Abstract
This study assesses the efficacy of three white-rot fungi—Bjerkandera adusta, Phanerochaete chrysosporium, and Trametes versicolor—in degrading synthetic dyes and lignin in pulp and paper mill effluents, which annually contribute around 40,000 million cubic meters of dyed waste. Exploiting the structural resemblance of dyes to lignin, the fungi utilize ligninolytic enzymes—lignin peroxidase, manganese peroxidase, and laccase—to break down the pollutants. Initial mycoremediation trials in synthetic dye solutions with Direct black 80, Direct yellow 11, Basic brown 1, Orange II, and Red 8 BLP achieved decolorization rates of 70–80% within 7 days, except for Red 8 BLP. Both soluble and insoluble lignin fractions were significantly reduced, with an overall removal rate of 80–90%. Contrary to prior beliefs about the recalcitrance of azo dyes, B. adusta demonstrated substantial biodegradation capabilities, even on non-lignocellulosic substrates, such as dairy waste. The decolorization efficacy varied with dye structure, suggesting that efficiency should not be judged solely on color reduction. Remarkably, B. adusta also effectively decolorized and removed lignin from actual mill effluents without pH alteration, indicating a viable low-cost bioremediation strategy. This invites further investigation into optimizing B. adusta for industrial wastewater biodecolorization, especially in the field of PAHs (Polycyclic Aromatic Hydrocarbons) and EDCs (Endocrine Disrupting Chemicals).
1. Introduction
Synthetic dyes are organic compounds with chromophore and auxochrome groups coupled to a benzene ring. The color-imparting property of a dye is due to the chromophore group, whereas the auxochrome group facilitates the electrolytic dissociation and interaction property. Synthetic dyes are extensively used in a number of industries [1]. They represent a large group of chemically different compounds, which are classified by their chromophore as azo, anthraquinone, triphenylmethane, heterocyclic, or phthalocyanine dyes [2]. From the second half of the nineteenth century on, synthetic dyes have progressively replaced those of natural origin due to their easy availability in ready-to-apply form, simple application processes, and lower costs for mass use [3].
Azo dyes were one of the first man-made compounds and are now the most common chemical family of dyes. With a yearly production estimated to be around 700,000 tons, they account for up to 70% of the yearly dye production [4]. Azo dyes are characterized by the presence of an azo bond (N=N) along with two or more aromatic rings [5]. The success of azo dyes is due to the simple and cost-effective synthetic procedures, great structural diversity, high chemical stability, and high molar extinction coefficient, making them widely used in textile, color photography, and paper printing, as well as in the food and cosmetic industry [6]. However, it is important to note that only 10–15% of azo dyes are permanently fixed to the material, while most end up in wastewater as effluents or directly discharged into the aquatic environment [7]. The presence of dyes in the receiving water body generates several undesirable environmental effects, such as interference with the photosynthetic activity of plants and algae and increasing levels of turbidity due to their xenobiotic nature [8]. At the same time, even trace amounts of azo dyes dispersed in the environment can lead to severe health hazards as some of them are toxic, carcinogenic, mutagenic, and can hinder various biological activities [9]. Moreover, they are modeled to resist fading on treatment with water, soap, sweat, light, or any type of oxidizing agent, making them recalcitrant and persistent in nature [10]. In the aquatic system, dyes hinder the photosynthetic activity of plants and algae. They are also responsible for an increased level of turbidity, biological oxygen demand (BOD), and chemical oxygen demand (COD) of water bodies due to the increase of organic charge [11]. Moreover, the formation of a thin layer of dye over the water surface blocks oxygen exchange between air and water and, therefore, causes hypoxic or anoxic conditions. Irrigation of agricultural crops with dye-containing wastewater leads to a diminution in the rate of germination and development of plant biomass and increases plants’ susceptibility to a variety of pathogens [12]. The negative effect, in terms of reduced soil fertility and integrity, is the result of the rigorous use of dye-containing effluents for agricultural land irrigation [13]. Azo dyes have also been known for potential health hazards. Several azo dyes have been the cause of DNA damage, which leads to malignant tumors [14]. When these compounds come in contact with the human body through skin or injection and ingestion, they are metabolized by intestinal bacteria and converted into aromatic amines and free radicals. The converted amines are often more toxic, mutagenic, and carcinogenic to humans and animals than the dyes themselves [15].
Dyes can be removed from wastewater by three methods, namely, physical, chemical, and biological [16]. There are several physical methods through which dyes are biodegraded, such as adsorption, membrane filters, coagulation/flocculation, ion exchange, and radiation [17]. In the chemical method, various types of chemical reagents and treatments are applied to degrade the dyes, such as ozone treatment, Fenton’s reagent, photochemical oxidation, sodium hypochlorite, and electrochemical [18]. Unfortunately, almost all of such conventional wastewater treatment systems are inefficient, and existing physical and chemical approaches could be, under certain conditions, discouraging because such treatments are often economically unfeasible, labor intensive, and with limited selectivity and efficiency, as well as generating a huge amount of sludge, which may cause secondary pollution [19,20]. In fact, the degradation of azo dyes produces several aromatic amines, which are more toxic and carcinogenic than the dyes themselves [21].
Therefore, biological methods, i.e., bioremediation, are increasingly welcomed as environmentally friendly treatment options for dye removal under different conditions [22]. These techniques have several benefits over conventional techniques, such as low cost, environment-friendly, safe operation, and less sludge production. Bioremediation is now considered an upcoming [4]. The bioremediation method can use natural and recombinant microorganisms to degrade toxic materials because of flexibility in operating conditions and design. Flexibility in this technique is understood by the fact that they could be employed off-site or on-site, and even plants and algae can be used (phytoremediation). Biodegradation could be defined as the biologically facilitated breakdown of dye molecules into several by-products of water and CO2 by the action of a variety of enzymes [23,24].
Fungi have shown more potential for bioremediation applications in comparison to bacteria due to their ability to overcome certain environmental factors, such as the toxicity of persistent organic compounds as well as heavy metals that might limit or prevent biodegradation by bacteria [25]. The method of using fungi in bioremediation is termed fungal bioremediation or mycoremediation [26].
The application of filamentous white-rot fungi, a renowned group of lignolytic organisms, in the mycoremediation of various xenobiotic substances, including synthetic dyes, has garnered interest for several years [24]. These fungi produce relatively non-specific lignin-degrading enzymes, which, in conjunction with H2O2-producing oxidases, play a pivotal role in the biodegradation of synthetic dyes and the biodecolorization of contaminated effluents [25]. White-rot fungi secrete these lignolytic enzymes extracellularly, initiating lignin oxidation outside the fungal cell. These enzymes are classified into two categories: peroxidases and laccases. Subject to variations in species and environmental conditions, white-rot fungi are capable of activating their extracellular ligninolytic systems to degrade a variety of refractory compounds, such as lignin, aromatic compounds, pharmaceutically active compounds (PhACs) [27], endocrine disruptor compounds (EDCs) [28], synthetic dyes [26], and even for the co-remediation of heavy metal and organic pollutants [29]. While some fungi achieve decolorization solely through a biosorption mechanism, white-rot fungi employ both biosorption and degradation, which can occur either simultaneously or sequentially, with degradation representing an oxidative process that can completely break down dye molecules into CO2 and water.
