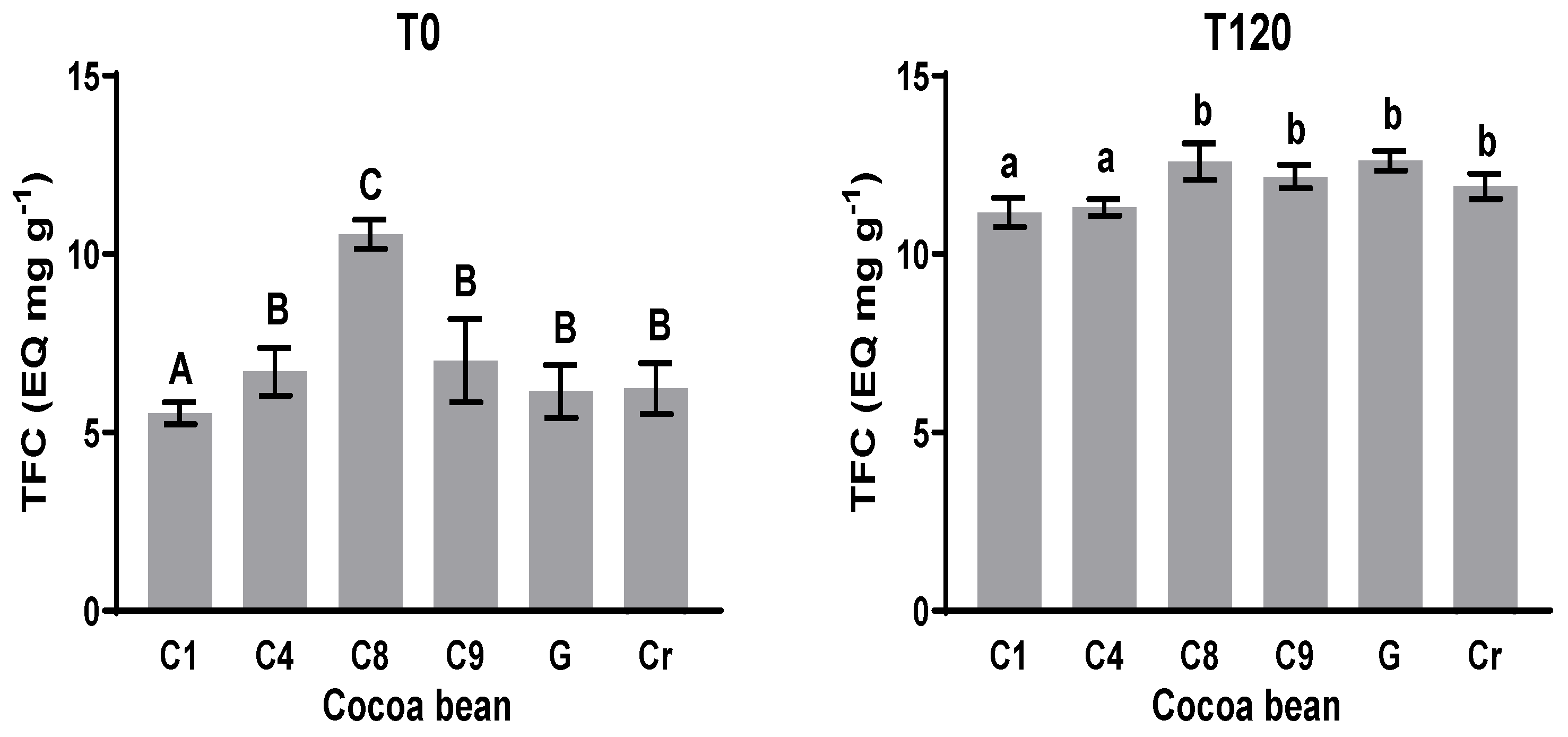
3.4. Total Flavonoids Content (TFC)
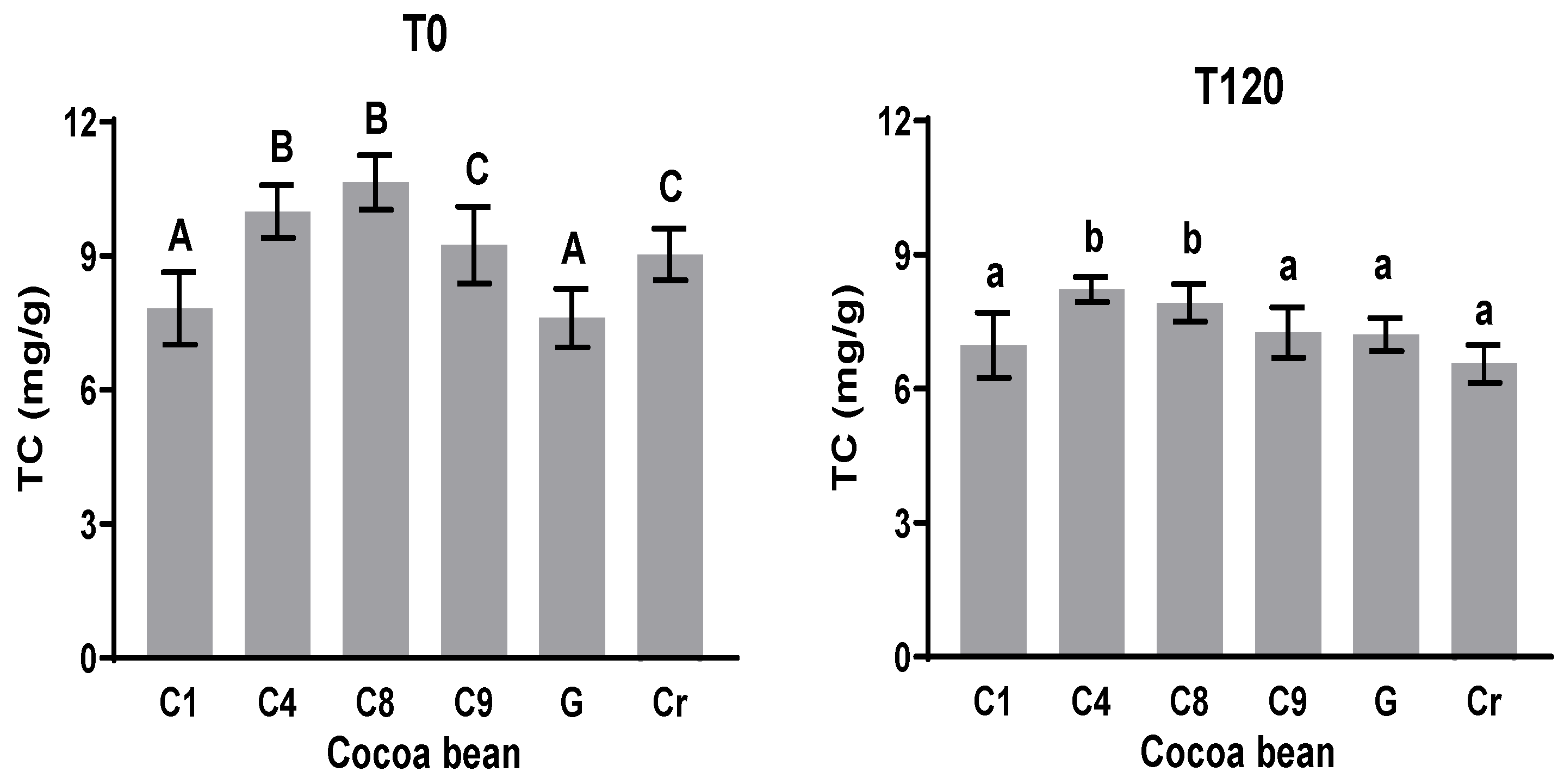
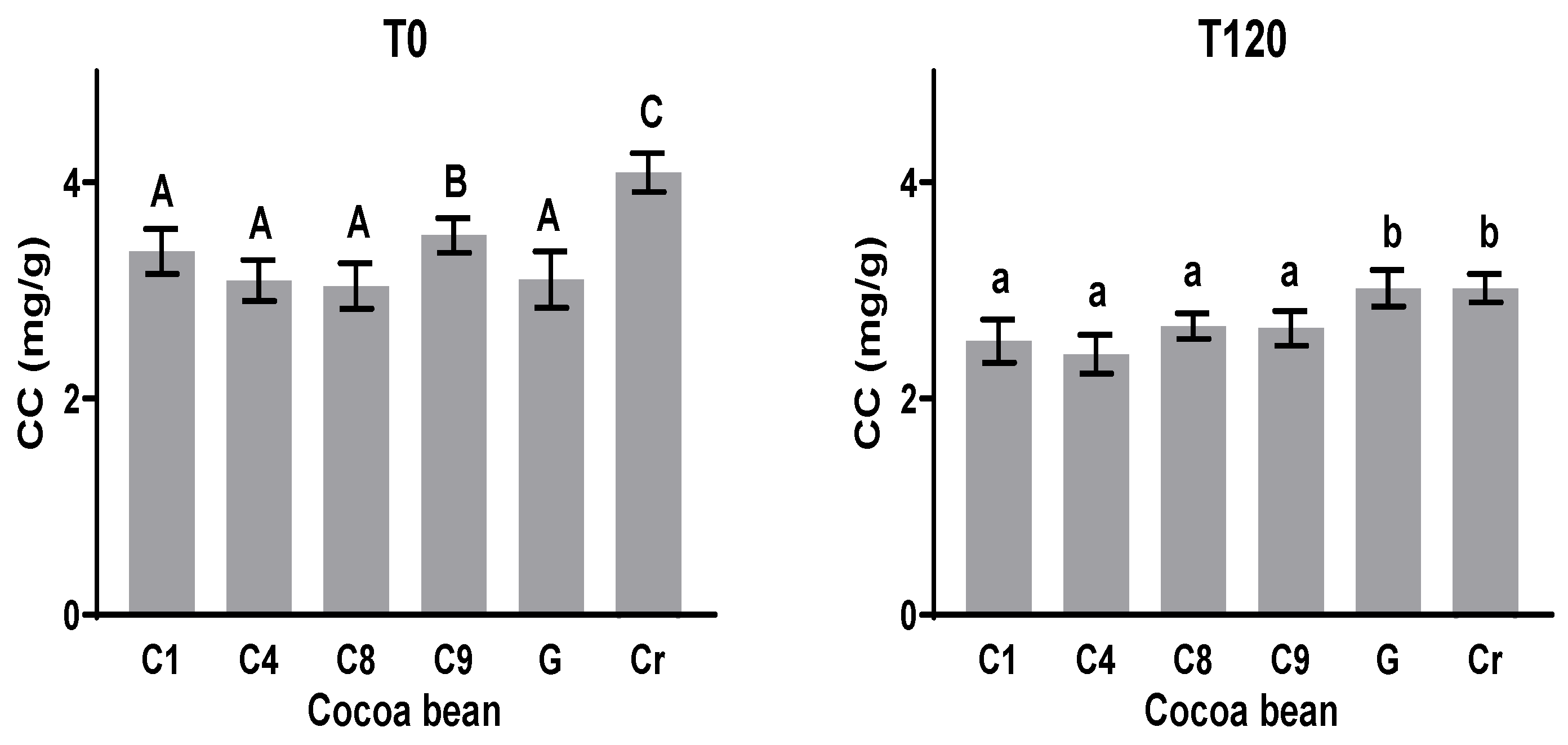
3.5. Methylxanthines (Theobromine (TC) and Caffeine (CC))
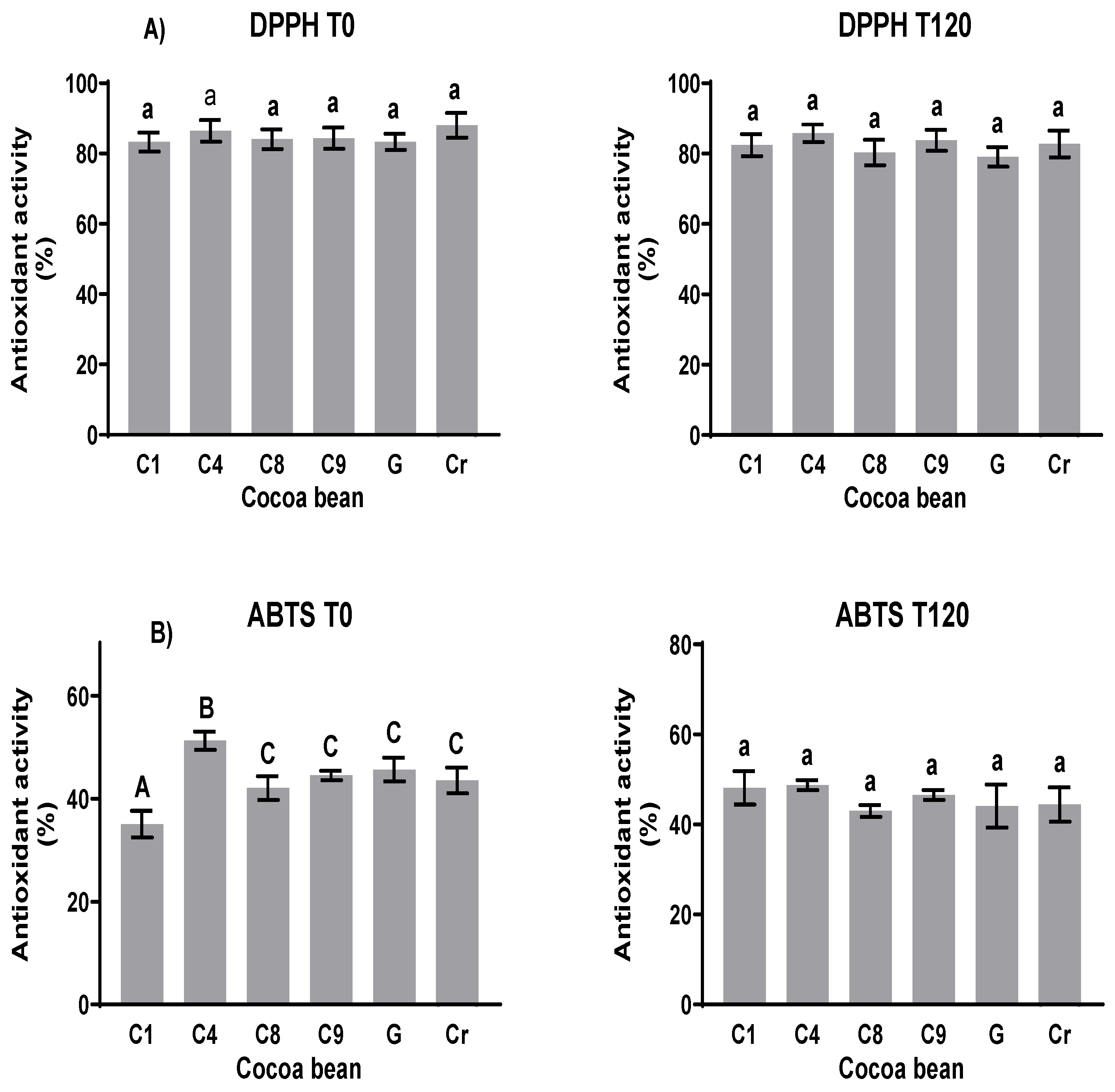
3.6. Antioxidant Capability Determination
3.7. FTIR-ATR Spectroscopy of Fat-Free Cocoa
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Voigt, J.; Heinrichs, H.; Voigt, G.; Biehl, B. Cocoa-Specific Aroma Precursors Are Generated by Proteolytic Digestion of the Vicilin-like Globulin of Cocoa Seeds. Food Chem. 1994, 50, 177–184. [Google Scholar] [CrossRef]
- Barišić, V.; Kopjar, M.; Jozinović, A.; Flanjak, I.; Ačkar, D.; Miličević, B.; Šubarić, D.; Jokić, S.; Babić, J. The Chemistry behind Chocolate Production. Molecules 2019, 24, 3163. [Google Scholar] [CrossRef] [PubMed]
- Schnermann, P.; Schieberle, P. Evaluation of Key Odorants in Milk Chocolate and Cocoa Mass by Aroma Extract Dilution Analyses. J. Agric. Food Chem. 1997, 45, 867–872. [Google Scholar] [CrossRef]
- Voight, J.; Lieberei, R. Biochemistry of Cocoa Fermentation. In Cocoa and Coffee Fermentations; Fleet, G.H., Schwan, R.F., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 194–216. Available online: https://www.worldcat.org/title/cocoa-and-coffee-fermentations/oclc/893680375 (accessed on 21 February 2023).
