Recycling Fermentation Strategy for Waste Cellular Residues in the Production of Polyunsaturated Fatty Acids
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains
2.2. Culture Conditions
2.2.1. Aurantiochytrium Culture and DHA Fermentation
2.2.2. Mortierella Culture and ARA Fermentation
2.3. Preparation of Waste Cellular Residues
2.3.1. Aurantiochytrium Cellular Residues (ACRs)
2.3.2. Mortierella Cellular Residues (MCRs)
2.4. Analytical Methods
2.4.1. Total Nitrogen, Glucose, and Cell Dry Weight
2.4.2. Total Lipids, DHA, and ARA Content
3. Results and Discussion
3.1. Nutrient Composition of Cellular Residues from PUFA Fermentation
3.2. Aurantiochytrium Cellular Residues as a Nitrogen Source for DHA and ARA Production
3.3. Mortierella Cellular Residues as a Nitrogen Source for DHA and ARA Production
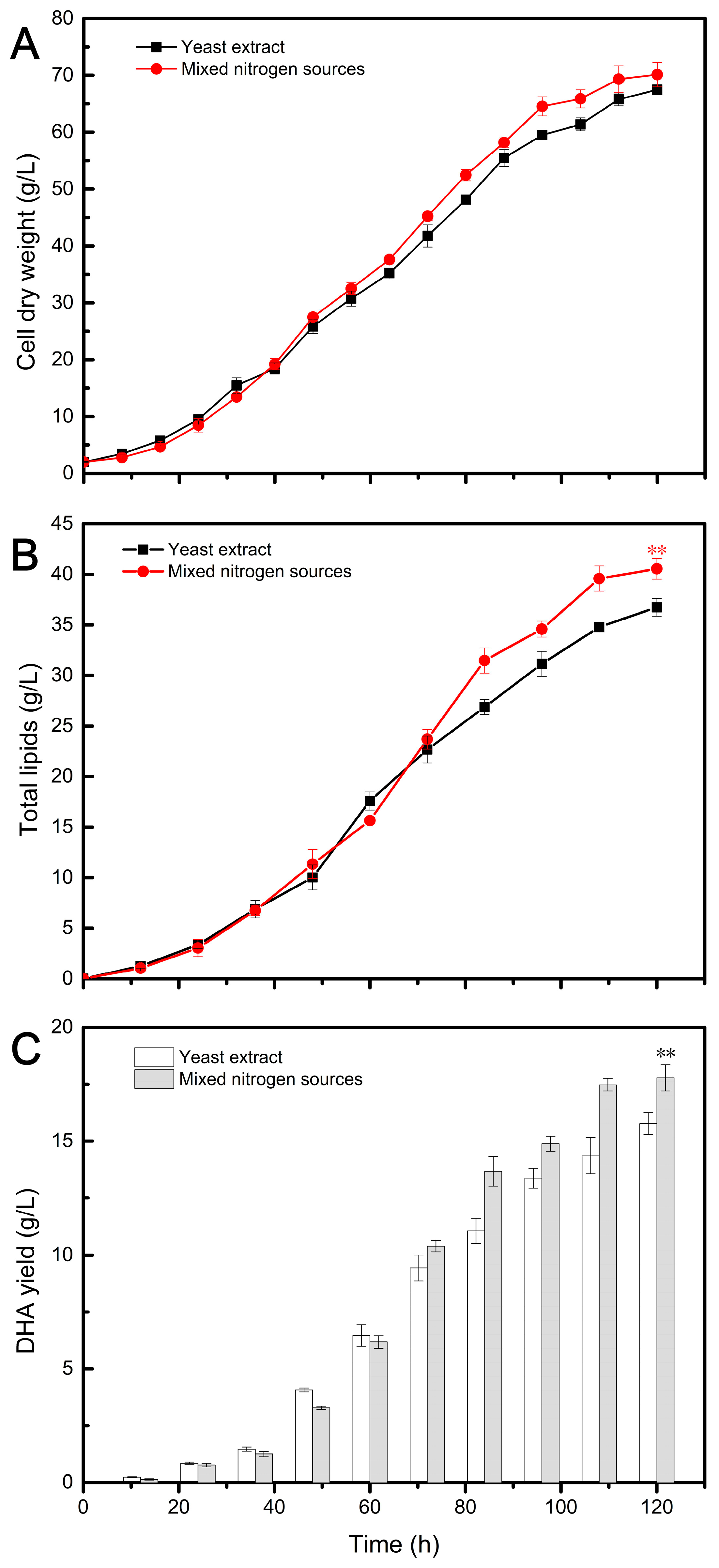
3.4. Fermentation of PUFAs Using Waste Cellular Residues
3.5. Nitrogen Source Cost Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ratledge, C. Fatty acid biosynthesis in microorganisms being used for Single Cell Oil production. Biochimie 2004, 86, 807–815. [Google Scholar] [CrossRef]
- Ji, X.-J.; Ledesma-Amaro, R. Microbial Lipid Biotechnology to Produce Polyunsaturated Fatty Acids. Trends Biotechnol. 2020, 38, 832–834. [Google Scholar] [CrossRef]
- Campoy, C.; Chisaguano Tonato, A.M.; de la Garza Puente, A.; Seenz de Pipaon, M.; Verduci, E.; Koletzko, B.; Gonzalez Casanova, I.; Larque, E.; Valenzuela, R.; Moreno Villares, J.M.; et al. Controversy about the critical role of long-chain pol-yunsaturated fatty acids, arachidonic acid (ARA) and docosahexaenoic acid (DHA), during infancy. Nutr. Hosp. 2021, 38, 1101–1112. [Google Scholar]
- Von Schacky, C. Importance of EPA and DHA Blood Levels in Brain Structure and Function. Nutrients 2021, 13, 1074. [Google Scholar] [CrossRef] [PubMed]
- Mironov, A.A.; Nemashkalov, V.A.; Stepanova, N.N.; Kamzolova, S.V.; Rymowicz, W.; Morgunov, I.G. The Effect of pH and Temperature on Arachidonic Acid Production by Glycerol-Grown Mortierella alpina NRRL-A-10995. Fermentation 2018, 4, 17. [Google Scholar] [CrossRef]
- Chang, L.; Chen, H.; Tang, X.; Zhao, J.; Zhang, H.; Chen, Y.Q.; Chen, W. Advances in improving the biotechnological application of oleaginous fungus Mortierella alpina. Appl. Microbiol. Biotechnol. 2021, 105, 6275–6289. [Google Scholar] [CrossRef]
- Xu, X.; Huang, C.; Xu, Z.; Xu, H.; Wang, Z.; Yu, X. The strategies to reduce cost and improve productivity in DHA production by Aurantiochytrium sp.: From biochemical to genetic respects. Appl. Microbiol. Biotechnol. 2020, 104, 9433–9447. [Google Scholar] [CrossRef]
