Abstract
A complex evaluation of antimicrobial activities of microalgae, including those relevant to wastewater treatment (WWT), in light of the integrated biorefinery concept, is performed. An example of this concept is linking a commercial microalgal system to plants, factories, or farms that emit polluted wastewater (WW). The microalgae would not only metabolize the pollutants—such as nitrogen (N) and phosphorus (P)—from the WW, thus fueling their biomass, but they would exert an antibacterial effect against the pathogenic bacteria there. The biomass then could be harvested and used for biofertilizers, biofuels, and bioplastics and might possibly be utilized as animal feed, antimicrobial and other pharmaceutical agents. A large amount of the research on the antimicrobial activity and WWT potential focuses on the families Chlorellaceae and Scenedesmaceae, which are also some of the most commercially used strains of microalgae. For that reason, they are the species chosen for the current review. Furthermore, the increasing antimicrobial resistance necessitates the search for antibiotic alternatives, and the antibacterial and antifungal activity of Chlorellaceae and Scenedesmaceae is very promising. Microalgae are rich in antibacterial compounds like polyunsaturated fatty acids (PUFAs), polysaccharides, carotenoids, proteins, etc., and for that reason, their extracts possess antimicrobial effects. The in vitro antimicrobial activity of Chlorellaceae and Scenedesmaceae families has varied in a broad range from low to strong activity or no effect. Several strains have fulfilled the criteria for outstanding and high activity, especially C. vulgaris and other Chlorellaceae spp., with an effect equal to or better than the control antibiotics. There were several strains with minimum inhibitory concentrations (MIC) below 80 µg/mL and even 10 and 1.5 µg/mL; some species also had inhibition zones (IZ) over 30 mm, even as high as 48 mm. In vivo results are also promising but scarce, and all this warrants further in vivo and in situ studies—from animal models to clinical and environmental trials. Altogether, important data in the light of the circle economy, the urgent necessity to decrease CO2 emissions to fight climate change, and to curb the harmful influence of future pandemics are presented. This review paves the way for further utilizing the total potential of a microalgal system.
1. Introduction
The first microalgal antimicrobial agent (chlorellin) was discovered in the 40s [1,2]; however, the antibiotic boom soon occurred, and probably for that reason, that knowledge seems to have been “abandoned” until recently. Then, the rising antimicrobial resistance led to the urgent need for the discovery of new antimicrobial chemotherapeutics. Scientists look for alternative sources for antibiotics other than (semi)synthesis or the classic producers, such as actinomycetes and other microorganisms. Research on the antimicrobial effect of microalgae was renewed and specifically intensified after approximately the year 2010, as a Scopus search shows. It led to promising in vitro and in vivo results. In regard to microalgal secondary metabolites, scholars find the discovery of specific compounds, apart from chlorellin, responsible for the antimicrobial action, to be a relatively emerging field of research [1,2]. It can be speculated that other promising species of terrestrial plants or algae are “rediscovered” today as scientists turn their interest towards ethnobotany and research on important plants treating infections in traditional medicine.
To date, natural products and their derivatives amount to nearly 70% of the FDA-approved antibacterial drugs. The majority (97%) comes from microbes (bacteria and fungi), while algae and higher plant products contribute to just 3% of them [3]. Plant products, in general, can be claimed to include algal products since most of the eukaryotic species that are named with the polyphyletic term (micro)algae are from the kingdom Plantae sensu lato [4,5,6,7].
These 3%, however, do not accurately reflect the potential of algal and higher plant natural products. As Quave (2016) has excellently summarized in her outline, “we have only uncovered the tip of the iceberg in our understanding of the chemical diversity and bioactivity of plant natural products” [3].
The reasons for such low numbers are, on the one hand, that plant natural products were likely being ignored during the golden era of antibiotics, and even today, their development would mean the diversion of resources from the development of conventional antibiotics [8]. On the other hand, there are some inherent difficulties in the development of plant natural products as antimicrobial drugs. Quave lists them as follows: plant extracts are very chemically complex, and the isolation of single antibacterial compounds can be time-consuming and requires a large amount of plant material. Rediscovery of known compounds from unexplored plant species slows the process. It can be challenging to access plant specimens, particularly in an international setting. Synergism between compounds in an extract poses particular challenges, as due to the lack of fully developed scientific technology, it is difficult to study multiple compounds acting simultaneously on multiple biological targets [3].
Particularly regarding microalgae, in addition, proved antimicrobial natural sources, including in ethnobotany, such as eucalyptus, clove, oregano, etc., were likely priorities for research and development.
Similarly, fatty acids, especially polyunsaturated fatty acids (PUFAs), which are abundant in microalgae, have an underappreciated antimicrobial therapeutic potential [9]. For example, increasing their concentration in the animal organism through diet or other means, suppressed infections and also had a prophylactic effect [10]. However, there are other specific deterrents to their application, as outlined in the respective sections of this review.
Based on the outline above, the conclusion that microalgae or fatty acids, perceived as food, have therapeutic potential for treating infections is reached. Chlorella has been known to have an antimicrobial effect for 80 years, but its crude biomass is mostly used as food. Fatty acids have been known for a long time as trivial and ubiquitous components of foods and human physiology and organisms (some longer-chain PUFAs are abundant in fish oil and also precursors of eicosanoids and structural components of mammal nerve tissue). As paradoxical as it sounds, there is accumulating research described below that has begun to unveil their promising therapeutic potential.
Chlorella is a microalgae that is approved by The Food and Drug Administration (FDA) as GRAS (Generally Recognized as Safe) and is consumed as a dietary supplement [11]. Indeed, a GRAS approval does not exclude a possibility for infection treatment use, since undoubtedly antibacterial plant products (even with certified antibacterial use in dentistry) such as clove oil and oregano oil are also GRAS.
This review analyzes the outline of algae process development under the integrated biorefinery concept [12,13]. An advantage of microalgae is that they do not require arable land, can be grown in many substrates with minimal requirements [13] in the frame of system analysis theory [14], and fulfill the idea of integral utilization of algae biomass and their metabolites. The visibility of microalgal technology, in fact, has to pass through conditional optimization of all unit operations (microalgae process development [15,16], photobioreactor (PBR) design and scaling up [17,18], and the downstream processing) for minimal time and with minimal scientific efforts at minimal costs. As a first step of the outline, analysis of the engineering approach of the strain isolation, identification, and its ability to synthesize HVP under given operational conditions [19] and nutrient medium is fundamental. Modern approaches to calculating macro- and micro elements can be considered as a base for an effective first step in the optimization of microalgal kinetics [20]. Its effective modeling, optimization, and design determine all the other steps of upstream process development. The techno-economic analysis should be taken into account under the microalgal biorefinery concept [21]. It is especially important to notice and to know how the production of metabolites relates to the growth of the strains. This is directly linked to the kinetics optimization procedure.
Both marine and freshwater microalgae produce, through various metabolic pathways, a wide variety of chemically active compounds. They act as a chemical defense against herbivores under environmental stress and high competition for space. These molecules have antibacterial, antialgal and antifungal properties, being effective also against bio-fouling [22].
Microalgae are commercial sources of some high-value products (HVP), mainly pigments (carotenoids and phycobiliproteins, which are both food colorants and antioxidants), vitamins, and PUFAs. They are developed in the nutrition and health markets because of their nutraceutical value [2]. For example, carotenoids and vitamin E are antioxidants and may be not approved drugs but are used as food supplements for anti-inflammatory and other effects. Carotenoids are also anticancer compounds [23]. PUFAs are important and fundamental for human metabolism, membrane fluidity, and electron and oxygen transport, and they have antibacterial and anti-inflammatory qualities, anti-carcinogenic effects, and improve cardiovascular diseases [22,24,25,26]. Two kinds of PUFAs are prescription drugs (alone or in combination)—Vascepa® (Amarin, Dublin, Ireland) and Lovaza® (Woodward Pharma Services LLC, Wixom, MI, USA)—for lowering triglyceride levels [27]. Marine microalgae are the main primary producers of ω-3 PUFAs, especially the longer chain ones [28].
Microalgae may have resulted in many food supplements and few approved drugs; however, supplements are also used for medical and pharmaceutical applications. They can also be part of therapeutic schemes for chronic diseases, e.g., saline solutions (alkaline citrates), unregistered as drugs, are prescribed for kidney oxalate stones [29].
There are other important medicinal benefits and potential uses of microalgae and their compounds. The mycosporin-like amino acids have ultraviolet light protective effects, the sterols have pharmaceutical applications, and the phycotoxins are cytostatic [23]. Other beneficial biological activities include immunomodulatory, anti-obesity, anti-angiogenic, and neuroprotective [22]. Microalgae are considered one of the most important sources of antimicrobial molecules, including fatty acids, proteins, vitamins, pigments, etc. [22,30,31].
This review reflects the current knowledge of the antimicrobial potential of many Chlorellaceae and Scenedesmaceae species and the features to produce HVP. Another reason for choosing these microalgal families is that, in one report, they generally exhibited more antibacterial activity than, e.g., Dunaliella [32]. The cited studies on the in vitro antimicrobial activity of the two genera can also be viewed as a means to reveal their and their metabolites’ wastewater (WW) decontamination potential. Notably, researchers have to have in mind the complexity of the microalgal system and its relationship with upstream and downstream processes in order to reach the final step of the market realization. The so-called integrated biorefinery concept gives a solid base of cost-effective and robust microalgal-based biotechnology.
2. Why Are Chlorellacea, e.g., C. vulgaris and Scenedesmaceae, among the Most Commercially and Industrially Used Microalgae?
2.1. Overview
Because these taxa are rich sources of HVP, they are appreciated and vastly exploited in biotechnology, although mainly as biomass and extracts from it, for protein-rich food, feed, cosmetics, and bioethanol. Examples of reviews covering that topic are those of Mobin et al. (2017) [33] and Bhalamurugan et al. (2018) [34].
Scientists are divided on whether cyanobacteria such as Spirulina (Arthrospira) and Nostoc are (micro)algae, as they would be nearly the only group of the Bacteria domain in this category. Many credible scientific sources still include them, as the tradition is since (micro)algae is an informal term and do not form a natural group that has descended from a common ancestor. Its eukaryotic members are species from multiple distinct clades, both from the kingdom Plantae sensu lato and not [4,5,6,7]. Cyanobacteria share many characteristics in terms of physiology and ecology, and, especially, many biotechnological applications with the eukaryotic microalgae [35]. For those reasons, they are considered microalgae in this paper.
Over the last 20 years, the biotechnological utilization has focused on four major microalgal species: Spirulina (Arthrospira platensis), Chlorella vulgaris, Dunaliella salina, and Haematococcus lacustris [33]. The most commonly exploited microalgae for the production of bioactive compounds of pharmaceutical interest include Arthrospira, Chlorella, Dunaliella, Haematococcus, and Nostoc [34].
The commercial cultivation of microalgae began in the 1960s in Japan, and Chlorella was the first targeted organism. Chlorella is the most cultivated eukaryotic alga since it is widely used as a feed supplement and health food highly rich in protein. It produces biomass fast—its photosynthetic efficiency can reach 8%, which surpasses that of crops with high efficiency, such as sugar cane [36]. Chlorella and Chlorellaceae are freshwater taxa, although there are exceptions, such as the marine species Chlorella salina.
Moreover, only a few from the approximate 40,000 species of algae have been used by the food industry and Chlorella vulgaris Beijerinck is among them. It is a nutrient-dense superfood containing, as percentages of its dry weight, 18 amino acids, 20 vitamins and minerals, 12–28% carbohydrate, 2–46% lipids, and, notably, it can reach 60% protein. In addition to being directly manufactured into health products like tablets and capsules, it is also used as a supplement to noodles, breads, biscuits, candies, ice cream, bean curd, and other common foods to enhance nutritive and health values [22], as well as coloring matters for food stuffs [33]. An additional reason for this alga to be used as an antimicrobial food supplement may be the presence of flavonoids. The special interest in promoting it in the form of a new medicinal food supplement also comes from its negligible side effects [37]. Until today, Chlorella (together with Spirulina) has been the most commonly sold food-microalgae because both of them can grow very fast. Chlorella is widely used in (health) beverage production [33]. Chlorella spp. e.g., C. vulgaris are also used as animal feed and for extraction of carbohydrates. The increase in global production of Chlorella (2000 t dry weight/year) suggests that in the years to come, microalgae may prove to be a fascinating source of food for humans. Commercial cultures of Chlorella spp. have been produced in PBRs, large circular tanks, and circular or mixed open ponds with paddle wheels. Chlorella’s commercial market value is over 1 billion USD. The production has a compound annual growth rate of 5.3% [34].
A nucleotide-peptide complex, the so-called Chlorella growth factor, is one of the unique properties of the species, allowing the high growth rate of one cell multiplying into four new cells approximately every 20 h. Chlorella is, arguably, the food substance known to be the richest in nucleic acids, and spraying pepper seedlings with Chlorella extract has increased plant height [38,39].
In regard to carotenoids, β-carotene is an antioxidant that works as a vitamin A precursor. Dunaliella spp., e.g., D. salina, are the best source of β-carotene (up to 14% of dry biomass) and sources of production. They are followed by Tetradesmus almeriensis (Scenedesmaceae), which is not industrially used for the purpose yet but is showing promise for production [34]. Scenedesmaceae is a fresh and brackish water family. The carotenoid compounds lutein, zeaxanthin, and canthaxanthin are used for pharmaceutical purposes mainly as antioxidants and for food colorants, e.g., in orange juice and curiously for chicken skin and plumage coloration [33]. The documented for carotenoid production microalgal species are D. salina, C. vulgaris, and Haematococcus pluvialis. Auxenochlorella protothecoides, H. lacustris, the mentioned T. almeriensis, etc., also show promise as sources [40]. When culture conditions are optimal, the biomass of C. vulgaris accumulates a high quantity of secondary carotenoids. This strain is characterized by high volume production of β-carotene [41]. Besides being rich in astaxanthin, the strongest antioxidant found in nature, [42] and lutein, Chlorella is a commercial source of canthaxanthin. All three carotenoids have been in regular use as pigments. For example, they have been added to the feed for salmonid fish, trout, and poultry to enhance the reddish color of the fish or the yellow color of the egg yolk [43]. Astaxanthin is mostly used in aquaculture, while lutein is also highly used as a source for animal nutrition [44]. In the present day, the marigold flower is the only commercial source for lutein [45]. However, because of some drawbacks, alternative sources are searched for, and T. almeriensis is a promising source for lutein production with an approximate content of 4.5 mg/g dry weight [46], followed by Chromochloris zofingiensis (Scenedesmaceae) and A. protothecoides. The cost of 1000 mg of lutein from an algae source is approximately USD 2.5. So far, H. pluvialis is the only microalgal commercial source of astaxanthin. However, similar reasons drive the exploration of alternative algal producers, such as C. zofingiensis [42]. The microalgae T. almeriensis and Nannochloropsis oculata are good candidates to produce zeaxanthin. For example, T. almeriensis yields 0.34 mg/g of zeaxanthin, and the cost of 1000 mg of zeaxanthin from an algae source is USD 10. Scenedesmaceae species could also be used as rich astaxanthin and vitamin sources (C, B1, and B2). The carotenoids β-carotene, lutein, astaxanthin, canthaxanthin, zeaxanthin, violaxanthin, neoxanthin, and antheraxanthin, etc. are among the biomolecular compounds of Chlorella spp., together with chlorophyll-a and b [47,48].
C. vulgaris also has a wound-healing ability which is better in comparison to calcium sodium alginate (brown algae) dressing [41]. Chlorella spp. contain an important compound known as β-1, 3-glucan. It works as a free-radical scavenger, boosting the immune system and reducing blood lipids like cholesterol. It also has antitumor properties and is effective for wound healing, body detoxifying, and treating stomach ulcers. Chlorella extracts have hepatoprotective, blood-sugar-lowering, and hemoglobin-boosting properties. Chlorella spp. are rich in α-carotene, β-carotene, lutein, ascorbic acid, and α-tocopherol, and these compounds can contribute to decreasing the incidence of certain cancers and preventing macular degeneration. They are also rich in chlorophyll, carbohydrates, lipids, and B vitamins (B1, B2, B6, and especially B12).
Chlorella, Dunaliella, and Scenedesmaceae are among the commonly used species of microalgae for farm and aquaculture feed. Scenedesmaceae species can be regarded as a source of monounsaturated, polyunsaturated, and saturated fatty acids in feed [34]; however, their high price is a drawback [25,26].
Various cosmetics frequently contain Spirulina, Chlorella, Dunaliella, and Haematococcus. These microalgae extracts are used to replenish face and body lotions and creams. Additionally, they are a component of shampoos, hair masks, and sun protection creams. The synthesis of skin collagen is reportedly stimulated by the C. vulgaris extract. They also prevent wrinkles from appearing on the skin’s surface and aid in the regeneration of fibers. Protein-enriched Spirulina extract slows down the aging process of the skin [33,34].
Chlorella spp., Scenedesmus spp., and Spirulina fusiformis are among the most commonly used microalgae for bioethanol production. Desmodesmus abundans and Tetradesmus obliquus (Turpin), M.J. Wynne (Scenedesmaceae), and Chlorella sorokiniana are among the microalgae commonly employed in the production of hydrogen [34].
The most common and important for human physiology ω-3 PUFAs are α-linolenic acid (ALA, 18:3, ω-3), eicosapentaenoic acid (EPA 20:5, ω-3), and docosahexaenoic acid (DHA, C22:6, ω-3). EPA acts as a precursor for eicosanoids, such as prostaglandins and leukotrienes. DHA is often among the fatty acids of phosphatidylserine, which, while not only limited to nerve tissues, is the major phospholipid of the cerebral cortex, thus making DHA an important component of the human brain and neuronal phospholipids, retina, etc. [49]. EPA and DHA are the prescription drugs (alone or in combination) VascepaTM and LovazaTM, used for lowering triglyceride levels [27].
