Abstract
Irradiation with ultraviolet A (UVA) plays an important role in the pathogenesis of skin photoaging since it increases oxidative stress and inflammation in the epidermis. There is an urgent need to screen, investigate, and apply the potential anti-photoaging active ingredients. Agaricus blazei Murill (ABM) polysaccharides have a wide range of promising pharmacological applications. Previous studies have confirmed their antioxidant effect, but whether it has an anti-photoaging effect is unclear. In this study, two ABM polysaccharides (AB-J and AB-K) were obtained to discuss the potential photodamage-protective capacity. The free radical scavenging abilities in vitro, the safety assessment, and their protective effects and mechanisms on UVA-induced human fibroblasts (HSFs) were evaluated. The intracellular antioxidant enzyme levels and extracellular matrix proteins, such as COL-I and ELN, were significantly accelerated, and metalloproteinases (MMP-1, and MMP-9) were decreased by AB-J and AB-K. The Keap-1-Nrf2/ARE signaling pathway was activated, thus inducing the upregulated expression of downstream genes (Ho-1 and Nqo-1). The suppression of P38 and Jnk1 by AB-J and AB-K was speculated to be the inducer of the activation of the Keap-1-Nrf2/ARE signaling pathway. Owing to the excellent exhibition of AB-J, its safety assessment and the structural characterization are discussed further.
1. Introduction
Agaricus Blazei Murill (A. blazei Murill, ABM), also named the Brazilian mushroom, is a kind of mushroom commonly used in medicine and human nutrition, typically grown in China and Japan [,]. Because of its functional characteristics, it is an excellent candidate for the auxiliary treatment of some chronic diseases, such as dyslipidemia and diabetes, and is also used as a nutritional supplement for cancers [,]. ABM is rich in polysaccharides, proteins, nucleic acids, fats, saponins, tannins, sterols, minerals, trace elements, and essential amino acids, among which, polysaccharides are the main active substances, and their functional characteristics are recognized worldwide []. In the past few decades, some studies have proved the biological characteristics of the chemical components in this mushroom’s fruiting body, such as anti-tumor, hypolipidemic, anti-herpetic and immunomodulatory properties [,,]. These properties are mainly due to the existence of the β (1→6) and α (1→4) polysaccharide configuration [,].
The antioxidant and anti-aging capacities of ABM polysaccharides have been proved at different levels. The free radical scavenging abilities have been studied in vitro; further, the antioxidant effects were also studied in vivo in different models. Lv et al. (2017) found ABM polysaccharides had antioxidant effect and could reduce the loss of oxygen free radicals in renal tissue in diabetic rats []. The anti-inflammatory and immunomodulatory activities of ABM polysaccharides were also observed. Liu et al. found that ABM polysaccharides could inhibit the secretion of tumor necrosis factor (TNF-α) and nitric oxide (NO) induced by lipopolysaccharide (LPS) in vivo []. In addition, based on RT-PCR results, the expression levels of JNK, ERK, and p38 were significantly reduced in RAW 264.7 cells induced by lipopolysaccharide (LPS) and treated with ABM polysaccharides to exert immune regulatory effects []. Not only from Agaricus Blazei Murill, polysaccharides from other medicinal fungi, such as Ganoderma lucidum [], Cordyceps cicadae [], Schizophyllum commune [], and Hericium erinaceus [], were also proved to exhibit antioxidant activities. The above achievements give ABM more possibilities in the food and drug industry. However, the studies and applications in the field of cosmetic and dermatology are limited.
Ultraviolet (UV) rays constantly bombard the human skin. Research has shown that UVA (315–400 nm) produces more oxidative stress than UVB (315–400 nm) []. Damage to DNA and cancer are some of the most serious effects associated with photoaging []. When UVA irradiation is applied to the dermis, excessive reactive oxygen species (ROS) are generated rapidly and cause oxidative stress, which do harm to many biological processes, including gene expression, protein production, and lipid oxidation [,,]. More and more attention has been paid to skin protection against UV rays. It is urgent to screen substances with potential photodamage-protective effects.
Collagens, such as COL-I and Elastin (ELN), are the vital components of the extracellular matrix (ECM), the lack of which show great relationships with skin relaxation and wrinkles. Matrix metalloproteinases (MMPs) such as MMP-1, MMP-3, and MMP-9, are a family of enzymes degrading different components of the ECM []. It is generally believed that the skin aging process can be improved by promoting ECM synthesis and reducing the activities of MMPs []. Some active ingredients extracted from mushrooms can play roles in the expression of the ECM and MMPs [].
A key mechanism of cellular defense against oxidative stress injury is the Keap1-Nrf2/ARE signaling pathway. The activation of nuclear factor-erythroid 2-related factor 2 (Nrf2) increases the expression of detoxification and antioxidant defense proteins. In order to scavenge excess ROS, antioxidant enzymes such as catalase (CAT), glutathione peroxidase (GSH-Px), haemoxygenase (Ho-1), and NAD(P)H quinone oxidoreductase 1 (Nqo1) are accelerated []. Studies have reported that the activation of P38 and JNK can induce the phosphorylation and acetylation of Nrf2 []. Whether ABM polysaccharides can also activate the Keap1-Nrf2/ARE signaling pathway through P38 and JNK remains to be further studied.
In this study, two kinds of strains of ABM were used to obtain the ABM polysaccharides AB-J and AB-K. A structure analysis was performed to find out the differences between them. The UVA-induced photodamage human skin fibroblast (HSF) model was established to discuss the potential photodamage-protective capacity of ABM polysaccharides. The Keap1-Nrf2/ARE signaling pathway and the related gene expressions are studied. The levels of antioxidant enzymes (GSH-Px and CAT), ECM components (COL-I and ELN), and MMPs (MMP-1, MMP-3, and MMP-9) are also discussed. It is hoped that the results of the study will offer a prospective basis for the in-depth development and the applications of ABM polysaccharides in cosmetics, foods, and pharmaceuticals.
2. Materials and Methods
2.1. Materials
The materials used in this study include human skin fibroblasts (HSFs), Zhejiang Meisen Cell Technology Co., Ltd., Pan’an, Zhejiang, China; Dulbecco’s Modified Eagle Medium (DMEM), fetal bovine serum (FBS), penicillin–streptomycin, 0.05% (containing EDTA) trypsin, Life Technologies (Carlsbad, CA, USA); Folin reagent, Sinopharm Group (Beijing, China); total sugar test kit, reducing sugar test kit, total phenol test kit, total antioxidant capacity detection kit (ABTS method), catalase detection kit, reactive oxygen species detection kit, GSH-Px detection kit, BCA assay kit, Trizol (total RNA extraction reagent), Beyotime Biotechnology Co., Ltd. (Shanghai, China); ELN, COL-I, MMP-1, MMP-3, and MMP-9 assay kits, Nanjing Jiancheng Institute of Bioengineering (Nanjing, China); EasyScript® One-Step gDNA Removal and cDNA Synthesis SuperMix and TransStart® Top Green qPCR SuperMix, TransGen Biotech Co., Ltd. (Beijing, China).
2.2. The Preparation of AB-J and AB-K
Two strains (CGMCC5.485 and CGMCC5.787) of ABM obtained from the China General Microbiological Culture Collection Center (CGMCC) were inoculated in potato glucose medium and fermented at 28 °C with shaking at 150 rpm for 5 days to obtain the fermentation broth. Two types of fermentation broth were concentrated, followed by alcohol precipitation using anhydrous ethanol, centrifugation, and the collection of the precipitate. The Sevage method was subsequently employed to eliminate residual proteins from the precipitate [,]. The resulting crude polysaccharides AB-J and AB-K, essential for this study, were obtained through freeze-drying.
