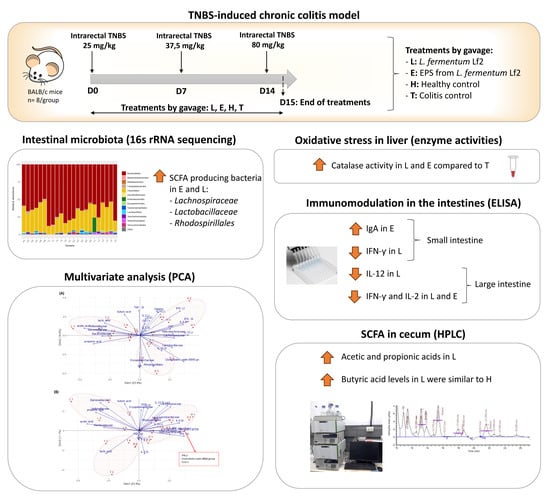
Protective Role of Limosilactobacillus fermentum Lf2 and Its Exopolysaccharides (EPS) in a TNBS-Induced Chronic Colitis Mouse Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Organisms and Growth Conditions
2.2. Production of Exopolysaccharides
2.3. Chronic Colitis Model
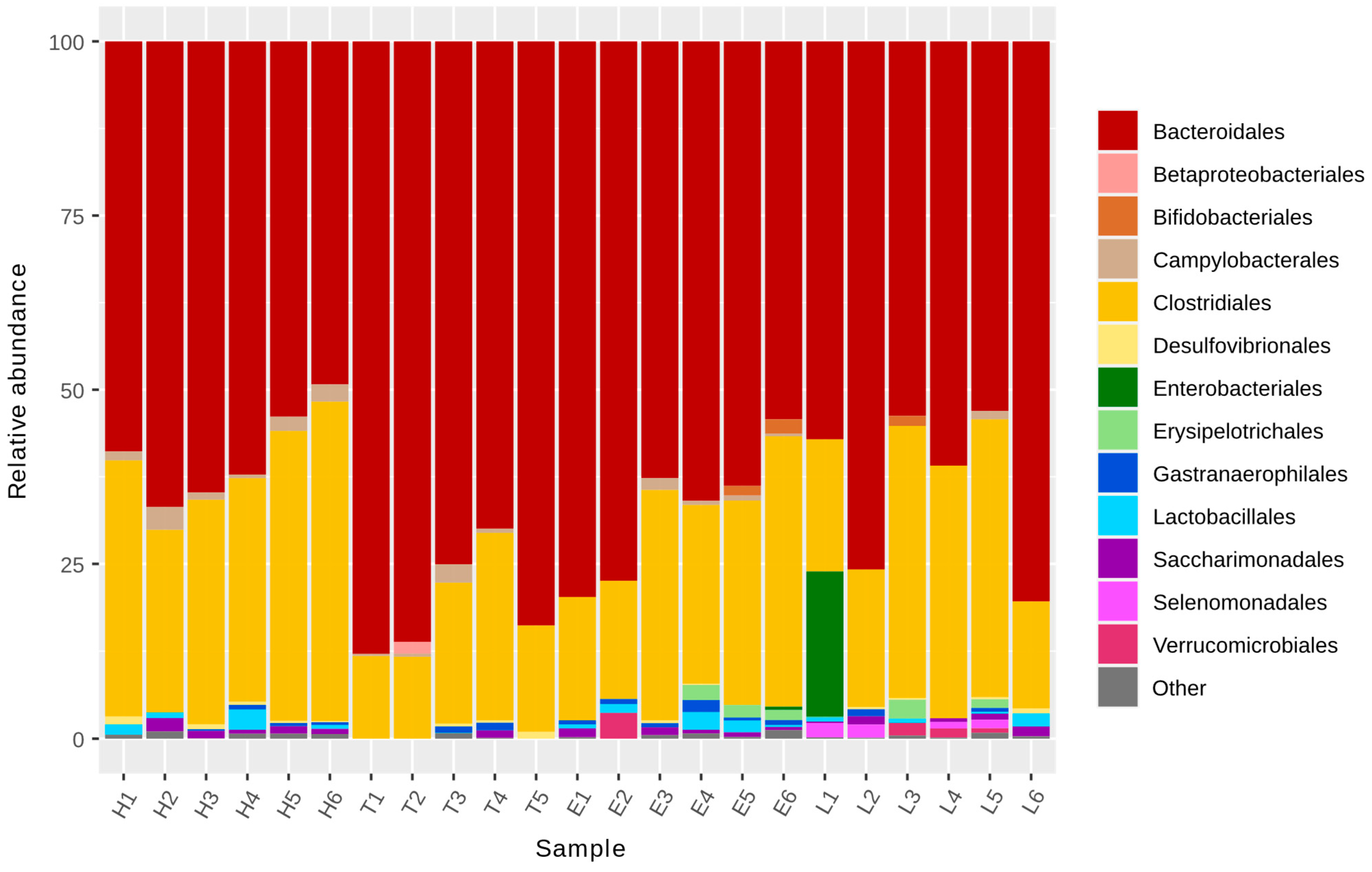
2.4. Microbiota Analysis
2.5. Determination of Translocation, s-IgA, and Cytokines
2.6. Oxidative Stress
2.7. Short-Chain Fatty Acids (SCFA) and Lactic Acid Determinations
2.8. Statistical Analysis
3. Results and Discussion
3.1. Microbiota Analysis
3.2. Determination of Translocation, s-IgA, and Cytokines
3.3. Oxidative Stress
3.4. Short-Chain Fatty Acids (SCFA) and Lactic Acid Determinations
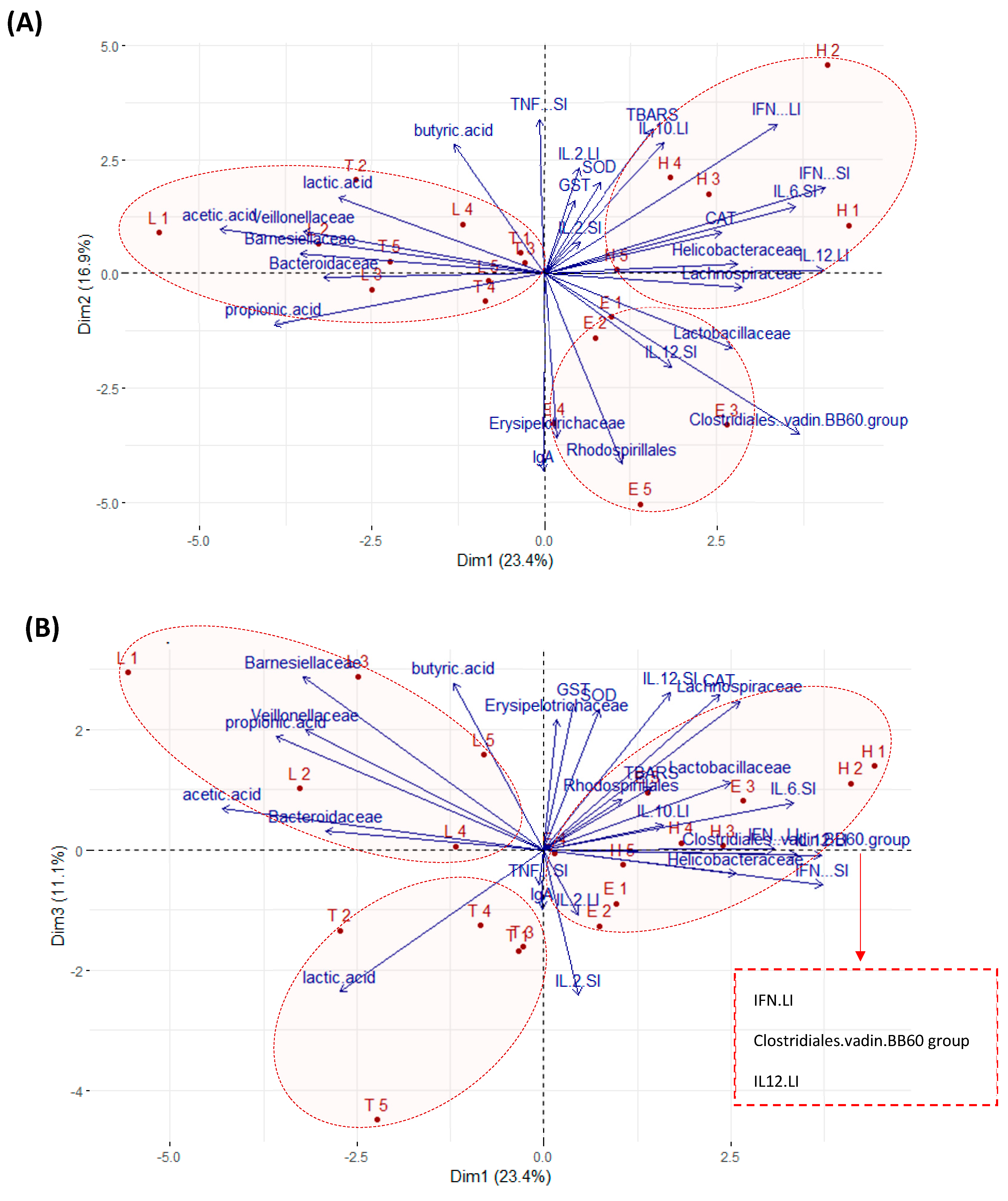
3.5. Multivariate Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaplan, G.G.; Windsor, J.W. The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Noble, A.J.; Nowak, J.K.; Adams, A.T.; Uhlig, H.H.; Satsangi, J. Defining interactions between the genome, epigenome, and the environment in inflammatory bowel disease: Progress and prospects. Gastroenterology 2023, 165, 44–60. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Wang, S.; Li, J. Treatment of inflammatory bowel disease: A Comprehensive Review. Front. Med. 2021, 8, 765474. [Google Scholar] [CrossRef] [PubMed]
- Mentella, M.C.; Scaldaferri, F.; Pizzoferrato, M.; Gasbarrini, A.; Miggiano, G.A.D. Nutrition, IBD and gut microbiota: A Review. Nutrients 2020, 12, 944. [Google Scholar] [CrossRef] [PubMed]
- Carboni, A.D.; Martins, G.N.; Gómez-Zavaglia, A.; Castilho, P.C. Lactic acid bacteria in the production of traditional fermented foods and beverages of Latin America. Fermentation 2023, 9, 315. [Google Scholar] [CrossRef]
- Khushboo; Karnwal, A.; Malik, T. Characterization and selection of probiotic lactic acid bacteria from different dietary sources for development of functional foods. Front. Microbiol. 2023, 14, 1170725. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Werning, M.L.; Hernández-Alcántara, A.M.; Ruiz, M.J.; Soto, L.P.; Dueñas, M.T.; López, P.; Frizzo, L.S. Biological functions of exopolysaccharides from lactic acid bacteria and their potential benefits for humans and farmed animals. Foods 2022, 11, 1284. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Pourjafar, H.; Ansari, F.; Sadeghi, A.; Samakkhah, S.A.; Jafari, S.M. Functional and health-promoting properties of probiotics’ exopolysaccharides; isolation, characterization, and applications in the food industry. Crit. Rev. Food Sci. Nutr. 2023, 63, 8194–8225. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Guan, X.-X.; Tang, Y.-J.; Sun, J.-F.; Wang, X.-K.; Wang, W.-D.; Fan, J.-M. Clinical effects and gut microbiota changes of using probiotics, prebiotics or synbiotics in inflammatory bowel disease: A systematic review and meta-analysis. Eur. J. Nutr. 2021, 60, 2855–2875. