Factors Affecting the Quality of Probiotic Plant-Based Frozen Desserts—The Authors’ Own Experiments in the Context of the Literature
Abstract
1. Introduction
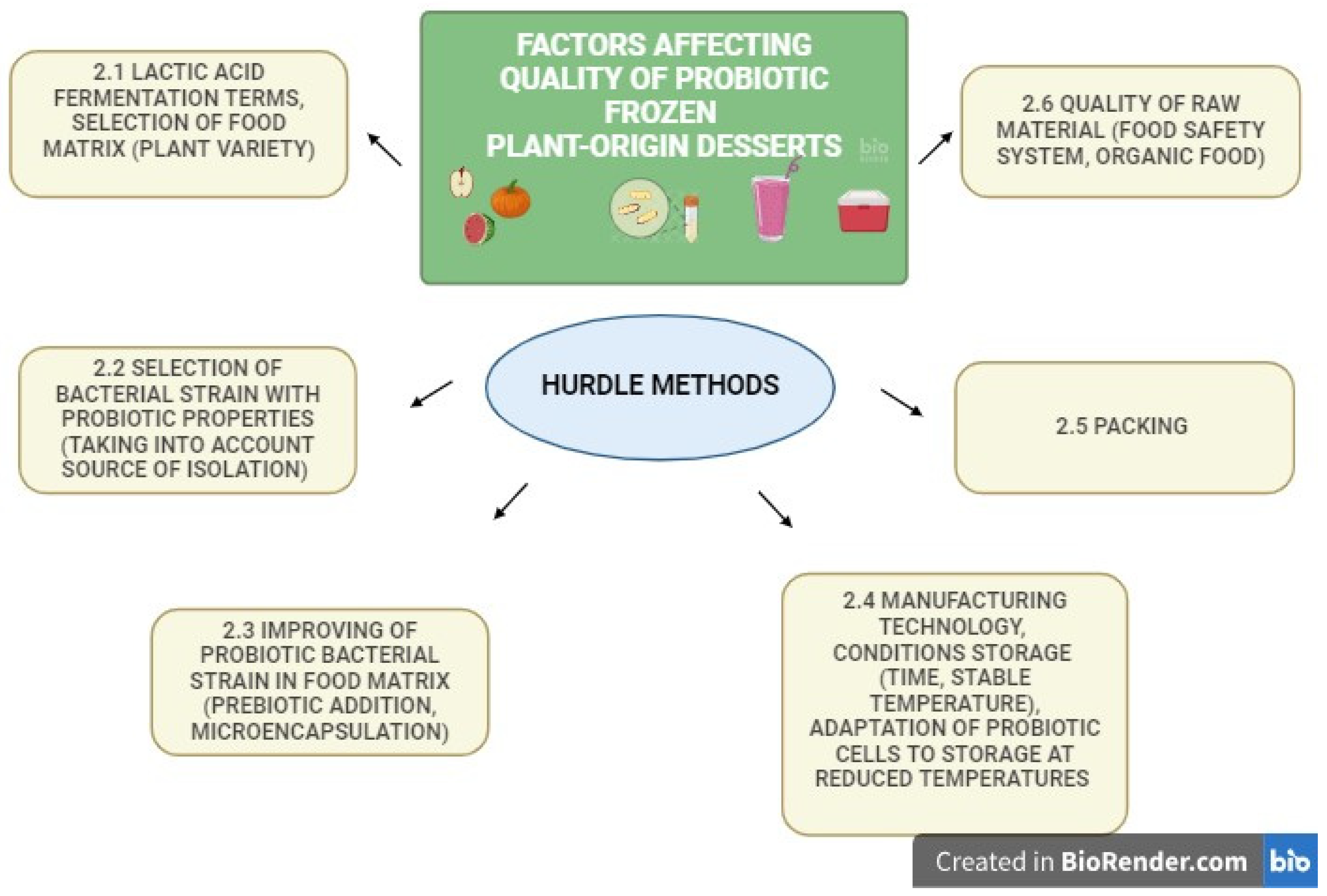
2. Factors Affecting Quality of Frozen Plant-Origin Desserts with Probiotic Bacteria
2.1. The Lactic Acid Fermentation Process and Choice of Food Matrix
2.2. Selection of Bacterial Strains with Probiotic Properties
2.3. Improving Probiotic Survival in Plant Matrix Food
2.3.1. Microencapsulation
2.3.2. Fortification with Prebiotics (Synbiotics)
2.4. Manufacturing Process and Storage Conditions
2.5. Packaging of Functional Products
2.6. Quality of Raw Food Material
3. Examples of Plant-Based Frozen Desserts Developed in the Authors’ Own Experiments
4. Conclusions and Future Trends
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frozen Food in Europe Market, Overview Report 2023–2027, August 2023. Available online: https://www.reportlinker.com/market-report/Frozen-Food/5387/Frozen-Food?term=frozen%20food%20industry&matchtype=b&loc_interest=&loc_physical=9061062&utm_group=standard&utm_term=frozen%20food%20industry&utm_campaign=ppc&utm_source=google_ads&utm_medium=paid_ads&utm_content=transactionnel (accessed on 8 January 2024).
- Krahl, T.; Fuhrmann, H.; Dimassi, S. Chapter 9—Ice cream. In Handbook on Natural Pigments in Food and Beverages; Industrial Applications for Improving Food Color; Woodhead Publishing: Cambridge, UK, 2016; pp. 197–207. [Google Scholar] [CrossRef]
- Goff, H.D.; Hartel, R.W. Ice Cream, 7th ed.; Springer Science + Business: New York, NY, USA, 2013; ISBN 978-1-4614-6095-4. [Google Scholar]
- Frozen Dessert Market Size, Share & Trends Analysis Report by Distribution Channel, by Product (Confectionaries & Candies, Ice Cream, Frozen Yogurt), by Region, and Segment Forecasts, 2019–2025. Available online: https://www.grandviewresearch.com/industry-analysis/frozen-dessert-market (accessed on 10 February 2024).