2.3. Physical and Chemical Properties of AB-J and AB-K
The determination of total sugar, reducing sugar, and total phenol was carried out according to the instructions of the kit. The content of flavonoids was determined using the nitrous acid chromogenic method according to the literature [,]. Rutin was used as the standard substance. The absorbance was measured at 510 nm (using deionized water as a blank control). The content of β-glucan was determined using the Congo red method according to the literature [,]. We entrusted Qingdao Kechuang with the detection of fat, protein, moisture, ash, and carbohydrates according to the Chinese national test standard GB5009.91-2017 [].
2.4. Determination of Antioxidant Activity In Vitro
AB-J and AB-K were prepared in different concentrations to examine their ability to scavenge free radicals. Ascorbic acid (VC) was used as a positive control. According to the references, the DPPH free radical scavenging activity and hydroxyl radical scavenging activity were measured [,]. The ABTS method was employed to determine the overall antioxidant capacity, utilizing a total antioxidant capacity determination kit in accordance with the manufacturer’s instructions.
2.5. Cell Culture and Cytotoxicity Assay
HSFs were grown in DMEM containing 10% fetal bovine serum (FBS) and 1% penicillin–streptomycin and cultured in a humidity environment of 37 °C with a concentration of 5% carbon dioxide [,,].
HSFs were cultivated in a 96-well plate for 12 h, followed by treatment with varying concentrations of crude polysaccharides AB-J and AB-K for 24 h. Cytotoxicity testing was conducted using the CCK-8 assay kit, as per the manufacturer’s guidelines.
2.6. Model and Sample Treatment
Model establishment: HSFs were plated at a density of 1 × 104 cells per well in a 96-well plate and incubated in a cell culture incubator for 12 h to assess cell confluence. Subsequently, the cells were rinsed with 1 mL PBS at a pH of 7.4 and a concentration of 0.01 M, followed by aspiration. Subsequently, 1 mL of PBS (pH 7.4, 0.01 M) was added and the cells were exposed to varying doses of UVA radiation ranging from 0 to 12 J/cm2. UVA lamps were utilized to emit UVA radiation and the intensity was quantified using a UV power meter. Subsequently, the viability of HSF cells was assessed utilizing the CCK-8 assay. Detailed establishment data are shown in Figure 1F.
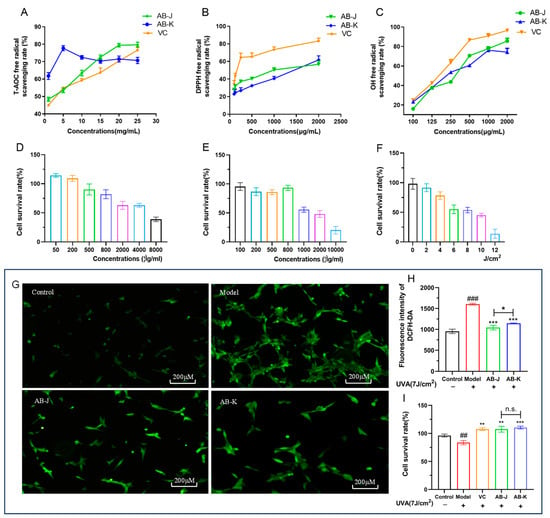
Figure 1.
The antioxidant capacities of AB-J and AB-K. (A) The total antioxidant capacity (ABTS method); (B) DPPH free radical scavenging ability; (C) hydroxyl radical scavenging ability; (D) cell survival rates of AB-J ranging from 50 to 8000 μg/mL; (E) cell survival rates of AB-K ranging from 100 to 10,000 μg/mL; (F) cell survival rates of different intensities of UVA (the UVA-induced model was established at 7 J/cm2); (G) the representative ROS fluorescence images; (H) the fluorescence intensity of DCFH-DA in different treatment; (I) the protective effects of AB-J and AB-K on HSFs. Ascorbic acid (VC) was chosen as the positive control. * p < 0.05, ** p < 0.01, *** p < 0.001, versus the model group; ## p <0.01, ### p <0.001, versus the control group. n.s., p > 0.05.
Sample treatment: HSFs were seeded in 6-well plates at 5 × 105 cells per well and grown in a complete DMEM medium for 12 h. Then, the HSFs were incubated with serum-free DMEM (the control group and model group) or different concentrations of samples for 24 h. Then, HSFs were washed twice with PBS. Another 1 mL PBS was added for further treatment with UVA with a total energy of 7 J/cm2, while the blank control group was not irradiated. Then, the cells were washed twice with PBS and cultured in serum-free DMEM for another 12 h. The supernatants or the cells were collected for subsequent testing.
2.7. Determination of Intracellular ROS, GSH-Px and CAT
The ROS assay kits were used to detect the ROS levels and quantify intracellular ROS production in accordance with the guidelines provided by the manufacturer. Fluorescence images were captured using a fluorescence microscope, and fluorescence intensity was quantified utilizing a fluorescence enzyme-linked immunosorbent assay (ELISA) reader. The fluorescence signal was measured at excitation and emission wavelengths of 488 nm and 525 nm, respectively.
Cell sample preparation and the catalase (CAT) and glutathione peroxidase (GSH-Px) detection steps were performed according to the manufacturer’s instructions. The protein contents in cell lysates were measured using the BCA assay kit to normalize each index.
2.8. Determination of COL-I, ELN, and Matrix Metalloproteinase (MMPs)
After the sample treatment, the cell supernatants were collected and centrifuged for 5 min. According to the manufacturer’s instructions, the contents of ELN, COL-I, and MMPs (MMP-1, MMP-3, and MMP-9) in HSFs after UVA injury were measured using ELISA kits. The protein contents in cell lysates were measured using the BCA assay kit to normalize each index.
2.9. Relative mRNA Expressions of Key Genes
Total RNA extraction and cDNA synthesis were performed using the TriQuick reagent and UEIris II RT-PCR system. The primer sequences used are shown in Table 1. Beta-actin served as an internal reference gene. The cycle parameter was 94 °C pre-denaturation for 30 s, followed by PCR reaction (45 cycles of 94 °C for 15 s, 60 °C for 15 s, 72 °C for 10 s), and fluorescence data were collected at 72 °C. The calculated relative gene expression was analyzed using the 2−ΔΔCT method [].

Table 1.
The primer sequences of key genes.
2.10. Red Blood Cell (RBC) Hemolysis Assay
The red blood cell (RBC) hemolysis assay was performed as previously described []. The samples were set up with three concentrations: high (9 mg/mL), medium (2.25 mg/mL), and low (0.56 mg/mL). The sample group (A1) was a 750 μL sample + 250 μL RBC. The negative control group (A2) was 750 μL PBS + 250 μL RBC, and the positive control group (A3) was 750 μL 0.1% sodium dodecyl sulfate (SDS) + 250 μL RBC. The absorbance value of each group was measured at 560 nm, and the hemolysis rate of the sample was calculated. Hemolysis rate = (A1 − A2)/(A3 − A2) × 100%.
2.11. Eye Irritation Determination: HET-CAM
The hen’s egg test-chorioallantoic membrane (HET-CAM) assay was performed to determine the degree of eye irritation from AB-J and AB-K []. A positive control sample (0.1 M NaOH), a negative control sample (0.9% NaCl), and sample groups (AB-J and AB-K) with different concentrations were discussed. After the removal of the eggshell, 0.3 mL of test solution was added to observe and record the vascular injury after 3 min with 4 groups of parallel experiments.
