Optimization of Fermentation Process of Wheat Germ Protein by Aspergillus niger and Analysis of Antioxidant Activity of Peptide
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Equipment
2.2. Preparation of Wheat Germ Protein Fermentation Medium
2.3. Activation of the Strain and Preparation of Seed Solution
2.4. Screening of Biomodified Strains
2.5. Single-Factor Experiments for Optimization of Wheat Germ Protein Fermentation Conditions
2.6. Response Surface Method for Optimization of Wheat Germ Protein Fermentation Conditions
2.7. Preparation of Peptides of Different Molecular Weights
2.8. Analysis of Antioxidant Effects of Peptides with Different Molecular Weights
2.8.1. DPPH Free Radical Scavenging Capacity
2.8.2. ·OH Free Radical Scavenging Capacity
2.8.3. ABTS Free Radical Scavenging Capacity
2.9. Statistical Analysis
3. Results
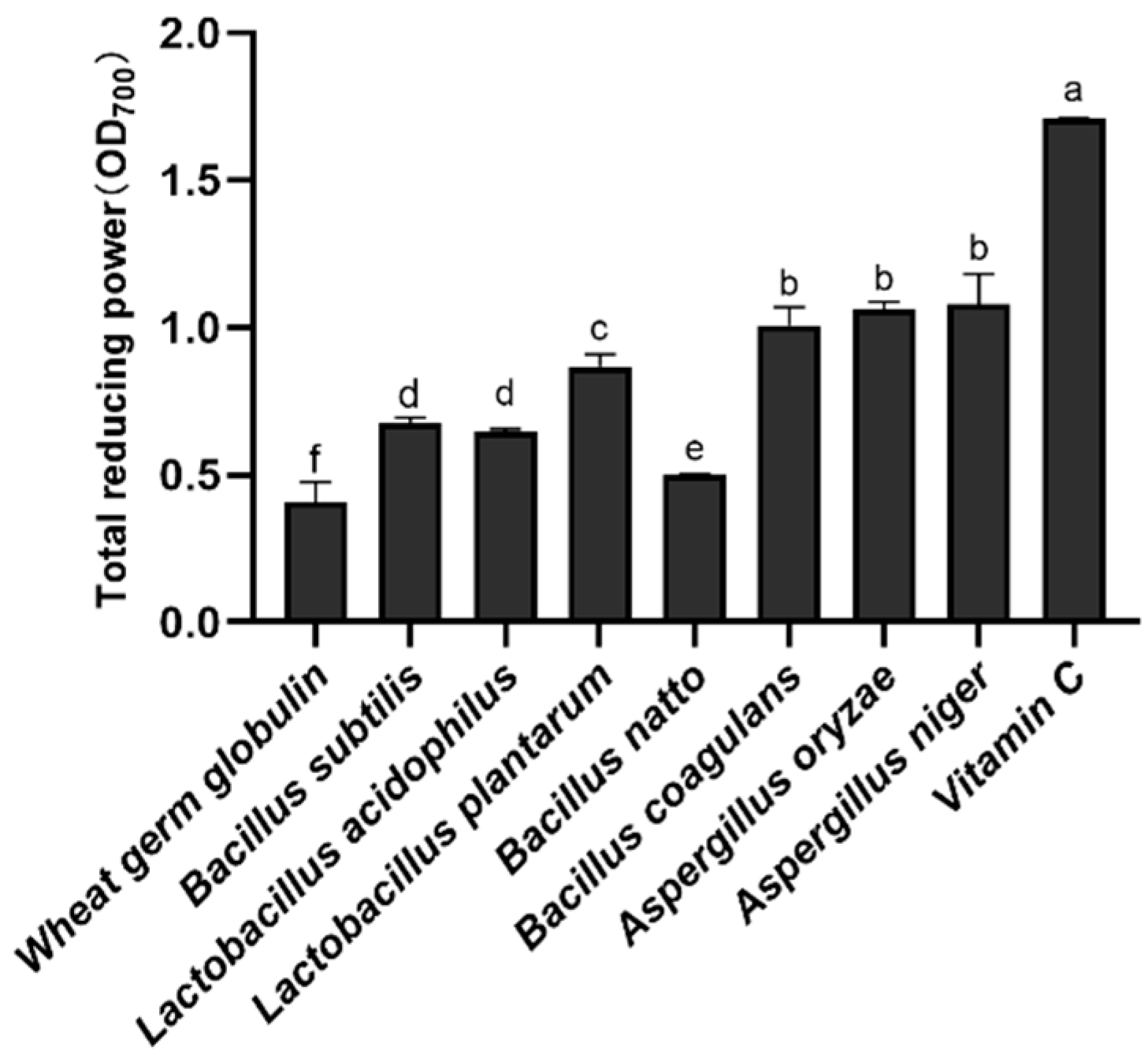
3.1. Comparison of the Reducing Power of Different Strains of Fermentation
3.2. Single-Factor Experiments for Optimization of Wheat Germ Protein Fermentation Conditions
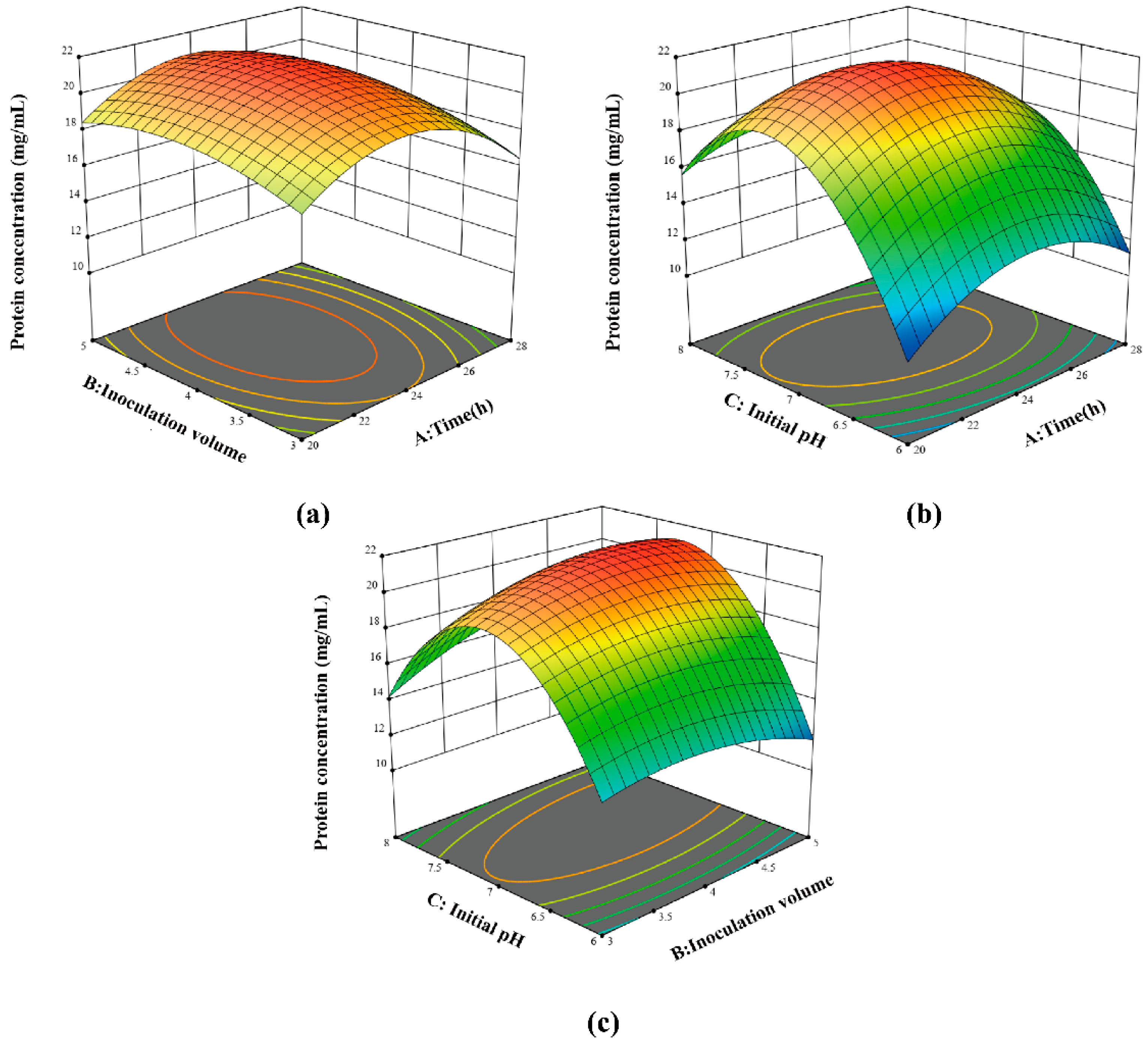
3.3. Response Surface Method for Optimization of Wheat Germ Protein Fermentation Conditions
3.4. Analysis of Antioxidant Effects of Peptides with Different Molecular Weights
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ge, Y.; Sun, A.; Ni, Y.; Cai, T. Some nutritional and functional properties of defatted wheat germ protein. J. Agric. Food Chem. 2000, 48, 6215–6218. [Google Scholar] [CrossRef] [PubMed]
- Brandolini, A.; Hidalgo, A. Wheat germ: Not only a by-product. Int. J. Food Sci. Nutr. 2011, 63, 71–74. [Google Scholar] [CrossRef]
- Gili, R.D.; Irigoyen, R.M.T.; Penci, M.C.; Giner, S.A.; Ribotta, P.D. Physical characterization and fluidization design parameters of wheat germ. J. Food Eng. 2017, 212, 29–37. [Google Scholar] [CrossRef]
- Zhu, K.-X.; Zhou, H.-M.; Qian, H.-F. Proteins Extracted from Defatted Wheat Germ: Nutritional and Structural Properties. Cereal Chem. 2006, 83, 69–75. [Google Scholar] [CrossRef]
- Rocha-Garza, A.E.; Zayas, J.F. Effect of wheat germ protein flour on the quality characteristics of beef patties cooked on a griddle. J. Food Process. Preserv. 1995, 19, 341–360. [Google Scholar] [CrossRef]
