Spore-Based Probiotic Bacillus subtilis: Current Applications in Humans and Future Perspectives
Abstract
1. Introduction
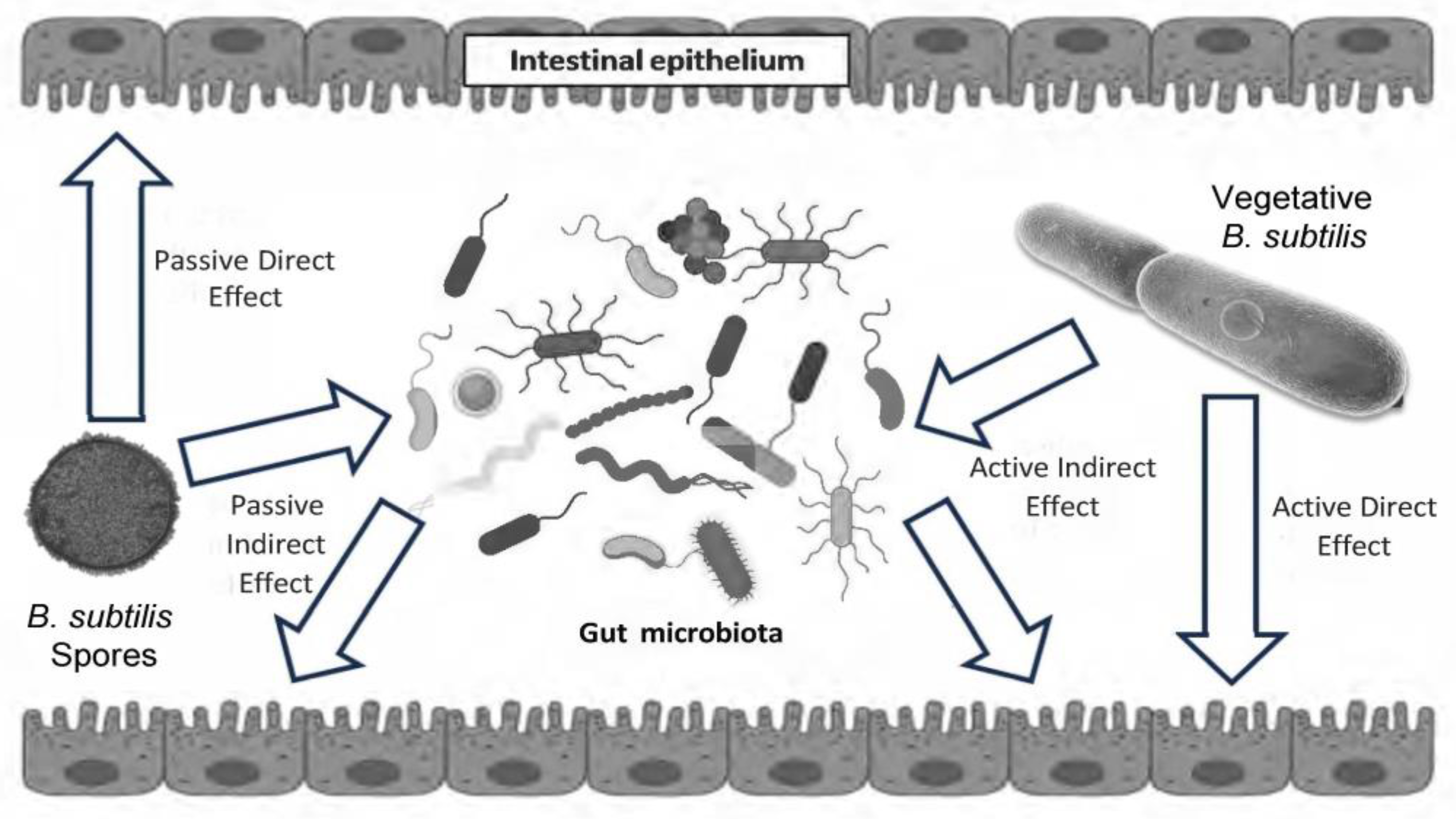
2. Life Cycle of Bacillus subtilis in the Gastrointestinal Tract
3. Effects of Different Strains of Probiotic Bacillus subtilis in Human Applications
3.1. Bacillus subtilis R0179
3.2. Bacillus subtilis DE111
3.3. Bacillus subtilis C-3102
3.4. Bacillus subtilis BS50
3.5. Bacillus subtilis MB4
3.6. Bacillus subtilis CU1
3.7. Bacillus subtilis-Containing Synbiotic Products
3.8. Bacillus subtilis as a Postbiotic
| Reference | Study Design Subjects/Models | Probiotic Dose/Duration | Results |
|---|---|---|---|
| Patch et al. 2023 [62] | Randomized, double-blind, placebo-controlled, parallel-arm trial. Healthy adults (aged 18–75), n = 67, with self-reported diagnosis of functional gastrointestinal disorders (FGID). | B. subtilis BG01-4™ 5 × 109 CFU Daily dose for 4 weeks | Constipation in the probiotic group was significantly improved compared to placebo (33% vs.15%, respectively). Clusters for constipation (18% improvement), indigestion (11%), and dyspepsia (10%) were significantly improved in the probiotic group compared to the placebo. |
| Garvey et al. 2022 [58] | Randomized, double-blind, placebo-controlled, parallel-arm clinical trial. Healthy adults (aged 30–65), n = 76, with at least minimal complaints of abdominal bloating, burping, or flatulence. | B. subtilis BS50 2 × 109 CFU 1 capsule/day for 6 weeks | Improvement of 2 or more points in the 7-day, 3-item composite score according to GITQ (composite score for flatulence, bloating, and burping) between baseline and week 6 (47.4% vs. 22.2%). Compared to placebo, the proportion of participants with an improvement of 1 or more points in GITQ for burping (44.7% vs. 22.2%) and bloating (31.6% vs. 13.9%). There were no significant differences between groups for flatulence (47.4% vs. 44.4%). No change in bowel habits, sleep quality, respiratory infections, and blood markers for intestinal permeability, inflammation, and lipid profile. |
| Kordowski et al. 2022 [11] | Open-label, single-arm real-life exploratory study. Healthy adults, n = 192. | B. subtilis DSM32315 2 × 109 CFU (+290 mg L-Alanyl-L-Glutamine) 2 capsules/day for 4 weeks | Fasting glucose significantly decreased from pre- to post-treatment (96.92 ± 8.29 mg/dL vs. 94.58 ± 9.27 mg/dL, respectively). HbA1c significantly decreased from pre- to post-treatment (5.72% ± 0.27 vs. 5.65% ± 0.30). Postprandial glycemic response improved. Body weight (and BMI) significantly decreased. Relative abundance of Bacteroidetes significantly increased and Firmicutes decreased at post-treatment. |
| Dieck et al. 2021 [61] | Open-label, single-arm pilot study. Healthy men (aged 18–40), n = 18. | B. subtilis DSM32315 2 × 109 CFU (+290 mg L-Alanyl-L-Glutamine) Daily dose for 4 weeks | DSM32315 increased levels of butyrate and butyrate-producing taxa in gut microbiota. Plasma LDL-, total cholesterol, and LDL/HDL cholesterol ratio significantly decreased. Fasting levels of PYY (Peptide YY) and GLP-1 (Glucagon-like Peptide 1) significantly decreased. |
| Freedman et al. 2021 [47] | Randomized, double-blind, placebo-controlled, parallel-arm clinical trial. Healthy adults (aged 20–65), n = 44. | B. subtilis DE111 1 × 109 CFU 1 capsule/day for 4 weeks | Increase in anti-inflammatory immune cell populations in response to ex vivo LPS stimulation of PBMCs in the DE111 group. Overall perceived gastrointestinal health, microbiota, and circulating and fecal markers of inflammation (Il-6, sIgA) and gut barrier function (plasma zonulin) were largely unaffected by DE111 intervention. |