The membrane was assessed for different conditions, including bleeding, coagulation, and vascular melting. The stimulus score (IS) is calculated using the following formula:
where SecL is the mean time for vascular melting to occur on the chick embryo chorioallantoic membrane; SecH is the mean time for hemorrhage to occur on the chick embryo chorioallantoic membrane; and SecC is the mean time for coagulation to occur on the chick embryo chorioallantoic membrane. Four levels of eye irritation were based on the IS values shown in Table 2.

Table 2.
Results of irritation evaluation score.
2.12. UV-Visible and FT-IR Spectrum Spectra
The AB-J solution with a concentration of 1 mg/mL was used for the UV-visible spectroscopy analysis in the range of 200~800 nm []. The sample (AB-J) was mixed with KBr (1:100), pressed and grounded, and analyzed using a Fourier Transform Infrared Spectrometer in the range of 4000–400 cm−1 [,].
2.13. Monosaccharide Composition Analysis, GPC, SEM and AFM
The monosaccharide composition analysis was conducted using a high-performance liquid chromatography (HPLC) system with an Xtimate C18 column (4.6 × 200 mm, 5 µm). Mannose, ribose, rhamnose, glucuronic acid, galacturonic acid, N-acetyl-glucosamine, glucose, N-acetyl-galactose, galactose, xylose, arabinose, and fucose were chosen as the standard controls for the comparative analysis. In summary, the samples underwent hydrolysis in a nitrogen environment using 2 M trifluoroacetic acid (TFA) for a duration of 4 h at a temperature of 120 °C. Subsequently, the TFA was removed through vacuum evaporation, after which the sample was reconstituted in 3 mL of water. A precise volume of 250 µL of hydrolysate or the control solution was aliquoted into a 5 mL EP tube. Subsequently, 250 µL of 0.6 M NaOH and 500 µL of 0.4 M 3-methyl-1-phenyl-2-pyrazoline-5-one (PMP)-methanol were added to initiate the reaction, which was carried out at 70 °C for a duration of 1 h. Following a cooling period of 10 min in cold water, 500 µL of 0.3 M HCl was introduced for the purpose of neutralization. Following the addition of one milliliter of chloroform for the extraction of the derivatized sample, the mobile phase utilized in the analysis comprised 83% 0.05 M dihydrogen phosphate solution (pH 6.70, adjusted with NaOH) and 17% acetonitrile. The analysis was conducted at a wavelength of 250 nm, with a flow rate of 1 mL/min, and a temperature of 30 °C.
The molecular weight (MW) was measured using gel permeation chromatography (GPC) combined with a laser light scattering instrument and a differential refractive index detector (GPC-LS-RI), as previously described [].
The morphological characteristics were investigated using scanning electron microscopy (SEM Nova Nano 450, FEI, Tokyo, Japan). The AB-J material was immobilized on the SEM stubs for 5 mg, coated with a 10 nm layer of gold, and observed across 250 to 1000 magnifications following a 5.0 kV acceleration.
A high-resolution imaging analysis was performed using a Dimension icon atomic force microscope (AFM, Brooke, Berlin, Germany) with a scanning range of 90 µm × 90 µm in the XY direction, and the overall noise is below an RMS value of 0.03 nm.
2.14. Statistics Analysis
The data analysis was conducted using SPSS 17.0 (SPSS, Armonk, NY, USA) and GraphPad Prism 9.0 (GraphPad, San Diego, CA, USA) in three replications of each experiment. (*, # p < 0.05, **, ## p < 0.01).
3. Results
3.1. Content Index of AB-J and AB-K and Their Antioxidant Capacities In Vitro
Typical compounds of AB-J and AB-K were measured, and the results are shown in Table 3. Among these active substances, both AB-J and AB-K had the highest proportion of carbohydrates, 51.3 ± 2.3% and 73.2 ± 4.1%, respectively. The proportions of protein (AB-J and AB-K) reached 23.7 ± 3.1% and 8.69 ± 2.1%, respectively.

Table 3.
Contents of AB-J and AB-K.
The in vitro free radical scavenging experiments can quickly and intuitively verify the antioxidant capacity of ABM polysaccharides. In the study, three kinds of free radicals (DPPH radicals, hydroxyl radicals, and ABTS) were used to measure the scavenging capacities (Figure 1A–C). The dose-dependent manners can be observed when discussing the scavenging capacities of DPPH and hydroxyl radicals. AB-J and AB-K both had lower scavenging abilities than VC at the same concentration, while AB-J had a higher scavenging ability than AB-K. The total antioxidant capacity was performed using the ABTS method. Differently, AB-K ranging from 1 to 15 mg/mL exhibited a relatively higher antioxidant capacity than AB-J and VC at the same concentration.
The intracellular antioxidant capacities were evaluated after the cell viability assays (Figure 1D,E). The survival rates were lower with the increase of ABM polysaccharides. The effective concentration of 80% (EC80) was used in the following experiments. Therefore, AB-J at 800 μg/mL and AB-K at 500 μg/mL were used to treat UVA-induced HSFs, respectively. Meanwhile, UVA at 7 J/cm2 was used to establish the damaged HSFs model (Figure 1F). The ROS levels and the protective effects of the two polysaccharides were further studied. As shown in Figure 1G,H, after UVA irradiation, the intracellular ROS content increased significantly in the model group, and decreased obviously when treated with AB-J and AB-K, respectively. Further, AB-J showed a relatively greater decrease than AB-K. The protective effects were also measured according to the cell viability data. All of AB-J, AB-K, and VC could increase the cell survival rates when compared to the model group. There was no significance between AB-J and AB-K.
3.2. AB-J and AB-K Can Accelerate Keap-1-Nrf2/ARE Signaling Pathway
It is believed that Nrf2 activity is partially controlled by Keap-1, a cytoplasm-related protein. Cellular homeostasis depends on the separation of Keap-1 and Nrf2. In the study, we determined the effects of AB-J and AB-K on Nrf2 acceleration (Figure 2). The pretreatment with AB-J and AB-K upregulated the mRNA expression of Nrf2 and downregulated the mRNA expression of Keap-1, thus causing the nuclear translocation of Nrf2 in the cells (Figure 2A). Reportedly, blocking the activation of upstream genes such as Jnk1 and p38 could promote Nrf2 translocation to the nucleus [,,]. AB-J and AB-K effectively relieved the UVA-induced high expression of p38 and Jnk1 in cells after UVA injury (Figure 2A).
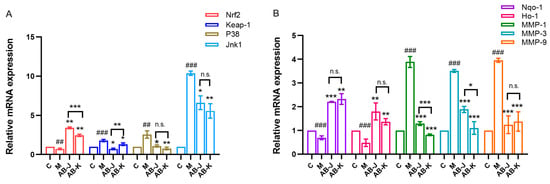
Figure 2.
The relative expressions of anti-aging related genes. (A): Nrf2, Keap-1, P38, Jnk1 ofmRNA expression levels. (B): Nqo1, Ho-1, MMP-1, MMP-3, and MMP-9 of mRNA expression levels. C represents the control group; M represents the UVA-induced model group; ## p < 0.01, ### p < 0.001, versus C (the control group); * p < 0.05, ** p < 0.01, *** p < 0.001, versus M (the model group); n.s. p > 0.05, AB-J versus AB-K.
The relative expressions of Nqo1 and Ho-1 were also measured using qRT-PCR (Figure 2B), which played important roles in ROS reduction []. As a downstream product of Nrf2, Nqo1 can be stimulated by the activation of Nrf2 []. As shown in Figure 2B, they were both decreased in the model group. The treatment of AB-J and AB-K, respectively, could accelerate their expressions significantly. There were no significant differences between the two samples. MMPs are proteolytic enzymes that degrade the extracellular matrix. In the study, MMP-1, MMP-3, and MMP-9 were detected all in sharp decrease by AB-J and AB-K, which were accelerated in the model. Between them, AB-K showed a relatively greater inhibition on MMP-1 and MMP-3.
