Abstract
Since ancient times, the placenta has been used to produce cosmetic and health food products, whereas fermentation is a technology that has been used to produce foods and cosmetics. For application in cosmetics, traditional placental extracts produced solely by proteolysis have not had enough moisturizing properties or the ability to stimulate the proliferation of epidermal keratinocytes. We combined these two traditional approaches to produce raw materials without such drawbacks that are suitable for cosmetic applications. Using a unique lactic acid bacterial strain, Enterococcus faecalis PR31, to directly ferment and digest both porcine and equine placentas, we produced the following liquid products: placenta ferment filtrates. The ferment filtrates stimulated the proliferation of not only normal human dermal fibroblasts but also epidermal keratinocytes. The ferments had higher equilibrium water content properties than traditional placental extracts, and the ferment derived from the porcine placenta maintained high stratum corneum water content levels for up to 6 h after its application on the skin. Metabolome analysis revealed various molecules that were increased by fermentation, among which lactic acid was assumed to play a central role in the high moisturizing properties. To conclude, the placenta ferment filtrates developed in this study are beneficial for cosmetic applications.
1. Introduction
The placenta plays an important and central role in nurturing mammalian fetuses until birth. After an animal has given birth, the placenta is delivered as an afterbirth, and according to anecdotage, the mother animal is said to eat it to clean the place of delivery and for nutritional purposes [1,2]. Because of the various beneficial components of the placenta, including proteins, peptides, amino acids, growth factors, hormones, vitamins, and minerals, it has been used for both nutritional and cosmetic purposes. The oldest manuscript describing the use of the placenta as an ingredient for external applications might be “Ebers papyrus”, dating back to 3000 BC, in which the use of cat placenta as an ingredient in the mixture recommended to prevent hair graying was described (Ebers 453) [3]. Several old documents describing the usage of the placenta as traditional medicines have been discovered in Asian countries; for example, a Chinese historical book, “Compendium of Materia Medica (Ben Cao Gang Mu)” mentioned the use of dried human placenta (Ze He Che) as a revitalizer [4]. Recently, placental extracts produced by digesting the placenta of several animals with proteases or acids have been used for both inner and outer beauty, especially in Asian countries, including Japan [5,6,7,8,9]. For application in cosmetics, although traditional placental extracts produced solely by proteolysis have been reported to have the property to stimulate the proliferation of dermal fibroblasts [10,11,12], their moisturizing effects were not sufficient, and they could not stimulate the proliferation of epidermal keratinocytes [10].
Fermentation is a traditional technology used worldwide since ancient times, mainly to produce fermented food. For cosmetic applications, a wax-based remedy containing fermented plant juice for wrinkle care and the use of sour milk for producing smooth skin were described in ancient Egypt [13]. The application of a rather sticky bread-dough-based face pack was described by Juvenal, an ancient Roman poet [14]. Recently, many cosmetic products have been produced using fermentation technologies since various molecules produced by fermentation have cosmetically beneficial effects [15]. To produce cosmetic ingredients, various microbes, such as lactic acid bacteria, bifidobacteria, yeasts, fungi, and mushrooms, are combined with various materials for fermentation [16].
In this study, we attempted to combine these two traditional approaches to solve the drawbacks of traditional placental extracts mentioned above; that is, we intended to directly ferment the placenta using microbes to produce cosmetically applicable raw materials. Although several studies described the fermentation of placental extract rather than the placenta [17,18,19], reports on direct fermentation of the placenta are rare, probably because in such fermentation, microbials need to digest the placenta to use its components as nutrients for their growth. To achieve this, we selected the lactic acid bacterium Enterococcus faecalis PR31 (PR31) as a unique strain with the ability to digest the placenta. As the placentas, we used porcine and equine ones, which have been widely used in Japan for producing cosmetically applicable placental extracts. Through the fermentation and digestion of porcine and equine placentas with PR31, we produced a novel placenta-derived liquid product suitable for cosmetic applications. In this paper, we describe the characteristics of these products for cosmetic applications.
2. Materials and Methods
2.1. Lactic Acid Bacteria
The lactic acid bacterium PR31 used in this study was isolated from Kiteck, a traditional fermented milk in Xinjiang Uyghur Autonomous Region (China), by Dr. Taku Miyamoto, an honorary professor at Okayama University, in 1997. This strain was selected from Miyamoto’s lactic acid bacteria library, which is derived from various traditional fermented foods worldwide, for its ability to directly digest the placenta. He generously permitted us to use his library and the strain PR31. This strain was identified as Enterococcus faecalis through sequence analysis of the DNA encoding 16S rRNA.
Regarding its virulence, PR31 is not hemolytic, it is vancomycin sensitive, and it lacks the gelatinase phenotype in standard biological assays [20,21]; however, it has the gelE gene in PCR analysis [22]. A PCR analysis [22] also showed that PR31 does not have the virulent genes, esp, agg, cylA, cylB, and cylM, although it has efaA, cpd, cob, ccf, and cad, which are common in strains of E. faecalis isolated from fermented foods [22]. These properties indicate that the practical virulence of PR31 is too low for use in industrial fermentation, although careful handling is necessary.
2.2. The Placenta
In this study, we used porcine and equine placentas obtained from a Japanese pig farmer and New Zealand ranches, respectively, which were collected for the industrial production of traditional placental extracts in Japan. In brief, placentas delivered from healthy mothers as afterbirth were collected, washed with water, and immediately frozen until use. Before usage, the placentas were thawed and minced into several centimeters in size. Because the minced pieces derived from multiple animals were pooled, each run of production uses a mixture of all parts of the placenta.
2.3. Bacterial Culture and Fermentation
For seed culture for fermentation, PR31 was grown in a medium containing 2.4% placental extract (Snowden Co., Ltd., Tokyo, Japan), 1% yeast extract, 0.1% Tween80, and 0.5% glucose. The placental extract used for seed culture was derived from the same animal species used for fermentation.
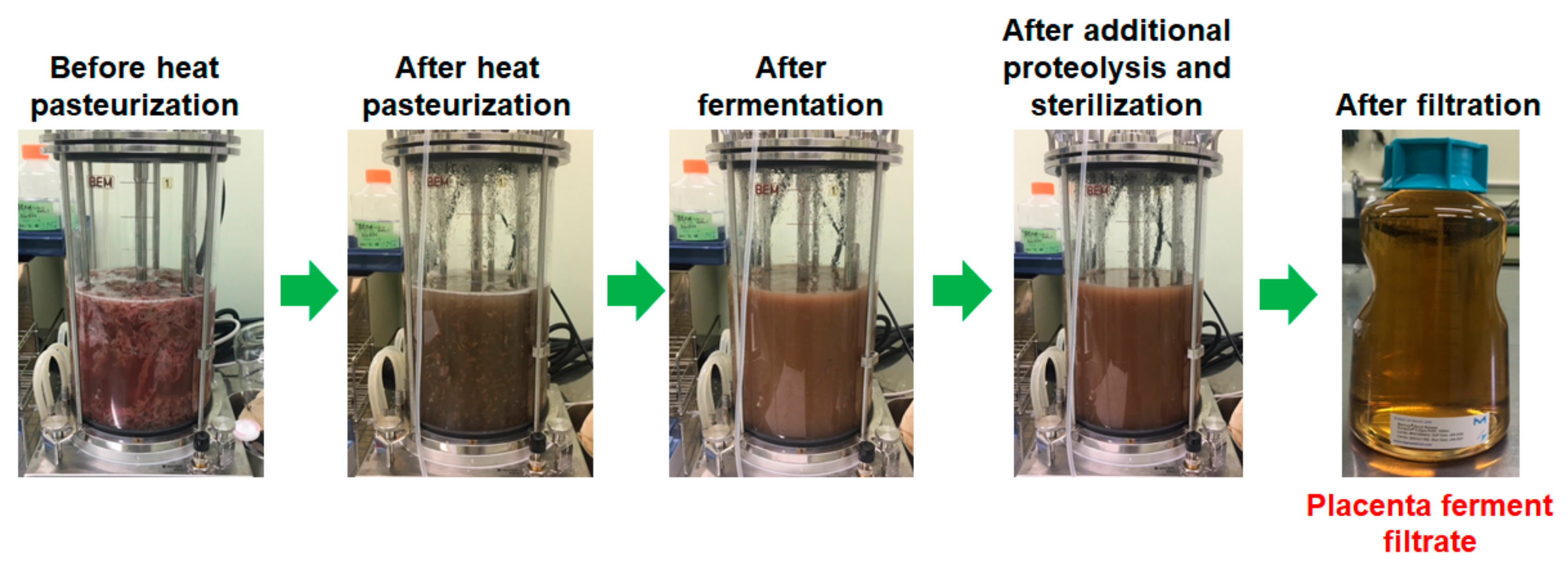
Placental fermentation was performed as follows: Minced porcine (1 kg) or equine (0.3 kg) placentas were mixed with water (2.4 kg in total), heated for pasteurization (75 °C, 1 h), and then a sterilized glucose solution was added (final glucose concentrations were 3 and 1.25% for porcine and equine, respectively). After the seed culture of PR31 (2%) was added, the mixture was fermented for 20 h at 37 °C using a 5 L jar fermenter (Bioneer-Neo, Marubishi Bioengineering, Tokyo, Japan) under pH-stat condition (pH 6.0) with a NaOH solution, and then the obtained fermented placenta suspension was treated with protease SE-4 (0.2% of the weight of placenta; SkinMedical, Tokyo, Japan) at 40 °C for 1 h for further digestion. Finally, the fermented placenta suspension was heat-sterilized (121 °C, 20 min) and filtered to obtain a clear solution of placenta ferment filtrate. For reference, the photos at each step of the production of porcine placenta ferment filtrate are depicted in Figure 1.
