Abstract
Wild ginseng is known to have better pharmacological effects than cultivated ginseng. Additionally, recently developed bioengineering technology has made it possible to produce cultured wild ginseng with the same genetic composition. In this study, we investigated the change in characteristics and the improvement of the intestinal barrier of cultured wild ginseng roots (CWG) and fermented cultured wild ginseng roots (FCWG). First, we screened nine strains of bacteria that are capable of growing on 5-brix CWG medium, and Limosilactobacillus fermentum HY7303 (HY7303) showed the highest growth. Second, changes in the characteristics of CWG due to fermentation using HY7303 showed that pH and total carbohydrates decreased, and reducing sugars increased. The contents of minor ginsenosides (Rg3(s), Rk1, and Rg5) increased. Third, extracellular vesicles (EVs) with a single peak at 493.7 nm were isolated from CWG, and EVs with three peaks at 9.0 nm, 155.6 nm, and 459.0 nm were isolated from FCWG, respectively. Finally, when we treated Caco-2 cells with FCWG and EVs, we confirmed the improvement of intestinal barrier functions, including recovery, permeability, and expression of tight-junction protein genes. In this study, we confirmed the potential pharmacological effects of minor ginsenosides and EVs derived from FCWG. In conclusion, this study suggests that CWG fermentation with HY7303 improves the intestinal barrier by increasing minor ginsenosides and producing EVs.
1. Introduction
Wild ginseng is a perennial herb belonging to the Araliaceae family and refers to ginseng grown naturally in the wild; it is reported that wild ginseng has superior medicinal properties to cultivated ginseng [1,2]. Despite the excellent efficacy of wild ginseng, its industrial application has many difficulties because it grows to at least 10 to 15 years old, and the cultivable area gradually decreases [2,3,4]. But recently, a large-capacity bioreactor using plant tissue culture technology, a biological technique, and mass-proliferation technology has been developed, and the cultured wild ginseng roots (CWG) grown by these bioengineering techniques are reported to have a stable genetic composition [5,6]. In addition, processed ginseng, reinforced with specific ingredients like saponin using fermentation technology, has recently attracted attention as a functional food [7]. For example, studies have reported that red ginseng or cultured wild ginseng have been developed as functional materials that ferment using microbial strains to increase the metabolic rate in the body of active ingredients such as enhancing antioxidant power [8,9,10].
Ginsenosides (ginseng saponins) are representative pharmacologically active compounds of wild ginseng [11]. Common ginsenosides are in the form of polymers and have three or more monosaccharides attached by a strong double bond [12]. Major ginsenosides, which account for more than 80% of ginsenosides, are deglycosylated; they become minor ginsenosides [13]. The decomposed minor ginsenosides are known to have increased body absorption and pharmacological effects [14,15,16]. However, naturally existing minor ginsenosides are very low in concentration. Therefore, enzyme treatment or fermentation is required to obtain them [13].
Probiotics have been defined as viable microorganisms which beneficially influence the health of the host [17,18]. Well-known benefits include the ability to favorably regulate the intestinal environment by changing the composition of gut microbiota or producing metabolites [19]. Recently, the role of extracellular vesicles (EVs) that mediate intracellular communication secreted by probiotics has been increasingly recognized [20]. EVs are lipid-bilayer-enclosed nano-sized particles (20–250 nm in diameter) that contain various proteins, lipids, nucleic acids, and polysaccharides [21,22,23]. Bacterial EVs exhibited various health-promoting effects including immune modulation, anti-inflammation, anti-allergy, and anti-cancer properties [24,25]. Gram-positive bacterial EVs from probiotic bacteria are also studied, drawing attention to their therapeutic efficacy [26]. EVs derived from the Lactobacillus strain enhanced intestinal barrier function by maintaining tight-junction proteins [27].
In this study, we investigated the change in components in fermented cultured wild ginseng roots (FCWG) using Limosilactobacillus fermentum HY7303 (HY7303) and its effects on intestinal health. This study aims to select strains that grow well in CWG medium and present enhanced beneficial effects of FCWG through fermentation. We present the ginsenoside contents and isolated EVs produced by the fermentation process in FCWG and confirm the possibility that FCWG and EVs improve intestinal permeability and tight-junction protein in Caco-2 cells.
2. Materials and Methods
2.1. Sample Preparation
The dried cultured wild ginseng roots (CWG) used in this experiment were purchased from BioFD&C (BioFD&C, Yeonsu-gu, Incheon, Republic of Korea). Dried CWG was extracted with 70% alcohol at 80 °C for 6 h three times. It was concentrated to 65 brix to remove alcohol and diluted with purified water for fermentation to make it to 5 brix [28]. The 5-brix cultured wild ginseng root extract dilution was sterilized at 121 °C for 15 min before use. This experiment used Limosilactobacillus fermentum HY7303 (HY7303) and 8 strains of lactic acid bacteria (LAB) provided by the manufacturing plant at Hy Co., Ltd. (Pyeongtaek-si, Gyeonggi-do, Republic of Korea), which were inoculated into De Man–Rogosa–Sharpe (MRS) broth (BD Difco, Franklin Lakes, NJ, USA) and cultured at 37 °C for 24 h to produce fermented strains.
2.2. Measurement of pH, Total Carbohydrates, and Reducing Sugar Contents
To investigate the alterations in the properties of CWG by fermentation, pH, total carbohydrates, and reducing sugar were measured. Activated L. fermentum HY7303 and 8 LAB were inoculated with 10 mL to 1 L of 5-brix CWG for fermentation. This was shaken at 100 rpm in a shaking incubator at 37 °C and fermented for 24 h, and then pH and the number of viable bacteria were measured. To inactivate lactic acid bacteria, fermented cultured wild ginseng roots (FCWG) were manufactured by treating them at 90 °C for 10 min.
The phenol–sulfuric acid method was used to check changes in total carbohydrate contents before and after the fermentation of CWG [29]. Briefly, 0.5 mL samples were mixed with 0.5 mL of 5% phenol solution, and then 2.5 mL of 98% H2SO4 was added. The reaction mixture was set at room temperature for 20 min, and the absorbance was measured at 490 nm. The dinitrosalicylic acid (DNS) method was used to check changes in reducing sugar contents before and after the fermentation of CWG [30]. A volume of 1 mL of diluted sample was mixed with 2 mL of DNS reagents (3,5-dinitrosalicylic acid, 2M NaOH, potassium sodium tartrate, sodium bisulfate, and phenol) and incubated in boiling water for 15 min. Absorbance was measured at 540 nm. Both total carbohydrates and reducing sugar were calculated based on the standard using D-glucose.