3.3. AB-J and AB-K Can Affect the Antioxidant Enzymes and Extracellular Matrix Levels
Oxidative stress damage can lead to a decrease in the ability of cells to scavenge ROS, and the antioxidant enzymes CAT and GSH-Px play important roles in the body. In the study, the antioxidant enzymes (GSH-Px and CAT), and the extracellular matrix (COL-I and ELN) were found to decrease, while the relative contents of MMP-1 and MMP-9 were accelerated when HSFs were treated with UVA, as shown in Figure 3. AB-J and AB-K pretreatment could reverse the tendencies. AB-J exhibited a relatively greater acceleration of CAT than AB-K. There were no differences between the two samples in other indexes. Based on the results, we can conclude that the transcriptional activation of Nrf2 upregulated the secretion of CAT, GSH-Px, ELN and COL-I, and downregulated the expression of MMPs (MMP-1 and MMP-9), which played important roles in protecting HSFs from oxidative damage. The results of MMPs were consistent with their mRNA expressions exhibited in Figure 2B.
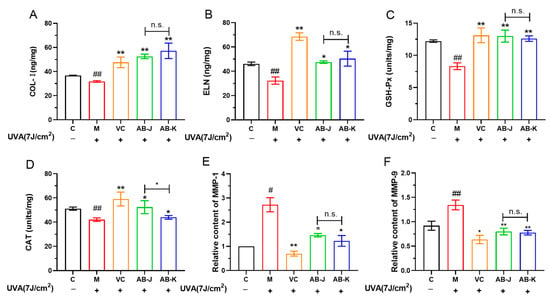
Figure 3.
The levels of COL-I (A), ELN (B), GSH-Px (C), CAT (D), MMP-1 (E), and MMP-9 (F) affected by AB-J and AB-K. C represents the control group; M represents the UVA-induced model group. The ascorbic acid (VC) was chosen as the positive control. * p < 0.05, ** p < 0.01, versus M (the model group). # p < 0.05, ## p < 0.01, versus C (the control group); n.s. p > 0.05, AB-J versus AB-K.
3.4. Safety Evaluation
The in vivo HET-CAM assay and RBC hemolysis assay can determine whether the measured substance is a stimulant by detecting vascular bleeding and hemolysis [].
Figure 4A,B show the RBC hemolysis results of AB-J and AB-K. Compared with the negative control (PBS), the hemolysis rate at high levels of AB-J or AB-K was significantly higher, but still less than 1%. As a result, we concluded that both AB-J and AB-K at different concentrations had negligible hemolytic effects on RBCs (erythrocyte hemolysis rate < 1%). The CAM assay was performed with 0.9% NaCl as the negative group and 0.1 mol/L NaOH as the positive group, respectively. Representative images of CAMs are shown in Figure 4C. Correspondingly, the classification of types of hemorrhagic effects is shown in Table 4. After 3 min of NaOH treatment, obvious bleeding, vascular coagulation, and vascular dissolution caused by severe stimulation occurred in the blood vessels on the membrane (IS = 17.42), while the above phenomena did not occur in the sample groups (IS = 0.08 for AB-J and IS = 0.08 for AB-K) and the negative control group (IS = 0.07). These results suggest that AB-J and AB-K do not cause eye irritation or hemolysis.
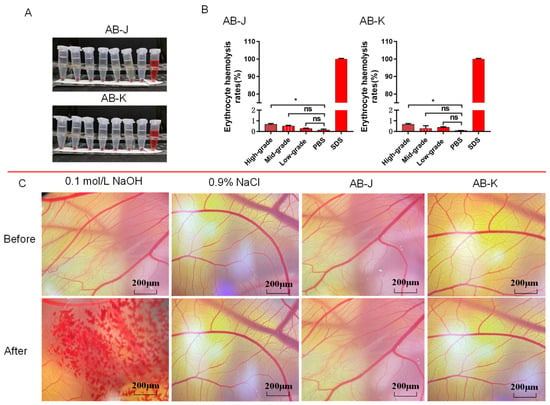
Figure 4.
Safety determination of AB-J and AB-K. (A) Erythrocyte hemolysis at different concentrations (9, 4.5, 2.25, 1.13, 0.56, 0.28 mg/mL) of AB-J and AB-K. Sodium dodecyl sulfate (0.1% SDS) was used as a positive control. PBS was chosen as a negative control. (B) Erythrocyte hemolysis rates of three levels of samples were calculated. (C) Vascular presentation and response plots before and after representative CAM spiking for AB-J and AB-K. NaOH at the concentration of 0.1 mol/L was chosen as the positive control, and NaCl at the concentration of 0.9% was chosen as the negative control. * p < 0.05, ns p > 0.05, versus PBS (the model group).

Table 4.
Classification of types of hemorrhagic effects of AB-J and AB-K on CAM.
3.5. AB-J Showed a Relatively Better Effect Than AB-K
Both AB-J and AB-K were non-toxic to HSFs, and both had certain promotion effects on cell proliferation. The extracellular free radical scavenging experiment exhibited a more effective effect of AB-J than AB-K. In addition, AB-J had a more significant effect in terms of its effects on the levels of antioxidant enzymes and extracellular matrix contents. Based on this comprehensive comparison, AB-J was chosen for further study.
3.6. Physical Properties of AB-J
The physical properties of AB-J were investigated. As shown in Figure 5A, there was no special UV absorption peak at 260–280 nm, indicating that AB-J did not contain proteins and nucleic acids. The infrared spectra of AB-J (Figure 5B) exhibited several typical absorption peaks characteristic of typical polysaccharides. C-H’s tensile vibration was responsible for the peak at 3006–2908 cm−1, and AB-J’s C = O stretching vibration was responsible for the peaks at 1669 and 1556 cm−1 [,]. The 1388 cm−1 peak suggests the existence of carboxyl (COOH) [], whereas the peaks at 1261 cm−1 and 1042 cm−1 corresponded to the stretching vibrations of C-O-C or C-O, indicating the presence of pyranose in the form of sugar or glycosidic bonds [].
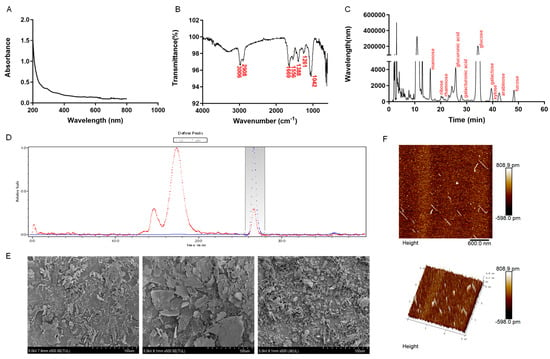
Figure 5.
Structural characterization of AB-J. (A) UV absorption peaks of AB-J in the range of 200–600. (B) IR spectrum of AB-K. (C) HPLC-based monosaccharide composition analysis of AB-J. (D) GPC-based molecular weight analysis of AB-J. (E) SEM analysis of AB-J at different magnifications (5×, 50×, and 100×). (F) Thermogravimetric analysis of AB-J.
The GPC and monosaccharides analysis showed that AB-J had an average molecular weight (Mw) of 5.439 × 102 (±63.146%) Da, and was mainly composed of glucose, glucuronic acid, mannose, and galactose at the ratio of 0.91:1.445:95.371:0.831.