| Penet et at. 2021 [59] | Randomized, double-blind, placebo-controlled, parallel-arm clinical trial. Healthy adults (aged 18–75), n = 100, with self-reported symptoms of bloating, abdominal discomfort, and gas. | B. subtilis MB40 5 × 109 CFU 1 capsule/day for 4 weeks | No significant differences in bloating intensity, number of days with and duration of bloating, abdominal discomfort, and gas between MB40 and placebo groups. Physical limitation, vitality, and social functioning were significantly improved from baseline to week 4 in the MB40 group. At 2 weeks, physical functioning significantly improved in the MB40 group versus placebo. Clinical, but not statistically significant (10%), reductions in bloating intensity, number of days with abdominal discomfort, gas, bloating, and duration of gas, and 10% improvement in general health score in male sub-group receiving MB40 compared to placebo. |
| Trotter et al. 2020 [53] | Randomized, double-blind, placebo-controlled, parallel-arm clinical trial. Healthy adults (aged 18–65), n = 88. | B. subtilis DE111 1 × 109 CFU 1 capsule/day for 4 weeks | Significant reduction in total cholesterol and non-high-density lipoprotein cholesterol in DE111 group. Improvements in endothelial function and in low-density lipoprotein cholesterol. |
| Paytuvi-Gallart et al. 2020 [48] | Randomized, double-blind, placebo-controlled, parallel arm study. Healthy children (aged 2–6), n = 101, attending daycare. | B. subtilis DE111 1 × 109 CFU 1 capsule/day for 8 weeks | Microbiome composition analysis: alpha diversity increased in probiotic group; no significant changes in the overall microbiome equilibrium; six taxa (at the genus level) significantly increased after probiotic intake, and three taxa significantly decreased. |
| Hatanaka et al. 2020 [56] | Randomized, double-blind, placebo-controlled, parallel-arm study. Healthy adults, n = 44. | B. subtilis C-3102 4.8 × 1010 CFU Daily dose for 4 weeks | Body fat percentage was significantly lower in the C-3102 group than in the placebo group at 2 weeks after probiotic. Mean corpuscular hemoglobin level was significantly higher, and cholinesterase, total cholesterol, and triglyceride levels were significantly lower 2 weeks after intake in the C-3102 group than in the placebo group. Direct bilirubin was significantly higher and total cholesterol significantly lower 4 weeks after intake in the C-3102 group than in the placebo group. No significant changes in other measured parameters. |
| Townsend et al. 2020 [52] | Randomized, double-blind, placebo-controlled, parallel-arm study. Recreationally active adults, n = 22. | B. subtilis DE111 1 × 109 CFU 1 capsule/day for 28 days | Supplementation with DE111 does not affect plasma amino acid response following acute whey protein ingestion. |
| Toohey et al. 2020 [51] | Randomized, double-blind, placebo-controlled, parallel-arm study. Division I college female athletes, n = 23. | B. subtilis DE111 1 × 109 CFU 1 capsule/day for 10 weeks | Significant reduction in body fat % in DE111 supplementation group (−2.05 ± 1.38%) compared with placebo (0.2 ± 1.6%). No other differences between probiotic and placebo groups were observed. |
| Townsend et al. 2018 [50] | Randomized, double-blind, placebo-controlled, parallel-arm study. Division I college male athletes, n = 25. | B. subtilis DE111 1 × 109 CFU 1 capsule/day for 12 weeks | TNF-α concentrations were significantly lower after DE111 compared to placebo. No significant group differences in any other measured biochemical markers. No effect on body composition, performance, hormonal status, or gut permeability. |
