Enhanced Anti-Inflammatory and Non-Alcoholic Fatty Liver Disease (NAFLD) Improvement Effects of Bacillus subtilis-Fermented Fagopyrum tataricum Gaertner
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
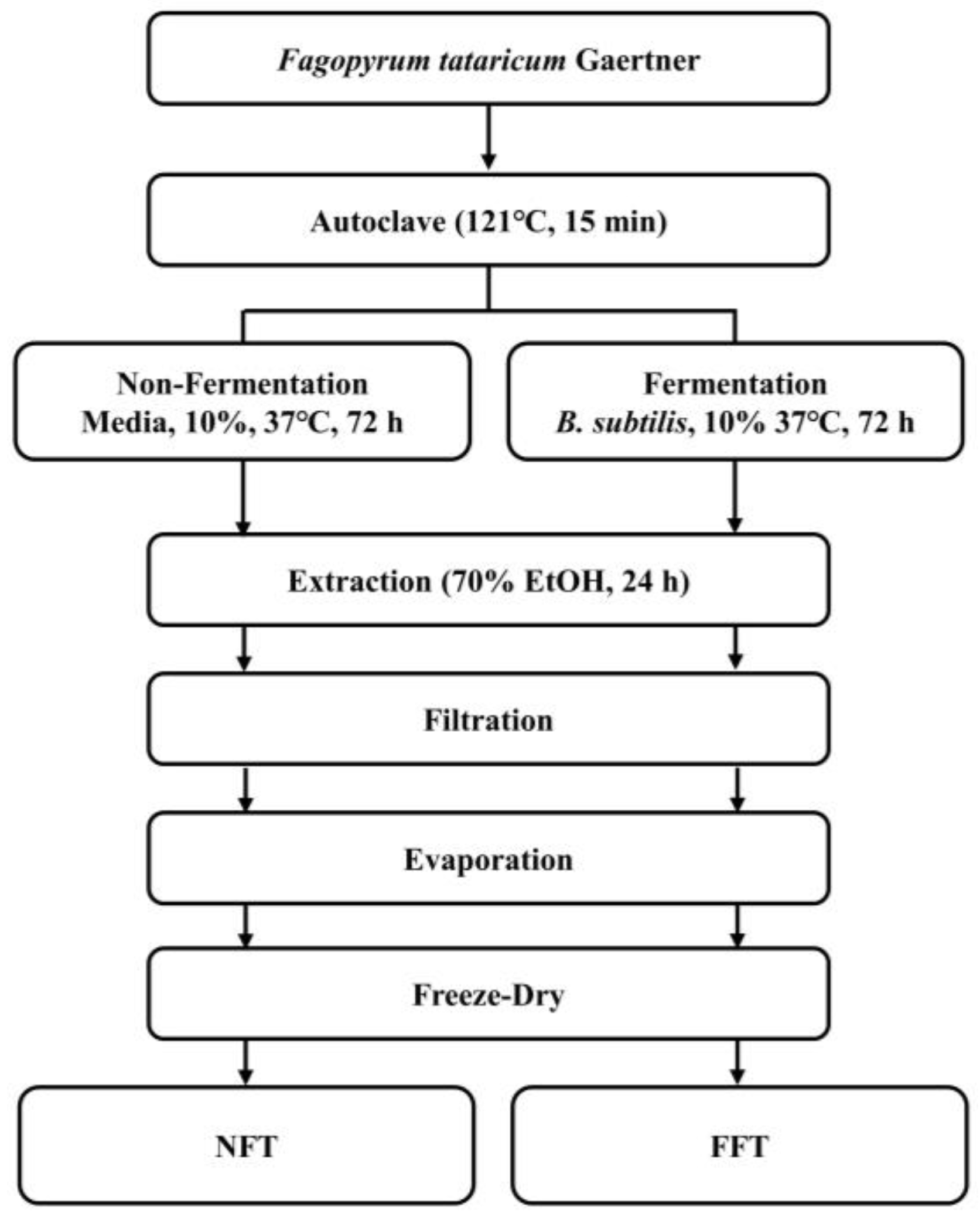
2.2. Preparation of NFT and FFT
2.3. High-Performance Liquid Chromatography (HPLC)
2.4. Cell Lines and Cultivation
2.5. Cell Viability Assessment
2.6. Nitric Oxide (NO) Production Assay
2.7. Reverse Transcription–Polymerase Chain Reaction (RT-PCR)
2.8. Western Blot
2.9. Oil Red O Stain
2.10. Statistical Analysis
3. Results
3.1. Rutin and Quercetin Content in NFT and FFT
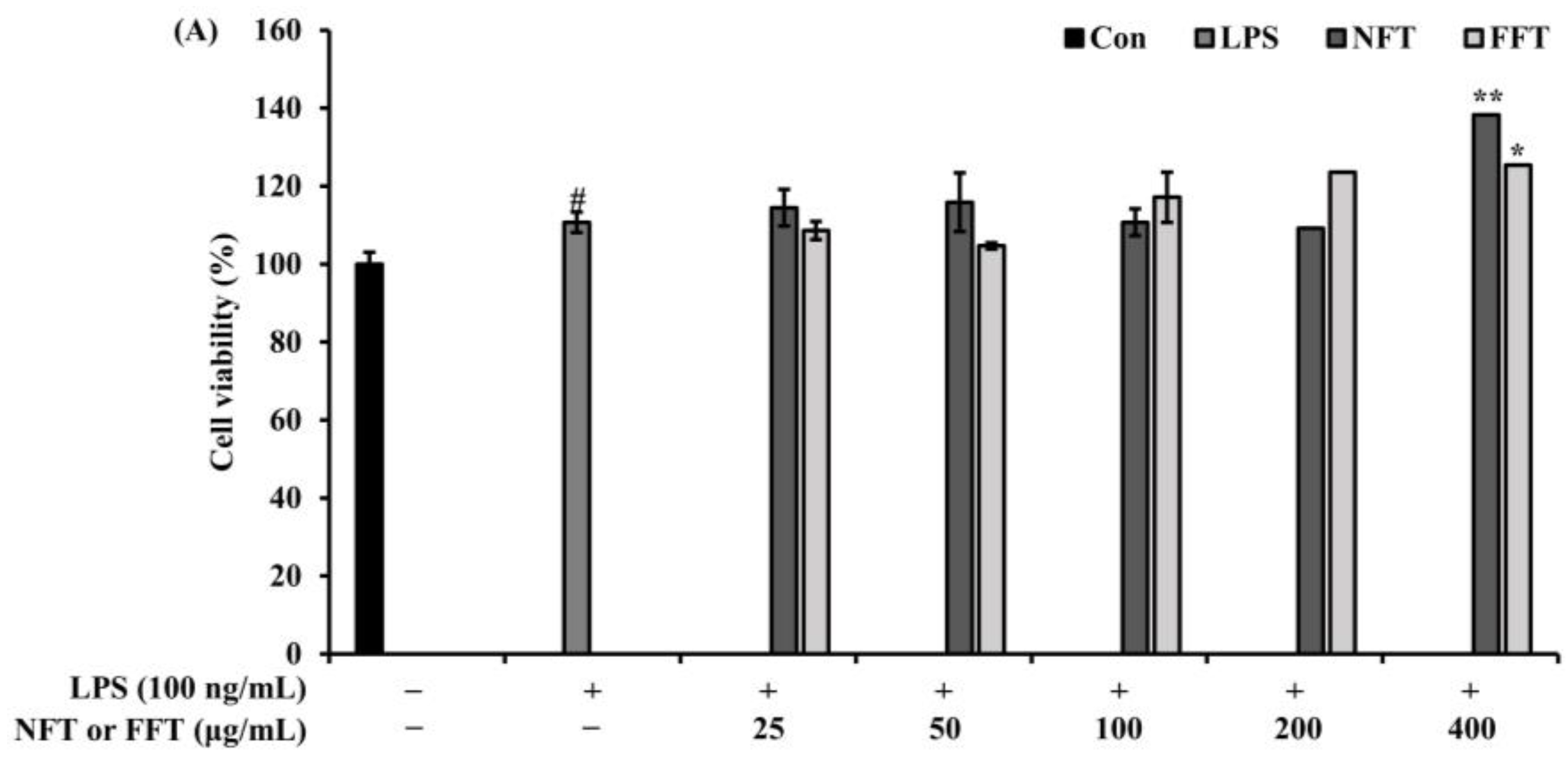
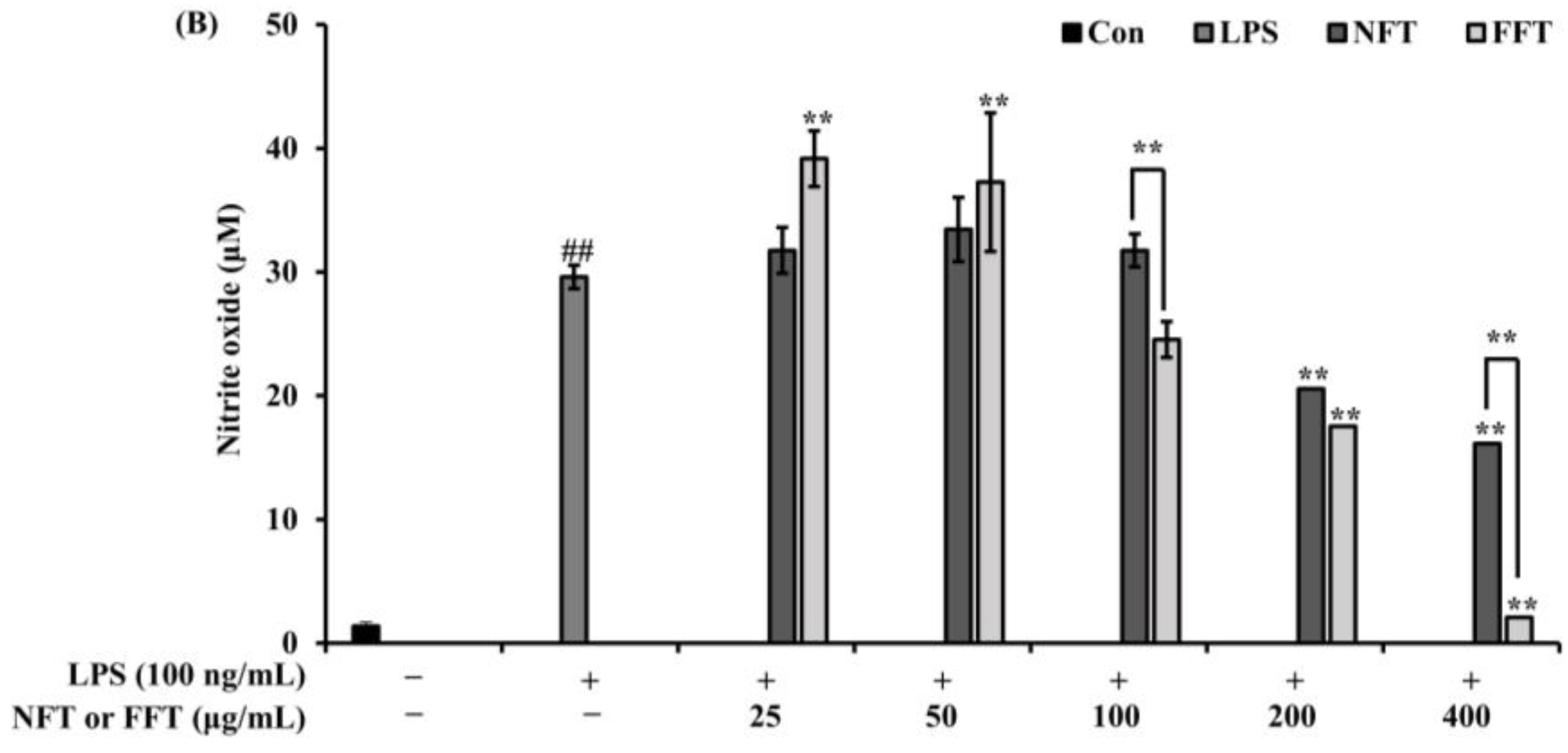
3.2. Anti-Inflammatory Activity by NFT and FFT in LPS-Induced RAW 264.7 Cells
3.3. Effect of FFT on iNOS and COX-2 Protein Expression
3.4. Effect of FFT on Inflammatory Cytokine Expression
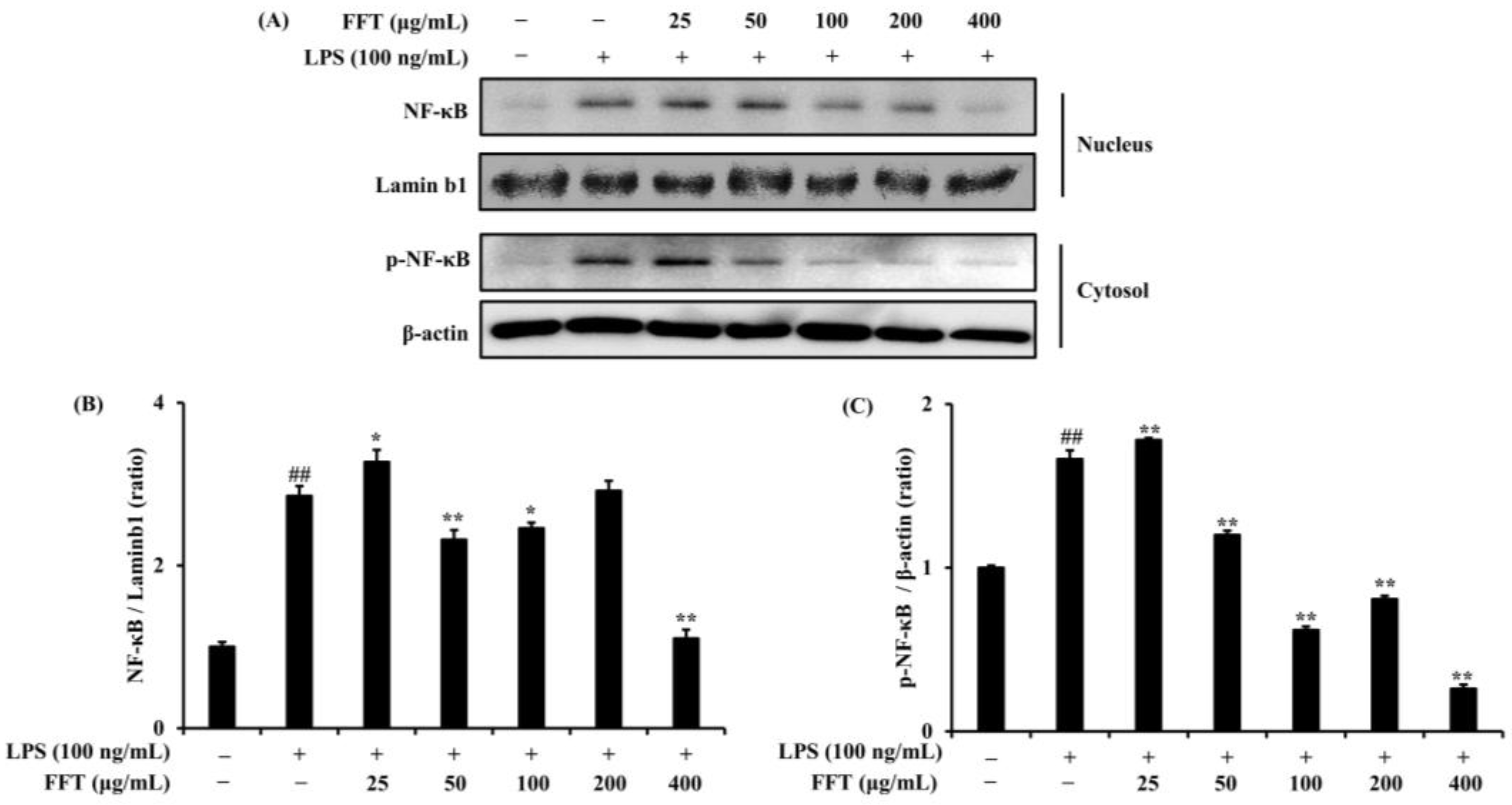
3.5. Effects of FFT on NF-κB Phosphorylation and Nuclear Translocation
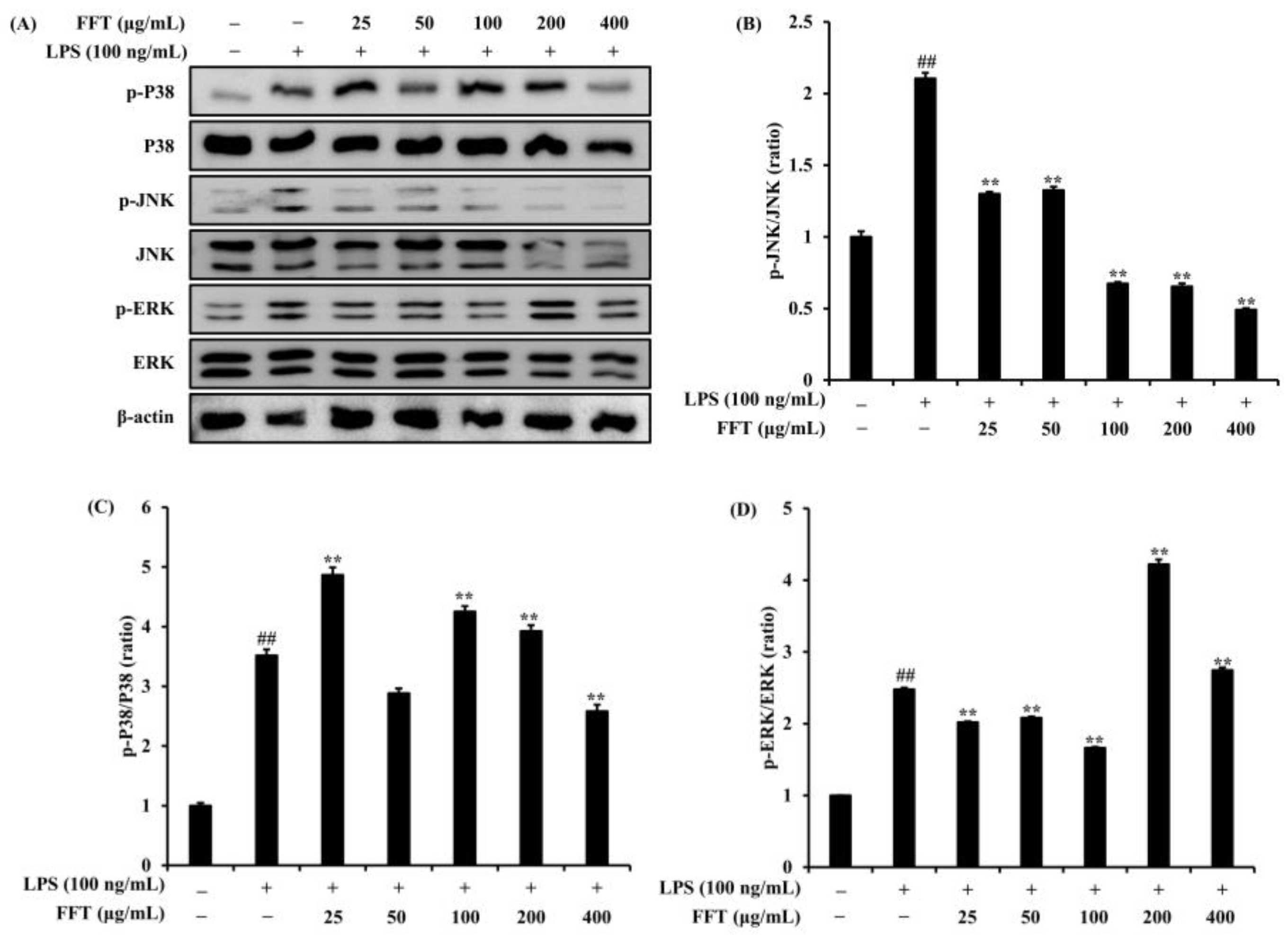
3.6. Effects of FFT on MAPK Signaling Pathway
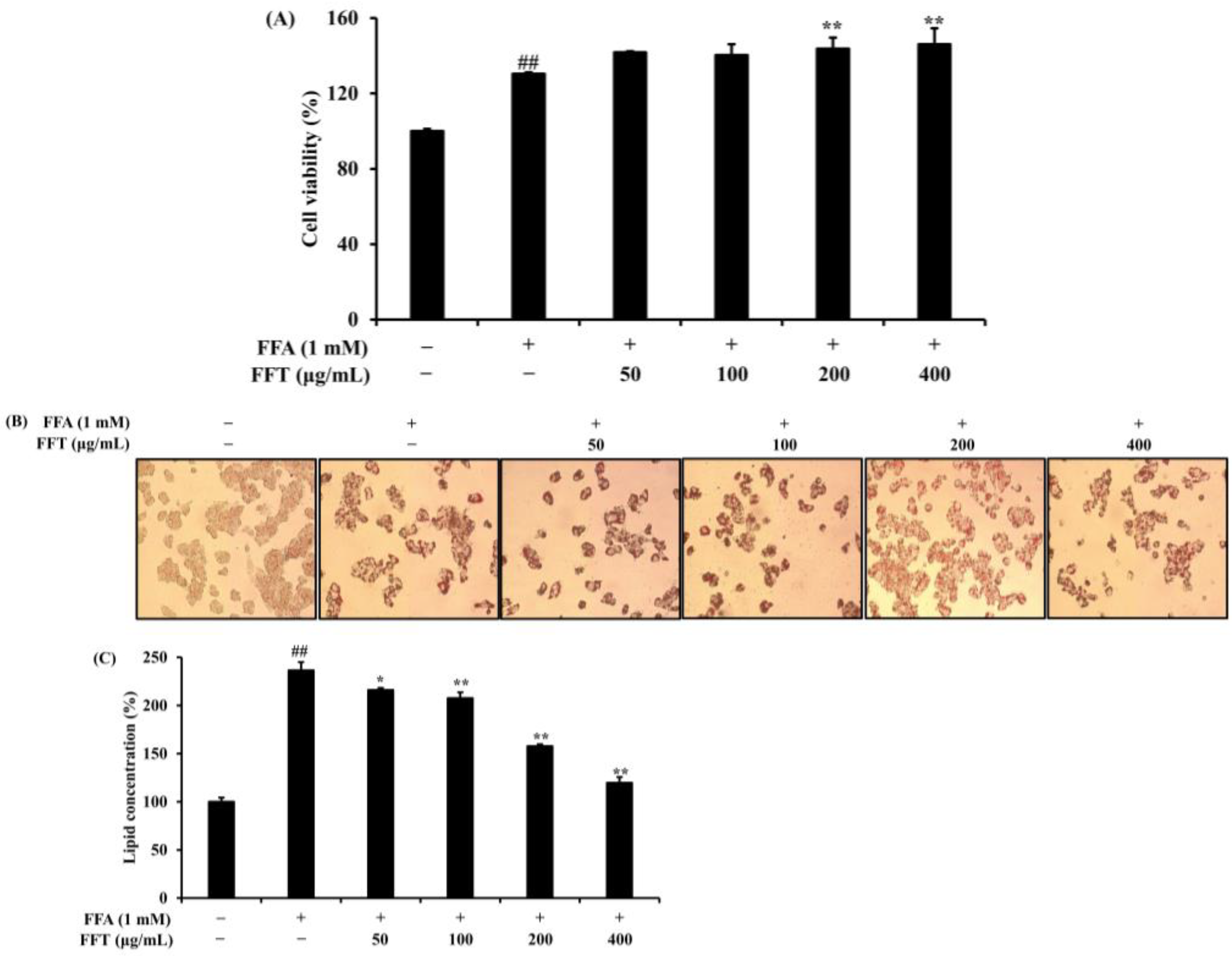
3.7. Effect of FFT on Lipid Accumulation in HepG2 Cells
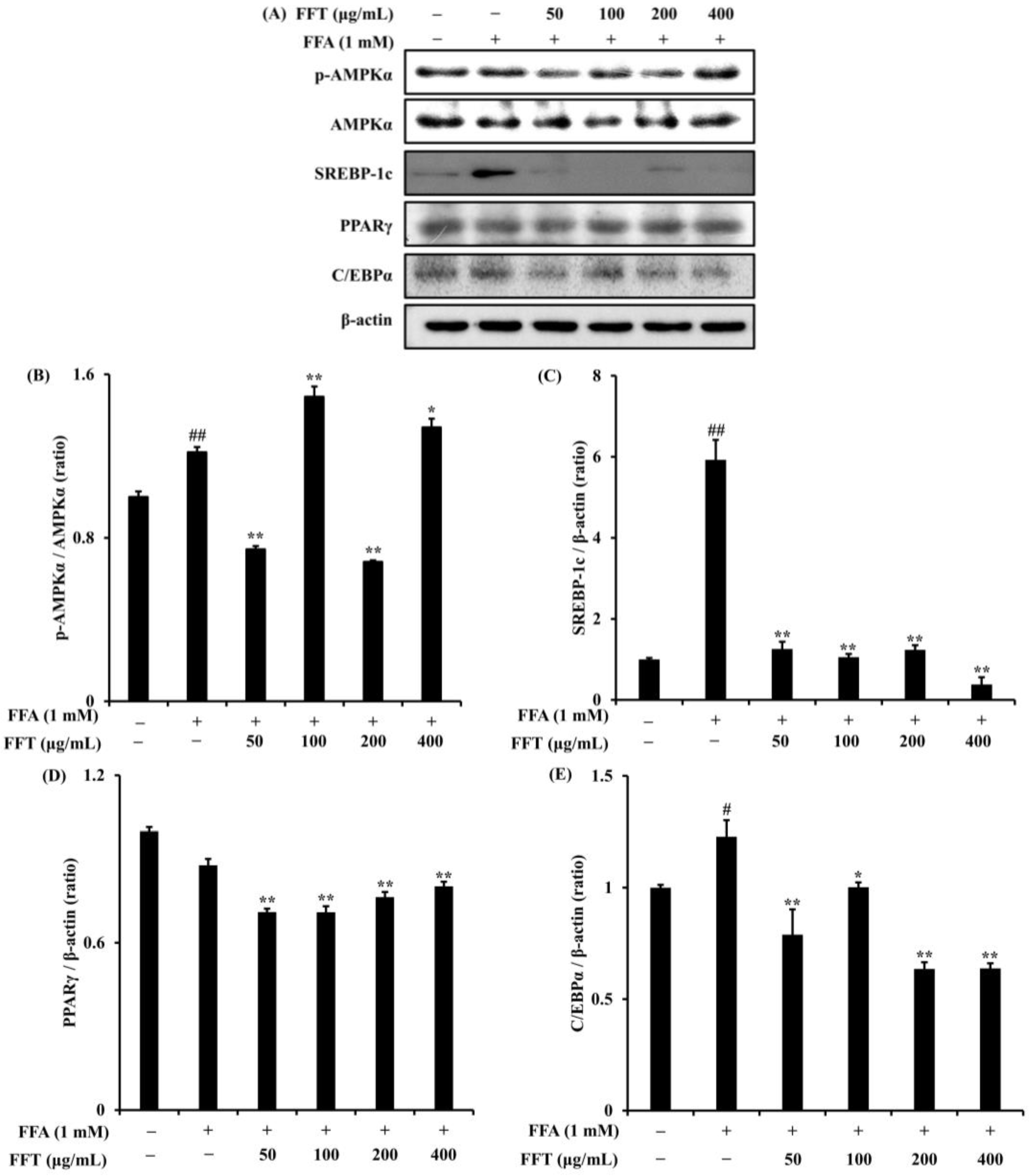
3.8. Regulation of Lipogenesis-Related Protein Expression by FFT
3.9. Effects of FFT on mRNA Expression of Lipogenesis-Related Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brunt, E.M. Nonalcoholic steatohepatitis: Definition and pathology. Semin. Liver Dis. 2001, 21, 3–16. [Google Scholar] [CrossRef]
- Browning, J.D.; Szczepaniak, L.S.; Dobbins, R.; Nuremberg, P.; Horton, J.D.; Cohen, J.C.; Grundy, S.M.; Hobbs, H.H. Prevalence of hepatic steatosis in an urban population in the United States: Impact of ethnicity. Hepatology 2004, 40, 1387–1395. [Google Scholar] [CrossRef]