- Ingredion. The Clean Label Guide to Europe; Ingredion: London, UK, 2014. [Google Scholar]
- Maruyama, S.; Streletskaya, N.A.; Lim, J. Clean label: Why this ingredient but not that one? Food Qual. Prefer. 2021, 87, 104062. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Yerlikaya, O. Starter cultures used in probiotic dairy product preparation and popular probiotic dairy drinks. Food Sci. Technol. 2014, 34, 221–229. [Google Scholar] [CrossRef]
- Williams, N.T. Probiotics. Am. J. Health Syst. Pharm. 2010, 67, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Karbowiak, M.; Zielińska, D. Postbiotyki–właściwości, zastosowanie i wpływ na zdrowie człowieka. Żywność Nauk. Technol. Jakość/Food Sci. Technol. Qual. 2020, 123, 22–37. [Google Scholar] [CrossRef]
- Garnier, L.; Mounier, J.; Lê, S.; Pawtowski, A.; Pinon, N.; Camier, B.; Chatel, M.; Garric, G.; Thierry, A.; Coton, E.; et al. Development of antifungal ingredients for dairy products: From in vitro screening to pilot scale application. Food Microbiol. 2018, 81, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Darwish, M.S.; Qiu, L.; Taher, M.A.; Zaki, A.A.; Abou-Zeid, N.A.; Dawood, D.H.; Shalabi, O.M.A.K.; Khojah, E.; Elawady, A.A. Health Benefits of Postbiotics Produced by E. coli Nissle 1917 in Functional Yogurt Enriched with Cape Gooseberry (Physalis peruviana L.). Fermentation 2022, 8, 128. [Google Scholar] [CrossRef]
- Ramos, I.M.; Rodríguez-Sánchez, S.; Seseña, S.; Palop, M.L.; Poveda, J.M. Assessment of safety characteristics, postbiotic potential, and technological stress response of Leuconostoc strains from different origins for their use in the production of functional dairy foods. LWT 2022, 165, 113722. [Google Scholar] [CrossRef]
- Rodríguez-Gómez, F.; Romero-Gil, V.; García-García, P.; Garrido-Fernández, A.; Arroyo-López, F.N. Fortification of table olive packing with the potential probiotic bacteria Lactobacillus pentosus TOMC-LAB2. Front. Microbiol. 2014, 5, 467. [Google Scholar]
- Szutowska, J. Functional properties of lactic acid bacteria in fermented fruit and vegetable juices: A systematic literature review. Eur. Food Res. Technol. 2020, 246, 357–372. [Google Scholar] [CrossRef]
- Marinho, J.F.U.; da Silva, M.P.; Mazzocato, M.C.; Tulini, F.L.; Favaro-Trindade, C.S. Probiotic and Synbiotic Sorbets Produced with Jussara (Euterpe edulis) Pulp: Evaluation Throughout the Storage Period and Effect of the Matrix on Probiotics Exposed to Simulated Gastrointestinal Fluids. Probiotics Antimicrob. Proteins 2019, 11, 264–272. [Google Scholar] [CrossRef]
- Szydłowska, A.; Zielińska, D. Wpływ wybranych technologii mrożenia na liczbę bakterii Lactobacillus casei ŁOCK 0900, aktywność przeciwutleniającą i cechy sensoryczne sorbetów na bazie fermentowanej pulpy dyniowej. Żywność Nauk. Technol. Jakość/Food. Sci. Technol. Qual. 2019, 26, 109–121. [Google Scholar] [CrossRef]
- Kemsawasd, V.; Chaikham, P. Effects of Frozen Storage on Viability of Probiotics and Antioxidant Capacities of Synbiotic Riceberry and Sesame-Riceberry Milk Ice Creams. Curr. Res. Nutr. Food Sci. 2020, 8, 107–121. [Google Scholar] [CrossRef]
- Da Silva Machado, C.C.; Carlos Fernandes, M.T.; Saori Ishii Mauro, C.; Silva Farinazzo, F.; Prudencio, S.H.; Garcia, S. Probiotic Juçara and Banana Sorbet: Cell Viability, Antioxidant Activity during Storage and Sensory Acceptability by Children. J. Culin. Sci. Technol. 2021, 19, 460–474. [Google Scholar] [CrossRef]
- Norouzi, S.; Pourjafar, H.; Ansari, F.; Homayouni, A. Survey the survival of Lactobacillus paracasei in fermented and non-fermented frozen soy dessert. Biocatal. Agric. Biotechnol. 2019, 21, 101297. [Google Scholar] [CrossRef]
- Gupta, S.; Abu-Ghannam, N. Probiotic Fermentation of Plant Based Products: Possibilities and Opportunities. Crit. Rev. Food Sci. Nutr. 2012, 52, 183–199. [Google Scholar] [CrossRef]
- Williams, J.; McKune, A.J.; Naumovski, N. Sorbets as Functional Food Products, Unexplored Food Matrices, Their Challenges, and Advancements. Appl. Sci. 2023, 13, 11945. [Google Scholar] [CrossRef]
- Tyutkov, N.; Zhernyakova, A.; Birchenko, A.; Eminova, E.; Nadtochii, L.; Baranenko, D. Probiotics viability in frozen food products. Food Biosci. 2022, 50, 101996. [Google Scholar] [CrossRef]
- Gaggia, F.; Di Gioia, D.; Baffoni, L.; Biavati, B. The role of protective and probiotic cultures in food and feed and their impact in food safety. Trends Food Sci. Technol. 2011, 22, S58–S66. [Google Scholar] [CrossRef]
- Dimidi, E.; Cox, S.R.; Rossi, M.; Whelan, K. Fermented Foods: Definitions and Characteristics, Impact on the Gut Microbiota and Effects on Gastrointestinal Health and Disease. Nutrients 2019, 11, 1806. [Google Scholar] [CrossRef]
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Gänzle, M.; Kort, R.; Pasin, G.; Pihlanto, A.; et al. Health benefits of fermented foods: Microbiota and beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Pontonio, E.; Dingeo, C.; Di Cagno, R.; Blandino, M.; Gobbetti, M.; Rizzello, C.G. Brans from hull-less barley, emmer and pigmented wheat varieties: From by-products to bread nutritional improvers using selected lactic acid bacteria and xylanase. Int. J. Food Microbiol. 2019, 313, 108384. [Google Scholar] [CrossRef] [PubMed]
- Filannino, P.; Bai, Y.; Di Cagno, R.; Gobbetti, M.; Gänzle, M.G. Metabolism of phenolic compounds by Lactobacillus spp. during fermentation of cherry juice and broccoli puree. Food Microbiol. 2015, 46, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Coulibaly, A.; Kouakou, B.; Chen, J. Phytic acid in cereal grains: Structure, healthy or harmful ways to reduce phytic acid in cereal grains and their effects on nutritional quality. Am. J. Plant Nutr. Fertil. Technol. 2011, 1, 1–22. [Google Scholar] [CrossRef]
- Abu-Salem, F.M.; Mohamed, R.K.; Gibriel, A.Y.; Rasmy, N.M. Levels of Some Antinutritional Factors in Tempeh Produced From Some Legumes and Jojobas Seeds. Int. J. Biol. Agric. Biosyst. Life Sci. Eng. 2014, 8, 280–285. [Google Scholar]
- Simwaka, J.E.; Chamba MV, M.; Huiming, Z.; Masamba, K.G.; Luo, Y. Effect of fermentation on physicochemical and antinutritional factors of complementary foods from millet, sorghum, pumpkin and amaranth seed flours. Int. Food Res. J. 2017, 24, 1869–1879. Available online: http://www.ifrj.upm.edu.my/24%20(05)%202017/(5).pdf (accessed on 1 March 2024).