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, C.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zheng, S.; Cui, J.; Guo, T.; Zhang, J.; Li, B. Alleviative effects of exopolysaccharide produced by Lactobacillus helveticus KLDS1.8701 on dextran sulfate sodium-induced colitis in mice. Microorganisms 2021, 9, 2086. [Google Scholar] [CrossRef] [PubMed]
- Min, Z.; Xiaona, H.; Aziz, T.; Jian, Z.; Zhennai, Y. Exopolysaccharides from Lactobacillus plantarum YW11 improve immune response and ameliorate inflammatory bowel disease symptoms. Acta Biochim. Pol. 2020, 67, 485–493. [Google Scholar] [CrossRef]
- Ale, E.C.; Batistela, V.; Correa Olivar, G.; Ferrado, J.; Sadiq, S.; Ahmed, H.; Reinheimer, J.; Vera-Candioti, L.; Laws, A.P.; Binetti, A.G. Statistical optimization of the exopolysaccharide extract produced by Lactobacillus fermentum Lf2, a potential functional food ingredient, and the impact on its chemical composition. Int. J. Dairy. Technol. 2019, 73, 76–87. [Google Scholar] [CrossRef]
- Ahmed, H.I.; Ransom-Jones, E.; Sadiq, S.; Vitlic, A.; McLay, N.; Rojas, M.F.; Ale, E.C.; Binetti, A.G.; Collett, A.; Humphreys, P.N.; et al. Structural characterisation of two medium molecular mass exopolysaccharides produced by the bacterium Lactobacillus fermentum Lf2. Carbohydr. Res. 2020, 488, 107909. [Google Scholar] [CrossRef]
- Vitlic, A.; Sadiq, S.; Ahmed, H.I.; Ale, E.C.; Binetti, A.G.; Collett, A.; Humpreys, P.N.; Laws, A.P. Isolation and characterization of a high molecular mass β-glucan from Lactobacillus fermentum Lf2 and evaluation of its immunomodulatory activity. Carbohydr. Res. 2019, 476, 44–52. [Google Scholar] [CrossRef]
- Ale, E.C.; Perezlindo, M.J.; Burns, P.; Tabacman, E.; Reinheimer, J.A.; Binetti, A.G. Exopolysaccharide from Lactobacillus fermentum Lf2 and its functional characterization as a yogurt additive. J. Dairy. Res. 2016, 83, 487–492. [Google Scholar] [CrossRef]
- Ale, E.C.; Bourin, M.J.B.; Peralta, G.H.; Burns, P.; Ávila, O.B.; Contini, L.; Reinheimer, J.; Binetti, A.G. Functional properties of exopolysaccharide (EPS) extract from Lactobacillus fermentum Lf2 and its impact when combined with Bifidobacterium animalis INL1 in yoghurt. Int. Dairy. J. 2019, 96, 114–125. [Google Scholar] [CrossRef]
- Ale, E.C.; Perezlindo, M.J.; Pavón, Y.; Peralta, G.H.; Costa, S.; Sabbag, N.; Bergamini, C.; Reinheimer, J.A.; Binetti, A.G. Technological, rheological and sensory characterizations of a yogurt containing an exopolysaccharide extract from Lactobacillus fermentum Lf2, a new food additive. Food Res. Int. 2016, 90, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Ale, E.C.; Ibáñez, R.A.; Wilbanks, D.J.; Peralta, G.H.; Ceylan, F.D.; Binetti, A.G.; Bolling, B.W.; Lucey, J.A. Technological role and metabolic profile of two probiotic EPS-producing strains with potential application in yoghurt: Impact on rheology and release of bioactive peptides. Int. Dairy. J. 2023, 137, 105533. [Google Scholar] [CrossRef]
