Therapeutic Applications of Native and Engineered Saccharomyces Yeasts
Abstract
1. Introduction
2. Health Benefits of Sb and Sc, and Their Modes of Action
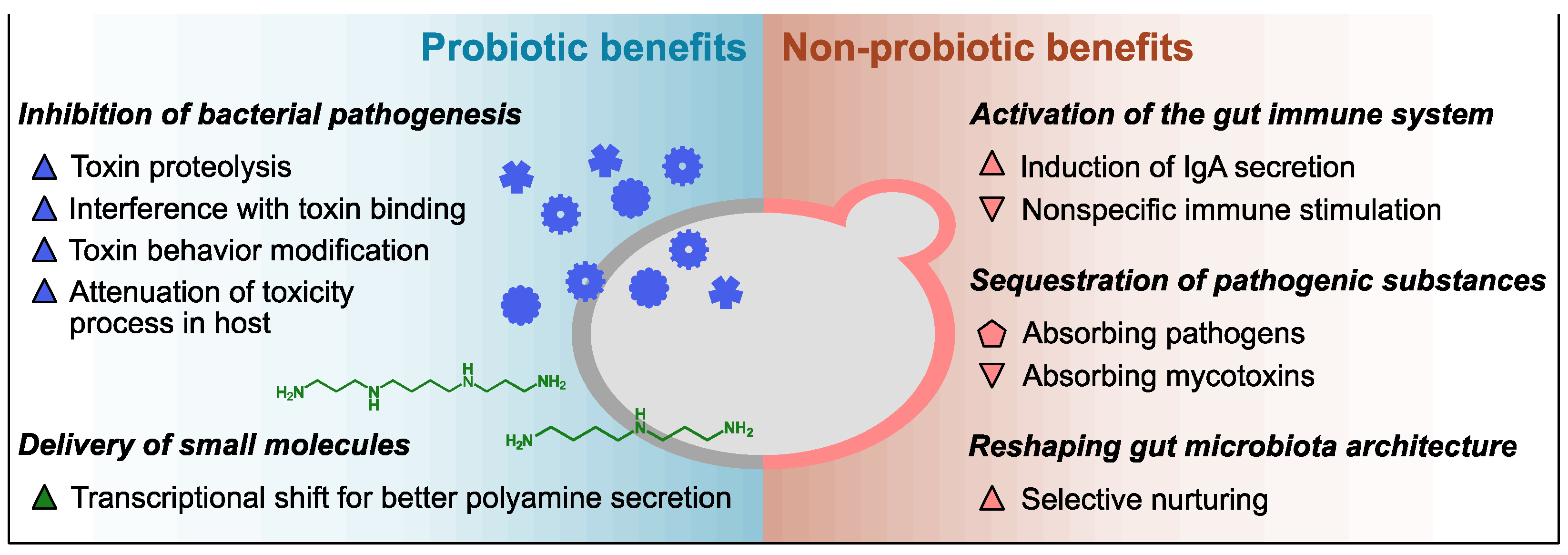
2.1. Innate Probiotic Benefits
2.2. Innate Non-Probiotic Benefits
3. Engineering of Saccharomyces Yeasts as Therapeutic Avenues
3.1. In Situ Delivery of Therapeutic Proteins
3.2. In Situ Delivery of Small Molecules
3.3. Biosensing and Expression Systems in Synthetic Probiotic Yeasts
3.4. Control of the Viability and Activity of Synthetic Yeasts
3.5. Engineering of Yeast Cell Wall Polysaccharides as Parabiotic and Prebiotic Biomaterials
4. Discussion
4.1. Controversies about the Potential of Sb as a Probiotic Chassis
4.2. Concerns about the Safety and Tractability of Sb
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kelesidis, T.; Pothoulakis, C. Efficacy and safety of the probiotic Saccharomyces boulardii for the prevention and therapy of gastrointestinal disorders. Ther. Adv. Gastroenterol. 2012, 5, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Pais, P.; Oliveira, J.; Almeida, V.; Yilmaz, M.; Monteiro, P.T.; Teixeira, M.C. Transcriptome-wide differences between Saccharomyces cerevisiae and Saccharomyces cerevisiae var. boulardii: Clues on host survival and probiotic activity based on promoter sequence variability. Genomics 2021, 113, 530–539. [Google Scholar] [CrossRef] [PubMed]
- McFarland, L.V. Saccharomyces boulardii is not Saccharomyces cerevisiae. Clin. Infect. Dis. 1996, 22, 200–201. [Google Scholar] [CrossRef][Green Version]
- Pais, P.; Almeida, V.; Yılmaz, M.; Teixeira, M.C. Saccharomyces boulardii: What Makes It Tick as Successful Probiotic? J. Fungi 2020, 6, 78. [Google Scholar] [CrossRef] [PubMed]
- Edwards-Ingram, L.; Gitsham, P.; Burton, N.; Warhurst, G.; Clarke, I.; Hoyle, D.; Oliver, S.G.; Stateva, L. Genotypic and physiological characterization of Saccharomyces boulardii, the probiotic strain of Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2007, 73, 2458–2467. [Google Scholar] [CrossRef]
- Fietto, J.L.R.; Araújo, R.S.; Valadão, F.N.; Fietto, L.G.; Brandão, R.L.; Neves, M.J.; Gomes, F.C.O.; Nicoli, J.R.; Castro, I.M. Molecular and physiological comparisons between Saccharomyces cerevisiae and Saccharomyces boulardii. Can. J. Microbiol. 2004, 50, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-J.; Zhang, G.-C.; Kong, I.I.; Yun, E.J.; Zheng, J.-Q.; Kweon, D.-H.; Jin, Y.-S. A Mutation in PGM2 Causing Inefficient Galactose Metabolism in the Probiotic Yeast Saccharomyces boulardii. Appl. Environ. Microbiol. 2018, 84, 2280–2287. [Google Scholar] [CrossRef]