| Takimoto et al. 2018 [54] | Randomized, double-blind, placebo-controlled, parallel-arm study. Healthy postmenopausal Japanese women (aged 50–69), n = 76. | B. subtilis C-3102 3.4 × 109 CFU Daily dose for 24 weeks | Significant increase in total hip BMD in probiotic group (placebo = 0.83 ± 0.63%, C-3102 = 2.53 ± 0.52%). Significantly lower uNTx probiotic vs. placebo group at 12 weeks of treatment. A trend of a decrease in the bone resorption marker TRACP-5b when compared with the placebo group at 12 weeks of treatment. No change in markers of bone formation, BAP and iPTH. Changes in microbiota composition after C-3102 supplementation. |
| Hatanaka et al. 2018 [55] | Randomized, double-blind, placebo-controlled, parallel-arm study. Healthy adults (aged 20–79), n = 82, with loose stools. | B. subtilis C-3102 2.2 × 109 CFU Daily dose for 8 weeks | Stool frequency per day significantly decreased after C-3102 treatment. Stool quality (measured by BBC scores) significantly improved. Abdominal sound symptoms (reported by GSRS) significantly decreased. Change in microbiota composition following C-3102 treatment. |
| Cuentas et al. 2017 [49] | Randomized, double-blind, placebo-controlled, parallel-arm clinical trial. Healthy adults (aged 18–65), n = 50, with occasional constipation and/or diarrhea. | B. subtilis DE111 1 × 109 CFU 1 capsule/day for 90 days | By day 90, the proportion of normal stools (43.1%) to non-normal stools (6.13%) in the DE111 group differed significantly from placebo group (evaluated by BSC). The proportion of normal stools increased from week 1 to the last week in DE111 group (37.36% to 43.1%) vs. no change in placebo (33.77% to 35.43%). |
| Lefevre et al. 2015 [60] | Randomized, double-blind, placebo-controlled, parallel-arm study. Healthy elderly (aged 60–74), n = 100. | B. subtilis CU1 2 × 109 CFU 1 capsule/day for 10 days, intermittent with 18 days, no ingestion, for 4 months | No significant decrease in mean number of days of reported for CID symptoms over the 4 months of study. B. subtilis CU1 significantly increased fecal and salivary secretory IgA concentrations compared to the placebo. No statistically significant differences in the plasma concentrations of cytokines (IL-1beta, IL-4, IL-6, IL-8, IL-10, IL-12p70, IgA, and TNF-alpha) between the probiotic and the placebo groups from pre- to post-supplementation. |
| Hanifi et al. 2015 [46] | Randomized, double-blind, placebo-controlled, parallel-arm clinical trial. Healthy adults, n = 81. | B. subtilis R0179 0.1 × 109, 1.0 × 109, or 10 × 109 CFU 1 capsule/day for 4 weeks | The scores of the GI distress syndrome between placebo, 0.1, 1.0, and 10 × 109 CFU groups were equivalent. The 0.1 × 109 CFU (0.3 ± 0.1) group was not equivalent to the 1 × 109 (0.6 ± 0.1). The abdominal pain, reflux, diarrhea, indigestion, and constipation syndrome were equivalent across all periods by treatment comparisons. Microbiota composition was affected by probiotic treatment. |
4. Future Perspectives for Probiotic Bacillus subtilis in Human Applications
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cutting, S.M. Bacillus probiotics. Food Microbiol. 2011, 28, 214–220. [Google Scholar] [CrossRef]
- Rudrappa, T.; Quinn, W.J.; Stanley-Wall, N.R.; Bais, H.P. A degradation product of the salicylic acid pathway triggers oxidative stress resulting in down-regulation of Bacillus subtilis biofilm formation on Arabidopsis thaliana roots. Planta 2007, 226, 283–297. [Google Scholar] [CrossRef]