- Escobar, S.; Santander, M.; Zuluaga, M.; Chacon, I.; Rodriguez, J.; Vaillant, F. Fine Cocoa Beans Production: Tracking Aroma Precursors through a Comprehensive Analysis of Flavor Attributes Formation. Food Chem. 2021, 365, 130627. [Google Scholar] [CrossRef]
- Ozturk, G.; Young, G.M. Food Evolution: The Impact of Society and Science on the Fermentation of Cocoa Beans. Compr. Rev. Food Sci. Food Saf. 2017, 16, 431–455. [Google Scholar] [CrossRef] [PubMed]
- Gibson, M.; Newsham, P. Chocolate/Cacao. In Food Science and the Culinary Arts; Academic Press: Cambridge, MA, USA, 2018; pp. 341–352. [Google Scholar] [CrossRef]
- Hernández, C. Análisis de La Composición Química Del Cacao, Extracción y Estudio de Compuestos Antioxidantes En Genotipos Del Banco de Germoplasma de México. Ph.D. Thesis, Universidad de Sevilla, Sevilla, Spain, 2018. Available online: https://idus.us.es/handle/11441/82298 (accessed on 1 July 2024).
- Kongor, J.E.; Hinneh, M.; de Walle, D.V.; Afoakwa, E.O.; Boeckx, P.; Dewettinck, K. Factors influencing quality variation in cocoa (Theobroma cacao) bean flavour profile—A review. Food Res. Int. 2016, 82, 44–52. [Google Scholar] [CrossRef]
- Dos Santos, C.M.; de Cerqueira e Silva, A.B.; de Lima Silva Marques, E.; Rezende, R.P.; Pirovani, C.P.; Ferreira, A.C.R.; Andrade, L.M.; Wellington da Cruz Santos, D.; Díaz, A.M.; Soares, M.R.; et al. Biotechnological Starter Potential for Cocoa Fermentation from Cabruca Systems. Braz. J. Dev. 2021, 7, 60739–60759. [Google Scholar] [CrossRef]
- Kadow, D. The Biochemistry of Cocoa Flavor—A Holistic Analysis of Its Development along the Processing Chain. J. Appl. Bot. Food Qual. 2020, 93, 300–312. [Google Scholar] [CrossRef]
- Ordoñez-Araque, R.H.; Landines-Vera, E.F.; Urresto-Villegas, J.C.; Caicedo-Jaramillo, C.F. Microorganisms during Cocoa Fermentation: Systematic Review. Foods Raw Mater. 2020, 8, 155–162. [Google Scholar] [CrossRef]
- Diaz-Muñoz, C.; De Vuyst, L. Functional Yeast Starter Cultures for Cocoa Fermentation. J. Appl. Microbiol. 2021, 133, 39–66. [Google Scholar] [CrossRef]
- Fernández-Niño, M.; Rodríguez-Cubillos, M.J.; Herrera-Rocha, F.; Anzola, J.M.; Cepeda-Hernández, M.L.; Mejía, J.L.A.; Chica, M.J.; Olarte, H.H.; Rodríguez-López, C.; Calderón, D.; et al. Dissecting Industrial Fermentations of Fine Flavour Cocoa through Metagenomic Analysis. Nat. Sci. Rep. 2021, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Othman, A.; Ismail, A.; Ghani, N.A.; Adenan, I. Antioxidant Capacity and Phenolic Content of Cocoa Beans. Food Chem. 2007, 100, 1523–1530. [Google Scholar] [CrossRef]
- Calixto-Cotos, M.R.; Chire-Fajardo, G.C.; Orihuela-Rivera, C.A. Antioxidants Properties of Chocolates Sold in Peru. Acta Agron. 2018, 67, 479–485. [Google Scholar] [CrossRef]
- Ramos-Escudero, F.; Casimiro-Gonzales, S.; Fernández-Prior, A.; Chávez, K.C.; Gómez-Mendoza, J.; de la Fuente-Carmelino, L.; Muñoz, A.M. Colour, Fatty Acids, Bioactive Compounds, and Total Antioxidant Capacity in Commercial Cocoa Beans (Theobroma Cacao L.). LWT Food Sci. Technol. 2021, 147, 111629. [Google Scholar] [CrossRef]
- Cueto-Moreno, J.; Aguirre-Medina, J.F.; Zamarripa-Colmenero, A.; Iracheta-Donjuan, L.; De los Santos, A.O. El Mejoramiento del Cultivo de Cacao (Theobroma cacao L.) en México; INIFAP: Tuxtla Chico, Mexico, 2007.