- Kwak, S.; Mahmud, B.; Dantas, G. A Tunable and Expandable Transactivation System in Probiotic Yeast Saccharomyces boulardii. ACS Synth. Biol. 2021, 11, 508–514. [Google Scholar] [CrossRef]
- Kwak, S.; Robinson, S.J.; Lee, J.W.; Lim, H.; Wallace, C.L.; Jin, Y.-S. Dissection and enhancement of prebiotic properties of yeast cell wall oligosaccharides through metabolic engineering. Biomaterials 2022, 282, 121379. [Google Scholar] [CrossRef]
- Hudson, L.E.; McDermott, C.D.; Stewart, T.P.; Hudson, W.H.; Rios, D.; Fasken, M.B.; Corbett, A.H.; Lamb, T.J. Characterization of the Probiotic Yeast Saccharomyces boulardii in the Healthy Mucosal Immune System. PLoS ONE 2016, 11, e0153351. [Google Scholar] [CrossRef]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Mechanisms of Action of Probiotics. Adv. Nutr. 2019, 10, S49–S66. [Google Scholar] [CrossRef] [PubMed]
- Czerucka, D.; Piche, T.; Rampal, P. Review article: Yeast as probiotics—Saccharomyces boulardii, Aliment. Pharmacol. Ther. 2007, 26, 767–778. [Google Scholar] [CrossRef]
- Nielsen, J. Yeast Systems Biology: Model Organism and Cell Factory. Biotechnol. J. 2019, 14, e1800421. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-J.; Kong, I.I.; Zhang, G.-C.; Jayakody, L.N.; Kim, H.; Xia, P.-F.; Kwak, S.; Sung, B.H.; Sohn, J.-H.; Walukiewicz, H.E.; et al. Metabolic Engineering of Probiotic Saccharomyces boulardii. Appl. Environ. Microbiol. 2016, 82, 2280–2287. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.J.; Langdon, A.E.; Dantas, G. Understanding the impact of antibiotic perturbation on the human microbiome. Genome Med. 2020, 12, 82. [Google Scholar] [CrossRef] [PubMed]
- Hudson, L.E.; Fasken, M.B.; McDermott, C.D.; McBride, S.M.; Kuiper, E.G.; Guiliano, D.B.; Corbett, A.H.; Lamb, T.J. Functional heterologous protein expression by genetically engineered probiotic yeast Saccharomyces boulardii. PLoS ONE 2014, 9, e112660. [Google Scholar] [CrossRef] [PubMed]
- Hamedi, H.; Misaghi, A.; Modarressi, M.H.; Salehi, T.Z.; Khorasanizadeh, D.; Khalaj, V. Generation of a Uracil Auxotroph Strain of the Probiotic Yeast Saccharomyces boulardii as a Host for the Recombinant Protein Production. Avicenna J. Med. Biotechnol. 2013, 5, 29–34. [Google Scholar] [PubMed]
- Wang, L.; Sun, H.; Zhang, J.; Liu, Q.; Wang, T.; Chen, P.; Li, H.; Xiao, Y.; Wang, F.; Zhao, X. Establishment and application of target gene disruption system in Saccharomyces boulardii. Biotechnol. Bioprocess Eng. 2015, 20, 26–36. [Google Scholar] [CrossRef]
- Moslehi-Jenabian, S.; Pedersen, L.L.; Jespersen, L. Beneficial effects of probiotic and food borne yeasts on human health. Nutrients 2010, 2, 449–473. [Google Scholar] [CrossRef]