- Fall, R.; Kinsinger, R.F.; Wheeler, K.A. A simple method to isolate biofilm-forming Bacillus subtilis and related species from plant roots. Syst. Appl. Microbiol. 2004, 27, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Ursino, E.; Albertini, A.M.; Fiorentino, G.; Gabrieli, P.; Scoffone, V.C.; Pellegrini, A.; Gasperi, G.; Di Cosimo, A.; Barbieri, G. Bacillus subtilis as a host for mosquitocidal toxins production. Microb. Biotechnol. 2020, 13, 1972–1982. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, A.; Mohan, S. Bacillus subtilis and B. licheniformis Isolated from Heterorhabditis indica Infected Apple Root Borer (Dorysthenes huegelii) Suppresses Nematode Production in Galleria mellonella. Acta Parasitol. 2021, 66, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.; Harshitha, M.; Disha, S.; Dubey, S.; Munang’andu, H.M.; Evensen, O.; Karunasagar, I.; Chakraborty, A.; Maiti, B. In vitro determination of probiotic efficacy of Bacillus subtilis TLDK301120C24 isolated from tilapia against warm water fish pathogens and in vivo validation using gnotobiotic zebrafish model. Microb. Pathog. 2023, 185, 106429. [Google Scholar] [CrossRef]
- Ushakova, N.A.; Voznesenskaya, V.V.; Kozlova, A.A.; Nifatov, A.V.; Samoylenko, V.A.; Nekrasov, R.V.; Egorov, I.A.; Pavlov, D.S. Release of a somatostatin-like peptide by cells of Bacillus subtilis B-8130, an intestinal symbiont of the wild bird Tetrao urogallus: The influence of the bacillus on the animal. Dokl. Biol. Sci. 2010, 434, 328–331. [Google Scholar] [CrossRef]
- Li, A.; Jiang, X.; Wang, Y.; Zhang, L.; Zhang, H.; Mehmood, K.; Li, Z.; Waqas, M.; Li, J. The impact of Bacillus subtilis 18 isolated from Tibetan yaks on growth performance and gut microbial community in mice. Microb. Pathog. 2018, 128, 153–161. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Dai, L.; Liu, Y.; Cheng, M.; Chen, L. Isolation and characterization of a novel gossypol-degrading bacteria Bacillus subtilis strain Rumen Bacillus subtilis. Asian-Australas. J. Anim. Sci. 2018, 31, 63–70. [Google Scholar] [CrossRef]
- Swain, M.R.; Ray, R.C. Biocontrol and other beneficial activities of Bacillus subtilis isolated from cowdung microflora. Microbiol. Res. 2009, 164, 121–130. [Google Scholar] [CrossRef]
- Kordowski, A.; Tetzlaff-Lelleck, V.V.; Speckmann, B.; Loh, G.; Kunstner, A.; Schulz, F.; Schroder, T.; Smollich, M.; Sina, C.; Tom Dieck, H. A nutritional supplement based on a synbiotic combination of Bacillus subtilis DSM 32315 and L-alanyl-L-glutamine improves glucose metabolism in healthy prediabetic subjects—A real-life post-marketing study. Front. Nutr. 2022, 9, 1001419. [Google Scholar] [CrossRef]
- Folmsbee, M.J.; McInerney, M.J.; Nagle, D.P. Anaerobic growth of Bacillus mojavensis and Bacillus subtilis requires deoxyribonucleosides or DNA. Appl. Environ. Microbiol. 2004, 70, 5252–5257. [Google Scholar] [CrossRef] [PubMed]
- Tam, N.K.; Uyen, N.Q.; Hong, H.A.; Duc, L.H.; Hoa, T.T.; Serra, C.R.; Henriques, A.O.; Cutting, S.M. The intestinal life cycle of Bacillus subtilis and close relatives. J. Bacteriol. 2006, 188, 2692–2700. [Google Scholar] [CrossRef] [PubMed]
- Leser, T.D.; Knarreborg, A.; Worm, J. Germination and outgrowth of Bacillus subtilis and Bacillus licheniformis spores in the gastrointestinal tract of pigs. J. Appl. Microbiol. 2008, 104, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Casula, G.; Cutting, S.M. Bacillus probiotics: Spore germination in the gastrointestinal tract. Appl. Environ. Microbiol. 2002, 68, 2344–2352. [Google Scholar] [CrossRef] [PubMed]
- Hoa, T.T.; Duc, L.H.; Isticato, R.; Baccigalupi, L.; Ricca, E.; Van, P.H.; Cutting, S.M. Fate and dissemination of Bacillus subtilis spores in a murine model. Appl. Environ. Microbiol. 2001, 67, 3819–3823. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, L.K.; Roberts, M.S.; Cohan, F.M. Relationship of Bacillus subtilis clades associated with strains 168 and W23: A proposal for Bacillus subtilis subsp. subtilis subsp. nov. and Bacillus subtilis subsp. spizizenii subsp. nov. Int. J. Syst. Bacteriol. 1999, 49 Pt 3, 1211–1215. [Google Scholar] [CrossRef] [PubMed]