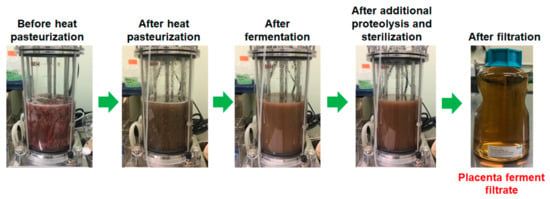
Figure 1.
Photos at each step of the production of porcine placenta ferment filtrate.
2.4. Cell Proliferation Assay with Normal Human Epidermal Keratinocytes and Dermal Fibroblasts
NHEK(NB) (Kurabo, Osaka, Japan) was used as a normal human epidermal keratinocyte. The cells were cultivated in DermaLife K keratinocyte medium (Lifeline Cell Technology, Frederick, MD, USA) at 37 °C under a 5% CO2 atmosphere in a humidified chamber. For the assay, cells were seeded in each well of a collagen-I-coated 96-well microplate at a density of 5.0 × 104 cells/mL in 100 µL. After 24 h, the medium was replaced with a medium containing 1/100 concentrations of supplements and test samples. After another 5 d of culture, the relative cell number was analyzed using the Cell Counting Kit WST-8 (Dojindo Laboratories, Kumamoto, Japan).
NHDF(NB) (Kurabo) was used as a normal human dermal fibroblast. The cells were cultivated in the FibroLife S2 Comp kit (Lifeline Cell Technology) at 37 °C under a 5% CO2 atmosphere in a humidified chamber. For the assay, cells were seeded in each well of collagen-I-coated 96-well microplate at a density of 1.5 × 104 cells/mL in 100 µL. After 24 h of culture, the medium was replaced with Dulbecco’s modified Eagle medium containing 3% fetal bovine serum and test samples. After another 3 d of culture, the relative cell number was analyzed using the Cell Counting Kit WST-8, as aforementioned.
2.5. Total Nitrogen Concentration
Total nitrogen concentrations were measured via the semi-micro Kjeldahl method using an AutoKjeldahl Unit K-370 and Digest Automat K-438 (Nihon BUCHI, Tokyo, Japan).
2.6. Size Exclusion Chromatography
Molecular weight distribution analyses of water-soluble fractions of samples were performed using an ultra-performance liquid chromatography (UPLC) system (ACQUITY UPLC H-Class, Waters, Milford, MA, USA) equipped with a size-exclusion column (ACQUITY UPLC Protein BEH SEC 125 Å, Waters). Samples were diluted in the mobile phase (100 mM phosphate buffer, pH 6.7), filtered using a 0.45 µm membrane, and injected into the system. The injection volume was 1 µL or 5 µL, the flow rate was 0.30 mL/min, and the monitoring wavelength was 220 nm. Molecular weights were calculated using a calibration curve constructed with uracil (112 Da), ribonuclease A (13.7 kDa), ovalbumin (44.2 kDa), and thyroglobulin (660 kDa).
2.7. Equilibrium Moisture Content
To determine the humectant potential of the materials, their equilibrium moisture contents (EMCs) were measured. The liquid sample was freeze-dried, and the obtained powder was placed on an aluminum plate, which was then placed in a chamber set at 40 °C and 75% relative humidity. After 48 h, the water content (WC) of the samples was determined using an infrared moisture analyzer (MA35, Sartorius, Gottingen, Germany). The EMC was calculated using the following equation:
EMC(%) = WC(%)/[100 − WC(%)].
An EMC of 100% indicates that the dried material can absorb the same amount of water as its dry weight.
2.8. Metabolome Analysis
Metabolome analyses were performed at Human Metabolome Technologies Inc. (HMT, Yamagata, Japan). Before the analysis, 80 µL of the sample was mixed with 20 µL of Milli-Q water containing internal standards (H3304-1002, HMT) to attain 1000 µM. The mixture was centrifugally filtered through a Millipore 5-kDa cutoff filter (ULTRAFREE MC PLHCC, HMT) at 9100× g, 4 °C for 60 min to remove macromolecules. The filtrate was then used for metabolome analysis according to the HMT Basic Scan package, using capillary electrophoresis time-of-flight mass spectrometry (CE-TOFMS) based on previously described methods [23,24]. Briefly, CE-TOFMS analysis was performed using an Agilent CE capillary electrophoresis system equipped with an Agilent 6210 time-of-flight mass spectrometer (Agilent Technologies, Inc., Santa Clara, CA, USA). The systems were controlled using Agilent G2201AA ChemStation software version B.03.01 (Agilent Technologies) and connected by a fused silica capillary (50 μm i.d. × 80 cm total length) with commercial electrophoresis buffer (H3301-1001 and I3302-1023 for cation and anion analyses, respectively, HMT) as the electrolyte. The spectrometer was scanned from a mass-to-charge ratio (m/z) of 50 to 1000 and peaks were extracted using the MasterHands automatic integration software (Keio University, Yamagata, Japan) to obtain peak information, including m/z, peak area, and migration time (MT) [25]. Signal peaks corresponding to isotopomers, adduct ions, and other product ions of known metabolites were excluded, and the remaining peaks were annotated according to the HMT metabolite database based on their m/z values and MTs. The areas of the annotated peaks were normalized to internal standards and sample amounts to obtain the relative levels of each metabolite. One hundred and ten primary metabolites were absolutely quantified based on one-point calibrations using their respective standard compounds.
2.9. Analysis of Stratum Corneum Water Content
To evaluate the skin-moisturizing effect of placenta ferments in humans, we employed a noninvasive method for stratum corneum water content by measuring the conductance at the skin surface at a high-frequency current of 3.5 MHz using SKICON-200EX-USB (Yayoi, Tokyo, Japan). This method was developed based on the positive relationship between the conductance at the skin surface and hydration [26,27].
We recruited five female volunteers aged 25–55 (average = 42.4; ages of each participant were 25, 37, 41, 54, and 55) years, without skin problems such as injuries and scratches at the testing sites (inner forearm), skin sensitivity, and skin allergy, and who were not undergoing any treatment or taking medication to treat skin diseases, not pregnant or possible to be pregnant, and not breastfeeding. Before testing, the safety aspects of the test material were guaranteed using the primary skin irritation test (alternative method) and 24 h occlusive patch testing. Written informed consent was obtained from all participants beforehand. This test was performed on 27–28 November 2023.
The sites of application of the test materials were 2 × 2 cm square areas on both inner forearms. Before testing, the test sites were washed gently with hand soap, and the participants were acclimated for 20 min in a room set at 22 °C and 50% relative humidity. The conductance levels at test sites were determined 10 times using SKICON-200EX-USB, and the averaged values were calculated as the values for “before application”. Then, 20 µL of the test material (water or porcine placenta-derived ferment) was applied at the test site on a randomly assigned right or left inner arm and gently spread until the materials were absorbed into the skin using a finger wearing a powder-free nitril glove. At 0.5, 1, 2, 4, and 6 h after the application of the samples, the conductance at the test sites was measured as described above. Before each measurement, the participants were acclimated for 20 min as aforementioned.