- López, P.; Monteserín, D.C.; Gueimonde, M.; de los Reyes-Gavilán, C.G.; Margolles, A.; Suárez, A.; Ruas-Madiedo, P. Exopolysaccharide-producing Bifidobacterium strains elicit different in vitro responses upon interaction with human cells. Food Res. Int. 2012, 46, 99–107. [Google Scholar] [CrossRef]
- Burns, P.; Alard, J.; Hrdỳ, J.; Boutillier, D.; Páez, R.; Reinheimer, J.; Pot, B.; Vinderola, G.; Grangette, C. Spray-drying process preserves the protective capacity of a breast milk-derived Bifidobacterium lactis strain on acute and chronic colitis in mice. Sci. Rep. 2017, 7, 43211. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef]
- Bacchetta, C.; Rossi, A.; Ale, A.; Campana, M.; Parma, M.J.; Cazenave, J. Combined toxicological effects of pesticides: A fish multi-biomarker approach. Ecol. Indic. 2014, 36, 532–538. [Google Scholar] [CrossRef]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. Available online: http://www.ncbi.nlm.nih.gov/pubmed/4623845 (accessed on 4 September 2023). [CrossRef]
- Beutler, E. Catalase. In Red Cell Metabolism, a Manual of Biochemical Methods; Beutler, E., Ed.; Grune and Stratton Inc.: New York, NY, USA, 1982; pp. 105–106. [Google Scholar]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. Available online: http://www.ncbi.nlm.nih.gov/pubmed/4436300 (accessed on 21 August 2023). [CrossRef]
- Yagi, K. A simple fluorometric assay for lipoperoxide in blood plasma. Biochem. Med. 1976, 15, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Ferrario, C.; Taverniti, V.; Milani, C.; Fiore, W.; Laureati, M.; De Noni, I.; Stuknyte, M.; Chouaia, B.; Riso, P.; Guglielmetti, S. Modulation of fecal Clostridiales bacteria and butyrate by probiotic intervention with Lactobacillus paracasei DG varies among healthy adults. J. Nutr. 2014, 144, 1787–1796. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Li, X.; Liu, C.; Su, L.; Xia, Z.; Li, X.; Li, Y.; Li, L.; Yan, T.; Feng, Q.; et al. Dysbiosis of the fecal microbiota in the TNBS-induced Crohn’s disease mouse model. Appl. Microbiol. Biotechnol. 2016, 100, 4485–4494. [Google Scholar] [CrossRef] [PubMed]
- Alkadhi, S.; Kunde, D.; Cheluvappa, R.; Randall-Demllo, S.; Eri, R. The murine appendiceal microbiome is altered in spontaneous colitis and its pathological progression. Gut Pathog. 2014, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Schwab, C.; Berry, D.; Rauch, I.; Rennisch, I.; Ramesmayer, J.; Hainzl, E.; Heider, S.; Decker, T.; Kenner, L.; Müller, M.; et al. Longitudinal study of murine microbiota activity and interactions with the host during acute inflammation and recovery. ISME J. 2014, 8, 1101–1114. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Cheng, L.; Zhao, Y.; Wang, L.; Wang, S.; Zhang, J. Biosynthesis and prebiotic activity of a linear levan from a new Paenibacillus isolate. Appl. Microbiol. Biotechnol. 2021, 105, 769–787. [Google Scholar] [CrossRef] [PubMed]