2.3. High-Performance Liquid Chromatography (HPLC) Analysis
The prepared CWG and FCWG were concentrated to 65 brix, dissolved in 70% methanol, filtered, and used as an analysis sample. Ginsenoside analysis was performed using an Agilent HPLC 1260 infinity, (Agilent Technologies, Santa Clara, CA, USA), and the column Discovery C18 (5 μm, 250 × 4.6 mm) was measured using acetonitrile (ACN) and water as mobile phases. The concentration gradient is shown in Table 1. For the quantitative method, the measured area value was substituted into the calibration curve equation for the ginsenoside standard product, and unit conversion was calculated by substituting the equation below.
Ginsenoside content (mg/g) =
Ginsenoside concentration (μg/mL) × (total amount of test solution × dilution factor)
/sample collection amount (g) × 1/1000-unit conversion factor

Table 1.
Concentration gradient conditions of mobile phase used in HPLC analysis.
2.4. Tangential Flow Filtration (TFF) System and Size Analyze of EVs
CWG and FCWG were pelleted by continuous centrifugation at 15,970× g at 10 °C for 15 min. Culture supernatants were separated once more by centrifugation and filtered using a KrosFlo® KR2i TFF system 0.2 μm membrane filter from Repligen (Spectrum Labs, Los Angeles, CA, USA) to remove cell residues. Afterward, extracellular vesicles (EVs) derived from CWG and FCWG were obtained using a tangential flow filtration (TFF) system 300 kDa membrane filter. The final volume of the retentive was concentrated to 20 mL for a 300 kDa size retentive at 50-fold concentration. Then, isolated EVs were diluted 1:1 in 15% maltodextrin solution with PBS and dried using a freeze dryer (FDT-8620, Operon, Gimpo, Republic of Korea). Afterward, bacterial-derived EVs in powder form were diluted in PBS to a 50 mg/mL concentration and stored at a refrigerated temperature (4 °C). The particle size of EVs isolated at a 50-fold concentration at 300 kDa was measured using a Zetasizer Nano ZS90 (Malvern Instruments Ltd., Malvern, Worcestershire, UK).
2.5. Evaluation of Barrier Improvement Efficacy
2.5.1. Cell Culture
Caco-2 cells were purchased from the Korean Cell Line Bank (Seoul, Republic of Korea) and cultured in Dulbecco’s modified Eagle’s medium (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin–streptomycin. Caco-2 cells were seeded at a concentration of 1 × 106 cells/well in used plates. Caco-2 cells were replaced with fresh medium every two days during cell growth at 37 °C in an atmosphere containing 5% CO2.
2.5.2. Transepithelial Electrical Resistance (TEER)
After the cells were grown to 100% confluence, the integrity of Caco-2 cell monolayers was assessed using a Millicell ERS volt–ohm meter (Merck Millipore Corporation, Darmstadt, Germany). Cell monolayers were used for experiments 21 days after seeding. Only cell monolayers with transepithelial electrical resistance (TEER) values exceeding 600 Ω/cm2 were selected for all co-culture experiments. This is a generally accepted value indicating the presence of a fully differentiated cell monolayer. Sample resistances were obtained using cell-free inserts as blanks, and blank values were subtracted from all samples. The final unit area resistance was then calculated by multiplying the sample resistance value by the effective transepithelial volume (0.33 cm2). To determine the effect of CWG and FCWG on cell barrier permeability, differentiated Caco-2 cell monolayers were washed twice with PBS and cultured in DMEM for 30 min. CWG and FCWG were diluted to a concentration of 10 μg/mL, and CWG-EVs and FCWG-EVs dissolved in DMSO were treated at 10 μg/mL with 1 μg/mL of lipopolysaccharides (LPSs, Sigma-Aldrich, St. Louis, MO, USA) for 24 h. In all subsequent experiments, cells were treated with the same concentration of LPSs and samples. TEER values were measured and recorded at selected time points (immediately after sample processing and 24 h after sample processing).
2.5.3. Determination of Paracellular Apparent Permeability
Caco-2 cells were cultured in Dulbecco’s modified Eagle’s medium (Gibco, Grand Island, NY, USA) containing 10% FBS (Gibco, Grand Island, NY, USA) and penicillin–streptomycin. A volume of 500 μL of Caco-2 cell suspension at a concentration of 1.0 was used. The growth medium was changed approximately every 3 days. After the monolayer of cells was formed, each well, except for the untreated group, was treated with LPSs at a concentration of 1 μg/mL for 1 day. Each experimental group was treated with FCWG and EVs derived from FCWG for 24 h at an appropriate concentration, and then an apparent permeability assay was performed and carried out. Briefly, a fluorescence reaction was generated by dispensing a medium containing 1 mg/mL of 4 kDa fluorescein isothiocyanate (FITC)-dextran. Afterward, 100 μL of the medium was collected every hour, and the fluorescence was measured at 485 nm and 520 nm, excitation and emission, respectively, using a Synergy HTX multimode reader (Bio-Tek, Winooski, VT, USA). The apparent permeability coefficient (Papp) is calculated from the permeability and compound concentration at 0 and 2 h, as shown in the equation below.
Papp = dQ/dt × 1/(A × C0)
- dQ/dt: The amount of product present in the basal (A-B) compartment as a function of time (nmol/s).
- A: Area (cm2).
- C0: Initial concentration (nmol/mL).
2.5.4. qRT-PCR
Caco-2 cell suspension was seeded in 6-well plates at a concentration of 1.0 × 106 cells/mL. As with the permeability assay, sample processing was performed followed by qRT-PCR. Briefly, total RNA was isolated from cells using the easy-spin total RNA extraction kit (iNtRON Biotechnology, Boston, MA, USA). The extracted total RNA samples were quantified using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) and stored at −20 °C until use. cDNA was synthesized using the Maxime R.T. premix kit (iNtRON Biotechnology, Boston, MA, USA). cDNA samples were amplified using the Quant Studio 6-flex real-time PCR system (Applied Biosystems, Foster City, CA, USA) and gene expression master mix (Applied Biosystems, Foster city, CA, USA). qRT-PCR was performed using mouse-specific TaqMan gene expression assays (Applied Biosystems, Carlsbad, CA, USA) for tight-junction protein 1 (TJP1; Hs01551861_m1), tight-junction protein 2 (TJP2; Hs00910541_m1), occludin (OCLN; Hs00170162_m1), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Hs99999905_m1). The mRNA expression level of each gene was calculated using the 2−ΔΔCt method and normalized to the GAPDH value. Information on primers is shown in Table 2.

Table 2.
Information on primers for qRT-PCR.
2.6. Statistical Analysis
All experimental results were expressed as mean ± standard deviation (S.D.) of independent experiments and analyzed using GraphPad Prism version 10.1.2 (GraphPad Software, San Diego, CA, USA). Statistical comparisons between groups were made using Student’s t-test.
