Effects of Fermented Goat Milk on Adiposity and Gut Microbiota in a Diet-Induced Obesity Murine Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
2.2. Elaboration of Fermented Goat Milk (FGM) Supplemented with the Mixes
2.3. Animals, Diets, and Experimental Design
2.4. Determination of Body Weight, Feed Consumption, and Feed Efficiency Ratio
2.5. Animal Sample Collection
2.6. Analysis of Inflammatory and Metabolic Parameters
2.7. Histological Analysis
2.8. Determination of Gut Microbiota Composition
2.9. Statistical Analysis
3. Results
3.1. Evaluation of Nutritional Parameters
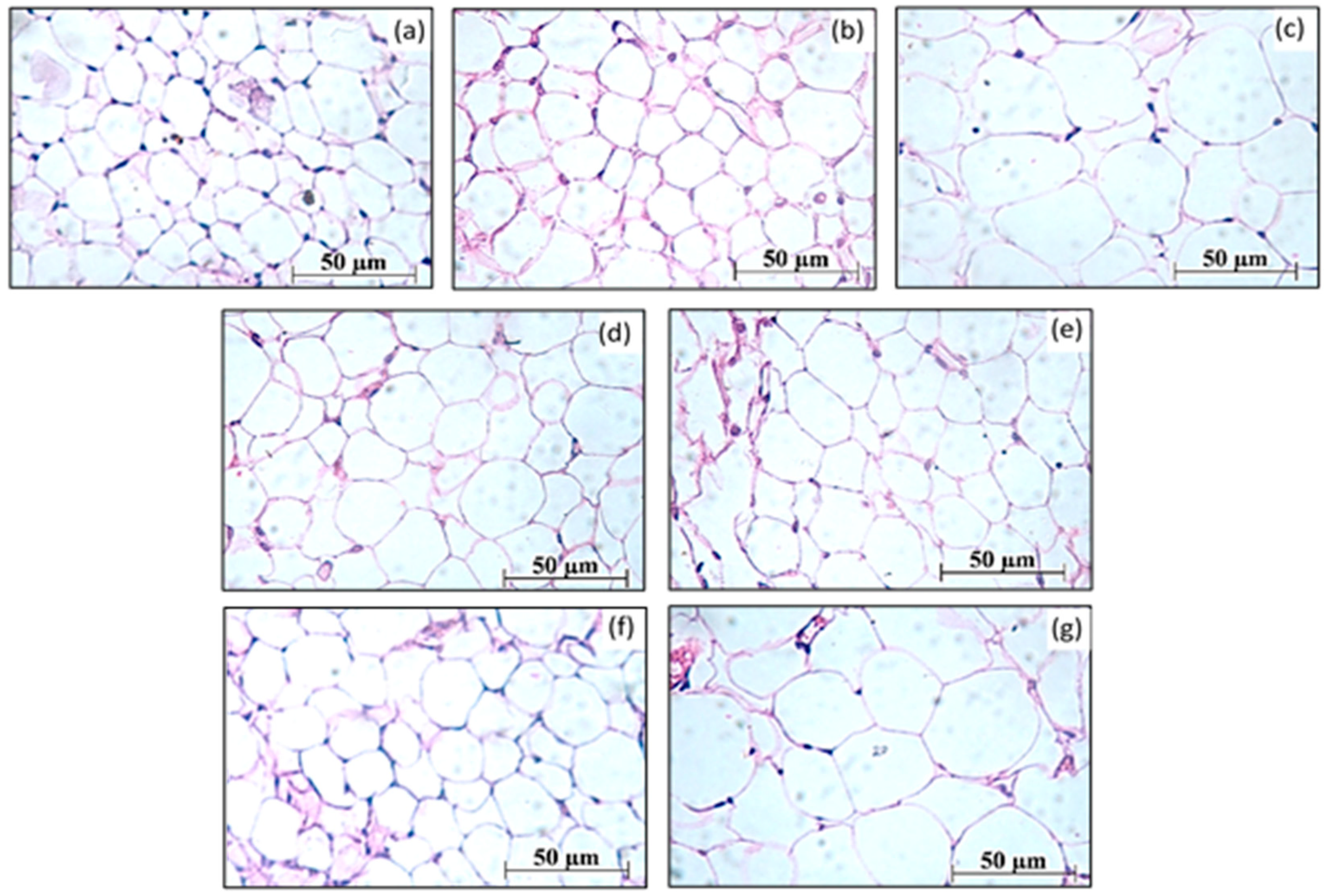
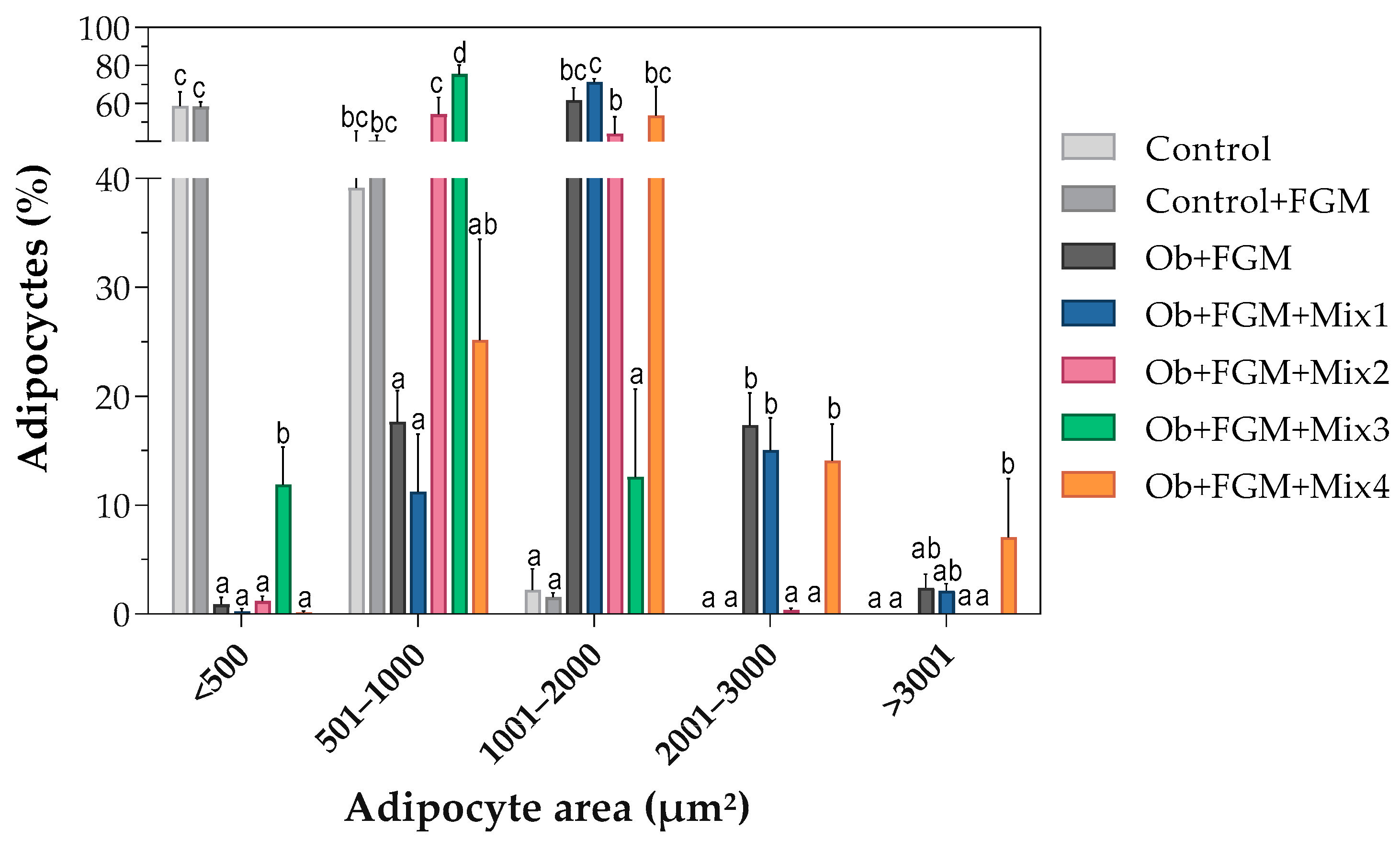
3.2. Assessment of the Structure of Adipocytes
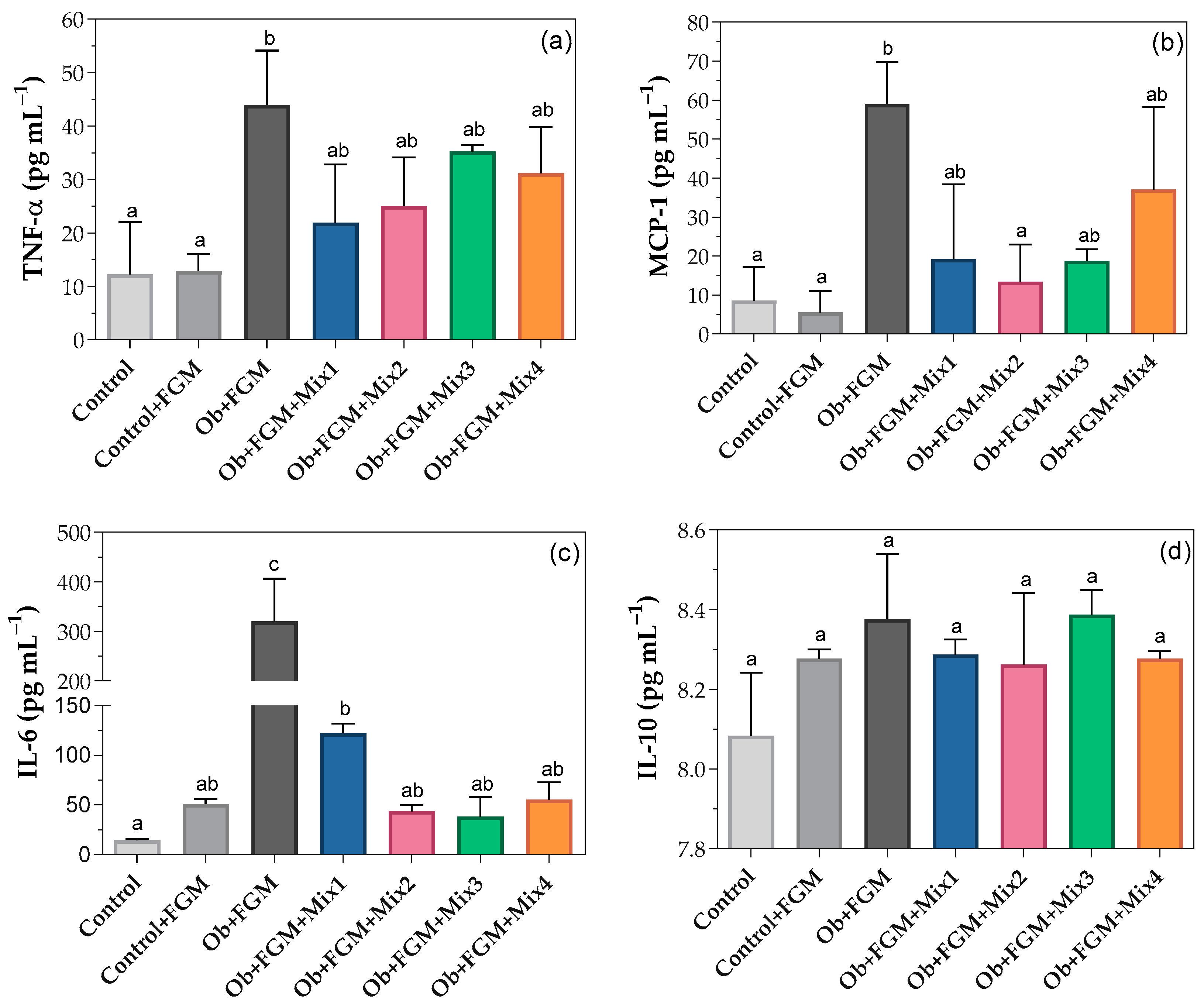
3.3. Inflammatory Status of Obese Mice after Administration of FGM
3.4. Biochemical Parameters of Obese Mice after Administration of FGM