- Kothri, M.; Mavrommati, M.; Elazzazy, A.M.; Baeshen, M.N.; Moussa, T.A.A.; Aggelis, G. Microbial sources of polyun-saturated fatty acids (PUFAs) and the prospect of organic residues and wastes as growth media for PUFA-producing microorganisms. FEMS Microbiol. Lett. 2020, 367, fnaa028. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Ong, C.-C.; Lin, T.-Y. Effect of Sea Salt and Taro Waste on Fungal Mortierella alpina Cultivation for Arachidonic Acid-Rich Lipid Production. Fermentation 2022, 8, 81. [Google Scholar] [CrossRef]
- Yin, F.-W.; Guo, D.-S.; Ren, L.-J.; Ji, X.-J.; Huang, H. Development of a method for the valorization of fermentation wastewater and algal-residue extract in docosahexaenoic acid production by Schizochytrium sp. Bioresour. Technol. 2018, 266, 482–487. [Google Scholar] [CrossRef]
- Park, W.-K.; Moon, M.; Shin, S.-E.; Cho, J.M.; Suh, W.I.; Chang, Y.K.; Lee, B. Economical DHA (Docosahexaenoic acid) production from Aurantiochytrium sp. KRS101 using orange peel extract and low cost nitrogen sources. Algal Res. Biomass Biofuels Bioprod. 2018, 29, 71–79. [Google Scholar] [CrossRef]
- Wang, S.-K.; Wang, X.; Tian, Y.-T.; Cui, Y.-H. Nutrient recovery from tofu whey wastewater for the economical production of docosahexaenoic acid by Schizochytrium sp. S31. Sci. Total. Environ. 2020, 710, 136448. [Google Scholar] [CrossRef]
- Goyzueta-Mamani, L.D.; de Carvalho, J.C.; Magalhaes, A.I., Jr.; Soccol, C.R. Production of arachidonic acid by Mortierella alpine using wastes from potato chips industry. J. Appl. Microbiol. 2021, 130, 1592–1601. [Google Scholar] [CrossRef]
- Asimakopoulou, G.; Karnaouri, A.; Staikos, S.; Stefanidis, S.D.; Kalogiannis, K.G.; Lappas, A.A.; Topakas, E. Production of Omega-3 Fatty Acids from the Microalga Crypthecodinium cohnii by Utilizing Both Pentose and Hexose Sugars from Agricultural Residues. Fermentation 2021, 7, 219. [Google Scholar] [CrossRef]
- Yin, F.-W.; Sun, X.-L.; Zheng, W.-L.; Yin, L.-F.; Luo, X.; Zhang, Y.-Y.; Wang, Y.-F.; Fu, Y.-Q. Development of a Strategy for L-Lactic Acid Production by Rhizopus oryzae Using Zizania latifolia Waste and Cane Molasses as Carbon Sources. Molecules 2023, 28, 6234. [Google Scholar] [CrossRef]
- Kumar, M.; Sun, Y.; Rathour, R.; Pandey, A.; Thakur, I.S.; Tsang, D.C. Algae as potential feedstock for the production of biofuels and value-added products: Opportunities and challenges. Sci. Total. Environ. 2020, 716, 137116. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.-S.; Tong, L.-L.; Ji, X.-J.; Ren, L.-J.; Ding, Q.-Q. Development of a Strategy to Improve the Stability of Culture Envi-ronment for Docosahexaenoic Acid Fermentation by Schizochytrium sp. Appl. Biochem. Biotechnol. 2020, 192, 881–894. [Google Scholar] [CrossRef]
- Shi, K.; Gao, Z.; Lin, L.; Wang, W.-J.; Shi, X.-Q.; Yu, X.; Song, P.; Ren, L.-J.; Huang, H.; Ji, X.-J. Manipulating the generation of reactive oxygen species through intermittent hypoxic stress for enhanced accumulation of arachidonic acid-rich lipids. Chem. Eng. Sci. 2018, 186, 36–43. [Google Scholar] [CrossRef]