- Earl, A.M.; Losick, R.; Kolter, R. Bacillus subtilis genome diversity. J. Bacteriol. 2007, 189, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Traag, B.A.; Pugliese, A.; Eisen, J.A.; Losick, R. Gene conservation among endospore-forming bacteria reveals additional sporulation genes in Bacillus subtilis. J. Bacteriol. 2013, 195, 253–260. [Google Scholar] [CrossRef]
- Ihekwaba, A.E.; Mura, I.; Barker, G.C. Computational modelling and analysis of the molecular network regulating sporulation initiation in Bacillus subtilis. BMC Syst. Biol. 2014, 8, 119. [Google Scholar] [CrossRef]
- Earl, A.M.; Losick, R.; Kolter, R. Ecology and genomics of Bacillus subtilis. Trends Microbiol. 2008, 16, 269–275. [Google Scholar] [CrossRef]
- Cartman, S.T.; La Ragione, R.M.; Woodward, M.J. Bacillus subtilis spores germinate in the chicken gastrointestinal tract. Appl. Environ. Microbiol. 2008, 74, 5254–5258. [Google Scholar] [CrossRef]
- Latorre, J.D.; Hernandez-Velasco, X.; Kallapura, G.; Menconi, A.; Pumford, N.R.; Morgan, M.J.; Layton, S.L.; Bielke, L.R.; Hargis, B.M.; Tellez, G. Evaluation of germination, distribution, and persistence of Bacillus subtilis spores through the gastrointestinal tract of chickens. Poult. Sci. 2014, 93, 1793–1800. [Google Scholar] [CrossRef]
- Bernardeau, M.; Lehtinen, M.J.; Forssten, S.D.; Nurminen, P. Importance of the gastrointestinal life cycle of Bacillus for probiotic functionality. J. Food Sci. Technol. 2017, 54, 2570–2584. [Google Scholar] [CrossRef] [PubMed]
- Sampath, V.; Duk Ha, B.; Kibria, S.; Kim, I.H. Effect of low-nutrient-density diet with probiotic mixture (Bacillus subtilis ms1, B. licheniformis SF5-1, and Saccharomyces cerevisiae) supplementation on performance of weaner pigs. J. Anim. Physiol. Anim. Nutr. 2022, 106, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Cho, S.B.; Song, M.H.; Lee, S.I.; Hong, S.M.; Yun, W.; Lee, J.H.; Oh, H.J.; Chang, S.Y.; An, J.W.; et al. Effects of different Bacillus licheniformis and Bacillus subtilis ratios on nutrient digestibility, fecal microflora, and gas emissions of growing pigs. J. Anim. Sci. Technol. 2022, 64, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Duc, L.H.; Hong, H.A.; Barbosa, T.M.; Henriques, A.O.; Cutting, S.M. Characterization of Bacillus probiotics available for human use. Appl. Environ. Microbiol. 2004, 70, 2161–2171. [Google Scholar] [CrossRef] [PubMed]
- Hatanaka, M.; Nakamura, Y.; Maathuis, A.J.; Venema, K.; Murota, I.; Yamamoto, N. Influence of Bacillus subtilis C-3102 on microbiota in a dynamic in vitro model of the gastrointestinal tract simulating human conditions. Benef. Microbes 2012, 3, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Colom, J.; Freitas, D.; Simon, A.; Brodkorb, A.; Buckley, M.; Deaton, J.; Winger, A.M. Presence and Germination of the Probiotic Bacillus subtilis DE111((R)) in the Human Small Intestinal Tract: A Randomized, Crossover, Double-Blind, and Placebo-Controlled Study. Front. Microbiol. 2021, 12, 715863. [Google Scholar] [CrossRef] [PubMed]
- Garvey, S.M.; Emami, N.K.; Guice, J.L.; Sriranganathan, N.; Penet, C.; Rhoads, R.P.; Spears, J.L.; Dalloul, R.A.; El-Kadi, S.W. The Probiotic Bacillus subtilis MB40 Improves Immunity in a Porcine Model of Listeriosis. Microorganisms 2023, 11, 2110. [Google Scholar] [CrossRef]
- Song, J.; Jeong, S.J.; Lim, C.B.; Kang, B.; Oh, S.S.; Yun, G.; Kim, I.H.; Cho, Y. Assessment of a 50:50 mixture of two Bacillus subtilis strains as growth promoters for finishing pigs: Productivity improvement and noxious gas reduction. J. Anim. Sci. 2023, 101, skad374. [Google Scholar] [CrossRef] [PubMed]