2.10. Statistics
Statistical differences compared to conditions without the addition of test samples in the cell growth assays were analyzed using the Dunnett method. A statistical comparison between the test sample and water in the stratum corneum water content assay at each time point was performed using the Student’s paired t-test. The difference was considered statistically significant if the significance probability was <0.05.
3. Results
3.1. Digestioin and Solubilization by Fermentation of the Placenta
To determine the extent of solubilization at each step of fermentation of the placentas, we measured the total nitrogen concentrations of the aqueous sample at each step after filtration with a 0.45 μm membrane filter. We employed the nitrogen concentration as a measure of the amount of variety of solubilized molecules, e.g., not only proteins but also other molecules containing nitrogen. The results are summarized in Table 1. As shown in Table 1, before heat pasteurization (Step 1 in Table 1), approximately 20% and 19% of nitrogen relative to the final products were already soluble in porcine and equine placental suspensions, respectively. By heat pasteurization (Step 2), approximately 40% and 21% of nitrogen were further solubilized, affording approximately 60% and 40% of soluble nitrogen relative to the final porcine and equine products, respectively. Fermentation with PR31 (Step 3) solubilized approximately 27% and 51% of the nitrogen, yielding approximately 87% and 91% of the final porcine and equine products, respectively. Additional proteolysis solubilized approximately 13% and 9% (Step 4). These results indicated that the contribution of the fermentation step was the largest in equine placenta solubilization, whereas that in porcine placenta solubilization was the second largest after the heat pasteurization step. Regardless of this difference, the contribution of fermentation exceeded that of additional proteolysis for both porcine (27.4% vs. 13.0%) and equine (51.2% vs. 8.7%) placental solubilization. When the same amounts of placentas for fermentation were solubilized solely by proteolysis without fermentation after heat pasteurization, the soluble nitrogen concentrations were approximately 111% and 121% relative to Step 4 for porcine and equine placentas, respectively.

Table 1.
Nitrogen concentration at each step of fermentation and proteolysis of placentas.
3.2. Molecular Weight Distribution at Each Step of the Fermentation of the Placenta
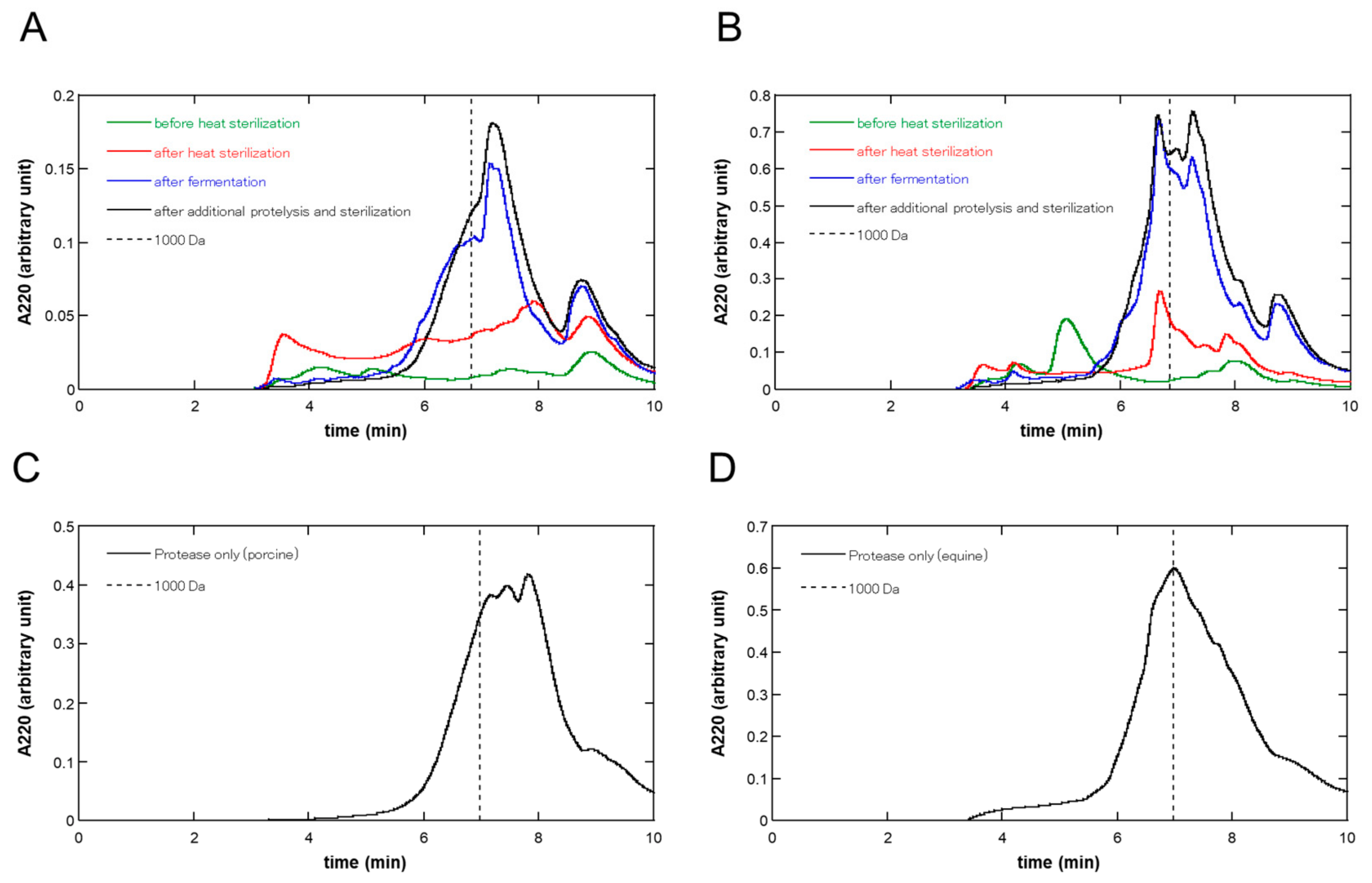
Figure 2 shows the size-exclusion UPLC chromatograms at each step of fermentation and proteolysis in the (A) porcine and (B) equine placentas, respectively. As shown in Figure 2, during fermentation, large amounts of molecules were solubilized, and the molecular weight distribution was shifted in the direction of low molecular weight in both in porcine and equine placentas. Additional proteolysis did not largely change the molecular weight distribution patterns; however, it brought about a slight shift in the low-molecular-weight direction.
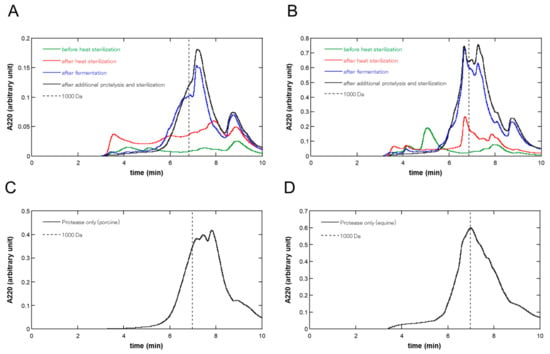
Figure 2.
Size exclusion UPLC chromatograms at each step of fermentation and proteolysis of (A) porcine and (B) equine placentas. At each step, the liquid phase of the sample was filtered using a 0.45 µm membrane filter and 1 or 5 µL was injected into the size exclusion column for porcine or equine preparation, respectively. Absorbance at 220 nm was monitored and the chromatograms before heat pasteurization, after heat pasteurization, after fermentation, and after additional proteolysis and sterilization were shown in green, red, blue, and black lines, respectively. Size exclusion UPLC chromatograms of placental extracts produced solely by proteolysis are also shown: (C) porcine and (D) equine placental extracts. Liquid phase of the sample was filtered and 5 µL was injected into the size exclusion column, and then analyzed as above. Each chromatogram shows the average of triplicate runs. The retention times at 1000 Da were indicated in broken lines.