White-rot fungi, such as Coriolus spp., Phlebia spp. [27], and Clitocybula dusenii [28] have successfully decolorized diluted artificial and real effluents. Notably, the genera Bjerkandera, Phanerochaete, and Trametes are recognized for their potent dye-degrading capabilities [29]. Furthermore, they have shown the ability to grow on waste-derived media, such as dairy residues [30].
This research investigates the potential of three white-rot fungal species—Bjerkandera adusta, Phanerochaete chrysosporium, and Trametes versicolor—cultivated on dairy side-product from cheese processing for biodecolorizing five azo dyes commonly found in industrial effluents (Orange II, Red 8BLP, Direct black 80, Direct yellow 11, Basic brown 1). Subsequently, we conducted preliminary tests on actual pulp and paper mill effluents containing a mixture of these dyes. Considering the nutritional value of dairy residues, this study proposes using these substrates for the large-scale cultivation of these microorganisms. The sustainable valorization of dairy wastewater could thus facilitate its use as a microbial growth medium [31,32]. This research advances toward the ultimate goal of creating a model for a circular and sustainable bioeconomy through the utilization of bioresources and waste valorization.
2. Materials and Methods
2.1. Materials, Microrganisms, and Reagents
The fungi Bjerkandera adusta (DSM 23426), Phanerochete chrysosporium (DSM 1547), and Trametes versicolor (DSM 3026) were provided by Leibniz-Institute DSMZ (Braunschweig, Germany) as agarized slants. Reagents were purchased from Sigma-Aldrich (St. Louis, UM, USA). Orange II, Red 8BLP, Direct yellow 11, and Basic brown 1 were in liquid form, whereas the Direct black 80 was in the form of solid fine powder (KAPP-CHEMIE GmbH, Miehlen, Germany) (Table 1). Dairy wastewater from cheese processing and pulp and paper mill effluent were supplied by local industries (Granarlo S.p.A., Bologna, Italy and NCR Biochemical, Castello D’argile (BO), Bologna, Italy).

Table 1.
Chemical, structural, and spectroscopic characteristics of different dyes.
Dye absorption peak detection was carried out by means of spectrophotometric measures (OndaUV-30 Scan, Giorgio Bormac, Modena, Italy) at proper absorption wavelenght.
2.2. Inoculum Preparation
An agar surface of about 5 × 5 mm was inoculated in 20 mL of malt extract medium (ME) (malt extract, 30 g/L; soy peptone, 3 g/L). The culture was then incubated under agitation (150 rpm) for 72 h at 24 °C. 1 mL of the broth containing fungal mycelia was added with 500 µL of glycerol and stored at −20 °C (master cell banks, MCB). Working cell banks (WCB) were obtained from the MCB following the same protocol and used for seed culture. The content of a whole cryovial was poured into 20 mL of sterilized ME medium and incubated under agitation (150 rpm) for 96 h at 24 °C. Once mycelia had grown, they were inoculated on a Petri dish containing agarized ME medium and incubated for 7 days at 24 °C.
An agar surface of about 5 × 5 mm was then used to inoculate 100 mL Erlenmeyer flasks containing process culture medium (PCM), consisting of dairy wastewater added with KH2PO4 (1 g/L), MgSO4·7H2O (0.5 g/L), CaCl2·2H2O (0.5 g/L) and ammonium tartrate (0.2 g/L). The chemical composition of dairy wastewater is reported elsewhere [30]. Before inoculation, media pH was corrected to 5.5 with HCl 1 M solution. Cell cultures were then incubated under agitation (60 rpm) for 7 days at 24 °C and used as pre-inocula for subsequent tests.
2.3. Decolorization of Artificial Dye-Baths by White-Rot Fungi
A single dye was added to a 1 L-Erlenmeyer flask containing 500 mL of distilled water to obtain a final concentration of 20 ppm. Then, the pH was adjusted to 5.5 with a HCl 1 M solution. The medium was inoculated using the pre-inoculum of B. adusta previously grown in dairy waste medium and incubated for 7 days under agitation (60 rpm) at 24 °C. Samples (1 mL) were daily withdrawn and analyzed by spectrophotometer at the specific wavelength for each dye. The same experimental procedures have been used for P. chrysosporium and T. versicolor. Blank experiments were performed using the medium containing each dye without the inoculum of fungi. All tests were performed in triplicate.
2.4. Lignin Degradation Capacity
In 250 mL of Erlenmeyer flasks, 100 mL of PCM was added with soluble and insoluble lignin in equal amounts to give a final concentration of 100 mg/L. The pH was adjusted to 5.5 with a solution of HCl 1 M. After thawing, 1 cryovial of each fungus was inoculated, and flasks incubated for 10 days at 35 °C under mild stirring (60 rpm) and 10 mL samples were withdrawn at 0, 3, 5, 7, and 10 days. All tests were performed in triplicate.
As a control, the same experiment was carried out using ME as a culture medium. Samples were analyzed using the INNVENTIA Test Methods L 2:2016 method to determine the lignin content.
2.5. Lignin Analysis
Lignin quantification was performed using the INNVENTIA method (Test Methods L 2:2016) based on the TAPPI method [31]. In a 50-mL Erlenmeyer flask, 100 mg of the sample is weighed (if the sample is wet, it needs to be dried to 90%), and H2SO4 72% (1 mL) is added. It is kept under stirring until the sample is completely dissolved at 30 °C for 1 h. Distilled water is added (28 mL) and autoclaved at 120 °C for 1 h. It is cooled to 80 °C and filtered through a previously weighed glass fiber filter. The filtrate, transferred to a second flask, is used for the determination of the soluble lignin fraction by measuring absorbance at λ = 205 nm. The precipitate is washed with warm water to a neutral pH. The filter is dried at 105 °C overnight, and the lignin fraction is determined by weight difference.
The weight of acid-insoluble lignin (AIR, acid-insoluble residue) expressed in mg will be:
where m is the weight of the lignin residue after drying expressed in g and M is the weight of the sample before acid hydrolysis expressed in g. The weight of acid-soluble lignin (ASL) expressed in mg will be:
where A is the absorbance at 205 nm; D is the dilution factor; V is the volume of the filtrate in L (here 0.029 L); a is the molar extinction coefficient of lignin, in g/L cm (here 110 g/L cm, in accordance with TAPPI UM 250); b is the optical path (here 1 cm); and M is the weight of the sample before acid hydrolysis, in g. AIR and ASL lignin weights were converted to mg, multiplying the result by 1000. The total lignin content (expressed in mg) has been expressed as:
TLC = AIR + ASL
2.6. Decoloration and Lignin Degradation of Pulp and Paper Mill Effluent: Preliminary Tests
500 mL of pulp and paper mill dark-colored effluent containing lignin and mixed synthetic dyes in unknown proportion and concentration was added to a 1 L-Erlenmeyer flask. Experimental assays were carried out with pH correction to 5.5 with a HCl 1 M solution and without correction (pH 7.5–7.8). As a negative control, the effluent was submitted to the same conditions, with and without pH correction and without being inoculated. Flasks were inoculated with B. adusta, P. chrysosporium, and T. versicolor pre-inoculum separately and incubated under mild agitation (60 rpm) for 7 days at 24 °C. Samples were withdrawn daily and analyzed by spectrophotometer at the specific wavelength for each dye. All tests were performed in triplicate, and blank experiments were performed using the pulp and paper mill dark-colored effluent without the inoculum of fungi.