Microalgae, in general, are abundant sources of the three acids. ALA is synthesized in high amounts also in terrestrial higher plants, but in regard to EPA and DHA, few animals (e.g., fishes) can synthesize them with low yield and accumulate them through consumption of microalgae and other microorganisms. The conversion of α-linolenic acid to EPA and DHA occurs at an even more limited rate, with inefficiency, in mammals, including humans. Therefore, ensuring a sufficient level of these acids on a diet that lacks them is very difficult; hence, they must be consumed through the diet, and all vertebrates largely rely on the intake of EPA and DHA from food.
Therefore, it is mainly microalgae that are the primary and original producers of ω-3 PUFAs, especially the longer chain ones, EPA and DHA, and marine microalgae are especially important in that regard [28].
Fish, being higher in the food chain, concentrate EPA from the microalgae they consume, and commercially available EPA is most often derived from fish oil. Nevertheless, the facts that fish oil tends to be one of the most expensive nutritional product on the market, the diminishing fish resources, the need for sustainability, the added risk of contamination such as mercury, the vegetarian preferences of some people, and even the unpleasant flavor and odor for others make microalgae an especially important alternative source of PUFAs [28,50]. Substituting algae for fish oil offers a great chance to develop alternative ω-3 PUFAa for pharmaceutical and nutraceutical uses [22].
PUFAs reach 35–40% of the total lipid content from Chlorella, comprising up to 24% linoleic acid and 27% ALA in C. vulgaris [51]. Some sources claim that chlorophytes (Chlorella, Dunaliella, and Scenedesmaceae spp.) have low and even trace amounts of EPA (up to 3%) and are low and even deficient in DHA [25,26,52], but that is not entirely true. Besides ALA, Chlorella spp. is a ω-3 source also in the form of EPA [53]. However, DHA was found in C. sorokiniana [54]. Auxenochlorella pyrenoidosa and Chloroidium ellipsoideum (Chlorellaceae) are claimed to be among the most common algae employed for the production of DHA-rich algal oil [34]. ALA is the dominant ω-3 fatty acid in T. obliquus, which was found to be deficient in EPA and DHA [55]. However, Scenedesmaceae members have shown high EPA content [56]. Nevertheless, the species rich in DHA are usually dinoflagellates and other marine microorganisms [57], and chlorophytes are not suitable for a single-species diet.
2.2. Production Costs and Economic Robustness of HVP from Chlorellaceae and Scenedesmaceae
World companies first started exploring microalgae for biodiesel and other fuels, but the production costs turned out to be higher than those of petroleum-based fuels. The knowledge about microalgal HVP and nutraceuticals is owed to this fact as it lead to the investment in their intensive research [58].
A detailed techno-economical analysis and a summary of the cost of microalgal low-, middle-, and high-value products, as well as the means of HVP extraction and the optimization for carbon emission reduction, are previously published by Kroumov et al. (2017) [21] and (2021) [59].
Chlorellaceae and Scenedesmaceae are commercially produced mainly as biomass at present. Although academia stresses that HVP from these two families, including antimicrobial ones, are very promising for industrial production [2,60,61,62], single HVP from Chlorellaceae and Scenedesmaceae are currently expected to be isolated and offered mostly on a pilot scale. Apart from the established commercial microalgal HVP production from certain other microalgal species, a number of companies have implemented the biorefinery techniques, enabling cultivating microalgae on a pilot scale and generating diverse products (primarily energy carriers, pigments, and PUFAs) from the same biomass [63].
The exact production costs for specific HVP from a defined species are difficult to calculate. For example, both synthetic (dominating the market) and natural (e.g., microalgal) astaxanthin have been produced for at least three decades. However, which system had the lower cost was claimed to be unknown up until 2011. The analysis showed that the production cost of astaxanthin and microalgae biomass can be as low as $718/kg and $18/kg. This cost might be lower than that of synthetic astaxanthin, and the lack of absolute certainty shows the difficulty in the calculation of production costs [64]. The market demand for natural astaxanthin, the slow growth rate, low biomass production, and ease of contamination with fast-growing microbes ensured the search for an alternative to H. pluvialis sources rich in astaxanthin, such as C. zofingiensis [42].
As mentioned, the expensive costs of harvesting marigold flowers, the commercial lutein source, the land and water use, and the seasonality of the growth [46] drove a comparative analysis. It showed that lutein from marigolds consumed more land and water but less nutrients and power. Therefore, the absolute cost of production is difficult to assess and depends on factors, i.e., microalgal lutein production is suitable for establishments with limited land and water resources and high personnel costs. Therefore, research and development for affordable microalgal commercial lutein production is needed [65].
The health market prefers natural β-carotene because it contains both trans and cis isomers. Although some sources claim that the production of microalgal carotenoids requires less labor and the harvesting process is easier, that production is still very expensive, with significant costs associated with labor, capital, and processing [66]. For those reasons, microalgal β-carotene can be purchased for as high as 700 €/kg, while synthetic forms cannot be purchased for more than half that amount [43].
Microalgal HVP commercialization, in general, undoubtedly has many benefits. As mentioned, algae have higher photosynthetic efficiency compared to terrestrial plants, hence high growth rate and higher yields. They do not require arable land and biomass can be harvested regardless of seasons and geographic area [67]. Microalgal HVPs are usually produced within a biorefinery model, which increases revenue, in essence, an extraction and yield of different co-products, in addition to the ecological benefit of diminishing anthropogenic waste [58,67].
Despite all the listed benefits, and even though microalgal HVP or other ingredients have high demand and price [58,68], their commercialization has not yet reached its peak. This fact is typically ascribed to the exorbitant expenses associated with the extraction and purification procedures currently in use. These high costs, which challenge the profitable commercialization of the compounds, are typically linked to the losses during purification steps and the complexity of the processes that require a lot of organic solvents, have a significant environmental impact or involve more specialized human resources and sophisticated equipment.
For instance, despite microalgae being the only vegetarian source of EPA and DHA, the high cost of fish oil, and the insufficiency of ω-3 products in the market, production costs of PUFAs from microalgal biomass are yet far from competitive, mainly because of the little proportions of PUFAs within the biomass [63].
The listed challenges require efficient and inexpensive extraction and purification processes in order to design new products, opening up new markets [68]. It is necessary to decrease product losses during purification steps [58] and to develop straightforward, efficient processes of hyperaccumulation of the greatest number of HVP [68]. Innovative strategies, such as system analysis theory and computational fluid dynamics [21], are currently being researched and applied; however, hyperaccumulating strain selection, culture condition optimization [67], and minimizing losses due to product degradation [58] are still the holdups to be currently resolved. Economic and environmental studies regarding HVP production from microalgae are needed, as it is a market-driven process. The successful establishment of microalgae products in general, as well as from Chlorellaceae and Scenedesmaceae, depends on all this to ensure a reduction in the cost of production [68].
3. Microalgae-Based Wastewater Treatment (WWT), Potential for Pathogen Removal and Utilization of Biomass
3.1. General Process
As an alternative to removing nutrients and heavy metals from WW and to stop the spread of microorganisms carrying water-borne diseases, microalgal treatment of WW has been studied for more than 50 years. Algal WWT systems are now generally acknowledged to be just as effective as conventional treatment systems [69].
WW-integrated algal biorefinery has recently attracted considerable interest and attention. The state of the art in the field provides evidence that municipal [70,71], agricultural [72], and industrial [73,74] WW can be sources of chemical elements that serve as nutrients for microalgal growth. Microalgae have been rigorously investigated because of their high metabolic flexibility as promising bioremediation agents [75] to be used in tertiary WW treatment for the removal of conventional pollutants, such as nitrogen (N) and phosphorus (P). Chemically- and physically-based technologies to remove excess nitrogen and P from WWs are expensive [76]. In this regard, microalgae are also a sustainable and promising technique for antibiotic removal [76,77]. In addition, microalgae could cooperate with some aerobic bacteria in wastewater, exchanging CO2 and O2. This process results in WWT, where the bacteria oxidize the organic compounds.
Furthermore, the ability of microalgae for multi-contaminant removal can be greatly improved using immobilization techniques. Using algal cells in fixed positions, such as biofilms or immobilized cells, leads to better and faster remediation. This accelerated reaction rate is due to an increased cell density and other factors [69].
Chlorella vulgaris has several advantages for WWT. It accumulates nutrients up to 60% of its biomass, and, as noted, they might possibly be processed to HVP. In addition, as Virdi (2019) lists, it grows very quickly, as noted, compared to other microalgal species. It has been found to grow well in WW and can grow dynamically in the presence of essential nutrients that can be readily obtained from any kind of WW. It has the capacity to eliminate organic compounds and heavy metals from its nutrient medium as well. The species is adaptable for WW remediation because it can grow in a variety of environments with different nutrient concentrations, temperatures, and pH levels. Furthermore, all three growth conditions—autotrophic, heterotrophic, and mixotrophic can support the growth of Chlorella spp. [69].
Chlorellaceae, Scenedesmaceae, and Arthrospira species are among the most researched single species for WWT, apart from mixed consortia of microalgae. Virdi (2019) lists several promising studies on WWT and the aforementioned Chlorophyta families [69], and we would like to highlight some more.
Olive mill and industrial WW can be effectively treated by microalgae, specifically Chlorella and Scenedesmaceae [72,78]. For instance, Tao et al. (2017) experimented with cultivating Tetradesmus lagerheimii using biosludge digestate from pulp and paper mills. The findings indicate that, at a biomass concentration of 2.9 g/L, Chlorella and Scenedesmaceae spp. could remove more than 99.99% of NH4+-N and more than 96.9% of PO₄−3-P. Furthermore, it has been reported that Chlorella sp. can withstand high CO2 concentrations and convert them into biomass [79]. Wu et al. (2017) showed that Chlorella sp. could grow using raw textile WW [72], and 84% and >60% of the NH4+-N removal efficiency and chemical oxygen demand (COD) removal efficiency were achieved with aeration at 0% and 10% dilution rate, respectively. Chlorella sp. cultivated on brewery WW was also found to remove 94% ammonia and 88.5% nitrogen with a decrease in COD [80]. Tetradesmus obliquus has proved to be able to remove ammonia and nitrogen from municipal WW effectively [81]. Heavy metals like Cd2+ have the ability to stimulate the production of extracellular sulfated polysaccharide (EPS) and protein by C. vulgaris at concentrations lower than 0.5 mg/L. This can lead to faster interactions between Cd2+ and the bacteria in EPS fractions, which in turn improves the ability of C. vulgaris to remove ammonia and phosphate [82].
Special attention has to be given to heavy metal ion removal from WWs and the main mechanisms of ion uptake by the microalgal cells [83]. Since green microalgae are the most widely used species and can adsorb metals, they have a great deal of potential for treating WW contaminated with them [76]. Desmodesmus communis (Scenedesmaceae) was able to reduce nickel and zinc from WWs by 99%, and Chlorella miniata reduced the amount of nickel by 70% [84]. A constraint to the removal process is the presence of organic carbon sources in the growth medium [85,86,87]. Because the WW from the dairy industry is typically high in COD and organic matter, mixotrophic microalgal strains—such as Scenedesmus sp. and Tetraselmis sp.—might be particularly effective at treating these waste streams [88]. While the produced water from the oil and gas industries may be toxic to many microalgal species, some of them, such as Chlorella sp., Dunaliella sp., Scenedesmus sp., etc., may be able to withstand and even thrive in the (pretreated) produced water [89]. EPS from Scenedesmus sp. and C. vulgaris played an important role in the settling of algal biomass via bioflocculation for alga harvesting from WWT for downstream applications [82].
A commercial microalgae system can also be linked to plants, factories, or farms, which emit flue gases, including CO2. The microalgae would not only metabolize the CO2, thus fueling their biomass, but this biomass could be used for inoculating WWs to achieve WWT. That process would help the environment to be preserved when using also atmospheric CO2 [77,90,91]. These two benefits of WW and CO2-integrated microalgae-based biorefinery are a realization of the clean circle and a profitable economy [91,92,93]. Such a type of microalgal integrated biorefinery concept is given in Figure 1.
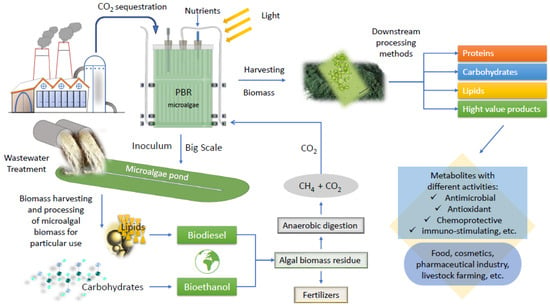
Figure 1.
Scheme of an integrated microalgal biorefinery concept. Biomass potential application.
3.2. Pathogen Inhibition
Even after the decrease in mortality from infectious diseases, they remain the third leading cause of death, responsible for 14% of the deaths globally in 2019. Among them, diarrheal diseases are second after pneumonia, claiming 1.5 million lives annually, or 2.7% of the deaths in 2019 [94]. According to an estimate by the World Health Organization and other estimates, the mortality of water borne diseases exceeded 3.4 million people in 2001 [95] and between 1.4 to 3.5 million people in 2019 [96,97], remaining one of the leading causes of human morbidity and mortality worldwide [98]. This occurs mainly in low-income countries, mostly due to anthropogenic pollution, and the victims are mainly children under 5 years old. Therefore, improving the global WWT capacity is warranted.
The antibacterial effect of microalgae from environments little affected by anthropogenic influence (natural biotopes) is weaker compared to cultures of microalgae from highly polluted waters (WWs). It is likely that this reflects a defense mechanism in a highly competitive environment of microalgae vs. bacteria [99]. For example, extracts of Dunaliella spp. isolated from clean versus polluted waters were studied by Lustigman (1988). Only species from contaminated water produce a heat-labile, non-proteinous substance that inhibits Escherichia coli [100].
This defense mechanism could be utilized in purifying the WW from pathogenic bacteria including those that have resistance to clinically established antibiotics. This presents the connection between the microalgal antimicrobial effect and WWT, leading to prevention or decreasing the risk of epidemics. This decontamination is a process close to the in vitro conditions of experiments testing the antibacterial activity of microalgae (but in a larger volume, which requires scale-up investigations).
Microalgae have been studied (although to a lesser extent than for conventional pollutants) as promising bioremediation agents in tertiary WW treatment for pathogen removal, such as coliforms [75]. An additional instance demonstrates that there were no direct effects observed when microalgae and Oligella, the predominant risk group 2 (RG2) pathogen, from piggery WW were co-cultivated. However, the mixotrophic (but not heterotrophic) microalgal cultivation induced a sudden change in the bacterial community, and the RG2 pathogens were reduced by 63% after the raw piggery WW underwent microalgal treatment. The findings demonstrated that this was an indirect effect of microalgae linked to bacteria—Oligella was indirectly connected with microalgal growth via other bacteria [101].
In fact, C. vulgaris was subjected to one of the first WW pathogen removal research. Interestingly, soon after discovering chlorellin, Pratt et al. (1951) described its practical application during World War II. The authors claimed that it was safe to release open sewage from the military installations into nearby streams due to its high C. vulgaris inoculation. Actually, the coliform count was often lower in effluent from pools heavily inoculated with C. vulgaris and not chlorinated than it was in effluent from chlorinated pools that contained no C. vulgaris. This happened primarily during the summer months when high light intensity and roughly vertical sun rays favored the growth of the algae [102]. More recently, C. vulgaris was able to remove phosphate-phosphorus (PO4–P) and reduce coliform proliferation and even the antibiotic-resistance genes the sul1 and blaTEM in the bacteria as well as the intl1 gene. The last is encoding the class 1 integrons integrase, found in pathogenic bacteria of humans and domestic animals and commonly used as a marker for anthropogenic pollution and related to sul1 as it integrates it in the bacterial genome. However, autochthonous microalgal species belonging to the classes Cyanophyceae, Chlorophyceae, and Bacillariophyceae proved to be more efficient [103]. For reasons already described, Virdi (2019) also selected C. vulgaris for his study related to WW remediation and pathogen inhibition of E. coli and Klebsiella pneumoniae. He chose these bacteria because they are frequently discovered in wastewater and notorious for harboring multiple water-borne illnesses, including pneumonia, dysentery, diarrhea, and acute gastroenteritis. Chlorella vulgaris excelled in both tasks, and the efficiency of phycoremediation was improved by immobilizing algal cells in beads of sodium alginate. However, the antimicrobial tests were of an extract in vitro and not in situ or co-cultivation in the WW [69].
The antimicrobial microalgal metabolites can be stored inside the cell (endometabolites) or secreted outside of the cell (exometabolites). Therefore, supernatant and pellet fractions differ in their biological activities [104]. Microalgal exometabolomes remain understudied, and some exometabolites were found to have antialgal effects, suggesting potential roles in allelopathy [105]. Nevertheless, practice shows that antibacterial and antifungal metabolites are more often stored inside than secreted, and the supernatants rarely have antimicrobial activity [106,107]. Another example illustrating this is the fact that the maximal content of free fatty acids (FFAs) in algae culture medium is reported to be in the stationary phase, as it is directly correlated to the process of autolysis when they are released. That could also happen in natural conditions in a water basin. Since FFAs have a general antibacterial effect, their release could secure the resistance and stability of the natural biocenose to the settlement of allochtonic pathogenic microbes. The rate of their elimination could rise with microalgae mass dying and this is one of the mechanisms of self-purification of the water basin. FFAs and other intracellular substances released during microalgal destruction could also play a role in the antibacterial effect of peloid (mud or clay often associated with water basins and used for therapeutic purposes) [32,108]. An example of such effect is the peloid containing extracts of C. vulgaris, T. obliquus, and Desmodesmus magnus var. magnus that had a pronounced antibacterial effect against four opportunistic bacteria—Staphylococcus aureus, E. coli, Klebsiella ozaenae, and Pseudomonas aeruginosa [32]. There is a hypothesis that other microalgal antibacterial compounds are also released after autolysis, or are alternatively induced by the presence of bacteria [109].