- Ariefdjohan, M.W.; Dilk, A.; Brown-Esters, O.N.; Savaiano, D.A. Intestinal Microbiota and Diet in Health. In Nutrition in the Prevention and Treatment of Disease, 4th ed.; Coulston, A.M., Boushey, C.J., Ferruzi, M.G., Delahanty, L.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 811–834. [Google Scholar]
- Berger, K.; Burleigh, S.; Lindahl, M.; Bhattacharya, A.; Patil, P.; Stålbrand, H.; Nordberg Karlsson, E.; Hållenius, F.; Nyman, M.; Adlercreutz, P. Xylooligosaccharides increase bifidobacteria and Lachnospiraceae in mice on a high-fat diet, with a concomitant increase in short-chain fatty acids, especially butyric acid. J. Agric. Food Chem. 2021, 69, 3617–3625. [Google Scholar] [CrossRef]
- Li, L.-L.; Wang, Y.-T.; Zhu, L.-M.; Liu, Z.-Y.; Ye, C.-Q.; Qin, S. Inulin with different degrees of polymerization protects against diet-induced endotoxemia and inflammation in association with gut microbiota regulation in mice. Sci. Rep. 2020, 10, 978. [Google Scholar] [CrossRef]
- Huang, G.; Wang, Z.; Wu, G.; Zhang, R.; Dong, L.; Huang, F.; Zhang, M.; Su, D. Lychee (Litchi chinensis Sonn.) pulp phenolics activate the short-chain fatty acid-free fatty acid receptor anti-inflammatory pathway by regulating microbiota and mitigate intestinal barrier damage in dextran sulfate sodium-induced colitis in mice. J. Agric. Food Chem. 2021, 69, 3326–3339. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Z.; Liu, J.; Zheng, Z.; Li, Q.; Wang, H.; Chen, Z.; Wang, K. Identification of the core active structure of a Dendrobium officinale polysaccharide and its protective effect against dextran sulfate sodium-induced colitis via alleviating gut microbiota dysbiosis. Food Res. Int. 2020, 137, 109641. [Google Scholar] [CrossRef] [PubMed]
- Qiao, N.; Wittouck, S.; Mattarelli, P.; Zheng, J.; Lebeer, S.; Felis, G.E.; Gänzle, M.G. After the storm—Perspectives on the taxonomy of Lactobacillaceae. JDS Commun. 2022, 3, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, P.; Liu, X.; Luo, L.; Lin, W. Bacterial dynamics and metabolite changes in solid-state acetic acid fermentation of Shanxi aged vinegar. Appl. Microbiol. Biotechnol. 2016, 100, 4395–4411. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.J.; Kim, W.-K.; Han, D.H.; Lee, K.; Ko, G. Lactobacillus fermentum species ameliorate dextran sulfate sodium-induced colitis by regulating the immune response and altering gut microbiota. Gut Microbes 2019, 10, 696–711. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.-C.; Ho, P.-Y.; Chen, W.-J.; Wu, S.-H.; Pan, M.-H. Lactobacillus fermentum V3 ameliorates colitis-associated tumorigenesis by modulating the gut microbiome. Am. J. Cancer Res. 2020, 10, 1170–1181. [Google Scholar] [PubMed]
