Biochemical and Biorefinery Platform for Second-Generation Bioethanol: Fermentative Strategies and Microorganisms
Abstract
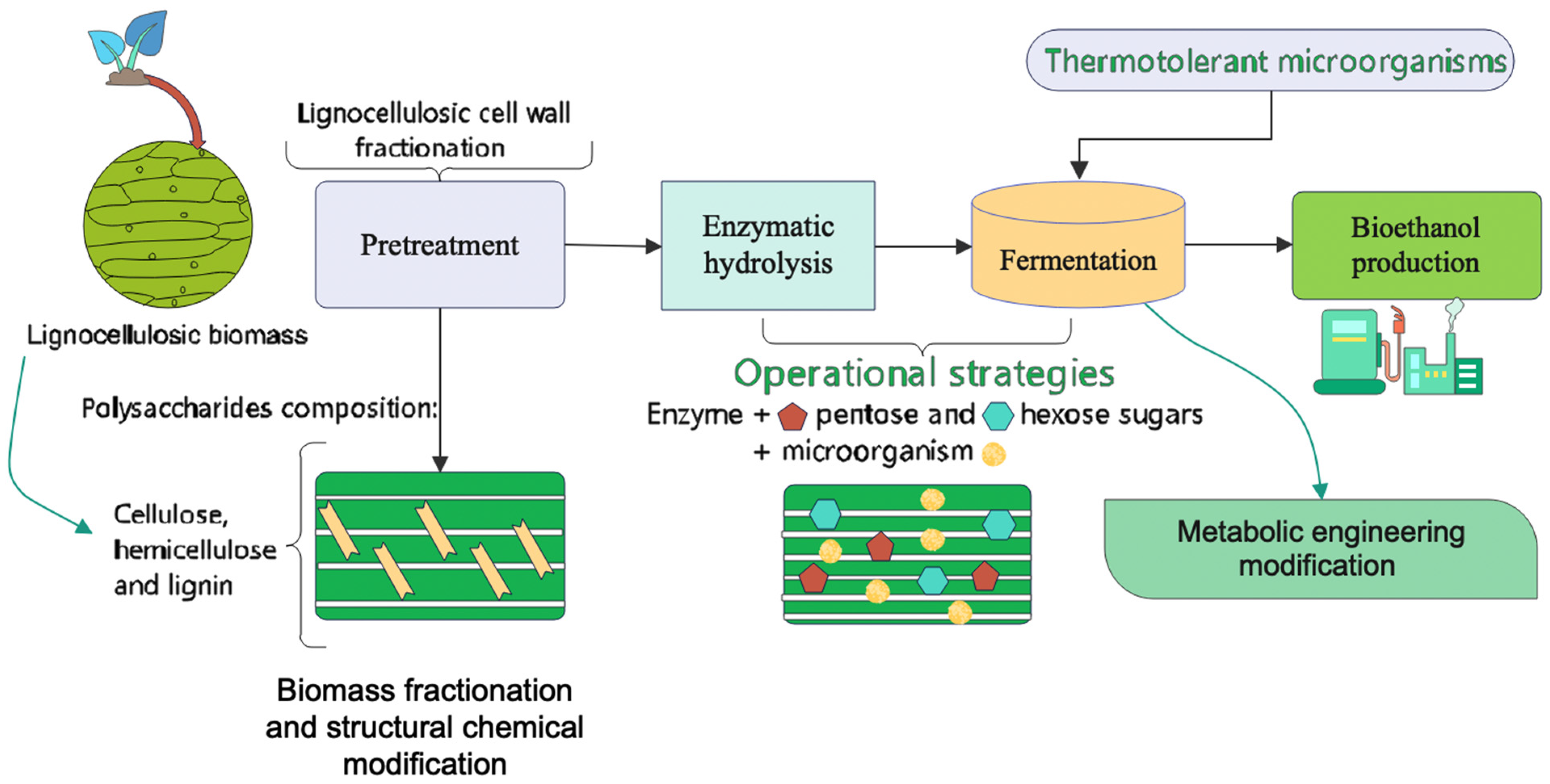
1. Introduction
2. Bioethanol Production
3. Ethanol-Producing Microorganisms
3.1. Yeast
Characteristics of Yeasts
3.2. Challenges to Overcome for the Fermenting Microorganism (High Temperature, Inhibitors, Mix of Sugars)
3.2.1. Thermotolerance Yeast
3.2.2. Inhibitors
3.2.3. Mix of Sugars
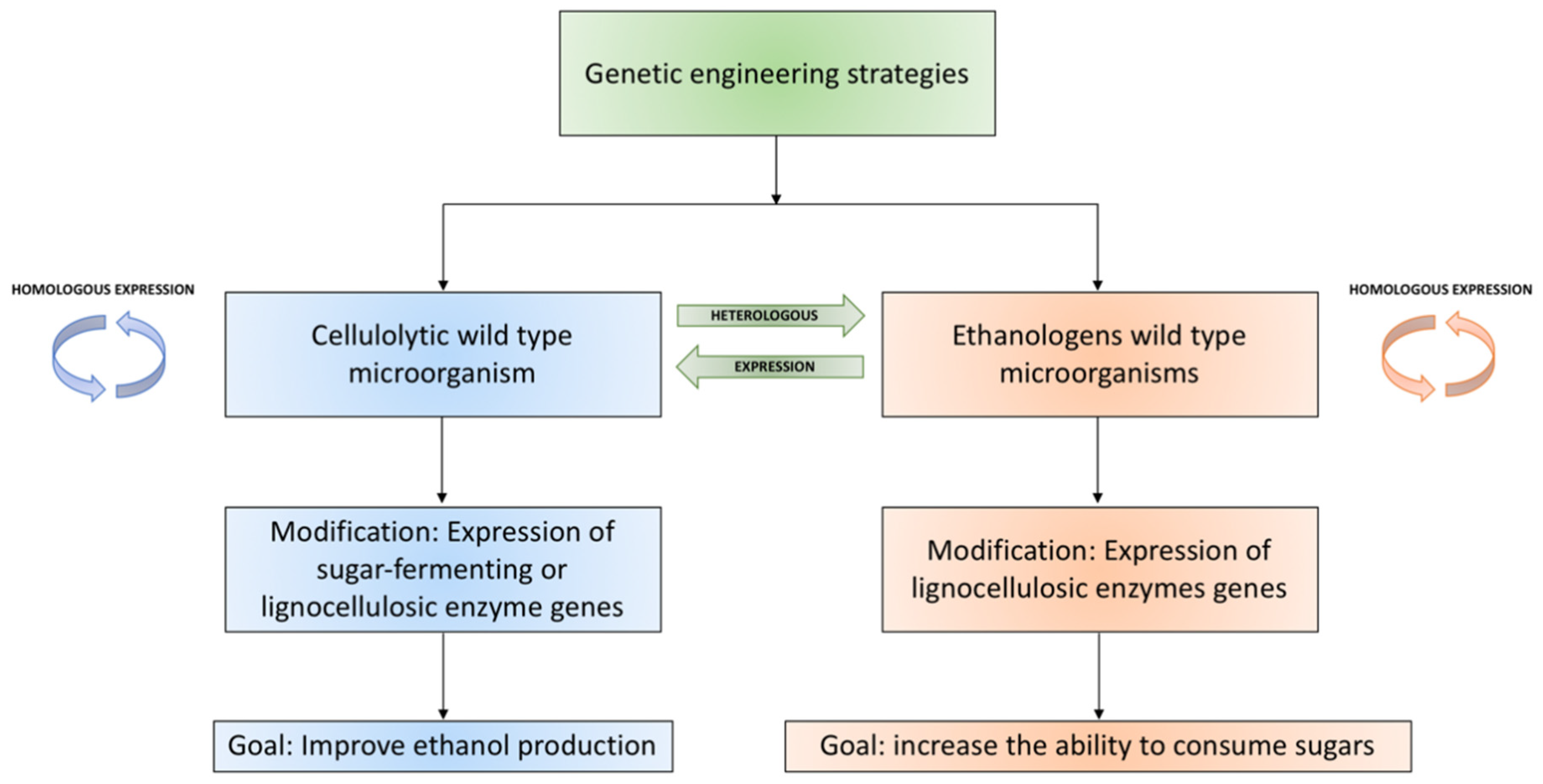
3.3. Genetically Modified Microorganisms
3.3.1. Yeast: S. cerevisiae
| Metabolic Engineering Strategies of Saccharomyces cerevisiae | |||
|---|---|---|---|
| Aim | Strategy | Ref. | |
| Improving xylose fermentation | To decrease xylitol formation | Incorporation of the NADH oxidase from Lactococcus lactis in the industrial polyploidy strain S. cerevisiae JHS200 | [46] |
| Structure-guided mutagenesis and directed evolution to provide a compilation of variants of XR and XDH with altered co-factor preferences | [47] | ||
| To increase xylose uptake/transport | Directed evolution of a glucose/xylose co-transporter from Candida intermedia significantly increases the xylose transport capacity in S. cerevisiae | [47,48] | |
| Mutation in a general co-repressor of CYC8 (Y353C) to modify hexose transporter expression and improve xylose metabolism in S. cerevisiae | [49] | ||
| Discovery of new xylose transporters, such as the ones identified in Aspergillus niger and T. reesei | [50] | ||
| Evolutionary engineering in engineered S. cerevisiae harboring genes for XR, XDH, and XK resulted in a mutation of chimeric transporter Hxt36p with an enhanced xylose uptake rate | [41] | ||
| Evolutionary engineering to obtain mutant HXT7(F79S), with a few single nucleotide polymorphisms showing improved xylose uptake rates | [21,51] | ||
| Incorporation of xylose transporter genes AT5G17010 and AT5G59250 from Arabidopsis thaliana to improve xylose transporter efficiency | [50,52] | ||
| Improving robustness toward lignocellulosic inhibitors | Modulation of spermidine (SPD) content by altered expression levels of the genes in the SPD biosynthetic pathway | [53,54] | |
| Overexpression of WHI2 in engineered yeast significantly improved glucose and xylose fermentation under acetic acid stress | [55,56] | ||
| Disruption of the SIZ1 gene in S. cerevisiae increases furfural tolerance | [57] | ||
| Obtaining hydrolyzing S. cerevisiae strains | Application of different yeast strains displaying a scaffoldin (mini CipA) and containing three cohesin domains, endoglucanase (CelA), exoglucanase (CBHII), or β-glucosidase (BGLI) | [58,59] | |
| Expression of two endoglucanases from T. reseei (Cel7B and Cel5A) and cellobiohydrolases from T. reseei, Aspergillus niger, and Phaenorachaete chrysosporium in S. cerevisiae Y294 | [60,61] | ||
| Multiple copy integration of cellulase genes into the delta (δ) repeat sites of transposable elements (Tn) in the S. cerevisiae chromosome | [62] | ||
| Use of constitutive promoters, such as TEF1 and PGK1, to significantly increase cellulase expression in S. cerevisiae | [63] | ||
| Assembly of trifunctional mini cellulosomes containing a mini-scaffoldinin S. cerevisiae | [64] | ||
3.3.2. Non-Conventional Yeast Species
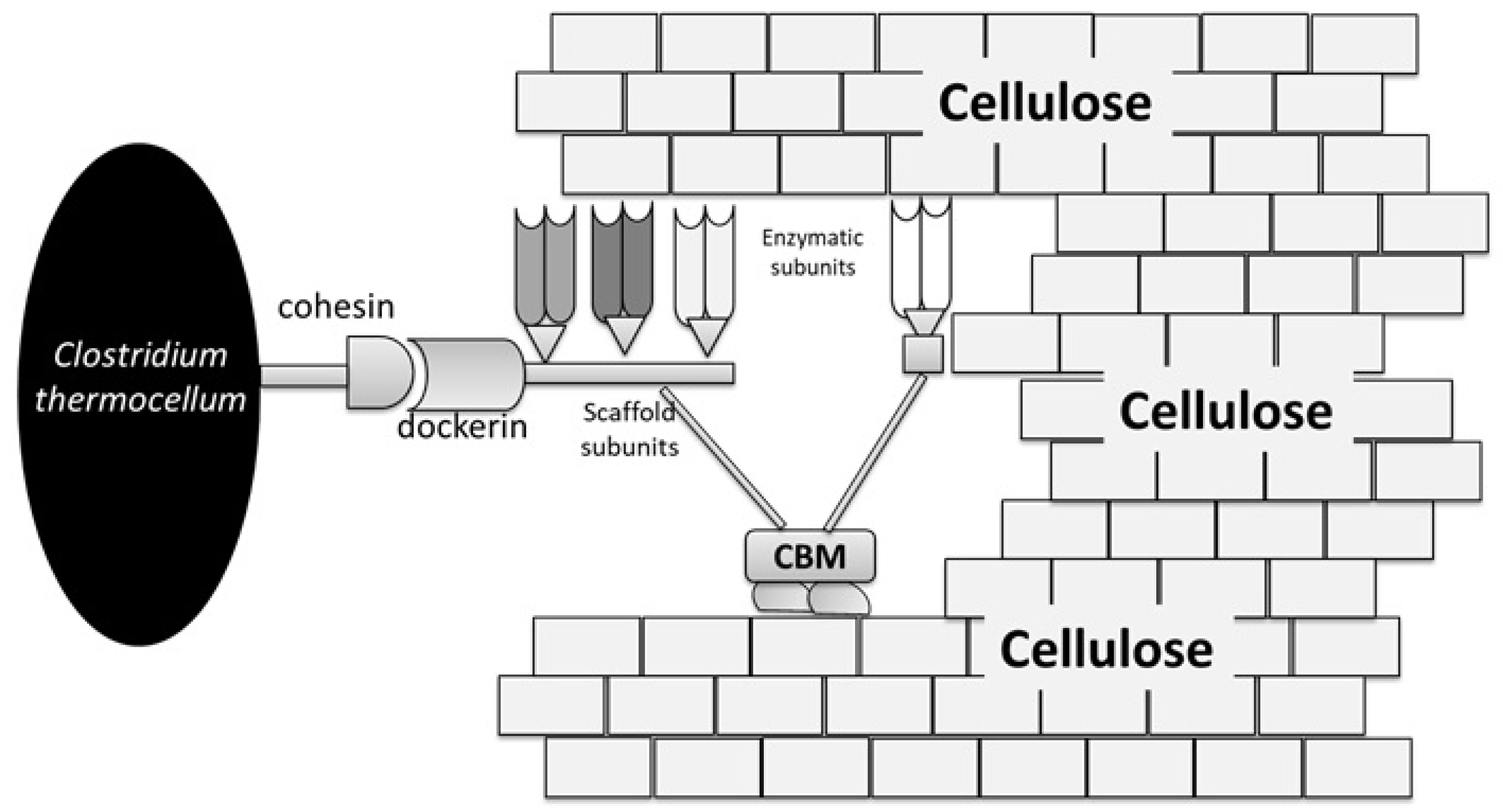
3.3.3. Bacteria
3.3.4. Fungi
| Enzymes | Microorganism (Source of Gene) | Host Microorganism | References |
|---|---|---|---|
| β-glucosidase | Penicillium decumbens | Trichoderma reesei | [90] |
| Endoglucanases | Trametes versicolor | Pichia pastoris | [91] |
| Xylanase | Orpinomyces sp. | Hypocrea jecorina | [92] |
| Endo-1,4-xylanase | Schizophyllum commune | Pichia pastoris | [93] |
| Lacasse | Pycnoporus cinnabarinus | Aspergillus niger | [94] |
| Lacasse | Trametes versicolor | Pichia methanolica | [95] |
| Lacasse | Trametes sp. | Trichoderma reesei | [95,96] |
| Peroxidase | Geotrichum candidum | Aspergillus oryzae | [83] |