3.3. Utilization of Microalgal Biomass
On the other hand, the harvested microalgal biomass could be used for several applications, and this, naturally, would reduce the cost of microalgal cultivation, as there would be little to no requirement for additional nutrients. There is a disagreement between published scientific opinions about these applications because of the obvious safety concerns with microalgae cultivated on WWs (MCW). For example, while some authors state they could not be used for animal feed or the cosmetic industry, others believe that their HVPs are able to enter human nutraceutical and pharmaceutical production, given that they meet the regulatory requirements in terms of toxicology and pathogenic bacteria [69,103,110,111]. If, in the future, MCW is processed into antimicrobial (and other) pharmaceutical agents, another link between the antimicrobial effect of microalgae, WWT with such algae, and antimicrobial MCW would be provided.
The European Union legislation explicitly includes some types of WWs as input materials for obtaining ingredients for fertilizers (EU2019/1009) [112], and the use of MGW is not explicitly prohibited for use in animal feed (EU 68/2013) [113] and cosmetics (1223/2009/EC) [114].
Su et al. (2023) and Al-Jabri et al. (2021) [89] state in their reviews that microalgae cultivated on WWs cannot be used for human applications but only in manners that do not directly affect the human food chain. They could be used as animal feed (except those grown on urban WW), biofertilizers and biostimulants [115], biofuels, bioplastics, bulk chemical production, nutrient recycling, and as ingredients for the cosmetic industry.
Among them, the animal feed application is actively researched. The same authors outline several studies where microalgae cultivated on WWs (MCW) stimulated animal growth performance when fed to fish and pigs [116]. Among them, a consortium of Chlorella sp. and Scenedesmus sp. from anaerobic digested piggery effluent and used for pig feedstock had meal approximately 1.9 value-adding use of ω-3 and ω-6 PUFAs [117]. C. vulgaris and T. obliquus grown on effluent from crabs seafood production and later used for fish diet supplement had high protein and high carotenoid content for attracting colors [118], supporting the view that MCW from aquaculture WW can be used for aquaculture feed [111]. Scenedesmus sp. cultivated in chicken slaughterhouse WW and later fed to fish showed high EPA content [56].
The authors stress that animal-fed utilization is possible only if the end algal product is safe, which would be ensured by processing if needed and strict microbiological control for pathogens [116]. The safety of some MCW, including Chlorella and Scenedesmaceae, in terms of toxicology evaluation and pathogenic bacteria load, suggests no issues for the use of this biomass as an animal feed and has been found to meet regulatory requirements of conventional animal feedstock [119].
In the review of Srimongkol et al., 2022 [103], several studies about species of the two families, alone or combined with other microalgal species, are outlined. They include C. vulgaris, C. zofingiensis, T. obliquus, etc., grown on WWs such as municipal domestic aquaculture, piggery, and the end products of MGW were biodiesel, ethanol, hydrogen, methane, proteins, lipids, and carbohydrates.
If used as fertilizers and biostimulants in the European Union, regulations for permitted levels of heavy metals should be met [116]. In order to produce biodiesel from MCW, the wet biomass needs to be dried, which could be very energy-intensive; therefore, biogas (methane) energy production is more cost- and energy-effective. Bulk chemical production includes alcohol, lactic acid, glycerol, and EPS which could be used as a gelling agent, thickener, stabilizer, etc. [89,116].
Limitations of MCW utilization exist. A success has been demonstrated mostly at a relatively small scale (mainly indoor) while longterm, large-scale demonstrations are limited, thus needed [76,89,116]. One of the major and practical limitations faced in microalgal WWT systems is the harvesting or separation of the algal biomass from the treated water discharge. In fact immobilization of algal cells has been proposed as one of the ways for circumventing that problem [69]. Nevertheless, the potential of microalgae under control conditions for WWT in order to purify WW and, at the same time, to be a source of biomass with economic benefits is extremely promising. Having experience with culturing methods, the researcher may direct the metabolism of microalgae by combining different engineering approaches and complex working conditions where stress factors can be controlled in the proper way, in particular PBR design. This is because the phycoremediation procedure includes physical, chemical, and biological influences for WWT. This field is very challenging and demands efforts in order to explore the antimicrobial potential of microalgae biomass and to identify, isolate, and optimize the production of potential valuable antimicrobial agents, which will dramatically decrease the cost of the overall microalgae technology for phycoremediation of wastewater which in fact represents the concept of integral biorefinery.
4. Antimicrobial Activity of Microalgae, with a Focus on Chlorellaceae and Scenedesmaceae
4.1. Antimicrobial Metabolites from Microalgae
The rising antimicrobial resistance to established antibiotics is an incentive for intensive research of their alternatives and plant or algae natural products provide immense opportunities. The significance of compounds originating from land plants and aquatic organisms is being increasingly acknowledged by the pharmaceutical industries [120]. Apart from oncology and immunoregulation, the therapeutic effects of natural products and drugs derived from them are mainly achieved in infectious diseases, as their targets are major crossroads in signaling pathways in cancer cells or pathogens [121]. Some of the benefits of natural products include their economic advantage and fewer side effects. The high cost and slow rate of the antibiotic drug discovery pipeline processes determine the need for fueling this pipeline with new agents [122]. Natural products often do not have the significant side effects of synthetic drugs, such as hypersensitivity, allergic reactions, and immunosuppression. In addition, substances from the plants used in traditional medicine have a proven effect and compatibility with the living organism [123,124,125]. Microalgae have been used as food from ancient times, such as Arthrospira—by Aztecs and natives of Chad—Nostoc—by ancient Chinese people—and Chlorella in the present time [126].
Another particular example is drugs for amoebic diseases, which have been reported to be mutagenic. It has been suggested that natural products have not only more potential to treat amoebic diseases but are less prone to develop mutagenic effects in the host; the amoebas have a smaller possibility of developing resistance [127]. Although microalgae may not always be as effective as chemical fungicides, they pose fewer environmental risks and fewer food safety problems [22].
Adhoni et al. (2016), after obtaining effects as high as those of gentamycin, called the organic extracts of different algal species, including C. vulgaris, “herbal antibiotics” and were even optimistic that they could be used in place of synthetic or chemically manufactured antibiotics [22]. We hope that, even if found not suitable to replace antibiotics, they will supplement them and acquire more deserved clinical presence. They can be utilized in combination and synergism with clinically approved chemotherapeutics so that the dose of the latter is lowered, leading to fewer adverse effects.
The antibacterial activity within the same species of microalgae can vary due to ecotypes that are tailored to suit distinct environmental conditions [109]. As noted, the antimicrobial metabolites of microalgae can be exometabolites or endometabolites released after the loss of algal integrity. Therefore, supernatant and pellet fractions differ in their biological activities [104]. Nevertheless, practice shows that they are more often stored inside than secreted, and the supernatants rarely have activity [106,107]. The yield of the antimicrobial compounds can be increased by recombinant techniques [30]. As noted, there is a hypothesis that these compounds can also be induced by the presence of bacteria [109].
Because of the new types of compounds discovered in recent years, the identification of the compounds directly responsible for the antimicrobial potential of algae remains a relatively nascent field of research. Furthermore, studies are still in their early stages from a commercial perspective [1,2]. Nevertheless, research has shed light on numerous antimicrobial compounds and their mechanism of action.
4.1.1. Fatty Acids
Generally, most fatty acids have high antibacterial properties. In fact, 10-undecylenic acid (C11:1n-1) is already used clinically to treat fungal infections of the skin and nails. In addition, there are numerous patents utilizing the antimicrobial effect of FFA for medical, agricultural, and other uses, as summarized by Desbois, 2012. Some studies have suggested that FFA may be used to treat systemic infections; however, the patented and most promising uses of FFAs in medicine are where conventional antibiotics are undesirable or forbidden, and especially topical and surface-based, such as preventing microbial colonization of medical equipment, eliminating drug-resistant pathogens from hospital patients’ skin and noses, and treating microbial infections of the skin, wounds, and mucosa. There are also patents for animals, e.g., for prevention and treatment of infections of plants, bees, hooves, mastitis, gastrointestinal infections, or as alternatives to antibiotics for increasing the growth performance of livestock. These substances could be helpful preservatives and disinfectants of food, feed, cosmetics, and medication. Desbois finds that, so far, the full commercial potential of antimicrobial fatty acids has yet to be realized [8]. This is likely because some of them can be unstable or bind nonspecifically to proteins [128], their other biological activities are a complication, their development would divert resources from the development of conventional antibiotics, there may be a perceived lack of patentable intellectual property, etc. However, there are solutions for these problems, and FFAs hold potential for commercial application, especially because they act through different mechanisms to most conventional antibiotics, and the evolution of inducible FFA-resistant bacteria is less problematic and frequent than with conventional antibiotics [8].
The short- and medium-chain saturated fatty acids are most often in the patent literature [128] but that does not exclude the PUFAs and other fatty acids from microalgae to be utilized in the future.
FFA, if extracted to their free form or resulting from autolysis, are among the most potent antibacterial compounds from microalgae, too, and the lipid composition of microalgal extracts influences their antimicrobial activity [1,24,44,129,130,131,132,133,134]. The Chlorophyta (green algae) phylum is known for its ability to synthesize a variety of PUFAs and other lipids. Other lipids, such as glycolipids, can also be antibacterial [135]. The microalgal properties against some specific human pathogens, such as E. coli, P. aeruginosa, S. aureus, Staphylococcus epidermidis, Klebsiella spp., and Bacillus spp., have been attributed to EPA, DHA, palmitoleic, lauric, oleic, lactic, arachidonic, γ-linolenic, stearic, and hexadecatrienoic acids [129]. These pathogens are significant since they are considered to cause water borne and vector diseases, nosocomial infections, pneumonia, etc., therefore causing drastic health impacts [37,99]. It is important to consider that certain compounds, such as cholesterol and vitamin E, can counteract the antimicrobial effects [130], for example, the antiprotozoal effect of PUFAs [10,136].
As FFAs are surfactants, they influence bacterial cell membranes. However, the precise mechanism of action of fatty acids is still not fully elucidated, as they may influence multiple cellular targets. The mechanism is discussed in detail, to the best of our knowledge, by Desbois and Smith, 2010 [128] and later by Chanda et al., 2018 [136]. Even small disruption of membrane integrity leads to electron leakage that may result in cell death. Greater destabilization and membrane damage likely lead to a change of cell surface charge, cell leakage, the entry of harmful components, and the reduction of nutrient uptake. It has been documented that algal FFAs inhibit electron transport systems and cellular respiration. The cell then undergoes lysis, after which peroxidation and auto-oxidation degradation products are formed [128,129,136,137,138,139]. Additionally, FFAs may start peroxidative reactions, impede bacterial fatty acid synthesis (block FabI enzymes) [128,136,138,140,141], and inhibit bacterial enzyme activity [128,136,139]. The antimicrobial mechanisms of PUFAs, including ω-3 ones, may also include the disruption of cell-to-cell communication and ATP production, alteration in membrane hydrophobicity, and increasing membrane poles, which causes cellular leakage [128,136].
Recent structure-function relationship studies suggest that fatty acids’ capacity to impede bacterial growth is dependent on both their chain length and degree of unsaturation [129,142]. It appears that fatty acids containing more than ten carbon atoms cause bacterial protoplasts to lyse. An example of this is EPA and linolenic acid from Ennalax costatus, which demonstrated activity against aquaculture bacteria [130].
4.1.2. Polyunsaturated Fatty Acids (PUFAs)
Polyunsaturated single-cell oils possess antimicrobial and anticancer properties [24]. Among FFAs, the longer-chain polyunsaturated ones, such as EPA and DHA, are especially active antimicrobials and are abundant in microalgae [1,24,44,129,130,131,132,133,134]. EPA and DHA have also demonstrated antibiofilm properties [143,144]. ALA has also shown an antibacterial effect by inhibiting methicillin-resistant S. aureus (MRSA) [47].
It is interesting that EPA, DHA, and other PUFAs are normally found in foods, e.g., fish oil and are components with a physiological role in the organism. Yet, in vivo results show that when increasing their concentration in the organism through consumption, genetic engineering, etc., they suppress infections and also have a prophylactic effect.
In their review, Alhusseiny and Beshbishihave cover the in vivo antimicrobial effect of fish oil or different ω-PUFAs such as DHA and EPA (ω-3), arachidonic and linoleic acid (ω-6), oleic acid (ω-9) against protozoa, such as Plasmodium spp., Trypanosoma, Toxoplasma, etc., in laboratory animals. Increasing these fatty acids in the administered diet or an intra-peritoneal intake route suppressed the infection, decreased parasitemia, and improved the survival of laboratory animals in comparison with the controls (with few exceptions). Specific effects such as decreased cerebral malaria, cardiac parasitism, caecal lesions, and number of Toxoplasma gondii infected cells, and increase in specific antibody levels, etc., were also observed [10].
In addition, increased production of EPA and DHA was achieved by expressing delta-6 desaturase and elongase genes in the liver of transgenic zebrafish. Interestingly, the survival rate of transgenic strains was 70%, while that of wild type was 20% 24 h post Vibrio vulnificus infection. Antimicrobial effects of DHA on Burkholderia cenocepacia using a Galleria mellonella caterpillar in vivo model also showed that ω-3 PUFAs have antimicrobial properties [136]. Transgenic mice that have large amounts of ω-3 PUFAs and low levels of ω-6 PUFAs in their tissues have demonstrated significant reductions in the T. gondii cyst burden in brain tissues [10].
Nevertheless, whether PUFAs exert that suppression of infection in vivo because of direct antimicrobial action, because of their anti-inflammatory effect, or because of both and to what extent is not known. For that reason, PUFAs are also looked into in the next section about immunomodulatory activities. Chanda et al. point out that ω-3-PUFA studies so far typically have focused on ameliorating inflammatory tissue damage rather than getting rid of the etiological agents. Since more evidence of the in vitro and in vivo broad-spectrum nature of fatty acids has accumulated, including direct antimicrobial effect, clinical studies are of utmost importance to substantiate the antimicrobial potential of ω-3 PUFAs, authors conclude [136]. Cheng et al. are of the opinion that EPA and DHA both inhibited bacterial growth directly, as well as enhanced the production of anti-inflammatory cytokines, thereby protecting zebrafish from V. vulnificus infection [145]. More studies aiming to understand the main pathway/s responsible for the activity of PUFAs are needed.
It is true that some drawbacks exist, for example, toxicity, the low oral bioavailability of PUFAs, the fact that vitamin E and cholesterol reverse their antiprotozoal effect, etc., but that could be overcome by technological approaches [10,136].
To date, unfortunately, as Firoozabad and Nasr conclude, the utilization of ω- and specifically ω-3 PUFAs as antimicrobial agents has not been widely appreciated, perhaps due to a lack of understanding of antimicrobial mechanisms, toxicity, and route of administration, but it is worthwhile to consider ω-3 PUFAs in the list of potential antimicrobial agents [9].
Altogether, PUFAs can be considered novel adjuvant drug candidates or alternative therapeutic agents for infections, but future studies are required [9,10,136]. Clinical studies on the effectiveness of ω-3 PUFAs against pathogenic microbes are lacking and, hence, required. Additionally, their benefits are that they have little effect on evolving antimicrobial resistance and are also safe for human use [9]. The incorporation of ω-3 FAs into chewing gums, tooth paste, etc., may provide other routes of treating and preventing oral diseases [136]. In addition, authors encourage dietary intake of PUFAs [10].
4.1.3. Other Compounds
In addition to fatty acids and PUFAs, another important antibacterial class of compounds is polysaccharides (PSs). They are produced and secreted by algae and eukaryotic microalgae, such as Chlorella spp. and Dunaliella spp., at relatively high levels [2,129]. The pharmaceutical and food industries have seen the successful utilization of algal PSs and sulfated PSs. EPS produced by microalgae has acidic properties and may be used as a medicinal agent [146]. The anti-adhesive characteristics of the EPS of certain microalgae against the adherence of microbes may account for the antibacterial action of PSs. Since other carbohydrates on cell surfaces have already been shown to serve as recognition sites for microbes to attach to, PSs may compete with them for this role. The EPS’s capacity to prevent the formation of biofilms may also be connected to its antibacterial action [147] and, therefore, to its anti-adhesive properties. The majority of data point to these compounds’ ability to alter biotic surfaces’ physical characteristics as their mode of action [148]. The distinct cell surfaces of Gram-positive and Gram-negative bacteria may account for the variations in the corresponding inhibitions detected by the EPS [146]. According to Rendueles and Ghigo (2012), PSs may alter the bacterial cell surfaces’ physical characteristics because they are surfactant molecules. PSs derived from microalgae may function similarly to exopolysaccharides from E. coli, which have the ability to prevent bacterial cell adhesins from causing autoaggregation [148,149]. Another mechanism might be the result of PSs binding with substances in the bacterial cell wall and membrane through glycoprotein receptors on the bacterial cell surface. This leads to increased cell membrane permeability, protein leakage, and bacterial DNA binding [137,150]. As sugar polymers, polysaccharides can also function as lectin inhibitors. Lectin is primarily found on the surface of bacterial cells, and it helps bacteria adhere to their hosts by attaching to the glycan substrates on the surface of those cells [151]. By competing with lectins’ sugar-binding domain, PSs prevent pathogen adherence that is dependent on lectin and the creation of biofilms, which lowers the risk of infection. Hydrocolloids’ mechanism of action could be linked to the fact that EPS wound dressings diminish scarring and wound secretions, which in turn lowers bacterial inflammation. [149]. Additionally, sulfated PSs have the ability to prevent infections by preventing their adherence to the gastric surface.
Antibacterial peptides are another class of compounds, although they are mainly cyanopeptides (from cyanobacteria, e.g., Oscillatoriales and Nostocales) [152,153,154]. Cyanopeptides are mainly non-ribosomal and cyclic peptides. Antibacterial microalgal peptides are considerably rarer, e.g., few peptides from Tetraselmis suecica and protein hydrolysates from two Chlorella species. The antibacterial mechanism of action of cyanobacterial and microalgae peptides is generally not known [152]. It has been suggested that Chlorella peptides induced bacterial cell wall destruction and cell growth inhibition [155]. The generally more potent effect against Gram-positive bacteria than against Gram-negative is observed for many compounds and is especially expressed for these peptides. An example is the pepsin hydrolysate and the peptide fractions from C. sorokiniana. This is likely due to hindered penetration of the large oligomeric structure of the peptide by the outer lipopolysaccharide bacterial membrane [156].