- Champagne, C.E.; Gomez da Cruz, A.; Daga, M. Strategies to improve the functionality of probiotics in supplements and foods. Curr. Opin. Food Sci. 2018, 22, 160–166. [Google Scholar] [CrossRef]
- Li, S.; Chen, C.; Ji, Y.; Lin, J.; Chen, X.; Qi, B. Improvement of nutritional value, bioactivity and volatile constituents of quinoa seeds by fermentation with Lactobacillus casei. J. Cereal Sci. 2018, 84, 83–89. [Google Scholar] [CrossRef]
- Behera, S.S.; Ray, R.C.; Zdolec, N. Lactobacillus plantarum with Functional Properties: An Approach to Increase Safety and Shelf-Life of Fermented Food. BioMed Res. Int. 2018, 2018, 9361614. [Google Scholar] [CrossRef]
- Siroli, L.; Patrignani, F.; Serrazanetti, D.I.; Gardini, F.; Lanciotti, R. Innovative strategies based on the use of bio-control agents to improve the safety, shelf-life and quality of minimally processed fruits and vegetables. Trends Food Sci. Technol. 2015, 46, 302–310. [Google Scholar] [CrossRef]
- Di Cagno, R.; Filannino, P.; Vincentini, O.; Lanera, A.; Cavoski, I.; Gobbetti, M. Exploitation of Leuconostoc mesenteroides strains to improve shelf life, rheological, sensory and functional features of prickly pear (Opuntia ficus-indica L.) fruit puree. Food Microbiol. 2016, 59, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Kaprasob, R.; Kerdchoechuen, O.; Laohakunjit, N.; Somboonpanyakul, P. B vitamins and prebiotic fructooligosaccharides of cashew apple fermented with probiotic strains Lactobacillus spp., Leuconostoc mesenteroides and Bifidobacterium longum. Process. Biochem. 2018, 70, 9–19. [Google Scholar] [CrossRef]
- Montemurro, M.; Pontonio, E.; Gobbetti, M.; Rizzello, C.G. Investigation of the nutritional, functional and technological effects of the sourdough fermentation of sprouted flours. Int. J. Food Microbiol. 2019, 302, 47–58. [Google Scholar] [CrossRef]
- Atallah, A.A.; Ismail, E.A.; Yehia, H.M.; Elkhadragy, M.F.; Aloufi, A.S.; Gemiel, D.G. Physicochemical, microbiological and microstructural characteristics of sucrose-free probiotic-frozen yogurt during storage. Foods 2022, 11, 1099. [Google Scholar] [CrossRef] [PubMed]
- Khubber, S.; Marti-Quijal, F.; Tomasevic, I.; Remize, F.; Barba, F. Lactic acid fermentation as a useful strategy to recover antimicrobial and antioxidant compounds for food and by-products. Curr. Opin. Food Sci. 2021, 43, 189–198. [Google Scholar] [CrossRef]
- Haas, R.; Schnepps, A.; Pichler, A.; Meixner, O. Cow milk versus plant-based milk substitutes: A comparison of product image and motivational structure of consumption. Sustainability 2019, 11, 5046. [Google Scholar] [CrossRef]
- De Carvalho Marchesin, J.; Celiberto, L.S.; Orlando, A.B.; de Medeiros, A.I.; Pinto, R.A.; Zuanon, J.A.S.; Spolidorio, L.C.; dos Santos, A.; Taranto, M.P.; Cavallini, D.C.U. A soy-based probiotic drink modulates the microbiota and reduces body weight gain in diet-induced obese mice. J. Funct. Foods 2018, 48, 302–313. [Google Scholar] [CrossRef]
- Rasika, D.M.D.; Vidanarachchi, J.K.; Luiz, S.F.; Azeredo, D.R.P.; Cruz, A.G.; Ranadheera, C.S. Probiotic Delivery through Non-Dairy Plant-Based Food Matrices. Agriculture 2021, 11, 599. [Google Scholar] [CrossRef]
- Rezac, S.; Kok, C.R.; Heermann, M.; Hutkins, R. Fermented Foods as a Dietary Source of Live Organisms. Front. Microbiol. 2018, 9, 1785. [Google Scholar] [CrossRef]
- Holzapfel, W.H. Appropriate starter culture technologies for small-scale fermentation in developing countries. Int. J. Food Microbiol. 2002, 75, 197–212. [Google Scholar] [CrossRef]
- Leroy, F.; De Vuyst, L. Lactic Acid Bacteria as Functional Starter Cultures for the Food Fermentation Industry. Food Sci. Technol. 2004, 15, 67–78. [Google Scholar] [CrossRef]
- Hipólito, C.; Ramalheira, R.; Beirão da Costa, S.; Moldão-Martins, M. The effect of fruit cultivar/origin and storage time on sorbets quality. LWT—Food Sci. Technol. 2016, 68, 462–469. [Google Scholar] [CrossRef]
- Krawęcka, A.; Libera, J.; Latoch, A. The Use of the Probiotic Lactiplantibacillus plantarum 299v in the Technology of Non-Dairy Ice Cream Based on Avocado. Foods 2021, 10, 2492. [Google Scholar] [CrossRef] [PubMed]
- Akalın, H.; Kınık, Ö.; Şatır, G. Manufacturing plant-based non-dairy and probiotic frozen desserts and their impact on physicochemical, sensory and functional aspects. J. Food Sci. Technol. 2024. [Google Scholar] [CrossRef]
- Shori, A.B.; AL Zahrani, A.J. Non-dairy plant-based milk products as alternatives to conventional dairy products for delivering probiotics. Food Sci. Technol. 2021, 42, e101321. [Google Scholar] [CrossRef]
- Leahu, A.; Ropciuc, S.; Ghinea, C. Plant-based milks: Alternatives to the manufacture and characterization of ice cream. Appl. Sci. 2022, 12, 1754. [Google Scholar] [CrossRef]
- Papadimitriou, K.; Alegría, Á.; Bron, P.A.; de Angelis, M.; Gobbetti, M.; Kleerebezem, M.; Lemos, J.A.; Linares, D.M.; Ross, P.; Stanton, C.; et al. Stress Physiology of Lactic Acid Bacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 837–890. [Google Scholar] [CrossRef] [PubMed]