| Manganese peroxidase, Lignin peroxidase, Versatile peroxidase | Trametes versicolor | Phanerochaete chrysosporium | [97] |
| Lignin peroxidase | Trametes versicolor | Phanerochaete chrysosporium | [98,99] |
| Manganese peroxidase | Pleurotus eryngii | Phanerochaete chrysosporium | [82] |
| Cellulases | Pyrococcus sp. | Talaromyces cellulolyticus | [96,100] |
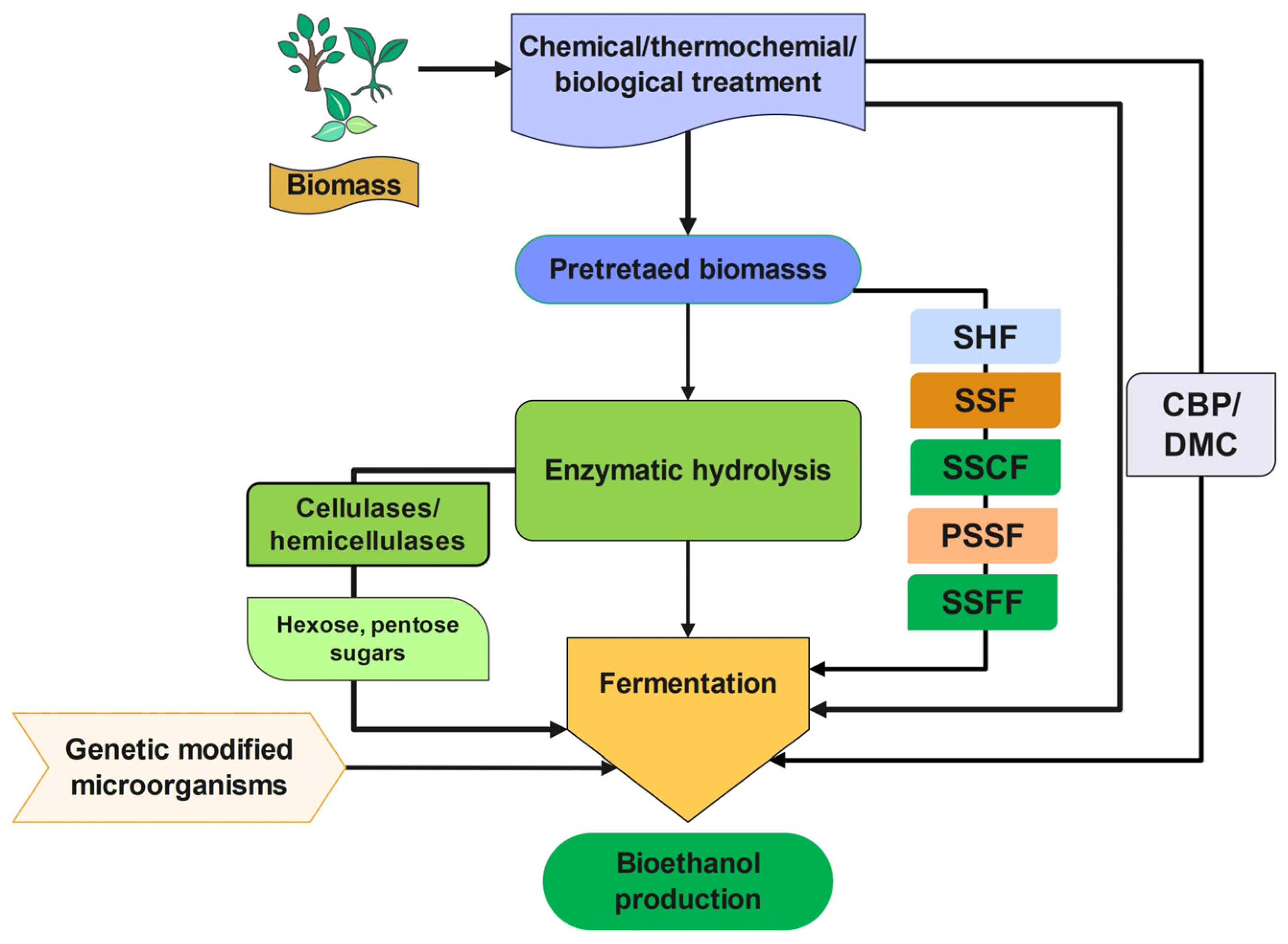
4. Operative Strategies of Bioethanol Production
| Process | Substrate | Hydrolytic Enzyme | Enzyme Loading | Fermenting Strain | Temperature (°C) | Reaction Time (h) | Ethanol Concentration (g/L) | Ethanol Yield (g/g-Sugar) | References |
|---|---|---|---|---|---|---|---|---|---|
| SHF | Steam-exploded SCB | Cellulase—Celluclast derived from Trichoderma reesei; β-Glucosidase Novozyme 188 | Cellulase—10 FPU/g cellulose, β-glucosidase 5% of cellulase | Saccharomyces cerevisiae UFPEDA 1238 | Hydrolysis—50 Fermentation—34 | 120 | 23.38 | 0.39 | [104,105,106] |
| Steam-exploded palm kernel cake | Galactomannan, Driselase from Basidiomycetes sp.; β mannanase from T. reesei; Cellulase—Cellic C Tec2 | Galactomannan—26.5; Driselase—53.1 U; Cellic CTec2—10.4 FPU/g | Geobacillus thermoglucosidasius TM242 | Hydrolysis—50 Fermentation—60 | 48 | 9.9 | 0.47 | [105] | |
| Cassava bagasse | Amylolytic Crude extract from Rhizopusoligo sporus | 1.5 U/mL | S. cerevisiae | Hydrolysis—50 Fermentation —32 | 24 | 39.5 | 0.45 | [107] | |
| Food waste | Cellulases derived from Aspergillus oryzae | Glucoamylas—13.5 U; Amylase—0.4 U; Cellulase—1 U | Zymomonas mobilis | Hydrolysis—50 Fermentation—30 | 54 | 71.8 | 0.50 | [108] | |
| KOH-pretreated corn cob | Endoxylanase derived from Streptomyces thermovulgaris TISTR1948; Commercial cellulase iKnowZyMe AC | 22.04 FPU/g corn cob | Candida glabrata KY618709 | Hydrolysis—50 Fermentation—40 | 168 | 21.92 | 0.37 | [109] | |
| SSF | NaOH-pretreated SCB | Cellulase | 20 FPU | S. cerevisiae | 30 | 120 | 11.810 | - | [110] |
| H2SO4-pretreated Arundodonax | Cellulase—CellicCTec2 | 0.6% (v/v) | Escherichia coli MS04 | 40 | 96 | 25 | - | [111] | |
| Steam-exploded triticale straw | Spezyme® CP | 15 FPU/g cellulose | S. cerevisiae | 37 | 144 | 29.6 | 0.41 | [112] | |
| Hydrothermolysis SCB | Cellulase—Accellerase 1500 | 30 FPU g/g cellulose | Kluyveromyces marxianus IMB3 | 45 | 72 | 29.2 | 0.30 | [113] | |
| Microwave NaOH-H2SO4-pretreated rice straw | Cellulase derived from Bacillus subtilis NA15 | CMCase—1.46 U/mL FPase—0.43 U/mL β-glucosidase—0.12 U/mL | S. cerevisiae | 30 | 48 | 25.2 | 0.38 | [114] | |
| KOH-pretreated corn cob | Xylanase derived from S. thermovulgaris TISTR1948; Cellulase—iKnowZyMe AC | 22.04 FPU/g corn cob | C. glabrata KY618709 | 40 | 72 | 31.32 | 0.27 | [109] | |
| SSSF | Water-microwave-pretreated oil palm fronts | Cellulase derived from A. niger | 70 FPU/g db | S. cerevisiae | Hydrolysis—50 Fermentation—37 | 120 | 4.313 | 0.32 | [115,116] |
| Soda lignin obtained from the spent liquor of the soda pulping of cedar wood chips | Cellulase—Genencor GC220 | 10 FPU/g pulp | S. cerevisiae | Hydrolysis—50 Fermentation—38 | 156 | 49.4 | 0.33 | [117] | |
| Liquid hot water-pretreated reed | Cellulase derived from T. longbrachiatum | 30–40 FPU/g db | S. cerevisiae | Hydrolysis—50 Fermentation—36 | 78 | 39.4 | - | [118] | |
| SSCF | Wood dust-pretreated | Cellulases/hemicellulases derived from A. niger and T. reesei | NR | Z. mobilis BCRC 10809 | Hydrolysis—50 Fermentation—30 | 14 | 0.51 | 0.18 | [119] |
| Hydrothermally pretreated wheat straw | Crude extract derived from Fusarium oxysporum; Cellulase—Cellic Ctec2 | Crude extract—0.7 FPU/g dm; Cellic CTec2—7 FPU/g dm | S. cerevisiae | 30 | 72 | 62 | 0.44 | [120] | |
| Hydrothermally pretreated corn flour and corn stover | Glucoamylase—Spirizyme® Fuel; Cellulolase—Accellerase 1500 | Spirizyme—75 FPU/g cellulose Accellerase 1500—5FPU/g cellulose | S. cerevisiae | 38 | 72 | 130.2 | - | [103] | |