Chlorophylls (such as chlorophyll-a derivatives) and carotenoids are some of the major pigments present in microalgae and are not only antioxidants but also effective microbial growth inhibitors, especially β-carotene among the carotenoids [157,158]. There is a hypothesis that most of the antibacterial compounds are synthesized as a result of chlorophyll and FFA oxidation [32,108]. There is also a patented method of using astaxanthin to prevent bacterial infections, such as from Helicobacter pylori [142,159,160].
Phenolic compounds from microalgae are both antibacterial and antifungal. At high concentrations, they can denature cell proteins, which involves the hydrogen bond formed between phenol and protein. Cell walls and cell membranes, which have high protein content, become disrupted by permeability, causing lysis due to leakage [129,130,132]. Phenols, at low concentrations, have been shown to impact the activity of enzymes, particularly those involved in energy production [135]. The antifungal effect may be due to the impact on spore germination [161]. For multicellular parasites, phenols have been suggested to bind to lipids in membranes of the cell and organelles and change their functional, thus killing the cell, a mechanism that could be valid to the eukaryotic microorganisms as well [162].
Other important identified antimicrobials are microalgal amino acids, terpenoids, sterols, saponins, acetogenins, sulfur-containing heterocyclic compounds, and other carbohydrates. Steroids, florotanins, halogenated ketones, and acrylic acid can be used as antimicrobial and anticancer compounds [1,44,129,130,131,132,133,134,139,141]. Steroids destroy the bacterial cell membrane, while saponins lower the surface tension of the cell wall entering the cell easily, then disrupting bacterial metabolism and eventually killing the cells [141]. Other chemical classes such as indoles, terpenoids, acetogenins, and hydrocarbons, such as volatile halogenated hydrocarbons, are accredited to give microalgal extracts not only antibacterial but also anti-protozoal and antiplasmodial effects [2,8,163]. Through the formation of a strong bond polymer, terpenoids have the ability to damage the transmembrane protein Porin in the outer membrane of bacterial cell walls. That would cause a reduction in the permeability of cell walls and, thus, nutritional deficiencies, leading to growth inhibition and even death [141].
Engineering approaches related to the optimization of PBR conditions, as well as gene and metabolic engineering, can be used to boost and maximize the production of antimicrobial or other bioactive compounds.
4.2. Immunomodulatory and Infection Preventing (Prophylactic) Activities
Since microalgae are used as food additives, the antibacterial effect contributes to their nutraceutical properties. Green microalgae have been suggested as a potential substitute for in-feed antibiotics in order to protect the human food chain by preventing disease in livestock and poultry and preserving the microbiological safety of animal products [137]. Pigments in fish are generally from the microalgae they consume. Fish with high levels of carotenoids are more resistant to bacterial and fungal infections [47]. Aquatic animals’ immunity could be strengthened by utilizing the antioxidants found in microalgae, which would solve the issue of antibiotic overuse. It was found that the large yellow croaker Pseudosciaena crocea showed an increase in lysozyme activity, an indicator of fish immunity, with an increase in astaxanthin levels. This suggests that fish immunity is positively correlated with the consumption of microalgal pigments (astaxanthin) [164]. Astaxanthin significantly improves human neutrophil phagocytic and microbicidal capacity [165]. C. vulgaris is a source of (1,3)-β-glucans and by promoting the synthesis and/or expression of interleukins (IL), dectin-1, and toll-like receptors-2 on macrophages and dendritic cells, respectively, those glucans produced antifungal and antibacterial responses in rats and some resistance to mammalian organisms against infections with E. coli. Only C. vulgaris contains homo-polysaccharides of galactose and glucose [149]. Likely as a consequence of this, patients treated with C. vulgaris tablets for non-alcoholic fatty liver disease had significantly lower serum levels of the pro-inflammatory cytokine TNF-α. Using various chemical triggers (such as low oxygen, NaCl, and nutrient starvation), Montero-Lobato et al. (2018) reported that some microalgal species (such as C. zofingiensis, D. salina, A. platensis, etc.) produced more anti-inflammatory molecules (carotenoids, fatty acids, and sulfated polysaccharides) [166]. Studies revealed that macrophages from the mouse peritoneal cavity could be activated to engage in phagocytic activity by extracts of Chlorella stigmatophora polysaccharide. A diet supplemented with C. vulgaris enhanced the natural killer activity of peripheral blood mononuclear cells isolated from the patients receiving treatment and raised the levels of interferon-gamma, IL-1, and IL-12 in the serum [167].
As already elaborated, whether PUFAs exert suppression of infection in vivo because of direct antimicrobial action, because of their anti-inflammatory effect (influencing cytokine and leukotriene production), or both is not known. As Chanda et al. point out, there are a lot of ω-3-PUFA studies that show ameliorating tissue damage caused by activated host response [136]. Alhusseiny and Beshbishihave also state that the protective effect against parasitic infections of ω-PUFAs is due to both anti-microbial properties and anti-inflammatory effect, but stress that they prevent the inflammatory and oxidant reactions harming the human body [10]. Cheng et al. also support the dual action of EPA and DHA [145]. Further studies are warranted. Ω-3-PUFA are potential agents of food preservatives, and their incorporation into products such as chewing gums, drinks, tea, milk, sugar, tooth paste, or dentifrice may provide the antimicrobial requirements for preventing oral diseases [136].
4.3. Chlorella vulgaris and Other Chlorellaceae Strains
The first discovery of the antibacterial effect of microalgae was in the 1940s when “chlorellin”, a mixture of fatty acids from C. vulgaris, was able to inhibit the growth of Gram-positive and Gram-negative bacteria (P. aeruginosa, S. aureus, Streptococcus pyogenes, and Bacillus subtilis) [168]. Although chlorellin was unsuitable for widespread commercial use, it announced the upcoming studies on algal antimicrobial inhibitors [137]. As mentioned, C. vulgaris also has a wound-healing ability better than calcium sodium alginate [41]. This species has the ability to deform E. coli cells, make their surfaces rough, and make depressions in some cell walls [169]. Another study found that E. coli was moderately susceptible to its acetone and ethanolic extracts, while S. aureus had low susceptibility to various solvent extracts of C. vulgaris [170]. The species was reported to reduce dramatically the biofilm of P. aeruginosa without inhibiting its growth and without high bactericidal activity. The effect was focused on the interference of bacterial signaling. Therefore, the extracts inhibited the quorum sensing not due to the reduction of “quorum” but due to the interruption of “sensing” [171]. Plaza et al. (2012) found that almost no differences in the effect of pressurized liquid extracts (PLE) and ultrasound-assisted extracts (UAE) of the species could be observed when changing the extraction temperature. Activity was mainly related to the polarity of the extraction solvent (organic solvent and water) [24].
In vivo activity is more important than in vitro, as an agent may have strong in vitro activity but fail in an in vivo test or show unacceptable side effects. There have been few papers covering the in vivo effect of Chlorella spp. but some of them show very promising results.
Dermatophytes are a highly specialized group of fungi that have adapted to colonize the keratinized tissues of animals. Twenty to twenty-five percent of people worldwide are affected, and the frequency is rising. Impetigo is a bacterial cutaneous infectious disease, and its treatment with antibacterial agents may cause serious problems. C. vulgaris ointment applied twice a day for a total period of one week for impetigo and two weeks for fungal infections was effective, and the histological examinations of the rat’s tissues revealed the fast healing of the skin layers and normal histological patterns of the skin, with minimal inflammatory cellular infiltrate. Positive controls of ciclopirox and nystatin creams were applied as antifungal agents, while mupirocin was applied as an antibacterial agent. These synthetic ointments illustrated partially cured lesions and inflammation (partial healing). The antimicrobial material was defined as a phenolic nitrile [161].
Amoebic dysentery is one of the tropic public health problems. An extract of Chlorella sp. completely eliminated the amoebic cysts from the feces of mice, but there was no information on the amount of extract consumed, only for its concentration. The therapeutic efficiency of the extract was 69%. It reverted to a high extent the pathological changes in the tissues of the large intestine caused by the amoebic trophozoite [162]. Unfortunately, no positive control was applied in order to compare the effect with an established drug.
In a different in vivo study, mice were intragastrically inoculated with H. pylori three times, separated by two days. Mice were given the whole carotenoid extract of Chlorella sp. (100 mg/kg body weight per day, 0.15–0.18 mL in corn oil) orally for two weeks following inoculation. During this time, the stomach and spleen were removed and prepared for additional histopathology analysis. It showed that Chlorella sp. treatment could delay the gastritis process because the denudation of the surface epithelium, hyperplasia of the gastric gland, and swollen lamina propria were observed on day 14 after treatment only. However, the extract did not lead to improvement. It is known that β-carotene accelerates the gastric healing [172]. However, Isochrysis sp. had fewer carotenoids than Chlorella sp. and better activity; therefore, the action regarding these two species was not correlated to carotenoid content [173].
Doses of twice the effective inhibitory concentrations used in the in vitro inhibition study did not kill H. pylori and AGS gastric epithelial cells. A polysaccharide agglutination assay showed that allowing porcine gastric mucin to interact with the polysaccharides before the addition of H. pylori did not prevent the agglutination of H. pylori. Similarly, the presence of mucin did not affect the polysaccharides in agglutinating H. pylori. Polysaccharides from A. platensis were confirmed to be effective carbohydrate-based antiadhesives in preventing H. pylori from adhering to gastric mucin and colonization without affecting the host [174].
Cepas et al. (2019) investigated the antibiofilm activity of 675 extracts from 225 microalgae, including Chlorophyta and cyanobacteria species, against clinically important bacterial and fungal pathogens [144]. Extracts from the phylum Chlorophyta showed higher minimal biofilm inhibitory concentration values than the other phyla. EPA and DHA were not evident, and the activity was not attributed to any particular compound [143,144].
In regard to the (presumably) active bioactive metabolites of proven antimicrobial C. vulgaris extracts, according to reports in the literature, as mentioned, this species is rich in PUFAs, and they act as antimicrobials [24]. Other antimicrobial compounds are fatty acids in general, carbohydrates, phenols including phytoalexins, phenolic glycosides, saponins, terpenes, unsaturated lactones, cyanogenic glycosides, sulfur-containing heterocyclic compounds, halogenated hydrocarbons, and acrylic acid. An aqueous extract with a concentration of 150 mg/mL was active against all tested organisms but Saccharomyces cerevisiae. The highest antimicrobial activity was observed for the protein fraction which indicated that protein compounds were responsible for the antifungal properties [175]. There was a high content of long-chain fatty acids, e.g., oleic acid, in the intracellular and extracellular extracts, which have been reported to inhibit S. aureus [176]. Phytochemical screening has also revealed the presence of flavonoids, tannins, alkaloids, and cardiac glycosides in various organic solvent extracts [37,170]. Tannins and phlobatannins have already been reported for their antibacterial and antiviral properties [170]. Flavonoids may be an additional reason for this alga to be used as an antimicrobial food supplement [37]. Hexane and chloroform extracts, particularly, have been shown to contain a large group of metabolites, followed by ethyl acetate and methanol extracts. Alkaloids, flavonoids, glycosides, carotenoids, phenols, lignins, saponins, sterols, tannins, reducing sugars, volatile oils, fats, amino acids, and carbohydrates were revealed [22]. The presence of triterpenes, condensed tannins, and saponins in an extract effective in an excision wound model was revealed. These have already been reported in the literature for antimicrobial activities and favor the wound-healing process. A hydrogel had a wound-healing effect by promoting the remodeling of damaged tissue, forming a barrier against and reducing the local bacterial population [11]. Twenty compounds were identified from PLE and UAE extracts; some of them have been described as carotenoid degradation products, such as neophytadiene and derivatives, which were detected in large amounts. This hydrocarbon, from the phytans family, had been identified in other microalgae and had previously demonstrated some antimicrobial activity. Phytol has also been described in different plant essential oils with proved antimicrobial properties [24]. Eight major compounds were found in ethanol and aqueous extracts. Diethyl phthalate and trimethyl(4-tertbutylphenoxy)silane, as the major compounds in the ethanol extract, have been reported to have antibacterial activity [171]. Heptanal, a known biomedical compound, and some other metabolites, such as 2-butanol and 4-methyldocosane, were found in a C. vulgaris extract [169].
Concerning the (presumably) active secondary metabolites of proven antimicrobial extracts of other or unidentified Chlorellaceae species, extracts have been found to contain phenolics, saponins, steroids, and triterpenoids [141]. Minimum inhibitory concentration (MIC) values of the best two extracts from the strain Chlorella SRD3 against Gram-negative bacteria (ethyl acetate and methanol) were tested. The compounds 2-(2-aminoethoxy)-ethanol and 9-octadecyne from the methanol extract had been previously reported to have antibacterial activity [177]. The phytochemical screening of the extract of C. sorokiniana revealed the presence of phytoconstituents, including anthraquinones, cardiac glycosides, flavonoids, saponins, tannins, and terpenoids [178]. Sterols, hydrocarbons, and fatty acid methyl esters are known antimicrobials. For example, polypropylene meshes are coated with a mixture of esterified ω-3 PUFAs to prevent microbial colonization and inflammation [8].
The extracts from A. pyrenoidosa had a broad spectrum of antimicrobial substances against Gram-negative bacteria. The high antimicrobial properties of the methanol extract may be due to the abundance of some lipophilic but polar compounds and corresponded to the highest percentages of the total phenolic and total flavonoid contents [179]. The main constituents detected in the ethanol extract of C. salina were organic acids like the antimicrobial palmitic acid (6%), octadecanoic acid, phytol, and the benzophenone ether octabenzone. When used in aquaculture (food), sea bass in the post-larval stage showed a decrease in the amount of S. aureus, so these algae could be used as a green water technique [131].
An overview of the literature data on the antimicrobial activity of C. vulgaris and different Chlorellaceae strains is given in Table 1 and Table 2, respectively.

Table 1.
A review of the research on the antimicrobial activity of Chlorella vulgaris Beijerinck.

Table 2.
A review of the research on the antimicrobial activity of different Chlorella spp., including unidentified ones.
4.4. Tetradesmus obliquus and Other Scenedesmaceae Strains
Extracts from Scenedesmaceae spp. have positive results but with varying values of IZs. They have higher antibacterial activity against Gram-positive bacteria than Gram-negative bacteria.
Phosphorus (P) is one of the most important nutritional factors that influence metabolism and cell growth. The optimum P concentration for the antibacterial properties of T. obliquus turned out to be 0.01 g/L P for S. aureus (IZ 26 mm) and 0.007 g/L P for E. coli [203]. MIC values of Desmodesmus subspicatus were more satisfactory using the dimethyl sulfoxide (DMSO) extract, which showed antibacterial activity against most bacteria tested, both Gram-negative and Gram-positive. However, the aqueous extract also had satisfactory activity, and employing an aqueous extract, if effective, is a cost-effective protocol that avoids the usage of toxic solvents [99]. The effect of five strains of Scenedesmus depended on the harvest time, strain-type, and extracting solvent. The majority of the strains had potent antimicrobial activity with MICs less than 1 mg/mL, but the MIC values for the majority of the PE extracts were not determined due to the insufficient yield from the extracting solvent [204].
Eleven compounds were identified in fractions from the diethyl ether crude extract (DEE) of T. obliquus, and most of them have been reported to possess biological activities, e.g., antimicrobial properties against B. subtilis, Enterococcus faecalis, E. coli, S. aureus, P. aeruginosa, K. pneumoniae, Bacillus cereus, Candida albicans, and several other species of mycotoxigenic fungi—Aspergillus niger, Aspergillus flavus, and Aspergillus fumigatus. The main compounds were nonadecane, butylated hydroxytoluene, 9-octadecadienoic acid (Z), and 7,3’,4’-trimethoxyquercetin. There were other compounds that had been reported to have antimicrobial activity when isolated from other plants, such as heptadecane, hexadecane, 2-hexadecenal octasiloxane, etc. [205]. Oleic and linoleic acids have been reported to inhibit S. Aureus, and they were abundantly found in the cells of different T. obliquus strains. The presence of high content of other long-chain fatty acids, such as EPA, may contribute to the antibacterial effect [176]. Nevertheless, there is yet to be a conclusive identification and characterization of specific metabolites responsible for the antibacterial activities of T. obliquus [142].
The most abundant compounds in the methanol extract of Tetradesmus dimorphus included phenols, carboxylic acids such as hexadecatrienoic acid n-4, esters, plasticizer compounds, and terpens, while in the ethanol, N-hexane and diethylether extracts, the most abundant compounds were found to be acyclic diterpene alcohols, plasticizers, carboxylic acids, hydrocarbons like limonene, azulene and eicosane and esters of phthalate and others. Terpenes, carbohydrates, phenols, eicosane, and palmitic acid may be reasons for the antimicrobial effects. Hexadecatrienoic acid n-4 has been found to be active against Gram-positive and Gram-negative bacteria and to be also highly active against MRSA [1]. Methyl esters are known antimicrobials. For example, polypropylene meshes are coated with a mixture of esterified ω-3 PUFAs to prevent microbial colonization and inflammation [8].
Since the crude pigment extract of a Scenedesmus sp. had high antibacterial properties, the authors concluded that the activity was most probably due to chlorophyll [157]. About 30 different fatty acid methyl esters (FAMEs) were identified in Desmodesmus bicellularis among which the methyl esters of DHA (2.3%), EPA (1.1%), linoleic (1.4%), linolenic (6.3%), stearic (0.6%), oleic (1%), and erucic acid (1%) acquire great importance as a result of their potent antimicrobial activity. It was previously established that via inhibiting FabI gene expression, this class of FAMEs inhibits the fatty acid biosynthesis pathway [206].