2.7. Visible Decolorization Efficiency
Decoloration in the liquid medium was followed by measuring the absorbance of filtered broth samples. Decolorization was measured as a percentage value and calculated as reported by Lopez et al. [32]. Briefly, the cell-free supernatant sample was read in the spectrophotometer at the reference absorbance wavelength (λmax) of each dye. The decolorization efficiency was calculated according to the following equation:
where:
Absinitial = initial absorbance after the inoculation of the fungus;
Absfinal = absorbance after the incubation period.
3. Results
3.1. Decoloration of Artificial Dye-Baths with B. adusta
The decolorization ability of B. adusta was tested in artificial aqueous dye baths corresponding to each dye type. Dyes decolorization was monitored daily, as evidenced by the change in absorbance values over time (Figure 1).
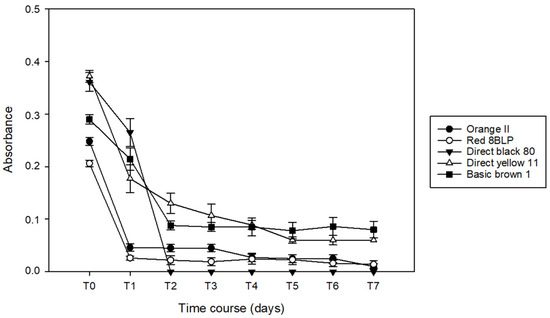
Figure 1.
Absorbance over time for dyes decolorization by B. adusta at 20 ppm in artificial single dye bath.
B. adusta showed a marked degradative capacity of Orange II and Red 8BLP on the first day of incubation (81.4% and 87.4%, respectively). In contrast, notable decolorization effects of the remaining three dyes occurred in 48 h. In particular, Direct black 80 was almost completely decolorized after two days (95.5%), and a reduction of 69.1% and 69.9% of Direct yellow 11 and Basic brown 1, respectively, was observed. Between days 1 and 2, the decolorization rate starts to slow down, almost completely in the case of Orange II, Red 8BLP, and Direct black 80. Direct yellow 11 and Basic brown 1 reach a maximum decolorization of 72.3% and 70.1%, respectively, from days 3 to 7.
For the Red 8BLP dye, a marked decolorization (87.38%) was observed after the first day of incubation, followed by a stationary phase reaching a reduction of 93.20% at the end of the process.
After 7 days of incubation with B. adusta, all dye solutions appeared cleared and transparent, except for the presence of grown fungal mycelia as shown in Figure 2for three dyes. As far as the blank experiment is concerned, no changes in absorbance, and consequently in concentration, were detected for the investigated dyes during the entire duration of the test.
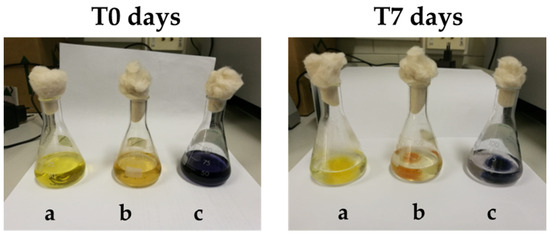
Figure 2.
The visual effect of decolorization after 7 days of treatment for artificial dye bath containing (a) Direct yellow 11; (b) Basic brown 1; (c) Direct black 80 treated with B. adusta.
3.2. Decoloration of Artificial Dye-Baths with P. chrysosporium
P. chrysosporium showed a different capacity for dye removal in comparison with B. adusta. Almost all dyes, except for the Basic brown 1 on the first day of incubation, did not change absorbance, indicating that decoloration did not occur (Figure 3). From day 2 on, decoloration proceeded, with a complete removal only of Direct black 80 on the 5th day.
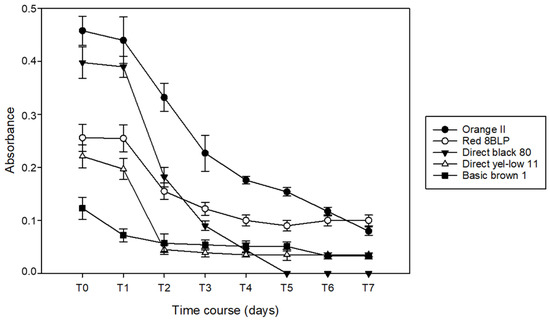
Figure 3.
Absorbance over time for dye decolorization by P. chrysosporium at 20 ppm in artificial single dye bath.
Orange II was removed at 83%, as well as Brown at 73%, whereas Red 8BLP reached a value of 61%. At the end of the 7th day, Direct yellow 11 was decolored at 84% efficiency, the majority of decolorization activity occurring on day 2.
After 7 days of incubation with P. chrysosporium, all dye solutions appeared almost completely cleared, except for the presence of grown fungal mycelia.
Overall, except in the case of Direct black 80, the decoloration capacity of P. chrysosporium reached a plateau after days 2–3. Probably, by waiting a few additional days, it would have been possible to further reduce the concentration of Orange II, until complete decoloration. Regarding the blank experiment, no change in absorbance was detected for the investigated dyes during the entire duration of the test.
3.3. Decoloration of Artifical Dye-Baths with T. versicolor
T. versicolor showed similar behavior considering the final decolorization capacity but with different removal rates. Except for Red 8BLP, the other dyes were removed in 7 days at more than 80%, in particular, Orange II at 95%, Direct black 80 at 100%, Direct yellow 11 at 81%, and Basic brown 1 at 90%. Red 8BLP was removed at 70%, and decolorization started only on days 2–3 (Figure 4).
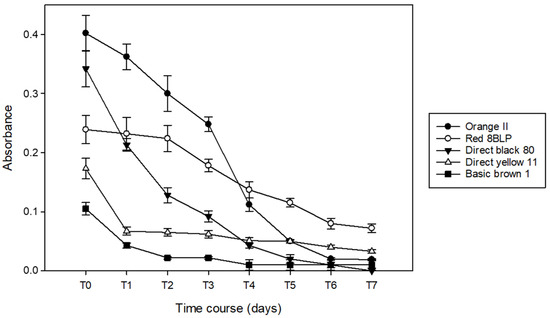
Figure 4.
Absorbance over time for dye decolorization by T. versicolor at 20 ppm in artificial single dye bath.
Otherwise, the decrease of absorbance associated with other dyes progressively proceeded from day 1 to day 7 and started immediately at the beginning of incubation.