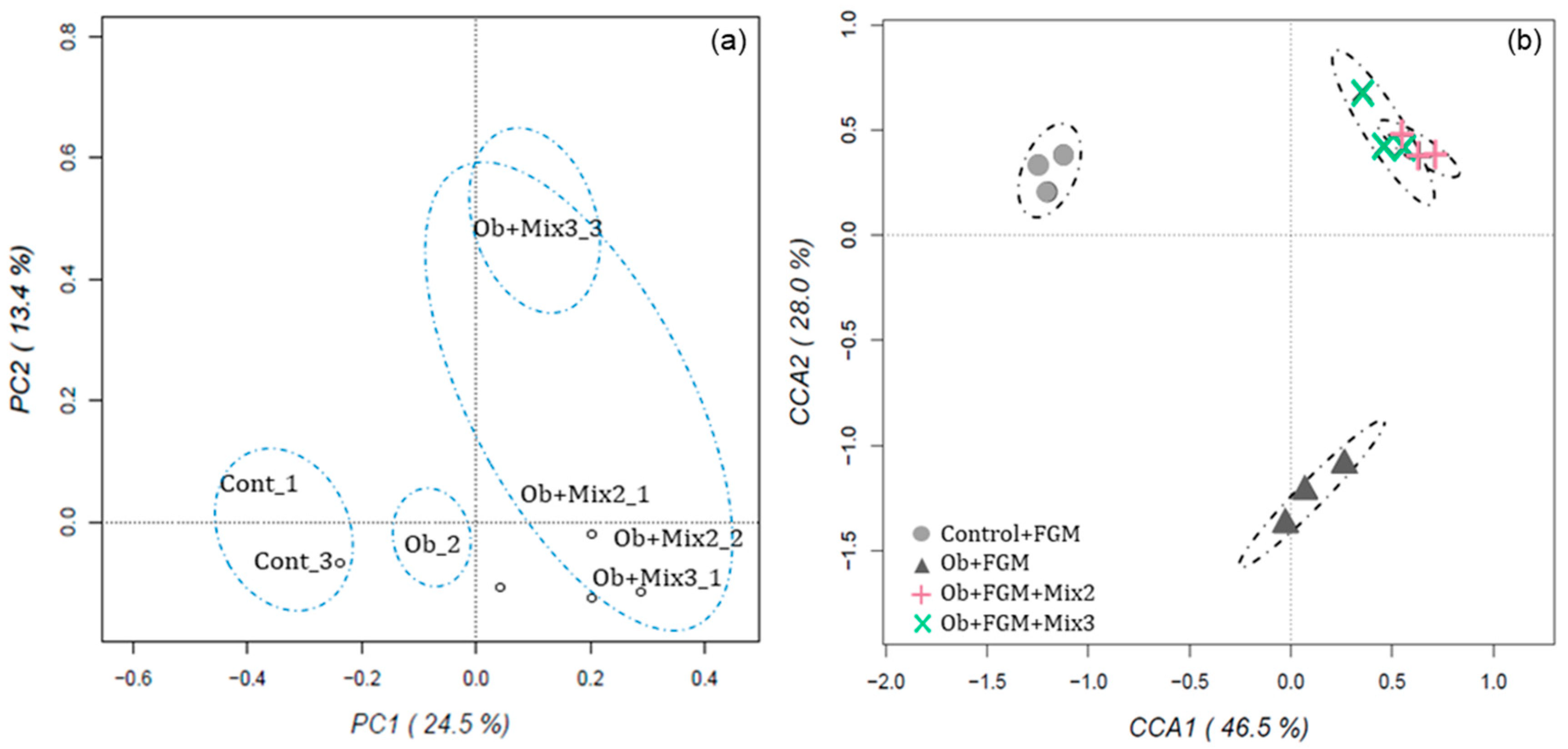
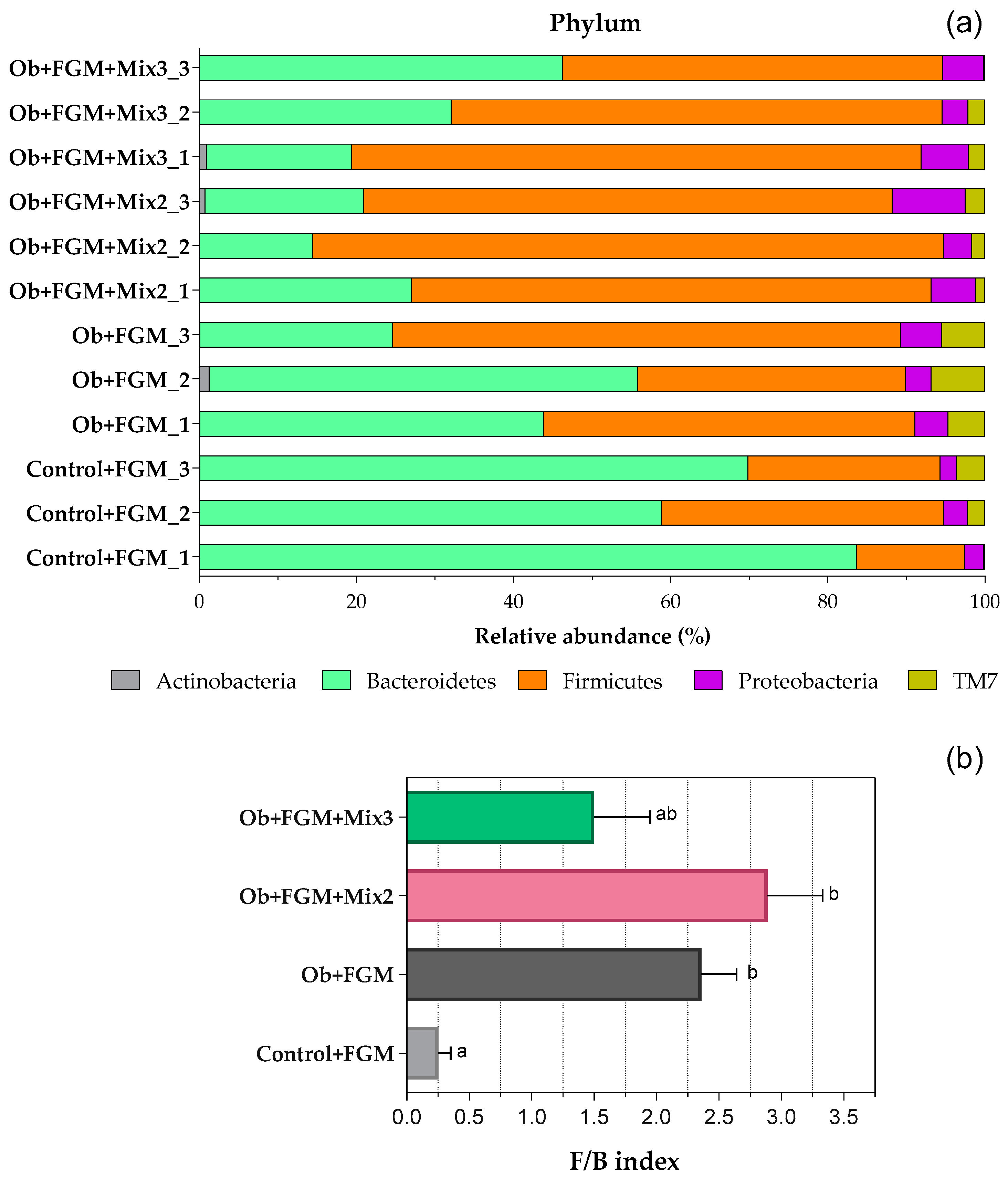
3.5. Determination of the Composition of GM of Obese Mice after Administration of FGM
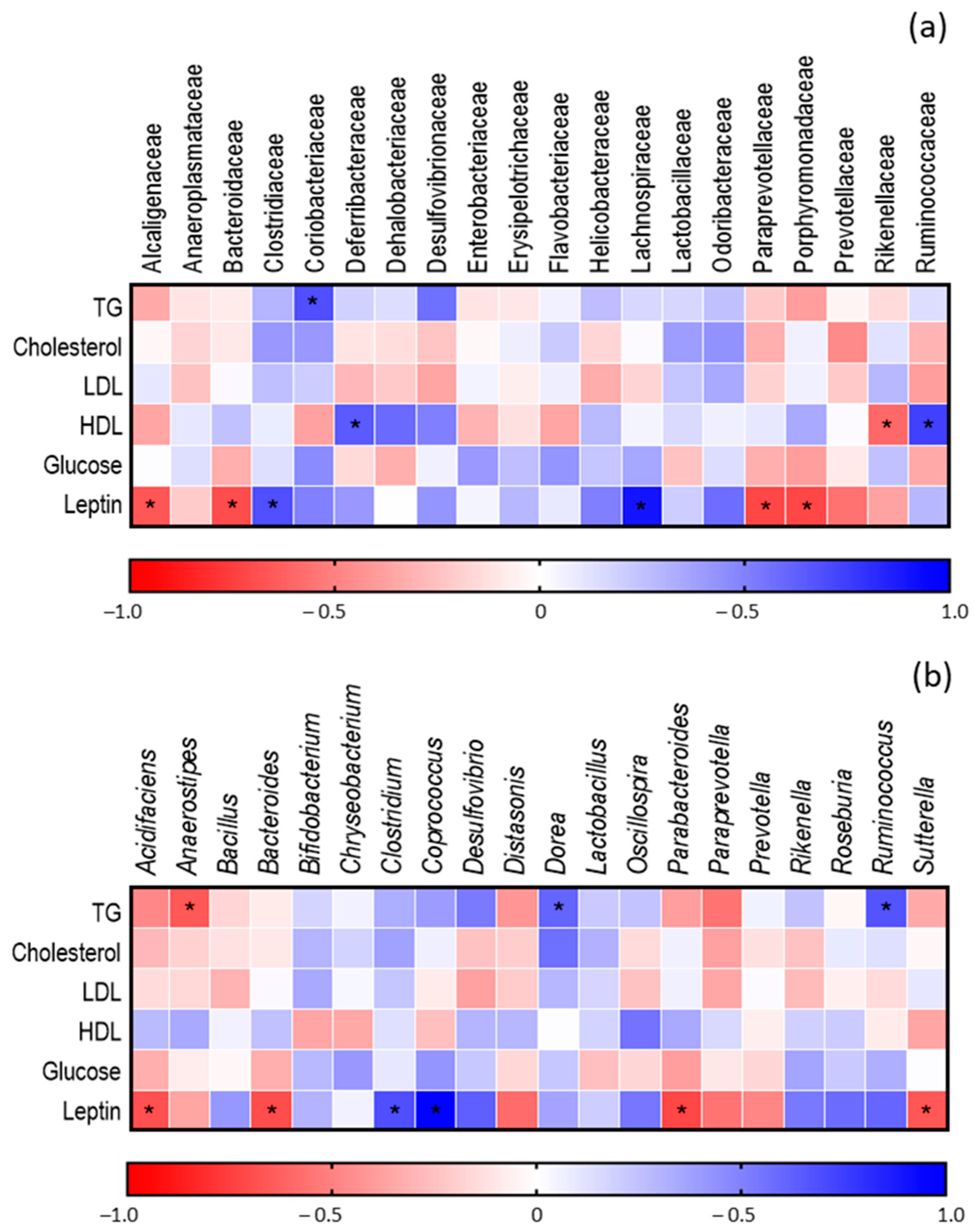
Correlation between GM and Metabolic Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 6 April 2020).