- Baxter, N.T.; Zackular, J.P.; Chen, G.Y.; Schloss, P.D. Structure of the gut microbiome following colonization with human feces determines colonic tumor burden. Microbiome 2014, 2, 20. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Nogales, A.; Algieri, F.; Garrido-Mesa, J.; Vezza, T.; Utrilla, M.P.; Chueca, N.; Garcia, F.; Olivares, M.; Rodríguez-Cabezas, M.E.; Gálvez, J. Differential intestinal anti-inflammatory effects of Lactobacillus fermentum and Lactobacillus salivarius in DSS mouse colitis: Impact on microRNAs expression and microbiota composition. Mol. Nutr. Food Res. 2017, 61, 1700144. [Google Scholar] [CrossRef]
- Wan, C.; Qian, W.; Liu, W.; Pi, X.; Tang, M.; Wang, X.; Gu, Q.; Li, P.; Zhou, T. Exopolysaccharide from Lactobacillus rhamnosus ZFM231 alleviates DSS- induced colitis in mice by regulating gut microbiota. J. Sci. Food Agric. 2022, 102, 7087–7097. [Google Scholar] [CrossRef]
- Yang, S.; Xu, X.; Peng, Q.; Ma, L.; Qiao, Y.; Shi, B. Exopolysaccharides from lactic acid bacteria, as an alternative to antibiotics, on regulation of intestinal health and the immune system. Anim. Nutr. 2023, 13, 78–89. [Google Scholar] [CrossRef]
- Rychen, G.; Aquilina, G.; Azimonti, G.; Bampidis, V.; de Lourdes Bastos, M.; Bories, G.; Chesson, A.; Cocconcelli, P.S.; Flachowsky, G.; Gropp, J.; et al. Guidance on the characterisation of microorganisms used as feed additives or as production organisms. EFSA J. 2018, 16, e05206. [Google Scholar] [CrossRef]
- Binda, S.; Hill, C.; Johansen, E.; Obis, D.; Pot, B.; Sanders, M.E.; Tremblay, A.; Ouwehand, A.C. Criteria to qualify microorganisms as “probiotic” in foods and dietary supplements. Front. Microbiol. 2020, 11, 1662. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, H.; Zhang, J.; Mu, J.; Zalan, Z.; Hegyi, F.; Takács, K.; Zhao, X.; Du, M. Protective effect of Lactobacillus fermentum CQPC04 on dextran sulfate sodium–induced colitis in mice is associated with modulation of the Nuclear Factor-κB signaling pathway. J. Dairy. Sci. 2019, 102, 9570–9585. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Yang, L.; Wu, Y.; Zhao, X. Protective effect of Limosilactobacillus fermentum HFY06 on dextran sulfate sodium-induced colitis in mice. Front. Microbiol. 2022, 13, 935792. [Google Scholar] [CrossRef]