- Mendoza, A.; Gallardo, R.; Avendaño, C. El Mundo del Cacao (Theobroma cacao L.). Agroproductividad 2011, 4, 2. [Google Scholar]
- Solis, J.L.; Zamarripa, A.; Pecina, V.; Garrido, E.; Hernández, E. Evaluación Agronomica de Híbridos de Cacao (Theobroma Cacao L.) Para Selección de Alto Rendimiento y Resistencia en Campo a Monoliasis. Rev. Mex. Cienc. Agrícolas 2015, 6, 71–82. [Google Scholar]
- Rivera-Torrez, D.L.; Lagunes-Galvez, L.M.; Azpeitia-Morales, A.; Garcia-Alamilla, P. Reproductive and Morphological Phenology of Eight Mexican Cacao Clones (Theobroma cacao L.). AgroProductividad 2022, 15, 157–167. [Google Scholar] [CrossRef]
- Bordiga, M.; Locatelli, M.; Travaglia, F.; Coïsson, J.D.; Mazza, G.; Arlorio, M. Evaluation of the Effect of Processing on Cocoa Polyphenols: Antiradical Activity, Anthocyanins and Procyanidins Profiling from Raw Beans to Chocolate. Food Sci. Technol. 2015, 50, 840–848. [Google Scholar] [CrossRef]
- Seguine, E.; Mills, D.; Marelli, J.-P.; Motomayor-Arias, J.-C.; Da Silvia Coelho, I. Micro-Fermentation of Cocoa. U.S. Patent 2,0140,199,437A1, 17 July 2014. WO 2013/025621 Al, issued 2013. [Google Scholar]
- Gourieva, K.B.; Tserrevitinov, O.B. Method of evaluating of degree of fermentation of cocoa beans. USRR Patent 646254, 5 February 1979. [Google Scholar]
- Ooi, T.S.; Ting, A.S.Y.; Siow, L.F. Influence of Selected Native Yeast Starter Cultures on the Antioxidant Activities, Fermentation Index and Total Soluble Solids of Malaysia Cocoa Beans: A Simulation Study. Food Sci. Technol. 2020, 122, 108977. [Google Scholar] [CrossRef]
- García-González, E.; Ochoa-Muñoz, A.; Montalvo-Rodriguez, C.; Ordoñez-Narváez, A.; Londoño-Hernández, L. Sucesión Microbiana Durante la Fermentación Espontánea de Cacao en Unidades Productivas. Cienc. Desarro. 2021, 12, 21–30. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitinicult. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The Determination of Flavonoid Contents in Mulberry and Their Scavenging Effects on Superoxide Radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Peralta-Jiménez, L.; Cañizares-Macías, M.P. Ultrasound-Assisted Method for Extraction of Theobromine and Caffeine from Cacao Seeds and Chocolate Products. Food Bioprocess Technol. 2013, 6, 3522–3529. [Google Scholar] [CrossRef]
- Lai, L.S.; Chou, S.T.; Chao, W.W. Studies on the Antioxidative Activities of Hsian-Tsao (Mesona procumbens Hemsl) leaf gum. J. Agric. Food Chem. 2001, 49, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved abts radical cation decolorization assay. EMPA Act. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Torres, O.; de Fariñas, L.G.; de Bertorelli, L.O.; Trujillo, A. Efecto Del Tiempo Transcurrido Entre La Cosecha y El Desgrane de La Mazorca Del Cacao Tipo Forastero de Cuyagua Sobre Características Del Grano En Fermentación. Agron. Trop. 2004, 54, 7430776. [Google Scholar]
- Horta-Tellez, H.; Sandoval-Aldana, A.; García-Muñoz, M.; Cerón-Salazar, I. Evaluation of the Fermentation Process and Final Quality of Five Cacao Clones from Departament Of Huila, Colombia. DYNA 2019, 86, 233–239. [Google Scholar] [CrossRef]