- Vogt, C.M.; Armua-Fernandez, M.T.; Tobler, K.; Hilbe, M.; Aguilar, C.; Ackermann, M.; Deplazes, P.; Eichwald, C. Oral Application of Recombinant Bacillus subtilis Spores to Dogs Results in a Humoral Response against Specific Echinococcus granulosus Paramyosin and Tropomyosin Antigens. Infect. Immun. 2018, 86, 10–128. [Google Scholar] [CrossRef] [PubMed]
- Matei, M.C.; Andrei, S.M.; Buza, V.; Cernea, M.S.; Dumitras, D.A.; Neagu, D.; Rafa, H.; Popovici, C.P.; Szakacs, A.R.; Catinean, A.; et al. Natural Endotoxemia in Dogs-A Hidden Condition That Can Be Treated with a Potential Probiotic Containing Bacillus subtilis, Bacillus licheniformis and Pediococcus acidilactici: A Study Model. Animals 2021, 11, 1367. [Google Scholar] [CrossRef] [PubMed]
- Allenspach, K.; Sung, C.H.; Ceron, J.J.; Peres Rubio, C.; Bourgois-Mochel, A.; Suchodolski, J.S.; Yuan, L.; Kundu, D.; Colom Comas, J.; Rea, K.; et al. Effect of the Probiotic Bacillus subtilis DE-CA9(TM) on Fecal Scores, Serum Oxidative Stress Markers and Fecal and Serum Metabolome in Healthy Dogs. Vet. Sci. 2023, 10, 566. [Google Scholar] [CrossRef] [PubMed]
- Isidori, M.; Rueca, F.; Massacci, F.R.; Diaferia, M.; Giontella, A.; Caldin, M.; Furlanello, T.; Corbee, R.J.; Mannucci, G.; Pezzotti, G.; et al. The Use of Ascophyllum nodosum and Bacillus subtilis C-3102 in the Management of Canine Chronic Inflammatory Enteropathy: A Pilot Study. Animals 2021, 11, 3417. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Yan, F.F.; Hu, J.Y.; Mohammed, A.; Cheng, H.W. Bacillus subtilis-Based Probiotic Improves Skeletal Health and Immunity in Broiler Chickens Exposed to Heat Stress. Animals 2021, 11, 1494. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Achard, C.; Barbe, F.; Chevaux, E.; Ronholm, J.; Zhao, X. Bacillus pumilus and Bacillus subtilis Promote Early Maturation of Cecal Microbiota in Broiler Chickens. Microorganisms 2021, 9, 1899. [Google Scholar] [CrossRef]
- Guo, M.; Li, M.; Zhang, C.; Zhang, X.; Wu, Y. Dietary Administration of the Bacillus subtilis Enhances Immune Responses and Disease Resistance in Chickens. Front. Microbiol. 2020, 11, 1768. [Google Scholar] [CrossRef]
- Mohamed, T.M.; Sun, W.; Bumbie, G.Z.; Elokil, A.A.; Mohammed, K.A.F.; Zebin, R.; Hu, P.; Wu, L.; Tang, Z. Feeding Bacillus subtilis ATCC19659 to Broiler Chickens Enhances Growth Performance and Immune Function by Modulating Intestinal Morphology and Cecum Microbiota. Front. Microbiol. 2021, 12, 798350. [Google Scholar] [CrossRef]
- Suva, M.; Sureja, V.; Kheni, D. Novel insight on probiotic Bacillus subtilis: Mechanism of action and clinical applications. J. Curr. Res. Sci. Med. 2016, 2, 65–72. [Google Scholar] [CrossRef]
- Tompkins, T.A.; Xu, X.; Ahmarani, J. A comprehensive review of post-market clinical studies performed in adults with an Asian probiotic formulation. Benef. Microbes 2010, 1, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Soman, R.J.; Swamy, M.V. A prospective, randomized, double-blind, placebo-controlled, parallel-group study to evaluate the efficacy and safety of SNZ TriBac, a three-strain Bacillus probiotic blend for undiagnosed gastrointestinal discomfort. Int. J. Colorectal. Dis. 2019, 34, 1971–1978. [Google Scholar] [CrossRef]
- Lv, L.; Ruan, G.; Ping, Y.; Cheng, Y.; Tian, Y.; Xiao, Z.; Zhao, X.; Chen, D.; Wei, Y. Clinical study on sequential treatment of severe diarrhea irritable bowel syndrome with precision probiotic strains transplantation capsules, fecal microbiota transplantation capsules and live combined Bacillus subtilis and enterococcus faecium capsules. Front. Cell. Infect. Microbiol. 2022, 12, 1025889. [Google Scholar] [CrossRef] [PubMed]