- Hou, C.L.; Zhang, J.; Liu, X.T.; Liu, H.; Zeng, X.F.; Qiao, S.Y. Superoxide dismutase recombinant Lactobacillus fermentum ameliorates intestinal oxidative stress through inhibiting NF- κ B activation in a trinitrobenzene sulphonic acid-induced colitis mouse model. J. Appl. Microbiol. 2014, 116, 1621–1631. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Huang, H.; Zhu, F.; Zhou, X.; Yang, Z.; Zhao, X. A Mixture of Lactobacillus fermentum HFY06 and Arabinoxylan ameliorates dextran sulfate sodium-induced acute ulcerative colitis in mice. J. Inflamm. Res. 2021, 14, 6575–6585. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, S.; Cao, Y.; Tian, X.; Zeng, R.; Liao, D.-F.; Cao, D. Oxidative stress and carbonyl lesions in ulcerative colitis and associated colorectal cancer. Oxid. Med. Cell Longev. 2016, 2016, 9875298. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS sources in physiological and pathological conditions. Oxid. Med. Cell Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef]
- Chauhan, R.; Sudhakaran Vasanthakumari, A.; Panwar, H.; Mallapa, R.H.; Duary, R.K.; Batish, V.K.; Grover, S. Amelioration of colitis in mouse model by exploring antioxidative potentials of an indigenous probiotic strain of Lactobacillus fermentum Lf1. Biomed. Res. Int. 2014, 2014, 206732. [Google Scholar] [CrossRef]
- Markowiak-Kopeć, P.; Śliżewska, K. The effect of probiotics on the production of short-chain fatty acids by human intestinal microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef]
- Kaczmarczyk, O.; Dąbek-Drobny, A.; Woźniakiewicz, M.; Paśko, P.; Dobrowolska-Iwanek, J.; Woźniakiewicz, A.; Piątek-Guziewicz, A.; Zagrodzki, P.; Mach, T.; Zwolińska-Wcisło, M. Fecal levels of lactic, succinic and short-chain fatty acids in patients with ulcerative colitis and Crohn disease: A pilot study. J. Clin. Med. 2021, 10, 4701. [Google Scholar] [CrossRef]
- Taylor, S.J.; Winter, M.G.; Gillis, C.C.; Silva, L.A.; da Dobbins, A.L.; Muramatsu, M.K.; Jimenez, A.G.; Chanin, R.B.; Spiga, L.; Llano, E.M.; et al. Colonocyte-derived lactate promotes E. coli fitness in the context of inflammation-associated gut microbiota dysbiosis. Microbiome 2022, 10, 200. [Google Scholar] [CrossRef] [PubMed]
- Grenda, T.; Grenda, A.; Domaradzki, P.; Krawczyk, P.; Kwiatek, K. Probiotic potential of Clostridium spp.—Advantages and Doubts. Curr. Issues Mol. Biol. 2022, 44, 3118–3130. [Google Scholar] [CrossRef] [PubMed]
- De Brito, L.P.; da Silva, E.C.; Silva, P.H.; de Freitas, L.D.B.R.; Pastrana, L.; Soares, M.T.C.V.; Porto, A.L.F. Exopolysaccharides in immunomodulation of ulcerative colitis: A systematic review and meta-analysis of randomized controlled trials in an animal model. Food Hydrocoll. Health 2023, 4, 100158. [Google Scholar] [CrossRef]
 ), TNF-α (
), TNF-α ( ), IFN-γ (
), IFN-γ ( ), IL-6 (
), IL-6 ( ), IL-2 (
), IL-2 ( ); (B) intestinal fluid; and (C) large intestine: IL-10 (
); (B) intestinal fluid; and (C) large intestine: IL-10 ( ), IL-12 (
), IL-12 ( ), IFN-γ (
), IFN-γ ( ), IL-2 (
), IL-2 ( ). H: healthy group; T: TNBS-induced colitis group; E: TNBS-induced colitis group which received the purified EPS from Lf2; L: TNBS-induced colitis group which received Lf2. Different letters indicate significant differences among treatments (p < 0.05).