A summary of the antimicrobial properties of T. obliquus and other Scenedesmaceae spp. is given in Table 3 and Table 4, respectively.

Table 3.
A review of the research on the antimicrobial activity of Tetradesmus obliquus (Turpin) M.J. Wynne.

Table 4.
A review of the research on the antimicrobial activity of different Scenedesmaceae spp.
4.5. Evaluation of the Antimicrobial Activity of Chlorellaceae and Scenedesmaceae according to Different Criteria
The criteria for assessing antibacterial or antimicrobial activity in vitro are mainly in consideration of the MIC. Eloff, an experienced researcher of antimicrobial activity of natural products, proposed the following criteria for MICs: outstanding activity <20 μg/mL, excellent activity 21–40 μg/mL, very good activity 41–80 μg/mL, good activity 81–160 μg/mL, moderate activity 161–320 μg/mL and weak activity >320 μg/mL. Higher MICs may still be effective in ethnopharmacological studies [212]. Porras et al., 2021 [213] proposed stricter MIC criteria: high activity ≤10 µg/mL and moderate activity 11–100 µg/mL.
There are no universal criteria for IZ. Still, practice shows that, in addition to consulting The European Committee on Antimicrobial Susceptibility Testing (EUCAST) [214], it can be tentatively assumed that zones of inhibition above 20-30 mm are relatively large, therefore the agent that produced it—with relatively strong antimicrobial effect. In addition, the quantity of the extract on the disc has to be considered. When comparing it with the reference antibiotic, we have to remember that extract or fraction is compared to a pure compound.
As Table 1, Table 2, Table 3 and Table 4 show, the antibacterial and antifungal activities of the two genera are in a broad range—from no activity to weak, moderate, strong, or very strong activity. This depends on the algal species, the pathogen, the kind of extract and the criteria used. Often, an extract is weak against a species of bacteria but is highly active against another. For example, a crude pigment extract from a Scenedesmus sp. did not inhibit Salmonella sp. at any concentration, but its MIC was as low as 80 µg/mL on S. aureus [157]. Frequently, one organic solvent produces extracts that are more inhibitory than another. For instance, the ethanol and acetone extracts of C. vulgaris had better antibacterial activity than the chloroform and aqueous ones [170].
The undoubtedly best activity, in terms of MIC, was observed for a strain of Chlorella (SRD3) with a MIC value of only 1.5 µg/mL against E. faecalis (ethyl acetate extract) [177]. Therefore, this strain is with outstanding effect according to the criteria of Eloff and high according to those of Porras et al. Lipid extracts of C. vulgaris, rich in palmitic and linoleic acid, exerted a MIC of 10 µg/mL against Propionibacterium acnes [188]. A chloroform extract of Scenedesmus bijuga also had a very significant effect on K. pneumoniae with a MIC of 16 µg/mL. While both these extracts had an outstanding activity, according to Eloff, the latter had moderate, according to Porras et al., which is valid for all agents described next [196]. C. ellipsoideum, A. protothecoides, and A. pyrenoidosa had a MIC of 20 µg/mL against P. acnes (outstanding effect, according to Eloff) [188]. Other exceptionally good results with C. vulgaris are MIC values as low as 25 to 40 µg/mL (excellent effect according to Eloff), where K. pneumoniae and S. aureus were more susceptible than B. subtilis and Micrococcus luteus [192]. A Scenedesmus sp. showed a MIC of 80 µg/mL (very good activity according to Eloff) against S. aureus [157]. It is obvious that all listed examples fulfill the criteria of [215].
Regarding the IZ, the best results belong to a methanol extract of A. pyrenoidosa with an IZ of 48 mm against E. coli [179]. C. vulgaris showed an exceptionally high antibacterial and antifungal effect because its acetone extract inhibited Sarcina lutea and Fusarium oxysporum with IZs of 30 and 45 mm, respectively. F. oxysporum was also inhibited by a 35.5 mm IZ by a Scenedesmus sp. [190].
Although T. obliquus does not show that significant effect, Table 3 shows that some of its in vitro tests have demonstrated IZs as large as 26–28 mm.
Naturally, that great activity of Chlorella sp. SRD3 predicted correctly that its IZ would be greater than that of chloramphenicol [177]. The IZ of the mentioned A. pyrenoidosa surpassed the IZ of the control antibiotic [179]. Other Chlorellaceae representatives have also shown better results than control antibiotics in an in vitro test. For instance, The IZ of an ethanol extract of C. vulgaris for E. coli surpassed the IZ of tetracycline [37]. Scenedesmaceae spp. have not been found to exceed the activity of antibiotics, but some of their effects are still comparable. Tetradesmus obliquus had IZs nearly as large as that of vancomycin against S. aureus [203]. All tested solvent extracts of Tetradesmus bajacalifornicus had IZs nearly as large as that of fluconazole against C. albicans; the ethanol extract had an IZ comparable to that of the standard against B. subtilis, and interestingly, that was also valid for the aqueous extract against A. niger [211].
5. Conclusions
The biomass of Chlorellaceae and Scenedesmaceae is widely commercialized; however, in order to begin production of specific HVP, efforts ought to be directed toward minimizing product loss as well as the energy and equipment expenses related to the extraction and purification steps. Furthermore, to achieve processes that are both environmentally friendly and economically viable, large-scale downstream processing should be further developed. Research and development for affordable HVP production from the two families, as well as from microalgae in general, is needed.
The antimicrobial activity of the two taxa could be utilized in WWT, diminishing the pathogenic microorganism’s load. Combining the knowledge, scientific, and research experience in the field of microalgal technology under the biorefinery concept, it is worth highlighting the perspectives of the microalgal potential to be an extremely competitive option for WWT and other ecological and industrial applications.
The in vitro antimicrobial activity of Chlorellaceae and Scenedesmaceae is in a broad range. The species could be inactive or have a low, medium, or strong effect. Several strains have fulfilled the criteria of Eloff and Porras et al. for outstanding and prominently high activity, especially those among C. vulgaris and other Chlorellaceae representatives. There were several strains with MICs below 80 µg/mL and even 10 and 1.5 µg/mL. Some strains also had IZs over 30 mm, even as high as 45 and 48 mm. Frequently, the two genera have better or nearly as good as the results of antibiotics against some pathogens.
The reason that the 80-year-old knowledge about the antimicrobial effect of microalgae, e.g., Chlorella, has not led to further development besides a protein-rich food does not accurately reflect their potential and is likely mainly due to the actinomycetes’ antibiotics prioritized for development. FFAs are also nutriments, including the long-chain ω-3 PUFAs that are precursors of eicosanoids and structural components of neural tissue; however, research shows some of them have an infection-suppressing potential when increased in the diet, and others have patents for topical antimicrobial applications. Microalgae, such as Chlorella, have been called “herbal antibiotics” based on vitro studies and the few in vivo studies of Chlorella are very promising. In vitro studies are the solid base for in vivo research and, ideally, the latter, in turn, support clinical trials. We hope that Chlorella and other microalgae will acquire more clinical presence, for example use in synergism with established antibiotics.
Author Contributions
Conceptualization, A.D.K.; methodology A.D.K.; writing—original draft preparation, Y.I., A.D.K. and M.M.Z.; writing—review and editing, Y.I. and H.N.; funding acquisition, H.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Research data can be obtained from the authors by e-mail.
Acknowledgments
The authors would like to express their gratitude to the Bulgarian Ministry of Education and Science, grant number DO1-217/30.11.2018 (National Research Program “Innovative Low-Toxic Bioactive Systems for Precision Medicine, BioActiveMed”), agreement number DO1-278/03.12.2021.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Habibi, Z.; Imanpour Namin, J.; Ramezanpour, Z. Evaluation of Antimicrobial Activities of Microalgae Scenedesmus Dimorphus Extracts against Bacterial Strains. Casp. J. Environ. Sci. 2018, 16, 23–34. [Google Scholar] [CrossRef]
- Saha, S.K.; Mchugh, E.; Murray, P.; Walsh, D.J. Microalgae as a Source of Nutraceuticals (Book Chapter). In Phycotoxins: Chemistry and Biochemistry; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 255–291. [Google Scholar]
- Antibiotics from Nature: Traditional Medicine as a Source of New Solutions for Combating Antimicrobial Resistance|AMR Control. Available online: http://resistancecontrol.info/rd-innovation/antibiotics-from-nature-traditional-medicine-as-a-source-of-new-solutions-for-combating-antimicrobial-resistance/ (accessed on 26 February 2024).
- Pereira, L. Macroalgae. Encyclopedia 2021, 1, 177–188. [Google Scholar] [CrossRef]
- Turland, N.J.; Wiersema, J.H.; Barrie, F.R.; Greuter, W.; Hawksworth, D.L.; Herendeen, P.S.; Knapp, S.; Kusber, W.-H.; Li, D.-Z.; Marhold, K.; et al. (Eds.) International Code of Nomenclature for Algae, Fungi, and Plants (Shenzhen Code) Adopted by the Nineteenth International Botanical Congress Shenzhen, China, July 2017, Regnum Vegetabile 159; Koeltz Botanical Books: Glashütten, Germany, 2018. [Google Scholar]
- Wehr, J.D. Freshwater Algae of North America: Ecology and Classification, 2nd ed.Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Raven, J.A.; Giordano, M. Algae. Curr. Biol. 2014, 24, R590–R595. [Google Scholar] [CrossRef] [PubMed]
- Desbois, A.P. Potential Applications of Antimicrobial Fatty Acids in Medicine, Agriculture and Other Industries. Recent Pat. Antiinfect. Drug Discov. 2012, 7, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Firoozabad, M.S.M.; Nasr, M.M. Antimicrobial Activities of Microbial Essential Fatty Acid against Foodborne Pathogenic Bacteria. Iran. J. Microbiol. 2022, 14, 214. [Google Scholar] [CrossRef]
- Alhusseiny, S.M.; El-Beshbishi, S.N. Omega Polyunsaturated Fatty Acids and Parasitic Infections: An Overview. Acta Trop. 2020, 207, 105466. [Google Scholar] [CrossRef] [PubMed]
- de Melo, R.G.; de Andrade, A.F.; Bezerra, R.P.; Viana Marques, D.D.A.; da Silva, V.A.; Paz, S.T.; de Lima Filho, J.L.; Porto, A.L.F. Hydrogel-Based Chlorella vulgaris Extracts: A New Topical Formulation for Wound Healing Treatment. J. Appl. Phycol. 2019, 31, 3653–3663. [Google Scholar] [CrossRef]
- Zaharieva, M.M.; Zheleva-Dimitrova, D.; Rusinova-Videva, S.; Ilieva, Y.; Brachkova, A.; Balabanova, V.; Gevrenova, R.; Kim, T.C.; Kaleva, M.; Georgieva, A.; et al. Antimicrobial and Antioxidant Potential of Scenedesmus obliquus Microalgae in the Context of Integral Biorefinery Concept. Molecules 2022, 27, 519. [Google Scholar] [CrossRef] [PubMed]
- Scheufele, F.B.; Hinterholz, C.L.; Zaharieva, M.M.; Najdenski, H.M.; Módenes, A.N.; Trigueros, D.E.G.; Borba, C.E.; Espinoza-Quiñones, F.R.; Kroumov, A.D. Complex Mathematical Analysis of Photobioreactor System. Eng. Life Sci. 2019, 19, 844–859. [Google Scholar] [CrossRef]
- Kroumov, A.D.; Módenes, A.N.; Trigueros, D.E.G.; Espinoza-Quiñones, F.R.; Borba, C.E.; Scheufele, F.B.; Hinterholz, C.L. A Systems Approach for CO2 Fixation from Flue Gas by Microalgae—Theory Review. Process Biochem. 2016, 51, 1817–1832. [Google Scholar] [CrossRef]
- Gonçalves, V.D.; Fagundes-Klen, M.R.; Goes Trigueros, D.E.; Kroumov, A.D.; Módenes, A.N. Statistical and Optimization Strategies to Carotenoids Production by Tetradesmus Acuminatus (LC192133.1) Cultivated in Photobioreactors. Biochem. Eng. J. 2019, 152, 107351. [Google Scholar] [CrossRef]
- Gonçalves, V.D.; Fagundes-Klen, M.R.; Trigueros, D.E.G.; Schuelter, A.R.; Kroumov, A.D.; Módenes, A.N. Combination of Light Emitting Diodes (LEDs) for Photostimulation of Carotenoids and Chlorophylls Synthesis in Tetradesmus sp. Algal Res. 2019, 43, 101649. [Google Scholar] [CrossRef]
- Hinterholz, C.; Schuelter, A.; Módenes, A.; Trigueros, D.; Borba, C.; Espinoza-Quiñones, F.; Kroumov, A. Microalgae Flat Plate-Photobioreactor (FP-PBR) System Development: Computational Tools to Improve Experimental Results. Acta Microbiol. Bulg. 2017, 33, 119–124. [Google Scholar]
- Hinterholz, C.L.; Trigueros, D.E.G.; Módenes, A.N.; Borba, C.E.; Scheufele, F.B.; Schuelter, A.R.; Kroumov, A.D. Computational Fluid Dynamics Applied for the Improvement of a Flat-Plate Photobioreactor towards High-Density Microalgae Cultures. Biochem. Eng. J. 2019, 151, 107257. [Google Scholar] [CrossRef]
- Schuelter, A.R.; Kroumov, A.D.; Hinterholz, C.L.; Fiorini, A.; Trigueros, D.E.G.; Vendruscolo, E.G.; Zaharieva, M.M.; Módenes, A.N. Isolation and Identification of New Microalgae Strains with Antibacterial Activity on Food-Borne Pathogens. Engineering Approach to Optimize Synthesis of Desired Metabolites. Biochem. Eng. J. 2019, 144, 28–39. [Google Scholar] [CrossRef]
- Kroumov, A.D.; Nivaldo Módenes, A.; Estelita Goes Trigueros, D. A Complex Theoretical Approach for Algal Medium Optimization for CO2 Fixation from Flue Gas. Acta Microbiol. Bulg. 2015, 31, 61–70. [Google Scholar]
- Kroumov, A.D.; Scheufele, F.B.; Trigueros, D.E.G.; Modenes, A.N.; Zaharieva, M.; Najdenski, H. Modeling and Technoeconomic Analysis of Algae for Bioenergy and Coproducts. In Algal Green Chemistry: Recent Progress in Biotechnology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 201–241. ISBN 9780444640413. [Google Scholar]
- Adhoni, S.A.; Thimmappa, S.C.; Kaliwal, B.B. Phytochemical Analysis and Antimicrobial Activity of Chorella Vulgaris Isolated from Unkal Lake. J. Coast. Life Med. 2016, 4, 368–373. [Google Scholar] [CrossRef]
- Sathasivam, R.; Radhakrishnan, R.; Hashem, A.; Abd_Allah, E.F. Microalgae Metabolites: A Rich Source for Food and Medicine. Saudi J. Biol. Sci. 2019, 26, 709–722. [Google Scholar] [CrossRef]
- Plaza, M.; Santoyo, S.; Jaime, L.; Avalo, B.; Cifuentes, A.; Reglero, G.; García-Blairsy Reina, G.; Señoráns, F.J.; Ibáñez, E. Comprehensive Characterization of the Functional Activities of Pressurized Liquid and Ultrasound-Assisted Extracts from Chlorella Vulgaris. LWT—Food Sci. Technol. 2012, 46, 245–253. [Google Scholar] [CrossRef]
- Jacob, J.P.; Mathew, S. Effect of Lipases from Candida cylinderacea on Enrichment of PUFA in Marine Microalgae. J. Food Process. Preserv. 2017, 41, e12928. [Google Scholar] [CrossRef]
- Kiuru, P.; Valeria D’Auria, M.; Muller, C.D.; Tammela, P.; Vuorela, H.; Yli-Kauhaluoma, J. Exploring Marine Resources for Bioactive Compounds. Planta Med. 2014, 80, 1234–1246. [Google Scholar] [CrossRef] [PubMed]
- Bays, H.E.; Tighe, A.P.; Sadovsky, R.; Davidson, M.H. Prescription Omega-3 Fatty Acids and Their Lipid Effects: Physiologic Mechanisms of Action and Clinical Implications. Expert Rev. Cardiovasc. Ther. 2008, 6, 391–409. [Google Scholar] [CrossRef] [PubMed]
- Kannan, N.; Rao, A.S.; Nair, A. Microbial Production of Omega-3 Fatty Acids: An Overview. J. Appl. Microbiol. 2021, 131, 2114–2130. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.; Ho, H.C.; Pietropaolo, A.; Somani, B.K. Guideline of Guidelines for Kidney and Bladder Stones. Turkish J. Urol. 2020, 46, S104. [Google Scholar] [CrossRef]
- Yarkent, Ç.; Aslanbay Güler, B.; Imamoglu, E.; Oncel, S.S. Microalgae-Factories as Potential Antimicrobial Agents: A Comprehensive Review. Biologia 2024, 79, 1237–1250. [Google Scholar] [CrossRef]
- Borowitzka, M.A. Microalgae in Medicine and Human Health: A Historical Perspective. In Microalgae in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2018; pp. 195–210. ISBN 9780128114056. [Google Scholar]
- Selivanova, E.A.; Ignatenko, M.E.; Nemtseva, N.V. Antagonistic Activity of Novel Green Microalgae Strain. Zh. Mikrobiol. 2014, 4, 72–76. [Google Scholar]
- Mobin, S.; Alam, F. Some Promising Microalgal Species for Commercial Applications: A Review. Energy Procedia 2017, 110, 510–517. [Google Scholar]
- Bhalamurugan, G.L.; Valerie, O.; Mark, L. Valuable Bioproducts Obtained from Microalgal Biomass and Their Commercial Applications: A Review. Environ. Eng. Res. 2018, 23, 229–241. [Google Scholar] [CrossRef]
- Thoré, E.S.J.; Muylaert, K.; Bertram, M.G.; Brodin, T. Microalgae. Curr. Biol. 2023, 33, R91–R95. [Google Scholar] [CrossRef]
- Chlorella—Sahha by Mark Morales—SHOP. Available online: https://www.markmorales.net/product/chlorella/ (accessed on 23 May 2023).