- Hildebrandt, X.; Ibrahim, M.; Peltzer, N. Cell death and inflammation during obesity: “Know my methods, WAT(son)”. Cell Death Differ. 2023, 30, 279–292. [Google Scholar] [CrossRef]
- Karczewski, J.; Śledzińska, E.; Baturo, A.; Jończyk, I.; Maleszko, A.; Samborski, P.; Begier-Krasińska, B.; Dobrowolska, A. Obesity and inflammation. Eur. Cytokine Netw. 2018, 29, 83–94. [Google Scholar] [CrossRef]
- Park, M.; Yoo, J.H.; Lee, Y.S.; Lee, H.J. Lonicera caerulea Extract Attenuates Non-Alcoholic Fatty Liver Disease in Free Fatty Acid-Induced HepG2 Hepatocytes and in High Fat Diet-Fed Mice. Nutrients 2019, 11, 494. [Google Scholar] [CrossRef]
- Cho, Y.K. Comorbodities and metabolic derangement of NAFLD/NASH. Clin. Mol. Hepatol. 2010, 16, 67–73. [Google Scholar]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- Lee, S.H.; Kwak, C.H.; Lee, S.K.; Ha, S.H.; Park, J.Y.; Chung, T.W.; Ha, K.T.; Suh, S.J.; Chang, Y.C.; Chang, H.W.; et al. Anti-Inflammatory Effect of Ascochlorinin LPS-Stimulated RAW 264.7 Macrophage Cells Is Accompanied with the Down-Regulation of iNOS, COX-2 and Proinflammatory Cytokines Through NF-κB, ERK1/2, and p38 Signaling Pathway. J. Cell Biochem. 2016, 117, 978–987. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Yang, L.; He, J. Plantanone C attenuates LPS-stimulated inflammation by inhibiting NF-κB/iNOS/COX-2/MAPKs/Akt pathways in RAW 264.7 macrophages. Biomed. Pharmacother. 2021, 143, 112104. [Google Scholar] [CrossRef]
- Lee, S.O.; Kim, M.J.; Kim, D.G.; Choi, H.J. Antioxidative Activities of Temperature-stepwise Water Extracts from Inonotus obliquus. J. Korean Soc. Food Sci. Nutr. 2005, 34, 139–147. [Google Scholar]
