Antioxidant and Anticancer Potential of Extracellular Polysaccharide from Porphyridium aerugineum (Rhodophyta)
Abstract
1. Introduction
2. Materials and Methods
2.1. Microalgal Strain
2.2. Microalgal Cultivation
2.3. Dry Weight Determination
2.4. Cell Count
2.5. Carbohydrate Analysis
2.6. Extracellular Polysaccharide Viscosity, Extraction and Purification
2.7. Evaluation of Antioxidant Activity
2.8. Anticancer Activity
2.8.1. Cell Cultivation
2.8.2. MTT Test (to Prove Cell Viability/Vitality/Proliferation)
2.9. Statistical Analysis
3. Results and Discussion
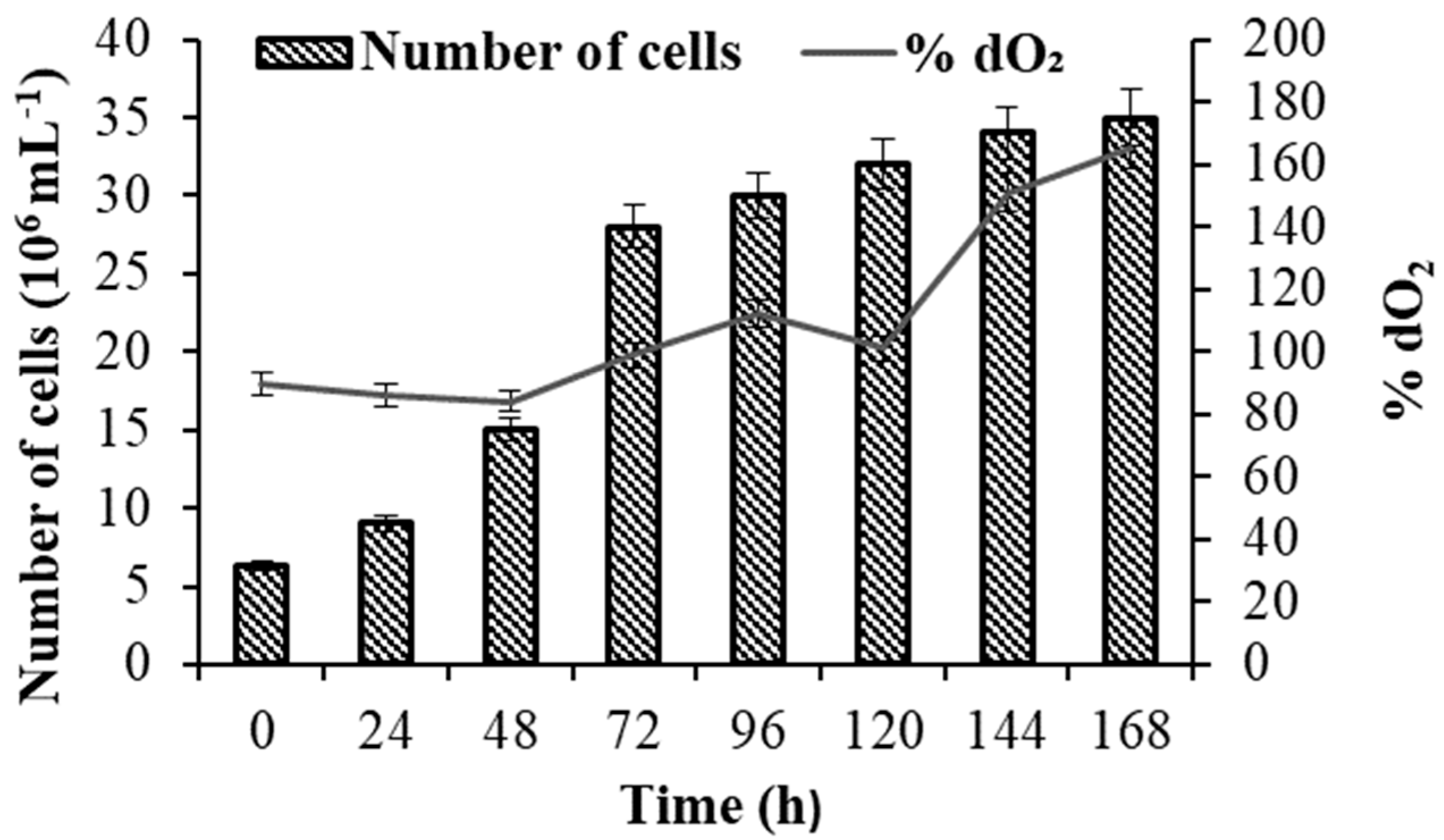
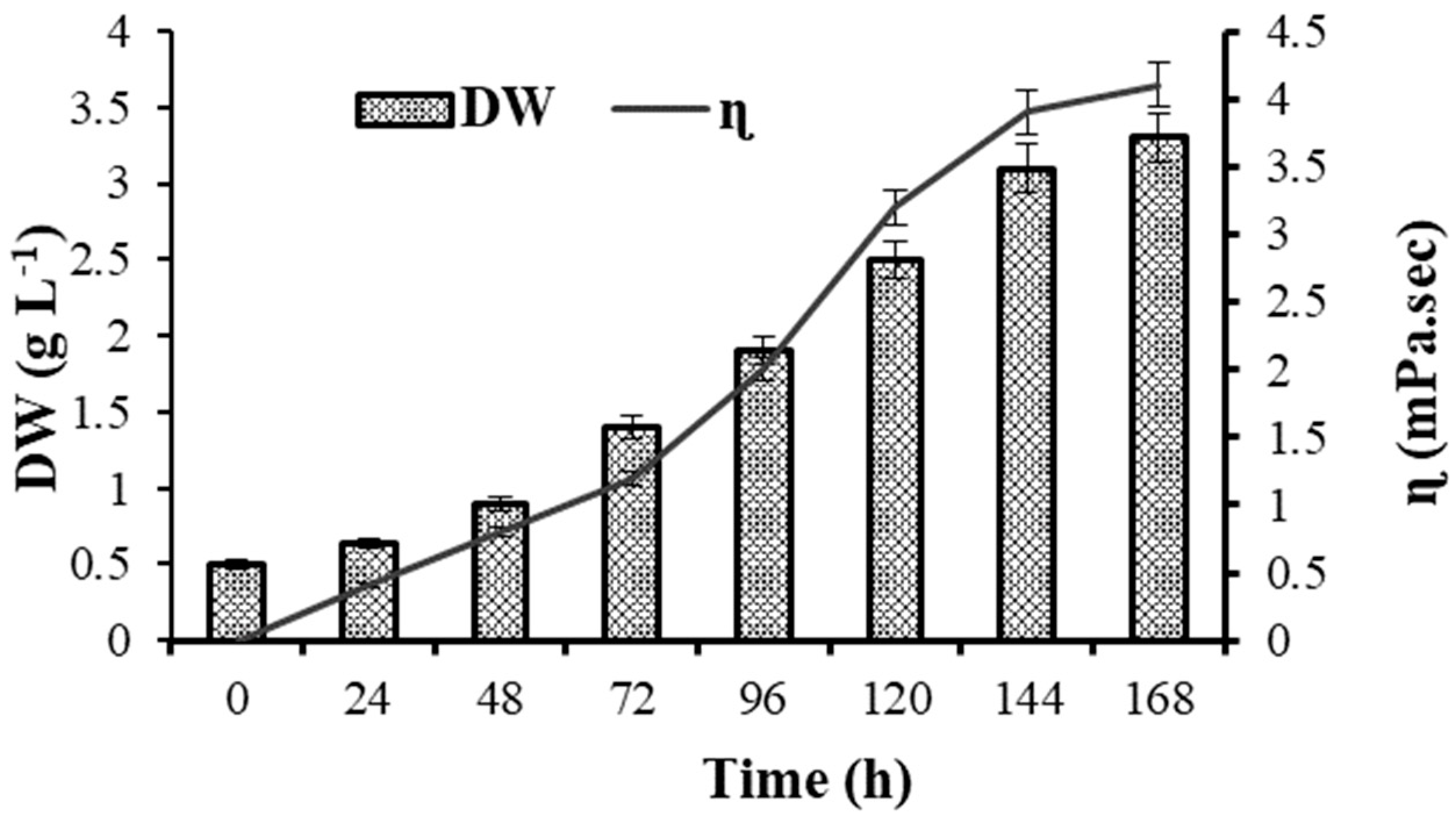
3.1. Growth and Polysaccharide Production
3.2. Antioxidant Activity
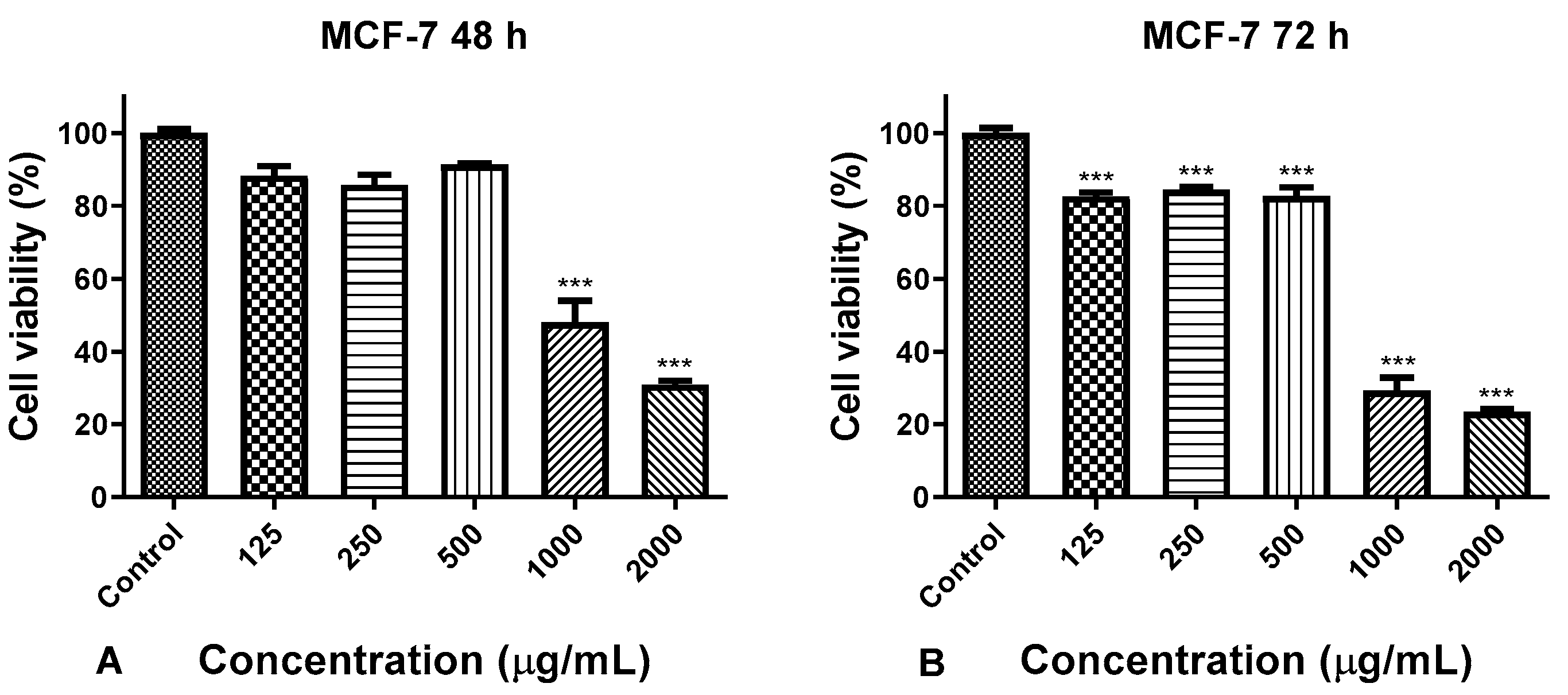
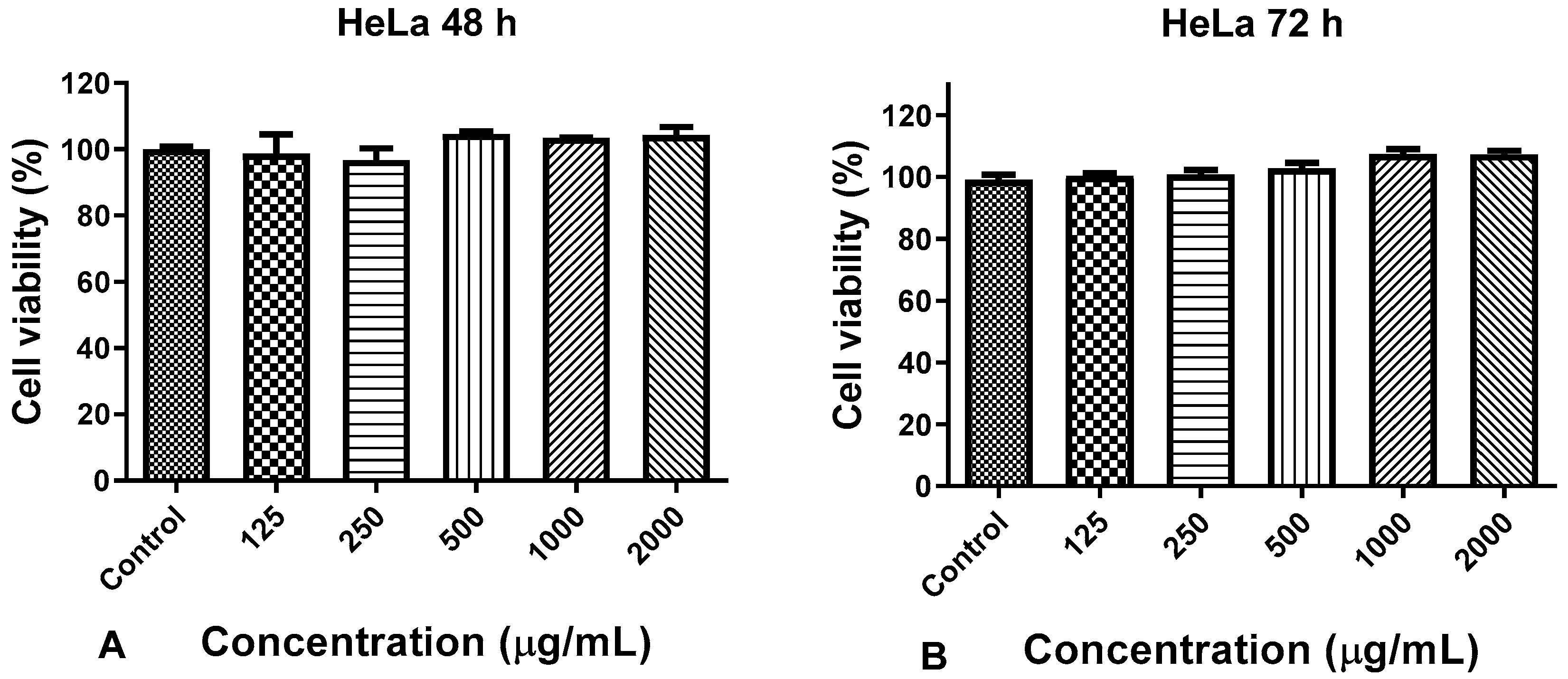
3.3. Anticancer Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lalibertè, G.; Noüie, J.D.L. Auto-, hetero-, and mixotrophic growth of Chlamydomonas humicola (Cmloroimiyckak) on acetate. J. Phycol. 2010, 29, 612–620. [Google Scholar] [CrossRef]
- Tan, J.S.; Lee, S.Y.; Chew, K.W.; Lam, M.K.; Lim, J.W.; Ho, S.H.; Show, P.L. A review on microalgae cultivation and harvesting, and their biomass extraction processing using ionic liquids. Bioengineered 2020, 11, 116–129. [Google Scholar] [CrossRef]
- Mahmood, T.; Hussain, N.; Shahbaz, A. Sustainable production of biofuels from the algae-derived biomass. Bioprocess Biosyst. Eng. 2023, 46, 1077–1097. [Google Scholar] [CrossRef] [PubMed]
- Esqueda, B.A.; Gardarin, C.; Laroche, C. Exploring the diversity of red microalgae for exopolysaccharide production. Mar. Drugs 2022, 20, 246. [Google Scholar] [CrossRef] [PubMed]