Table 2 shows the molecular weight balance by discriminating at 1000 Da and the areas under the curves (AUCs) at each step of fermentation and proteolysis based on the size exclusion UPLC analysis, as shown in Figure 1. As seen in Table 2, the shifts to a low-molecular-weight direction were observed at the fermentation step (Step 3) in both porcine and equine (≤1000 Da: shift from 54.8% to 65.8% and 51.7% to 62.5% in porcine and equine, respectively). Additional proteolysis (Step 4) brought about a further slight low-molecular-weight shift (≤1000 Da: shift from 65.8% to 74.0% and 62.5% to 67.9% in porcine and equine, respectively). Although in porcine placentas, almost no low-molecular-weight shift was observed during the heat pasteurization step (Step 2), a shift from 33.9% to 51.7% was observed in the equine placenta. Regarding the amount of solubilization, as judged by the stepwise increase in AUCs, the contribution of fermentation was the largest in equine placentas (53.1%), and in porcine placentas, the contribution of heat pasteurization (43.3%) exceeded that of fermentation (26.0%), similar to the results judged by the nitrogen concentration in Table 1. Regardless of this difference, the contribution of fermentation was greater than that of additional proteolysis in both porcine and equine placentas (26.0% vs. 8.6% and 53.1% vs. 14.6%, respectively).

Table 2.
Molecular weight analysis with size exclusion UPLC at each step of fermentation and proteolysis of placentas.
For comparison, we analyzed the molecular weight distribution of placental extracts prepared solely with protease after heat pasteurization, which were independent runs using different placentas. The results are shown in Figure 2C,D. The ratios of AUCs corresponding to ≤1000 Da were 75.6% and 62.1% for porcine and equine placental extracts, respectively, which were approximately the same as those of placenta ferments, i.e., 74.0% and 67.9% for porcine and equine placenta-derived preparations (Table 2), respectively.
3.3. Changes in the Amounts of Metabolites Due to Fermentation
The results of the metabolome analyses focusing on the changes in the amounts of metabolites due to fermentation are summarized in Table 3, Table 4, Table 5 and Table 6. Table 3 and Table 4 present the metabolites that were 4-fold or more in porcine and equine placenta-derived ferment filtrates, respectively, compared to the corresponding proteolysate filtrates (produced solely by proteolysis), where metabolites with relative areas in fermentations larger than 1 × 10−4 were picked up. Table 5 and Table 6 show the lists of metabolites that were 0.25-fold or less in porcine and equine placenta-derived ferments, respectively, compared to the corresponding proteolysates, where the metabolites with relative areas in the proteolysates larger than 1 × 10−4 were picked up. The quantification results for certain metabolites are shown in the tables.

Table 3.
Compounds that are 4-fold or more in the ferment filtrate than the proteolysate filtrate of porcine placenta.

Table 4.
Compounds that are 4-fold or more in the ferment filtrate than proteolysate filtrate of equine placenta.

Table 5.
Compounds that are 0.25-fold or less in the ferment filtrate than the proteolysate filtrate of porcine placenta.

Table 6.
Compounds that are 0.25-fold or less in the ferment filtrate than the proteolysate filtrate of the equine placenta.
As seen in Table 3 and Table 4, the most abundant and largely increased metabolite by fermentation was lactic acid, which increased from 1452 to 412,140 µM (283.8-fold) and from 651 to 188,642 µM (289.8-fold) in the porcine and equine ferment filtrates, respectively. This was followed by pyruvic acid (68.3 to 2322 µM and 40.3 to 2453 µM, ornithine (122 to 1002 µM and 12.0 to 637 µM), and tyramine (5.06 to 538 µM and 12.9 to 388 µM) in porcine and equine placenta ferment filtrates, respectively. Other metabolites that increased following fermentation and were quantified were citrulline, succinic acid, hydroxyproline, glucose 6-phosphate, gluconic acid, AMP, UMP, and homoserine in both porcine and equine placenta fermentations; in porcine GMP, spermidine, fructose 6-phosphate, and CMP were also quantified. Certain metabolites, such as 2-phenylethylamine, 2-hydrozy-4 (or 3)-methylvaleric acid, 3-amino-2-piperidone, and β-hydroxyisovaleric acid (or 2-hydroxyvaleric acid, or 2-hydroxyisovaleric acid), lacked quantification data.
As seen in Table 5 and Table 6, the first- and second-ranking metabolites in abundance decreased following fermentation were the two amino acids, arginine and tyrosine, respectively, in both fermented porcine and equine placentas. Arginine decreased from 1455 to 361 µM and from 866 to 195 µM in porcine and equine placenta ferments, respectively, and tyrosine decreased from 808 to 107 µM and from 260 to 19.5 µM, respectively. Other metabolites that were decreased by fermentation in the porcine placenta ferments were ten nucleic acids and one amino acid cystine, whereas in equine placenta ferments, one nucleic acid guanosine and one amino acid cystine were found to have been decreased by fermentation.
3.4. Effect on the Proliferation of Normal Human Dermal Fibroblasts
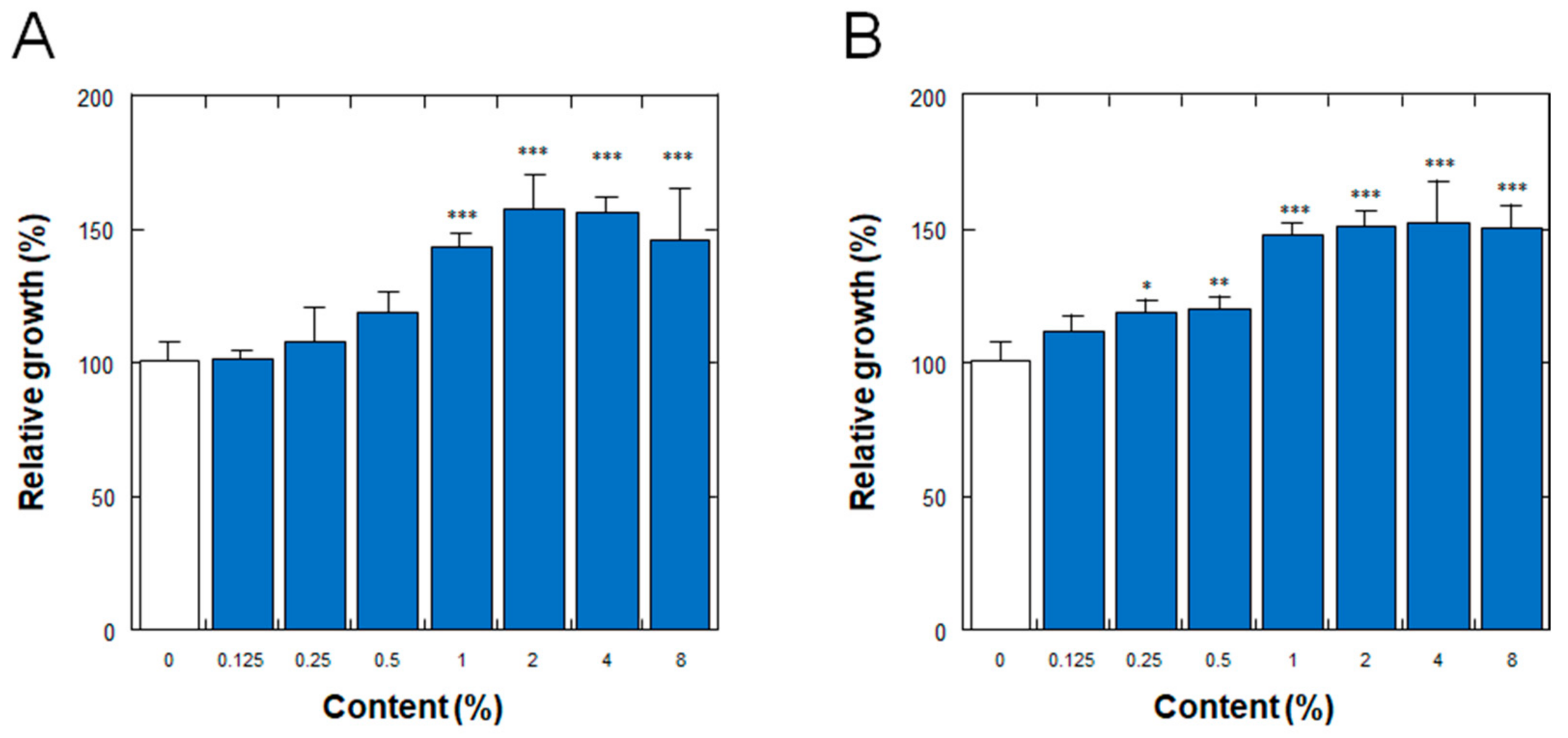
Figure 3 shows the effects on the proliferation of normal human dermal fibroblasts (NHDFs) of the placenta ferment filtrates produced by fermentation of the porcine or equine placenta followed by additional proteolysis, as described in Section 2. As shown in Figure 3, the ferment filtrates derived from both porcine and equine placentas stimulated the proliferation of NHDFs in a dose-dependent manner, with minimum effective concentrations of 1% and 0.25% for the porcine and equine placenta ferment filtrates, respectively.