The surface morphology of AB-J was determined using SEM at different magnifications (5×, 50×, and 100×). The microstructure of AB-J was an irregular rod or cloud, with a smooth and delicate surface and looser pores, which conformed to the light appearance of the powder. AB-J was predicted to have good water retention and rheological properties.
The atomic force microscopy reveals intermolecular forces and structural changes, providing the surface morphology and microstructure of polysaccharides (Figure 5F). Accordingly, for the roughness observed on the surface, the higher the roughness, the greater the potential biological activity. The three-dimensional characterization results indicated that AB-J polysaccharides exhibited a dense grain pile-like structure with obvious pointed and irregular protrusions. More importantly, the thickness of the polysaccharide chains, ranging from 0.1 to 0.8 nm, exceeded the size of single chain polysaccharide molecules (0.1–1.0 nm), respectively. This observation indicated that the interwoven forces within and between molecules, including van der Waals forces and hydrogen bonds, contributed to the formation of the polymer structures. This indicated that AB-J had a multi-branched structure and probably had many hydroxyl and carboxyl groups, which may promote the interaction between molecules [].
4. Discussion
The health effects of medicinal and edible fungi have been widely recognized and used for food and drug and skin care [,]. A. blazei Murill from Brazil is a Chinese traditional medicinal material with nutraceutical and medicinal applications [,,]. Several studies have reported the antioxidant and anti-inflammatory effects of Agaricus blazei [,,]. However, research on skin UV damage models are limited. More systematic and in-depth research on how A. blazei Murill affects skin cells when dealing with oxidative stress is required. UVA irradiation on the dermis stimulates the production of ROS by dermal cells, mainly manifested by an increase in ROS content. Excessive ROS will pass through the cell membrane and react with most biological molecules (DNA, protein, lipid, etc.), which destroy cell structure and function, and thus induce skin aging [,]. Further, the increasing oxidative stress induced by ROS can lead to inflammation []. Whether the excess ROS can be removed is one of the important indexes to evaluate the antioxidant capacity of the active substances.
More and more researchers have been committed to screening functional components to resist photoaging. Some fermentation active substances are reported to have the abilities to scavenge ROS []. Some expressions of genes (e.g., c-jun and c-fos) and proteins, such as collagens and MMPs [], are involved in the process, thereby exerting antioxidant effects.
Similarly, medicinal fungi can also exert antioxidant effects against UVA radiation. Ganoderma lucidum extract can resist photodamage by regulating the cell cycle and decreasing the number of apoptotic cells [,]. However, there is relatively little research on A. blazei Murill.
In this study, AB-J and AB-K were obtained from two strains of Agaricus Blazei Murill. Both of them could repress the ROS levels accelerated by UVA. The antioxidant enzymes and their mRNAs were found in high levels after the pretreatment. All these observations demonstrate the antioxidant and anti-UV damage abilities in HSFs. However, AB-J and AB-K are not identical in degree, which may be related to the differences in strains.
The Nrf2-Keap-1/ARE pathway plays a role in the response to oxidative stress. The transcription factor Nrf2 regulates the activation of antioxidant response elements (AREs) and induces the expression of antioxidant enzymes [,]. Our results detected an increased Nrf2 expression, indicating that AB-J and AB-K can dissociate the Nrf2-keap-1 complex in the cytoplasm and transport it to the nucleus, thus activating the genes in the Nrf2-Keap-1/ARE pathway. The downstream antioxidant biomarkers named Ho-1 and Nqo1 are playing roles in scavenging ROS and protecting cells from oxidative damage [,]. In the study, apart from the enzyme levels of GSH-Px and CAT, the relative expression of Ho-1 and Nqo1 were accelerated by AB-J and AB-K, thus playing roles in oxidative response.
Further, MMPs were also decreased by the active substances in the study. In general, the increased ROS stimulates the synthesis of MMPs. MMP-1 is an interstitial collagenase that hydrolyzes the main components of dermal type I and type III collagen, and plays a decisive role in the destruction of tissue and the degradation of the dermal extracellular matrix. Among this MMP family, MMP-9 belongs to gelatinases and is the largest and the most complex member. Together with MMP-2, MMP-9 is also one of the major metalloproteinases responsible for the chronic inflammation []. MMP-3 is an activator of the precursor of MMP-9 (pro-MMP-9) []. In the study, the mRNA expression of MMP-1, MMP-3, and MMP-9 was upregulated after UVA irradiation, which suggests that the intracellular photoaging and inflammatory process existed. The pretreatment of AB-J and AB-K, respectively, could downregulate their mRNA expressions (Figure 2), which were consistent with the changes in the contents of MMP-1 and MMP-9, except for MMP-3.
MAPK has three classic pathways, with different types of MAPK (p38, JNK, ERK5) []. The p38 and JNK pathways have physiological effects which are mainly associated with inflammation and apoptosis as stress responses. Studies in the literature have reported the relationships between their inhibition and Nrf2 [,]. MAPK family members are sensitive to cellular oxidative stress. The secretion of some pro-inflammatory cytokines and the response to ROS are accelerated by MAPK [,,,]. In the study, the expression of Jnk1 and P38 were both inhibited by AB-J and AB-K. Despite the lack of proof for up-stream pathway regulation, Jnk1 and P38 may play a crucial role in Keap-1-Nrf2/ARE signaling pathway acceleration. There may, however, be other ways of affecting Nrf2 besides those mentioned here.
It is expected that high-throughput technologies, such as transcriptomes, will lead to a better understanding of the mechanism underlying the dynamic regulation of fungal polysaccharides against photoaging in the coming years. It is also necessary to continue working on structural identification. We hope to make efforts on the application of ABM in cosmetics and healthy products.
5. Conclusions
Both AB-J and AB-K achieved good intracellular and extracellular antioxidant effects in vitro by scavenging free radicals, but to different degrees. The UVA-induced HSFs were established to study the mechanism of their anti-photoaging effects. The decreased intracellular ROS levels and MMPs and accelerated antioxidant enzymes were observed when HSFs were pretreated with AB-J and AB-K. The acceleration of the Keap-1-Nrf2/ARE signaling pathway may be the reason for the response to photodamage. The inhibition of Jnk1 and P38 might be playing roles in the acceleration of Nrf2.
A safety assessment was conducted in the study and both AB-J and AB-K showed no eye irritation or hemolysis. In addition, a structure analysis was performed. All the above results can be used as the basis for the further application of A. blazei Murill polysaccharides.