- Rodenes-Gavidia, A.; Lamelas, A.; Bloor, S.; Hobson, A.; Treadway, S.; Haworth, J.; Vijayakumar, V.; Naghibi, M.; Day, R.; Chenoll, E. An insight into the functional alterations in the gut microbiome of healthy adults in response to a multi-strain probiotic intake: A single arm open label trial. Front. Cell Infect. Microbiol. 2023, 13, 1240267. [Google Scholar] [CrossRef] [PubMed]
- Rea, K.; Colom, J.; Simon, E.A.; Khokhlova, E.; Mazhar, S.; Barrena, M.; Enrique, M.; Martorell, P.; Perez, B.A.; Tortajada, M.; et al. Evaluation of Bacillus clausii CSI08, Bacillus megaterium MIT411 and a Bacillus cocktail on gastrointestinal health: A randomised, double-blind, placebo-controlled pilot study. Benef. Microbes 2023, 14, 165–182. [Google Scholar] [CrossRef]
- Hanifi, A.; Culpepper, T.; Mai, V.; Anand, A.; Ford, A.L.; Ukhanova, M.; Christman, M.; Tompkins, T.A.; Dahl, W.J. Evaluation of Bacillus subtilis R0179 on gastrointestinal viability and general wellness: A randomised, double-blind, placebo-controlled trial in healthy adults. Benef. Microbes 2015, 6, 19–27. [Google Scholar] [CrossRef]
- Freedman, K.E.; Hill, J.L.; Wei, Y.; Vazquez, A.R.; Grubb, D.S.; Trotter, R.E.; Wrigley, S.D.; Johnson, S.A.; Foster, M.T.; Weir, T.L. Examining the Gastrointestinal and Immunomodulatory Effects of the Novel Probiotic Bacillus subtilis DE111. Int. J. Mol. Sci. 2021, 22, 2453. [Google Scholar] [CrossRef]
- Paytuvi-Gallart, A.; Sanseverino, W.; Winger, A.M. Daily intake of probiotic strain Bacillus subtilis DE111 supports a healthy microbiome in children attending day-care. Benef. Microbes 2020, 11, 611–620. [Google Scholar] [CrossRef]
- Cuentas, A.; Deaton, J.; Davidson, J.; Ardita, C. The effect of Bacillus subtilis DE111 on the daily bowel movement profile for people with occasional gastrointestinal irregularity. J. Probiotics Health 2017, 5, 4. [Google Scholar] [CrossRef]
- Townsend, J.R.; Bender, D.; Vantrease, W.C.; Sapp, P.A.; Toy, A.M.; Woods, C.A.; Johnson, K.D. Effects of Probiotic (Bacillus subtilis DE111) Supplementation on Immune Function, Hormonal Status, and Physical Performance in Division I Baseball Players. Sports 2018, 6, 70. [Google Scholar] [CrossRef]
- Toohey, J.C.; Townsend, J.R.; Johnson, S.B.; Toy, A.M.; Vantrease, W.C.; Bender, D.; Crimi, C.C.; Stowers, K.L.; Ruiz, M.D.; VanDusseldorp, T.A.; et al. Effects of Probiotic (Bacillus subtilis) Supplementation During Offseason Resistance Training in Female Division I Athletes. J. Strength Cond. Res. 2020, 34, 3173–3181. [Google Scholar] [CrossRef] [PubMed]
- Townsend, J.R.; Vantrease, W.C.; Jones, M.D.; Sapp, P.A.; Johnson, K.D.; Beuning, C.N.; Haase, A.A.; Boot, C.M. Plasma Amino Acid Response to Whey Protein Ingestion Following 28 Days of Probiotic (Bacillus subtilis DE111) Supplementation in Active Men and Women. J. Funct. Morphol. Kinesiol. 2020, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Trotter, R.E.; Vazquez, A.R.; Grubb, D.S.; Freedman, K.E.; Grabos, L.E.; Jones, S.; Gentile, C.L.; Melby, C.L.; Johnson, S.A.; Weir, T.L. Bacillus subtilis DE111 intake may improve blood lipids and endothelial function in healthy adults. Benef. Microbes 2020, 11, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Takimoto, T.; Hatanaka, M.; Hoshino, T.; Takara, T.; Tanaka, K.; Shimizu, A.; Morita, H.; Nakamura, T. Effect of Bacillus subtilis C-3102 on bone mineral density in healthy postmenopausal Japanese women: A randomized, placebo-controlled, double-blind clinical trial. Biosci. Microbiota Food Health 2018, 37, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Hatanaka, M.; Yamamoto, K.; Suzuki, N.; Iio, S.; Takara, T.; Morita, H.; Takimoto, T.; Nakamura, T. Effect of Bacillus subtilis C-3102 on loose stools in healthy volunteers. Benef. Microbes 2018, 9, 357–365. [Google Scholar] [CrossRef]