- Arad, S.M.; Levy-Ontman, O. Red microalgal cell-wall polysaccharides: Biotechnological aspects. Curr. Opin. Biotechnol. 2010, 21, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Fact. 2018, 17, 36–57. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, Z.; Xiong, S.; Zhang, H.; Li, N.; Zhou, S.; Liu, Y.; Huang, Z. Pilot-scale isolation of bioactive extracellular polymeric substances from cell-free media of mass microalgal cultures using tangential-flow ultrafiltration. Process Biochem. 2011, 46, 1104–1109. [Google Scholar] [CrossRef]
- Liberman, G.N.; Ochbaum, G.; Mejubovsky-Mikhelis, M.; Bitton, R.; Arad, S.M. Physico-chemical characteristics of the sulfated polysaccharides of the red microalgae Dixoniella grisea and Porphyridium aerugineum. Int. J. Biol. Macromol. 2020, 145, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- Prybylski, N.; Toucheteau, C.; El Alaoui, H.; Bridiau, N.; Maugard, T.; Abdelkafi, S.; Fendri, I.; Delattre, C.; Dubessay, P.; Pierre, G.; et al. Chapter 20—Bioactive polysaccharides from microalgae. In Handbook of Microalgae-Based Processes and Products; Jacob-Lopes, E., Maroneze, M., Queiroz, M., Zepka, L., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 533–571. [Google Scholar]
- Raposo, M.F.; de Morais, R.M.S.C.; de Morais, A.M.M.B. Bioactivity and applications of sulphated polysaccharides from marine microalgae. Mar. Drugs 2013, 11, 233–252. [Google Scholar] [CrossRef] [PubMed]
- Ramus, J. The production of extracellular polysaccharide by the unicellular red alga Porphyridium aerugineum. J. Phycol. 1972, 8, 97–111. [Google Scholar] [CrossRef]
- Gardeva, E.; Toshkova, R.; Minkova, K.; Gigova, L. Cancer protective action of polysaccharide, derived from red microalga Porphyridium cruentum—A biological background. Biotechnol. Biotechnol. Equip. 2009, 23, 783–787. [Google Scholar] [CrossRef]