Author Contributions
Methodology, F.D., W.C. and L.L.; Software, W.C.; Validation, C.P., R.S. and J.Z.; Investigation, R.S.; Resources, L.L.; Data curation, C.P.; Writing—original draft, F.D.; Writing—review & editing, F.D.; Funding acquisition, J.Z., C.W. and M.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gonzaga, M.L.; Ricardo, N.M.; Heatley, F.; Soares, S. Isolation and characterization of polysaccharides from Agaricus blazei Murill. Carbohydr. Polym. 2005, 60, 43–49. [Google Scholar] [CrossRef]
- Stojkovi, D.; Reis, F.; Glamolija, J.; Ciric, A.; Barros, L.; Van Griensven, L.J.; Ferreira, I.C.; Sokovic, M. Cultivated strains of Agaricus bisporus and A. brasiliensis: Chemical characterization and evaluation of antioxidant and antimicrobial properties for the final healthy product – natural preservatives in yoghurt. Food Funct. 2014, 5, 1602–1612. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.C.; Correa, V.; Gonalves, G.; Soares, A.; Bracht, A.; Peralta, R. Agaricus blazei bioactive compounds and their effects on human health: Benefits and controversies. Curr. Pharm. Des. 2017, 23, 2807–2834. [Google Scholar] [CrossRef]
- De Sá-Nakanishi, A.B.; Soares, A.A.; Natali, M.R.M.; Comar, J.F.; Peralta, R.M.; Bracht, A. Effects of the Continuous Administration of an Agaricus blazei Extract to Rats on Oxidative Parameters of the Brain and Liver during Aging. Molecules 2014, 19, 18590–18603. [Google Scholar] [CrossRef]
- Liu, P.; Yuan, J.; Jiang, Z.; Wang, Y.; Wen, B.; Li, G. A lower cadmium accumulating strain of Agaricus brasiliensis produced by 60 Co-γ-irradiation. LWT 2024, 114, 108370. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, D.; Fang, L.; Zhao, X.; Zhou, A.; Xie, J. A galactomannoglucan derived from Agaricus brasiliensis: Purification, characterization and macrophage activation via MAPK and I kappa B/NF kappa B pathways. Food Chem. 2018, 239, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Galhardi, L.C.; Rincao, V.; Soares, S.; Vieira, I.G.; Ricardo, N.M.; Nozawa, C.; Linhares, R.E. Antiherpetic activity of an Agaricus brasiliensis polysaccharide, its sulfated derivative and fractions. Int. J. Biol. Macromol. 2013, 52, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Lu, X.; Yuan, G.; Zhang, Q.; An, L. Effects of Agaricus blazei polypeptide on cell senescence by regulation of Keap1/Nrf2/ARE and TLR4/NF-κBp65 signaling pathways and its mechanism in D-gal-induced NIH/3T3 cells. J. Funct. Foods 2020, 72, 104037. [Google Scholar] [CrossRef]
- Gonzaga, M.L.; Campelo, M.; Saraiva, K.; Santos, A.Q.; Leal, L.K.; Ricardo, N.M.; Soares, S.; Riberiro, M.E. Chitosan and Agaricus brasiliensis Polysaccharides Films: A Preliminary Study. J. Braz. Chem. Soc. 2020, 31, 990–998. [Google Scholar] [CrossRef]
- Liu, J.; Sun, Y. Structural analysis of an alkali-extractable and water-soluble polysaccharide (ABP-AW1) from the fruiting bodies of Agaricus blazei Murill. Carbohydr. Polym. 2011, 86, 429–432. [Google Scholar] [CrossRef]
- Lv, J.; Bai, F.; Wei, P. Effects of Agaricus blazei polysaccharide on oxygen free radicals and inflammatory related factors in diabetes rats. Guizhou Med. 2017, 41, 2. [Google Scholar] [CrossRef]
- Liu, W.; Yang, J.; Ren, J.; Zhang, D.; Ning, Z. Structural characterization and anti-inflammatory activity of ABD polysaccharides from Agaricus blazei. Mod. Food Technol. 2017, 33, 7. [Google Scholar] [CrossRef]
- Cheng, F.; Yan, X.; Zhang, M.; Chang, M.; Yun, S.; Meng, J.; Liu, J.; Feng, C. Regulation of RAW 264.7 cell-mediated immunity by polysaccharides from Agaricus blazei Murill via the MAPK signal transduction pathway. Food Funct. 2017, 8, 1475–1480. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Cheng, W.; Wang, Q.; Zhang, J.; Wang, C.; Li, M.; Zhao, D.; Wang, D.; An, Q. Exploring the Protective and Reparative Mechanisms of G. lucidum Polysaccharides Against H2O2-Induced Oxidative Stress in Human Skin Fibroblasts. Clin. Cosmet. Investig. Dermatol. 2021, 14, 1481–1496. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Gong, X.; Wei, D.; Liu, Y.; Zhao, M.; Yu, X.; Olatunji, O.; Jiao, X.; Zhen, O. Antioxidant Activity and Preliminary Structure Analysis of Polysaccharides from Cordyceps cicadas. Food Sci. 2016, 37, 19–24. [Google Scholar] [CrossRef]
- Cheng, W.; Yang, Y.; Zhang, J.; Di, F.; Li, L.; Wang, C.; Li, M.; Zhao, D.; Shi, X.; Huo, T.; et al. Protective mechanisms of intra- and extracellular polysaccharides from Schizophyllum commune on H2O2-induced oxidative damage of human skin fibroblasts. Food Agric. Immunol. 2023, 34, 2203407. [Google Scholar] [CrossRef]
- Wang, M. Recent developments in Hericium erinaceus polysaccharides: Extraction, purification, structural characteristics and biological activities. Crit. Rev. Food Sci. Nutr. 2019, 59, 96–115. [Google Scholar] [CrossRef] [PubMed]
- Florence, D.; Cedric, L.; Alix, V.; Olivier, T. UV, stress and aging. Derm. Endocrinol. 2014, 4, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Agar, N.; Halliday, G.; Barnetson, R.; Ananthaswamy, H.; Wheeler, M.; Jones, A. The basal layer in human squamous tumors harbors more UVA than UVB fingerprint mutations: A role for UVA in human skin carcinogenesis. Proc. Natl. Acad. Sci. USA 2004, 101, 4954–4959. [Google Scholar] [CrossRef]
- Li, Q.; Bai, D.; Qin, L.; Shao, M.; Zhang, S.; Yan, C.; Yu, G.; Hao, J. Protective effect of D-tetramannuronic acid tetrasodium salt on UVA-induced photo-aging in HaCaT cells. Biomed. Pharmacother. Biomed. Pharmacother. 2020, 126, 110094. [Google Scholar] [CrossRef]
- Jaszewska, E.; Soin, M.; Filipek, A.; Naruszewicz, M. UVA-induced ROS generation inhibition by Oenothera paradoxa defatted seeds extract and subsequent cell death in human dermal fibroblasts. J. Photochem. Photobiol. B Biol. 2013, 126, 42–46. [Google Scholar] [CrossRef]
- Lee, H.; Hong, Y.; Tran, Q.; Cho, H.; Kim, M.; Kim, C.; Kwon, S.; Park, S.; Park, J.; Park, J. A new role for the ginsenoside RG3 in antiaging via mitochondria function in ultraviolet-irradiated human dermal fibroblasts. J. Ginseng. Res. 2019, 43, 431–441. [Google Scholar] [CrossRef]
- Varani, J.; Warner, R.; Gharaee-Kermani, M.; Phan, S.; Kang, S.; Chung, J.; Wang, Z.; Datta, S.; Fisher, S.; Voorhees, J. Vitamin A Antagonizes Decreased Cell Growth and Elevated Collagen-Degrading Matrix Metalloproteinases and Stimulates Collagen Accumulation in Naturally Aged Human Skin1. J. Investig. Dermatol. 2000, 114, 480–486. [Google Scholar] [CrossRef]