- Cho, S.H.; Choi, Y.J.; Rho, C.W.; Choi, C.Y.; Kim, D.S.; Cho, S.H. Reactive Oxygen Species and Cytotoxicity of Bamboo (Phyllostachys pubescens) Sap. Korean J. Food Preserv. 2008, 15, 105–110. [Google Scholar]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Lee, B.W. Diabetes Mellitus and Non-Alcoholic Fatty Liver Disease: Diagnosis and Treatment. J. Korean Diabetes 2021, 22, 38–45. [Google Scholar] [CrossRef]
- Sturza, A.; Paucean, A.; Chis, M.S.; Muresan, V.; Vodnar, D.C.; Man, S.M.; Urcan, A.C.; Rusu, I.E.; Fostoc, G.; Muste, S. Influence of buckwheat and buckwheat sprouts flours on the nutritional and textural parameters of wheat buns. Appl. Sci. 2020, 10, 7969. [Google Scholar] [CrossRef]
- Li, Z.; Ren, Z.; Zhao, L.; Chen, L.; Yu, Y.; Wang, D.; Mao, X.; Cao, G.; Zhao, Z.; Yang, H. Unique roles in health promotion of dietary flavonoids through gut microbiota regulation: Current understanding and future perspectives. Food Chem. 2022, 399, 133959. [Google Scholar] [CrossRef]
- Kim, J.E.; Joo, S.I.; Seo, J.H.; Lee, S.P. Antioxidant and α-Glucosidase Inhibitory Effect of Tartary Buckwheat Extract Obtained by the Treatment of Different Solvents and Enzymes. J. Korean Soc. Food Sci. Nutr. 2009, 38, 989–995. [Google Scholar] [CrossRef]
- Yoon, B.R.; Cho, B.J.; Lee, H.K.; Kim, D.J.; Rhee, S.K.; Hong, H.D.; Lee, O.H. Antioxidant and Anti-Adipogenic Effects of Ethanolic Extracts fro Tartary and Common Buckwheats. Korean J. Food Preserv. 2012, 19, 122–130. [Google Scholar] [CrossRef]
- Lee, S.G.; Park, S.Y.; Hwang, I.C.; Kang, H. Antioxidant and Anti-inflammatory Activities of Ethanol Extracts from Fagopyrum tataricum. J. Naturop. 2016, 5, 9–14. [Google Scholar]
- Do, J.R.; Heo, I.S.; Back, S.Y.; Yoon, H.S.; Jo, J.H.; Kim, Y.M.; Kim, K.J.; Kim, S.K. Antihypertensive, Antimicrobial and Antifungal Activities of Buckwheat Hydrolysate. Korean J. Food Sci. Technol. 2016, 38, 268–272. [Google Scholar]
- Han, N.K.; Park, C.M.; Gwon, J.C.; Joung, M.S.; Choi, J.W. Whitening Effect of Fagopyrum tataricum Extract. J. Soc. Cosmet. Sci. Korea 2014, 40, 179–186. [Google Scholar]