3. Results
3.1. Screening of Bacteria in CWG
We evaluated and selected which bacteria can grow in CWG. Before incubation, the CWG extract was diluted to 5 brix using sterilized distilled water. To confirm that nine types of bacteria were growing in CWG, we measured the colony-forming unit (CFU), as shown in Figure 1. The initial inoculation CFU of bacteria was 1.78 × 106 on average and cultured for 24 h. After incubation, the CFU of all bacteria used in this experiment increased in 5-brix CWG. Among the bacteria used in the experiment, the strain with the lowest growth was Lactiplantibacillus plantarum strain KLDS (37.04% increase), and the one with the highest growth was Limosilactobacillus fermentum HY7303 (1265.38% increase). We selected Limosilactobacillus fermentum HY7303 (HY7303), which represented significant growth compared to the others in CWG medium, and used this strain in all subsequent experiments.
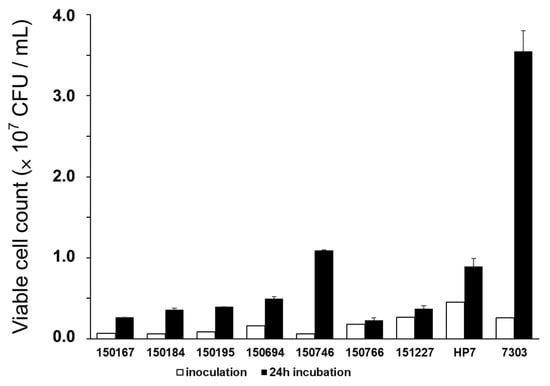
Figure 1.
Growth of 9 types of bacteria in 5-brix CWG. 150167, Lactiplantibacillus plantarum strain CSI9; 150184, Lactiplantibacillus plantarum subsp. Plantarum; 150195, Lactobacillus plantarum subsp. Plantarum; 150694, Lactiplantibacillus plantarum strain RS7; 150746, Limosilactobacillus reuteri strain MF1572; 150766, Lactiplantibacillus plantarum strain H1S1L1; 151227, Lactiplantibacillus plantarum strain KLDS; HP7, Lactobacillus paracasei HP7; HY7303, Limosilactobacillus fermentum HY7303. Error bars represent standard deviation of the mean (n = 3).
3.2. Physiological Changes of CWG by Fermentation Using HY7303
Changes by fermentation, including pH, total carbohydrates, and reducing sugar, are shown in Table 3. Before fermentation began, the pH of CWG was 5.27. After fermentation for 24 h, the pH of FCWG decreased to 4.24. Likewise, total carbohydrates were 4.86 before fermentation but decreased to 4.47 after 24 h of fermentation. On the other hand, reducing sugar was 4.35 before fermentation but significantly increased to 4.86 after fermentation.We analyzed a total of eleven types of ginsenosides, and among them, the seven ginsenosides that showed the most notable changes were divided into four major ginsenosides and three minor ginsenosides and quantified (Figure 2 and Table 4). To quantify the ginsenosides of 65 brix of CWG and FCWG, we multiplied the dilution factor and calculated it, which is shown in Table 3. The total concentration of ginsenosides in 65-brix CWG was 111.15 ± 10.17 mg/g. Four major ginsenosides (Rb1, Rc, Rb2, and Rd) were detected in relatively large amounts, but three minor ginsenosides were detected in trace amounts (Rg3(s), Rk1, and Rg5). The total concentration of ginsenosides in 65-brix FCWG was 130.37 ± 2.29 mg/g. The levels of major ginsenosides were decreased in FCWG compared to the CWG, while the levels of minor ginsenosides were increased in FCWG. Overall, major ginsenosides tended to decrease (Rb1, Rc1, Rb2, and Rd), and minor ginsenosides (Rg3(s), Rk1, and Rg5) tended to increase by fermentation.

Table 3.
Changes in pH, total carbohydrate content, and reducing sugar content in CWG medium for 24 h fermentation by L. fermentum HY7303 compared with before fermentation.
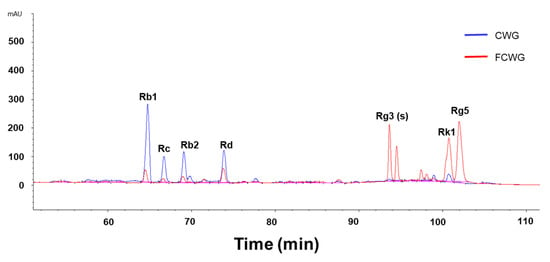
Figure 2.
Representative HPLC chromatograms of the products analyzed in this study. Major ginsenosides Rb1, Rc, Rb2, and Rd were detected in high levels in CWG, and minor ginsenosides Rg3(s), Rk1, and Rg5 were detected in high levels in FCWG. CWG, cultured wild ginseng roots; FCWG, fermented cultured wild ginseng roots using HY7303.

Table 4.
Quantitative analysis of ginsenosides in CWG and FCWG using HPLC.
3.3. Size Difference between CWG- and FCWG-Derived EVs
In this study, we used a nanoparticle size analyzer with zeta potential to determine the particle size of extracellular vesicles (EVs). We investigated the particle size distribution isolated from CWG (Figure 3A) and FCWG (Figure 3B). The size of nanoparticles isolated from CWG was distributed at 493.7 ± 435.9 (Table 5). And the size of nanoparticles isolated from FCWG was distributed with three peaks, 9.065 ± 1.98, 155.6 ± 51.5, and 459 ± 161.3. Thus, considering EVs have a size of 20 to 250 nm, relatively more EVs were detected in nanoparticles isolated from FCWG compared to CWG.
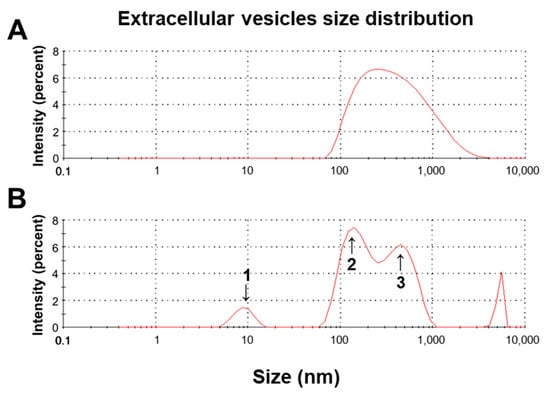
Figure 3.
Size distribution profile of (A) CWG-EVs and (B) FCWG-EVs by intensity using a Zetasizer. CWG, cultured wild ginseng roots; FCWG, fermented wild ginseng roots using HY7303; EVs, extracellular vesicles.

Table 5.
Quantitative size analysis and distribution rate analysis of CWG-EVs and FCWG-EVs using a Zetasizer.