- Zapata, S.; Tamayo, A.; Rojano, B. Efecto de La Fermentación Sobre la Actividad Antioxidante de Diferentes Clones de Cacao Colombiano. Rev. Cuba. Plantas Med. 2013, 18, 391–404. [Google Scholar]
- Teneda-Llerena, W.F. Mejoramiento del Proceso de Fermentación del Cacao (Theobroma cacao L.) Variedad Nacional y Variedad CCN51; Editorial; Universidad Internacional de Andalucía: Sevilla, Spain, 2016. [Google Scholar] [CrossRef]
- Elwers, S.; Zambrano, A.; Rohsius, C.; Lieberei, R. Differences between the Content of Phenolic Compounds in Criollo, Forastero and Trinitario Cocoa Seed (Theobroma cacao L.). Eur. Food Res. Technol. 2009, 229, 937–948. [Google Scholar] [CrossRef]
- Niemenak, N.; Rohsius, C.; Elwers, S.; Ndoumou, D.O.; Lieberei, R. Comparative Study of Different Cocoa (Theobroma cacao L.) Clones in Terms of Their Phenolics and Anthocyanins Contents. J. Food Compos. Anal. 2006, 19, 612–619. [Google Scholar] [CrossRef]
- Wollgast, J.; Anklam, E. Review on Polyphenols in Theobroma cacao: Changes in Composition during the Manufacture of Chocolate and Methodology for Identification and Quantification. Food Res. Int. 2000, 33, 423–447. [Google Scholar] [CrossRef]
- Melo, T.S.; Pires, T.C.; Engelmann, J.V.P.; Monteiro, A.L.O.; Maciel, L.F.; da Silva Bispo, E. Evaluation of the Content of Bioactive Compounds in Cocoa Beans during the Fermentation Process. J. Food Sci. Technol. 2021, 58, 1947–1957. [Google Scholar] [CrossRef] [PubMed]
- Camino, C.; Espin, S.; Samaniego, I.; Carpio, C. Comparación de los Niveles de Grasa, Alcaloides y Polifenoles Totales en Almendras de Cacao Nacional Fino de Aroma de Diferentes Zonas del Litoral Ecuatoriano. Aliment. Cienc. Ing. 2014, 22, 34–40. Available online: https://repositorio.iniap.gob.ec/bitstream/41000/3280/1/iniapscCD69.pdf (accessed on 23 July 2024).
- Rojas-Rojas, K.; Hernández-Aguirre, C.; Mencía-Guevara, A. Transformaciones Bioquímicas Del Cacao (Theobroma cacao L.) Durante Un Proceso de Fermentación Controlada. Agron. Costarric. 2021, 45, 53–65. [Google Scholar] [CrossRef]
- Vázquez-Ovando, A.; Ovando-Medina, I.; Adriano-Anaya, L.; Betancur-Ancona, D.; Salvador-Figueroa, M. Alcaloides y Polifenoles del Cacao, Mecanismos que Regulan su Biosíntesis y sus Implicaciones en el Sabor y Aroma. Arch. Latinoam. Nutr. 2016, 656, 239–254. [Google Scholar]
- Lima, L.J.R.; Almeida, M.H.; Nout, M.J.R.; Zwietering, M.H. Theobroma cacao L., ‘the Food of the Gods’: Quality Determinants of Commercial Cocoa Beans, with Particular Reference to the Impact of Fermentation. Crit. Rev. Food Sci. Nutr. 2011, 51, 731–761. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, J.; Chungara, M.; Ibieta, G.; Alejo, I.; Tejeda, L.; Peralta, C.; Aliaga-Rossel, E.; Mollinedo, P.; Peñarrieta, J.M. Determinación de Teobromina, Catequina, Capacidad Antioxidante Total y Contenido Fenólico Total en Muestras Representativas de Cacao Amazónico Boliviano y su Comparación antes y después del Proceso de Fermentación. Rev. Boliv. Química 2019, 36, 40–50. [Google Scholar] [CrossRef]
- Rajurkar, N.S.; Hande, S.M. Estimation of phytochemical content and antioxidant activity of some selected traditional indian medicinal plants. Indian J. Pham. Sci. 2011, 73, 146–151. [Google Scholar] [CrossRef]