- Ammar, E.M.; Arora, N.; Philippidis, G.P. The Prospects of Agricultural and Food Residue Hydrolysates for Sustainable Production of Algal Products. Energies 2020, 13, 6427. [Google Scholar] [CrossRef]
- Jaseera, K.V.; Ebeneezar, S.; Sayooj, P.; Nair, A.V.; Kaladharan, P. Dietary supplementation of microalgae, Aurantiochytrium sp. and co-feeding with Artemia enhances the growth, stress tolerance and survival in Penaeus monodon (Fabricius, 1798) post larvae. Aquaculture 2021, 533, 736176. [Google Scholar]
- Uggetti, E.; Sialve, B.; Trably, E.; Steyer, J.-P. Integrating microalgae production with anaerobic digestion: A biorefinery approach. Biofuels Bioprod. Biorefining-Biofpr 2014, 8, 516–529. [Google Scholar] [CrossRef]
- Karthik, V.; Kumar, P.S.; Vo, D.-V.N.; Sindhu, J.; Sneka, D.; Subhashini, B.; Saravanan, K.; Jeyanthi, J. Hydrothermal pro-duction of algal biochar for environmental and fertilizer applications: A review. Environ. Chem. Lett. 2021, 19, 1025–1042. [Google Scholar] [CrossRef]
- Hui, X.; Fang, W.; Wang, G.; Liu, H.; Dai, X. Waste recycling of antibiotic mycelial residue: The feasible harmless treatment and source control of antibiotic resistance. J. Clean. Prod. 2023, 401, 136786. [Google Scholar] [CrossRef]
- Nobrega, R.O.; Batista, R.O.; Correa, C.F.; Mattioni, B.; Filer, K.; Pettigrew, J.E.; Fracalossi, D.M. Dietary supplementation of Aurantiochytrium sp. meal, a docosahexaenoic-acid source, promotes growth of Nile tilapia at a suboptimal low temperature. Aquaculture 2019, 507, 500–509. [Google Scholar] [CrossRef]
- Reboleira, J.; Felix, R.; Felix, C.; de Melo, M.M.R.; Silva, C.M.; Saraiva, J.A.; Bandarra, N.M.; Teixeira, B.; Mendes, R.; Paulo, M.C.; et al. Evaluating the Potential of the Defatted By-Product of Aurantiochytrium sp. Industrial Cultivation as a Functional Food. Foods 2021, 10, 3058. [Google Scholar] [CrossRef]
- Trovao, M.; Pereira, H.; Costa, M.; Machado, A.; Barros, A.; Soares, M.; Carvalho, B.; Silva, J.T.; Varela, J.; Silva, J. Lab-Scale Optimization of Aurantiochytrium sp. Culture Medium for Improved Growth and DHA Production. Appl. Sci. 2020, 10, 2500. [Google Scholar] [CrossRef]
- Guimaraes, A.M.; Schleder, D.D.; Nagata, M.; Nobrega, R.O.; Fracalossi, D.M.; Seiffert, W.Q.; Vieira, F.D.N. Aurantiochytrium sp. meal can replace fish oil in practical diets for the juvenile Pacific white shrimp. Aquac. Nutr. 2019, 25, 798–807. [Google Scholar] [CrossRef]
- Yu, X.-J.; Huang, C.-Y.; Chen, H.; Wang, D.-S.; Chen, J.-L.; Li, H.-J.; Liu, X.-Y.; Wang, Z.; Sun, J.; Wang, Z.-P. High-Throughput Biochemical Fingerprinting of Oleaginous Aurantiochytrium sp. Strains by Fourier Transform Infrared Spectroscopy (FT-IR) for Lipid and Carbohydrate Productions. Molecules 2019, 24, 1593. [Google Scholar] [CrossRef]