3.4. Effect of Gut Health for Treatment of FCWG and EVs
We studied the effects of FCWG and its EVs on inflammatory epithelial cell monolayers. The goal was to evaluate whether it supports the healing and recovery of damaged cell monolayers. Intact monolayers were first treated with LPSs, and damage was recorded through TEER measurements. Afterward, CWG, FCWG, CWG-EVs, and FCWG-EVs were each treated at a concentration of 10 µg/mL for 24 h, and TEER was measured. As shown in Figure 4A, untreated monolayers maintained a constant TEER level (average value of 608.19 Ωcm2) throughout the experiment. However, in the LPS-treated control group, TEER levels were significantly decreased (TEER levels decreased by 23% compared to the normal group, a significant difference compared to the normal group, p < 0.001). Surprisingly, TEER levels tended to recover in all experimental groups treated with CWG, FCWG, and FCWG-EVs. Especially in FCWG and FCWG-EVs, TEER levels were significantly restored to normal group levels.
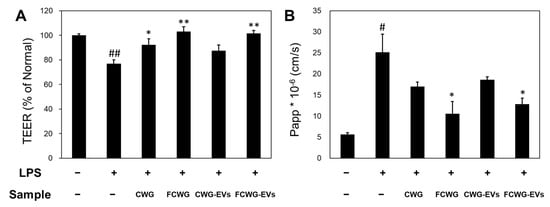
Figure 4.
Evaluation of gut barrier improvement of FCWG and its EVs using HY7303. (A) Effect of FCWG and FCWG-EVs on the transepithelial electrical resistance (TEER) in Caco-2 cells’ monolayers. (B) Effect of intestinal apparent permeability of FCWG and FCWG-EVs on apparent permeability of Caco-2 cells to FITC-dextran. Cells without any treatment were used as normal. Error bars represent standard deviation of the mean (n = 3). Significant differences are indicated by * p < 0.05 and ** p < 0.01, relative to the control group. # p < 0.05, ## p < 0.001 compared with the normal group. In order from left to right: normal, LPS, CWG, FCWG, CWG-EV, and FCWG-EV group (LPSs; 1 μg/mL, CWG; 10 μg/mL, FCWG; 10 μg/mL, CWG-EVs; 10 μg/mL, FCWG-EVs; 10 μg/mL). CWG, cultured wild ginseng roots; FCWG, fermented cultured wild ginseng roots using HY7303; EVs, extracellular vesicles.
To identify the effect of probiotics and their EVs on the tight junction of intestinal cells, the permeability of the Caco-2 cell layer to FITC-dextran was confirmed (Figure 4B). Compared to the normal group with a value of 5.63, the LPS-treated group showed higher Papp values of 25.16, indicating that intestinal cell function was weakened. In the FCWG and FCWG-EV treatment groups, it was identified that the increased Papp value after LPS treatment was recovered similarly to normal cells. On the other hand, in the CWG and CWG-EV treatment, the Papp was similar to the value of the LPS treatment group. These results showed that the FCWG and its EVs can improve intestinal wall function by preventing membrane permeability.
LPSs emitted by harmful bacteria play an essential pathogenic role in intestinal inflammation and other inflammatory diseases. Increased LPSs is known to cause impaired intestinal permeability. We identified the expressions of ZO-1, ZO-2, and OCLN, which are associated with the intestinal leakage phenomenon, using qRT-PCR (Figure 5). As a result, we confirmed that the mRNA levels of ZO-1 and ZO-2 were 0.758 and 0.609 times lower in LPS-treated cells than in control cells, and in FCWG- and EV-treated cells, mRNA levels of ZO-1 significantly increased 1.433-fold and 1.141-fold, while mRNA levels of ZO-2 increased 1.516-fold and 1.505-fold. Regarding OCLN gene expression, we confirmed that the level of OCLN was significantly decreased in LPS cells by 0.60-fold, but it significantly improved in FCWG- and EV-treated cells by 1.706-fold and 1.282-fold.
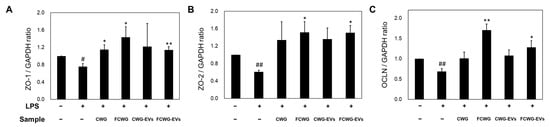
Figure 5.
The effect of FCWG and its EVs on tight-junction proteins. The mRNA levels of (A) zonula occludens-1 (ZO-1), (B) zonula occludens-2 (ZO-2), and (C) occludin (OCLN) in cells were normalized to the levels of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA and calculated as a relative-fold value. Error bars represent standard deviation of the mean (n = 3). Significant differences are indicated by * p < 0.05 and ** p < 0.01, relative to the LPS group. # p < 0.05, ## p < 0.001 compared with normal group. In order from left to right: normal, LPS, CWG, FCWG, CWG-EV, and FCWG-EV group (LPSs; 1 μg/mL, CWG; 10 μg/mL, FCWG; 10 μg/mL, CWG-EVs; 10 μg/mL, FCWG-EVs; 10 μg/mL). CWG, cultured wild ginseng roots; FCWG, fermented cultured wild ginseng roots using HY7303; EVs, extracellular vesicles.
4. Discussion
For thousands of years, wild ginseng (Panax ginseng, C.A. Meyer) has been used as a tonic, antifatigue, sedative, and anti-gastric ulcer drug [31,32]. However, growing wild ginseng in fields takes several years and requires very sophisticated care because its growth conditions (i.e., soil, climate, and pathogenesis) are challenging to control. For these reasons, low yields and high costs hamper efforts to meet increasing market demand. However, with the cell culture technique, fastidious and complicated conditions for producing ginseng and ginsenosides can be overcome and optimized. Fortunately, wild ginseng roots produced through in vitro culture have genetic DNA similar to wild ginseng and are known to have a high saponin content [33,34].
Recently, substantial advances in the understanding of the molecular pathogenesis of inflammatory bowel disease (IBD) have been made owing to some investigation [35]. A defective intestinal epithelial tight-junction barrier is an important pathogenic factor to determine gut permeability by allowing paracellular permeation of luminal antigens that elicit and promote inflammatory response [36,37]. The prevalence of IBD is likely to steadily increase in newly industrialized countries [38]. Therefore, there have been various attempts to treat IBD [39,40,41,42,43]. Recently, there has been growing interest in the use of probiotics and their fermentations as an adjunct to standard anti-inflammatory and immune-suppressing therapy, as evidence suggests disruption of the gastrointestinal microbiome and intestinal immune balance as potential triggers for IBD [44].