- McFarland, L.V.; Surawicz, C.M.; Greenberg, R.N.; Fekety, R.; Elmer, G.W.; Moyer, K.A.; Melcher, S.A.; Bowen, K.E.; Cox, J.L.; Noorani, Z. A randomized placebo-controlled trial of Saccharomyces boulardii in combination with standard antibiotics for Clostridium difficile disease. JAMA 1994, 271, 1913–1918. [Google Scholar] [CrossRef]
- Surawicz, C.M.; McFarland, L.V.; Greenberg, R.N.; Rubin, M.; Fekety, R.; Mulligan, M.E.; Garcia, R.J.; Brandmarker, S.; Bowen, K.; Borjal, D.; et al. The search for a better treatment for recurrent Clostridium difficile disease: Use of high-dose vancomycin combined with Saccharomyces boulardii. Clin. Infect. Dis. 2000, 31, 1012–1017. [Google Scholar] [CrossRef]
- Elmer, G.W.; Corthier, G. Modulation of Clostridium difficile induced mortality as a function of the dose and the viability of the Saccharomyces boulardii used as a preventative agent in gnotobiotic mice. Can. J. Microbiol. 1991, 37, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Castagliuolo, I.; LaMont, J.T.; Nikulasson, S.T.; Pothoulakis, C. Saccharomyces boulardii protease inhibits Clostridium difficile toxin A effects in the rat ileum. Infect. Immun. 1996, 64, 5225–5232. [Google Scholar] [CrossRef] [PubMed]
- Castagliuolo, I.; Riegler, M.F.; Valenick, L.; LaMont, J.T.; Pothoulakis, C. Saccharomyces boulardii protease inhibits the effects of Clostridium difficile toxins A and B in human colonic mucosa. Infect. Immun. 1999, 67, 302–307. [Google Scholar] [CrossRef]
- Pontier-Bres, R.; Rampal, P.; Peyron, J.-F.; Munro, P.; Lemichez, E.; Czerucka, D. The Saccharomyces boulardii CNCM I-745 strain shows protective effects against the B. anthracis LT toxin. Toxins 2015, 7, 4455–4467. [Google Scholar] [CrossRef] [PubMed]
- Buts, J.-P.; Dekeyser, N.; Stilmant, C.; Delem, E.; Smets, F.; Sokal, E. Saccharomyces boulardii produces in rat small intestine a novel protein phosphatase that inhibits Escherichia coli endotoxin by dephosphorylation. Pediatr. Res. 2006, 60, 24–29. [Google Scholar] [CrossRef]
- Czerucka, D.; Roux, I.; Rampal, P. Saccharomyces boulardii inhibits secretagogue-mediated adenosine 3′,5′-cyclic monophosphate induction in intestinal cells. Gastroenterology 1994, 106, 65–72. [Google Scholar] [CrossRef]
- Buts, J.P.; De Keyser, N.; Marandi, S.; Hermans, D.; Sokal, E.M.; Chae, Y.H.; Lambotte, L.; Chanteux, H.; Tulkens, P.M. Saccharomyces boulardii upgrades cellular adaptation after proximal enterectomy in rats. Gut 1999, 45, 89–96. [Google Scholar] [CrossRef]
- Buts, J.P.; Bernasconi, P.; Vaerman, J.P.; Dive, C. Stimulation of secretory IgA and secretory component of immunoglobulins in small intestine of rats treated with Saccharomyces boulardii. Dig. Dis. Sci. 1990, 35, 251–256. [Google Scholar] [CrossRef]