- Ji, X.; Han, L.; Liu, F.; Yin, S.; Peng, O.; Wang, M. A mini-review of isolation, chemical properties and bioactivities of polysaccharides from buckwheat (Fagopyrum Mill). Int. J. Biol. Macromol. 2019, 127, 204–209. [Google Scholar] [CrossRef]
- Huda, M.N.; Lu, S.; Jahan, T.; Ding, M.; Jha, R.; Zhang, K.; Zhang, W.; Georgiev, M.I.; Park, S.U.; Zhou, M. Treasure from garden: Bioactive compounds of buckwheat. Food Chem. 2021, 335, 127653. [Google Scholar] [CrossRef]
- Morales, D.; Miguel, M.; Garcés-Rimón, M. Pseudocereals: A novel source of biologically active peptides. Crit. Rev. Food Sci. Nutr. 2020, 61, 1537–1544. [Google Scholar] [CrossRef]
- Lijuan, L.; Yuan, X.; Dezhi, Z.; Huarui, H.; Yingli, W.; Huiyong, F.; Minhui, L. Fagopyrum tataricum (L.) Gaertn.: A Review on its Traditional Uses, Phytochemical and Pharmacology. Food Sci. Technol. Res. 2017, 23, 1–7. [Google Scholar]
- Plumb, J.A. Cell sensitivity assays: The MTT assay. Methods Mol. Med. 1999, 28, 25–30. [Google Scholar]
- Yao, H.R.; Liu, J.; Plumeri, D.; Cao, Y.B.; He, T.; Lin, L.; Li, Y.; Jiang, Y.Y.; Li, J.; Shang, J. Lipotoxicity in HepG2 cells triggered by free fatty acids. Am. J. Transl. Res. 2011, 3, 284–291. [Google Scholar] [PubMed]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Rio, D.C. Reverse Transcription-Polymerase Chain Reaction. Cold Spring Harb. Protoc. 2014, 11, 1207–1216. [Google Scholar] [CrossRef] [PubMed]
- Kim, B. Western blot techniques. In Molecular Profiling; Humana Press: New York, NY, USA, 2017; pp. 133–139. [Google Scholar]
- Kim, H.R.; Jung, D.Y.; Kim, S.; Jung, M.H. Preventive Effect of Poricoic Acid against Nonalcoholic Steatohepatitis. J. Life Sci. 2022, 32, 962–970. [Google Scholar]