- Nikolova, B.; Semkova, S.; Tsoneva, I.; Antov, G.; Ivanova, J.; Vasileva, I.; Kardaleva, P.; Stoineva, I.; Christova, N.; Nacheva, L.; et al. Characterization and potential antitumor effect of a heteropolysaccharide produced by the red alga Porphyridium sordidum. Eng. Life Sci. 2019, 19, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wan, H.; Wang, R.; Hao, D. Sulfated polysaccharides from Phaeodactylum tricornutum: Isolation, structural characteristics, and inhibiting HepG2 growth activity in vitro. PeerJ 2019, 7, e6409. [Google Scholar] [CrossRef] [PubMed]
- Gargouch, N.; Elleuch, F.; Karkouch, I.; Tabbene, O.; Pichon, C.; Gardarin, C.; Rihouey, C.; Picton Abdelkafi, L.S.; Fendri, I.; Laroche, C. Potential of exopolysaccharide from Porphyridium marinum to contend with bacterial proliferation, biofilm formation, and breast cancer. Mar. Drugs 2021, 19, 66. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, J.; Konstantinidou, A.; Kabaivanova, L. Examination of exopolysaccharides from Porphyridium cruentum for estimation of their potential antitumour activity in vitro. C. R. Acad. Bulg. Sci. 2022, 75, 1146–1155. [Google Scholar] [CrossRef]
- Mousavian, Z.; Safavi, M.; Azizmohseni, F.; Hadizadeh, M.; Mirdamadi, S. Characterization, antioxidant and anticoagulant properties of exopolysaccharide from marine microalgae. AMB Express 2022, 12, 27. [Google Scholar] [CrossRef] [PubMed]
- Burg, A.; Oshrat, L.O. Salt effect on the antioxidant activity of red microalgal sulfated polysaccharides in soy-bean formula. Mar. Drugs 2015, 13, 6425–6439. [Google Scholar] [CrossRef] [PubMed]
- Pekarkova, B.; Hindak, F.; Smarda, J. Morphological characteristics and physiological properties of a coccoid rhodophycean alga Rhodella grisea from thermal springs at Piestany, Czechoslovakia. Arch. Protistenkd. 1988, 135, 69–83. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Abbas, K.A.; Abdulkarim, S.M.; Saleh, A.M.; Ebrahimian, M.M. Suitability of viscosity measurement methods for liquid food variety and applicability in food industry—A review. J. Food Agric. Environ. 2010, 8, 100–107. [Google Scholar]