- Arshad, M.U.; Anjum, F.M.; Zahoor, T. Nutritional assessment of cookies supplemented with defatted wheat germ. Food Chem. 2007, 102, 123–128. [Google Scholar] [CrossRef]
- Feng, Y.-X.; Ruan, G.-R.; Jin, F.; Xu, J.; Wang, F.-J. Purification, identification, and synthesis of five novel antioxidant peptides from Chinese chestnut (Castanea mollissima Blume) protein hydrolysates. LWT 2018, 92, 40–46. [Google Scholar] [CrossRef]
- Ayim, I.; Ma, H.; Ali, Z.; Alenyorege, E.A.; Donkor, P.O. Preparation of antioxidant peptides from tea (Camellia sinensis L.) residue. J. Food Meas. Charact. 2018, 12, 2128–2137. [Google Scholar] [CrossRef]
- Power, O.; Jakeman, P.; Fitzgerald, R.J. Antioxidative peptides: Enzymatic production, in vitro and in vivo antioxidant activity and potential applications of milk-derived antioxidative peptides. Amino Acids 2013, 44, 797–820. [Google Scholar] [CrossRef]
- Li, N.; Wen, L.; Wang, F.; Wang, T.; Li, T.; Qiao, M.; Song, L.; Bukyei, E.; Huang, X. Mechanism of mitigating effect of wheat germ peptides on lead-induced oxidative damage in PC12 cells. Ecotoxicol. Environ. Saf. 2022, 246, 114190. [Google Scholar] [CrossRef]
- Zheng, X.-Q.; Li, L.-T.; Liu, X.-L.; Wang, X.-J.; Lin, J.; Li, D. Production of hydrolysate with antioxidative activity by enzymatic hydrolysis of extruded corn gluten. Appl. Microbiol. Biotechnol. 2006, 73, 763–770. [Google Scholar] [CrossRef]
- Zhang, J.; Wen, C.; Li, C.; Duan, Y.; Zhang, H.; Ma, H. Antioxidant Peptide Fractions Isolated from Wheat Germ Protein with Subcritical Water Extraction and Its Transport Across Caco-2 Cells. J. Food Sci. 2019, 84, 2139–2146. [Google Scholar] [CrossRef]
- Wang, F.; Weng, Z.; Lyu, Y.; Bao, Y.; Liu, J.; Zhang, Y.; Sui, X.; Fang, Y.; Tang, X.; Shen, X. Wheat germ-derived peptide ADWGGPLPH abolishes high glucose-induced oxidative stress via modulation of the PKCζ/AMPK/NOX4 pathway. Food Funct. 2020, 11, 6843–6854. [Google Scholar] [CrossRef]