- Jeon, J.H.; Park, K.G. Definition, Pathogenesis, and Natural Progress of Non-alcoholic Fatty Liver Disease. J. Korean Diabetes 2014, 15, 65–70. [Google Scholar] [CrossRef]
- Park, C.H.; Choi, Y.R.; Yum, K.J.; Kim, M.J. A Novel Strain of Saccharomyces servazzii, Ceb-kc-011 from Young Radish Kimchi: Optimization of the Growth Condition and Inhibition of α-Glucosidase by Bioconverted Rhynchosia nulubilis Using Ceb-kc-011. J. Korean Soc. Food. Sci. Nutr. 2020, 49, 236–243. [Google Scholar] [CrossRef]
- Park, K.Y. Increased Health Functionality of Fermented Foods. Food Ind. Nutr. 2012, 17, 1–8. [Google Scholar]
- Shin, D.H. Globalization Trends and Prospect of Korean Traditional Fermented Foods. Food Sci. Ind. 2010, 43, 69–82. [Google Scholar]
- Hur, S.J.; Lee, S.Y.; Kim, Y.C.; Choi, I.; Kim, G.B. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, M.; Zheng, J.; Gänzle, M.G. Bacillus species in food fermentations: An underappreciated group of organisms for safe use in food fermentations. Curr. Opin. Food Sci. 2023, 50, 101007. [Google Scholar] [CrossRef]
- Do, E.S.; Chang, J.P.; Kil, K.J.; Choi, M.S.; Yang, J.K.; Yun, C.W.; Jeong, S.M.; Jung, Y.H.; Lee, G.H. Antioxidative activity and cytotoxicity of fermented Allium victorialis L. extract. Korean J. Plant Res. 2011, 24, 30–39. [Google Scholar]
- An, S.; Lee, C.M.; Halie, D.H.; Yun, S.J. Inactivation of Rutin Degrading Enzymes in Buckwheat Groats by Roasting and Steaming. Korean J. Med. Crop. Sci. 2019, 27, 108–114. [Google Scholar] [CrossRef]
- Jang, K.S.; Lee, S.J.; Kim, S.J.; Han, M.S. Changes of Rutin and Quercetin in Commercial Gochujang Prepared with Buckwheat Flour during Fermentation. J. Korean Soc. Food Sci. Nutr. 2005, 34, 509–512. [Google Scholar]
- Spiller, F.; Formiga, R.O.; Coimbra, F.S.J.; Alves-Filho, J.C.; Cunha, T.M.; Cunha, F.Q. Targeting nitric oxide as a key modulator of sepsis, arthritis and pain. Nitric Oxide 2019, 89, 32–40. [Google Scholar] [CrossRef]