| Steam-exploded corn stover | Celluclast, β-glucosidase— Novozyme 188 | Celluclast—15 FPU/g dm β-glucosidase—25 IU/g db | S. cerevisiae TMB3400 | 35 | 96 | 17.2 | 0.33 | [121] | |
| H2O2-pretreated corn stover | Trichoderma reesei extracted cellulase | 1% (v/v) | S. cerevisiae; C. tropicalis | 32 | 144 | 109.24 | 0.48 | [38] | |
| Ethylenedi-amine-pretreated corn stover | Cellic Ctec2; Cellic Htec2 | 10 mg/g glucan | S. cerevisiae SyBE005 | Prehydrolysis—50 Fermentation—34 | 108 | 37.8 | 0.38 | [122] | |
| CBP | NaOH-pretreated SCB | - | - | Phlebia sp. MG—60 | 28 | 240 | 4.5 | 0.33 | [45] |
| Waste newspaper | Crude extract of Phlebia sp. MG—60 | NR | Phlebia sp. MG—60 | 30 | 216 | 4.2 | 0.20 | [123] | |
| H2O2-pretreated rice bran | Cellic CTec2 | 6% w/w | S. cerevisiae M2n [TLG1-SFA1] | 30 | 60 | 42.06 | 0.47 | [124] | |
| Hydrothermally pretreated wheat straw | Crude extract derived from F. oxysporum F3; Cellic Ctec2 | 1.23 FPU/g dm; Cellic CTec 2—7 FPU/g dm | S. cerevisiae | 30 | 72 | 58 | 0.41 | [125] |
4.1. Separate Hydrolysis and Fermentation (SHF)
4.2. Simultaneous Saccharification and Fermentation (SSF)
4.3. Simultaneous Saccharification and Co-Fermentation (SSCF)
4.4. Semi or Pre-Simultaneous Saccharification and Fermentation (SSSF or PSSF)
4.5. Simultaneous Saccharification, Filtration, and Fermentation (SSFF)
4.6. Direct Microbial Conversion/Consolidated Bioprocessing (DMC/CBP)
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ndubuisi, I.A.; Amadi, C.O.; Nwagu, T.N.; Murata, Y.; Ogbonna, J.C. Novel Thermotolerant Yeast Suitable for Industrial Bioethanol Production. Biofuels 2024, 15, 545–554. [Google Scholar] [CrossRef]
- López-Sandin, I.; Gutiérrez-Soto, G.; Gutiérrez-Díez, A.; Medina-Herrera, N.; Gutiérrez-Castorena, E.; Zavala-García, F. Evaluation of the Use of Energy in the Production of Sweet Sorghum (Sorghum bicolor (L.) Moench) under Different Production Systems. Energies 2019, 12, 1713. [Google Scholar] [CrossRef]
- Mankar, A.R.; Pandey, A.; Modak, A.; Pant, K.K. Pretreatment of lignocellulosic biomass: A review on recent advances. Bioresour. Technol. 2021, 334, 125235. [Google Scholar] [CrossRef] [PubMed]
- González-Gloria, K.D.; Rodríguez-Jasso, R.M.; Rosero-Chasoy, G.; Shiva; Kostas, E.T.; Aparicio, E.; Sanchez, A.; López-Sandin, I.; Ruiz, H.A. Scale-up of Hydrothermal Processing: Liquid Hot Water and Pilot-Scale Tubular Steam Explosion Batch Reactor for Bioethanol Production Using Macroalgae Sargassum spp. Biomass. Bioresour. Technol. 2023, 369, 128448. [Google Scholar] [CrossRef] [PubMed]
- Zabed, H.; Sahu, J.N.; Suely, A.; Boyce, A.N.; Faruq, G. Bioethanol Production from Renewable Sources: Current Perspectives and Technological Progress. Renew. Sustain. Energy Rev. 2017, 71, 475–501. [Google Scholar] [CrossRef]
- Ubando, A.T.; Felix, C.B.; Chen, W.H. Biorefineries in Circular Bioeconomy: A Comprehensive Review. Bioresour. Technol. 2020, 299, 122585. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, B.; Luo, L.; Zhang, F.; Yi, Y.; Shan, Y.; Liu, B.; Zhou, Y.; Wang, X.; Lü, X. A Review on Recycling Techniques for Bioethanol Production from Lignocellulosic Biomass. Renew. Sustain. Energy Rev. 2021, 149, 111370. [Google Scholar] [CrossRef]
- RFA. Industry Statistics. Available online: http://www.ethanolrfa.org/resources/industry/statistics (accessed on 30 April 2023).
- Tse, T.J.; Wiens, D.J.; Reaney, M.J.T. Production of Bioethanol—A Review of Factors Affecting Ethanol Yield. Fermentation 2021, 7, 268. [Google Scholar] [CrossRef]
- Or-Chen, D.; Gerchman, Y.; Mamane, H.; Peretz, R. Paper-Mill Wastes for Bioethanol Production in Relation to Circular Economy Concepts: A Review. Appl. Sci. 2024, 14, 1081. [Google Scholar] [CrossRef]
- RFA Ethanol Producers Magazine. Available online: https://ethanolproducer.com/plants/list/ethanol (accessed on 8 May 2024).