- Barlow, G.M.; Lin, E.A.; Mathur, R. Chapter 6—An Overview of the Roles of the Gut Microbiome in Obesity and Diabetes. In Nutritional and Therapeutic Interventions for Diabetes and Metabolic Syndrome, 2nd ed.; Bagchi, D., Nair, S., Eds.; Academic Press: Los Angeles, CA, USA, 2018; pp. 65–91. ISBN 978-0-12-812019-4. [Google Scholar]
- Sivamaruthi, B.S.; Kesika, P.; Suganthy, N.; Chaiyasut, C. A Review on Role of Microbiome in Obesity and Antiobesity Properties of Probiotic Supplements. BioMed Res. Int. 2019, 2019, 3291367. [Google Scholar] [CrossRef]
- Zsálig, D.; Berta, A.; Tóth, V.; Szabó, Z.; Simon, K.; Figler, M.; Pusztafalvi, H.; Polyák, É. A Review of the Relationship between Gut Microbiome and Obesity. Appl. Sci. 2023, 13, 610. [Google Scholar] [CrossRef]
- FAO/WHO. Probiotics in Food Health and Nutritional Properties and Guidelines for Evaluation. 2001. ISBN 92-5-105513-0. Available online: http://www.fao.org/3/a0512e/a0512e.pdf (accessed on 6 April 2020).
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, W.M.; Guimarães Gomes, A.C.; de Caldas Nobre, M.S.; de Souza Pereira, Á.M.; dos Santos Pereira, E.V.; dos Santos, K.M.O.; Florentino, E.R.; Alonso Buriti, F.C. Goat Milk as a Natural Source of Bioactive Compounds and Strategies to Enhance the Amount of These Beneficial Components. Int. Dairy J. 2023, 137, 105515. [Google Scholar] [CrossRef]
- Ranadheera, C.; Evans, C.A.; Baines, S.K.; Balthazar, C.F.; Cruz, A.G.; Esmerino, E.A.; Freitas, M.Q.; Pimentel, T.C.; Wittwer, A.E.; Naumovski, N.; et al. Probiotics in Goat Milk Products: Delivery Capacity and Ability to Improve Sensory Attributes. Compr. Rev. Food Sci. Food Saf. 2019, 18, 867–882. [Google Scholar] [CrossRef] [PubMed]
- Andrada, E.; Marquez, A.; Russo, M.; Gauffin-Cano, P.; Medina, R. Fermented Goat Milk as a Functional Food for Obesity Prevention or Treatment: A Narrative Review. Front. Food Sci. Technol. 2024, 3, 1329037. [Google Scholar] [CrossRef]
- Chen, L.; Bagnicka, E.; Chen, H.; Shu, G. Health Potential of Fermented Goat Dairy Products: Composition Comparison with Fermented Cow Milk, Probiotics Selection, Health Benefits and Mechanisms. Food Funct. 2023, 14, 3423–3436. [Google Scholar] [CrossRef]
- Marquez, A.; Andrada, E.; Russo, M.; Bolondi, M.L.; Fabersani, E.; Medina, R.; Gauffin-Cano, P. Characterization of Autochthonous Lactobacilli from Goat Dairy Products with Probiotic Potential for Metabolic Diseases. Heliyon 2022, 8, e10462. [Google Scholar] [CrossRef]
- de Carvalho Marchesin, J.; Celiberto, L.S.; Orlando, A.B.; de Medeiros, A.I.; Pinto, R.A.; Zuanon, J.A.S.; Spolidorio, L.C.; dos Santos, A.; Taranto, M.P.; Cavallini, D.C.U. A Soy-Based Probiotic Drink Modulates the Microbiota and Reduces Body Weight Gain in Diet-Induced Obese Mice. J. Funct. Foods 2018, 48, 302–313. [Google Scholar] [CrossRef]
- Gauffin Cano, M.P.; Santacruz, A.; Trejo, F.M.; Sanz, Y. Bifidobacterium CECT 7765 Improves Metabolic and Immunological Alterations Associated with Obesity in High-Fat Diet-Fed Mice. Obesity 2013, 21, 2310–2321. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global Patterns of 16S rRNA Diversity at a Depth of Millions of Sequences per Sample. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4516–4522. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-High-Throughput Microbial Community Analysis on the Illumina HiSeq and MiSeq Platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef]
- Vallianou, N.G.; Kounatidis, D.; Tsilingiris, D.; Panagopoulos, F.; Christodoulatos, G.S.; Evangelopoulos, A.; Karampela, I.; Dalamaga, M. The Role of Next-Generation Probiotics in Obesity and Obesity-Associated Disorders: Current Knowledge and Future Perspectives. Int. J. Mol. Sci. 2023, 24, 6755. [Google Scholar] [CrossRef] [PubMed]
- Buyukdere, Y.; Gulec, A.; Akyol, A. Cafeteria Diet Increased Adiposity in Comparison to High Fat Diet in Young Male Rats. PeerJ 2019, 7, e6656. [Google Scholar] [CrossRef]
- Hariri, N.; Thibault, L. High-Fat Diet-Induced Obesity in Animal Models. Nutr. Res. Rev. 2010, 23, 270–299. [Google Scholar] [CrossRef]