- Kim, J.M.; Jeon, Y.H.; Jeong, Y.J.; Yoon, K.Y. Comparison of bioactive composition, antioxidant activity, and nitric oxide inhibition effect of enzyme-treated and commercial noni juice. Korean J. Food Sci. Technol. 2020, 52, 75–80. [Google Scholar]
- Zhang, X.; Song, Y.; Ci, X.; An, N.; Ju, Y.; Li, H.; Wang, X.; Han, C.; Cui, J.; Deng, X. Ivermectin inhibits LPS-induced production of inflammatory cytokines and improves LPS-induced survival in mice. Inflamm. Res. 2008, 57, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Zhang, X.; Edwards, J.P.; Mosser, D.M. NF-kappaB1 (p50) homodimers differentially regulate pro- and anti-inflammatory cytokines in macrophages. J. Biol. Chem. 2006, 281, 26041–26050. [Google Scholar] [CrossRef] [PubMed]
- Magnani, M.; Crinelli, R.; Bianchi, M.; Antonelli, A. The ubiquitin-dependent proteolytic system and other potential targets for the modulation of nuclear factor-kB (NF-kB). Curr. Drug Targets 2000, 1, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Weng, J.; Yu, L.; Huang, Q.; Jiang, Y.; Guo, X. Role of TLR4-p38 MAPK-Hsp27 signal pathway in LPS-induced pulmonary epithelial hyperpermeability. BMC Pulm. Med. 2018, 18, 178. [Google Scholar] [CrossRef]
- Ko, W.M.; Sohn, J.H.; Jang, J.H.; Ahn, J.S.; Kang, D.G.; Lee, H.S.; Kim, J.S.; Kim, Y.C.; Oh, H.C. Inhibitory effects of alternaramide on inflammatory mediator expression through TLR4-MyD88-mediated inhibition of NF-кB and MAPK pathway signaling in lipopolysaccharide-stimulated RAW264.7 and BV2 cells. Chem. Biol. Interact. 2016, 244, 16–26. [Google Scholar] [CrossRef]
- Choi, H.C. Effects of AMP-activated Protein Kinase Activating Compounds and Its Mechanism. Yeungnam Univ. J. Med. 2012, 29, 77–82. [Google Scholar] [CrossRef]