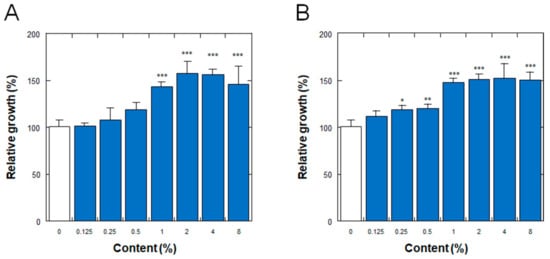
Figure 3.
Effect of (A) porcine and (B) equine placenta-derived ferment filtrates on the proliferation of normal human dermal fibroblasts. Relative growths of the cells were determined by the amount of soluble formazan from tetrazolium salt using Cell Counting Kit WST-8. The results were expressed with % values for the condition without ferments (indicated as 0%). Each bar represents the mean of 6 and 3 determinations for 0% and others, respectively, with standard deviations. * p < 0.05, ** p < 0.01, and *** p < 0.001 vs. 0%.
3.5. Effect on the Proliferation of Normal Human Epidermal Keratinocytes
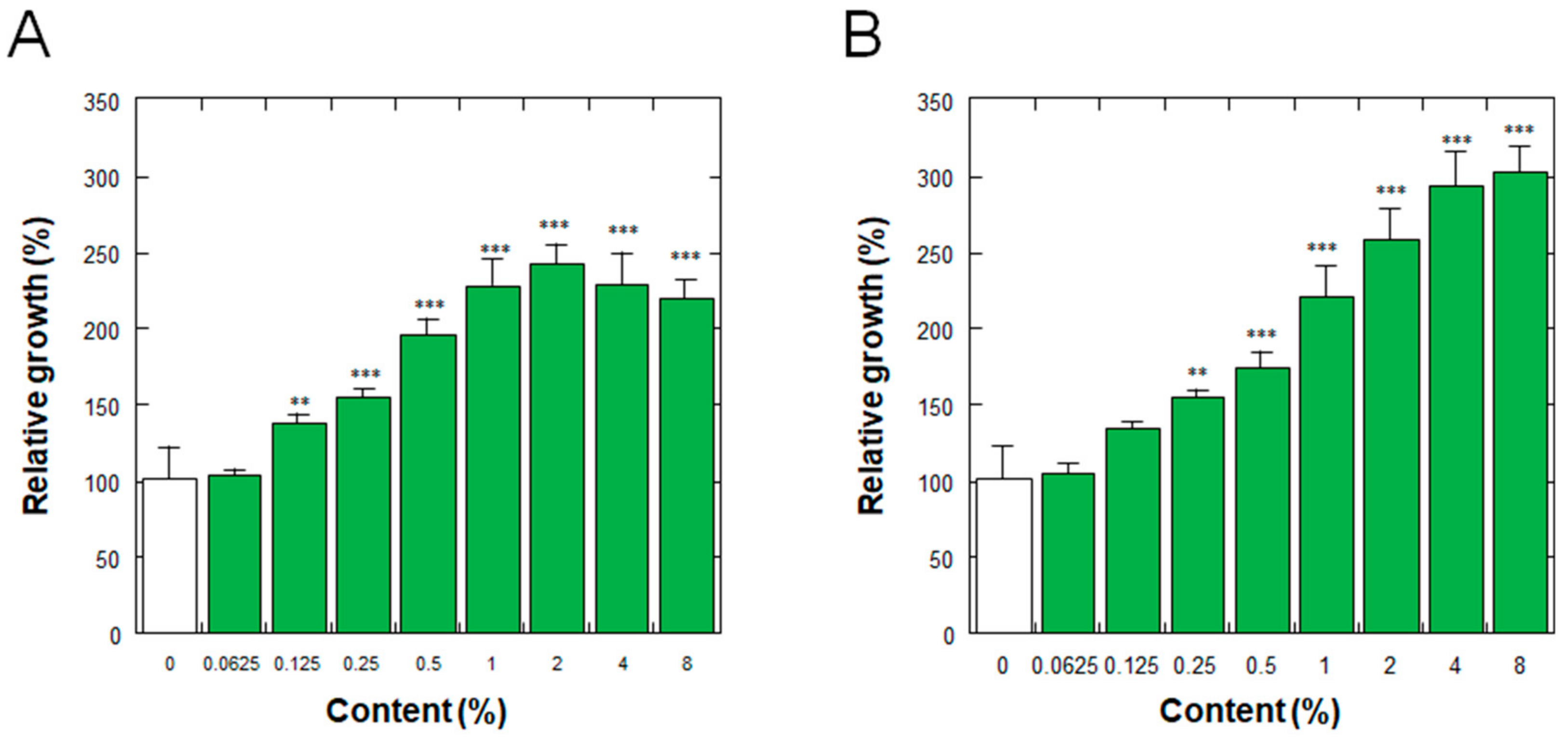
Figure 4 shows the effects on the proliferation of normal human epidermal keratinocytes (NHEKs) of the placenta ferment filtrates as aforementioned. As shown in Figure 4, the ferment filtrates derived from both porcine and equine placentas stimulated the proliferation of NHEKs in a dose-dependent manner, with minimum effective concentrations of 0.125% and 0.25% for porcine and equine placenta ferment filtrates, respectively.
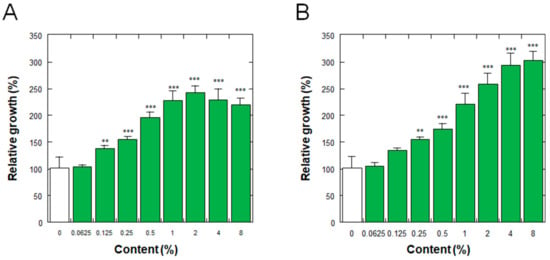
Figure 4.
Effect of (A) porcine and (B) equine placenta-derived ferment filtrates on the proliferation of normal human epidermal keratinocytes. Relative growths of the cells were determined by the amount of soluble formazan from tetrazolium salt using Cell Counting Kit WST-8. The results were expressed with % values for the condition without ferments (indicated as 0%). Each bar represents the mean of 8 and 3 determinations for 0% and others, respectively, with standard deviations. ** p < 0.01 and *** p < 0.001 vs. 0%.
3.6. Equilibrium Moisture Content
Table 7 shows the equilibrium moisture contents (EMCs) at 40 °C and 75% relative humidity of placenta ferment filtrates and corresponding placenta proteolysate filtrates derived from porcine and equine placentas after their lyophilization. As shown in Table 7, the EMCs of ferment filtrates (80.3% and 79.3% for porcine and equine, respectively) were greater than those of the corresponding proteolysate filtrates (34.5% and 38.0% for porcine and equine, respectively) by 2-fold or more. As a reference, we also measured the EMCs of glycerol and sodium hyaluronate (average molecular weight = 2000 kDa) under the same conditions and observed that the values were 78% and 37%, respectively, suggesting that the EMCs of ferment filtrates and proteolysate filtrates were comparable to those of glycerol and sodium hyaluronate, respectively.

Table 7.
Equilibrium moisture content of placenta-derived samples.