- Shinmen, Y.; Kawashima, H.; Shimizu, S.; Yamada, H. Concentration of eicosapentaenoic acid and docosahexaenoic acid in an arachidonic acid-producing fungus, Mortierella alpina 1S-4, grown with fish oil. Appl. Microbiol. Biotechnol. 1992, 38, 301–304. [Google Scholar] [CrossRef]
- Yin, F.-W.; Zhan, C.-T.; Huang, J.; Sun, X.-L.; Yin, L.-F.; Zheng, W.-L.; Luo, X.; Zhang, Y.-Y.; Fu, Y.-Q. Efficient Co-production of Docosahexaenoic Acid Oil and Carotenoids in Aurantiochytrium sp. Using a Light Intensity Gradient Strategy. Appl. Biochem. Biotechnol. 2022, 195, 623–638. [Google Scholar] [CrossRef]
- Ji, X.-J.; Zhang, A.-H.; Nie, Z.-K.; Wu, W.-J.; Ren, L.-J.; Huang, H. Efficient arachidonic acid-rich oil production by Mortierella alpina through a repeated fed-batch fermentation strategy. Bioresour. Technol. 2014, 170, 356–360. [Google Scholar] [CrossRef]
- Xiangyu, L.; Chao, Y.; Jianming, Y.; Zhiming, W.; Shuhuan, L. An Online Respiratory Quotient-Feedback Strategy of Feeding Yeast Extract for Efficient Arachidonic Acid Production by Mortierella alpina. Front. Bioeng. Biotechnol. 2017, 5, 83. [Google Scholar]
- Yin, F.-W.; Zhang, Y.-T.; Jiang, J.-Y.; Guo, D.-S.; Gao, S.; Gao, Z. Efficient docosahexaenoic acid production by Schizochytrium sp. via a two-phase pH control strategy using ammonia and citric acid as pH regulators. Process. Biochem. 2019, 77, 1–7. [Google Scholar] [CrossRef]
- Dzurendova, S.; Losada, C.B.; Dupuy-Galet, B.X.; Fjær, K.; Shapaval, V. Mucoromycota fungi as powerful cell factories for modern biorefinery. Appl. Microbiol. Biotechnol. 2022, 106, 101–115. [Google Scholar] [CrossRef]
- Campos-Takaki, G.M.; Dietrich, S.M.C. Characterization of Cell Walls from Mucoralean Fungi by Biochemical Composition, Transmission Electron Microscopy and X-Ray Microanalysis. In Current Research Topics in Applied Microbiology and Microbial Biotechnology; World Scientific: Singapore, 2009; pp. 121–125. [Google Scholar]
- Glencross, B.; Huyben, D.; Schrama, J. The Application of Single-Cell Ingredients in Aquaculture Feeds—A Review. Fishes 2020, 5, 22. [Google Scholar] [CrossRef]
- Yen, S.-W.; Nagarajan, D.; Chen, W.-H.; Lee, D.-J.; Chang, J.-S. Fermentative production of astaxanthin from sorghum distillery residue by an indigenous Aurantiochytrium sp. CJ6 strain using a continuous-feeding fed-batch process. Bioresour. Technol. 2023, 376, 128817. [Google Scholar] [CrossRef]
- Chang, L.; Lu, H.; Chen, H.; Tang, X.; Zhao, J.; Zhang, H.; Chen, Y.Q.; Chen, W. Lipid metabolism research in oleaginous fungus Mortierella alpina: Current progress and future prospects. Biotechnol. Adv. 2022, 54, 107794. [Google Scholar] [CrossRef]
- Prabhakaran, P.; Raethong, N.; Thananusak, R.; Nazir, M.Y.M.; Sapkaew, C.; Soommat, P.; Kingkaw, A.; Hamid, A.A.; Vongsangnak, W.; Song, Y. Revealing holistic metabolic responses associated with lipid and docosahexaenoic acid (DHA) production in Aurantiochytrium sp. SW1. Biochim. Et Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2023, 1868, 159306. [Google Scholar] [CrossRef]