During fermentation, the enzymatic activity of the raw material and the metabolic activity of microorganisms can change the nutritional and bioactive properties of the food matrix in ways that have beneficial consequences for human health [18,45]. We screened about 50 LAB from the bacteria library provided by hy Co., Ltd. Finally, we selected nine strains of lactic acid bacteria whose CFU increased on CWG medium (Figure 1). The growth rate of HY7303 was significantly higher than others on CWG medium. And then, we investigated the changes that occurred as a result of fermenting CWG with HY7303 and found that pH and total carbohydrates are decreased, and reducing sugars increased (Table 2). The glycoside hydrolase activity of lactic acid bacteria plays a very important role in hydrolysis of plant metabolites [46,47]. These results showed similarity with previous research results in which reducing sugars increased due to glycoside hydrolases as a result of fermenting wild ginseng [48]. β-glucosidase, belonging to family-3 GHs, bioconverts polysaccharides such as cellulose into glucose [49,50]. Carbohydrates in CWG were converted to monosaccharides such as glucose by β-glucosidase of HY7303, which caused an increase in reducing sugars.
HPLC analysis of the FCWG revealed the presence of increased concentrations of minor ginsenosides such as Rg3(s), Rk1, and Rg5, which may be attributable to the bacterial conversion of major ginsenosides (Figure 2 and Table 3). However, compared to the major ginsenosides found in abundance in CWG, minor ginsenosides are present in very low amounts or are not present at all [51]. In previous studies, minor ginsenosides, such as Rg3(s), Rk1, and Rg5, were produced through the loss of the glycosyl moieties at the C20 position of the major ginsenosides [52]. In addition, it has been confirmed through many studies that microbes which have β-glucosidase activity show a strong ability to convert ginsenoside Rb1 or Rd into Rg3(S) [52,53]. In our study, we found a decrease in major ginsenosides including Rb1 and Rd and an increase in minor ginsenosides. This is suggested to be related to the β-glucosidase activity of HY7303. And it has been confirmed that minor ginsenosides can more easily be absorbed by the human body [54]. Rg3(s) maintains the stability of intestinal barrier function by effectively regulating the intestinal flora that interact with intestinal epithelial cells [55]. Rk1 exhibits anti-permeability activity through enhanced stability of tight-junction proteins at the boundary between cells [56]. Rg5 treatment effectively improved intestinal pathological morphology and repaired intestinal barrier function in diabetic db/db mice [57]. Therefore, it was expected that the increased trace amounts of ginsenosides in FCWG would improve anti-permeability activity by regulating tight-junction proteins.
EVs are membrane-derived lipid bilayers produced by a process known as vesiculogenesis [58]. It can package cellular compounds that microbial metabolism produces, such as proteins, nucleic acids, and polysaccharides [59]. It is well known that bacteria, eukarya, and archaea can release EVs [58]. In many cases, EV administration may be safer and more effective than probiotics [60]. Interestingly, EVs can succeed when intact bacteria fail to produce beneficial effects. This may be because EVs can penetrate the intestinal epithelial barrier and migrate to other organs [26]. In this study, we used HY7303, an L. fermentum strain already known to produce EVs. Since HY7303 can use CWG as a nutrient source, CWG can be used as a culture medium for EV isolation. We separated EVs using the TFF method, one of the well-known methods for separating EVs, and identified the peak of EV size (20 to 250 nm) in FCWG using a size analyzer (Figure 3 and Table 4). While the size of EVs isolated from CWG is normally distributed with a single peak, the multiple peaks of FCWG indicate the isolation of various types of extracellular vesicles, including exosomes, which have been shown to have various positive functions [23]. There have been studies to isolate EVs produced as by-products of fermentation, but to the best of our knowledge, this is the first study to examine the differences in EVs before and after fermentation [24].
The importance of the intestinal epithelial barrier in disease pathogenesis has recently become an interesting topic [61]. The intestinal barrier protects the host from pathogenic microbes, food antigens, and chemical toxins in the intestinal tract [62]. Therefore, regulating the permeability of the intestinal epithelial barrier has become very important. Previous studies have shown that saponins can inhibit intestinal inflammation, promote intestinal barrier repair, maintain the diversity of intestinal flora, and reduce the incidence of colon cancer associated with colonic inflammation [63]. However, this study showed that CWG did not have a significant effect on improving the barrier (Figure 4). Meanwhile, FCWG and FCWG-EVs were found to be effective in recovery of Caco-2 cells. This may have effectively contributed to the recovery of the intestinal epithelial barrier because the high components of minor ginsenosides in FCWG were more easily absorbed into the cell. Intestinal wound healing is dependent on the precise balance of migration, proliferation, and differentiation of the epithelial cells adjacent to the wounded area [64]. Previous studies have shown that milk-derived extracellular vesicles (mEVs) alleviate epithelial tight-junction disruption and have therapeutic potential in metabolic diseases related to the gut–liver axis [65]. In previous studies, cellular uptake of mEVs by Caco-2 cells was confirmed, supporting the remarkable functionality shown by FCWG-EVs in our study. Additionally, by comparing the results of CWG-EVs and FCWG-EVs, it suggests that fermentation using HY7303 makes a significant difference in the production of EVs of cultured wild ginseng roots and the resulting ability to improve the intestinal barrier. We have not yet determined at what stage of repair the minor ginsenosides operate, and in further study, it will be necessary to investigate the molecular mechanism by which minor ginsenosides affect intestinal epithelial cells.
The loosened barrier of LPS-stimulated enterocytes can be restored as FCWG and FCWG-EVs (Figure 5). The tight-junction proteins such as zonula occludens 1 (ZO-1), zonula occludens 2 (ZO-2), claudins, and occludins (OCLN) are pivotal for maintaining epithelial barrier integrity [66]. The intestinal barrier can be disrupted by inflammation, leading to various pathological processes. Loosening intestinal permeability is also a significant feature of IBD pathogenesis [67]. Fermented wild ginseng upregulated the expression of tight-junction proteins. Ginsenosides such as Rg1 and Rk1 derived from ginseng showed anti-permeability with increased stability of tight-junction proteins. These studies indicate that ginsenosides from wild ginseng can protect gut permeability by modulating tight-junction proteins [56,68]. Surprisingly, FCWG-EVs were shown to significantly contribute to both the recovery and the upregulation of tight-junction proteins of Caco-2 cells (Figure 4 and Figure 5). [64]. In a previous study, the recovery of ZO-1 lost when fermented wild ginseng was orally administered to mice with colitis was reported through fluorescent staining [69]. Our study provides additional evidence for the intestinal barrier recovery ability of fermented wild ginseng by not only confirming the recovery of ZO-1, as confirmed in previous studies, but also the recovery of ZO-2 and OCLN. In addition, we ruled out the possibility of external factors affecting protein expression and confirmed that FCWG has an effect on intestinal cells at the gene level by confirming the expression of mRNA. Our study is, to the best of our knowledge, the first study on the intestinal barrier improvements of EVs derived from fermented wild ginseng. We suggest that minor ginsenosides and EVs, complex biological molecules mediating interactions between the gut microbiota and the host, can be potent components for regulating the intestinal barrier [70]. This suggests that the benefits of fermentation are not limited to increasing the absorption rate of ginsenosides but also produce by-products that can exhibit new functionality.