After 7 days of incubation with T. versicolor, all dye solutions appeared almost completely cleared, except for the presence of grown fungal mycelia. Also, in this case, the blank experiment showed no change in absorbance in the samples taken throughout the duration of the test.
3.4. Lignin Degradation Capacity
The three fungi showed a significant capacity to remove AIR and any effect on ASL (Figure 5). Insoluble lignin fraction (AIR) was eliminated almost completely by B. adusta and T. versicolor (97% and 90% reduction, respectively), achieving a 74% by P. chrysosporium as shown in Figure 5a. Otherwise, soluble fraction (ASL) remains unchanged throughout the entire test (Figure 5b).
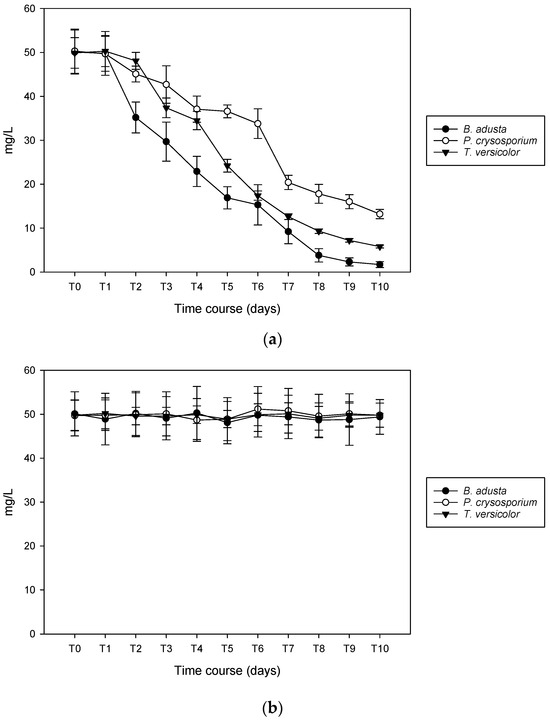
Figure 5.
Biodegradation of AIR (a) and ASL (b) fraction of lignin by B. adusta, P. chrysosporium, and T. versicolor.
AIR removal showed a better efficiency for all three fungi when grown in the PCM than in ME. In fact, in this case, removal efficiency does not exceed 55% in any of the cases of B. adusta and T. versicolor and 50% in the case of P. chrysosporium.
3.5. Mycoremediation and Lignin Removal of Colored Pulp and Paper Mill Effluent: Preliminary Test
Following the same conditions applied in the tests carried out in aqueous solutions of separated dyes, mycoremediation and lignin removal capacity of B. adusta, P. chrysosporium, and T. versicolor were evaluated in real samples of pulp and paper mill effluent, containing a mix of the five dyes previously tested, but at unknown proportion and concentrations.
Total lignin content (TLC) in pulp and paper mill sample was quantified as 10.2 ± 2.6 mg/L, where ASL corresponded to 0.46 ± 0.01 mg/L.
The effluent was markedly black-colored. Absorbance has been measured at λ = 599 nm, corresponding to the peak of black. Based on the absorbance value found for the effluent, compared with the absorbance at 20 ppm dye concentration in aqueous solution, the concentration of the dye in the effluent has been arbitrarily estimated to be about 40–50 ppm. The decolorization rate at corrected pH = 5.5 and without correction at pH = 7.8 are reported in Figure 6.
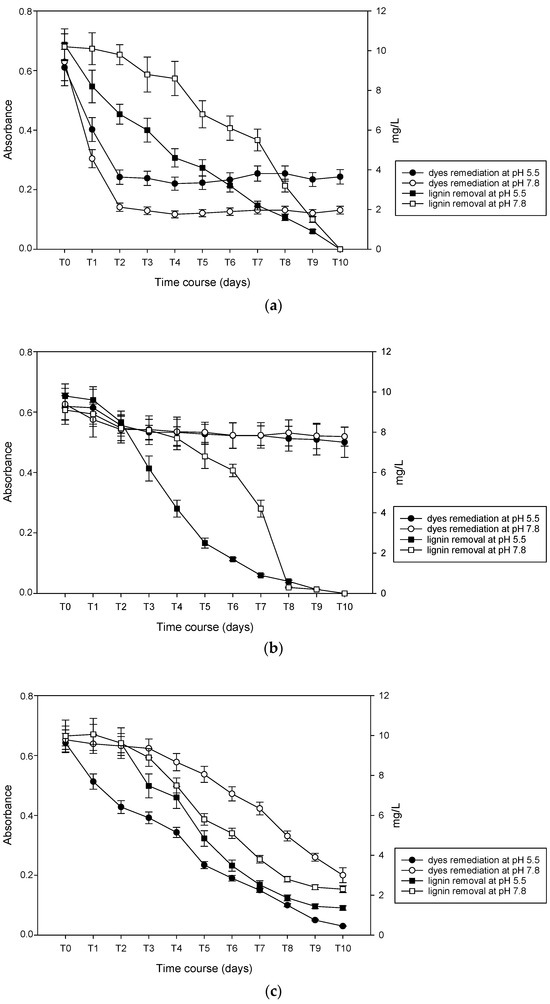
Figure 6.
Mycoremediation and total lignin content (TLC) removal by (a) B. adusta; (b) P. chrysosporium; and (c) T. versicolor. Lignin degradation values are referred to as the secondary axis (on the right).
The fungi showed diverse courses of dye remediation and lignin removal. B. adusta has been shown to be able to significantly decolorize pulp-and-paper mill effluent containing a mixture of dyes. The sharpest fall in dye concentration was evident after 2 days (77.6% and 60.1%, at pH = 5.5 and 7.8, respectively), and a significant decrease in decolorization occurred till the end of the experiment. The final decolorization was 79.2% at pH = 5.5 and 63.4% at pH = 7.8, respectively, in 7 days of treatment. The decolorization trend is similar in both cases to those obtained for Direct yellow 11 and Basic brown 1 in an aqueous solution. TLC removal was complete (100%) after 10 days of treatment in both pH conditions, with different trends at pH = 5.5 and 7.8, respectively. At pH = 7.8, it seems to have a sort of lag phase before starting to remove lignin. P. chysosporium inoculated in pulp and paper mill effluent decolorized at a very low percentage compared with artificial dye baths (around 20%) at both pH conditions, whereas lignin removal proceeded up to a complete clearing (100%) from day 2 to day 8. At pH = 5.5, removal kinetic proceeded constantly, whereas at higher pH, as B. adusta, it showed a long lag phase. Finally, T. versicolor exhibits good capacity for lignin removal, 86.3% at pH = 5.5 and 76.9% at pH = 7.8.
Dyes remediation run with diverse trends depending on pH, achieving almost complete decolorization at pH = 5.5 (95.3%), lowering the efficiency to 69.4% at pH = 7.8.
4. Discussion
Large quantities of effluent-containing synthetic dyes are produced annually from industries, pulp and paper mills being one of them as a result of paper dyeing processes [33].