- Yin, F.-W.; Zhu, S.-Y.; Guo, D.-S.; Ren, L.-J.; Ji, X.-J.; Huang, H.; Gao, Z. Development of a strategy for the production of do-cosahexaenoic acid by Schizochytrium sp. from cane molasses and algae-residue. Bioresour. Technol. 2019, 271, 118–124. [Google Scholar] [CrossRef]
- Koizumi, K.; Higashiyama, K.; Park, E.Y. Effects of amino acid on morphological development and nucleus formation of arachidonic acid-producing filamentous micro-organism, Mortierella alpina. J. Appl. Microbiol. 2006, 100, 885–892. [Google Scholar] [CrossRef]
- Valdebenito, D.; Urrutia, S.; Leyton, A.; Chisti, Y.; Asenjo, J.A.; Shene, C. Nitrogen sources affect the long-chain polyunsatu-rated fatty acids content in Thraustochytrium sp. RT2316-16. Mar. Drugs 2022, 21, 15. [Google Scholar] [CrossRef]
- Lu, H.; Chen, H.; Tang, X.; Yang, Q.; Zhang, H.; Chen, Y.Q.; Chen, W. Time-resolved multi-omics analysis reveals the role of nutrient stress-induced resource reallocation for TAG accumulation in oleaginous fungus Mortierella alpina. Biotechnol. Biofuels 2020, 13, 117. [Google Scholar] [CrossRef]
- Ling, X.-P.; Zeng, S.-Y.; Chen, C.-X.; Liu, X.-T.; Lu, Y.-H. Enhanced arachidonic acid production using a bioreactor culture of Mortierella alpina with a combined organic nitrogen source. Bioresour. Bioprocess. 2016, 3, 43. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, L.; Li, T.; Wu, N.; Jiang, L.; Ji, X.; Huang, H. How nitrogen sources influence Mortierella alpina aging: From the lipid droplet proteome to the whole-cell proteome and metabolome. J. Proteom. 2018, 179, 140–149. [Google Scholar] [CrossRef]
- Lu, J.; Peng, C.; Ji, X.-J.; You, J.; Cong, L.; Ouyang, P.; Huang, H. Fermentation Characteristics of Mortierella alpina in Response to Different Nitrogen Sources. Appl. Biochem. Biotechnol. 2011, 164, 979–990. [Google Scholar] [CrossRef] [PubMed]
- Nisha, A.; Venkateswaran, G. Effect of Culture Variables on Mycelial Arachidonic acid Production by Mortierella alpina. Food Bioprocess Technol. 2008, 4, 232–240. [Google Scholar] [CrossRef]
- Li, C.; Zhang, G.; Zhang, Z.; Ma, D.; Xu, G. Alkaline thermal pretreatment at mild temperatures for biogas production from anaerobic digestion of antibiotic mycelial residue. Bioresour. Technol. 2016, 208, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhu, X.; Ye, H.; Wen, Y.; Sen, B.; Wang, G. Low dissolved oxygen supply functions as a global regulator of the growth and metabolism of Aurantiochytrium sp. PKU#Mn16 in the early stages of docosahexaenoic acid fermentation. Microb. Cell Factories 2023, 22, 52. [Google Scholar]