5. Conclusions
In conclusion, the fermentation of CWG by HY7303 showed a significant improvement in the intestinal environment. FCWG and EVs recovered the thickness and permeability of Caco-2 cells and increased the expression of tight-junction protein genes. In our study, their functionality was evaluated separately, but if the synergistic effect of FCWG and FCWG-EVs is revealed, we believe that consumption of fermented wild ginseng cultured roots will further improve the intestinal environment. A limitation of our study is that more research is needed to explain the complex intestinal environment through an in vitro model. Additionally, an understanding of the molecular mechanisms of minor ginsenosides and FCWG-derived EVs is needed. Nevertheless, our study suggests that CWG fermentation by HY7303 has the potential to induce bioconversion from major ginsenosides to minor ginsenosides, generate EVs as beneficial by-products, and consequently effectively improve the inflammatory intestinal environment.
Author Contributions
Conceptualization, S.-J.M., E.-J.K., S.-D.P., J.-J.S. and J.-L.L.; formal analysis, S.-J.M. and E.-J.K.; investigation, S.-J.M. and E.-J.K.; methodology, S.-J.M. and E.-J.K.; project administration, S.-D.P.; software, S.-J.M.; supervision, J.-J.S.; validation, S.-J.M.; visualization, E.-J.K.; writing—original draft preparation, S.-J.M., E.-J.K. and Y.-H.L.; writing—review and editing, J.-H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article. The raw data are available upon reasonable request from the corresponding author.
Conflicts of Interest
All authors are affiliated with a company, but no conflicts of interest are declared regarding this study. The company has not been involved in our research, which was conducted strictly according to the authors’ protocol. We would like to thank the manufacturing plant at Hy Co., Ltd. (Pyeongtaek-si, Gyeonggi-do, Republic of Korea) for the kind gift of the bacteria library.
References
- Yoo, Y.-G.; Joung, M.-S.; Lee, Y.-H.; Kim, J.-Y.; Joong-Hoi, K.; Kee-Yoeup, P. A Study on the Effect of Mountain Ginseng Adventitious Roots Extract. J. Soc. Cosmet. Scientists Korea 2004, 30, 337–383. [Google Scholar]
- Hong, M.L.; Lim, H.K.; Ji-Eun, P.; Neung, J.J.; Young, J.L.; Moonjae, C.; Somi Kim, C. The Antihypertensive and Vasodilating Effects of Adventitious Root Extracts of Wild Ginseng. J. Korean Soc. Appl. Biol. Chem. 2009, 5, 102–107. [Google Scholar]
- Park, H.P.S.; Sang, H. Production and quality of mountain ginseng. In Proceedings of the 8th International Symposium on Ginseng, Korean Society of Ginseng, Seoul, Republic of Korea, 28–31 October 2002; pp. 456–466. [Google Scholar]
- Kim, E.-L.; Lee, H.Y.; Lee, H.R.; Kim, E.Y.; Yoon, M.C.; Shin, S.S. Mountain cultivated ginseng water boiled extract decreases blood glucose level and improves lipid metabolism in male db/db mice. Korean J. Herbol. 2012, 27, 69–75. [Google Scholar] [CrossRef][Green Version]
- Lee, Y.W. Mass production of wild ginseng (Panax ginseng C. A. Meyer) roots in a bioreactor and enhancement of ginsenoside contents using an elicitor, methyl jasmonate. Ph.D. Thesis, Seoul National University, Seoul, Republic of Korea, 2007; pp. 57–68. [Google Scholar]
- Chang-Sik, S.; Do-Hyun, L.; Sung-Han, K.; Min-Ho, S.; Chang-Ho, J.; Ki-Hwan, S. Ginsenoside Contents and Antioxidative Activities from Red Ginseng Treated with High Hydrostatic Pressure. J. Agric. Life Sci. 2010, 44, 133–140. [Google Scholar]
- Cha, B.-C.; Yoon, H.C.; Dae-Ho, L.; Jae-Seuk, P.; Ki-Rok, K. Component analysis of cultivated ginseng and mountain ginseng to the change of ginsenoside components in the process of heating and fermentation. J. Pharmacopunct. 2010, 13, 33–49. [Google Scholar] [CrossRef]
- Akao, T.; Kanaoka, M.; Kobashi, K. Appearance of compound K, a major metabolite of ginsenoside Rb1 by intestinal bacteria, in rat plasma after oral administration--measurement of compound K by enzyme immunoassay. Biol. Pharm. Bull. 1998, 21, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.J.; Cosmetics, H.H.; Seong, E.S.; Yoo, J.H.; Lee, J.G.; Kim, N.J.; Choi, S.K.; Lim, J.D.; Yu, C.Y. Biological Activity of Panax ginseng C. A. Meyer Culture Roots. Fermented with Microorganisms. Korean J. Med. Crop. Sci. 2016, 24, 191–197. [Google Scholar] [CrossRef]
- Kang, S.-W.; Min, H.-Y. Ginseng, the ‘immunity boost’: The effects of panax ginseng on immune system. J. Ginseng Res. 2012, 36, 354. [Google Scholar] [CrossRef]
- Yuan, C.-S.; Wang, C.-Z.; Wicks, S.M.; Qi, L.-W. Chemical and pharmacological studies of saponins with a focus on American ginseng. J. Ginseng Res. 2010, 34, 160–167. [Google Scholar] [CrossRef]
- Huang, X.; Liu, Y.; Zhang, Y.; Li, S.-P.; Yue, H.; Chen, C.-B.; Liu, S.-Y. Multicomponent assessment and ginsenoside conversions of Panax quinquefolium L. roots before and after steaming by HPLC-MSn. J. Ginseng Res. 2010, 43, 27–37. [Google Scholar] [CrossRef]
- Cui, C.-H.; Kim, J.-K.; Kim, S.-C.; Im, W.-T. Characterization of a ginsenoside-transforming β-glucosidase from Paenibacillus mucilaginosus and its application for enhanced production of minor ginsenoside F2. PLoS ONE 2014, 9, e85727. [Google Scholar] [CrossRef] [PubMed]
- Park, E.-H.; Kim, Y.-J.; Yamabe, N.; Park, S.-H.; Kim, H.-K.; Jang, H.-J.; Kim, J.H.; Cheon, G.J.; Ham, J.; Kang, K.S. Stereospecific anticancer effects of ginsenoside Rg3 epimers isolated from heat-processed American ginseng on human gastric cancer cell. J. Ginseng Res. 2014, 38, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, M.Z.; Ahn, S.; Kang, S.; Kim, Y.; Veerappan, K.; Yang, D.; Yang, D. Stimulative effect of ginsenosides Rg5:Rk1 on murine osteoblastic MC3T3-E1 cells. Phytother. Res. 2014, 28, 1447–1455. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Si, M.; Wang, Y.; Liu, L.; Zhang, Y.; Zhou, A.; Wei, W. Ginsenoside metabolite compound K exerts anti-inflammatory and analgesic effects via downregulating COX2. Inflammopharmacology 2019, 27, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Schrezenmeir, J.; de Vrese, M. Probiotics, prebiotics, and synbiotics—Approaching a definition. Am. J. Clin. Nutr. 2001, 73, 361s–364s. [Google Scholar] [CrossRef]