- Ha, C.H.; Yun, C.W.; Paik, H.D.; Kim, S.W.; Kang, C.W.; Hwang, H.J.; Chang, H.I. Preparation and analysis of yeast cell wall mannoproteins, immune enhancing materials, from cell wall mutant Saccharomyces cerevisiae. J. Microbiol. Biotechnol. 2006, 16, 247–255. [Google Scholar]
- Gedek, B.R. Adherence of Escherichia coli serogroup O 157 and the Salmonella typhimurium mutant DT 104 to the surface of Saccharomyces boulardii. Mycoses 1999, 42, 261–264. [Google Scholar] [CrossRef]
- Tiago, F.C.P.; Martins, F.S.; Souza, E.L.S.; Pimenta, P.F.P.; Araujo, H.R.C.; Castro, I.M.; Brandão, R.L.; Nicoli, J.R. Adhesion to the yeast cell surface as a mechanism for trapping pathogenic bacteria by Saccharomyces probiotics. J. Med. Microbiol. 2012, 61, 1194–1207. [Google Scholar] [CrossRef]
- Pizzolitto, R.P.; Armando, M.R.; Salvano, M.A.; Dalcero, A.M.; Rosa, C.A. Evaluation of Saccharomyces cerevisiae as an antiaflatoxicogenic agent in broiler feedstuffs. Poult. Sci. 2013, 92, 1655–1663. [Google Scholar] [CrossRef]
- Baptista, A.S.; Horii, J.; Calori-Domingues, M.A.; da Glória, E.M.; Salgado, J.M.; Vizioli, M.R. The capacity of mannooligosaccharides thermolysed yeast and active yeast to attenuate aflatoxicosis. World J. Microbiol. Biotechnol. 2004, 20, 475–481. [Google Scholar] [CrossRef]
- Everard, A.; Matamoros, S.; Geurts, L.; Delzenne, N.M.; Cani, P.D. Saccharomyces boulardii administration changes gut microbiota and reduces hepatic steatosis, low-grade inflammation, and fat mass in obese and type 2 diabetic db/db mice. MBio 2014, 5, e01011–e01014. [Google Scholar] [CrossRef]
- Toothaker, R.D.; Elmer, G.W. Prevention of clindamycin-induced mortality in hamsters by Saccharomyces boulardii. Antimicrob. Agents Chemother. 1984, 26, 552–556. [Google Scholar] [CrossRef]
- Corthier, G.; Dubos, F.; Ducluzeau, R. Prevention of Clostridium difficile induced mortality in gnotobiotic mice by Saccharomyces boulardii. Can. J. Microbiol. 1986, 32, 894–896. [Google Scholar] [CrossRef]
- Castex, F.; Corthier, G.; Jouvert, S.; Elmer, G.W.; Lucas, F.; Bastide, M. Prevention of Clostridium difficile-induced experimental pseudomembranous colitis by Saccharomyces boulardii: A scanning electron microscopic and microbiological study. J. Gen. Microbiol. 1990, 136, 1085–1089. [Google Scholar] [CrossRef]
- Pothoulakis, C.; Kelly, C.P.; Joshi, M.A.; Gao, N.; O’Keane, C.J.; Castagliuolo, I.; Lamont, J.T. Saccharomyces boulardii inhibits Clostridium difficile toxin A binding and enterotoxicity in rat ileum. Gastroenterology 1993, 104, 1108–1115. [Google Scholar] [CrossRef]