- Moore, C.F.; Schlain, G.S.; Mancino, S.; Sabino, V.; Cottone, P. A Behavioral and Pharmacological Characterization of Palatable Diet Alternation in Mice. Pharmacol. Biochem. Behav. 2017, 163, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Wang, L.; Yang, D.; Li, L.; Togo, J.; Wu, Y.; Liu, Q.; Li, B.; Li, M.; Wang, G.; et al. Dietary Fat, but Not Protein or Carbohydrate, Regulates Energy Intake and Causes Adiposity in Mice. Cell Metab. 2018, 28, 415–431.e4. [Google Scholar] [CrossRef]
- Patience, J.F.; Rossoni-Serão, M.C.; Gutiérrez, N.A. A Review of Feed Efficiency in Swine: Biology and Application. J. Anim. Sci. Biotechnol. 2015, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Sárvári, A.K.; Van Hauwaert, E.L.; Markussen, L.K.; Gammelmark, E.; Marcher, A.-B.; Ebbesen, M.F.; Nielsen, R.; Brewer, J.R.; Madsen, J.G.S.; Mandrup, S. Plasticity of Epididymal Adipose Tissue in Response to Diet-Induced Obesity at Single-Nucleus Resolution. Cell Metab. 2021, 33, 437–453.e5. [Google Scholar] [CrossRef]
- Fabersani, E.; Marquez, A.; Russo, M.; Ross, R.; Torres, S.; Fontana, C.; Puglisi, E.; Medina, R.; Gauffin-Cano, P. Lactic Acid Bacteria Strains Differently Modulate Gut Microbiota and Metabolic and Immunological Parameters in High-Fat Diet-Fed Mice. Front. Nutr. 2021, 8, 718564. [Google Scholar] [CrossRef]
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.A.K.; Sarker, M.; Wan, D. Immunomodulatory Effects of Probiotics on Cytokine Profiles. BioMed Res. Int. 2018, 2018, 8063647. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Liu, Y.; Kwok, L.-Y.; Cai, T.; Zhang, W. The Immune Regulatory Role of Lactobacillus Acidophilus: An Updated Meta-Analysis of Randomized Controlled Trials. Food Biosci. 2020, 36, 100656. [Google Scholar] [CrossRef]
- Jung, U.; Choi, M.-S. Obesity and Its Metabolic Complications: The Role of Adipokines and the Relationship between Obesity, Inflammation, Insulin Resistance, Dyslipidemia and Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2014, 15, 6184–6223. [Google Scholar] [CrossRef] [PubMed]
- Pothuraju, R.; Sharma, R.K.; Kavadi, P.K.; Chagalamarri, J.; Jangra, S.; Bhakri, G.; De, S. Anti-Obesity Effect of Milk Fermented by Lactobacillus Plantarum NCDC 625 Alone and in Combination with Herbs on High Fat Diet Fed C57BL/6J Mice. Benef. Microbes 2016, 7, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Torres, S.; Fabersani, E.; Marquez, A.; Gauffin-Cano, P. Adipose Tissue Inflammation and Metabolic Syndrome. The Proactive Role of Probiotics. Eur. J. Nutr. 2019, 58, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, A.G.; Crujeiras, A.B.; Casanueva, F.F.; Carreira, M.C. Leptin, Obesity, and Leptin Resistance: Where Are We 25 Years Later? Nutrients 2019, 11, 2704. [Google Scholar] [CrossRef] [PubMed]
- Roselli, M.; Finamore, A.; Brasili, E.; Rami, R.; Nobili, F.; Orsi, C.; Zambrini, A.V.; Mengheri, E. Beneficial Effects of a Selected Probiotic Mixture Administered to High Fat-Fed Mice before and after the Development of Obesity. J. Funct. Foods 2018, 45, 321–329. [Google Scholar] [CrossRef]
- Nakata, M.; Yamamoto, S.; Okada, T.; Gantulga, D.; Okano, H.; Ozawa, K.; Yada, T. IL-10 Gene Transfer Upregulates Arcuate POMC and Ameliorates Hyperphagia, Obesity and Diabetes by Substituting for Leptin. Int. J. Obes. 2016, 40, 425–433. [Google Scholar] [CrossRef]
- Russo, M.; Marquez, A.; Herrera, H.; Abeijon-Mukdsi, C.; Saavedra, L.; Hebert, E.; Gauffin-Cano, P.; Medina, R. Oral Administration of Lactobacillus fermentum CRL1446 Improves Biomarkers of Metabolic Syndrome in Mice Fed a High-Fat Diet Supplemented with Wheat Bran. Food Funct. 2020, 11, 3879–3894. [Google Scholar] [CrossRef]
- Namekawa, J.; Takagi, Y.; Wakabayashi, K.; Nakamura, Y.; Watanabe, A.; Nagakubo, D.; Shirai, M.; Asai, F. Effects of High-Fat Diet and Fructose-Rich Diet on Obesity, Dyslipidemia and Hyperglycemia in the WBN/Kob-Leprfa Rat, a New Model of Type 2 Diabetes Mellitus. J. Vet. Med. Sci. 2017, 79, 988–991. [Google Scholar] [CrossRef]