- Figueroa-González, I.; Cruz-Guerrero, A.; Quijano, G. The benefits of probiotics on human health. J. Microbial Biochem. Technol. S 2011, 1, 1948–5948. [Google Scholar]
- Parvez, S.; Malik, K.A.; Ah Kang, S.; Kim, H.-Y. Probiotics and their fermented food products are beneficial for health. J. Appl. Microbiol. 2006, 100, 1171–1185. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lee, K.; Hsu, M.; Nau, G.; Mylonakis, E.; Ramratnam, B. Lactobacillus-derived extracellular vesicles enhance host immune responses against vancomycin-resistant enterococci. BMC Microbiol. 2017, 17, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Tkach, M.; Théry, C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell 2016, 164, 1226–1232. [Google Scholar] [CrossRef]
- Sel, A.; Mäger, I.; Breakefield, X.O.; Wood, M.J. Extracellular vesicles: Biology and emerging therapeutic opportunities. Nat. Rev. Drug Discov. 2013, 12, 347–357. [Google Scholar]
- Kim, J.-H.; Jeun, E.-J.; Hong, C.-P.; Kim, S.-H.; Jang, M.S.; Lee, E.-J.; Moon, S.J.; Yun, C.H.; Im, S.-H.; Jeong, S.-G.; et al. Extracellular vesicle–derived protein from Bifidobacterium longum alleviates food allergy through mast cell suppression. J. Allergy Clin. Immunol. 2016, 137, 507–516.e8. [Google Scholar] [CrossRef] [PubMed]
- Behzadi, E.; Mahmoodzadeh Hosseini, H.; Imani Fooladi, A.A. The inhibitory impacts of Lactobacillus rhamnosus GG-derived extracellular vesicles on the growth of hepatic cancer cells. Microb. Pathog. 2017, 110, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Defourny, K.A.Y.; Smid, E.J.; Abee, T. Gram-Positive Bacterial Extracellular Vesicles and Their Impact on Health and Disease. Front. Microbiol. 2018, 9, 1502. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.A.; Choi, H.-I.; Hong, S.W.; Kang, S.; Jegal, H.-Y.; Choi, E.W.; Park, B.-S.; Kim, J.S. Extracellular Vesicles Derived from Kefir Grain Lactobacillus Ameliorate Intestinal Inflammation via Regulation of Proinflammatory Pathway and Tight Junction Integrity. Biomedicines 2020, 8, 522. [Google Scholar] [CrossRef] [PubMed]
- Mo, S.-J.; Nam, B.; Bae, C.-H.; Park, S.-D.; Shim, J.-J.; Lee, J.-L. Characterization of novel Lactobacillus paracasei HY7017 capable of improving physiological properties and immune enhancing effects using red ginseng extract. Fermentation 2021, 7, 238. [Google Scholar] [CrossRef]
- Nielsen, S.S. Total carbohydrate by phenol-sulfuric acid method. In Food Analysis Laboratory Manual; Springer: Cham, Switzerland, 2017; pp. 137–141. ISBN 978-1-4419-1462-0. [Google Scholar]
- Miller, G.L. Use of Dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Kim, H.A.; Kim, S.; Chang, S.H.; Hwang, H.J.; Choi, Y.-N. Anti-arthritic effect of ginsenoside Rb1 on collagen induced arthritis in mice. Int. Immunopharmacol. 2007, 7, 1286–1291. [Google Scholar] [CrossRef]
- Yun, T.-K. Experimental and epidemiological evidence on non-organ specific cancer preventive effect of Korean ginseng and identification of active compounds. Mutat. Res. Mol. Mech. Mutagen. 2003, 523–524, 63–74. [Google Scholar] [CrossRef]
- An, K.S.; Choi, Y.O.; Lee, S.M.; Ryu, H.Y.; Kang, S.J.; Yeon, Y.; Kim, Y.R.; Lee, J.G.; Kim, C.J.; Lee, Y.J.; et al. Ginsenosides Rg5 and Rk1 Enriched Cultured Wild Ginseng Root Extract Bioconversion of Pediococcus pentosaceus HLJG0702: Effect on Scopolamine-Induced Memory Dysfunction in Mice. Nutrients 2019, 11, 1120. [Google Scholar] [CrossRef]
- Jang, W.; Jang, Y.; Kim, N.-H.; Waminal, N.E.; Kim, Y.C.; Lee, J.W.; Yang, T.-J. Genetic diversity among cultivated and wild Panax ginseng populations revealed by high-resolution microsatellite markers. J. Ginseng Res. 2020, 44, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Xavier, R.J.; Podolsky, D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007, 448, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Al-Sadi, R.; Said, H.M.; Ma, T.Y. Lipopolysaccharide causes an increase in intestinal tight junction permeability in vitro and in vivo by inducing enterocyte membrane expression and localization of TLR-4 and CD14. Am. J. Pathol. 2013, 182, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Rahimi, R.; Nikfar, S.; Abdollahi, M. Induction of clinical response and remission of inflammatory bowel disease by use of herbal medicines: A meta-analysis. World J. Gastroenterol. WJG 2013, 19, 5738. [Google Scholar] [CrossRef]
- Nielsen, O.H.; Munck, L.K. Drug Insight: Aminosalicylates for the treatment of IBD. Nat. Clin. Pract. Gastroenterol. Hepatol. 2007, 4, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Magro, F.; Portela, F. Management of inflammatory bowel disease with infliximab and other anti-tumor necrosis factor alpha therapies. BioDrugs 2010, 24, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Pithadia, A.B.; Jain, S. Treatment of inflammatory bowel disease (IBD). Pharmacol. Rep. 2011, 63, 629–642. [Google Scholar] [CrossRef]
- Jakubczyk, D.; Leszczyńska, K.; Górska, S. The effectiveness of probiotics in the treatment of inflammatory bowel disease (IBD)—A critical review. Nutrients 2020, 12, 1973. [Google Scholar] [CrossRef]