3.7. Stratum Corneum Water Content
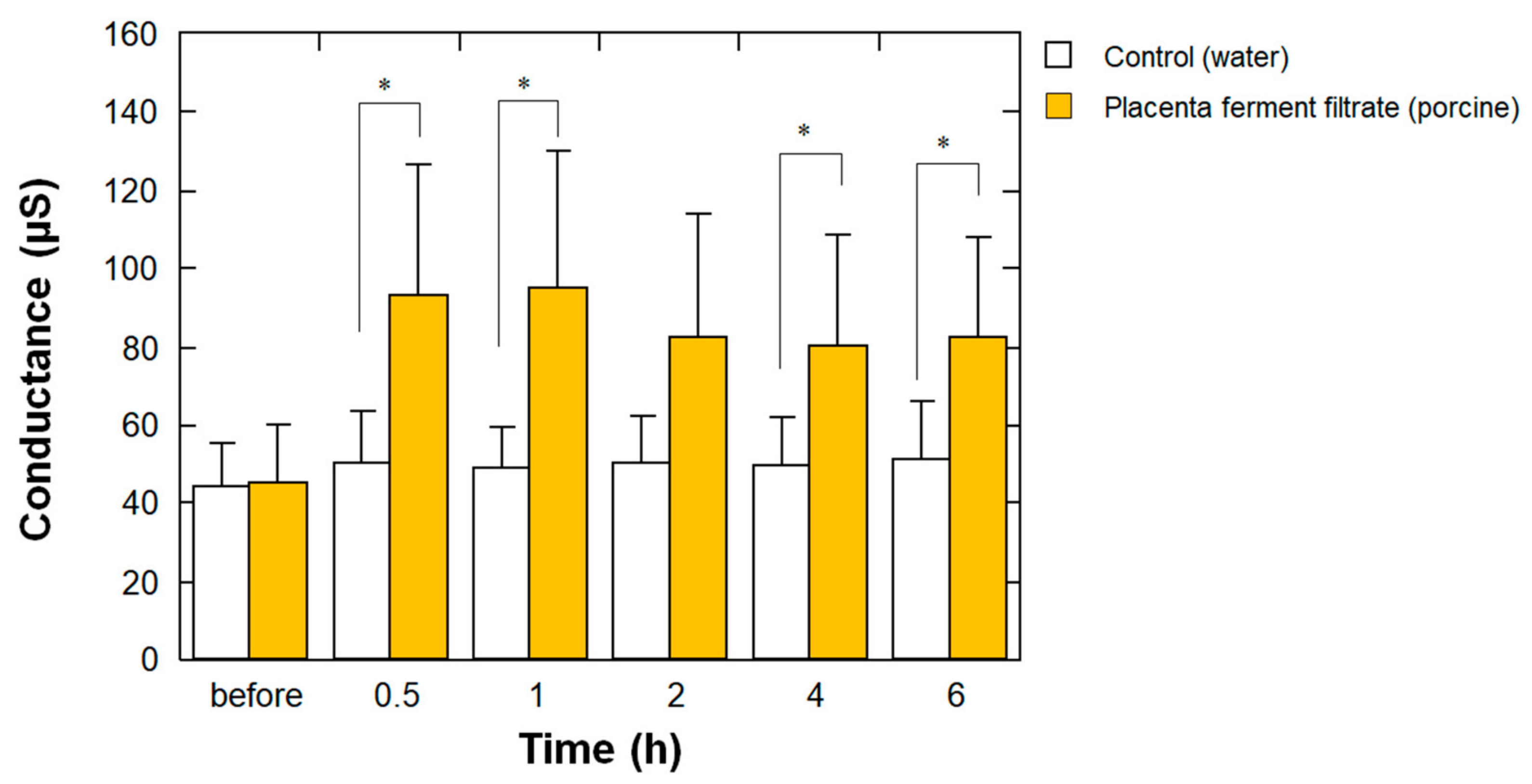
To determine whether the aforementioned high EMC properties of placenta ferment filtrates bring about a high moisturizing effect on human skin, we examined the effect of porcine-derived placenta fermentation on stratum corneum water content. As shown in Figure 5, at 0.5, 1, 4, and 6 h after the application of the samples, the skin surface conductance at the site of placenta ferment application was significantly higher than that of water application. This result clearly indicates that the high EMC value of placenta ferments results in their high moisturizing properties on the skin.
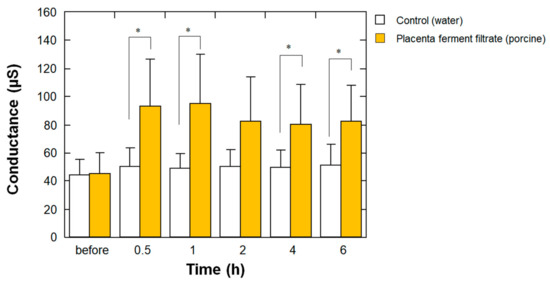
Figure 5.
Effect of porcine-derived placenta ferment filtrates on stratum corneum water content. Before and after 0.5, 1, 2, 4, and 6 h from the topical application of 20 μL of the ferment (closed bars) or water (open bars) on each forearm, the stratum corneum water content was measured using SKICON-200EX-USB as described in Materials and Methods. Each bar represents the mean with standard deviation of the measurements in five subjects. * p < 0.05 vs. control at each time point.
4. Discussion
In the present study, we produced a placenta ferment filtrate using a strain of lactic acid bacteria PR31 to ferment and directly digest both porcine- and equine-derived placentas. The placenta ferments produced by this method contained various molecules derived from placentas and fermentation, stimulated the proliferation of both normal human dermal fibroblasts and normal human epidermal keratinocytes, and had high EMC values. Moreover, the stratum corneum water content after topical application of the ferment derived from porcine placenta to the human skin was significantly higher than that of water until 6 h after application. These properties suggest that these placenta ferment filtrates are the materials preferred for cosmetic applications. The porcine- and equine-derived placenta ferments have been assigned the following product names as cosmetic ingredients: Enterococcus faecalis/Pig Placenta Ferment Filtrate and Enterococcus faecalis/Horse Placenta Ferment Filtrate, respectively, according to the International Nomenclature of Cosmetic Ingredients. We propose these materials as novel raw materials for cosmetic applications.
Regarding the application of fermentation to produce placenta-derived products, several reports have described methods for fermenting placental extracts (not the placenta itself) using yeast [17,18,19]. In other words, in these studies, yeast grew in a nutrient medium containing placental extract as a component, where the placental extract (not the placenta itself) was produced beforehand by proteolysis or other non-biological methods. An examination of the possible use of selected yeast strains for deodorizing sheep placental extract solutions has also been reported [28]. We have provided a type of porcine placental extract produced by combining fermentation and proteolysis to researchers in universities to examine its effects on lipid metabolism [29,30,31], where the porcine placenta was fermented with yeast and lactic acid bacteria, and then the fraction not solubilized by the fermentation was proteolyzed [29]. However, in such a preparation, over 50% of the nitrogen was solubilized by proteolysis, indicating that the contribution of fermentation was much smaller when digesting the placenta than in the present study. In this study, a unique lactic acid bacterium strain, PR31, enabled the direct and rapid digestion of placentas to obtain liquid materials for use in cosmetics. Although we employed additional proteolysis to thoroughly digest placentas in the last step, the gains from the final proteolysis were marginal, that is, approximately 10% for the solubilization of nitrogen (Table 1 and Table 2) and approximately 5% to 8% for the shift to low molecular weight (Table 2). In the preparations produced solely by proteolysis, the solubilized nitrogen concentrations were slightly higher than those in the corresponding ferments by 10–20%. This might be because, in the ferments, some components containing nitrogen were assumed to be used by PR31 as nutrients.
We found that the placenta ferment filtrates derived from both porcine and equine placentas stimulated the proliferation of both normal human dermal fibroblasts (Figure 3) and epidermal keratinocytes (Figure 4). These properties are preferable for cosmetic applications because they may promote both dermal and epidermal skin turnover. In previous studies, porcine placental extract was reported to stimulate the proliferation of dermal fibroblasts [10,11,12]; however, it was also reported to have no effect on the proliferation of epidermal keratinocytes [10]. Although there are some reports describing the growth-promoting activity of human placental extracts produced without heat treatment in keratinocytes [32,33], heat-labile and/or human-specific molecules may have been responsible for such an effect. The detailed molecular mechanisms underlying the effects of placenta ferment on dermal fibroblast and epidermal keratinocyte proliferation should be elucidated in future studies. In this study, since the placenta ferments were heat-sterilized at 121 °C, assuming the contribution of growth factors possibly contained in the raw placentas is unrealistic. In dermal fibroblasts, the growth-promoting effect of porcine placental extract was reported to occur via fibroblast growth factor (FGF) receptors [12], and the porcine placental extract stimulated the expression of FGF in dermal fibroblasts [10]. Therefore, assuming that certain factors in placenta ferments derived from the placenta promote FGF expression and affect dermal fibroblasts in an autocrine manner is reasonable. However, some molecules produced by fermentation might be responsible for the proliferation-promoting activity in keratinocytes of placenta ferments because placental extracts produced solely by proteolysis do not have such an effect [10]. Although detailed identification of such molecules remains a future challenge, one of the candidates might be pyruvic acid, as described below.