- Kim, J.; Kim, D.; Kim, H.; Jang, A. Protection effect of donkey hide gelatin hydrolysates on UVB-induced photoaging of human skin fibroblasts. Process Biochem. 2018, 67, 118–126. [Google Scholar] [CrossRef]
- Lee, K.; Park, J.; Jung, E.; Ryu, J.; Kim, Y.; Youm, J.; Kang, S. A study of facial wrinkles improvement effect of veratric acid from cauliflower mushroom through photo-protective mechanisms against UVB irradiation. Arch. Dermatol. Res. 2016, 308, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Hseu, Y.; Chang, C.; Gowrisankar, Y.; Chen, X.; Lin, H.; Yen, H.; Yang, H. Zerumbone Exhibits Antiphotoaging and Dermatoprotective Properties in Ultraviolet A-Irradiated Human Skin Fibroblast Cells via the Activation of Nrf2/ARE Defensive Pathway. Oxidative Med. Cell. Longev. 2019, 4098674. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Zheng, C.; Zheng, Q.; Chen, S.; Li, W.; Shang, Z.; Zhang, H. Salvianolic acid A attenuates early brain injury after subarachnoid hemorrhage in rats by regulating ERK/P38/Nrf2 signaling. Am. J. Transl. Res. 2017, 9, 5643–5652. [Google Scholar]
- Sevag, M.; Lackman, D.; Smolens, J. The isolation of components of Streptococcal nucleoproteins in serologically active form. J. Biol. Chem. 1938, 124, 425–436. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Q.; Zheng, Y.; He, Z.; Guan, P.; He, X.; Hui, L.; Dai, Y. Study of Schiff base formation between dialdehyde cellulose and proteins, and its application for the deproteinization of crude polysaccharide extracts. Ind. Crops Prod. 2018, 112, 532–540. [Google Scholar] [CrossRef]
- You, S.; Wang, C.; Zhang, J.; Li, M.; Zhao, D.; An, Q. An Preliminary purification and anti-aging activity evaluation of total flavonoids from seabuckthorn seed meal prepared by fermentation method. Dly. Chem. Ind. 2019, 49, 7. [Google Scholar]
- Mu, H.; Gao, H.; Chen, H.; Fang, X.; Ge, L. Determination of total flavanoid content in Asparagus officinalis Linn by spectrofluorimetry. J. Chin. Inst. Food Sci. Technol. 2010, 10, 201–205. [Google Scholar] [CrossRef]
- Cao, H.; Liu, C.; Li, S. Congo Red Method for Quantitative Detection of Yeast β- Research on the Methods of Dextran in the Food and Fermentation Industry. Food Ferment. Ind. 2022, 48, 261–266. [Google Scholar] [CrossRef]
- Tu, J.; Zhang, X.; Yu, L.; Liu, H.; Wu, F.; Liu, X.; Ma, X. Studies on rapid determination of beta-glucan content in grain products by flow-injecting Congo red spectrophotometry. Cereal Feed. Ind. 2009, 46–48. [Google Scholar]
- GB5009.91-2017; Food Safety National Standard—Determination of Potassium and Sodium in Food. Standardization Administration of China: Beijing, China, 2017.
- Liu, Z.; Jiao, Y.; Lu, H.; Shu, X.; Chen, Q. Chemical characterization, antioxidant properties and anticancer activity of exopolysaccharides from Floccularia luteovirens. Carbohydr. Polym. 2019, 229, 115432. [Google Scholar] [CrossRef]
- Min, W.; Fang, X.; Wu, T.; Fang, L.; Liu, C.; Wang, J. Characterization and antioxidant activity of an acidic exopolysaccharide from Lactobacillus plantarum JLAU103. J. Bioence Bioeng. 2018, 127, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Han, A.; Park, S.; Cho, C.; Rhee, Y.; Hong, H. Effect of enzyme-assisted extraction on the physicochemical properties and bioactive potential of lotus leaf polysaccharides. Int. J. Biol. Macromol. 2020, 153, 169–179. [Google Scholar] [CrossRef]
- Hseu, Y.; Lo, H.; Korivi, M.; Tsai, Y.; Tang, M.; Yang, H. Dermato-protective properties of ergothioneine through induction of Nrf2/ARE-mediated antioxidant genes in UVA-irradiated Human keratinocytes. Free Radic. Biol. Med. 2015, 86, 102–117. [Google Scholar] [CrossRef]
- Livak, K.; Schmittgen, T. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Singleton, B.; Libretto, P.; Sibley, C.V.; Andrews, C. An in vitro haemolysis test as an alternative to the draize test for ocular irritation. Comp. Haematol. Int. 1994, 4, 49–54. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, X.; Zhang, W.; Cai, J.; Xue, J.; Yang, G.; Tan, X.; Xie, X.; Xiong, X.; Huang, J. Combined in vitro tests as an alternative to in vivo eye irritation tests. Altern. Lab. Anim. Atla 2010, 38, 303–314. [Google Scholar] [CrossRef]
- Chen, Z.; Yin, C.; Fan, X.; Ma, K.; Yao, F.; Zhou, R.; Shi, D.; Cheng, W.; Gao, H. Characterization of physicochemical and biological properties of Schizophyllum commune polysaccharide extracted with different methods. Int. J. Biol. Macromol. 2020, 156, 1425–1434. [Google Scholar] [CrossRef] [PubMed]
- Safaryan, M.; Ganjloo, A.; Bimakr, M.; Zarringhalami, S. Optimization of Ultrasound-Assisted Extraction, Preliminary Characterization and In Vitro Antioxidant Activity of Polysaccharides from Green Pea Pods. Foods 2016, 5, 78. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; You, S.; Wang, D.; Zhao, D.; Zhang, J.; An, Q.; Li, M.; Wang, C. Fermented Dendrobium officinale polysaccharides protect UVA-induced photoaging of human skin fibroblasts. Food Sci. Nutr. 2022, 10, 1275–1288. [Google Scholar] [CrossRef] [PubMed]
- Boo, Y.C. Natural Nrf2 Modulators for Skin Protection. Antioxidants 2020, 9, 812. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Mockabee-macias, A.; Jiang, C.; Falzone, A.; Prieto-Farigua, N.; Stone, E.; Harris, I.; Denicola, G.; Mockabee-Macias, A.; Jiang, C.; et al. Non-canonical glutamate-cysteine ligase activity protects against ferroptosis. Cell Metab. 2020, 33, 174. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Jinlin, D.; Wang, F.; Yuan, Z.; Xue, J.; Lu, T.; Huang, W.; Liu, Y.; Zhang, Y. GSTM3 deficiency impedes DNA mismatch repair to promote gastric tumorigenesis via CAND1/NRF2-KEAP1 signaling. Cancer Lett. 2022, 538, 215692. [Google Scholar] [CrossRef] [PubMed]
- Kaspar, J.; Jaiswal, A. Antioxidant-induced Phosphorylation of Tyrosine 486 Leads to Rapid Nuclear Export of Bach1 That Allows Nrf2 to Bind to the Antioxidant Response Element and Activate Defensive Gene Expression. J. Biol. Chem. 2010, 285, 153–162. [Google Scholar] [CrossRef]
- Xu, Y.; Cui, Y.; Wang, X.; Yue, F.; Shan, Y.; Liu, B.; Zhou, Y.; Yi, Y.; Lu, X. Purification, characterization and bioactivity of exopolysaccharides produced by Lactobacillus plantarum KX041. Int. J. Biol. Macromol. 2019, 128, 480–492. [Google Scholar] [CrossRef]
- Zhao, D.; Jiang, J.; Du, R.; Guo, S.; Ping, W.; Ling, H.; Ge, J. Purification and characterization of an exopolysaccharide from Leuconostoc lactis L2. Int. J. Biol. Macromol. Struct. Funct. Interact. 2019, 139, 1224–1231. [Google Scholar] [CrossRef]