- Vaithilingam, M.; Chandrasekaran, S.; Mehra, A.; Prakash, S.; Agarwal, A.; Ethiraj, S.; Vaithiyanathan, S. Fermentation of beet juice using lactic acid bacteria and its cytotoxic activity against human liver cancer cell lines HepG2. Curr. Bioact. Compd. 2016, 12, 258–263. [Google Scholar] [CrossRef]
- Cirlini, M.; Ricci, A.; Galaverna, G.; Lazzi, C. Application of lactic acid fermentation to elderberry juice: Changes in acidic and glucidic fractions. LWT 2019, 118, 108779. [Google Scholar] [CrossRef]
- Gao, H.; Wen, J.-J.; Hu, J.-L.; Nie, Q.-X.; Chen, H.-H.; Nie, S.-P.; Xiong, T.; Xie, M.-Y. Momordica charantia juice with Lactobacillus plantarum fermentation: Chemical composition, antioxidant properties and aroma profile. Food Biosci. 2019, 29, 62–72. [Google Scholar] [CrossRef]
- Ryu, J.Y.; Kang, H.R.; Cho, S.K. Changes over the fermentation period in phenolic compounds and antioxidant and anticancer activities of blueberries fermented by Lactobacillus plantarum. J. Food Sci. 2019, 84, 2347–2356. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Banat, F.; Taher, H. A review on the lactic acid fermentation from low-cost renewable materials: Recent developments and challenges. Environ. Technol. Innov. 2020, 20, 101138. [Google Scholar] [CrossRef]
- Chen, C.C.; Lan, C.C.; Pan, C.L.; Huang, M.Y.; Chew, C.H.; Hung, C.C.; Chen, P.H.; Lin, H.T.V. Repeated-batch lactic acid fermentation using a novel bacterial immobilization technique based on a microtube array membrane. Process Biochem. 2019, 87, 25–32. [Google Scholar] [CrossRef]
- Jampaphaeng, K.; Cocolin, L.; Maneerat, S. Selection and evaluation of functional characteristics of autochthonous lactic acid bacteria isolated from traditional fermented stinky bean (Sataw-Dong). Ann. Microbiol. 2016, 67, 25–36. [Google Scholar] [CrossRef]
- Uriot, O.; Denis SJuniua, M.; Roussel, Y.; Dary-Mourot, A.; Blanquet-Diot, S. Streptococcus thermophilus: From yogurt starter to a new promising probiotic candidate? J. Funct. Food 2017, 37, 74–89. [Google Scholar] [CrossRef]
- Aspri, M.; Leni, G.; Galaverna, G.; Papademas, P. Bioactive properties of fermented donkey milk, before and after in vitro simulated gastrointestinal digestion. Food Chem. 2018, 268, 476–484. [Google Scholar] [CrossRef]
- Terpou, A.; Papadaki, A.; Lappa, I.K.; Kachrimanidou, V.; Bosnea, L.A.; Kopsahelis, N. Probiotics in food systems: Significance and emerging strategies towards improved viability and delivery of enhanced beneficial value. Nutrients 2019, 11, 1591. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.B.C.; Marcolino, V.A.; Silva, C.; Barão, C.E.; Pimentel, T.C. Potentially synbiotic fermented beverages processed with water-soluble extract of Baru almond. Food Biosci. 2021, 42, 101200. [Google Scholar] [CrossRef]
- Sathyabama, S.; Vijayabharathi, R.; Priyadarisini, V.B. Screening for probiotic properties of strains isolated from feces of various human groups. J. Microbiol. 2012, 50, 603–661. [Google Scholar] [CrossRef]
- Sornplang, P.; Piyadeatsoontorn, S. Probiotic isolates from unconventional sources: A review. J. Anim. Sci. Technol. 2016, 58, 26. [Google Scholar] [CrossRef]
- Ołdak, A.; Zielińska, D.; Rzepkowska, A.; Kołożyn-Krajewska, D. Comparison of Antibacterial Activity of Lactobacillus plantarum Strains Isolated from Two Different Kinds of Regional Cheeses from Poland: Oscypek and Korycinski Cheese. BioMed Res. Int. 2017, 2017, 6820369. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, D.; Kolożyn-Krajewska, D. Food-Origin Lactic Acid Bacteria May Exhibit Probiotic Properties: Review. BioMed Res. Int. 2018, 2018, 5063185. [Google Scholar] [CrossRef] [PubMed]
- Jitpakdee, J.; Kantachote, D.; Kanzaki, H.; Nitoda, T. Selected probiotic lactic acid bacteria isolated from fermented foods for functional milk production: Lower cholesterol with more beneficial compounds. LWT 2020, 135, 110061. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, T.; Tang, H.; Li, X.; Chen, Y.; Zhang, L.; Zhang, J. Probiotic potential and amylolytic properties of lactic acid bacteria isolated from Chinese fermented cereal foods. Food Control. 2020, 111, 107057. [Google Scholar] [CrossRef]
- Tamang, J.P.; Watanabe, K.; Holzapfel, W.H. Review: Diversity of Microorganisms in Global Fermented Foods and Beverages. Front. Microbiol. 2016, 7, 377. [Google Scholar] [CrossRef] [PubMed]
- Yann, D.; Pauline, G. Usefulness of Natural Starters in Food Industry: The Example of Cheeses and Bread. Food Nutr. Sci. 2014, 5, 1679–1691. [Google Scholar] [CrossRef]
- Ly, D.; Mayrhofer, S.; Domig, K.J. Significance of traditional fermented foods in the lower Mekong subregion: A focus on lactic acid bacteria. Food Biosci. 2018, 26, 113–125. [Google Scholar] [CrossRef]
- Bosnea, L.A.; Moschakis, T.; Nigam, P.S.; Biliaderis, C.G. Growth adaptation of probiotics in biopolymer-based coacervate structures to enhance cell viability. LWT 2017, 77, 282–289. [Google Scholar] [CrossRef]