- Chen, S.; Lin, D.; Gao, Y.; Cao, X.; Shen, X. A novel antioxidant peptide derived from wheat germ prevents high glucose-induced oxidative stress in vascular smooth muscle cells in vitro. Food Funct. 2016, 8, 142–150. [Google Scholar] [CrossRef]
- Karami, Z.; Peighambardoust, S.H.; Hesari, J.; Akbari-Adergani, B. Response Surface Methodology to Optimize Hydrolysis Parameters in Production of Antioxidant Peptides from Wheat Germ Protein by Alcalase Digestion and Identification of Antioxidant Peptides by LC-MS/MS. J. Agric. Sci. Technol. 2019, 21, 829–844. [Google Scholar]
- Ali, N.M.; Yeap, S.-K.; Yusof, H.M.; Beh, B.-K.; Ho, W.-Y.; Koh, S.-P.; Abdullah, M.P.; Alitheen, N.B.; Long, K. Comparison of free amino acids, antioxidants, soluble phenolic acids, cytotoxicity and immunomodulation of fermented mung bean and soybean. J. Sci. Food Agric. 2015, 96, 1648–1658. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Chen, Z.; Shao, J.; Wang, C.; Zhan, C. Effect of fermentation on the peptide content, phenolics and antioxidant activity of defatted wheat germ. Food Biosci. 2017, 20, 141–148. [Google Scholar] [CrossRef]
- Song, Y.; Jeong, H.-Y.; Lee, J.-K.; Choi, Y.-S.; Kim, D.-O.; Jang, D.; Park, C.-S.; Maeng, S.; Kang, H. Enzyme Treatment Alters the Anti-Inflammatory Activity of the Water Extract of Wheat Germ In Vitro and In Vivo. Nutrients 2019, 11, 2490. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Mueller, T.; Coda, R.; Reipsch, F.; Nionelli, L.; Curiel, J.A.; Gobbetti, M. Synthesis of 2-methoxy benzoquinone and 2,6-dimethoxybenzoquinone by selected lactic acid bacteria during sourdough fermentation of wheat germ. Microb. Cell Factories 2013, 12, 105. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Kim, H.O.; Kim, G.-N.; Song, J.-H. Anti-Oxidant and Anti-Adipogenic Effects of Ethanol Extracts from Wheat Germ and Wheat Germ Fermented with Aspergillus oryzae. Prev. Nutr. Food Sci. 2015, 20, 29–37. [Google Scholar] [CrossRef][Green Version]
- Zhao, Y.; Liao, A.-M.; Liu, N.; Huang, J.-H.; Lv, X.; Yang, C.-R.; Chen, W.-J.; Hou, Y.-C.; Ma, L.-J.; Hui, M. Potential anti-aging effects of fermented wheat germ in aging mice. Food Biosci. 2021, 42, 101182. [Google Scholar] [CrossRef]