- Beatty, M.E.; Ashford, D.A.; Griffin, P.M.; Tauxe, R.V.; Sobel, J. Gastrointestinal anthrax: Review of the literature. Arch. Intern. Med. 2003, 163, 2527–2531. [Google Scholar] [CrossRef]
- Czerucka, D.; Rampal, P. Experimental effects of Saccharomyces boulardii on diarrheal pathogens. Microbes Infect. 2002, 4, 733–739. [Google Scholar] [CrossRef]
- Conner, J.G.; Teschler, J.K.; Jones, C.J.; Yildiz, F.H. Staying Alive: Vibrio cholerae’s Cycle of Environmental Survival, Transmission, and Dissemination. Microbiol. Spectr. 2016, 4, 593–633. [Google Scholar] [CrossRef]
- Khatri, I.; Akhtar, A.; Kaur, K.; Tomar, R.; Prasad, G.S.; Ramya, T.N.C.; Subramanian, S. Gleaning evolutionary insights from the genome sequence of a probiotic yeast Saccharomyces boulardii. Gut Pathog. 2013, 5, 30. [Google Scholar] [CrossRef]
- Buts, J.P.; De Keyser, N.; De Raedemaeker, L. Saccharomyces boulardii enhances rat intestinal enzyme expression by endoluminal release of polyamines. Pediatr. Res. 1994, 36, 522–527. [Google Scholar] [CrossRef]
- Rao, J.N.; Xiao, L.; Wang, J.-Y. Polyamines in Gut Epithelial Renewal and Barrier Function. Physiology 2020, 35, 328–337. [Google Scholar] [CrossRef]
- Ray, R.M.; McCormack, S.A.; Covington, C.; Viar, M.J.; Zheng, Y.; Johnson, L.R. The requirement for polyamines for intestinal epithelial cell migration is mediated through Rac1. J. Biol. Chem. 2003, 278, 13039–13046. [Google Scholar] [CrossRef]
- Gómez-Verduzco, G.; Cortes-Cuevas, A.; López-Coello, C.; Avila-González, E.; Nava, G.M. Dietary supplementation of mannan-oligosaccharide enhances neonatal immune responses in chickens during natural exposure to Eimeria spp. Acta Vet. Scand. 2009, 51, 11. [Google Scholar] [CrossRef]
- Kudoh, K.; Shimizu, J.; Ishiyama, A.; Wada, M.; Takita, T.; Kanke, Y.; Innami, S. Secretion and excretion of immunoglobulin A to cecum and feces differ with type of indigestible saccharides. J. Nutr. Sci. Vitaminol. 1999, 45, 173–181. [Google Scholar] [CrossRef]
- Swanson, K.S.; Grieshop, C.M.; Flickinger, E.A.; Bauer, L.L.; Healy, H.-P.; Dawson, K.A.; Merchen, N.R.; Fahey, G.C. Supplemental fructooligosaccharides and mannanoligosaccharides influence immune function, ileal and total tract nutrient digestibilities, microbial populations and concentrations of protein catabolites in the large bowel of dogs. J. Nutr. 2002, 132, 980–989. [Google Scholar] [CrossRef]
- Gut, A.M.; Vasiljevic, T.; Yeager, T.; Donkor, O.N. Salmonella infection—Prevention and treatment by antibiotics and probiotic yeasts: A review. Microbiology 2018, 164, 1327–1344. [Google Scholar] [CrossRef]