- Morelli, L. In vitro assessment of probiotic bacteria: From survival to functionality. Int. Dairy J. 2007, 17, 1278–1283. [Google Scholar] [CrossRef]
- Nagpal, R.; Kumar, A.; Kumar, M.; Behare, P.V.; Jain, S.; Yadav, H. Probiotics, their health benefits and applications for developing healthier foods: A review. FEMS Microbiol. Lett. 2012, 334, 1–15. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Munekata, P.E.S.; McClements, D.J.; Lorenzo, J.M. Encapsulation of Bioactive Phytochemicals in Plant-Based Matrices and Application as Additives in Meat and Meat Products. Molecules 2021, 26, 3984. [Google Scholar] [CrossRef] [PubMed]
- Zaeim, D.; Sarabi-Jamab, M.; Ghorani, B.; Kadkhodaee, R.; Liu, W.; Tromp, R.H. Microencapsulation of probiotics in multi-polysaccharide microcapsules by electro-hydrodynamic atomization and incorporation into ice-cream formulation. Food Struct. 2020, 25, 100147. [Google Scholar] [CrossRef]
- Călinoiu, L.F.; Ştefănescu, B.E.; Pop, I.D.; Muntean, L.; Vodnar, D.C. Chitosan Coating Applications in Probiotic Microencapsulation. Coatings 2019, 9, 194. [Google Scholar] [CrossRef]
- Homayouni, A.; Azizi, A.; Ehsani, M.R.; Yarmand, M.S.; Razavi, S.H. Effect of microencapsulation and resistant starch on the probiotic survival and sensory properties of synbiotic ice cream. Food Chem. 2008, 111, 50–55. [Google Scholar] [CrossRef]
- Song, D.; Khouryieh, H.; AbuGhazaleh, A.A.; Salem, M.M.E.; Hassan, O.; Ibrahim, S.A. Sensory properties and viability of probiotic microorganisms in chocolate ice cream supplemented with omega-3 fatty acids. Milchwissenschaft 2011, 66, 172–175. [Google Scholar]
- Pinto, S.S.; Fritzen-Freire, C.B.; Muñoz, I.B.; Barreto, P.L.M.; Prudêncio, E.S.; Amboni, R.D.M.C. Effects of the addition of microencapsulated Bifidobacterium BB-12 on the properties of frozen yogurt. J. Food Eng. 2012, 111, 563–569. [Google Scholar] [CrossRef]
- Coghetto, C.C.; Brinques, G.B.; Machado Siqueira, N.; Pletsch, J.; Duarte Soares, R.M.; Záchia Ayub, M.A. Electrospraying microencapsulation of Lactobacillus plantarum enhances cell viability under refrigeration storage and simulated gastric and intestinal fluids. J. Funct. Foods 2016, 24, 316–326. [Google Scholar] [CrossRef]
- Gomez-Mascaraque, L.G.; Morfin, R.C.; Pérez-Masiá, R.; Sanchez, G.; Lopez-Rubio, A. Optimization of electrospraying conditions for the microencapsulation of probiotics and evaluation of their resistance during storage and in-vitro digestion. LWT-Food Sci. Technol. 2016, 69, 438–446. [Google Scholar] [CrossRef]
- Feng, K.; Huangfu, L.; Liu, C.; Bonfili, L.; Xiang, Q.; Wu, H.; Bai, Y. Electrospinning and Electrospraying: Emerging Techniques for Probiotic Stabilization and Application. Polymers 2023, 15, 2402. [Google Scholar] [CrossRef]
- Frakolaki, G.; Katsouli, M.; Giannou, V.; Tzia, C. Novel encapsulation approach for Bifidobacterium subsp. lactis (BB-12) viability enhancement through its incorporation into a double emulsion prior to the extrusion process. Lebensm.-Wiss. Und-Technol.-Food Sci. Technol. 2020, 130, 109671. [Google Scholar] [CrossRef]
- Sultana, M.; Chan, E.-S.; Pushpamalar, J.; Choo, W.S. Advances in extru-sion-dripping encapsulation of probiotics and omega-3 rich oils. Trends Food Sci. Technol. 2022, 123, 69–86. [Google Scholar] [CrossRef]
- Liu, B.; Hu, J.; Yao, H.; Zhang, L.; Liu, H. Improved viability of probiotics encapsu-lated by layer-by-layer assembly using zein nanoparticles and pectin. Food Hydrocolloids 2023, 143, 108899. [Google Scholar] [CrossRef]
- Anselmo, A.C.; McHugh, K.J.; Webster, J.; Langer, R.; Jaklenec, A. Layer-by-layer en-capsulation of probiotics for delivery to the microbiome. Adv. Mater. 2016, 28, 9486–9490. [Google Scholar] [CrossRef]
- Song, H.; Yu, W.; Gao, M.; Liu, X.; Ma, X. Microencapsulated probiotics using emul-sification technique coupled with internal or external gelation process. Carbohydr. Polym. 2013, 96, 181–189. [Google Scholar] [CrossRef]
- Ji, R.; Wu, J.; Zhang, J.; Wang, T.; Zhang, X.; Shao, L.; Chen, D.; Wang, J. Extending Viabil-ity of Bifidobacterium longum in Chitosan-Coated Alginate Microcapsules Using Emulsifi-cation and Internal Gelation Encapsulation Technology. Front. Microbiol. 2019, 10, 1389. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Ji, Y.R.; Cho, Y.; Choi, M.J. Effects of lyoprotectant and encapsulated Lacto-bacillus acidophilus KBL409 on freeze-drying and storage stability. LWT 2023, 182, 114846. [Google Scholar] [CrossRef]
- Pudziuvelyte, L.; Marksa, M.; Sosnowska, K.; Winnicka, K.; Morkuniene, R.; Berna-toniene, J. Freeze-Drying Technique for Microencapsulation of Elsholtzia ciliata Ethanolic Extract Using Different Coating Materials. Molecules 2020, 25, 2237. [Google Scholar] [CrossRef] [PubMed]
- Silva de Farias, T.G.; Leite Ladislau, H.F.; Montenegro Stamford, T.C.; Costa Medeiros, J.A.; Mendonça Soares, B.L.; Stamford Arnaud, T.M.; Montenegro Stamford, T.L. Viabilities of Lactobacillus rhamnosus ASCC 290 and Lactobacillus casei ATCC 334 (in free form or encapsulated with calcium alginate-chitosan) in yellow mombin ice cream. LWT 2019, 100, 391–396. [Google Scholar] [CrossRef]