- Simon, B.; Geresh, S.; Arad, S. Degradation products of the cell wall polysaccharide of the red microalga Porphyridium sp. (Rhodophyta) by means of enzymatic activity of its predator the dinoflagellate Gymnodinium sp. (Gymnodiales). J. Phycol. 1992, 28, 460–465. [Google Scholar] [CrossRef]
- Benzie, I.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”. The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Mossmann, T. Rapid colorimetric assay of cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Hossain, S.Z.; Alnoaimi, A.; Razzak, S.A.; Ezuber, H.; Al-Bastaki, N.; Safdar, M.; Hossain, M.M. Multiobjective optimization of microalgae (Chlorella sp.) growth in a photobioreactor using Box-Behnken design approach. Can. J. Chem. Eng. 2018, 96, 1903–1910. [Google Scholar] [CrossRef]
- Kazbar, A.; Cogne, G.; Urbain, B.; Marec, H.; Le-Gouic, B.; Tallec, J.; Takache, H.; Ismail, A.; Pruvost, J. Effect of dissolved oxygen concentration on microalgal culture in photobioreactors. Algal Res. 2019, 39, 101432. [Google Scholar] [CrossRef]
- Foyer, C.H.; Bloom, A.J.; Queval, G.; Noctor, G. Photorespiratory metabolism: Genes, mutants, energetics, and redox signaling. Annu. Rev. Plant Biol. 2009, 60, 455–484. [Google Scholar] [CrossRef] [PubMed]
- Darveheia, P.; Bahria, P.A.; Moheimanib, N.R. Model development for the growth of microalgae: A review. Renew. Sustain. Energy Rev. 2018, 97, 233–258. [Google Scholar] [CrossRef]
- López Muñoz, I.; Bernard, O. Modeling the influence of temperature, light intensity and oxygen concentration on microalgal growth rate. Processes 2021, 9, 496. [Google Scholar] [CrossRef]