- Park, D.B. Non-Alcoholic Fatty Liver Disease and Metabolic Syndrome. Pediatr. Endocrinol. 2010, 15, 77–84. [Google Scholar]
- Lee, H.J.; Park, S.E.; Kim, S. Cudrania tricuspidata Fruit Extract Ameliorates Free Fatty Acid-induced Lipid Accumulation in HepG2 Cells. Korean J. Life Sci. 2019, 29, 1144–1151. [Google Scholar]
- Gauthier, M.S.; Miyoshi, H.; Souza, S.C.; Cacicedo, J.M.; Saha, A.K.; Greenberg, A.S.; Ruderman, N.B. AMP-activated protein kinase is activated as a consequence of lipolysis in the adipocyte: Potential mechanism and physiological relevance. J. Biol. Chem. 2008, 283, 16514–16524. [Google Scholar] [CrossRef]
- Lee, J.H. Special Review: Peroxisome Proliferator-Activated Receptor (PPAR) α/γ Agonist. Korean J. Med. 2014, 87, 19–25. [Google Scholar] [CrossRef]
- Tanigawa, K.; Luo, Y.; Kawashima, A.; Kiriya, M.; Nakamura, Y.; Karasawa, K.; Suzuki, K. Essential Roles of PPARs in Lipid Metabolism during Mycobacterial Infection. Int. J. Mol. Sci. 2021, 22, 7597. [Google Scholar] [CrossRef] [PubMed]




| Instrument | Conditions | |||
|---|---|---|---|---|
| Flow rate | 1.0 mL/min | |||
| Injection volume | 10 μL | |||
| Detector | Diode Array Detector (254 nm) | |||
| Mobile phase (Gradient) | Time (min) | Quercetin | 0.1% Phosphoric acid in DW | Acetonitrile |
| Rutin | Methanol | 0.5% Phosphoric acid in DW | ||
| 0 | 30 | 70 | ||
| 5 | 35 | 65 | ||
| 10 | 40 | 60 | ||
| 15 | 50 | 50 | ||
| 18 | 30 | 70 | ||
| 25 | Stop | |||
| Gene | Origin | Sequence | |
|---|---|---|---|
| iNOS | mouse | Forward | 5′-CTTGCAAGTCCAAGTCTTGC-3′ |
| Reverse | 5′-GTATGTGTCTGCAGATGTGCTG-3′ | ||
| COX-2 | mouse | Forward | 5′-ACATCCCTGAGAACCTGCAGT-3′ |
| Reverse | 5′-CCAGGAGGATGGAGTTGTTGT-3′ | ||
| IL-1β | mouse | Forward | 5′-CATATGAGCTGAAAGCTCTCCA-3′ |
| Reverse | 5′-GACACAGATTCCATGGTGAAGTC-3′ | ||
| IL-6 | mouse | Forward | 5′-GGAGGCTTAAITACACATGTT-3′ |