- Veerappan, G.R.; Betteridge, J.; Young, P.E. Probiotics for the treatment of inflammatory bowel disease. Curr. Gastroenterol. Rep. 2012, 14, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Gänzle, M.; Kort, R.; Pasin, G.; Pihlanto, A.; et al. Health benefits of fermented foods: Microbiota and beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Michlmayr, H.; Kneifel, W. β-Glucosidase activities of lactic acid bacteria: Mechanisms, impact on fermented food and human health. FEMS Microbiol. Lett. 2014, 352, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Filannino, P.; Di Cagno, R.; Gobbetti, M. Metabolic and functional paths of lactic acid bacteria in plant foods: Get out of the labyrinth. Curr. Opin. Biotechnol. 2018, 49, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.M.; Lee, H.Y.; Lee, Y.M.; Seo, E.Y.; Kim, D.H.; Son, K.-H.; Lee, J.; Cho, D.Y.; Lee, J.H. Comparative assessment of compositional constituents and antioxidant effects in ginseng sprouts (Panax ginseng) through aging and fermentation processes. LWT 2022, 164, 113644. [Google Scholar] [CrossRef]
- Singhania, R.R.; Patel, A.K.; Sukumaran, R.K.; Larroche, C.; Pandey, A. Role and significance of beta-glucosidases in the hydrolysis of cellulose for bioethanol production. Bioresour. Technol. 2013, 127, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Faure, D. The Family-3 glycoside hydrolases: From housekeeping functions to host-microbe interactions. Appl. Environ. Microbiol. 2002, 68, 1485–1490. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Hwang, H.; Lee, J.; Sohn, S.-O.; Lee, S.H.; Jung, M.Y.; Lim, H.I.; Park, H.W.; Lee, J.-H. Evaluation of ginsenoside bioconversion of lactic acid bacteria isolated from kimchi. J. Ginseng Res. 2017, 41, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-K.; Cui, C.-H.; Liu, Q.; Yoon, M.-H.; Kim, S.-C.; Im, W.-T. Mass production of the ginsenoside Rg3(S) through the combinative use of two glycoside hydrolases. Food Chem. 2013, 141, 1369–1377. [Google Scholar] [CrossRef]
- Cheng, L.-Q.; Na, J.R.; Bang, M.H.; Kim, M.K.; Yang, D.-C. Conversion of major ginsenoside Rb1 to 20 (S)-ginsenoside Rg3 by Microbacterium sp. GS514. Phytochemistry 2008, 69, 218–224. [Google Scholar] [CrossRef]
- Ke, Y.; Huang, L.; Song, Y.; Liu, Z.; Liang, L.; Wang, L.; Wang, T. Preparation and pharmacological effects of minor ginsenoside nanoparticles: A review. Front. Pharmacol. 2022, 13, 974274. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Cai, H.; Hu, T.; Lin, L.; Zeng, L.; Wang, H.; Cao, L.; Li, X. Ginsenoside Rg3 treats acute radiation proctitis through the TLR4/MyD88/NF-κB pathway and regulation of intestinal flora. Front. Cell. Infect. Microbiol. 2022, 12, 1028576. [Google Scholar] [CrossRef] [PubMed]
- Maeng, Y.-S.; Maharjan, S.; Kim, J.-H.; Park, J.-H.; Yu, Y.S.; Kim, Y.-M.; Kwon, Y.-G. Rk1, a Ginsenoside, is a new blocker of vascular leakage acting through actin structure remodeling. PLoS ONE 2013, 8, e68659. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Yang, H.; Zhu, C.; Deng, J.; Fan, D. Hypoglycemic Effect of Ginsenoside Rg5 Mediated Partly by Modulating Gut Microbiota Dysbiosis in Diabetic db/db Mice. J. Agric. Food Chem. 2020, 68, 5107–5117. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.; Catchpole, R.; Forterre, P. Extracellular membrane vesicles in the three domains of life and beyond. FEMS Microbiol. Rev. 2019, 43, 273–303. [Google Scholar] [CrossRef] [PubMed]
- Zomer, A.; Vendrig, T.; Hopmans, E.S.; van Eijndhoven, M.; Middeldorp, J.M.; Pegtel, D.M. Exosomes: Fit to deliver small RNA. Commun. Integr. Biol. 2010, 3, 447–450. [Google Scholar] [CrossRef] [PubMed]
- Martínez, G.P.; Giner-Pérez, L.; Castillo-Romero, K.F. Bacterial extracellular vesicles and associated functional proteins in fermented dairy products with Lacticaseibacillus paracasei. Front. Microbiol. 2023, 14, 1165202. [Google Scholar] [CrossRef] [PubMed]
- Odenwald, M.A.; Turner, J.R. Intestinal permeability defects: Is. it time to treat? Clin. Gastroenterol. Hepatol. 2013, 11, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Martel, J.; Chang, S.H.; Ko, Y.F.; Hwang, T.L.; Young, J.D.; Ojcius, D.M. Gut barrier disruption and chronic disease. Trends Endocrinol. Metab. TEM 2022, 33, 247–265. [Google Scholar] [CrossRef]
- Dong, J.; Liang, W.; Wang, T.; Sui, J.; Wang, J.; Deng, Z.; Chen, D. Saponins regulate intestinal inflammation in colon cancer and IBD. Pharmacol. Res. 2019, 144, 66–72. [Google Scholar] [CrossRef]
- Iizuka, M.; Konno, S. Wound healing of intestinal epithelial cells. World J. Gastroenterol. WJG 2011, 17, 2161. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Zhang, S.; Liu, Q.; Huang, C.; Hao, H.; Tan, M.S.; Yu, X.; Lou, C.K.L.; Huang, R.; Zhang, Z.; et al. Milk-derived extracellular vesicles protect intestinal barrier integrity in the gut-liver axis. Sci. Adv. 2023, 9, ade5041. [Google Scholar] [CrossRef] [PubMed]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Salim, S.Y.; Söderholm, J.D. Importance of disrupted intestinal barrier in inflammatory bowel diseases. Inflamm. Bowel Dis. 2011, 17, 362–381. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Fan, D.; Zhou, Z.; Wang, Y.; Huang, X.; Zhang, L.; Wu, D.; Ren, Y.; Lu, F.; Gao, X. Ginsenoside protects intestinal barrier function and improves epithelium injury in sepsis by regulating the miR-30e-5p/FBXO11 axis. Electron. J. Biotechnol. 2023, 66, 67–74. [Google Scholar] [CrossRef]
- Seong, M.A.; Woo, J.K.; Kang, J.-H.; Jang, Y.S.; Choi, S.; Jang, Y.S.; Lee, T.H.; Jung, K.H.; Kang, D.K.; Hurh, B.S.; et al. Oral administration of fermented wild ginseng ameliorates DSS-induced acute colitis by inhibiting NF-κB signaling and protects intestinal epithelial barrier. BMB Rep. 2015, 48, 419. [Google Scholar] [CrossRef]
- Ratajczak, J.; Wysoczynski, M.; Hayek, F.; Janowskawieczorek, A.; Ratajczak, M.Z. Membrane-derived microvesicles: Important and underappreciated mediators of cell-to-cell communication. Leukemia 2006, 20, 1487–1495. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).