- Iyer, A.; Brown, L. Fermented Wheat Germ Extract (Avemar) in the Treatment of Cardiac Remodeling and Metabolic Symptoms in Rats. Evid. -Based Complement. Altern. Med. 2011, 2011, 508957. [Google Scholar] [CrossRef]
- Starzyńska-Janiszewska, A.; Stodolak, B.; Mickowska, B.; Socha, R. Fermentation with edible Rhizopus strains as a beneficial alternative method in wheat germ cake processing. J. Cereal Sci. 2021, 102, 103309. [Google Scholar] [CrossRef]
- Niu, L.-Y.; Jiang, S.-T.; Pan, L.-J. Preparation and evaluation of antioxidant activities of peptides obtained from defatted wheat germ by fermentation. J. Food Sci. Technol. 2013, 50, 53–61. [Google Scholar] [CrossRef]
- Wu, J.; Cheng, Y.; Dong, Y. Antioxidant Activity of Lactobacillus plantarum DY-1 Fermented Wheat Germ Extract and Its Influence on Lipid Oxidation and Texture Properties of Emulsified Sausages. J. Food Qual. 2020, 2020, 8885886. [Google Scholar] [CrossRef]
- Ngoh, Y.-Y.; Gan, C.-Y. Enzyme-assisted extraction and identification of antioxidative and α-amylase inhibitory peptides from Pinto beans (Phaseolus vulgaris cv. Pinto). Food Chem. 2016, 190, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Siddhuraju, P.; Mohan, P.S.; Becker, K. Studies on the antioxidant activity of Indian Laburnum (Cassia fistula L.): A preliminary assessment of crude extracts from stem bark, leaves, flowers and fruit pulp. Food Chem. 2002, 79, 61–67. [Google Scholar] [CrossRef]
- Dorman, H.J.D.; Koşar, M.; Kahlos, K.; Holm, Y.; Hiltunen, R. Antioxidant Properties and Composition of Aqueous Extracts from Mentha Species, Hybrids, Varieties, and Cultivars. J. Agric. Food Chem. 2003, 51, 4563–4569. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, J.; Ye, J.; Vanga, S.K.; Raghavan, V. Influence of high-intensity ultrasound on bioactive compounds of strawberry juice: Profiles of ascorbic acid, phenolics, antioxidant activity and microstructure. Food Control 2019, 96, 128–136. [Google Scholar] [CrossRef]
- Yang, Q.; Cai, X.; Yan, A.; Tian, Y.; Du, M.; Wang, S. A specific antioxidant peptide: Its properties in controlling oxidation and possible action mechanism. Food Chem. 2020, 327, 126984. [Google Scholar] [CrossRef]