- Tao, Z.; Yuan, H.; Liu, M.; Liu, Q.; Zhang, S.; Liu, H.; Jiang, Y.; Huang, D.; Wang, T. Yeast Extract: Characteristics, Production, Applications and Future Perspectives. J. Microbiol. Biotechnol. 2023, 33, 151. [Google Scholar] [CrossRef]
- Asachi, R.; Karimi, K. Enhanced ethanol and chitosan production from wheat straw by Mucor indicus with minimal nutrient consumption. Process. Biochem. 2013, 48, 1524–1531. [Google Scholar] [CrossRef]
- Zhang, H.; Cui, Q.; Song, X. Research advances on arachidonic acid production by fermentation and genetic modification of Mortierella alpina. World J. Microbiol. Biotechnol. 2021, 37, 4. [Google Scholar] [CrossRef]
- Liu, L.; Wang, F.; Yang, J.; Li, X.; Cui, J.; Liu, J.; Shi, M.; Wang, K.; Chen, L.; Zhang, W. Nitrogen Feeding Strategies and Metabolomic Analysis To Alleviate High-Nitrogen Inhibition on Docosahexaenoic Acid Production in Crypthecodinium cohnii. J. Agric. Food Chem. 2018, 66, 10640–10650. [Google Scholar] [CrossRef]
- Song, X.; Zang, X.; Zhang, X. Production of High Docosahexaenoic Acid by Schizochytrium sp. Using Low-cost Raw Materials from Food Industry. J. Oleo Sci. 2015, 64, 197–204. [Google Scholar] [CrossRef]
- Cai, G.; Moghaddam, L.; O’Hara, I.M.; Zhang, Z. Microbial oil production from acidified glycerol pretreated sugarcane bagasse by Mortierella isabellina. RSC Adv. 2019, 9, 2539–2550. [Google Scholar] [CrossRef]
- Samadlouie, H.R.; Nurmohamadi, S.; Moradpoor, F.; Gharanjik, S. Effect of low-cost substrate on the fatty acid profiles of Mortierella alpina CBS 754.68 and Wickerhamomyces siamensis SAKSG. Biotechnol. Biotechnol. Equip. 2018, 32, 1228–1235. [Google Scholar] [CrossRef]
- Xu, J.; Zhu, Y.; Li, H.; Chen, L.; Chen, W.; Cui, M.; Han, L.; Hou, W.; Li, D. Alanine mother liquor as a nitrogen source for docosahexaenoic acid production by Schizochytrium sp. B4D1. Electron. J. Biotechnol. 2018, 35, 10–17. [Google Scholar] [CrossRef]
- Russo, G.L.; Langellotti, A.L.; Sacchi, R.; Masi, P. Techno-economic assessment of DHA-rich Aurantiochytrium sp. production using food industry by-products and waste streams as alternative growth media. Bioresour. Technol. Rep. 2022, 18, 100997. [Google Scholar] [CrossRef]



| Components | ACRs (g/L) | Nutrient Equivalence | MCRs (g/L) | Nutrient Equivalence |
|---|---|---|---|---|
| TN | 6.37 ± 0.27 | 54.26 (g YE/L) | 10.43 ± 0.46 | 88.84 (g YE/L) |
| FA | 2.44 ± 0.07 | 48.61 (g YE/L) | 3.50 ± 0.09 | 69.72 (g YE/L) |
| K | 0.87 ± 0.03 | 3.03 (g KH2PO4/L) | 1.26 ± 0.03 | 4.39 (g KH2PO4/L) |
| P | 1.52 ± 0.04 | 6.67 (g KH2PO4/L) | 2.33 ± 0.02 | 10.22 (g KH2PO4/L) |
| Mg | 0.64 ± 0.02 | 3.20 (g MgSO4/L) | 0.31 ± 0.01 | 1.55 (g MgSO4/L) |
| Ca | 0.17 ± 0.01 | 0.47 (g CaCl2/L) | 0.04 ± 0.00 | 0.11 (g CaCl2/L) |
| Nitrogen Source | CDW (g/L) | TL (g/L) | DHA (g/L) | TL/CDW (%) | DHA/CDW (%) | DHA/TL (%) | DHA Productivity (g/L·h) |
|---|---|---|---|---|---|---|---|