According to FAOSTAT [34], it has been estimated that the total effluent produced annually from these mills is about 40,000 million cubic meters, assuming an average of 200 cubic meters of effluent per ton of pulp and paper produced. This study aimed to investigate the possibility of purifying these effluents by using white-rot fungi, which have shown promising degradative abilities toward polycyclic aromatic compounds [35]. Specifically, the ability of B. adusta, P. chysosporium, and T. versicolor to degrade five different dyes in artificial dye baths, Orange II, Red 8BLP, and Direct yellow 11. Basic brown 1 and Direct black 80, currently used in paper industries, have been tested, as well as their capacity to remove lignin. The main findings of this research are displayed in Table 2.

Table 2.
Decolorization and delignification performance of the three white-rot fungi examined in this research (C = complete; NC = not complete).
The lignolytic enzyme activities of white rot fungi have already been investigated in a previous study [30]. The main enzymes associated with its ability to degrade lignin are lignin-peroxidase (LiP), manganese-peroxidase (MnP), and laccase [36]. As these enzymes are non-specific, they can degrade both lignin and various other pollutants, including dyes. In particular, the similarity of the chemical structure of commercial dyes with the structure of lignin could underlie the biodegradation capacity [37]. Although the presence of synthetic dyes often reduced the mycelial growth rate of fungi, we found no or only slight growth reduction in all cases (data not reported). The time courses of treatments in a single artificial dye bath have evidenced similar trends when treated with B. adusta. In the first 24 h, more than 80% of decolorization has occurred, except for Direct yellow 11 and Basic brown 1, the decolorization of which was slower, and after 7 days of treatment, it does not reach values above 75%, substantially maintaining a plateau from day 2 to day 7. Differently, P. chysosporium exhibited a lag phase of 24 h before starting dye degradation, proceeding almost constantly in degrading capacity up to day 5. T. versicolor, similar to the other two white rot fungi considered in this study, had a high capacity to remove Direct black 80 and Orange II; in particular, Direct black 80 is the only dye that was completely degraded by all three fungi, even though, interestingly, with different trends in the 7 days. Overall, the three fungi have shown a significant mycoremediation capacity on selected dyes, achieving a decolorization capacity that is always more than 80% for Orange II and more than 70% for Direct Yellow 11 and Basic brown 1. The results obtained with red 8BLP showed a certain variability with discoloration yields ranging from 90% with B. adusta to 61% using P. chrysosporium.
Easily decolorization obtained in our experiments was rather unexpected because azo dyes are all usually considered to be recalcitrant to decolorization due to their chemical structures [38]. Moreira et al. [39] thoroughly studied decolorization by white rot fungi, concluding that the dye concentration strongly affected the decoloration activity of the strain. Here, the dye concentration was relatively low compared with other studies [28,29], but they were chosen based on the same order of magnitude of the pulp and paper effluent to be treated. Different from other studies [40] reporting that fungal activity started on the 6th day of incubation, in our study, decolorization started in all cases on days 1 and 2. Riegas-Villalobos et al. [41] have noticed that better dye removal efficiency could be obtained if fungi are grown on a lignocellulosic carbon source before being inoculated in the colored mixture. A possible explanation given by those authors is that lignocellulosic carbon source could stimulate extracellular laccase activity, improving by about 30% removal in the first 24 h. Otherwise, in our experiments, all fungi started mycoremediation activity at the latest after 48 h since inoculum (P. chrysosporium) or immediately after inoculum. They have been grown on liquid dairy waste, a non-lignocellulosic carbon source, but nevertheless, high dye removal percentages were obtained in the very first days of treatment. Feijoo et al. [42] reported that peroxidase activity in Bjerkandera spp. and P. chrysosporium was stimulated by growing in cheese whey, and Nawaz and Gunasekaran [43] have demonstrated how lactose in culture media induced the production of hydrolytic and cellulolytic enzymes in white rot fungi. Although it certainly deserves more in deep studies, this may mean that, for example, in B. adusta, decolorization activity was exerted, firstly activating peroxidase and then laccase, which explains its activity only on day 2. This is consistent with the visible rapid decolorization trends found in the artificial dye baths and 1 day of lag in AIR removal by B. adusta (see Figure 6a). The mechanisms of enzymes involved in dye degradation are not obvious, and very little is known about their combined role and their interactions during the degradation process [44], but it is known that laccase normally oxidizes the azo interconnections, whereas peroxidases proceed with further degradations involving aromatic cleavage, desulfonation, deamination, and phenolic oxidation. Peroxidase activity could be performed in the first 24 h, being responsible for the rapid degradation of Orange II and Red 8BLP structures. In 48 h, Direct black 80 was completely degraded, probably by the peroxidase’s activity in the first 24 h on the three sulfonic groups, as well as on the two amino groups and phenolic. In the following 24 h, laccase acts on the three azo bonds. In 48 h, decolorization also occurred for Direct brown 1, which has two azo bonds, and slower for Direct yellow 11, which has only one azo bond. It seems that peroxidase activity occurred in the first 24 h and almost completely degraded Orange and Red, then laccase activity took place, completing the degradation with the cleavage of the azo bond at a rate depending on the number of N=N interconnections in the dye molecules.
P. chrysosporium cultivated on dairy waste needed 1–2 days of adaptation to process media conditions, both in artificial dye baths and real effluent, to start acting. T. versicolor showed intermediate behavior, degrading all dyes except for Red 8BLP, which is characterized by the simplest molecular structure.
It is worth noting that the overall complexity alone is not an indication of the decolorization of a particular dye by enzymes, and even small structural differences can largely affect the decolorization process. Moreover, on the basis of our results, it is obvious that the efficiency of decolorization is difficult to judge by comparing the percentage of color reduction since the results vary widely with the dye and its concentration. In addition, during decolorization, the effluent color can change, and reduced absorbance at the initial λmax does not guarantee lower values in the whole absorption spectrum.
The three fungi showed similar behavior towards lignin removal, both soluble and insoluble. Surprisingly, they are able to remove a very high amount of AIR, leaving the concentration of ASL almost unchanged. An explanation for such a strange behavior can be found in the mechanism of lignin removal. It is well-known that white rot fungi degrade lignin by means of oxidative enzymatic pathways, but several studies [38,45,46] reported that lignin degradation due to lignolytic enzyme activities started after 8–10 days since inoculum. In this case, it is not impossible that a sort of absorption of AIR on hyphae could have occurred before starting degradation, being extracellular enzymes involved in decoloration.
When B. adusta was inoculated on pulp and paper mill effluent samples, it confirmed the rapid decolorization activity even in dye mixtures. pH correction was set up to allow the fungus to develop in its optimal growth conditions. Although B. adusta is quite versatile (it was established that the fungus grows in a pH range of 3.5–7.5 [47]), pH = 7.8 is borderline, and this could explain the worst decolorization performance.