Regarding the differences between porcine and equine placentas, we assume that placenta ferments with the same properties were produced. However, in porcine placentas, larger amounts of materials were solubilized during heat pasteurization than in equine placentas (Table 1 and Table 2), possibly owing to the difference in the placental structures. In practice, approximately 3-fold of the wet weight of porcine placenta is required to attain the same order of nitrogen concentration of placenta ferment in our laboratory. Moreover, porcine placentas are assumed to have a looser structure compared to equine placentas. Such a loose structure might have enabled us to solubilize larger amounts of materials as a kind of soup by heat pasteurization. The loose nature of the porcine placenta might also be the cause of the difference in the final molecular weight balances; that is, the ratio of low molecular weights tended to be larger in the porcine placenta-derived ferment than in the equine placenta-derived ferment (Table 2).
Basic skincare cosmetics must possess excellent moisturizing properties. As shown in the EMC analysis, placenta ferments had high EMC values comparable to those of glycerol (Table 3). Moreover, the high skin moisturizing properties of the stratum corneum were confirmed (Figure 5). As their EMC values were higher than those of the corresponding traditional placental extracts produced solely by proteolysis (Table 3), these high moisturizing properties may be assumed to have been derived from molecules produced by fermentation. One of the molecules supporting the high moisturizing properties of placental ferments might be lactic acid, a natural moisturizing factor (NMF) [34]. In this study, lactic acid was largely increased by fermentation and was the most abundant metabolite in ferment by metabolome analysis (>100 mM in both porcine and equine placenta ferments). In addition to lactic acid, the metabolome analysis revealed that pyruvic acid, ornithine, tyramine, and citrulline largely increased to >100 µM by fermentation in both porcine and equine placenta ferments. Ornithine and citrulline are free amino acids that are not involved in protein structure but are the central molecules in the urea cycle. In the skin, since they are thought to be NMFs derived from filaggrin by degradation and conversion [35,36,37], these amino acids may also act as NMFs. As both ferments and proteolysates contain various free amino acids, these molecules may also function as NMFs. Although in this study biological analyses were performed only with placenta ferments, head-to-head comparisons of the biological activities between placenta ferments and corresponding proteolysates will be helpful to elucidate the roles of metabolites derived from fermentation in future studies.
Regarding other metabolites that were found to increase during fermentation, pyruvic acid is an important molecule in the citric acid cycle. It enters the citric acid cycle after being transformed into acetyl-CoA or oxaloacetate [38]. Since the citric acid cycle contributes to energy production in cells, this molecule may participate in promoting cell growth. Tyramine is an aromatic amine derived from tyrosine via decarboxylation. Although tyramine is a possible substrate for tyrosinase, a key enzyme in melanogenesis, tyramine inhibits tyrosinase activity when measured with tyrosine as its substrate [39]. Therefore, this molecule may be beneficial for lightening the skin; however, further research is necessary to test this hypothesis. Although we found various molecules that were increased by fermentation other than those aforementioned (Table 3 and Table 4), the elucidation of the biological implications of these molecules is open to future studies. Regarding the metabolites found to be decreased in the ferments (Table 5 and Table 6), two amino acids, arginine and tyrosine, were the top one and two, respectively, in both porcine and equine. Cystine and several other nucleic acids are also listed. We believe that these molecules were consumed by the lactic acid bacteria during fermentation.
5. Conclusions
By directly fermenting and digesting porcine or equine placentas using a unique lactic acid bacterial strain, PR31, we produced a liquid material, placenta ferment filtrate, that is suitable for cosmetic application. Ferment filtrates derived from both porcine and equine placentas stimulated the proliferation of normal human dermal fibroblasts and epidermal keratinocytes at a concentration of 1% or higher. Such a property of ferment filtrates was superior to that of traditional placental extracts, which could not stimulate the proliferation of epidermal keratinocytes. The ferment filtrates also had 2-fold or higher equilibrium water content properties than those of the corresponding proteolysate filtrates, and the expected high skin-moisturizing properties were confirmed by stratum corneum water content analysis with the ferment filtrate derived from porcine placenta, i.e., the ferment filtrate maintained high stratum corneum water content levels for up to 6 h after its application on the skin. Using metabolome analysis, several candidate molecules that support these effects can be hypothesized. These properties of placenta ferment filtrates derived from both porcine and equine placentas support their beneficial application in cosmetics. In a future study, the molecular basis of the biological effects of the placenta ferments should be elucidated in more detail, and other biological analyses related to their cosmetic applications should be performed as well.
Author Contributions
K.M. and Y.K. performed the experiments and wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study on stratum corneum water content was conducted without formal approval from the institutional review board, because the test sample, ferment filtrate derived from porcine placenta, had been already commercially available from January 2023 in Japan, and its safety aspects had been guaranteed by a primary skin irritation test (alternative method) and a 24 h occlusive patch testing. Despite the test material was thought safe as mentioned and the setting of the test was within short time-period (within 6 h), we obtained written informed consent from all participants beforehand.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study for their participation in the study on stratum corneum water content.
Data Availability Statement
The data underlying this article will be shared at the reasonable request to the corresponding author.
Acknowledgments
We thank Taku Miyamoto for allowing us to use his lactic acid bacteria collection. We also thank Human Metabolome Technologies Inc. (Niigata, Japan) and Clinimedic Inc. (Tokyo, Japan) for performing metabolome analysis and the study on stratum corneum water content, respectively.
Conflicts of Interest
K.M. and Y.K. are employees of Snowden Co., Ltd. This study was funded by Snowden Co., Ltd. No external funding was used.
References
- Soykova-Pachnerova, E.; Brutar, V.; Golova, B.; Zvolska, E. Placenta as a lactagogon. Gynaecologia 1954, 138, 617–627. [Google Scholar] [CrossRef]
- Kristal, M.B. Placentophagia: A biobehavioral enigma (or De gustibus non disputandum est). Neurosci. Biobehav. Rev. 1980, 4, 141–150. [Google Scholar] [CrossRef]
- Nunn, J.F. Ancient Gyptian Medicine; University of Oklahoma Press: Norman, OK, USA, 2002. [Google Scholar]
- Silini, A.R.; Cargnoni, A.; Magatti, M.; Pianta, S.; Parolini, O. The Long Path of Human Placenta, and Its Derivatives, in Regenerative Medicine. Front. Bioeng. Biotechnol. 2015, 3, 162. [Google Scholar] [CrossRef]
- Barat, T.; Abdollahimajd, F.; Dadkhahfar, S.; Moravvej, H. Evaluation of the efficacy and safety of cow placenta extract lotion versus minoxidil 2% in the treatment of female pattern androgenetic alopecia. Int. J. Womens Dermatol. 2020, 6, 318–321. [Google Scholar] [CrossRef]
- Nagae, M.; Nagata, M.; Teramoto, M.; Yamakawa, M.; Matsuki, T.; Ohnuki, K.; Shimizu, K. Effect of Porcine Placenta Extract Supplement on Skin Condition in Healthy Adult Women: A Randomized, Double-Blind Placebo-Controlled Study. Nutrients 2020, 12, 1671. [Google Scholar] [CrossRef]