- Chun, S.-S.; Vattem, D.A.; Lin, Y.-T.; Shetty, K. Phenolic antioxidants from clonal oregano (Origanum vulgare) with antimicrobial activity against Helicobacter pylori. Process. Biochem. 2005, 40, 809–816. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Chen, H.-M.; Muramoto, K.; Yamauchi, F.; Fujimoto, K.; Nokihara, K. Antioxidative Properties of Histidine-Containing Peptides Designed from Peptide Fragments Found in the Digests of a Soybean Protein. J. Agric. Food Chem. 1998, 46, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Ghassem, M.; Arihara, K.; Mohammadi, S.; Sani, N.A.; Babji, A.S. Identification of two novel antioxidant peptides from edible bird’s nest (Aerodramus fuciphagus) protein hydrolysates. Food Funct. 2017, 8, 2046–2052. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Ci, A.-T.; Wang, H.; Zhang, Y.-Y.; Zhang, J.-G.; Thakur, K.; Wei, Z.-J. Identification and hydrolysis kinetic of a novel antioxidant peptide from pecan meal using Alcalase. Food Chem. 2018, 261, 301–310. [Google Scholar] [CrossRef]
- Liu, D.; Chen, X.; Huang, J.; Huang, M.; Zhou, G. Generation of bioactive peptides from duck meat during post-mortem aging. Food Chem. 2017, 237, 408–415. [Google Scholar] [CrossRef]
- Samaranayaka, A.G.P.; Li-Chan, E.C.Y. Food-derived peptidic antioxidants: A review of their production, assessment, and potential applications. J. Funct. Foods 2011, 3, 229–254. [Google Scholar] [CrossRef]
- Teixeira, B.; Pires, C.; Nunes, M.L.; Batista, I. Effect of in vitro gastrointestinal digestion on the antioxidant activity of protein hydrolysates prepared from Cape hake by-products. Int. J. Food Sci. Technol. 2016, 51, 2528–2536. [Google Scholar] [CrossRef]
- Nwachukwu, I.D.; Aluko, R.E. Structural and functional properties of food protein-derived antioxidant peptides. J. Food Biochem. 2019, 43, e12761. [Google Scholar] [CrossRef]
- César, A.P.C.; Lopes, F.E.S.; Azevedo, F.F.N.; Pinto, Y.O.; Andrade, C.R.; Mesquita, F.P.; Silva, G.O.; Freitas, C.D.T.; Souza, P.F.N. Antioxidant peptides from plants: A review. Phytochem. Rev. 2023, 22, 1–10. [Google Scholar] [CrossRef]