- Joshi, G.; Pandey, J.K.; Rana, S.; Rawat, D.S. Challenges and Opportunities for the Application of Biofuel. Renew. Sustain. Energy Rev. 2017, 79, 850–866. [Google Scholar] [CrossRef]
- Carrillo-Nieves, D.; Rostro Alanís, M.J.; de la Cruz Quiroz, R.; Ruiz, H.A.; Iqbal, H.M.N.; Parra-Saldívar, R. Current Status and Future Trends of Bioethanol Production from Agro-Industrial Wastes in Mexico. Renew. Sustain. Energy Rev. 2019, 102, 63–74. [Google Scholar] [CrossRef]
- Barrera, I.; Amezcua-Allieri, M.A.; Estupiñan, L.; Martínez, T.; Aburto, J. Technical and Economical Evaluation of Bioethanol Production from Lignocellulosic Residues in Mexico: Case of Sugarcane and Blue Agave Bagasses. Chem. Eng. Res. Des. 2016, 107, 91–101. [Google Scholar] [CrossRef]
- Quintero, J.A.; Moncada, J.; Cardona, C.A. Techno-economic analysis of bioethanol production from lignocellulosic residues in Colombia: A process simulation approach. Bioresour. Technol. 2013, 139, 300–307. [Google Scholar] [CrossRef]
- Hasanly, A.; Khajeh Talkhoncheh, M.; Karimi Alavijeh, M. Techno-Economic Assessment of Bioethanol Production from Wheat Straw: A Case Study of Iran. Clean Technol. Environ. Policy 2017, 20, 357–377. [Google Scholar] [CrossRef]
- Souza, M.F.; Devriendt, N.; Willems, B.; Guisson, R.; Biswas, J.K.; Meers, E. Techno-Economic Feasibility of Extrusion as a Pretreatment Step for Biogas Production from Grass. Bioenergy Res. 2021, 15, 1232–1239. [Google Scholar] [CrossRef]
- Sanchez, A.; Sevilla-Güitrón, V.; Magaña, G.; Gutierrez, L. Parametric Analysis of Total Costs and Energy Efficiency of 2G Enzymatic Ethanol Production. Fuel 2013, 113, 165–179. [Google Scholar] [CrossRef]
- Santos, C.I.; Silva, C.C.; Mussatto, S.I.; Osseweijer, P.; van der Wielen, L.A.M.; Posada, J.A. Integrated 1st and 2nd Generation Sugarcane Bio-Refinery for Jet Fuel Production in Brazil: Techno-Economic and Greenhouse Gas Emissions Assessment. Renew. Energy 2018, 129, 733–747. [Google Scholar] [CrossRef]
- Ruiz, H.A.; Sganzerla, W.G.; Larnaudie, V.; Veersma, R.J.; van Erven, G.; Shiva; Ríos-González, L.J.; Rodríguez-Jasso, R.M.; Rosero-Chasoy, G.; Ferrari, M.D.; et al. Advances in Process Design, Techno-Economic Assessment and Environmental Aspects for Hydrothermal Pretreatment in the Fractionation of Biomass under Biorefinery Concept. Bioresour. Technol. 2023, 369, 128469. [Google Scholar] [CrossRef]
- Topaloğlu, A.; Esen, Ö.; Turanlı-Yıldız, B.; Arslan, M.; Çakar, Z.P. From Saccharomyces cerevisiae to Ethanol: Unlocking the Power of Evolutionary Engineering in Metabolic Engineering Applications. J. Fungi 2023, 9, 984. [Google Scholar] [CrossRef]
- Robak, K.; Balcerek, M. Current state-of-the-art in ethanol production from lignocellulosic feedstocks. Microbiol. Res. 2020, 240, 126534. [Google Scholar] [CrossRef]
- Jacobus, A.P.; Gross, J.; Evans, J.H.; Ceccato-Antonini, S.R.; Gombert, A.K. Saccharomyces cerevisiae Strains Used Industrially for Bioethanol Production. Essays Biochem. 2021, 65, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Eardley, J.; Timson, D.J. Yeast Cellular Stress: Impacts on Bioethanol Production. Fermentation 2020, 6, 109. [Google Scholar] [CrossRef]
- Stewart, G.G. Harvesting and Cropping Yeast: Flocculation and Centrifugation. In Brewing and Distilling Yeasts; Springer International Publishing: Cham, Switzerland, 2017; pp. 259–308. ISBN 978-3-319-69126-8. [Google Scholar]
- Mehta, D.V.; Curtis, S.J.; Rudolph, A.B.; Mary, C.S.; Goodrich, R.; Schneider, K.R.; MacIntosh, A.J. A Mini Review: The History of Yeast Flocculation with an Emphasis on Measurement Techniques. J. Am. Soc. Brew. Chem. 2021, 79, 333–339. [Google Scholar] [CrossRef]
- dos Santos, C.O.; Silva, M.C.S.; Castiglioni, G.L. Industrial Yeast Strains Competence in Mixed Culture with Wild Flocculent Yeast. Biocatal. Agric. Biotechnol. 2021, 36, 102144. [Google Scholar] [CrossRef]
- Mohd Azhar, S.H.; Abdulla, R.; Jambo, S.A.; Marbawi, H.; Gansau, J.A.; Mohd Faik, A.A.; Rodrigues, K.F. Yeasts in Sustainable Bioethanol Production: A Review. Biochem. Biophys. Rep. 2017, 10, 52–61. [Google Scholar] [CrossRef]
- Choudhary, J.; Singh, S.; Nain, L. Thermotolerant Fermenting Yeasts for Simultaneous Saccharification Fermentation of Lignocellulosic Biomass. Electron. J. Biotechnol. 2016, 21, 82–92. [Google Scholar] [CrossRef]
- Li, Y.-C.; Gou, Z.-X.; Zhang, Y.; Xia, Z.-Y.; Tang, Y.-Q.; Kida, K. Inhibitor Tolerance of a Recombinant Flocculating Industrial Saccharomyces cerevisiae Strain during Glucose and Xylose Co-Fermentation. Braz. J. Microbiol. 2017, 48, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, L.J.; Martín, C. Pretreatment of Lignocellulose: Formation of Inhibitory by-Products and Strategies for Minimizing Their Effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, L.J.; Alriksson, B.; Nilvebrant, N.-O. Bioconversion of Lignocellulose: Inhibitors and Detoxification. Biotechnol. Biofuels 2013, 6, 16. [Google Scholar] [CrossRef]
- Marulanda, V.A.; Gutierrez, C.D.B.; Alzate, C.A.C. Thermochemical, Biological, Biochemical, and Hybrid Conversion Methods of Bio-Derived Molecules into Renewable Fuels. In Advanced Bioprocessing for Alternative Fuels, Biobased Chemicals, and Bioproducts; Elsevier: Amsterdam, The Netherlands, 2019; pp. 59–81. [Google Scholar]
- Vanmarcke, G.; Demeke, M.M.; Foulquié-Moreno, M.R.; Thevelein, J.M. Identification of the Major Fermentation Inhibitors of Recombinant 2G Yeasts in Diverse Lignocellulose Hydrolysates. Biotechnol. Biofuels 2021, 14, 92. [Google Scholar] [CrossRef]
- Sjulander, N.; Kikas, T. Origin, Impact and Control of Lignocellulosic Inhibitors in Bioethanol Production—A Review. Energies 2020, 13, 4751. [Google Scholar] [CrossRef]
- Soares, L.B.; da Silveira, J.M.; Biazi, L.E.; Longo, L.; de Oliveira, D.; Furigo Júnior, A.; Ienczak, J.L. An Overview on Fermentation Strategies to Overcome Lignocellulosic Inhibitors in Second-Generation Ethanol Production Using Cell Immobilization. Crit. Rev. Biotechnol. 2023, 43, 1150–1171. [Google Scholar] [CrossRef] [PubMed]
- Cámara, E.; Olsson, L.; Zrimec, J.; Zelezniak, A.; Geijer, C.; Nygård, Y. Data Mining of Saccharomyces cerevisiae Mutants Engineered for Increased Tolerance towards Inhibitors in Lignocellulosic Hydrolysates. Biotechnol. Adv. 2022, 57, 107947. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, Z.; Wang, J.; Fan, Y.; Shi, W.; Liu, X.; Shun, Q. Simultaneous Saccharification and Co-Fermentation of Corn Stover Pretreated by H2O2 Oxidative Degradation for Ethanol Production. Energy 2019, 168, 946–952. [Google Scholar] [CrossRef]
- Jansen, M.L.A.; Bracher, J.M.; Papapetridis, I.; Verhoeven, M.D.; de Bruijn, H.; de Waal, P.P.; van Maris, A.J.A.; Klaassen, P.; Pronk, J.T. Saccharomyces cerevisiae Strains for Second-Generation Ethanol Production: From Academic Exploration to Industrial Implementation. FEMS Yeast Res. 2017, 17, fox044. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Peng, B.; Huang, S.; Geng, A. Recombinant Xylose-Fermenting Yeast Construction for the Co-Production of Ethanol and Cis, Cis-Muconic Acid from Lignocellulosic Biomass. Bioresour. Technol. Rep. 2020, 9, 100395. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Nielsen, J. Harnessing Xylose Pathways for Biofuels Production. Curr. Opin. Biotechnol. 2019, 57, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, L.E.; Albuini, F.M.; Castro, A.G.; Campos, V.J.; de Souza, G.B.; Mendonça, J.G.P.; Rosa, C.A.; Mendes, T.A.O.; Santana, M.F.; da Silveira, W.B.; et al. Influence of Glucose on Xylose Metabolization by Spathaspora passalidarum. Fungal Genet. Biol. 2021, 157, 103624. [Google Scholar] [CrossRef] [PubMed]
- Kadeba, A.; Wilgers, D.J. Enhanced Mutation through Exposure to EMS Affects the Evolution of Ethanol Tolerance in Saccharomyces cerevisiae. BIOS 2020, 91, 106–110. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, X.; Liu, W.; Xu, H.; Cao, Y. Consolidated Bioprocess for Bioethanol Production from Lignocellulosic Biomass Using Clostridium thermocellum DSM 1237. Bioresources 2020, 15, 8355–8368. [Google Scholar] [CrossRef]