- Asgher, M.; Urooj, Y.; Qamar, S.; Khalid, N. Improved exopolysaccharide production from Bacillus licheniformis MS3: Optimization and structural/functional characterization. Int. J. Biol. Macromol. 2020, 151, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Bhandary, T.; Kurian, C.; Muthu, M.; Anand, A.; Anand, T.; Paari, K. Exopolysaccharides Derived from Probiotic Bacteria and their Health Benefits. J. Pure Appl. Microbiol. 2023, 17, 35–40. [Google Scholar] [CrossRef]
- Shao, L.; Wu, Z.; Zhang, H.; Chen, W.; Guo, B. Observation of Exopolysaccharide S2 from Lactobacillus rhamnosus KF5 Using Atomic Force Microscopy. Food Sci. 2015, 36, 43–47. [Google Scholar] [CrossRef]
- Hyde, K.; Bahkali, A.; Moslem, M. Fungi-an unusual source for cosmetics. Fungal Divers. 2010, 43, 1–9. [Google Scholar] [CrossRef]
- Jiang, C.; Ge, J.; He, B.; Zeng, B. Glycosphingolipids in Filamentous Fungi: Biological Roles and Potential Applications in Cosmetics and Health Foods. Front. Microbiol. 2021, 12, 690211. [Google Scholar] [CrossRef]
- Taofiq, O.; Rodrigues, F.; Barros, L.; Peralta, R.; Barreiro, M.; Feeewira, I.C.; Oliveira, M.B. Agaricus blazei Murrill from Brazil: An ingredient for nutraceutical and cosmeceutical applications. Food Funct. 2019, 10, 565–572. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, R.; Xie, Y.; Wang, H.; Ge, M. The Protective Effects of Polysaccharides from Agaricus blazei Murill Against Cadmium-Induced Oxidant Stress and Inflammatory Damage in Chicken Livers. Biol. Trace Elem. Res. 2016, 178, 117–126. [Google Scholar] [CrossRef]
- Al-Dbass, A.; Al-Daihan, S.; Bhat, R. Agaricus blazei Murill as an efficient hepatoprotective and antioxidant agent against CCl4 -induced liver injury in rats. Saudi J. Biol. Sci. 2012, 19, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, O.M.; Vellosa, J.C.; Fernandes, A.; Buffa-Fiho, W.; Hakime-Silva, R.; Furlan, M.; Brunetti, I. Antioxidant activity of Agaricus blazei. Fitoterapia 2007, 78, 263–264. [Google Scholar] [CrossRef]
- Gu, Y.; Han, J.; Jiang, C.; Zhang, Y. Biomarkers, oxidative stress and autophagy in skin aging. Ageing Res. Rev. 2020, 59, 101036. [Google Scholar] [CrossRef]
- Bhatti, J.; Bhatti, G.; Reddy, P. Mitochondrial dysfunction and oxidative stress in metabolic disorders — A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta (BBA) - Mol. Basis Dis. 2016, 1863, 1066–1077. [Google Scholar] [CrossRef]
- Xu, H. The role of oxidative stress in the pathogenesis of diabetes related periodontitis. Int. J. Stomatol. 2011, 38, 4. [Google Scholar] [CrossRef]
- Fang, J.; Sun, Q.; Wang, Z.; Song, Z.; Wang, C.; Li, M.; Wang, D. Enhancement of Human Epidermal Cell Defense against UVB Damage by Fermentation of Passiflora edulis Sims Peel with Saccharomyces cerevisiae. Nutrients 2023, 15, 501. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, Y.; Shao, Y.; Wu, Y.; Hsu, W.; Cheng, K.; Yu, C.; Chou, C.; Hsieh, C. Physicochemical properties and protective effects on UVA-induced photoaging in Hs68 cells of Pleurotus ostreatus polysaccharides by fractional precipitation. Int. J. Biol. Macromol. 2023, 228, 537–547. [Google Scholar] [CrossRef]
- Chen, B.; Huang, H.; Tsai, K.; Wu, J.; Chang, Y.; Chang, M.; Lu, C.; Yang, S.; Huang, H. Protective Effect of a Water-Soluble Carotenoid-Rich Extract of Cordyceps militaris against Light-Evoked Functional Vision Deterioration in Mice. Nutrients 2022, 14, 1675. [Google Scholar] [CrossRef]
- Suzuki, T.; Yamamoto, M. Stress-sensing mechanisms and the physiological roles of the Keap1–Nrf2 system during cellular stress. J. Biol. Chem. 2017, 292, 16817–16824. [Google Scholar] [CrossRef] [PubMed]
- Canning, P.; Sorrell, F.; Bullock, A. Structural basis of Keap1 interactions with Nrf2. Free Radic. Biol. Med. 2015, 88, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Yamamoto, M. Molecular basis of the Keap1-Nrf2 system. Free Radic. Biol. Med. 2015, 88, 93–100. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, W.; Feng, X.; Yang, F.; Qin, H.; Wu, S.; Hou, D.; Chen, J. Nrf2-ARE Signaling Acts as Master Pathway for the Cellular Antioxidant Activity of Fisetin. Molecules 2019, 24, 708. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, J.; Sanapalli, B.K.; Bano, M.; Singh, S.; Gulati, M.; Karri, V.V. Nanostructured Lipid Carriers of Pioglitazone Loaded Collagen/Chitosan Composite Scaffold for Diabetic Wound Healing. Adv. Wound Care 2019, 8, 499–513. [Google Scholar] [CrossRef]
- Aluri, H.; Kublin, C.; Thotakura, S.; Armaos, H.; Samizadeh, M.; Hawley, D.; Thomas, W.; Leavis, P.; Makarenkova, H.; Zoukhri, D. Role of Matrix Metalloproteinases 2 and 9 in Lacrimal Gland Disease in Animal Models of Sjögren's Syndrome. Investig. Opthalmology Vis. Sci. 2015, 56, 5218–5228. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.; Kim, S.; Chung, H.; Pae, H. Chapter Two - Reactive Oxygen Species in the Activation of MAP Kinases. Methods Enzymol. 2013, 528, 27–48. [Google Scholar] [PubMed]
- Liu, Q.; Xiao, X.; Hu, L.; Jie, H.; Wang, Y.; Ye, W.; Li, M.; Liu, Z.; Anhuienoside, C. Ameliorates Collagen-Induced Arthritis through Inhibition of MAPK and NF-κB Signaling Pathways. Front. Pharmacol. 2017, 8, 299. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Guo, W.; Zhang, W.; Xu, J.; Qian, M.; Bai, W.; Zhang, Y.; Rao, P.; Ni, L.; Lv, X. Grifola frondosa polysaccharides ameliorate lipid metabolic disorders and gut microbiota dysbiosis in high-fat diet fed rats. Food Funct. 2019, 10, 2560–2572. [Google Scholar] [CrossRef] [PubMed]
- Shih, J.; Tsai, Y.; Li, I.; Chen, M.; Huang, Y. Hp-s1 Ganglioside Suppresses Proinflammatory Responses by Inhibiting MyD88-Dependent NF-κB and JNK/p38 MAPK Pathways in Lipopolysaccharide-Stimulated Microglial Cells. Mar. Drugs 2020, 18. [Google Scholar] [CrossRef] [PubMed]
- Shih, P.H.; Yen, G.C. Differential expressions of antioxidant status in aging rats: The role of transcriptional factor Nrf2 and MAPK signaling pathway. Biogerontroly 2007, 8, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Sun, Y.; Sun, J.; Ma, J.; Cheng, C. Protective role of quercetin against lead-induced inflammatory response in rat kidney through the ROS-mediated MAPKs and NF-κB pathway. Biochim. Biophys. Acta 2012, 1820, 1693–1703. [Google Scholar] [CrossRef]
- Wyatt, L.; Luz, A.; Cao, X.; Maurer, L.; Blawas, A.; Aballay, A.; Pan, W.K.; Meyer, J. Effects of methyl and inorganic mercury exposure on genome homeostasis and mitochondrial function in Caenorhabditis elegans. DNA Repair. 2017, 52, 31–48. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).