- Syed, S.; Arasu, A.; Ponnuswamy, I. The Uses of Chlorella vulgaris as Antimicrobial Agent and as a Diet: The Presence of Bio-Active Compounds Which Caters the Vitamins, Minerals in General. Int. J. Bio-Sci. Bio-Technol. 2015, 7, 185–190. [Google Scholar] [CrossRef]
- Jayshree, A.; Jayashree, S.; Thangaraju, N. Chlorella vulgaris and Chlamydomonas reinhardtii: Effective Antioxidant, Antibacterial and Anticancer Mediators. Indian J. Pharm. Sci. 2016, 78, 575–581. [Google Scholar] [CrossRef]
- Tian, S.L.; Khan, A.; Zheng, W.N.; Song, L.; Liu, J.H.; Wang, X.Q.; Li, L. Effects of Chlorella Extracts on Growth of Capsicum annuum L. Seedlings. Sci. Rep. 2022, 12, 15455. [Google Scholar] [CrossRef]
- Gong, M.; Bassi, A. Carotenoids from Microalgae: A Review of Recent Developments. Biotechnol. Adv. 2016, 34, 1396–1412. [Google Scholar] [CrossRef]
- Kamalnizat, I.; Ramliza, R.; Rashid, A.; Halim, A.; Yusof, M.; Anum, Y. Antimicrobial Property of Water and Ethanol Extract of Chlorella vulgaris: A Value-Added Advantage for a New Wound Dressing Material. Int. Med. J. 2015, 22, 399–401. [Google Scholar]
- Zhang, Y.; Ye, Y.; Bai, F.; Liu, J. The Oleaginous Astaxanthin-Producing Alga Chromochloris zofingiensis: Potential from Production to an Emerging Model for Studying Lipid Metabolism and Carotenogenesis. Biotechnol. Biofuels 2021, 14, 119. [Google Scholar] [CrossRef]
- Guedes, A.C.; Amaro, H.M.; Malcata, F.X. Microalgae as Sources of Carotenoids. Mar. Drugs 2011, 9, 625. [Google Scholar] [CrossRef]
- González-Davis, O.; Ponce-Rivas, E.; Sánchez-Saavedra, M.D.P.; Muñoz-Márquez, M.E.; Gerwick, W.H. Bioprospection of Microalgae and Cyanobacteria as Biocontrol Agents Against Vibrio campbellii and Their Use in White Shrimp Litopenaeus vannamei Culture. J. World Aquac. Soc. 2012, 43, 387–399. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, Y.; Li, S.; Nagarajan, D.; Varjani, S.; Lee, D.J.; Chang, J.S. Recent Advances in Lutein Production from Microalgae. Renew. Sustain. Energy Rev. 2022, 153, 111795. [Google Scholar] [CrossRef]
- Casella, P.; Marino, T.; Iovine, A.; Larocca, V.; Balducchi, R.; Musmarra, D.; Molino, A. Optimization of Lutein Extraction from Scenedesmus Almeriensis Using Pressurized Liquid Extraction. Chem. Eng. Trans. 2021, 87, 475–480. [Google Scholar] [CrossRef]
- Ahmad, M.T.; Shariff, M.; Yusoff, F.M.; Goh, Y.M.; Banerjee, S. Applications of Microalga Chlorella vulgaris in Aquaculture. Rev. Aquac. 2020, 12, 328–346. [Google Scholar] [CrossRef]
- Gouveia, L.; Veloso, V.; Reis, A.; Fernandes, H.; Novais, J.; Empis, J. Evolution of Pigment Composition in Chlorella vulgaris. Bioresour. Technol. 1996, 57, 157–163. [Google Scholar] [CrossRef]
- Canhada, S.; Castro, K.; Perry, I.S.; Luft, V.C. Omega-3 Fatty Acids’ Supplementation in Alzheimer’s Disease: A Systematic Review. Nutr. Neurosci. 2018, 21, 529–538. [Google Scholar] [CrossRef]
- Amjad Khan, W.; Chun-Mei, H.; Khan, N.; Iqbal, A.; Lyu, S.W.; Shah, F. Bioengineered Plants Can Be a Useful Source of Omega-3 Fatty Acids. Biomed Res. Int. 2017, 2017, 7348919. [Google Scholar] [CrossRef]
- Freitas, H.R. Chlorella vulgaris as a Source of Essential Fatty Acids and Micronutrients: A Brief Commentary. Open Plant Sci. J. 2017, 10, 92–99. [Google Scholar]
- Peltomaa, E.; Johnson, M.D.; Taipale, S.J. Marine Cryptophytes Are Great Sources of EPA and DHA. Mar. Drugs 2018, 16, 3. [Google Scholar] [CrossRef]
- Shim, S.J.; Hong, M.E.; Chang, W.S.; Sim, S.J. Repeated-Batch Production of Omega-3 Enriched Biomass of Chlorella sorokiniana via Calcium-Induced Homeoviscous Adaptation. Bioresour. Technol. 2020, 303, 122944. [Google Scholar] [CrossRef]
- Toumi, A.; Politaeva, N.; Ðurović, S.; Mukhametova, L.; Ilyashenko, S. Obtaining DHA–EPA Oil Concentrates from the Biomass of Microalga Chlorella sorokiniana. Resources 2022, 11, 20. [Google Scholar] [CrossRef]
- Masclaux, H.; Bec, A.; Kainz, M.J.; Perrière, F.; Desvilettes, C.; Bourdier, G. Accumulation of Polyunsaturated Fatty Acids by Cladocerans: Effects of Taxonomy, Temperature and Food. Freshw. Biol. 2012, 57, 696–703. [Google Scholar] [CrossRef]
- Li, J.; Otero-Gonzalez, L.; Michiels, J.; Lens, P.N.L.; Du Laing, G.; Ferrer, I. Production of Selenium-Enriched Microalgae as Potential Feed Supplement in High-Rate Algae Ponds Treating Domestic Wastewater. Bioresour. Technol. 2021, 333, 125239. [Google Scholar] [CrossRef]
- Mansour, M.P.; Frampton, D.M.F.; Nichols, P.D.; Volkman, J.K.; Blackburn, S.I. Lipid and Fatty Acid Yield of Nine Stationary-Phase Microalgae: Applications and Unusual C24–C28 Polyunsaturated Fatty Acids. J. Appl. Phycol. 2005, 17, 287–300. [Google Scholar] [CrossRef]
- Cuellar-Bermudez, S.P.; Aguilar-Hernandez, I.; Cardenas-Chavez, D.L.; Ornelas-Soto, N.; Romero-Ogawa, M.A.; Parra-Saldivar, R. Extraction and Purification of High-Value Metabolites from Microalgae: Essential Lipids, Astaxanthin and Phycobiliproteins. Microb. Biotechnol. 2015, 8, 190–209. [Google Scholar] [CrossRef]
- Kroumov, A.D.; Zaharieva, M.M.; Scheufele, F.B.; Balabanova, V.; Najdenski, H. Engineering Challenges of Carbon Dioxide Capture and Sequestration by Cyanobacteria. In Ecophysiology and Biochemistry of Cyanobacteria; Springer: Singapore, 2021; pp. 351–372. ISBN 978-981-16-4873-1. [Google Scholar]
- da Silva, M.E.T.; Martins, M.A.; de Oliveira Leite, M.; Milião, G.L.; dos Reis Coimbra, J.S. Microalga Scenedesmus obliquus: Extraction of Bioactive Compounds and Antioxidant Activity. Rev. Cienc. Agron. 2021, 52, e20196848. [Google Scholar] [CrossRef]
- Udayan, A.; Pandey, A.K.; Sirohi, R.; Sreekumar, N.; Sang, B.I.; Sim, S.J.; Kim, S.H.; Pandey, A. Production of Microalgae with High Lipid Content and Their Potential as Sources of Nutraceuticals. Phytochem. Rev. 2022, 22, 833–860. [Google Scholar] [CrossRef]
- El-fayoumy, E.A.; Ali, H.E.A.; Elsaid, K.; Elkhatat, A.; Al-Meer, S.; Rozaini, M.Z.H.; Abdullah, M.A. Co-Production of High Density Biomass and High-Value Compounds via Two-Stage Cultivation of Chlorella vulgaris Using Light Intensity and a Combination of Salt Stressors. Biomass Convers. Biorefinery 2023, 1, 1–14. [Google Scholar] [CrossRef]
- Kumar, R.R.; Rao, P.H.; Arumugam, M. Lipid Extraction Methods from Microalgae: A Comprehensive Review. Front. Energy Res. 2015, 2, 61. [Google Scholar] [CrossRef]
- Li, J.; Zhu, D.; Niu, J.; Shen, S.; Wang, G. An Economic Assessment of Astaxanthin Production by Large Scale Cultivation of Haematococcus pluvialis. Biotechnol. Adv. 2011, 29, 568–574. [Google Scholar] [CrossRef]
- Lin, J.H.; Lee, D.J.; Chang, J.S. Lutein Production from Biomass: Marigold Flowers versus Microalgae. Bioresour. Technol. 2015, 184, 421–428. [Google Scholar] [CrossRef]
- Pirwitz, K.; Flassig, R.J.; Rihko-Struckmann, L.K.; Sundmacher, K. Energy and Operating Cost Assessment of Competing Harvesting Methods for D. Salina in a β-Carotene Production Process. Algal Res. 2015, 12, 161–169. [Google Scholar] [CrossRef]
- Ren, Y.; Sun, H.; Deng, J.; Huang, J.; Chen, F. Carotenoid Production from Microalgae: Biosynthesis, Salinity Responses and Novel Biotechnologies. Mar. Drugs 2021, 19, 713. [Google Scholar] [CrossRef]
- Ventura, S.P.M.; Nobre, B.P.; Ertekin, F.; Hayes, M.; Garciá-Vaquero, M.; Vieira, F.; Koc, M.; Gouveia, L.; Aires-Barros, M.R.; Palavra, A.M.F. Extraction of Value-Added Compounds from Microalgae. In Microalgae-Based Biofuels and Bioproducts: From Feedstock Cultivation to End-Products; Woodhead Publishing: Sawston, UK, 2017; pp. 461–483. ISBN 9780081010273. [Google Scholar]
- Virdi, S.K. Role of Chlorella vulgaris Beijerinck in the Remediation of Wastewater and as Anti-Bacterial Agent. Harvest 2019, 4, 34–69. [Google Scholar]
- Liu, Y.; Yildiz, I. The Effect of Salinity Concentration on Algal Biomass Production and Nutrient Removal from Municipal Wastewater by Dunaliella salina. Int. J. Energy Res. 2018, 42, 2997–3006. [Google Scholar] [CrossRef]
- Han, W.; Jin, W.; Li, Z.; Wei, Y.; He, Z.; Chen, C.; Qin, C.; Chen, Y.; Tu, R.; Zhou, X. Cultivation of Microalgae for Lipid Production Using Municipal Wastewater. Process Saf. Environ. Prot. 2021, 155, 155–165. [Google Scholar] [CrossRef]
- Wu, J.Y.; Lay, C.H.; Chen, C.C.; Wu, S.Y. Lipid Accumulating Microalgae Cultivation in Textile Wastewater: Environmental Parameters Optimization. J. Taiwan Inst. Chem. Eng. 2017, 79, 1–6. [Google Scholar] [CrossRef]
- Goswami, R.K.; Mehariya, S.; Verma, P.; Lavecchia, R.; Zuorro, A. Microalgae-Based Biorefineries for Sustainable Resource Recovery from Wastewater. J. Water Process Eng. 2021, 40, 101747. [Google Scholar] [CrossRef]
- Liu, X.Y.; Hong, Y. Microalgae-Based Wastewater Treatment and Recovery with Biomass and Value-Added Products: A Brief Review. Curr. Pollut. Rep. 2021, 7, 227–245. [Google Scholar] [CrossRef]
- Amaro, H.M.; Salgado, E.M.; Nunes, O.C.; Pires, J.C.M.; Esteves, A.F. Microalgae Systems—Environmental Agents for Wastewater Treatment and Further Potential Biomass Valorisation. J. Environ. Manag. 2023, 337, 117678. [Google Scholar] [CrossRef]
- Xiao, R.; Zheng, Y. Overview of Microalgal Extracellular Polymeric Substances (EPS) and Their Applications. Biotechnol. Adv. 2016, 34, 1225–1244. [Google Scholar] [CrossRef]
- Li, S.; Show, P.L.; Ngo, H.H.; Ho, S.H. Algae-Mediated Antibiotic Wastewater Treatment: A Critical Review. Environ. Sci. Ecotechnol. 2022, 9, 100145. [Google Scholar] [CrossRef]
- Tao, R.; Kinnunen, V.; Praveenkumar, R.; Lakaniemi, A.M.; Rintala, J.A. Comparison of Scenedesmus Acuminatus and Chlorella vulgaris Cultivation in Liquid Digestates from Anaerobic Digestion of Pulp and Paper Industry and Municipal Wastewater Treatment Sludge. J. Appl. Phycol. 2017, 29, 2845–2856. [Google Scholar] [CrossRef]
- Chiu, S.Y.; Kao, C.Y.; Chen, C.H.; Kuan, T.C.; Ong, S.C.; Lin, C.S. Reduction of CO2 by a High-Density Culture of Chlorella sp. in a Semicontinuous Photobioreactor. Bioresour. Technol. 2008, 99, 3389–3396. [Google Scholar] [CrossRef]
- Goswami, R.K.; Agrawal, K.; Shah, M.P.; Verma, P. Bioremediation of Heavy Metals from Wastewater: A Current Perspective on Microalgae-Based Future. Lett. Appl. Microbiol. 2022, 75, 701–717. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Banat, F.; Alsafar, H.; Hasan, S.W. Algae Biotechnology for Industrial Wastewater Treatment, Bioenergy Production, and High-Value Bioproducts. Sci. Total Environ. 2022, 806, 150585. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Li, F.; Liu, N.; Ge, F.; Xiao, H.; Yang, Y. Role of Extracellular Polymeric Substances from Chlorella vulgaris in the Removal of Ammonium and Orthophosphate under the Stress of Cadmium. Bioresour. Technol. 2015, 190, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.F.; Mofijur, M.; Parisa, T.A.; Islam, N.; Kusumo, F.; Inayat, A.; Le, V.G.; Badruddin, I.A.; Khan, T.M.Y.; Ong, H.C. Progress and Challenges of Contaminate Removal from Wastewater Using Microalgae Biomass. Chemosphere 2022, 286, 131656. [Google Scholar] [CrossRef] [PubMed]
- Bellucci, M.; Marazzi, F.; Stefano Naddeo, L.; Piergiacomo, F.; Beneduce, L.; Ficara, E.; Mezzanotte, V. Disinfection and Nutrient Removal in Laboratory-Scale Photobioreactors for Wastewater Tertiary Treatment. J. Chem. Technol. Biotechnol. 2019, 2020, 959–966. [Google Scholar] [CrossRef]
- Suresh Kumar, K.; Dahms, H.U.; Won, E.J.; Lee, J.S.; Shin, K.H. Microalgae—A Promising Tool for Heavy Metal Remediation. Ecotoxicol. Environ. Saf. 2015, 113, 329–352. [Google Scholar] [CrossRef] [PubMed]
- Jais, N.M.; Mohamed, R.M.S.R.; Al-Gheethi, A.A.; Hashim, M.K.A. The Dual Roles of Phycoremediation of Wet Market Wastewater for Nutrients and Heavy Metals Removal and Microalgae Biomass Production. Clean Technol. Environ. Policy 2017, 19, 37–52. [Google Scholar] [CrossRef]
- Kanamarlapudi, S.L.R.K.; Kumar; Chintalpudi, V.; Muddada, S. Application of Biosorption for Removal of Heavy Metals from Wastewater. In Biosorption; IntechOpen: London, UK, 2018; ISBN 978-1-78923-473-2. [Google Scholar]
- Daneshvar, E.; Zarrinmehr, M.J.; Hashtjin, A.M.; Farhadian, O.; Bhatnagar, A. Versatile Applications of Freshwater and Marine Water Microalgae in Dairy Wastewater Treatment, Lipid Extraction and Tetracycline Biosorption. Bioresour. Technol. 2018, 268, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Al-Jabri, H.; Das, P.; Khan, S.; Thaher, M.; Abdulquadir, M. Treatment of Wastewaters by Microalgae and the Potential Applications of the Produced Biomass—A Review. Water 2020, 13, 27. [Google Scholar] [CrossRef]
- Wollmann, F.; Dietze, S.; Ackermann, J.U.; Bley, T.; Walther, T.; Steingroewer, J.; Krujatz, F. Microalgae Wastewater Treatment: Biological and Technological Approaches. Eng. Life Sci. 2019, 19, 860–871. [Google Scholar] [CrossRef]
- Chew, K.W.; Khoo, K.S.; Foo, H.T.; Chia, S.R.; Walvekar, R.; Lim, S.S. Algae Utilization and Its Role in the Development of Green Cities. Chemosphere 2021, 268, 129322. [Google Scholar] [CrossRef] [PubMed]
- Xiaogang, H.; Jalalah, M.; Jingyuan, W.; Zheng, Y.; Li, X.; Salama, E.S. Microalgal Growth Coupled with Wastewater Treatment in Open and Closed Systems for Advanced Biofuel Generation. Biomass Convers. Biorefinery 2020, 12, 1939–1958. [Google Scholar] [CrossRef]
- Kadir, W.N.A.; Lam, M.K.; Uemura, Y.; Lim, J.W.; Lee, K.T. Harvesting and Pre-Treatment of Microalgae Cultivated in Wastewater for Biodiesel Production: A Review. Energy Convers. Manag. 2018, 171, 1416–1429. [Google Scholar] [CrossRef]
- Causes of Death—Our World in Data. Available online: https://ourworldindata.org/causes-of-death (accessed on 22 April 2024).
- Water for Health Taking Charge; 2001. Available online: https://iris.who.int/handle/10665/66717 (accessed on 27 June 2024).