- Lee, Y.-G.; Jin, Y.-S.; Cha, Y.-L.; Seo, J.-H. Bioethanol Production from Cellulosic Hydrolysates by Engineered Industrial Saccharomyces cerevisiae. Bioresour. Technol. 2017, 228, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Geijer, C.; Faria-Oliveira, F.; Moreno, A.D.; Stenberg, S.; Mazurkewich, S.; Olsson, L. Genomic and Transcriptomic Analysis of Candida Intermedia Reveals the Genetic Determinants for Its Xylose-Converting Capacity. Biotechnol. Biofuels 2020, 13, 48. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.D.; Carbone, A.; Pavone, R.; Olsson, L.; Geijer, C. Evolutionary Engineered Candida Intermedia Exhibits Improved Xylose Utilization and Robustness to Lignocellulose-Derived Inhibitors and Ethanol. Appl. Microbiol. Biotechnol. 2019, 103, 1405–1416. [Google Scholar] [CrossRef]
- Nijland, J.G.; Shin, H.Y.; Boender, L.G.M.; de Waal, P.P.; Klaassen, P.; Driessen, A.J.M. Improved Xylose Metabolism by a CYC8 Mutant of Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2017, 83, AEM-00095. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Shen, Y.; Gu, L.; Wang, Z.; Su, N.; Niu, K.; Guo, W.; Hou, S.; Bao, X.; Tian, C.; et al. Identification and Characterization of an Efficient d-Xylose Transporter in Saccharomyces cerevisiae. J. Agric. Food Chem. 2020, 68, 2702–2710. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.K.; Behera, S.; Arora, R.; Kumar, S.; Sani, R.K. Xylose Transport in Yeast for Lignocellulosic Ethanol Production: Current Status. J. Biosci. Bioeng. 2018, 125, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Vargas, B.d.O.; dos Santos, J.R.; Pereira, G.A.G.; de Mello, F.d.S.B. An Atlas of Rational Genetic Engineering Strategies for Improved Xylose Metabolism in Saccharomyces cerevisiae. PeerJ 2023, 11, e16340. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Hu, Q.; Pu, Q.; Chen, M.; Zhu, X.; Gao, C.; Zhou, G.; Liu, L.; Wang, Z.; Yang, J.; et al. Spermidine Enhanced Free Polyamine Levels and Expression of Polyamine Biosynthesis Enzyme Gene in Rice Spikelets under Heat Tolerance before Heading. Sci. Rep. 2020, 10, 8976. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-K.; Jo, J.-H.; Jin, Y.-S.; Seo, J.-H. Enhanced Ethanol Fermentation by Engineered Saccharomyces cerevisiae Strains with High Spermidine Contents. Bioprocess Biosyst. Eng. 2017, 40, 683–691. [Google Scholar] [CrossRef]
- Chen, Y.; Stabryla, L.; Wei, N. Improved Acetic Acid Resistance in Saccharomyces cerevisiae by Overexpression of the WHI2 Gene Identified through Inverse Metabolic Engineering. Appl. Environ. Microbiol. 2016, 82, 2156–2166. [Google Scholar] [CrossRef]
- Cunha, J.T.; Costa, C.E.; Ferraz, L.; Romaní, A.; Johansson, B.; Sá-Correia, I.; Domingues, L. HAA1 and PRS3 Overexpression Boosts Yeast Tolerance towards Acetic Acid Improving Xylose or Glucose Consumption: Unravelling the Underlying Mechanisms. Appl. Microbiol. Biotechnol. 2018, 102, 4589–4600. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; HamediRad, M.; Xue, P.; Xiao, H.; Tasan, I.; Chao, R.; Liang, J.; Zhao, H. Genome-Scale Engineering of Saccharomyces cerevisiae with Single-Nucleotide Precision. Nat. Biotechnol. 2018, 36, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Zhu, M. Reconstitution of Cellulosome: Research Progress and Its Application in Biorefinery. Biotechnol. Appl. Biochem. 2019, 66, 720–730. [Google Scholar] [CrossRef]
- Kim, S.; Baek, S.-H.; Lee, K.; Hahn, J.-S. Cellulosic Ethanol Production Using a Yeast Consortium Displaying a Minicellulosome and β-Glucosidase. Microb. Cell Factories 2013, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Du Plessis, L.; Rose, S.H.; van Zyl, W.H. Exploring Improved Endoglucanase Expression in Saccharomyces cerevisiae Strains. Appl. Microbiol. Biotechnol. 2010, 86, 1503–1511. [Google Scholar] [CrossRef] [PubMed]
- Den Haan, R.; Mcbride, J.E.; La Grange, D.C.; Lynd, L.R.; Van Zyl, W.H. Functional Expression of Cellobiohydrolases in Saccharomyces cerevisiae towards One-Step Conversion of Cellulose to Ethanol. Enzyme Microb. Technol. 2007, 40, 1291–1299. [Google Scholar] [CrossRef]
- Mei, Y.-Z.; Zhu, Y.-L.; Huang, P.-W.; Yang, Q.; Dai, C.-C. Strategies for Gene Disruption and Expression in Filamentous Fungi. Appl. Microbiol. Biotechnol. 2019, 103, 6041–6059. [Google Scholar] [CrossRef] [PubMed]
- Adnan, M.; Ma, X.; Olsson, S.; Wang, J.; Liu, G. Promoter Regulation and Genetic Engineering Strategies for Enhanced Cellulase Expression in Trichoderma Reesei. Microbiol. Res. 2022, 259, 127011. [Google Scholar] [CrossRef]
- Tsai, S.-L.; Sun, Q.; Chen, W. Advances in Consolidated Bioprocessing Using Synthetic Cellulosomes. Curr. Opin. Biotechnol. 2022, 78, 102840. [Google Scholar] [CrossRef]
- Leonel, L.V.; Arruda, P.V.; Chandel, A.K.; Felipe, M.G.A.; Sene, L. Kluyveromyces marxianus: A Potential Biocatalyst of Renewable Chemicals and Lignocellulosic Ethanol Production. Crit. Rev. Biotechnol. 2021, 41, 1131–1152. [Google Scholar] [CrossRef]
- Zhang, B.; Ren, L.; Zhao, Z.; Zhang, S.; Xu, D.; Zeng, X.; Li, F. High Temperature Xylitol Production through Simultaneous Co-Utilization of Glucose and Xylose by Engineered Kluyveromyces marxianus. Biochem. Eng. J. 2021, 165, 107820. [Google Scholar] [CrossRef]
- Han, Y.; Tafur Rangel, A.; Pomraning, K.R.; Kerkhoven, E.J.; Kim, J. Advances in Genome-Scale Metabolic Models of Industrially Important Fungi. Curr. Opin. Biotechnol. 2023, 84, 103005. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Chen, J.; Jiao, X.; Gong, H.; Pan, D.; Liu, L.; Zhang, Y.; Tan, T. Genome-Scale Metabolic Network Models for Industrial Microorganisms Metabolic Engineering: Current Advances and Future Prospects. Biotechnol. Adv. 2024, 72, 108319. [Google Scholar] [CrossRef] [PubMed]
- Zuliani, L.; Serpico, A.; De Simone, M.; Frison, N.; Fusco, S. Biorefinery Gets Hot: Thermophilic Enzymes and Microorganisms for Second-Generation Bioethanol Production. Processes 2021, 9, 1583. [Google Scholar] [CrossRef]
- Rambabu, K.; Show, P.-L.; Bharath, G.; Banat, F.; Naushad, M.; Chang, J.-S. Enhanced Biohydrogen Production from Date Seeds by Clostridium thermocellum ATCC 27405. Int. J. Hydrogen Energy 2020, 45, 22271–22280. [Google Scholar] [CrossRef]
- Hamann, P.R.V.; de MB Silva, L.; Gomes, T.C.; Noronha, E.F. Assembling Mini-Xylanosomes with Clostridium thermocellum XynA, and Their Properties in Lignocellulose Deconstruction. Enzyme Microb. Technol. 2021, 150, 109887. [Google Scholar] [CrossRef] [PubMed]
- da Silva, G.A.R.; Oliveira, S.S.d.S.; Lima, S.F.; do Nascimento, R.P.; Baptista, A.R.d.S.; Fiaux, S.B. The Industrial Versatility of Gluconobacter Oxydans: Current Applications and Future Perspectives. World J. Microbiol. Biotechnol. 2022, 38, 134. [Google Scholar] [CrossRef]
- Liu, J.; Han, X.; Tao, F.; Xu, P. Metabolic Engineering of Geobacillus Thermoglucosidasius for Polymer-Grade Lactic Acid Production at High Temperature. Bioresour. Technol. 2024, 393, 130164. [Google Scholar] [CrossRef] [PubMed]
- Ntaikou, I.; Menis, N.; Alexandropoulou, M.; Antonopoulou, G.; Lyberatos, G. Valorization of Kitchen Biowaste for Ethanol Production via Simultaneous Saccharification and Fermentation Using Co-Cultures of the Yeasts Saccharomyces cerevisiae and Pichia stipitis. Bioresour. Technol. 2018, 263, 75–83. [Google Scholar] [CrossRef]
- Yan, Z.; Zhang, J.; Bao, J. Increasing Cellulosic Ethanol Production by Enhancing Phenolic Tolerance of Zymomonas mobilis in Adaptive Evolution. Bioresour. Technol. 2021, 329, 124926. [Google Scholar] [CrossRef]
- Yang, Z.; Sun, Q.; Tan, G.; Zhang, Q.; Wang, Z.; Li, C.; Qi, F.; Wang, W.; Zhang, L.; Li, Z. Engineering Thermophilic Geobacillus thermoglucosidasius for Riboflavin Production. Microb. Biotechnol. 2021, 14, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Beri, D.; Herring, C.D.; Blahova, S.; Poudel, S.; Giannone, R.J.; Hettich, R.L.; Lynd, L.R. Coculture with Hemicellulose-Fermenting Microbes Reverses Inhibition of Corn Fiber Solubilization by Clostridium thermocellum at Elevated Solids Loadings. Biotechnol. Biofuels 2021, 14, 24. [Google Scholar] [CrossRef] [PubMed]