- Vasileva, I.; Ivanova, J. Biochemical profile of green and red algae—A key for understanding their potential application as food additives. Trakia J. Sci. 2019, 17, 1–7. [Google Scholar] [CrossRef]
- Arad, S.; Friedman, O.; Rotem, A. Effect of nitrogen on polysaccharide production in a Porphyridium sp. Appl. Environ. Microbiol. 1988, 54, 2411–2414. [Google Scholar] [CrossRef] [PubMed]
- Laroche, C. Exopolysaccharides from microalgae and cyanobacteria: Diversity of strains, production strategies, and applications. Mar. Drugs 2022, 20, 336. [Google Scholar] [CrossRef] [PubMed]
- Goiris, K.; De Vreese, P.; De Cooman, L.; Muylaert, K. Rapid screening and guided extraction of antioxidants from microalgae using voltammetric methods. J. Agric. Food Chem. 2012, 60, 7359–7366. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, J.; Kabaivanova, L.; Vasileva, I. Assessment of the production potential of valuable compounds with antioxidant properties of different green microalgae. Oxid. Commun. 2021, 44, 27. [Google Scholar]
- Falcao, B.; Vishwakarma, J.; Jadav, H.; Vavilala, S.L. In vitro evaluation of the antioxidant and anti-skin aging properties of green algal sulfated polysaccharides. Arch. Microbiol. Immunol. 2020, 4, 75–90. [Google Scholar] [CrossRef]
- Dogra, B.; Amna, S.; Park, Y.; Park, J.K. Biochemical properties of water soluble polysaccharides from photosynthetic marine microalgae Tetraselmis Species. Macromol. Res. 2017, 25, 172–179. [Google Scholar] [CrossRef]
- Liberti, D.; Imbimbo, P.; Giustino, E.; D’Elia, L.; Silva, M.; Barreira, L.; Monti, D.M. Shedding light on the hidden benefit of Porphyridium cruentum culture. Antioxidants 2023, 12, 337. [Google Scholar] [CrossRef]