- Water, Sanitation and Hygiene: Burden of Disease. Available online: https://www.who.int/data/gho/data/themes/topics/water-sanitation-and-hygiene-burden-of-disease (accessed on 19 April 2024).
- Deaths from Dirty Water. Available online: https://www.theworldcounts.com/challenges/planet-earth/freshwater/deaths-from-dirty-water (accessed on 19 April 2024).
- Griffiths, J.K. Waterborne Diseases. In International Encyclopedia of Public Health; Academic Press: Cambridge, MA, USA, 2008; pp. 551–563. ISBN 9780123739605. [Google Scholar]
- Dantas, D.M.D.M.; Oliveira, C.Y.B.D.; Costa, R.M.P.B.; Carneiro-da-Cunha, M.D.G.; Gálvez, A.O.; Bezerra, R.D.S. Evaluation of Antioxidant and Antibacterial Capacity of Green Microalgae Scenedesmus subspicatus. Food Sci. Technol. Int. 2019, 25, 318–326. [Google Scholar] [CrossRef]
- Lustigman, B. Comparison of Antibiotic Production from Four Ecotypes of the Marine Alga, Dunaliella. Bull. Environ. Contam. Toxicol. 1988, 40, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Kim, M.; Kim, H.S.; Ahn, C.Y. Extra Benefit of Microalgae in Raw Piggery Wastewater Treatment: Pathogen Reduction. Microbiome 2022, 10, 142. [Google Scholar] [CrossRef] [PubMed]
- Pratt, R.; Mautner, H.; Gardner, G.M.; Sha, Y.; Dufrenoy, J. Report on Antibiotic Activity of Seaweed Extracts. J. Am. Pharm. Assoc. 1951, 40, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Srimongkol, P.; Sangtanoo, P.; Songserm, P.; Watsuntorn, W.; Karnchanatat, A. Microalgae-Based Wastewater Treatment for Developing Economic and Environmental Sustainability: Current Status and Future Prospects. Front. Bioeng. Biotechnol. 2022, 10, 904046. [Google Scholar] [CrossRef]
- Bhattacharjee, M. Pharmaceutically Valuable Bioactive Compounds of Algae. Asian J. Pharm. Clin. Res. 2016, 9, 43–47. [Google Scholar] [CrossRef]
- Brisson, V.; Mayali, X.; Bowen, B.; Golini, A.; Thelen, M.; Stuart, R.K.; Northen, T.R. Identification of Effector Metabolites Using Exometabolite Profiling of Diverse Microalgae. mSystems 2021, 6, e00835-21. [Google Scholar] [CrossRef]
- Kellam, S.J.; Walker, J.M. Antibacterial Activity from Marine Microalgae in Laboratory Culture. Br. Phycol. J. 1989, 24, 191–194. [Google Scholar] [CrossRef]
- Mudimu, O.; Rybalka, N.; Bauersachs, T.; Born, J.; Friedl, T.; Schulz, R. Biotechnological Screening of Microalgal and Cyanobacterial Strains for Biogas Production and Antibacterial and Antifungal Effects. Metabolites 2014, 4, 373–393. [Google Scholar] [CrossRef] [PubMed]
- Maksimova, I.V.; Sidorova, O.A. Light-Dependent Antibacterial Effect of Algae and Its Ecological Significance (a Review). Gidrobiol. žurnal 1986, 22, 3–11. [Google Scholar]
- Falaise, C.; François, C.; Travers, M.A.; Morga, B.; Haure, J.; Tremblay, R.; Turcotte, F.; Pasetto, P.; Gastineau, R.; Hardivillier, Y.; et al. Antimicrobial Compounds from Eukaryotic Microalgae against Human Pathogens and Diseases in Aquaculture. Mar. Drugs 2016, 14, 159. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Ashraf, S.S. Sustainable Food and Feed Sources from Microalgae: Food Security and the Circular Bioeconomy. Algal Res. 2023, 74, 103185. [Google Scholar] [CrossRef]
- Rawat, I.; Gupta, S.K.; Shriwastav, A.; Singh, P.; Kumari, S.; Bux, F. Microalgae Applications in Wastewater Treatment. In Algae Biotechnology; Springer: Cham, Switzerland, 2016; pp. 249–268. ISBN 978-3-319-12334-9. [Google Scholar]
- Regulation—2019/1009—EN—EUR-Lex. Available online: https://eur-lex.europa.eu/eli/reg/2019/1009/oj (accessed on 14 March 2024).
- Regulation—68/2013—EN—EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32013R0068 (accessed on 14 March 2024).
- Regulation—1223/2009—EN—Cosmetic Products Regulation—EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32009R1223 (accessed on 14 March 2024).
- Álvarez-González, A.; Greque de Morais, E.; Planas-Carbonell, A.; Uggetti, E. Enhancing Sustainability through Microalgae Cultivation in Urban Wastewater for Biostimulant Production and Nutrient Recovery. Sci. Total Environ. 2023, 904, 166878. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Bastiaens, L.; Verspreet, J.; Hayes, M. Applications of Microalgae in Foods, Pharma and Feeds and Their Use as Fertilizers and Biostimulants: Legislation and Regulatory Aspects for Consideration. Foods 2023, 12, 3878. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Gouveia, L.; Gonçalves, M. Aquaculture Wastewater Treatment through Microalgal. Biomass Potential Applications on Animal Feed, Agriculture, and Energy. J. Environ. Manag. 2021, 286, 112187. [Google Scholar] [CrossRef] [PubMed]
- Yaakob, M.A.; Mohamed, R.M.S.R.; Al-Gheethi, A.; Tiey, A.; Kassim, A.H.M. Optimising of Scenedesmus sp. Biomass Production in Chicken Slaughterhouse Wastewater Using Response Surface Methodology and Potential Utilisation as Fish Feeds. Environ. Sci. Pollut. Res. Int. 2019, 26, 12089–12108. [Google Scholar] [CrossRef]
- Moheimani, N.R.; Vadiveloo, A.; Ayre, J.M.; Pluske, J.R. Nutritional Profile and in Vitro Digestibility of Microalgae Grown in Anaerobically Digested Piggery Effluent. Algal Res. 2018, 35, 362–369. [Google Scholar] [CrossRef]
- McGee, P. Natural Products Re-Emerge. Drug Disc. Dev. 2006, 9, 18–26. [Google Scholar]
- Hoelder, S.; Clarke, P.A.; Workman, P. Discovery of Small Molecule Cancer Drugs: Successes, Challenges and Opportunities. Mol. Oncol. 2012, 6, 155–176. [Google Scholar] [CrossRef] [PubMed]
- Chassagne, F.; Samarakoon, T.; Porras, G.; Lyles, J.T.; Dettweiler, M.; Marquez, L.; Salam, A.M.; Shabih, S.; Farrokhi, D.R.; Quave, C.L. A Systematic Review of Plants With Antibacterial Activities: A Taxonomic and Phylogenetic Perspective. Front. Pharmacol. 2021, 11, 2069. [Google Scholar] [CrossRef] [PubMed]
- Iwu, M.M.; Duncan, A.R.; Okunji, C.O. New Antimicrobials of Plant Origin. In Perspectives on New Crops and New Uses; ASHS Press: Alexandria, VA, USA, 1999; pp. 457–462. [Google Scholar]
- Parimelazhagan, T. Pharmacological Assays of Plant-Based Natural Products; Progress in Drug Research; Springer International Publishing: Cham, Switzerland, 2016; Volume 71, ISBN 978-3-319-26810-1. [Google Scholar]
- Kumarasamy, Y.; Cox, P.J.; Jaspars, M.; Nahar, L.; Sarker, S.D. Screening Seeds of Scottish Plants for Antibacterial Activity. J. Ethnopharmacol. 2002, 83, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, J.; Curt, M.D.; Robert, N.; Fernández, J. Capter Two-Biomass Resources. In The Role of Bioenergy in the Bioeconomy: Resources, Technologies, Sustainability and Policy; Academic Press: Cambridge, MA, USA, 2019; pp. 25–111. ISBN 9780128130568. [Google Scholar]
- Rani, D. Plant Extracts with Antiamoebic Properties: A Theoretical Study with Reference to Entamoeba histolytica. Int. J. Pharm. Tech. Res. 2011, 3, 1113–1117. [Google Scholar]
- Desbois, A.P.; Smith, V.J. Antibacterial Free Fatty Acids: Activities, Mechanisms of Action and Biotechnological Potential. Appl. Microbiol. Biotechnol. 2010, 85, 1629–1642. [Google Scholar] [CrossRef] [PubMed]
- De Morais, M.G.; Vaz, B.D.S.; De Morais, E.G.; Costa, J.A.V. Biologically Active Metabolites Synthesized by Microalgae. Biomed Res. Int. 2015, 2015, 835761. [Google Scholar] [CrossRef] [PubMed]
- Pratita, A.T.K.; Fathurohman, M.; Ruswanto, R.; Khusnul; Suhartati, R. Potential of Autotroph Microalgae (Spirulina plantentis) as Antimicrobial Agent. J. Phys. Conf. Ser. 2019, 1179, 012173. [Google Scholar] [CrossRef]
- El-Sayed, H.S.; Ibrahim, H.A.H.; Beltagy, E.A.; Khairy, H.M. Effects of Short Term Feeding of Some Marine Microalgae on the Microbial Profile Associated with Dicentrarchus labrax Post Larvae. Egypt. J. Aquat. Res. 2014, 40, 251–260. [Google Scholar] [CrossRef][Green Version]
- Li, A.; Zhang, L.; Zhao, Z.Y.; Ma, S.S.; Wang, M.; Liu, P.H. Prescreening, Identification and Harvesting of Microalgae with Antibacterial Activity. Biologia 2016, 71, 1111–1118. [Google Scholar] [CrossRef]
- Kokou, F.; Makridis, P.; Kentouri, M.; Divanach, P. Antibacterial Activity in Microalgae Cultures. Aquac. Res. 2012, 43, 1520–1527. [Google Scholar] [CrossRef]
- Sayegh, F.; Elazzazy, A.; Bellou, S.; Moustogianni, A.; Elkady, A.I.; Baeshen, M.N.; Aggelis, G. Production of Polyunsaturated Single Cell Oils Possessing Antimicrobial and Anticancer Properties. Ann. Microbiol. 2016, 66, 937–948. [Google Scholar] [CrossRef]
- Rao, A.R.; Reddy, A.H.; Aradhya, S.M. Antibacterial Properties of Spirulina platensis, Haematococcus pluvialis, Botryococcus braunii Micro Algal Extracts. Curr. Trends Biotechnol. Pharm. 2010, 4, 809–819. [Google Scholar]
- Chanda, W.; Joseph, T.P.; Guo, X.F.; Wang, W.D.; Liu, M.; Vuai, M.S.; Padhiar, A.A.; Zhong, M.T. Effectiveness of Omega-3 Polyunsaturated Fatty Acids against Microbial Pathogens. J. Zhejiang Univ. Sci. B 2018, 19, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Shannon, E.; Abu-Ghannam, N. Antibacterial Derivatives of Marine Algae: An Overview of Pharmacological Mechanisms and Applications. Mar. Drugs 2016, 14, 81. [Google Scholar] [CrossRef]
- Maadane, A.; Merghoub, N.; El Mernissi, N.; Ainane, T.; Amzazi, S.; Wahby, I.; Bakri, Y. Antimicrobial Activity of Marine Microalgae Isolated from Moroccan Coastlines. J. Microbiol. Biotechnol. Food Sci. 2017, 6, 1257–1260. [Google Scholar] [CrossRef]
- Yoon, B.K.; Jackman, J.A.; Valle-González, E.R.; Cho, N.J. Antibacterial Free Fatty Acids and Monoglycerides: Biological Activities, Experimental Testing, and Therapeutic Applications. Int. J. Mol. Sci. 2018, 19, 1114. [Google Scholar] [CrossRef] [PubMed]
- Najdenski, H.M.; Gigova, L.G.; Iliev, I.I.; Pilarski, P.S.; Lukavský, J.; Tsvetkova, I.V.; Ninova, M.S.; Kussovski, V.K. Antibacterial and Antifungal Activities of Selected Microalgae and Cyanobacteria. Int. J. Food Sci. Technol. 2013, 48, 1533–1540. [Google Scholar] [CrossRef]
- Chaidir, Z.; Syafrizayanti; Hillman, P.F.; Zainul, R. Isolation and Identification of Freshwater Microalgae Potentially as Antibacterial from Talago Biru, Koto Baru, West Sumatera. Der Pharm. Lett. 2016, 8, 157–165. [Google Scholar]
- Ishaq, A.; Monica, H.; Peralta, M.; Basri, H. Bioactive Compounds from Green Microalga-Scenedesmus and Its Potential Applications: A Brief Review. Artic. Pertanika J. Trop. Agric. Sci. 2016, 39, 1–16. [Google Scholar]
- López, Y.; Soto, S.M. The Usefulness of Microalgae Compounds for Preventing Biofilm Infections. Antibiotics 2020, 9, 9. [Google Scholar] [CrossRef]
- Cepas, V.; López, Y.; Gabasa, Y.; Martins, C.B.; Ferreira, J.D.; Correia, M.J.; Santos, L.M.A.; Oliveira, F.; Ramos, V.; Reis, M.; et al. Inhibition of Bacterial and Fungal Biofilm Formation by 675 Extracts from Microalgae and Cyanobacteria. Antibiotics 2019, 8, 77. [Google Scholar] [CrossRef]
- Cheng, C.L.; Huang, S.J.; Wu, C.L.; Gong, H.Y.; Ken, C.F.; Hu, S.Y.; Wu, J.L. Transgenic Expression of Omega-3 PUFA Synthesis Genes Improves Zebrafish Survival during Vibrio vulnificus Infection. J. Biomed. Sci. 2015, 22, 103. [Google Scholar] [CrossRef]
- de Jesus Raposo, M.F.; de Morais, A.M.M.B.; de Morais, R.M.S.C. Bioactivity and Applications of Polysaccharides from Marine Microalgae. In Polysaccharides: Bioactivity and Biotechnology; Springer International Publishing: Cham, Switzerland, 2014; pp. 1–38. [Google Scholar]
- Bernal, P.; Llamas, M.A. Promising Biotechnological Applications of Antibiofilm Exopolysaccharides. Microb. Biotechnol. 2012, 5, 670. [Google Scholar] [CrossRef]
- Rendueles, O.; Ghigo, J.M. Multi-Species Biofilms: How to Avoid Unfriendly Neighbors. FEMS Microbiol. Rev. 2012, 36, 972–989. [Google Scholar] [CrossRef]
- De Jesus Raposo, M.F.; De Morais, A.M.B.; De Morais, R.M.S.C. Marine Polysaccharides from Algae with Potential Biomedical Applications. Mar. Drugs 2015, 13, 2967–3028. [Google Scholar] [CrossRef]
- Rodrigo das Neves dos Santos, A.; Rodrigues, J.A.G.; Holanda, M.L.; Quinderé, A.L.G.; de Paula, R.C.M.; Melo, V.M.M.; Benevides, N.M.B. Antimicrobial Effect of a Crude Sulfated Polysaccharide from the Red Seaweed Gracilaria Ornata. Brazilian Arch. Biol. Technol. 2012, 55, 171–181. [Google Scholar] [CrossRef]
- Esko, J.D.; Sharon, N. Microbial Lectins: Hemagglutinins, Adhesins, and Toxins. In Essentials of Glycobiology, 2nd ed.; Varki, A., Cumminhgs, R.D., Esko, J.D., Freeze, H.H., Stanley, P., Bertozzi, C.R., Hart, G.W., Etzler, M.E., Eds.; Cold Spring Harbor Laboratory Press: New York City, NY, USA, 2009. [Google Scholar]
- Rojas, V.; Rivas, L.; Cárdenas, C.; Guzmán, F. Cyanobacteria and Eukaryotic Microalgae as Emerging Sources of Antibacterial Peptides. Molecules 2020, 25, 5804. [Google Scholar] [CrossRef]
- Lykov, A.; Salmin, A.; Gevorgiz, R.; Zheleznova, S.; Rachkovskaya, L.; Surovtseva, M.; Poveshchenko, O. Study of the Antimicrobial Potential of the Arthrospira platensis, Planktothrix agardhii, Leptolyngbya cf. ectocarpi, Roholtiella mixta Nov., Tetraselmis viridis, and Nanofrustulum shiloi against Gram-Positive, Gram-Negative Bacteria, and Mycobacteria. Mar. Drugs 2023, 21, 492. [Google Scholar] [CrossRef]
- Ayswaria, R.; Vijayan, J.; Nathan, V.K. Antimicrobial Peptides Derived from Microalgae for Combating Antibiotic Resistance: Current Status and Prospects. Cell Biochem. Funct. 2023, 41, 142–151. [Google Scholar] [CrossRef]
- Sedighi, M.; Jalili, H.; Ranaei-Siadat, S.O.; Amrane, A. Potential Health Effects of Enzymatic Protein Hydrolysates from Chlorella vulgaris. Appl. Food Biotechnol. 2016, 3, 160–169. [Google Scholar] [CrossRef]
- Tejano, L.A.; Peralta, J.P.; Yap, E.E.S.; Chang, Y.W. Bioactivities of Enzymatic Protein Hydrolysates Derived from Chlorella sorokiniana. Food Sci. Nutr. 2019, 7, 2381–2390. [Google Scholar] [CrossRef]
- Ishaq, A.G.; Matias-Peralta, H.M.; Basri, H.; Muhammad, M.N. Antibacterial Activity of Freshwater Microalga Scenedesmus sp. on Foodborne Pathogens Staphylococcus aureus and Salmonella sp. J. Sci. Technol. 2015, 7. Available online: https://publisher.uthm.edu.my/ojs/index.php/JST/article/view/1144 (accessed on 18 May 2024).