- Yu, R.; Liu, J.; Wang, Y.; Wang, H.; Zhang, H. Aspergillus niger as a Secondary Metabolite Factory. Front. Chem. 2021, 9, 701022. [Google Scholar] [CrossRef] [PubMed]
- Duarte, J.C.; Costa-Ferreira, M. Aspergilli and lignocellulosics: Enzymology and biotechnological applications. FEMS Microbiol. Rev. 1994, 13, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Chaijan, M.; Rodsamai, T.; Charoenlappanit, S.; Roytrakul, S.; Panya, A.; Phonsatta, N.; Cheong, L.-Z.; Panpipat, W. Charac-terization of Antioxidant Peptides from Thai Traditional Semi-Dried Fermented Catfish. Fermentation 2021, 7, 262. [Google Scholar] [CrossRef]
- Liao, A.M.; Li, X.X.; Gu, Z.; He, J.Y.; Hou, Y.; Pan, L.; Zheng, S.N.; Zhang, J.; Peng, P.; Hui, M.; et al. Preparation and identification of an antioxidant peptide from wheat embryo albumin and characterization of its Maillard reaction products. J. Food Sci. 2022, 87, 2549–2562. [Google Scholar] [CrossRef]
- Tian, S.; Meng, F.; Du, K.; Chen, Y. Biological activity evaluation and identification of different molecular weight peptides from wheat germ albumin. LWT 2023, 189, 115556. [Google Scholar] [CrossRef]
- Pan, M.; Liu, K.; Yang, J.; Liu, S.; Wang, S.; Wang, S. Advances on Food-Derived Peptidic Antioxidants—A Review. Antioxidants 2020, 9, 799. [Google Scholar] [CrossRef]


| Level | A Fermentation Time/(h) | B Inoculation Volume/% | C Initial pH |
|---|---|---|---|
| −1 | 20 | 3 | 6 |
| 0 | 24 | 4 | 7 |
| 1 | 28 | 5 | 8 |
| Level | A Fermentation Time/(h) | B Inoculation Volume/% | C Initial pH | Protein Concentration (mg/mL) |
|---|---|---|---|---|
| 1 | 20 | 3 | 7 | 17.4849 |
| 2 | 28 | 3 | 7 | 15.9335 |
| 3 | 20 | 5 | 7 | 19.009 |
| 4 | 28 | 5 | 7 | 17.7434 |
| 5 | 20 | 4 | 6 | 10.15 |
| 6 | 28 | 4 | 6 | 11.5516 |
| 7 | 20 | 4 | 8 | 15.3892 |
| 8 | 28 | 4 | 8 | 12.9941 |
| 9 | 24 | 3 | 6 | 13.3071 |
| 10 | 24 | 5 | 6 | 11.4019 |
| 11 | 24 | 3 | 8 | 14.6271 |
| 12 | 24 | 5 | 8 | 17.2671 |
| 13 | 24 | 4 | 7 | 21.2544 |
| 14 | 24 | 4 | 7 | 20.7509 |
| 15 | 24 | 4 | 7 | 21.7715 |
| 16 | 24 | 4 | 7 | 21.023 |
| 17 | 24 | 4 | 7 | 21.7443 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 241.58 | 9 | 26.84 | 89.84 | <0.0001 |