- Casas-Arrojo, V.; Decara, J.; de los Ángeles Arrojo-Agudo, M.; Pérez-Manríquez, C.; Abdala-Díaz, R.T. Immunomodulatory, antioxidant activity and cytotoxic effect of sulfated polysaccharides from Porphyridium cruentum (S.F.Gray) Nägeli. Biomolecules 2021, 11, 488. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-N.; Li, Y.; Zhang, Y.; Xiang, W.-Z.; Li, A.-F.; Li, T. Comparison on characterization and antioxidant activity of exopolysaccharides from two Porphyridium strains. J. Appl. Phycol. 2021, 33, 2983–2994. [Google Scholar] [CrossRef]
- Wang, N.; Dai, L.; Chen, Z.; Li, T.; Wu, J.; Wu, H.; Wu, H.; Xiang, W. Extraction optimization, physicochemical characterization, and antioxidant activity of polysaccharides from Rhodosorus sp. SCSIO-45730. J. Appl. Phycol. 2022, 34, 285–299. [Google Scholar] [CrossRef]
- Trifan, A.; Sava, D.; Bucur, L.; Vasincu, A.; Vasincu, I.; Aprotosoaie, A.C.; Cioancă, O.; Miron, A. Isolation, characterization and antioxidant activity of the crude polysaccharide from Phyllophora pseudoceranoides. Rev. Med. Chir. Soc. Med. Nat. Iaşi 2015, 119, 603–609. [Google Scholar] [PubMed]
- Wang, W.N.; Li, T.; Li, Y.; Zhang, Y.; Wu, H.L.; Xiang, W.Z.; Li, A.F. Exopolysaccharides from the energy microalga strain Botryococcus braunii: Purification, characterization, and antioxidant activity. Foods 2022, 11, 110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, L.; Ren, Y.; Chen, F. Characterization of exopolysaccharides produced by microalgae with antitumor activity on human colon cancer cells. Int. J. Biol. Macromol. 2019, 128, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Shao, P.; Xiaoxiao, C.; Peilong, S. In vitro antioxidant and antitumor activities of different sulfated polysaccharides isolated from three algae. Int. J. Biol. Macromol. 2013, 62, 155–161. [Google Scholar] [CrossRef]
- Sun, L.; Wang, C.; Shi, Q.; Ma, C. Preparation of different molecular weight polysaccharides from Porphyridium cruentum and their antioxidant activities. Int. J. Biol. Macromol. 2009, 45, 42–47. [Google Scholar] [CrossRef]