- Gámbaroa, A.; Mc Sweeneyb, M.B. Chapter Eight—Sensory methods applied to the development of probiotic and prebiotic foods. Adv. Food Nutr. Res. 2020, 94, 295–337. [Google Scholar] [CrossRef]
- Hurtado-Romero, A.; Del Toro-Barbosa, M.; Garcia-Amezquita, L.E.; García-Cayuela, T. Innovative technologies for the production of food ingredients with prebiotic potential: Modifications, applications, and validation methods. Trends Food Sci. Technol. 2020, 104, 117–131. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Sharma, M. Cereal polysaccharides as sources of functional ingredient for reformulation of meat products: A review. J. Funct. Foods. 2019, 62, 103527. [Google Scholar] [CrossRef]
- Homayouni, A.; Azizi, A.; Javadi, M.; Mahdipour, S.; Ejtahed, H. Factors influencing probiotic survival in ice cream: A review. Int. J. Dairy Sci. 2012, 7, 1–10. [Google Scholar] [CrossRef]
- Akin, M.B.; Akin, M.S.; Kirmaci, Z. Effects of inulin and sugar levels on the viability of yogurt and probiotic bacteria and the physical and sensory characteristics in probiotic ice-cream. Food Chem. 2007, 104, 93–99. [Google Scholar] [CrossRef]
- Balthazar, C.F.; Silva HL, A.; Esmerino, E.A.; Rocha, R.S.; Moraes, J.; Carmo MA, V.; Cruz, A.G. The addition of inulin and Lactobacillus casei 01 in sheep milk ice cream. Food Chem. 2018, 246, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Homayouni, A.; Javadi, M.; Ansari, F.; Pourjafar, H.; Jafarzadeh, M.; Barzegar, A. Advanced methods in ice cream analysis: A review. Food Anal. Methods 2018, 11, 3224–3234. [Google Scholar] [CrossRef]
- Homayouni, A.; Norouzi, S. Evaluation of physicochemical traits, sensory properties and survival of lactobacillus casei in fermented soy-based ice cream. J. Food Process. Preserv. 2016, 40, 681–687. [Google Scholar] [CrossRef]
- Szydłowska, A.; Kołożyn-Krajewska, D. Development of potentially probiotic and synbiotic pumpkin frozen desserts. CyTA—J. Food 2019, 17, 251–259. [Google Scholar] [CrossRef]
- Diez-Gutiérrez, L.; Vicente, L.S.; Barrón, L.J.R.; del Carmen Villarán, M.; Chavarri, M. Gamma-aminobutyric acid and probiotics: Multiple health benefits and their future in the global functional food and nutraceuticals market. J. Funct. Foods 2019, 64, 103669. [Google Scholar] [CrossRef]
- Pessione, E.; Cirrincione, S. Bioactive molecules released in food by lactic acid bacteria: Encrypted peptides and biogenic amines. Front. Microbiol. 2016, 7, 876. [Google Scholar] [CrossRef]
- Lin, Q.; Li, D.; Qin, H. Molecular cloning, expression, and immobilization of glutamate decarboxylase from Lactobacillus fermentum YS2. Electron. J. Biotechnol. 2017, 27, 8–13. [Google Scholar] [CrossRef]
- Lim, H.S.; Seo, D.H.; Cha, I.T.; Lee, H.; Nam, Y.D.; Seo, M.J. Expression and characterization of glutamate decarboxylase from Lactobacillus brevis HYE1 isolated from kimchi. World J. Microbiol. Biotechnol. 2018, 34, 44. [Google Scholar] [CrossRef]
- Guimarães, J.T.; Balthazar, C.F.; Silva, R.; Rocha, R.S.; Graça, J.R.; Esmerino, E.A.; Márcia CSilva Sant’Ana, A.S.; KH Duarte, M.C.; Freitas, M.Q.; Cruz, A.G. Impact of probiotics and prebiotics on food texture. Curr. Opin. Food Sci. 2020, 33, 38–44. [Google Scholar] [CrossRef]
- Jeltema, M.; Beckley, J.; Vahalik, J. Food texture assessment and preference based on mouth behavior. Food Qual Prefer. 2016, 52, 160–171. [Google Scholar] [CrossRef]
- Szydłowska, A.; Kołożyn-Krajewska, D. Effect of oligofructose as additive on selected distinguishing features of quality of probiotic fruit-tea sorbets. Żywność Nauk. Technol. Jakość/Food Sci. Technol. Qual. 2016, 5, 82–94. [Google Scholar]
- Martins da Silva, J.; Klososki, S.J.; Silva, R.; Raices, R.S.L.; Silva, M.C.; Queiroz Freitas, M.; Barão, C.E.; Colombo Pimentel, T. Passion fruit-flavored ice cream processed with water-soluble extract of rice by-product: What is the impact of the addition of different prebiotic components? LWT 2020, 128, 109472. [Google Scholar] [CrossRef]
- de Souza, R.C.; Magnani, M.; de Medeiros, V.P.B.; Marcolino, V.A.; Klososki, S.J.; Lima, M.d.S.; Feihrmann, A.C.; Barão, C.E.; Pimentel, T.C. Lacticaseibacillus casei improves textural, functional, and sensory properties and phenolics’ bioaccessibility of frozen desserts prepared using water-soluble extract of rice by-product and Spirulina platensis. LWT 2023, 183, 114794. [Google Scholar] [CrossRef]
- Motyl, W.; Dziugan, P.; Motyl, I.; Jóźwiak, A.; Nowak, S. Functional ice cream with a “clean label”. Biotechnol. Food Sci. 2019, 83, 121–134. [Google Scholar] [CrossRef]
- Pavlyuk, R.; Pogarska, V.; Timofeyeva, N.; Bilenko, L.; Stukonozhenko, T. Exploring the processes of cryomechanodestruction and mechanochemistry when devising nano-technologies for the frozen carotenoid plant supplements. East. -Eur. J. Enterp. Technol. 2016, 6, 39–46. [Google Scholar] [CrossRef]