- Crosby, J.R.; Laemthong, T.; Bing, R.G.; Zhang, K.; Tanwee, T.N.N.; Lipscomb, G.L.; Rodionov, D.A.; Zhang, Y.; Adams, M.W.W.; Kelly, R.M. Biochemical and Regulatory Analyses of Xylanolytic Regulons in Caldicellulosiruptor bescii Reveal Genus-Wide Features of Hemicellulose Utilization. Appl. Environ. Microbiol. 2022, 88, e01302-22. [Google Scholar] [CrossRef] [PubMed]
- Sloothaak, J.; Tamayo-Ramos, J.A.; Odoni, D.I.; Laothanachareon, T.; Derntl, C.; Mach-Aigner, A.R.; Dos Santos, V.A.P.M.; Schaap, P.J. Identification and Functional Characterization of Novel Xylose Transporters from the Cell Factories Aspergillus niger and Trichoderma reesei. Biotechnol. Biofuels 2016, 9, 148. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Zhu, Y.; Li, B.; Jin, H.; Dong, Y. Increased Enzyme Activities and Fungal Degraders by Gloeophyllum trabeum Inoculation Improve Lignocellulose Degradation Efficiency during Manure-Straw Composting. Bioresour. Technol. 2021, 337, 125427. [Google Scholar] [CrossRef] [PubMed]
- Arimoto, M.; Yamagishi, K.; Wang, J.; Tanaka, K.; Miyoshi, T.; Kamei, I.; Kondo, R.; Mori, T.; Kawagishi, H.; Hirai, H. Molecular Breeding of Lignin-Degrading Brown-Rot Fungus Gloeophyllum trabeum by Homologous Expression of Laccase Gene. AMB Express 2015, 5, 81. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Sun, J.; Chang, J.-S.; Shukla, P. Engineering Microbes for Direct Fermentation of Cellulose to Bioethanol. Crit. Rev. Biotechnol. 2018, 38, 1089–1105. [Google Scholar] [CrossRef]
- Coconi-linares, N.; Magaña-ortíz, D.; Guzmán-ortiz, D.A.; Fernández, F. High-Yield Production of Manganese Peroxidase, Lignin Peroxidase, and Versatile Peroxidase in Phanerochaete chrysosporium. Appl. Microbiol. Biotechnol. 2014, 98, 9283–9294. [Google Scholar] [CrossRef]
- Coconi Linares, N.; Fernández, F.; Loske, A.M.; Gómez-Lim, M.A. Enhanced Delignification of Lignocellulosic Biomass by Recombinant Fungus Phanerochaete chrysosporium Overexpressing Laccases and Peroxidases. Microb. Physiol. 2018, 28, 1–13. [Google Scholar] [CrossRef]
- Moon, H.Y.; Sim, G.H.; Kim, H.J.; Kim, K.; Kang, H.A. Assessment of Cre-Lox and CRISPR-Cas9 as Tools for Recycling of Multiple-Integrated Selection Markers in Saccharomyces cerevisiae. J. Microbiol. 2022, 60, 18–30. [Google Scholar] [CrossRef]
- Long, C.; Cheng, Y.; Cui, J.; Liu, J.; Gan, L.; Zeng, B.; Long, M. Enhancing Cellulase and Hemicellulase Production in Trichoderma Orientalis EU7-22 via Knockout of the CreA. Mol. Biotechnol. 2018, 60, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Tong, S.; Amin, F.R.; Chen, W.; Cai, J.; Li, D. Heterologous Expression and Biochemical Characterization of a Thermostable Endoglucanase (MtEG5-1) from Myceliophthora thermophila. Fermentation 2023, 9, 462. [Google Scholar] [CrossRef]
- Scown, C.D.; Baral, N.R.; Yang, M.; Vora, N.; Huntington, T. Technoeconomic Analysis for Biofuels and Bioproducts. Curr. Opin. Biotechnol. 2021, 67, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Zou, G.; Bao, D.; Wang, Y.; Zhou, S.; Xiao, M.; Yang, Z.; Wang, Y.; Zhou, Z. Alleviating Product Inhibition of Trichoderma reesei Cellulase Complex with a Product-Activated Mushroom Endoglucanase. Bioresour. Technol. 2021, 319, 124119. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, K.M.V.; de Paula, R.G.; Antoniêto, A.C.C.; Reis, T.F.; Carraro, C.B.; Silva, A.C.; Almeida, F.; Rechia, C.G.V.; Goldman, G.H.; Silva, R.N. Characterization of a Novel Sugar Transporter Involved in Sugarcane Bagasse Degradation in Trichoderma reesei. Biotechnol. Biofuels 2018, 11, 84. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Trametes Versicolor (Synn. Coriolus versicolor) Polysaccharides in Cancer Therapy: Targets and Efficacy. Biomedicines 2020, 8, 135. [Google Scholar] [CrossRef]
- Passarinho, A.T.P.; Ventorim, R.Z.; Maitan-Alfenas, G.P.; de Oliveira, E.B.; Guimarães, V.M. Engineered GH11 Xylanases from Orpinomyces sp. PC-2 Improve Techno-functional Properties of Bread Dough. J. Sci. Food Agric. 2019, 99, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Tovar-Herrera, O.E.; Martha-Paz, A.M.; Pérez-LLano, Y.; Aranda, E.; Tacoronte-Morales, J.E.; Pedroso-Cabrera, M.T.; Arévalo-Niño, K.; Folch-Mallol, J.L.; Batista-García, R.A. Schizophyllum commune: An Unexploited Source for Lignocellulose Degrading Enzymes. Microbiologyopen 2018, 7, e00637. [Google Scholar] [CrossRef] [PubMed]
- Piumi, F.; Levasseur, A.; Navarro, D.; Zhou, S.; Mathieu, Y.; Ropartz, D.; Ludwig, R.; Faulds, C.B.; Record, E. A Novel Glucose Dehydrogenase from the White-Rot Fungus Pycnoporus cinnabarinus: Production in Aspergillus niger and Physicochemical Characterization of the Recombinant Enzyme. Appl. Microbiol. Biotechnol. 2014, 98, 10105–10118. [Google Scholar] [CrossRef]
- Bronikowski, A.; Hagedoorn, P.-L.; Koschorreck, K.; Urlacher, V.B. Expression of a New Laccase from Moniliophthora roreri at High Levels in Pichia pastoris and Its Potential Application in Micropollutant Degradation. AMB Express 2017, 7, 73. [Google Scholar] [CrossRef]
- Omeje, K.O.; Nnolim, N.E.; Ezema, B.O.; Ozioko, J.N.; Eze, S.O.O. Synthetic Dyes Decolorization Potential of Agroindustrial Waste-Derived Thermo-Active Laccase from Aspergillus Species. Biocatal. Agric. Biotechnol. 2020, 29, 101800. [Google Scholar] [CrossRef]
- Kishishita, S.; Fujii, T.; Ishikawa, K. Heterologous Expression of Hyperthermophilic Cellulases of Archaea pyrococcus sp. by Fungus Talaromyces cellulolyticus. J. Ind. Microbiol. Biotechnol. 2015, 42, 137–141. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sadaqat, B.; Khatoon, N.; Malik, A.Y.; Jamal, A.; Farooq, U.; Ali, M.I.; He, H.; Liu, F.-J.; Guo, H.; Urynowicz, M.; et al. Enzymatic Decolorization of Melanin by Lignin Peroxidase from Phanerochaete chrysosporium. Sci. Rep. 2020, 10, 20240. [Google Scholar] [CrossRef] [PubMed]
- Akpinar, M.; Ozturk Urek, R. Direct Utilization of Peach Wastes for Enhancements of Lignocellulolytic Enzymes Productions by Pleurotus eryngii under Solid-State Fermentation Conditions. Chem. Pap. 2022, 76, 6699–6712. [Google Scholar] [CrossRef]
- Tišma, M.; Žnidaršič-Plazl, P.; Šelo, G.; Tolj, I.; Šperanda, M.; Bucić-Kojić, A.; Planinić, M. Trametes versicolor in lignocellulose-based bioeconomy: State of the art, challenges and opportunities. Bioresour. Technol. 2021, 330, 124997. [Google Scholar] [CrossRef]
- Fernandes-Klajn, F.; Romero-García, J.M.; Díaz, M.J.; Castro, E. Comparison of fermentation strategies for ethanol production from olive tree pruning biomass. Ind. Crops Prod. 2018, 122, 98–106. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, M.; Roozeboom, K.; Wang, D. Integrated Bioethanol Production to Boost Low-Concentrated Cellulosic Ethanol without Sacrificing Ethanol Yield. Bioresour. Technol. 2018, 250, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; You, Y.; Lei, F.; Li, P.; Jiang, J.; Zhu, L. Enhancement of Enzymatic Hydrolysis of Sugarcane Bagasse by Pretreatment Combined Green Liquor and Sulfite. Fuel 2017, 203, 707–714. [Google Scholar] [CrossRef]
- Raita, M.; Ibenegbu, C.; Champreda, V.; Leak, D.J. Production of Ethanol by Thermophilic Oligosaccharide Utilising Geobacillus thermoglucosidasius TM242 Using Palm Kernel Cake as a Renewable Feedstock. Biomass Bioenergy 2016, 95, 45–54. [Google Scholar] [CrossRef]
- Wanderley, M.C.d.A.; Martín, C.; Rocha, G.J.d.M.; Gouveia, E.R. Increase in Ethanol Production from Sugarcane Bagasse Based on Combined Pretreatments and Fed-Batch Enzymatic Hydrolysis. Bioresour. Technol. 2013, 128, 448–453. [Google Scholar] [CrossRef]
- Escaramboni, B.; Núñez, E.G.F.; Carvalho, A.F.A.; de Oliva Neto, P. Ethanol Biosynthesis by Fast Hydrolysis of Cassava Bagasse Using Fungal Amylases Produced in Optimized Conditions. Ind. Crops Prod. 2018, 112, 368–377. [Google Scholar] [CrossRef]
- Ma, Y.; Cai, W.; Liu, Y. An Integrated Engineering System for Maximizing Bioenergy Production from Food Waste. Appl. Energy 2017, 206, 83–89. [Google Scholar] [CrossRef]