). H: healthy group; T: TNBS-induced colitis group; E: TNBS-induced colitis group which received the purified EPS from Lf2; L: TNBS-induced colitis group which received Lf2. Different letters indicate significant differences among treatments (p < 0.05).
 ), TNF-α (
), TNF-α ( ), IFN-γ (
), IFN-γ ( ), IL-6 (
), IL-6 ( ), IL-2 (
), IL-2 ( ); (B) intestinal fluid; and (C) large intestine: IL-10 (
); (B) intestinal fluid; and (C) large intestine: IL-10 ( ), IL-12 (
), IL-12 ( ), IFN-γ (
), IFN-γ ( ), IL-2 (
), IL-2 ( ). H: healthy group; T: TNBS-induced colitis group; E: TNBS-induced colitis group which received the purified EPS from Lf2; L: TNBS-induced colitis group which received Lf2. Different letters indicate significant differences among treatments (p < 0.05).
). H: healthy group; T: TNBS-induced colitis group; E: TNBS-induced colitis group which received the purified EPS from Lf2; L: TNBS-induced colitis group which received Lf2. Different letters indicate significant differences among treatments (p < 0.05).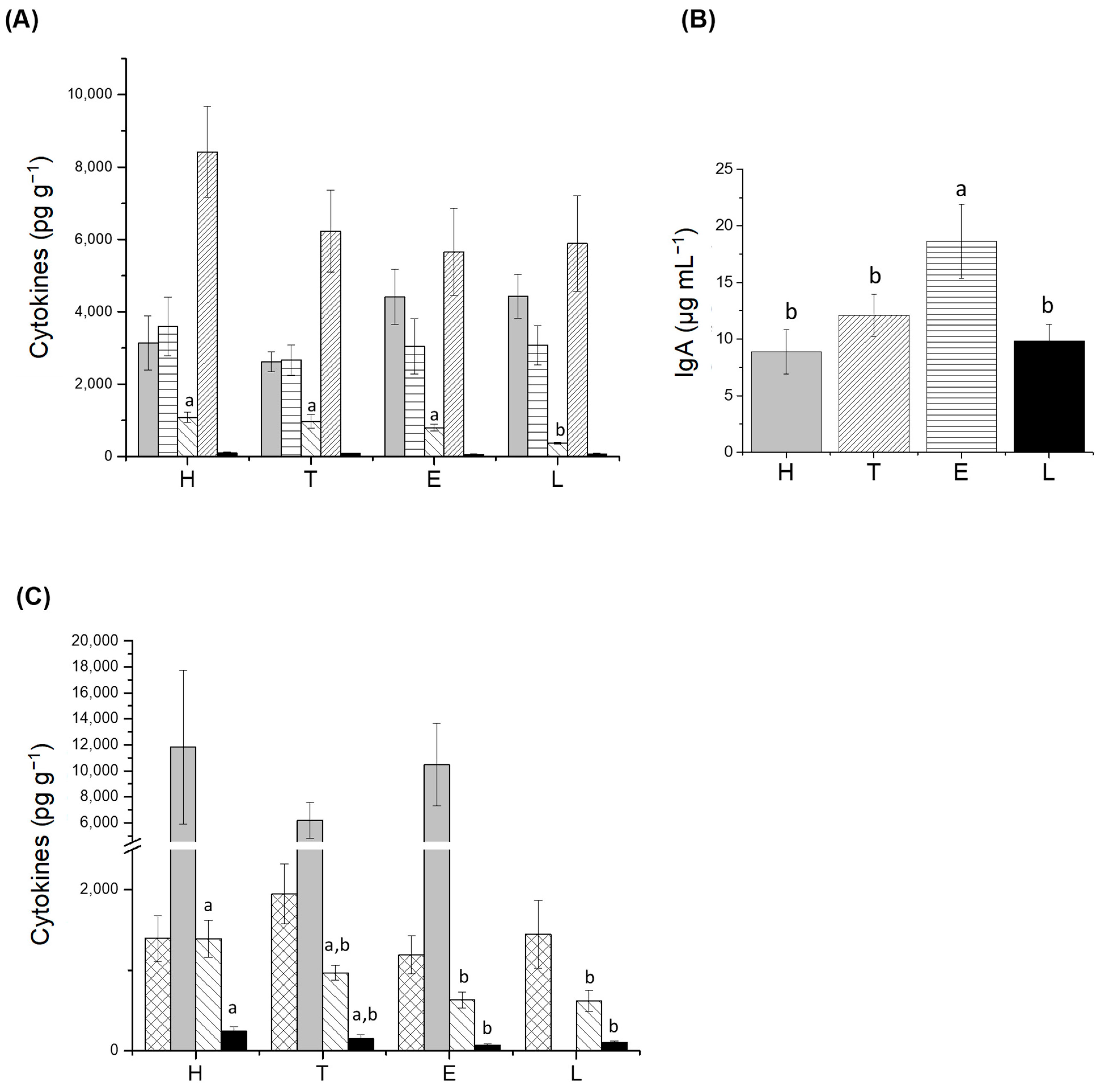
 ), CAT (
), CAT ( ), and SOD (
), and SOD ( ) activities; (B) lipid peroxidation (LPO) levels in liver. H: healthy group; T: TNBS-induced colitis group; E: TNBS-induced colitis group which received the purified EPS from Lf2; L: TNBS-induced colitis group which received Lf2. Different letters indicate significant differences among treatments (p < 0.05).
) activities; (B) lipid peroxidation (LPO) levels in liver. H: healthy group; T: TNBS-induced colitis group; E: TNBS-induced colitis group which received the purified EPS from Lf2; L: TNBS-induced colitis group which received Lf2. Different letters indicate significant differences among treatments (p < 0.05).
 ), CAT (
), CAT ( ), and SOD (
), and SOD ( ) activities; (B) lipid peroxidation (LPO) levels in liver. H: healthy group; T: TNBS-induced colitis group; E: TNBS-induced colitis group which received the purified EPS from Lf2; L: TNBS-induced colitis group which received Lf2. Different letters indicate significant differences among treatments (p < 0.05).
) activities; (B) lipid peroxidation (LPO) levels in liver. H: healthy group; T: TNBS-induced colitis group; E: TNBS-induced colitis group which received the purified EPS from Lf2; L: TNBS-induced colitis group which received Lf2. Different letters indicate significant differences among treatments (p < 0.05).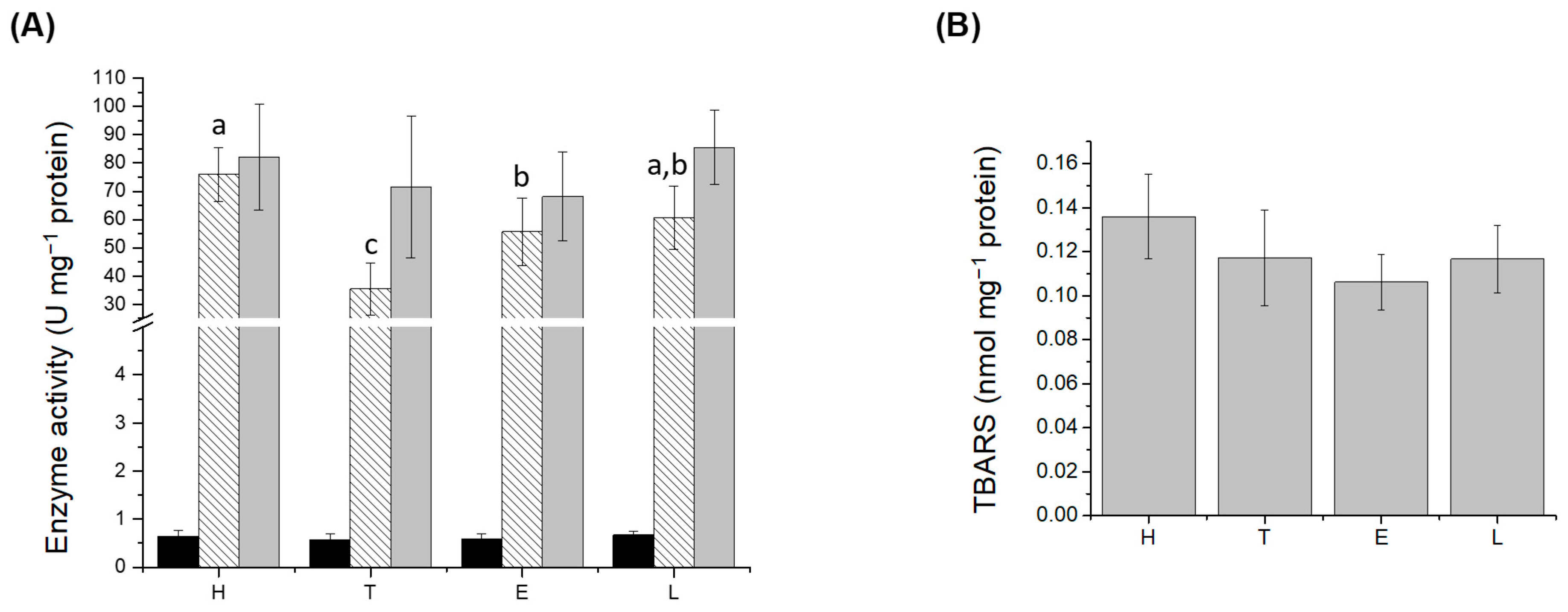
 ), acetic acid (
), acetic acid ( ), propionic acid (
), propionic acid ( ), and butyric acid (
), and butyric acid ( ). H: healthy group; T: TNBS-induced colitis group; E: TNBS-induced colitis group which received the purified EPS from Lf2; L: TNBS-induced colitis group which received Lf2. Different letters indicate significant differences among treatments (p < 0.05).