- Bhagavathy, S.; Sumathi, P.; Jancy Sherene Bell, I. Green Algae Chlorococcum humicola-a New Source of Bioactive Compounds with Antimicrobial Activity. Asian Pac. J. Trop. Biomed. 2011, 1, S1–S7. [Google Scholar] [CrossRef]
- Amaro, H.M.; Barros, R.; Guedes, A.C.; Sousa-Pinto, I.; Malcata, F.X. Microalgal Compounds Modulate Carcinogenesis in the Gastrointestinal Tract. Trends Biotechnol. 2013, 31, 92–98. [Google Scholar] [CrossRef]
- Bennedsen, M.; Wang, X.; Willén, R.; Wadström, T.; Andersen, L.P. Treatment of H. Pylori Infected Mice with Antioxidant Astaxanthin Reduces Gastric Inflammation, Bacterial Load and Modulates Cytokine Release by Splenocytes. Immunol. Lett. 2000, 70, 185–189. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; El-Shafay, S.M.; El-Ballat, E.M. In Vivo Evaluation of Antimicrobial Effect of Methanolic Extract of Chlorella vulgaris on Impetigo and Some Dermatophytes. Egypt. J. Bot. 2016, 56, 423–437. [Google Scholar] [CrossRef]
- Khadija, A.T.; Abd-Wahab, R.A.; Fadhil, A.M. Effect of Ethanolic Extract of Chlorella sp. on Entamoeba histolytica Parasite In Vivo. Plant Arch. 2020, 20, 1975–1978. [Google Scholar]
- Krishnakumar, S.; Dooslin, V.; Bai, M.; Rajan, A.R.A. Evaluation of bioactive metabolites from halophilic microalgae Dunaliella salina by GC-MS analysis. Int. J. Pharm. Pharm. Sci. 2013, 5, 296–303. [Google Scholar]
- Han, P.; Lu, Q.; Fan, L.; Zhou, W. A Review on the Use of Microalgae for Sustainable Aquaculture. Appl. Sci. 2019, 9, 2377. [Google Scholar] [CrossRef]
- MacEdo, R.C.; Bolin, A.P.; Marin, D.P.; Otton, R. Astaxanthin Addition Improves Human Neutrophils Function: In Vitro Study. Eur. J. Nutr. 2010, 49, 447–457. [Google Scholar] [CrossRef]
- Montero-Lobato, Z.; Vázquez, M.; Navarro, F.; Fuentes, J.L.; Bermejo, E.; Garbayo, I.; Vílchez, C.; Cuaresma, M. Chemically-Induced Production of Anti-Inflammatory Molecules in Microalgae. Mar. Drugs 2018, 16, 478. [Google Scholar] [CrossRef]
- Riccio, G.; Lauritano, C. Microalgae with Immunomodulatory Activities. Mar. Drugs 2020, 18, 2. [Google Scholar] [CrossRef]
- Pratt, R.; Daniels, T.C.; Eiler, J.J.; Gunnison, J.B.; Kumler, W.D.; Oneto, J.F.; Strait, L.A.; Spoehr, H.A.; Hardin, G.J.; Milner, H.W.; et al. Chlorellin, an Antibacterial Substance from Chlorella. Science 1944, 99, 351–352. [Google Scholar] [CrossRef]
- Alwathnani, H.; Perveen, K. Antibacterial Activity and Morphological Changes in Human Pathogenic Bacteria Caused by Chlorella vulgaris Extracts. Biomed. Res. 2017, 28, 1610–1614. [Google Scholar]
- Annamalai, J.; Jayashree, S.; Nallamuthu, T. Phytochemical Screening and Antimicrobial Activity of Chlorella vulgaris Beijerinck. Int. J. Curr. Res. Rev. 2012, 4, 33–38. [Google Scholar] [CrossRef]
- Gayatri, K.V.; Soundhari, C.; Pavithra, B.P. Biofilm Inhibitory Effect of Chlorella Extracts on Pseudomonas aeruginosa. Int. J. Pharm. Sci. Res. 2019, 10, 1966–1971. [Google Scholar] [CrossRef]
- Jang, S.H.; Lim, J.W.; Kim, H. β-Carotene Inhibits Helicobacter Pylori-Induced Expression of Inducible Nitrix Oxide Synthase and Cyclooxygenase-2 in Human Gastric Epithelial Cells. J. Physiol. Pharmacol. 2009, 60, 131–137. [Google Scholar]
- Ahmad, S.A.; Yong, J.F.S.; Syamsumir, D.F.; Zin, N.A.M.; Radzi, S.A.M.; Mohd Kassim, M.N.I.; Muzamel, M.A.; Yusof, M.R.; Segaran, T.C. The Potential of Carotenoids from Marine Tropical Microalgae in the Healing Process of Gastritis. J. Sustain. Sci. Manag. 2015, 10, 92–106. [Google Scholar]
- Loke, M.F.; Lui, S.Y.; Ng, B.L.; Gong, M.; Ho, B. Antiadhesive Property of Microalgal Polysaccharide Extract on the Binding of Helicobacter Pylori to Gastric Mucin. FEMS Immunol. Med. Microbiol. 2007, 50, 231–238. [Google Scholar] [CrossRef]
- Zielinski, D.; Fraczyk, J.; Debowski, M.; Zielinski, M.; Kaminski, Z.J.; Kregiel, D.; Jacob, C.; Kolesinska, B.; Rakotondraibe, H.L.; Delattre, C.; et al. Biological Activity of Hydrophilic Extract of Chlorella vulgaris Grown on Post-Fermentation Leachate from a Biogas Plant Supplied with Stillage and Maize Silage. Molecules 2020, 25, 1790. [Google Scholar] [CrossRef]
- Catarina Guedes, A.; Barbosa, C.R.; Amaro, H.M.; Pereira, C.I.; Xavier Malcata, F. Microalgal and Cyanobacterial Cell Extracts for Use as Natural Antibacterial Additives against Food Pathogens. Int. J. Food Sci. Technol. 2011, 46, 862–870. [Google Scholar] [CrossRef]
- Santhosh, S.; Manivannan, N.; Ragavendran, C.; Mathivanan, N.; Natarajan, D.; Hemalatha, N.; Dhandapani, R. Growth Optimization, Free Radical Scavenging and Antibacterial Potential of Chlorella sp. SRD3 Extracts against Clinical Isolates. J. Appl. Microbiol. 2019, 127, 481–494. [Google Scholar] [CrossRef]
- Asadi, S.; Doudi, M.; Darki, B.Z. An In-Vitro Investigation of the Antibacterial Effects of the Methanol and Aqueous Extracts and the Supernatant of the Algae Chlorella vulgaris CCATM 210-1 on Multiantibiotic-Resistant Staphylococcus aureus Isolates Causing Urinary Tract Infections. Int. J. Adv. Biotechnol. Res. 2016, 7, 806–814. [Google Scholar]
- Hamouda, I.A.; Doumandji, A. Comparative Phytochemical Analysis and in Vitro Antimicrobial Activities of the Cyanobacterium spirulina Platensis and the Green Alga Chlorella pyrenoidosa: Potential Application of Bioactive Components as an Alternative to Infectious Diseases. Bull. l’Institut Sci. 2017, 39, 41–49. [Google Scholar]
- Alghanmi, H.A.; Omran, A.S. Antibacterial Activity of Ethanol Extracts of Two Algae Species against Some Pathogenic Bacteria Isolated from Hospital Patients. EurAsian J. Biosci. 2020, 14, 383–394. [Google Scholar]
- Velichkova, K.; Sirakov, I.; Denev, S. In Vitro Antibacterial Effect of Lemna minuta, Chlorella vulgaris and Spirulina sp. Extracts against Fish Pathogen Aeromonas hydrophila. AACL Bioflux 2019, 12, 936–940. [Google Scholar]
- Silva-Júnior, J.N.; de Aguiar, E.M.; Mota, R.A.; Bezerra, R.P.; Porto, A.L.F.; Herculano, P.N.; Marques, D.D.A.V. Antimicrobial Activity of Photosynthetic Microorganisms Biomass Extract against Bacterial Isolates Causing Mastitis. J. Dairy Vet. Sci. 2019, 10, 1–7. [Google Scholar] [CrossRef]
- Lai, Y.C.; Chang, C.H.; Chen, C.Y.; Chang, J.S.; Ng, I.S. Towards Protein Production and Application by Using Chlorella Species as Circular Economy. Bioresour. Technol. 2019, 289, 121625. [Google Scholar] [CrossRef]
- Jafari, S.; Mobasher, M.A.; Najafipour, S.; Ghasemi, Y.; Mohkam, M.; Ebrahimi, M.A.; Mobasher, N. Antibacterial Potential of Chlorella vulgaris and Dunaliella Salina Extracts against Streptococcus mutans. Jundishapur J. Nat. Pharm. Prod. 2018, 13, e13226. [Google Scholar] [CrossRef]
- Velichkova, K.; Sirakov, I.; Rusenova, N.; Beev, G.; Denev, S.; Valcheva, N.; Dinev, T. In Vitro Antimicrobial Activity on Lemna minuta, Chlorella vulgaris and Spirulina sp. Extracts. Fresenius Environ. Bull. 2018, 27, 5736–5741. [Google Scholar]
- Hussein, H.J.; Naji, S.S.; Al-Khafaji, N.M.S. Antibacterial Properties of the Chlorella vulgaris Isolated from Polluted Water in Iraq. J. Pharm. Sci. Res. 2018, 10, 2457–2460. [Google Scholar]
- Elshouny, W.A.E.F.; El-Sheekh, M.M.; Sabae, S.Z.; Khalil, M.A.; Badr, H.M. Antimicrobial Activity of Spirulina platensis against Aquatic Bacterial Isolates. J. Microbiol. Biotechnol. Food Sci. 2017, 6, 1203–1208. [Google Scholar] [CrossRef]
- Sibi, G. Inhibition of Lipase and Inflammatory Mediators by Chlorella Lipid Extracts for Antiacne Treatment. J. Adv. Pharm. Technol. Res. 2015, 6, 7–12. [Google Scholar] [CrossRef]
- El-Sheekh Mostafa, M.; Abou-El-Souod, G.W. Extraction and Characterization of Antimicrobial Active Substances from Green Alga Chlorella vulgaris and the Cyanobacterium Pseudanabaena sp. J. Biol. Chem. Res. 2015, 32, 267–276. [Google Scholar]
- Salem, O.M.A.; Hoballah, E.M.; Ghazi, S.M.; Hanna, S.N. Antimicrobial Activity of Microalgal Extracts with Special Emphasize on Nostoc sp. Life Sci. J. 2014, 11, 752–758. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; El-Shafaay, S.M.; Abou-Shady, A.M.; El-Ballat, E.M. Antibacterial Activities of Different Extracts of Some Fresh and Marine Algae. Egypt. J. Exp. Biol. 2014, 10, 75–85. [Google Scholar]
- Priya, S. Analysis of Value–Added Biochemical Compounds and Antimicrobial Activity of Green Algae Chlorella vulgaris. J. Chem. Pharm. Res. 2012, 4, 2577–2579. [Google Scholar]
- Uma, R.; Sivasubramanian, V.; Devaraj, S.N. Preliminary Phycochemical Analysis and in Vitro Antibacterial Screening of Green Micro Algae, Desmococcus olivaceous, Chlorococcum humicola and Chlorella vulgaris. J. Algal Biomass Utln. 2011, 2, 74–81. [Google Scholar]
- Prakash, S.; Sasikala, S.L.; Aldous, V.H.J. Isolation and Identification of MDR-Mycobacterium Tuberculosis and Screening of Partially Characterised Antimycobacterial Compounds from Chosen Marine Micro Algae. Asian Pac. J. Trop. Med. 2010, 3, 655–661. [Google Scholar] [CrossRef]
- Pradhan, J.; Das, B.K. Antibacterial Properties of Selected Freshwater Microalgae against Pathogenic Bacteria. Ind. J. Fish. 2010, 57, 6166. [Google Scholar]
- Santhakumaran, P.; Ayyappan, S.M.; Ray, J.G. Nutraceutical Applications of Twenty-Five Species of Rapid-Growing Green-Microalgae as Indicated by Their Antibacterial, Antioxidant and Mineral Content. Algal Res. 2020, 47, 101878. [Google Scholar] [CrossRef]
- Mc Gee, D.; Archer, L.; Smyth, T.J.; Fleming, G.T.A.; Touzet, N. Bioprospecting and LED-Based Spectral Enhancement of Antimicrobial Activity of Microalgae Isolated from the West of Ireland. Algal Res. 2020, 45, 101704. [Google Scholar] [CrossRef]
- Saad, M.G.; Abdu, M.; Shafik, H.M.; Marwa, C.; Saad, G. Phytochemical Screening and Antimicrobial Activities of Some Green Algae from Egypt. J. Med. Plants Stud. 2019, 7, 12–16. [Google Scholar]
- Acurio, L.P.; Salazar, D.M.; Valencia, A.F.; Robalino, D.R.; Barona, A.C.; Alvarez, F.C.; Rodriguez, C.A. Antimicrobial Potential of Chlorella Algae Isolated from Stacked Waters of the Andean Region of Ecuador. In Proceedings of the IOP Conference Series: Earth and Environmental Science; Institute of Physics Publishing: Bristol, UK, 2018; Volume 151. [Google Scholar]
- Bashir, K.M.I.; Lee, J.H.; Petermann, M.J.; Shah, A.A.; Jeong, S.J.; Kim, M.S.; Park, N.G.; Cho, M.G. Estimation of Antibacterial Properties of Chlorophyta, Rhodophyta and Haptophyta Microalgae Species. Microbiol. Biotechnol. Lett. 2018, 46, 225–233. [Google Scholar] [CrossRef]
- Montalvão, S.; Demirel, Z.; Devi, P.; Lombardi, V.; Hongisto, V.; Perälä, M.; Hattara, J.; Imamoglu, E.; Tilvi, S.S.; Turan, G.; et al. Large-Scale Bioprospecting of Cyanobacteria, Micro- and Macroalgae from the Aegean Sea. New Biotechnol. 2016, 33, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.M.H.; Kubra, K.; Hossain, M.B.; Mustafa, M.G.; Jainab, T.; Karim, M.R.; Mehedy, M.E. Screening of Antibacterial and Antifungal Activity of Freshwater and Marine Algae as a Prominent Natural Antibiotic Available in Bangladesh. Int. J. Pharmacol. 2015, 11, 828–833. [Google Scholar] [CrossRef]
- Hamouda, R.A.E.; Abou-El-Souod, G.W. Influence of Various Concentrations of Phosphorus on the Antibacterial, Antioxidant and Bioactive Components of Green Microalgae Scenedesmus obliquus. Int. J. Pharmacol. 2018, 14, 99–107. [Google Scholar] [CrossRef]
- Aremu, A.O.; Masondo, N.A.; Stirk, W.A.; Ördög, V.; Van Staden, J. Influence of Culture Age on the Phytochemical Content and Pharmacological Activities of Five Scenedesmus Strains. J. Appl. Phycol. 2014, 26, 407–415. [Google Scholar] [CrossRef]
- Marrez, D.A.; Naguib, M.M.; Sultan, Y.Y.; Higazy, A.M. Antimicrobial and Anticancer Activities of Scenedesmus obliquus Metabolites. Heliyon 2019, 5, e01404. [Google Scholar] [CrossRef]
- Mubarakali, D.; Praveenkumar, R.; Shenbagavalli, T.; Mari Nivetha, T.; Parveez Ahamed, A.; Al-Dhabi, N.A.; Thajuddin, N. New Reports on Anti-Bacterial and Anti-Candidal Activities of Fatty Acid Methyl Esters (FAME) Obtained from Scenedesmus bijugatus Var. bicellularis biomass. RSC Adv. 2012, 2, 11552–11556. [Google Scholar] [CrossRef]
- Scholz, B.; Liebezeit, G. Screening for Biological Activities and Toxicological Effects of 63 Phytoplankton Species Isolated from Freshwater, Marine and Brackish Water Habitats. Harmful Algae 2012, 20, 58–70. [Google Scholar] [CrossRef]
- Senhorinho, G.N.A.; Laamanen, C.A.; Scott, J.A. Bioprospecting Freshwater Microalgae for Antibacterial Activity from Water Bodies Associated with Abandoned Mine Sites. Phycologia 2018, 57, 432–439. [Google Scholar] [CrossRef]
- Nair, B.B.; Krishnika, A.; Amm, S.; Chettiar, M. Antibacterial Activity of Freshwater Microalga (Scenedesmus sp.) against Three Bacterial Strains. J. Biosci. Resour. 2011, 2, 160–165. [Google Scholar]
- Chaidir, Z.; Rahmayuni, R.; Djamaan, A. Isolation and Selection of Growth Medium for Microalgae of Lake Biru Sawahlunto West Sumatra and Antibacterial Activity Test. J. Pure Appl. Microbiol. 2019, 13, 1689–1696. [Google Scholar] [CrossRef]
- Patil, L.; Kaliwal, B.B. Microalga Scenedesmus Bajacalifornicus BBKLP-07, a New Source of Bioactive Compounds with in Vitro Pharmacological Applications. Bioprocess Biosyst. Eng. 2019, 42, 979–994. [Google Scholar] [CrossRef]
- Eloff, J.N. A Proposal towards a Rational Classification of the Antimicrobial Activity of Acetone Tree Leaf Extracts in a Search for New Antimicrobials#. Planta Med. 2021, 87, 836–840. [Google Scholar] [CrossRef]
- Porras, G.; Chassagne, F.; Lyles, J.T.; Marquez, L.; Dettweiler, M.; Salam, A.M.; Samarakoon, T.; Shabih, S.; Farrokhi, D.R.; Quave, C.L. Ethnobotany and the Role of Plant Natural Products in Antibiotic Drug Discovery. Chem. Rev. 2021, 121, 3495. [Google Scholar] [CrossRef]
- Eucast: Clinical Breakpoints and Dosing of Antibiotics. Available online: https://www.eucast.org/clinical_breakpoints (accessed on 27 May 2024).
- Eloff, J.N. Quantification the Bioactivity of Plant Extracts during Screening and Bioassay Guided Fractionation. Phytomedicine 2004, 11, 370–371. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).