- Hatanaka, M.; Kanzato, H.; Tsuda, R.; Nadaoka, I.; Yasue, M.; Hoshino, T.; Iio, S.I.; Takara, T. Safety evaluation of the excessive intake of Bacillus subtilis C-3102 in healthy Japanese adults: A randomized, placebo-controlled, double-blind, parallel-group, comparison trial. Toxicol. Rep. 2020, 7, 46–58. [Google Scholar] [CrossRef]
- Brutscher, L.M.; Borgmeier, C.; Garvey, S.M.; Spears, J.L. Preclinical Safety Assessment of Bacillus subtilis BS50 for Probiotic and Food Applications. Microorganisms 2022, 10, 1038. [Google Scholar] [CrossRef] [PubMed]
- Garvey, S.M.; Mah, E.; Blonquist, T.M.; Kaden, V.N.; Spears, J.L. The probiotic Bacillus subtilis BS50 decreases gastrointestinal symptoms in healthy adults: A randomized, double-blind, placebo-controlled trial. Gut Microbes 2022, 14, 2122668. [Google Scholar] [CrossRef]
- Penet, C.; Kramer, R.; Little, R.; Spears, J.L.; Parker, J.; Iyer, J.K.; Guthrie, N.; Evans, M. A Randomized, Double-blind, Placebo-controlled, Parallel Study Evaluating the Efficacy of Bacillus subtilis MB40 to Reduce Abdominal Discomfort, Gas, and Bloating. Altern. Ther. Health Med. 2021, 27, 146–157. [Google Scholar]
- Lefevre, M.; Racedo, S.M.; Ripert, G.; Housez, B.; Cazaubiel, M.; Maudet, C.; Justen, P.; Marteau, P.; Urdaci, M.C. Probiotic strain Bacillus subtilis CU1 stimulates immune system of elderly during common infectious disease period: A randomized, double-blind placebo-controlled study. Immun. Ageing 2015, 12, 24. [Google Scholar] [CrossRef]
- Tom Dieck, H.; Schon, C.; Wagner, T.; Pankoke, H.C.; Fluegel, M.; Speckmann, B. A Synbiotic Formulation Comprising Bacillus subtilis DSM 32315 and L-Alanyl-L-Glutamine Improves Intestinal Butyrate Levels and Lipid Metabolism in Healthy Humans. Nutrients 2021, 14, 143. [Google Scholar] [CrossRef] [PubMed]
- Patch, C.; Pearce, A.J.; Cheng, M.; Boyapati, R.; Brenna, J.T. Bacillus subtilis (BG01-4(TM)) Improves Self-Reported Symptoms for Constipation, Indigestion, and Dyspepsia: A Phase 1/2A Randomized Controlled Trial. Nutrients 2023, 15, 4490. [Google Scholar] [CrossRef]
- Mandarino, F.V.; Sinagra, E.; Barchi, A.; Verga, M.C.; Brinch, D.; Raimondo, D.; Danese, S. Gastroparesis: The Complex Interplay with Microbiota and the Role of Exogenous Infections in the Pathogenesis of the Disease. Microorganisms 2023, 11, 1122. [Google Scholar] [CrossRef] [PubMed]
- Salmeri, N.; Sinagra, E.; Dolci, C.; Buzzaccarini, G.; Sozzi, G.; Sutera, M.; Candiani, M.; Ungaro, F.; Massimino, L.; Danese, S.; et al. Microbiota in Irritable Bowel Syndrome and Endometriosis: Birds of a Feather Flock Together—A Review. Microorganisms 2023, 11, 2089. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Williams, N.; Weir, T.L. Spore-Based Probiotic Bacillus subtilis: Current Applications in Humans and Future Perspectives. Fermentation 2024, 10, 78. https://doi.org/10.3390/fermentation10020078
Williams N, Weir TL. Spore-Based Probiotic Bacillus subtilis: Current Applications in Humans and Future Perspectives. Fermentation. 2024; 10(2):78. https://doi.org/10.3390/fermentation10020078
Chicago/Turabian StyleWilliams, Natasha, and Tiffany L. Weir. 2024. "Spore-Based Probiotic Bacillus subtilis: Current Applications in Humans and Future Perspectives" Fermentation 10, no. 2: 78. https://doi.org/10.3390/fermentation10020078
APA StyleWilliams, N., & Weir, T. L. (2024). Spore-Based Probiotic Bacillus subtilis: Current Applications in Humans and Future Perspectives. Fermentation, 10(2), 78. https://doi.org/10.3390/fermentation10020078