- Kitanohara, M.; Yamamoto, T.; Masunaga, S.; Ohishi, M.; Komatsu, Y.; Nagase, M. Effect of porcine placental extract on the mild menopausal symptoms of climacteric women. Climacteric 2017, 20, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Nagase, M.; Watanabe, C.; Kitanohara, M.; Nishiya, M.; Okada, T.; Ohishi, M.; Komatsu, Y. Improvement of Aspects of Subjective Sleep Quality of Healthy Volunteers by Ingestion of Porcine Placental Extract: A Randomized Cross-Over Pilot Study. Front. Nutr. 2020, 7, 550287. [Google Scholar] [CrossRef] [PubMed]
- Nagae, M.; Nishio, T.; Ohnuki, K.; Shimizu, K. Effects of oral administration of equine placental extract supplement on the facial skin of healthy adult women: A randomized, double-blind, placebo-controlled study. Health Sci. Rep. 2022, 5, e522. [Google Scholar] [CrossRef] [PubMed]
- Tebakari, M.; Katoh, S.; Hashimoto, H.; Kawashima, J.; Takano, F. Porcine placenta extract modulates the expression of skin functional proteins in cultured human fibroblasts and keratinocytes. Pharmacometrics 2015, 88, 1–6. [Google Scholar]
- Cho, H.R.; Ryou, J.H.; Lee, J.W.; Lee, M.H. The effects of placental extract on fibroblast proliferation. J. Cosmet. Sci. 2008, 59, 195–202. [Google Scholar] [PubMed]
- Ito, K.; Yamada, R.; Matsumoto, N.; Imamura, T. Evaluation of fibroblast growth factor activity exerted by placental extract used as a cosmetic ingredient. J. Cosmet. Dermatol. 2018, 17, 821–829. [Google Scholar] [CrossRef] [PubMed]
- El-Kilany, E.; Raoof, E. Facial Cosmetics in Ancient Egypt. Egypt. J. Tour. Stud. 2017, 16, 1–19. [Google Scholar]
- Stewart, S. Cosmetics & Perfumes in the Roman World; Tempus Publishing Limited: Stroud, UK, 2007. [Google Scholar]
- Pérez-Rivero, C.; López-Gómez, J.P. Unlocking the Potential of Fermentation in Cosmetics: A Review. Fermentation 2023, 9, 463. [Google Scholar] [CrossRef]
- Duarte, M.; Oliveira, A.L.; Oliveira, C.; Pintado, M.; Amaro, A.; Madureira, A.R. Current postbiotics in the cosmetic market-an update and development opportunities. Appl. Microbiol. Biotechnol. 2022, 106, 5879–5891. [Google Scholar] [CrossRef] [PubMed]
- Yoon, D.H.; Han, G.Y.; Hwang, S.S.; Lee, D.W.; Kim, J.S.; Kim, K.; Kim, J.; Song, W. The Effect of Fermented Porcine Placental Extract on Fatigue-Related Parameters in Healthy Adults: A Double-Blind, Randomized, Placebo-Controlled Trial. Nutrients 2020, 12, 3086. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Han, N.R.; Kim, N.R.; Lee, M.; Kim, J.; Kim, C.J.; Jeong, H.J.; Kim, H.M. Effect of fermented porcine placenta on physical fatigue in mice. Exp. Biol. Med. 2016, 241, 1985–1996. [Google Scholar] [CrossRef]
- Mitsui, Y.; Bagchi, M.; Marone, P.A.; Moriyama, H.; Bagchi, D. Safety and toxicological evaluation of a novel, fermented, peptide-enriched, hydrolyzed swine placenta extract powder. Toxicol. Mech. Methods 2015, 25, 13–20. [Google Scholar] [CrossRef]
- Anderson, A.C.; Jonas, D.; Huber, I.; Karygianni, L.; Wolber, J.; Hellwig, E.; Arweiler, N.; Vach, K.; Wittmer, A.; Al-Ahmad, A. Enterococcus faecalis from Food, Clinical Specimens, and Oral Sites: Prevalence of Virulence Factors in Association with Biofilm Formation. Front. Microbiol. 2015, 6, 1534. [Google Scholar] [CrossRef]
- Strzelecki, J.; Hryniewicz, W.; Sadowy, E. Gelatinase-associated phenotypes and genotypes among clinical isolates of Enterococcus faecalis in Poland. Pol. J. Microbiol. 2011, 60, 287–292. [Google Scholar] [CrossRef]
- Eaton, T.J.; Gasson, M.J. Molecular screening of Enterococcus virulence determinants and potential for genetic exchange between food and medical isolates. Appl. Environ. Microbiol. 2001, 67, 1628–1635. [Google Scholar] [CrossRef]
- Ohashi, Y.; Hirayama, A.; Ishikawa, T.; Nakamura, S.; Shimizu, K.; Ueno, Y.; Tomita, M.; Soga, T. Depiction of metabolome changes in histidine-starved Escherichia coli by CE-TOFMS. Mol. Biosyst. 2008, 4, 135–147. [Google Scholar] [CrossRef]
- Ooga, T.; Sato, H.; Nagashima, A.; Sasaki, K.; Tomita, M.; Soga, T.; Ohashi, Y. Metabolomic anatomy of an animal model revealing homeostatic imbalances in dyslipidaemia. Mol. Biosyst. 2011, 7, 1217–1223. [Google Scholar] [CrossRef]
- Sugimoto, M.; Wong, D.T.; Hirayama, A.; Soga, T.; Tomita, M. Capillary electrophoresis mass spectrometry-based saliva metabolomics identified oral, breast and pancreatic cancer-specific profiles. Metabolomics 2010, 6, 78–95. [Google Scholar] [CrossRef]
- Tagami, H.; Ohi, M.; Iwatsuki, K.; Kanamaru, Y.; Yamada, M.; Ichijo, B. Evaluation of the skin surface hydration in vivo by electrical measurement. J. Investig. Dermatol. 1980, 75, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto-Kumasaka, K.; Takahashi, K.; Tagami, H. Electrical measurement of the water content of the stratum corneum in vivo and in vitro under various conditions: Comparison between skin surface hygrometer and corneometer in evaluation of the skin surface hydration state. Acta Derm. Venereol. 1993, 73, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Ha, K.Y.; Xu, X.Y.; Kang, H.C.; Kim, H.; Kim, Y.J. Off-Flavor Removal from Sheep Placenta via Fermentation with Novel Yeast Strain Brettanomyces deamine kh3 Isolated from Traditional Apple Vinegar. Molecules 2021, 26, 5835. [Google Scholar] [CrossRef]
- Xu, L.; Nagata, N.; Nagashimada, M.; Zhuge, F.; Ni, Y.; Chen, G.; Kamei, J.; Ishikawa, H.; Komatsu, Y.; Kaneko, S.; et al. A porcine placental extract prevents steatohepatitis by suppressing activation of macrophages and stellate cells in mice. Oncotarget 2018, 9, 15047–15060. [Google Scholar] [CrossRef]
- Ando, Y.; Sato, F.; Fukunaga, H.; Iwasaki, Y.; Chiba, Y.; Tebakari, M.; Daigo, Y.; Kawashima, J.; Kamei, J. Placental extract suppresses differentiation of 3T3-L1 preadipocytes to mature adipocytes via accelerated activation of p38 MAPK during the early phase of adipogenesis. Nutr. Metab. 2019, 16, 32. [Google Scholar] [CrossRef] [PubMed]
- Ando, Y.; Odawara, E.; Sakai, H.; Sato, F.; Kamei, J. Placental extract suppresses lipid droplet accumulation by autophagy during the differentiation of adipose-derived mesenchymal stromal/stem cells into mature adipocytes. BMC Res. Notes 2023, 16, 338. [Google Scholar] [CrossRef]
- O’Keefe, E.J.; Chiu, M.L. Stimulation of thymidine incorporation in keratinocytes by insulin, epidermal growth factor, and placental extract: Comparison with cell number to assess growth. J. Investig. Dermatol. 1988, 90, 2–7. [Google Scholar] [CrossRef]
- O’Keefe, E.J.; Payne, R.E.; Russell, N. Keratinocyte growth-promoting activity from human placenta. J. Cell Physiol. 1985, 124, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, A.V.; Matts, P.J. Stratum corneum moisturization at the molecular level: An update in relation to the dry skin cycle. J. Investig. Dermatol. 2005, 124, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
- Sandilands, A.; Sutherland, C.; Irvine, A.D.; McLean, W.H. Filaggrin in the frontline: Role in skin barrier function and disease. J. Cell Sci. 2009, 122, 1285–1294. [Google Scholar] [CrossRef]
- Mlitz, V.; Latreille, J.; Gardinier, S.; Jdid, R.; Drouault, Y.; Hufnagl, P.; Eckhart, L.; Guinot, C.; Tschachler, E. Impact of filaggrin mutations on Raman spectra and biophysical properties of the stratum corneum in mild to moderate atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 983–990. [Google Scholar] [CrossRef]
- Caspers, P.J.; Lucassen, G.W.; Carter, E.A.; Bruining, H.A.; Puppels, G.J. In vivo confocal Raman microspectroscopy of the skin: Noninvasive determination of molecular concentration profiles. J. Investig. Dermatol. 2001, 116, 434–442. [Google Scholar] [CrossRef]
- Haddad, A.; Mohiuddin, S.S. Biochemistry, Citric Acid Cycle; StatPearls: Tampa, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK541072/ (accessed on 11 December 2023).
- Howe, J.; Costantino, R.; Slominski, A. On the putative mechanism of induction and regulation of melanogenesis by L-tyrosine. Acta Derm. Venereol. 1991, 71, 150–152. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).