). H: healthy group; T: TNBS-induced colitis group; E: TNBS-induced colitis group which received the purified EPS from Lf2; L: TNBS-induced colitis group which received Lf2. Different letters indicate significant differences among treatments (p < 0.05).
 ), acetic acid (
), acetic acid ( ), propionic acid (
), propionic acid ( ), and butyric acid (
), and butyric acid ( ). H: healthy group; T: TNBS-induced colitis group; E: TNBS-induced colitis group which received the purified EPS from Lf2; L: TNBS-induced colitis group which received Lf2. Different letters indicate significant differences among treatments (p < 0.05).
). H: healthy group; T: TNBS-induced colitis group; E: TNBS-induced colitis group which received the purified EPS from Lf2; L: TNBS-induced colitis group which received Lf2. Different letters indicate significant differences among treatments (p < 0.05).
| Phylum | Order | Family | H | T | E | L | p-Value |
|---|---|---|---|---|---|---|---|
| Bacteroidetes | Bacteroidales | Bacteroidaceae | 220.33 b | 457.60 a | 318.17 a | 383.67 a | 0.012 |
| Bacteroidetes | Bacteroidales | Barnesiellaceae | 0.00 b | 0.00 b | 0.67 b | 21.17 a | 0.016 |
| Epsilonbacteraeota | Campylobacterales | Helicobacteraceae | 71.33 a | 28.60 b | 27.83 b | 7.00 c | 0.018 |
| Firmicutes | Clostridiales | Clostridiales, vadin BB60 group | 81.00 b | 48.20 b | 109.67 a | 21.50 b | 0.015 |
| Firmicutes | Selenomonadales | Veillonellaceae | 0.00 b | 0.00 b | 0.00 | 34.33 a | 0.026 |
| Firmicutes | Clostridiales | Lachnospiraceae | 861.33 a | 279.40 b | 679.17 a | 648.33 a | 0.055 |
| Firmicutes | Erysipelotrichales | Erysipelotrichaceae | 0.00 b | 0.00 b | 51.00 a | 30.50 a | 0.074 |
| Firmicutes | Lactobacillales | Lactobacillaceae | 36.50 a | 0.40 b | 50.50 a | 17.67 a | 0.079 |
| Proteobacteria | Rhodospirillales | Uncultured bacteria | 0.00 b | 0.00 b | 13.17 a | 0.83 b | 0.014 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ale, E.C.; Irazoqui, J.M.; Ale, A.; Peralta, G.H.; Puntillo, M.; Burns, P.; Correa Olivar, G.; Cazenave, J.; Bergamini, C.V.; Amadio, A.F.; et al. Protective Role of Limosilactobacillus fermentum Lf2 and Its Exopolysaccharides (EPS) in a TNBS-Induced Chronic Colitis Mouse Model. Fermentation 2024, 10, 77. https://doi.org/10.3390/fermentation10020077
Ale EC, Irazoqui JM, Ale A, Peralta GH, Puntillo M, Burns P, Correa Olivar G, Cazenave J, Bergamini CV, Amadio AF, et al. Protective Role of Limosilactobacillus fermentum Lf2 and Its Exopolysaccharides (EPS) in a TNBS-Induced Chronic Colitis Mouse Model. Fermentation. 2024; 10(2):77. https://doi.org/10.3390/fermentation10020077
Chicago/Turabian StyleAle, Elisa C., José M. Irazoqui, Analía Ale, Guillermo H. Peralta, Melisa Puntillo, Patricia Burns, Gabriela Correa Olivar, Jimena Cazenave, Carina V. Bergamini, Ariel F. Amadio, and et al. 2024. "Protective Role of Limosilactobacillus fermentum Lf2 and Its Exopolysaccharides (EPS) in a TNBS-Induced Chronic Colitis Mouse Model" Fermentation 10, no. 2: 77. https://doi.org/10.3390/fermentation10020077
APA StyleAle, E. C., Irazoqui, J. M., Ale, A., Peralta, G. H., Puntillo, M., Burns, P., Correa Olivar, G., Cazenave, J., Bergamini, C. V., Amadio, A. F., & Binetti, A. G. (2024). Protective Role of Limosilactobacillus fermentum Lf2 and Its Exopolysaccharides (EPS) in a TNBS-Induced Chronic Colitis Mouse Model. Fermentation, 10(2), 77. https://doi.org/10.3390/fermentation10020077