| A-Time | 1.81 | 1 | 1.81 | 6.07 | 0.0432 |
| B-Inoculum Volume | 2.07 | 1 | 2.07 | 6.93 | 0.0338 |
| C-pH | 24.04 | 1 | 24.04 | 80.45 | <0.0001 |
| AB | 0.0204 | 1 | 0.0204 | 0.0683 | 0.8013 |
| AC | 3.6 | 1 | 3.6 | 12.06 | 0.0104 |
| BC | 5.16 | 1 | 5.16 | 17.29 | 0.0043 |
| A² | 30.65 | 1 | 30.65 | 102.57 | <0.0001 |
| B² | 4.8 | 1 | 4.8 | 16.08 | 0.0051 |
| C² | 156.15 | 1 | 156.15 | 522.62 | <0.0001 |
| Residual | 2.09 | 7 | 0.2988 | ||
| Lack of Fit | 1.29 | 3 | 0.4306 | 2.15 | 0.2362 |
| Pure Error | 0.7996 | 4 | 0.1999 | ||
| Cor Total | 243.67 | 16 |
| Clearance Rate | Fermentation Supernatant (%) | F6 (%) | Comparison (%) |
|---|---|---|---|
| DPPH | 54.79 ± 0.13 | 78.71 ± 0.12 | 43.66 |
| OH | 79.70 ± 0.08 | 100.00 ± 0.01 | 25.47 |
| ABTS | 54.00 ± 0.01 | 90.2 ± 0.01 | 67.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhou, Y.; Zhu, C.; Meng, Y.; Wang, J.; Chen, X.; Hou, Y.; Liao, A.; Pan, L.; Huang, J. Optimization of Fermentation Process of Wheat Germ Protein by Aspergillus niger and Analysis of Antioxidant Activity of Peptide. Fermentation 2024, 10, 121. https://doi.org/10.3390/fermentation10030121
Liu Y, Zhou Y, Zhu C, Meng Y, Wang J, Chen X, Hou Y, Liao A, Pan L, Huang J. Optimization of Fermentation Process of Wheat Germ Protein by Aspergillus niger and Analysis of Antioxidant Activity of Peptide. Fermentation. 2024; 10(3):121. https://doi.org/10.3390/fermentation10030121
Chicago/Turabian StyleLiu, Yingying, Yu Zhou, Chaohong Zhu, Yanglin Meng, Jingjing Wang, Xinyang Chen, Yinchen Hou, Aimei Liao, Long Pan, and Jihong Huang. 2024. "Optimization of Fermentation Process of Wheat Germ Protein by Aspergillus niger and Analysis of Antioxidant Activity of Peptide" Fermentation 10, no. 3: 121. https://doi.org/10.3390/fermentation10030121
APA StyleLiu, Y., Zhou, Y., Zhu, C., Meng, Y., Wang, J., Chen, X., Hou, Y., Liao, A., Pan, L., & Huang, J. (2024). Optimization of Fermentation Process of Wheat Germ Protein by Aspergillus niger and Analysis of Antioxidant Activity of Peptide. Fermentation, 10(3), 121. https://doi.org/10.3390/fermentation10030121