- Lye, H.-S.; Rahmat-Ali, G.R.; Liong, M.-T. Mechanisms of Cholesterol Removal by Lactobacilli under Conditions That Mimic the Human Gastrointestinal Tract. Int. Dairy J. 2010, 20, 169–175. [Google Scholar] [CrossRef]
- Reis, S.A.; Conceição, L.L.; Rosa, D.D.; Siqueira, N.P.; Peluzio, M.C.G. Mechanisms Responsible for the Hypocholesterolaemic Effect of Regular Consumption of Probiotics. Nutr. Res. Rev. 2017, 30, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Rawat, S.; Kumar, S.; Arora, M.; Iqbal, S.; Batra, J.; Sharma, S.; Sah, S.P. Assessment of Atherogenic Index of Plasma, Non-HDL Cholesterol and Other Cardiac Indices as Risk Factors for CVD in Type II Diabetes. Int. J. Health Clin. Res. 2020, 3, 137–141. [Google Scholar]
- Choi, I.-D.; Kim, S.-H.; Jeong, J.-W.; Lee, D.E.; Huh, C.-S.; Hong, S.S.; Sim, J.-H.; Ahn, Y.-T. Triglyceride-Lowering Effects of Two Probiotics, Lactobacillus Plantarum KY1032 and Lactobacillus Curvatus HY7601, in a Rat Model of High-Fat Diet-Induced Hypertriglyceridemia. J. Microbiol. Biotechnol. 2016, 26, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Garg, A.; Rout, A.; Lavie, C.J. Association of Obesity with More Critical Illness in COVID-19. Mayo Clin. Proc. 2020, 95, 2040–2042. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Park, S.; Sin, H.-S.; Jang, S.-H.; Lee, S.-W.; Kim, S.-Y.; Kwon, B.; Yu, K.-Y.; Kim, S.; Yang, D. Hypocholesterolemic Effects of Probiotic Mixture on Diet-Induced Hypercholesterolemic Rats. Nutrients 2017, 9, 293. [Google Scholar] [CrossRef] [PubMed]
- Darwish, A.M.G.; Allam, M.G.; Shokery, E.S.; Ayad, E.H.E. Functional Products Fortified with Probiotic LAB Isolated from Egyptian Dairy Sources Showed Hypolipidemic Effects in Albino Rats. PLoS ONE 2022, 17, e0263241. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, H.; Yang, J.; Mu, J.; Wang, R.; Zhao, X. Effect of Soybean Milk Fermented with Lactobacillus Plantarum HFY01 Isolated from Yak Yogurt on Weight Loss and Lipid Reduction in Mice with Obesity Induced by a High-Fat Diet. RSC Adv. 2020, 10, 34276–34289. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Chiu, C.-C.; Hung, S.-W.; Huang, W.-C.; Lee, Y.-P.; Liu, J.-Y.; Huang, Y.-T.; Chen, T.-H.; Chuang, H.-L. Gnotobiotic Mice Inoculated with Firmicutes, but Not Bacteroidetes, Deteriorate Nonalcoholic Fatty Liver Disease Severity by Modulating Hepatic Lipid Metabolism. Nutr. Res. 2019, 69, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Neves Casarotti, S.; Fernanda Borgonovi, T.; de Mello Tieghi, T.; Sivieri, K.; Lúcia Barretto Penna, A. Probiotic Low-Fat Fermented Goat Milk with Passion Fruit by-Product: In Vitro Effect on Obese Individuals’ Microbiota and on Metabolites Production. Food Res. Int. 2020, 136, 109453. [Google Scholar] [CrossRef] [PubMed]
- Jian, C.; Silvestre, M.P.; Middleton, D.; Korpela, K.; Jalo, E.; Broderick, D.; de Vos, W.M.; Fogelholm, M.; Taylor, M.W.; Raben, A.; et al. Gut Microbiota Predicts Body Fat Change Following a Low-Energy Diet: A PREVIEW Intervention Study. Genome Med. 2022, 14, 54. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chang, S.K.C.; Zhang, Y.; Hsu, C.-Y.; Nannapaneni, R. Gut Microbiota and Short Chain Fatty Acid Composition as Affected by Legume Type and Processing Methods as Assessed by Simulated in Vitro Digestion Assays. Food Chem. 2020, 312, 126040. [Google Scholar] [CrossRef] [PubMed]
- Nadal, I.; Santacruz, A.; Marcos, A.; Warnberg, J.; Garagorri, J.M.; Moreno, L.A.; Martin-Matillas, M.; Campoy, C.; Martí, A.; Moleres, A.; et al. Shifts in Clostridia, Bacteroides and Immunoglobulin-Coating Fecal Bacteria Associated with Weight Loss in Obese Adolescents. Int. J. Obes. 2009, 33, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Joung, H.; Chu, J.; Kim, B.-K.; Choi, I.-S.; Kim, W.; Park, T.-S. Probiotics Ameliorate Chronic Low-Grade Inflammation and Fat Accumulation with Gut Microbiota Composition Change in Diet-Induced Obese Mice Models. Appl. Microbiol. Biotechnol. 2021, 105, 1203–1213. [Google Scholar] [CrossRef] [PubMed]