| Reverse | 5′-TGATTCAAGATGAATTGGAT-3′ | ||
| TNF-α | mouse | Forward | 5′-TTCGAGTGACAAGCCTGTAGC-3′ |
| Reverse | 5′-AGATTGACCTCAGCGCTGAGT-3′ | ||
| GAPDH | mouse | Forward | 5′-CCAGTATGACTCCACTCACG-3′ |
| Reverse | 5′-CCTTCCACAATGCCAAGTT-3′ | ||
| SREBP-1 c | Human | Forward | 5′-CAGTGGAGGGAACACAGACG- 3′ |
| Reverse | 5′-AAAGACTGGGCTGTCAGGCT- 3′ | ||
| PPARγ | Human | Forward | 5′-CAGGAGCAGAGCAAAGAGGTG-3′ |
| Reverse | 5′-CAAACTCAAACTTGGGCTCCA-3′ | ||
| ACC1 | Human | Forward | 5′-GGAACAGTGTGCGGTGAAAC- 3′ |
| Reverse | 5′-TCACTAGTGATCCGAGCAGC-3′ | ||
| GAPDH | Human | Forward | 5′-CGGAGTCAACGGATTTGGTCGTAT-3′ |
| Reverse | 5′-AGCCTTCTCCATGGTGGTGAAGAC-3′ | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, C.-H.; Kang, H.; Lee, S.-G. Enhanced Anti-Inflammatory and Non-Alcoholic Fatty Liver Disease (NAFLD) Improvement Effects of Bacillus subtilis-Fermented Fagopyrum tataricum Gaertner. Fermentation 2024, 10, 116. https://doi.org/10.3390/fermentation10030116
Park C-H, Kang H, Lee S-G. Enhanced Anti-Inflammatory and Non-Alcoholic Fatty Liver Disease (NAFLD) Improvement Effects of Bacillus subtilis-Fermented Fagopyrum tataricum Gaertner. Fermentation. 2024; 10(3):116. https://doi.org/10.3390/fermentation10030116
Chicago/Turabian StylePark, Chan-Hwi, Hyun Kang, and Sung-Gyu Lee. 2024. "Enhanced Anti-Inflammatory and Non-Alcoholic Fatty Liver Disease (NAFLD) Improvement Effects of Bacillus subtilis-Fermented Fagopyrum tataricum Gaertner" Fermentation 10, no. 3: 116. https://doi.org/10.3390/fermentation10030116
APA StylePark, C.-H., Kang, H., & Lee, S.-G. (2024). Enhanced Anti-Inflammatory and Non-Alcoholic Fatty Liver Disease (NAFLD) Improvement Effects of Bacillus subtilis-Fermented Fagopyrum tataricum Gaertner. Fermentation, 10(3), 116. https://doi.org/10.3390/fermentation10030116