- Boonchuay, P.; Techapun, C.; Leksawasdi, N.; Seesuriyachan, P.; Hanmoungjai, P.; Watanabe, M.; Takenaka, S.; Chaiyaso, T. An Integrated Process for Xylooligosaccharide and Bioethanol Production from Corncob. Bioresour. Technol. 2018, 256, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Wahono, S.K.; Rosyida, V.T.; Darsih, C.; Pratiwi, D.; Frediansyah, A. Optimization of Simultaneous Saccharification and Fermentation Incubation Time Using Cellulose Enzyme for Sugarcane Bagasse on the Second-Generation Bioethanol Production Technology. Energy Procedia 2015, 65, 331–336. [Google Scholar] [CrossRef][Green Version]
- Loaces, I.; Schein, S.; Noya, F. Ethanol Production by Escherichia Coli from Arundo Donax Biomass under SSF, SHF or CBP Process Configurations and in Situ Production of a Multifunctional Glucanase and Xylanase. Bioresour. Technol. 2017, 224, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Kossatz, H.L.; Rose, S.H.; Viljoen-Bloom, M.; van Zyl, W.H. Production of Ethanol from Steam Exploded Triticale Straw in a Simultaneous Saccharification and Fermentation Process. Process Biochem. 2017, 53, 10–16. [Google Scholar] [CrossRef]
- Silva, G.M.; Giordano, R.L.C.; Cruz, A.J.G.; Ramachandriya, K.D.; Banat, I.M.; Wilkins, M.R. Ethanol Production from Sugarcane Bagasse Using SSF Process and Thermotolerant Yeast. Trans. ASABE 2015, 58, 193–200. [Google Scholar]
- Sakdaronnarong, C.; Jiratanakittiwat, K.; Tangkitthanasakul, T.; Laosiripojana, N. Ionosolv Pretreatment of Sugarcane Bagasse and Rice Straw Assisted by Catalytic Hydrothermal and Microwave Heating for Biorefining. Food Bioprod. Process 2017, 105, 104–116. [Google Scholar] [CrossRef]
- Akhtar, N.; Goyal, D.; Goyal, A. Characterization of Microwave-Alkali-Acid Pre-Treated Rice Straw for Optimization of Ethanol Production via Simultaneous Saccharification and Fermentation (SSF). Energy Convers. Manag. 2017, 141, 133–144. [Google Scholar] [CrossRef]
- Srimachai, T.; Thonglimp, V.; Sompong, O. Ethanol and Methane Production from Oil Palm Frond by Two Stage SSF. Energy Procedia 2014, 52, 352–361. [Google Scholar] [CrossRef]
- Cheng, N.; Yamamoto, Y.; Koda, K.; Tamai, Y.; Uraki, Y. Amphipathic Lignin Derivatives to Accelerate Simultaneous Saccharification and Fermentation of Unbleached Softwood Pulp for Bioethanol Production. Bioresour. Technol. 2014, 173, 104–109. [Google Scholar] [CrossRef]
- Lu, J.; Song, F.; Liu, H.; Chang, C.; Cheng, Y.; Wang, H. Production of High Concentration Bioethanol from Reed by Combined Liquid Hot Water and Sodium Carbonate-Oxygen Pretreatment. Energy 2021, 217, 119332. [Google Scholar] [CrossRef]
- Chen, W.-C.; Lin, Y.-C.; Ciou, Y.-L.; Chu, I.-M.; Tsai, S.-L.; Lan, J.C.-W.; Chang, Y.-K.; Wei, Y.-H. Producing Bioethanol from Pretreated-Wood Dust by Simultaneous Saccharification and Co-Fermentation Process. J. Taiwan Inst. Chem. Eng. 2017, 79, 43–48. [Google Scholar] [CrossRef]
- Paschos, T.; Xiros, C.; Christakopoulos, P. Simultaneous Saccharification and Fermentation by Co-Cultures of Fusarium oxysporum and Saccharomyces cerevisiae Enhances Ethanol Production from Liquefied Wheat Straw at High Solid Content. Ind. Crops Prod. 2015, 76, 793–802. [Google Scholar] [CrossRef]
- Koppram, R.; Olsson, L. Combined Substrate, Enzyme and Yeast Feed in Simultaneous Saccharification and Fermentation Allow Bioethanol Production from Pretreated Spruce Biomass at High Solids Loadings. Biotechnol. Biofuels 2014, 7, 54. [Google Scholar] [CrossRef]
- Qin, L.; Zhao, X.; Li, W.-C.; Zhu, J.-Q.; Liu, L.; Li, B.-Z.; Yuan, Y.-J. Process Analysis and Optimization of Simultaneous Saccharification and Co-Fermentation of Ethylenediamine-Pretreated Corn Stover for Ethanol Production. Biotechnol. Biofuels 2018, 11, 118. [Google Scholar] [CrossRef] [PubMed]
- Favaro, L.; Viktor, M.J.; Rose, S.H.; Viljoen-Bloom, M.; van Zyl, W.H.; Basaglia, M.; Cagnin, L.; Casella, S. Consolidated Bioprocessing of Starchy Substrates into Ethanol by Industrial Saccharomyces cerevisiae Strains Secreting Fungal Amylases. Biotechnol. Bioeng. 2015, 112, 1751–1760. [Google Scholar] [CrossRef] [PubMed]
- Favaro, L.; Cagnin, L.; Basaglia, M.; Pizzocchero, V.; van Zyl, W.H.; Casella, S. Production of Bioethanol from Multiple Waste Streams of Rice Milling. Bioresour. Technol. 2017, 244, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Marques, N.P.; de Cassia Pereira, J.; Gomes, E.; da Silva, R.; Araújo, A.R.; Ferreira, H.; Rodrigues, A.; Dussán, K.J.; Bocchini, D.A. Cellulases and Xylanases Production by Endophytic Fungi by Solid State Fermentation Using Lignocellulosic Substrates and Enzymatic Saccharification of Pretreated Sugarcane Bagasse. Ind. Crops Prod. 2018, 122, 66–75. [Google Scholar] [CrossRef]
- Iram, M.; Asghar, U.; Irfan, M.; Huma, Z.; Jamil, S.; Nadeem, M.; Syed, Q. Production of Bioethanol from Sugarcane Bagasse Using Yeast Strains: A Kinetic Study. Energy Sources Part A Recovery Util. Environ. Eff. 2018, 40, 364–372. [Google Scholar] [CrossRef]
- Dotsenko, A.S.; Dotsenko, G.S.; Senko, O.V.; Stepanov, N.A.; Lyagin, I.V.; Efremenko, E.N.; Gusakov, A.V.; Zorov, I.N.; Rubtsova, E.A. Complex Effect of Lignocellulosic Biomass Pretreatment with 1-Butyl-3-Methylimidazolium Chloride Ionic Liquid on Various Aspects of Ethanol and Fumaric Acid Production by Immobilized Cells within SSF. Bioresour. Technol. 2018, 250, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Zurawski, J.V.; Khatibi, P.A.; Akinosho, H.O.; Straub, C.T.; Compton, S.H.; Conway, J.M.; Lee, L.L.; Ragauskas, A.J.; Davison, B.H.; Adams, M.W.W.; et al. Bioavailability of Carbohydrate Content in Natural and Transgenic Switchgrasses for the Extreme Thermophile Caldicellulosiruptor bescii. Appl. Environ. Microbiol. 2017, 83, e00969-17. [Google Scholar] [CrossRef] [PubMed]
- Shang, S.M.; Qian, L.; Zhang, X.; Li, K.Z.; Chagan, I. Themoanaerobacterium calidifontis sp. Nov., a Novel Anaerobic, Thermophilic, Ethanol-Producing Bacterium from Hot Springs in China. Arch. Microbiol. 2013, 195, 439–445. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, I.J.; Bornscheuer, U.T.; Nam, K.H. Biochemical and Structural Analysis of a Glucose-Tolerant β-Glucosidase from the Hemicellulose-Degrading Thermoanaerobacterium saccharolyticum. Molecules 2022, 27, 290. [Google Scholar] [CrossRef] [PubMed]
- Pino, M.S.; Michelin, M.; Rodríguez-Jasso, R.M.; Oliva-Taravilla, A.; Teixeira, J.A.; Ruiz, H.A. Hot Compressed Water Pretreatment and Surfactant Effect on Enzymatic Hydrolysis Using Agave Bagasse. Energies 2021, 14, 4746. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, Y.; Zhang, J.; Wang, D.; Sun, L.; Hong, J. Engineered Kluyveromyces marxianus for Pyruvate Production at Elevated Temperature with Simultaneous Consumption of Xylose and Glucose. Bioresour. Technol. 2017, 224, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhu, M.-J. Enhanced Bioethanol Production by Fed-Batch Simultaneous Saccharification and Co-Fermentation at High Solid Loading of Fenton Reaction and Sodium Hydroxide Sequentially Pretreated Sugarcane Bagasse. Bioresour. Technol. 2017, 229, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Câmara-Salim, I.; González-García, S.; Feijoo, G.; Moreira, M.T. Screening the Environmental Sustainability of Microbial Production of Butyric Acid Produced from Lignocellulosic Waste Streams. Ind. Crops Prod. 2021, 162, 113280. [Google Scholar] [CrossRef]
- Ruchala, J.; Sibirny, A.A. Pentose Metabolism and Conversion to Biofuels and High-Value Chemicals in Yeasts. FEMS Microbiol. Rev. 2021, 45, fuaa069. [Google Scholar] [CrossRef]
- Hope, E.A.; Amorosi, C.J.; Miller, A.W.; Dang, K.; Heil, C.S.; Dunham, M.J. Experimental Evolution Reveals Favored Adaptive Routes to Cell Aggregation in Yeast. Genetics 2017, 206, 1153–1167. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, J.; Wang, D.; Han, R.; Ding, R.; Gao, X.; Sun, L.; Hong, J. Simultaneous Fermentation of Glucose and Xylose at Elevated Temperatures Co-Produces Ethanol and Xylitol through Overexpression of a Xylose-Specific Transporter in Engineered Kluyveromyces marxianus. Bioresour. Technol. 2016, 216, 227–237. [Google Scholar] [CrossRef]