- Pavlyuk, R.; Pogarska, V.; Kakadii, I.; Pogarskiy, A.; Stukonozhenko, T. Influence of the processes of steam-thermal cryogenic treatment and mechanolysis on biopolymers and biologically active substances in the course of obtaining health promoting nanoproducts. East. -Eur. J. Enterp. Technol. 2017, 6, 41–47. [Google Scholar] [CrossRef]
- Song, S.; Bae, D.W.; Lim, K.; Griffiths, M.W.; Oh, S. Cold stress improves the ability of Lactobacillus plantarum L67 to survive freezing. Int. J. Food. Microbiol. 2014, 191, 135–143. [Google Scholar] [CrossRef]
- Derzelle, S.; Hallet, B.; Ferain, T.; Delcour, J.; Hols, P. Improved adaptation to cold-shock, stationary-phase, and freezing stresses in Lactobacillus plantarum overproducing cold-shock proteins. Appl. Environ. Microbiol. 2003, 69, 4285–4290. [Google Scholar] [CrossRef]
- Ullah, J.; Takhar, P.S.; Sablani, S.S. Effect of temperature fluctuations on ice-crystal growth in frozen potatoes during storage. LWT—Food Sci. Technol. 2014, 59, 1186–1190. [Google Scholar] [CrossRef]
- Tan, M.; Mei, J.; Xie, J. The Formation and Control of Ice Crystal and Its Impact on the Quality of Frozen Aquatic Products: A Review. Crystals 2021, 11, 68. [Google Scholar] [CrossRef]
- Arellano, M.; JGonzalez, E.; Alvarez, G.; Benkhelifa, H.; Flick, D.; Leducq, D. Online ice crystal size measurements by the focused beam reflectance method (FBRM) during sorbet freezing. Procedia Food Sci. 2012; 1, 1256–1264. [Google Scholar] [CrossRef]
- Leducq, D.; NDoye, F.T.; Alvarez, G. Phase change material for the thermal protection of ice cream during storage and transportation. Int. J. Refrig. 2015, 52, 133–139. [Google Scholar] [CrossRef]
- Syed, Q.A.; Anwar, S.; Shukat, R.; Zahoor, T. Effects of different ingredients on texture of ice cream. J. Nutr. Health Food Eng. 2018, 8, 422–435. [Google Scholar] [CrossRef]
- Bahramparvar, M.; Mazaheri Tehrani, M. Application and functions of stabilizers in ice cream. Food Rev. Int. 2011, 27, 389–407. [Google Scholar] [CrossRef]
- Kamińska-Dwórznicka, A.; Janczewska-Dupczyk, A.; Kot, A.; Łaba, S.; Samborska, K. The impact of ι- and κ-carrageenan addition on freezing process and ice crystals structure of strawberry sorbet frozen by various methods. J. Food Sci. 2020, 85, 50–56. [Google Scholar] [CrossRef]
- Arai, N.; Fujiwara, A.; Wakuda, M.; Fujimoto, T.; Nambu, Y.; Ishii, T.; Matsumiya, K.; YMatsumura, Y.; Kawahara, H.; Ogino, K. Anti-freeze effect of Enoki mushroom extract on the quality preservation of frozen whipped cream. J. Food Eng. 2021, 291, 110285. [Google Scholar] [CrossRef]
- Masselot, V.; Bosc, V.; Benkhelifa, H. Analyzing the microstructure of a fresh sorbet with X-ray micro-computed tomography: Sampling, acquisition, and image processing. J. Food Eng. 2021, 29, 110347. [Google Scholar] [CrossRef]
- Muncke, J.; Backhaus, T.; Geueke, B.; Maffini, M.V.; Martin, O.V.; Myers, J.P.; Soto, A.M.; Trasande, L.; Trier, X.; Scheringer, M. Scientific challenges in the risk assessment of food contact materials. Environ. Health Perspect. 2017, 125, 09500. [Google Scholar] [CrossRef]
- Laličić-Petronijević, J.; Popov-Raljić, J.; Lazić, V.; Pezo, L.; Nedović, V. Synergistic effect of three encapsulated strains of probiotic bacteria on quality parameters of chocolates with different composition. J. Funct. Foods 2017, 38, 329–337. [Google Scholar] [CrossRef]
- Oró, E.; De Gracia, A.; Cabeza, L.F. Active phase change material package for thermal protection of ice cream containers. Int. J. Refrig. 2013, 36, 102–109. [Google Scholar] [CrossRef]
- Du, K.; Calautit, J.; Wang, Z.; Wu, Y.; Liu, H. A review of the applications of phase change materials in cooling, heating and power generation in different temperature ranges. Appl. Energy 2018, 220, 242–273. [Google Scholar] [CrossRef]
- Konstantas, A.; Stamford, L.; Azapagic, A. Environmental impacts of ice cream. J. Clean. Prod. 2019, 209, 259–272. [Google Scholar] [CrossRef]
- Wang, S.; Liu, X.; Yang, M.; Zhang, Y.; Xiang, K.; Tang, R. Review of Time Temperature Indicators as Quality Monitors in Food Packaging. Packag. Technol. Sci. 2015, 28, 839–867. [Google Scholar] [CrossRef]
- Walia, A.; Mehra, R.; Kumar, N.; Singh, T.P.; Kumar, H. Good Manufacturing Practices and Safety Issues in Functional Foods and Nutraceuticals. In Bioactive Components; Thakur, M., Belwal, T., Eds.; Springer: Singapore, 2023. [Google Scholar] [CrossRef]
- Bernacchia, R.; Preti, R.; Vinci, G. Organic and conventional foods: Differences in nutrients. Ital. J. Food Sci. 2016, 28, 565–578. [Google Scholar]
- Çakmakçı, S.; Çakmakçı, R. Quality and Nutritional Parameters of Food in Agri-Food Production Systems. Foods 2023, 12, 351. [Google Scholar] [CrossRef]
- Council Regulation (EC) No 834/2007 of 28; June 2007 on Organic Production and Labelling of Organic Products and Repealing Regulation (EEC) No. 2092/91. Available online: https://eur-lex.europa.eu/eli/reg/2007/834/oj (accessed on 1 March 2024).