P. chrysosporium in real pulp and paper mill effluent showed a very low decoloration capacity (less than 20%); maybe some kind of inhibition occurred in enzyme induction. This seems to confirm the fact that mycoremediation proceeds as a degradation mechanism, whereas lignin as adsorption, without enzyme intervention, at least in the first 7–10 days of treatments. Further investigations are surely needed. Adsorption, which is more effective when biomass is under optimal growth conditions, pH = 5.5. T. versicolor exerted decolorization activity with significant results but followed different trends depending on pH, which seems not to affect lignin removal capacity.
Another indication that we can deduce from the results is that, to a general extent, the dye-removal activity of white rot fungi depends on biomass growth-correlated factors, being higher when biomass grows in optimal conditions. In view of industrial applications, the attempt to avoid pH correction has been conducted from the perspective of operational simplification and cost reduction. The final condition will result in a trade-off between costs and decolorization efficiency, and surely, although promising, preliminary results obtained will deserve further experiments.
Unfortunately, the principal limitation to the full-scale industrial application of fungal-assisted processes is still the high dependence on operational parameters, such as optimal nutrient composition, medium agitation, pH, oxygen, and temperature conditions, as well as dye concentration. Additionally, more careful investigations are needed on fungal pathways of degradation in order to understand why certain dyes are easily degraded whereas others are not, or on the potential toxicity of the degradation metabolites. On the other hand, excessive growth of fungi causing reactor-clogging [48], bacterial contamination inhibiting fungal decoloration [49], and loss of the extracellular enzymes and mediators essential for dye degradation [50] have so far hampered the industrial development of fungal bioreactors.
In this study, white rot fungi were grown on dairy wastewater as a carbon source. Microbial biotechnology is essential for the development of circular bioeconomy by integrating wastewater valorization and resource recovery into the production of biomass to be used in its turn, as in this case, in effluents bioremediation. Nowadays, there is an urgent need to provide technological solutions that can adopt the principles of the circular economy—eliminate as much waste and pollution as possible, circulate products and materials, and regenerate nature. A sustainable circular bioeconomy is an element of the circular economy that is connected to all processes, products, and technologies that are “bio”, and aims at sustainability.
Finally, even though it was confirmed that white-rot fungi have great potential in mycoremediation and lignin removal, B. adusta seems to be the most promising to be applied in pulp and paper effluent treatments. Our study lay the ground to an integration of two different supply chain, that can be linked through B. adusta, able both to growth on a carbon source as dairy wastewater and to efficiently decolorize pulp and paper mill wastewater.
5. Conclusions
In conclusion, this study has demonstrated the potential of white-rot fungi, particularly Bjerkandera adusta, Phanerochaete chrysosporium, and Trametes versicolor, to significantly contribute to the purification of industrial effluents laden with synthetic dyes. The impressive decolorization capabilities observed in our experiments challenge the traditionally held view of azo dye recalcitrance, showcasing the fungi’s ability to degrade dyes rapidly and effectively. This rapid decolorization, especially by B. adusta, suggests a promising approach for the treatment of pulp and paper mill effluents without the need for pH adjustments, which could lead to cost-effective mycoremediation strategies.
Our findings also suggest that the structural complexity of dyes alone is not a definitive indicator of their susceptibility to enzymatic decolorization. Even slight structural differences among dyes significantly influence the decolorization process, rendering the assessment of efficiency based on color reduction percentages alone inadequate. The study has further revealed that white-rot fungi can decolorize effluents and degrade lignin effectively, with a noteworthy preference for Acid-Insoluble Residue (AIR) over Acid-Soluble Lignin (ASL). This predilection may be attributed to the absorption of AIR onto fungal hyphae prior to enzymatic degradation, highlighting a complex interplay between physical and biochemical processes in fungal bioremediation.
The utilization of dairy waste as a growth medium for these fungi not only underscores the versatility and adaptability of these organisms but also represents an innovative step towards the sustainable valorization of waste. This approach aligns with the principles of a circular bioeconomy, which aims to minimize waste and pollution, circulate products and materials at their highest utility, and regenerate natural systems. By integrating wastewater valorization into microbial biotechnology, we can advance toward a more sustainable, circular approach to industrial processes.
Although further research is required to optimize these processes for industrial application, the preliminary results of this study are promising. There is a need for more concentrated research effort in this area, especially on decolorization and detoxification of real wastewater by white rot fungi or enzymes. Future work on the fungal degradation of dyes should focus on minimizing the limitation factors that affect fungal activities. To develop fungal activities for better performance, the re-examination of recent and early successful studies should be carried out. An effective biodegradation process should consider degradation pathways, environmental factors, degradation rate, and degradation mechanisms that affect the removal of dye pollutants. It is highly imperative to ensure that the degraded products are not toxic to aquatic life or plants. They pave the way for a potential symbiotic relationship between the dairy industry and pulp and paper mills through the application of B. adusta, which can thrive on dairy waste while efficiently decolorizing paper mill wastewater. Such integration could significantly contribute to a more sustainable and circular bioeconomy, addressing the urgent need for technological solutions that embrace the principles of circularity and sustainability.
Author Contributions
Conceptualization, S.C., S.M. and E.T.; methodology, D.S., F.M. and S.C.; investigation, D.S. and F.M., data curation, I.G.; writing—original draft preparation, I.G., S.C. and E.T.; writing—review and editing, S.C. and E.T.; supervision, S.M. and S.V.; Funding: S.M. All authors have read and agreed to the published version of the manuscript.