| YE | 67.47 ± 1.3 | 36.74 ± 1.0 | 15.77 ± 0.6 | 54.45 ± 1.2 | 23.37 ± 0.4 | 42.92 ± 1.1 | 0.13 ± 0.02 |
| MNS | 70.12 ± 2.2 | 40.55 ± 1.4 | 17.78 ± 0.4 | 57.83 ± 1.0 | 25.36 ± 0.8 | 43.85 ± 1.3 | 0.15 ± 0.01 |
| Improvement | 3.93% | 10.37% | 12.75% | 6.20% | 8.48% | 2.15% | 12.75% |
| Nitrogen saving | 80% | ||||||
| Nitrogen Source | CDW (g/L) | TL (g/L) | ARA (g/L) | TL/CDW (%) | ARA/CDW (%) | ARA/TL (%) | ARA Productivity (g/L·d) |
|---|---|---|---|---|---|---|---|
| YE | 30.44 ± 1.0 | 13.39 ± 0.8 | 5.28 ± 0.3 | 0.44 ± 0.03 | 17.35 ± 0.1 | 39.43 ± 0.4 | 0.75 ± 0.02 |
| MNS | 33.29 ± 1.2 | 15.35 ± 0.2 | 5.77 ± 0.1 | 0.46 ± 0.02 | 17.33 ± 0.2 | 37.59 ± 0.8 | 0.82 ± 0.01 |
| Improvement | 9.36% | 14.64% | 9.28% | 4.82% | −0.08% | −4.67% | 9.28% |
| Nitrogen saving | 60% | ||||||
| YE | Waste Cellular Residues | Total Cost ($) | DHA (kg) | DHA Cost ($/kg) | |||
|---|---|---|---|---|---|---|---|
| Alkaline Protease | Cellulase | Chitinase | |||||
| Price ($/kg) | 5.70 | 6.26 | 6.26 | 82.04 | |||
| Traditional fermentation (kg) | 10 | 0 | 0 | 0 | 57 | 15.77 | 3.61 |
| Novel fermentation (kg) | 2 | 0.6415 | 0.0901 | 0.0600 | 20.90 | 17.78 | 1.18 |
| Cost saving (%) | 67.31 | ||||||
| YE | Waste Cellular Residues | Total Cost ($) | ARA (kg) | ARA Cost ($/kg) | |||
|---|---|---|---|---|---|---|---|
| Alkaline Protease | Cellulase | Chitinase | |||||
| Price ($/kg) | 5.70 | 6.26 | 6.26 | 82.04 | |||
| Traditional fermentation (kg) | 20 | 0 | 0 | 0 | 114 | 5.28 | 21.59 |
| Novel fermentation (kg) | 8 | 0.8475 | 0.2431 | 0.1621 | 65.73 | 5.77 | 11.39 |
| Cost saving (%) | 47.24 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, F.-W.; Huang, J.; Zhan, C.-T.; Sun, X.-L.; Zheng, W.-L.; Luo, X.; Zhang, Y.-Y.; Yin, L.-F.; Fu, Y.-Q. Recycling Fermentation Strategy for Waste Cellular Residues in the Production of Polyunsaturated Fatty Acids. Fermentation 2024, 10, 81. https://doi.org/10.3390/fermentation10020081
Yin F-W, Huang J, Zhan C-T, Sun X-L, Zheng W-L, Luo X, Zhang Y-Y, Yin L-F, Fu Y-Q. Recycling Fermentation Strategy for Waste Cellular Residues in the Production of Polyunsaturated Fatty Acids. Fermentation. 2024; 10(2):81. https://doi.org/10.3390/fermentation10020081
Chicago/Turabian StyleYin, Feng-Wei, Jiao Huang, Ci-Tong Zhan, Xiao-Long Sun, Wei-Long Zheng, Xi Luo, Ying-Ying Zhang, Long-Fei Yin, and Yong-Qian Fu. 2024. "Recycling Fermentation Strategy for Waste Cellular Residues in the Production of Polyunsaturated Fatty Acids" Fermentation 10, no. 2: 81. https://doi.org/10.3390/fermentation10020081
APA StyleYin, F.-W., Huang, J., Zhan, C.-T., Sun, X.-L., Zheng, W.-L., Luo, X., Zhang, Y.-Y., Yin, L.-F., & Fu, Y.-Q. (2024). Recycling Fermentation Strategy for Waste Cellular Residues in the Production of Polyunsaturated Fatty Acids. Fermentation, 10(2), 81. https://doi.org/10.3390/fermentation10020081