- Regulation (EU) 2018/848 of the European Parliament and of the Council on Organic Production and Labelling of Organic Products and Repealing Council Regulation (EC) No. 834/2007. Available online: https://eur-lex.europa.eu/eli/reg/2018/848/oj (accessed on 1 March 2024).
- Bosona, T.; Gebresenbet, G. Food traceability as an integral part of logistics management in food and agricultural supply chain. Food Control 2013, 33, 32–48. [Google Scholar] [CrossRef]
- Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 Laying Down the General Principles and Requirements of Food Law, Establishing the European Food Safety Authority and Laying Down Procedures in Matters of Food Safety. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32002R0178 (accessed on 1 March 2024).
- Azanedo, L.; Garcia-Garcia, G.; Stone, J.; Rahimifard, S. An Overview of Current Challenges in New Food Product Development. Sustainability 2020, 12, 3364. [Google Scholar] [CrossRef]
- The Lancet Planetary Health Sustainable food for a sustainable planet. Lancet Planet. Health 2017, 1, e123. [CrossRef] [PubMed]
- Szydłowska, A.; Zielińska, D. Effect of selected freezing technologies on count of Lactobacillus casei ŁOCK 0900, antioxidant activity and sensory characteristics of fermented pumpkin—Based sorbets. Żywność Nauk. Technol. Jakość/Food Sci. Technol. Qual. 2019, 26, 109–121. [Google Scholar]
- Szydłowska, A.; Zielińska, D.; Kołożyn-Krajewska, D. Effect of Pumpkin Cultivar on the Selected Quality Parameters of Functional Non-Dairy Frozen Desserts. Appl. Sci. 2022, 12, 8063. [Google Scholar] [CrossRef]
- Kołożyn-Krajewska, D.; Szydłowska, A.P.L. Frozen Dessert and Its Preparation. The Patent Office of the Republic of. Poland. Patent No. 213822 B1, 27 September 2010. [Google Scholar]
| Method | Practical Application of Method | Features of the Method | Reference |
|---|---|---|---|
| Electrospraying | L. plantarum | Convenient and fast, mild conditions, strong adaptability and easy to scale up. | [82,83] |
| Extrusion | Bifidobacterium subsp. lactis (BB-12) | Low cost, simple operation, mild conditions and uniform size, but the production yield is small and the particle size is larger; difficult to use in large scale productions. | [84,85] |
| Layer-by-layer | L. plantarum 550 | High controllability and adjustability. | [86,87] |
| Emulsification | Bifidobacterium longum | The production is easy to scale up, suitable for industrialization and the particle size is smaller, but there may be residual oil and the droplet size distribution is not uniform. | [88,89] |
| Freeze-drying | Lactobacillus acidophilus KBL409 | The product stability is good, suitable for embedding thermosensitive materials, but it is expensive, has a complicated operation and the surface of the product may wrinkle and shrink. | [90,91] |
| Frozen Dessert | Applied Prebiotic | Main Effect | Reference |
|---|---|---|---|
| Fruit tea sorbet | Oligofructose (added after fermentation) | The prebiotic used caused the improvement of the overall sensory quality of fruit tea sorbets. Sorbets with 2% of oligofructose added were characterized by a smoother texture compared to the control sample. After 12 weeks of storage, the synbiotic sorbets were rated more highly than the probiotic sorbet. | [110] |
| Pumpkin dessert | Inulin (added before fermentation) | Hollow spaces were observed on the surface of pumpkin–pineapple desserts with 2% and 3% inulin addition, which could be the consequence of ice sublimation on the internal walls of the containers. The appearance of a gritty structure in the products tested in the course of the storage process could be explained by the sugar crystallization process. Pumpkin–pineapple sorbets and sorbets with 1% inulin addition were characterized by a detectable pumpkin taste and smooth consistency and the highest overall sensory quality marks. | [103] |
| Passion fruit-flavored dessert | Polydextrose/Oligofructose (added when combining all ingredients, technology without fermentation) | These prebiotics resulted in products with a higher melting rate and altered fruit-flavor, oligofructose texture and color parameters, with greater functionality (bioactive compounds) of ice cream and and decreased health indices for the hypercholesterolemic saturated fatty acid index cream processed (and increased desired fatty acid index). Ice cream with long-chain inulin presented a lower consistency while products with medium-chain inulin had improved volatility and profile (appearance of 2-butanone,3-methyl, fruity aroma). | [111] |
| Riceberry and Sesame-Riceberry Milk Ice Creams | Inulin (added when combining all ingredients, technology without fermentation) | The prebiotic addition caused protective effects towards probiotic growth and metabolism; the final products showed higher resistance to simulated human gastric conditions. | [18] |
| Frozen desserts based on Soluble extract of rice by—product and Spirulina platensis | Polydextrose (added when combining all ingredients, technology without fermentation) | The use of prebiotics resulted in a more consistent and cohesive texture and higher melting rates. | [112] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szydłowska, A.; Zielińska, D.; Sionek, B.; Kołożyn-Krajewska, D. Factors Affecting the Quality of Probiotic Plant-Based Frozen Desserts—The Authors’ Own Experiments in the Context of the Literature. Fermentation 2024, 10, 291. https://doi.org/10.3390/fermentation10060291
Szydłowska A, Zielińska D, Sionek B, Kołożyn-Krajewska D. Factors Affecting the Quality of Probiotic Plant-Based Frozen Desserts—The Authors’ Own Experiments in the Context of the Literature. Fermentation. 2024; 10(6):291. https://doi.org/10.3390/fermentation10060291
Chicago/Turabian StyleSzydłowska, Aleksandra, Dorota Zielińska, Barbara Sionek, and Danuta Kołożyn-Krajewska. 2024. "Factors Affecting the Quality of Probiotic Plant-Based Frozen Desserts—The Authors’ Own Experiments in the Context of the Literature" Fermentation 10, no. 6: 291. https://doi.org/10.3390/fermentation10060291
APA StyleSzydłowska, A., Zielińska, D., Sionek, B., & Kołożyn-Krajewska, D. (2024). Factors Affecting the Quality of Probiotic Plant-Based Frozen Desserts—The Authors’ Own Experiments in the Context of the Literature. Fermentation, 10(6), 291. https://doi.org/10.3390/fermentation10060291