Funding
University of Ferrara, Grant to Stefano Manfredini (SM-FAR-2022).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors want to acknowledge Simone Pellizzari, Riccardo Blo, and Saverio Ganzerli (NCR Biochemical, Castello D’Argile, BO, Italy) for the kind support and precious suggestions during all the stages of the experimental research and conceptualization.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Slama, H.B.; Chenari Bouket, A.; Pourhassan, Z.; Alenezi, F.N.; Silini, A.; Cherif-Silini, H.; Oszako, T.; Luptakova, L.; Golińska, P.; Belbahri, L. Diversity of Synthetic Dyes from Textile Industries, Discharge Impacts and Treatment Methods. Appl. Sci. 2021, 11, 6255. [Google Scholar] [CrossRef]
- Gürses, A.; Açıkyıldız, M.; Güneş, K.; Gürses, M.S. Classification of Dye and Pigments. In Dyes and Pigments; Gürses, A., Açıkyıldız, M., Güneş, K., Gürses, M.S., Eds.; SpringerBriefs in Molecular Science; Springer International Publishing: Cham, Switzerland, 2016; pp. 31–45. ISBN 978-3-319-33892-7. [Google Scholar]
- Tamburini, D.; Breitung, E.; Mori, C.; Kotajima, T.; Clarke, M.L.; McCarthy, B. Exploring the Transition from Natural to Synthetic Dyes in the Production of 19th-Century Central Asian Ikat Textiles. Herit. Sci. 2020, 8, 114. [Google Scholar] [CrossRef]
- Akansha, K.; Kaur, T.; Yadav, A.; Kour, D.; Rai, A.K.; Singh, S.; Mishra, S.; Kumar, L.; Miglani, K.; Singh, K.; et al. Microbe-Mediated Remediation of Dyes: Current Status and Future Challenges. J. Appl. Biol. Biotech 2023, 11, 1–23. [Google Scholar] [CrossRef]
- Omar, A.Z.; El-Rahman, M.A.; Hamed, E.A.; El-Sadany, S.K.; El-atawy, M.A. Synthesis, Spectroscopic Characterization and Dyeing Performance of Novel Bis Azo Dyes Derived from Benzidine. Sci. Rep. 2023, 13, 7826. [Google Scholar] [CrossRef]
- Barciela, P.; Perez-Vazquez, A.; Prieto, M.A. Azo Dyes in the Food Industry: Features, Classification, Toxicity, Alternatives, and Regulation. Food Chem. Toxicol. 2023, 178, 113935. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Yang, Z.; Xing, L.; Zhang, X.; Li, X.; Zhang, D. Recent Advances in the Biodegradation of Azo Dyes. World J. Microbiol. Biotechnol. 2021, 37, 137. [Google Scholar] [CrossRef]
- Hashemi, S.H.; Kaykhaii, M. Chapter 15-Azo Dyes: Sources, Occurrence, Toxicity, Sampling, Analysis, and Their Removal Methods. In Emerging Freshwater Pollutants; Dalu, T., Tavengwa, N.T., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 267–287. ISBN 978-0-12-822850-0. [Google Scholar]
- Chung, K.-T. Azo Dyes and Human Health: A Review. J. Environ. Sci. Health Part C 2016, 34, 233–261. [Google Scholar] [CrossRef]
- Waghmode, T.R.; Kurade, M.B.; Sapkal, R.T.; Bhosale, C.H.; Jeon, B.-H.; Govindwar, S.P. Sequential Photocatalysis and Biological Treatment for the Enhanced Degradation of the Persistent Azo Dye Methyl Red. J. Hazard. Mater. 2019, 371, 115–122. [Google Scholar] [CrossRef]
- Islam, T.; Repon, M.R.; Islam, T.; Sarwar, Z.; Rahman, M.M. Impact of Textile Dyes on Health and Ecosystem: A Review of Structure, Causes, and Potential Solutions. Environ. Sci. Pollut. Res. 2023, 30, 9207–9242. [Google Scholar] [CrossRef]
- Samchetshabam, G.; Hussan, A.; Gon Choudhury, T.; Gita, S.; Soholars, P.; Hussan, A. Impact of Textile Dyes Waste on Aquatic Environments and Its Treatment. Environ. Ecol. 2017, 35, 2349–2353. [Google Scholar]
- Saha, J.K.; Selladurai, R.; Coumar, M.V.; Dotaniya, M.L.; Kundu, S.; Patra, A.K. Impact of Different Developmental Projects on Soil Fertility. In Soil Pollution-An Emerging Threat to Agriculture; Saha, J.K., Selladurai, R., Coumar, M.V., Dotaniya, M.L., Kundu, S., Patra, A.K., Eds.; Environmental Chemistry for a Sustainable World; Springer: Singapore, 2017; pp. 251–269. ISBN 978-981-10-4274-4. [Google Scholar]
- Kumari, R.; Sunil, D.; Ningthoujam, R.S.; Kumar, N.A. Azodyes as Markers for Tumor Hypoxia Imaging and Therapy: An up-to-Date Review. Chem.-Biol. Interact. 2019, 307, 91–104. [Google Scholar] [CrossRef]
- Siddiqui, S.I.; Allehyani, E.S.; Al-Harbi, S.A.; Hasan, Z.; Abomuti, M.A.; Rajor, H.K.; Oh, S. Investigation of Congo Red Toxicity towards Different Living Organisms: A Review. Processes 2023, 11, 807. [Google Scholar] [CrossRef]
- Donkadokula, N.Y.; Kola, A.K.; Naz, I.; Saroj, D. A Review on Advanced Physico-Chemical and Biological Textile Dye Wastewater Treatment Techniques. Rev. Environ. Sci. Biotechnol. 2020, 19, 543–560. [Google Scholar] [CrossRef]
- Vishnu, D.; Dhandapani, B.; Authilingam, S.; Sivakumar, S.V. A Comprehensive Review of Effective Adsorbents Used for the Removal of Dyes from Wastewater. Curr. Anal. Chem. 2022, 18, 255–268. [Google Scholar] [CrossRef]
- Asaithambi, P.; Yesuf, M.B.; Govindarajan, R.; Hariharan, N.M.; Thangavelu, P.; Alemayehu, E. A Review of Hybrid Process Development Based on Electrochemical and Advanced Oxidation Processes for the Treatment of Industrial Wastewater. Int. J. Chem. Eng. 2022, 2022, e1105376. [Google Scholar] [CrossRef]
- Piaskowski, K.; Świderska-Dąbrowska, R.; Zarzycki, P.K. Dye Removal from Water and Wastewater Using Various Physical, Chemical, and Biological Processes. J. AOAC Int. 2018, 101, 1371–1384. [Google Scholar] [CrossRef]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of Various Recent Wastewater Dye Removal Methods: A Review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Takahashi, H.; Hashimoto, Y. Formaldehyde-Mediated Modification of Natural Deoxyguanosine with Amines: One-Pot Cyclization as a Molecular Model for Genotoxicity. Bioorg. Med. Chem. Lett. 2001, 11, 729–731. [Google Scholar] [CrossRef]
- Covino, S.; Stella, T.; Cajthaml, T. Mycoremediation of Organic Pollutants: Principles, Opportunities, and Pitfalls. In Fungal Applications in Sustainable Environmental Biotechnology; Purchase, D., Ed.; Fungal Biology; Springer International Publishing: Cham, Switzerland, 2016; pp. 185–231. ISBN 978-3-319-42852-9. [Google Scholar]
- Ajaz, M.; Shakeel, S.; Rehman, A. Microbial Use for Azo Dye Degradation—A Strategy for Dye Bioremediation. Int. Microbiol. 2020, 23, 149–159. [Google Scholar] [CrossRef]
- Pinheiro, L.R.S.; Gradíssimo, D.G.; Xavier, L.P.; Santos, A.V. Degradation of Azo Dyes: Bacterial Potential for Bioremediation. Sustainability 2022, 14, 1510. [Google Scholar] [CrossRef]
- Noman, E.; Al-Gheethi, A.; Mohamed, R.M.S.R.; Talip, B.A. Myco-Remediation of Xenobiotic Organic Compounds for a Sustainable Environment: A Critical Review. Top. Curr. Chem. 2019, 377, 17. [Google Scholar] [CrossRef]
- Rajhans, G.; Barik, A.; Sen, S.K.; Raut, S. Degradation of Dyes by Fungi: An Insight into Mycoremediation. BioTechnologia 2021, 102, 445–455. [Google Scholar] [CrossRef]
- Zhuo, R.; Fan, F. A Comprehensive Insight into the Application of White Rot Fungi and Their Lignocellulolytic Enzymes in the Removal of Organic Pollutants. Sci. Total Environ. 2021, 778, 146132. [Google Scholar] [CrossRef]
- Chen, S.; Zhu, M.; Guo, X.; Yang, B.; Zhuo, R. Coupling of Fenton Reaction and White Rot Fungi for the Degradation of Organic Pollutants. Ecotoxicol. Environ. Saf. 2023, 254, 114697. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, X.; Zhang, M.; Zhu, Y.; Zhuo, R. Removal of Heavy-Metal Pollutants by White Rot Fungi: Mechanisms, Achievements, and Perspectives. J. Clean. Prod. 2022, 354, 131681. [Google Scholar] [CrossRef]
- Costa, S.; Dedola, D.G.; Pellizzari, S.; Blo, R.; Rugiero, I.; Pedrini, P.; Tamburini, E. Lignin Biodegradation in Pulp-and-Paper Mill Wastewater by Selected White Rot Fungi. Water 2017, 9, 935. [Google Scholar] [CrossRef]
- Schwanninger, M.; Hinterstoisser, B. Klason Lignin: Modifications to Improve the Precision of the Standardized Determination. Holzforschung 2002, 56, 161–166. [Google Scholar] [CrossRef]
- López, M.J.; Guisado, G.; Vargas-García, M.C.; Suárez-Estrella, F.; Moreno, J. Decolorization of Industrial Dyes by Ligninolytic Microorganisms Isolated from Composting Environment. Enzym. Microb. Technol. 2006, 40, 42–45. [Google Scholar] [CrossRef]
- Sanz, J.L.; Köchling, T. Next-Generation Sequencing and Waste/Wastewater Treatment: A Comprehensive Overview. Rev. Environ. Sci. Bio/Technol. 2019, 18, 635–680. [Google Scholar] [CrossRef]
- FAOSTAT: Forestry Production and Trade-Google Scholar. Available online: https://scholar.google.com/scholar_lookup?title=FAOSTAT%20-%20Forestry%20Production%20and%20Trade&publication_year=2021&author=FAO%20(Food%20and%20Agriculture%20Organization%20of%20the%20United%20Nations (accessed on 3 November 2023).