- Vacca, M.; Celano, G.; Calabrese, F.M.; Portincasa, P.; Gobbetti, M.; De Angelis, M. The Controversial Role of Human Gut Lachnospiraceae. Microorganisms 2020, 8, 573. [Google Scholar] [CrossRef] [PubMed]
- Tims, S.; Derom, C.; Jonkers, D.M.; Vlietinck, R.; Saris, W.H.; Kleerebezem, M.; de Vos, W.M.; Zoetendal, E.G. Microbiota Conservation and BMI Signatures in Adult Monozygotic Twins. ISME J. 2013, 7, 707–717. [Google Scholar] [CrossRef]
- Goodrich, J.K.; Waters, J.L.; Poole, A.C.; Sutter, J.L.; Koren, O.; Blekhman, R.; Beaumont, M.; Van Treuren, W.; Knight, R.; Bell, J.T.; et al. Human Genetics Shape the Gut Microbiome. Cell 2014, 159, 789–799. [Google Scholar] [CrossRef]
- Singer-Englar, T.; Barlow, G.; Mathur, R. Obesity, Diabetes, and the Gut Microbiome: An Updated Review. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 3–15. [Google Scholar] [CrossRef]
- Fabersani, E.; Portune, K.; Campillo, I.; López-Almela, I.; La Paz, S.M.; Romaní-Pérez, M.; Benítez-Páez, A.; Sanz, Y. Bacteroides Uniformis CECT 7771 Alleviates Inflammation within the Gut-Adipose Tissue Axis Involving TLR5 Signaling in Obese Mice. Sci. Rep. 2021, 11, 11788. [Google Scholar] [CrossRef] [PubMed]
- Palmas, V.; Pisanu, S.; Madau, V.; Casula, E.; Deledda, A.; Cusano, R.; Uva, P.; Vascellari, S.; Loviselli, A.; Manzin, A.; et al. Gut Microbiota Markers Associated with Obesity and Overweight in Italian Adults. Sci. Rep. 2021, 11, 5532. [Google Scholar] [CrossRef] [PubMed]
- Vojinovic, D.; Radjabzadeh, D.; Kurilshikov, A.; Amin, N.; Wijmenga, C.; Franke, L.; Ikram, M.A.; Uitterlinden, A.G.; Zhernakova, A.; Fu, J.; et al. Relationship between Gut Microbiota and Circulating Metabolites in Population-Based Cohorts. Nat. Commun. 2019, 10, 5813. [Google Scholar] [CrossRef] [PubMed]


| Groups | BWG | DFC | FER | AI |
|---|---|---|---|---|
| Control | 6.88 ± 0.11 a | 3.42 ± 0.13 c | 0.026 ± 0.001 a | 1.12 ± 0.15 ab |
| Control+FGM | 8.70 ± 0.61 ab | 3.37 ± 0.16 c | 0.037 ± 0.003 ab | 0.99 ± 0.09 a |
| Ob+FGM | 11.99 ± 0.93 c | 2.48 ± 0.10 a | 0.063 ± 0.005 c | 3.48 ± 0.46 d |
| Ob+FGM+Mix1 | 10.44 ± 1.55 bc | 2.88 ± 0.14 b | 0.062 ± 0.005 c | 3.25 ± 0.36 cd |
| Ob+FGM+Mix2 | 8.23 ± 1.38 ab | 2.55 ± 0.10 a | 0.042 ± 0.007 b | 2.25 ± 0.51 bc |
| Ob+FGM+Mix3 | 7.76 ± 0.82 ab | 2.54 ± 0.08 a | 0.052 ± 0.003 bc | 2.07 ± 0.42 ab |
| Ob+FGM+Mix4 | 10.29 ± 0.87 bc | 2.36 ± 0.08 a | 0.058 ± 0.002 c | 3.70 ± 0.30 d |
| Groups | Sobs | Chao | Shannon | Simpson |
|---|---|---|---|---|
| Control | 6469 ± 265 a | 39,238 ± 2981 a | 5.59 ± 0.06 a | 40.69 ± 3.4 a |
| Ob+FGM | 6889 ± 392 ab | 42,161 ± 1086 a | 5.95 ± 0.07 b | 59.51 ± 8.3 ab |
| Ob+FGM+Mix2 | 6987 ± 516 ab | 39,232 ± 2280 a | 6.02 ± 0.16 bc | 63.26 ± 9.5 b |
| Ob+FGM+Mix3 | 8027 ± 161 b | 44,138 ± 2016 a | 6.26 ± 0.05 c | 74.91 ± 3.4 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marquez, A.; Russo, M.; Tomei, C.; Castellano, P.; Puglisi, E.; Medina, R.; Gauffin-Cano, P. Effects of Fermented Goat Milk on Adiposity and Gut Microbiota in a Diet-Induced Obesity Murine Model. Fermentation 2024, 10, 155. https://doi.org/10.3390/fermentation10030155
Marquez A, Russo M, Tomei C, Castellano P, Puglisi E, Medina R, Gauffin-Cano P. Effects of Fermented Goat Milk on Adiposity and Gut Microbiota in a Diet-Induced Obesity Murine Model. Fermentation. 2024; 10(3):155. https://doi.org/10.3390/fermentation10030155
Chicago/Turabian StyleMarquez, Antonela, Matías Russo, Carlos Tomei, Patricia Castellano, Edoardo Puglisi, Roxana Medina, and Paola Gauffin-Cano. 2024. "Effects of Fermented Goat Milk on Adiposity and Gut Microbiota in a Diet-Induced Obesity Murine Model" Fermentation 10, no. 3: 155. https://doi.org/10.3390/fermentation10030155
APA StyleMarquez, A., Russo, M., Tomei, C., Castellano, P., Puglisi, E., Medina, R., & Gauffin-Cano, P. (2024). Effects of Fermented Goat Milk on Adiposity and Gut Microbiota in a Diet-Induced Obesity Murine Model. Fermentation, 10(3), 155. https://doi.org/10.3390/fermentation10030155