- Gubicza, K.; Nieves, I.U.; Sagues, W.J.; Barta, Z.; Shanmugam, K.T.; Ingram, L.O. Techno-Economic Analysis of Ethanol Production from Sugarcane Bagasse Using a Liquefaction plus Simultaneous Saccharification and Co-Fermentation Process. Bioresour. Technol. 2016, 208, 42–48. [Google Scholar] [CrossRef]
- Cotana, F.; Cavalaglio, G.; Gelosia, M.; Coccia, V.; Petrozzi, A.; Ingles, D.; Pompili, E. A Comparison between SHF and SSSF Processes from Cardoon for Ethanol Production. Ind. Crops Prod. 2015, 69, 424–432. [Google Scholar] [CrossRef]
- Bertacchi, S.; Jayaprakash, P.; Morrissey, J.P.; Branduardi, P. Interdependence between Lignocellulosic Biomasses, Enzymatic Hydrolysis and Yeast Cell Factories in Biorefineries. Microb. Biotechnol. 2022, 15, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Ceaser, R.; Montané, D.; Constantí, M.; Medina, F. Current Progress on Lignocellulosic Bioethanol Including a Technological and Economical Perspective. Environ. Dev. Sustain. 2024. [Google Scholar] [CrossRef]
- Jawad Kadhum, H.; Murthy, G.S. Novel System Design for High Solid Lignocellulosic Biomass Conversion. Bioresour. Technol. 2022, 350, 126897. [Google Scholar] [CrossRef]
- Sharma, S.; Nair, A.; Sarma, S.J. Biorefinery Concept of Simultaneous Saccharification and Co-Fermentation: Challenges and Improvements. Chem. Eng. Process. Process Intensif. 2021, 169, 108634. [Google Scholar] [CrossRef]
- Olguin-Maciel, E.; Singh, A.; Chable-Villacis, R.; Tapia-Tussell, R.; Ruiz, H.A. Consolidated Bioprocessing, an Innovative Strategy towards Sustainability for Biofuels Production from Crop Residues: An Overview. Agronomy 2020, 10, 1834. [Google Scholar] [CrossRef]
- Ding, J.; Huang, X.; Zhang, L.; Zhao, N.; Yang, D.; Zhang, K. Tolerance and Stress Response to Ethanol in the Yeast Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2009, 85, 253. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Arumugam, S.M.; Kumar, S.; Mahala, S.; Devi, B.; Elumalai, S. Updated Technologies for Sugar Fermentation to Bioethanol. In Biomass, Biofuels, Biochemicals; Elsevier: Amsterdam, The Netherlands, 2022; pp. 95–116. [Google Scholar]
- Li, Z.; Waghmare, P.R.; Dijkhuizen, L.; Meng, X.; Liu, W. Research Advances on the Consolidated Bioprocessing of Lignocellulosic Biomass. Eng. Microbiol. 2024, 4, 100139. [Google Scholar] [CrossRef]
- Kim, I.J.; Kim, S.R.; Kim, K.H.; Bornscheuer, U.T.; Nam, K.H. Characterization and Structural Analysis of the Endo-1,4-β-Xylanase GH11 from the Hemicellulose-Degrading Thermoanaerobacterium saccharolyticum Useful for Lignocellulose Saccharification. Sci. Rep. 2023, 13, 17332. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, T.B.; Korosh, T.K.; Stevenson, D.M.; Foster, C.; Maranas, C.; Olson, D.G.; Lynd, L.R.; Amador-Noguez, D. In Vivo Thermodynamic Analysis of Glycolysis in Clostridium thermocellum and Thermoanaerobacterium saccharolyticum Using 13C and 2H Tracers. mSystems 2020, 5, 10-1128. [Google Scholar] [CrossRef] [PubMed]
| Type | Microorganism | Characteristics | Refernces |
|---|---|---|---|
| Yeasts | Saccharomyces cerevisiae | Facultative anaerobic yeast, high tolerance to ethanol | [1] |
| Kluyveromyces marxianus | Thermophilic yeast is able to grow at high temperatures of 52 °C | [66] | |
| Candida sp. | Ethanologenic yeast, ferments xylose | [28] | |
| Komagataella pastoris | Possesses cellulase enzymes favorable to the SSF process | [74] | |
| Bacteria | Zymomonas mobilis | Ethanologenic, high ethanol productivity | [75] |
| Clostridium thermocellum Geobacillus thermoglucosidasius Clostridium cellulovorans Clostridium phytofermentans Thermoanaerobacterium calidifontis | Suitable for CBP Processing Ferments hexose, pentose, and oligomers Amenability for genetic modification Secretes individual enzymes instead of cellulosomes. Hemicellulolytic, xylanases | [45,76] | |
| Thermophilic bacteria | Thermoanaerobacterium saccharolyticum | Resistance to extremely high-temperature, ferments xylan | [77] |
| Caldicellulosiruptor bescii Thermoanaerobacter ethanolicus | Resistance to an extremely high-temperature of 70 °C Have celluloytic activity under high temperature | [77,78] | |
| Fungi | Trichoderma reesei | Produce high-level cellulase | [63] |
| Aspergillus niger | Ability to produce plant biomass-degrading enzymes | [79] |
| Fungal Strain (Receiver Organism) | Genetic Modification | Remarks | References |
|---|---|---|---|
| Gloeophyllum trabeum KU-41 | Homologous overexpression of an endogenous gene encoding a put-ative laccase activity gene (Gtlcc3) | Clone G. trabeum L#61 showed higher laccase activity (2.7% of lignin degradation) than the G. trabeum KU-41 and 45% more ethanol production than the wild type | [80,81] |
| Phanerochaete sordida YK-624 | Homologous overexpression of an extra pyruvate decarboxylase gene | Clone P. sordida GP7 produced 1.41 times more ethanol than the wild-type P. sordida YK-624 | [82] |
| Phanerochaete chrysosporium | Constitutive co-expression of 4 oxidoreductases: manganese peroxidase (MnP), lignin peroxidase (LiP), versatile peroxidase (VP) from Trametes versicolor, and laccase (Lac) from P. eryngii | Constitutive co-expression of four oxidoreductases in a basidiomycete P. chrysosporium in functional form (culture in minimal medium) | [83,84] |
| Phanerochaete chrysosporium | Constitutive co-overexpression of 4 oxidoreductases: MnP, LiP, VP, and Lac from Pleurotus eryngii | Lignin depolymerization of sugarcane bagasse and wheat straw was enhanced by up to 25% in the presence of recombinant fungi in comparison with the wild-type strain. Sugar release on lignocellulose was 2- to 6-fold higher by recombinant fungi as compared with the control | [84] |
| Trichoderma reseei | Double overexpression of endogenous egl2 and bgl1 genes (endoglucanase and β-glucosidase enzymes) using pyrG marker (orotidine-5′-monophosphate decarboxylase gene) | The EG2–BGL1 double overexpression strain QEB4 displayed a remarkable enhancement of cellulolytic ability on pretreated corncob residues. Cellulose conversion (94.2%) was found for the delignified corncob residues after 48 h enzymatic saccharification | [85,86] |
| Thermothelomyces thermophilus | Heterologous expression of an alcohol dehydrogenase (ScAdhl1) and cellodextrin transporters CDT-1/-2 | The engineered strain JY518 increased ethanol production by 200% from cellobiose compared to the wild-type strain | [87] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Gloria, K.D.; Tomás-Pejó, E.; Amaya-Delgado, L.; Rodríguez-Jasso, R.M.; Loredo-Treviño, A.; Singh, A.; Hans, M.; Martín, C.; Kumar, S.; Ruiz, H.A. Biochemical and Biorefinery Platform for Second-Generation Bioethanol: Fermentative Strategies and Microorganisms. Fermentation 2024, 10, 361. https://doi.org/10.3390/fermentation10070361
González-Gloria KD, Tomás-Pejó E, Amaya-Delgado L, Rodríguez-Jasso RM, Loredo-Treviño A, Singh A, Hans M, Martín C, Kumar S, Ruiz HA. Biochemical and Biorefinery Platform for Second-Generation Bioethanol: Fermentative Strategies and Microorganisms. Fermentation. 2024; 10(7):361. https://doi.org/10.3390/fermentation10070361
Chicago/Turabian StyleGonzález-Gloria, Karla D., Elia Tomás-Pejó, Lorena Amaya-Delgado, Rosa M. Rodríguez-Jasso, Araceli Loredo-Treviño, Anusuiya Singh, Meenu Hans, Carlos Martín, Sachin Kumar, and Héctor A. Ruiz. 2024. "Biochemical and Biorefinery Platform for Second-Generation Bioethanol: Fermentative Strategies and Microorganisms" Fermentation 10, no. 7: 361. https://doi.org/10.3390/fermentation10070361
APA StyleGonzález-Gloria, K. D., Tomás-Pejó, E., Amaya-Delgado, L., Rodríguez-Jasso, R. M., Loredo-Treviño, A., Singh, A., Hans, M., Martín, C., Kumar, S., & Ruiz, H. A. (2024). Biochemical and Biorefinery Platform for Second-Generation Bioethanol: Fermentative Strategies and Microorganisms. Fermentation, 10(7), 361. https://doi.org/10.3390/fermentation10070361