- Echezonachi, S.O. Chapter 16-The Role of White Rot Fungi in Bioremediation. In Microbes and Microbial Biotechnology for Green Remediation; Malik, J.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 305–320. ISBN 978-0-323-90452-0. [Google Scholar]
- Kumar, A.; Chandra, R. Ligninolytic Enzymes and Its Mechanisms for Degradation of Lignocellulosic Waste in Environment. Heliyon 2020, 6, e03170. [Google Scholar] [CrossRef]
- Sun, S.; Liu, P.; Ullah, M. Efficient Azo Dye Biodecolorization System Using Lignin-Co-Cultured White-Rot Fungus. J. Fungi 2023, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.-H.; Liu, W.-Z.; Tang, Z.-E.; Cui, D. Recent Advancements in Azo Dye Decolorization in Bio-Electrochemical Systems (BESs): Insights into Decolorization Mechanism and Practical Application. Water Res. 2021, 203, 117512. [Google Scholar] [CrossRef]
- Moreira, P.R.; Almeida-Vara, E.; Sena-Martins, G.; Polónia, I.; Xavier Malcata, F.; Cardoso Duarte, J. Decolourisation of Remazol Brilliant Blue R via a Novel Bjerkandera Sp. Strain. J. Biotechnol. 2001, 89, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-S.; Seo, J.-Y.; Lee, H.; Yoo, J.; Jung, J.; Kim, J.-J.; Kim, G.-H. Decolorization and Detoxification of Wastewater Containing Industrial Dyes by Bjerkandera adusta KUC9065. Water Air Soil. Pollut. 2013, 225, 1801. [Google Scholar] [CrossRef]
- Riegas-Villalobos, A.; Martínez-Morales, F.; Tinoco-Valencia, R.; Serrano-Carreón, L.; Bertrand, B.; Trejo-Hernández, M.R. Efficient Removal of Azo-Dye Orange II by Fungal Biomass Absorption and Laccase Enzymatic Treatment. 3 Biotech 2020, 10, 146. [Google Scholar] [CrossRef]
- Feijoo, G.; Moreira, M.T.; Roca, E.; Lema, J.M. Use of Cheese Whey as a Substrate to Produce Manganese Peroxidase by Bjerkandera Sp BOS55. J. Ind. Microbiol. Biotech 1999, 23, 86–90. [Google Scholar] [CrossRef]
- Nawaz, M.; Gunasekaran, M. Effect of Peat Extract on the Hydrolytic Enzymes of Phanerochaete chrysosporium. Resour. Conserv. Recycl. 1988, 1, 197–205. [Google Scholar] [CrossRef]
- Singh, G.; Dwivedi, S.K.; Mishra, J. Role of Fungal Enzymes in the Removal of Azo Dyes. In Microbial Enzymes: Roles and Applications in Industries; Arora, N.K., Mishra, J., Mishra, V., Eds.; Microorganisms for Sustainability; Springer: Singapore, 2020; pp. 231–257. ISBN 9789811517105. [Google Scholar]
- Qi-he, C.; Krügener, S.; Hirth, T.; Rupp, S.; Zibek, S. Co-Cultured Production of Lignin-Modifying Enzymes with White-Rot Fungi. Appl. Biochem. Biotechnol. 2011, 165, 700–718. [Google Scholar] [CrossRef]
- Fackler, K.; Gradinger, C.; Hinterstoisser, B.; Messner, K.; Schwanninger, M. Lignin Degradation by White Rot Fungi on Spruce Wood Shavings during Short-Time Solid-State Fermentations Monitored by near Infrared Spectroscopy. Enzym. Microb. Technol. 2006, 39, 1476–1483. [Google Scholar] [CrossRef]
- Korniłłowicz-Kowalska, T.; Rybczyńska-Tkaczyk, K. Growth Conditions, Physiological Properties, and Selection of Optimal Parameters of Biodegradation of Anticancer Drug Daunomycin in Industrial Effluents by Bjerkandera adusta CCBAS930. Int. Microbiol. 2020, 23, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Madhushika, H.G.; Ariyadasa, T.U.; Gunawardena, S.H.P. Textile Dye Removal from Industrial Wastewater by Biological Methods and Impact on Environment. In Environmental Degradation: Challenges and Strategies for Mitigation; Singh, V.P., Yadav, S., Yadav, K.K., Yadava, R.N., Eds.; Water Science and Technology Library; Springer International Publishing: Cham, Switzerland, 2022; pp. 181–210. ISBN 978-3-030-95542-7. [Google Scholar]
- Wang, Y.; Chen, S.; Zhou, J.; Fan, X.; He, L.; Fan, G. Enhanced Degradation Capability of White-Rot Fungi after Short-Term Pre-Exposure to Silver Ion: Performance and Selectively Antimicrobial Mechanisms. Sci. Total Environ. 2022, 818, 151672. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.K.; Raut, S.; Bandyopadhyay, P.; Raut, S. Fungal Decolouration and Degradation of Azo Dyes: A Review. Fungal Biol. Rev. 2016, 30, 112–133. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).