| Clone | Origin |
|---|---|
| INIFAP 1 (C1) | The parents are RIM 76A X EET 400. The RIM 76A genotype corresponds to a clonal selection of a Criollo genotype from Mexico, and with respect to the EET 400 genotype, it is a commercial clonal hybrid from Ecuador. |
| INIFAP 4 (C4) | The parents are RIM 75 X POUND 7. The RIM 75 genotype corresponds to a clonal selection of a Criollo genotype from Mexico, and with respect to the POUND 7 genotype, it corresponds to a clonal selection from Trinidad and Tobago. |
| INIFAP 8 (C8) | The parents are RIM 76A X EET 48. The RIM 76A genotype corresponds to a clonal selection of a Criollo genotype from Mexico, and with respect to the EET 48 genotype, it corresponds to a clonal selection from Ecuador |
| INIFAP 9 (C9) | The parents are RIM 75 X SPA 9. The RIM 75 genotype corresponds to a clonal selection of a Criollo genotype from Mexico, and with respect to the SPA 9 genotype, it corresponds to a clonal selection from Colombia. |
| Number of Wavenumbers | FTIR Range Peaks (cm−1) | Functional Groups | T0 Peaks | T120 Peaks |
|---|---|---|---|---|
| 1 | 3000–3350 | O-H and N-H stretching | 3280 | 3280↓ |
| 2 | 2853–2928 | CH2 and CH3 stretching vibrations | 2918 | 2852↑ |
| 2852 | 2852↑ | |||
| 3 | 1634–1744 | N-H bending vibrations, C=O bending vibrations | 1744 | 1744↑ |
| 1646 | 1646↓ | |||
| 4 | 1540–1550 | Aromaticity | 1550 | 1550↓ |
| 5 | 1399–1457 | CH3 lipids/proteins and COO- of amino acids | 1447 | 1447↓ |
| 6 | 1377–1407 | Primary or secondary O-H bending (in-plane), and phenol or tertiary alcohol (O-H bend) | 1380 | 1380↓ |
| 1224 | 1224↓ | |||
| 7 | 1048–1160 | C-O stretching vibrations | 1143 | 1143↓ |
| 1048 | 1048↓ | |||
| 8 | 721–997 | C-H bending vibrations | 763 | 763↓ |
| 9 | 400–700 | 513 | 513↓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Santiago, F.L.; Márquez-Rocha, F.J.; García-Alamilla, P.; Carrera-Lanestosa, A.; Ramírez-López, C.; Ocaranza-Sánchez, E.; Jiménez-Rodríguez, D.J. Physicochemical and Biochemical Changes in Cocoa during the Fermentation Step. Fermentation 2024, 10, 405. https://doi.org/10.3390/fermentation10080405
Ruiz-Santiago FL, Márquez-Rocha FJ, García-Alamilla P, Carrera-Lanestosa A, Ramírez-López C, Ocaranza-Sánchez E, Jiménez-Rodríguez DJ. Physicochemical and Biochemical Changes in Cocoa during the Fermentation Step. Fermentation. 2024; 10(8):405. https://doi.org/10.3390/fermentation10080405
Chicago/Turabian StyleRuiz-Santiago, Franco Lucio, Facundo Joaquín Márquez-Rocha, Pedro García-Alamilla, Areli Carrera-Lanestosa, Carolina Ramírez-López, Erik Ocaranza-Sánchez, and David Jesús Jiménez-Rodríguez. 2024. "Physicochemical and Biochemical Changes in Cocoa during the Fermentation Step" Fermentation 10, no. 8: 405. https://doi.org/10.3390/fermentation10080405
APA StyleRuiz-Santiago, F. L., Márquez-Rocha, F. J., García-Alamilla, P., Carrera-Lanestosa, A., Ramírez-López, C., Ocaranza-Sánchez, E., & Jiménez-Rodríguez, D. J. (2024). Physicochemical and Biochemical Changes in Cocoa during the Fermentation Step. Fermentation, 10(8), 405. https://doi.org/10.3390/fermentation10080405