| Time | 48 h | 72 h | 96 h | 120 h | 144 h | 168 h |
|---|---|---|---|---|---|---|
| Polysaccharide concentration (g L−1) | 0.08 ± 0.04 | 0.2 ± 0.03 | 0.4 ± 0.02 | 0.68 ± 0.02 | 1.0 ± 0.01 | 1.2 ± 0.02 |
| Polysaccharide | μmol TE g−1 DW |
|---|---|
| 0.5 mg mL−1 | 10 ± 0.15 |
| 1.0 mg mL−1 | 18 ± 0.07 |
| 1.5 mg mL−1 | 25 ± 0.10 |
| Polysaccharide (mg mL−1) | Inhibition (%) |
|---|---|
| 0.1 | 11 ± 0.20 |
| 0.5 | 27 ± 0.10 |
| 1.0 | 47 ± 0.07 |
| 1.5 | 55 ± 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanova, J.G.; Toshkova-Yotova, T.S.; Toshkova, R.A.; Deleva, V.R.; Georgieva, A.K.; Gigova, L.G. Antioxidant and Anticancer Potential of Extracellular Polysaccharide from Porphyridium aerugineum (Rhodophyta). Fermentation 2024, 10, 259. https://doi.org/10.3390/fermentation10050259
Ivanova JG, Toshkova-Yotova TS, Toshkova RA, Deleva VR, Georgieva AK, Gigova LG. Antioxidant and Anticancer Potential of Extracellular Polysaccharide from Porphyridium aerugineum (Rhodophyta). Fermentation. 2024; 10(5):259. https://doi.org/10.3390/fermentation10050259
Chicago/Turabian StyleIvanova, Juliana G., Tanya S. Toshkova-Yotova, Reneta A. Toshkova, Veronika R. Deleva, Ani K. Georgieva, and Liliana G. Gigova. 2024. "Antioxidant and Anticancer Potential of Extracellular Polysaccharide from Porphyridium aerugineum (Rhodophyta)" Fermentation 10, no. 5: 259. https://doi.org/10.3390/fermentation10050259
APA StyleIvanova, J. G., Toshkova-Yotova, T. S., Toshkova, R. A., Deleva, V. R., Georgieva, A. K., & Gigova, L. G. (2024). Antioxidant and Anticancer Potential of Extracellular Polysaccharide from Porphyridium aerugineum (Rhodophyta). Fermentation, 10(5), 259. https://doi.org/10.3390/fermentation10050259