- Qamar, A.; Aboudola, S.; Warny, M.; Michetti, P.; Pothoulakis, C.; LaMont, J.T.; Kelly, C.P. Saccharomyces boulardii stimulates intestinal immunoglobulin A immune response to Clostridium difficile toxin A in mice. Infect. Immun. 2001, 69, 2762–2765. [Google Scholar] [CrossRef]
- Pontier-Bres, R.; Munro, P.; Boyer, L.; Anty, R.; Imbert, V.; Terciolo, C.; André, F.; Rampal, P.; Lemichez, E.; Peyron, J.-F.; et al. Saccharomyces boulardii modifies Salmonella typhimurium traffic and host immune responses along the intestinal tract. PLoS ONE 2014, 9, e103069. [Google Scholar] [CrossRef]
- Posadas, G.A.; Broadway, P.R.; Thornton, J.A.; Carroll, J.A.; Lawrence, A.; Corley, J.R.; Thompson, A.; Donaldson, J.R. Yeast pro- and paraprobiotics have the capability to bind pathogenic bacteria associated with animal disease. Transl. Anim. Sci. 2017, 1, 60–68. [Google Scholar] [CrossRef]
- Martins, F.S.; Dalmasso, G.; Arantes, R.M.E.; Doye, A.; Lemichez, E.; Lagadec, P.; Imbert, V.; Peyron, J.-F.; Rampal, P.; Nicoli, J.R.; et al. Interaction of Saccharomyces boulardii with Salmonella enterica serovar Typhimurium protects mice and modifies T84 cell response to the infection. PLoS ONE 2010, 5, e8925. [Google Scholar] [CrossRef]
- Fàbrega, A.; Vila, J. Salmonella enterica serovar Typhimurium skills to succeed in the host: Virulence and regulation. Clin. Microbiol. Rev. 2013, 26, 308–341. [Google Scholar] [CrossRef]
- Sharon, N.; Eshdat, Y.; Silverblatt, F.J.; Ofek, I. Bacterial adherence to cell surface sugars. Ciba Found. Symp. 1981, 80, 119–141. [Google Scholar]
- Shetty, P.H.; Jespersen, L. Saccharomyces cerevisiae and lactic acid bacteria as potential mycotoxin decontaminating agents. Trends Food Sci. Technol. 2006, 17, 48–55. [Google Scholar] [CrossRef]
- Hassan, Z.U.; Al Thani, R.; Atia, F.A.; Alsafran, M.; Migheli, Q.; Jaoua, S. Application of yeasts and yeast derivatives for the biological control of toxigenic fungi and their toxic metabolites. Environ. Technol. Innov. 2021, 22, 101447. [Google Scholar] [CrossRef]
- Tanihiro, R.; Sakano, K.; Oba, S.; Nakamura, C.; Ohki, K.; Hirota, T.; Sugiyama, H.; Ebihara, S.; Nakamura, Y. Effects of Yeast Mannan Which Promotes Beneficial Bacteroides on the Intestinal Environment and Skin Condition: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2020, 12, E3673. [Google Scholar] [CrossRef] [PubMed]
- Abbott, D.W.; Martens, E.C.; Gilbert, H.J.; Cuskin, F.; Lowe, E.C. Coevolution of yeast mannan digestion: Convergence of the civilized human diet, distal gut microbiome, and host immunity. Gut Microbes 2015, 6, 334–339. [Google Scholar] [CrossRef]
- Cuskin, F.; Lowe, E.C.; Temple, M.J.; Zhu, Y.; Cameron, E.A.; Pudlo, N.A.; Porter, N.T.; Urs, K.; Thompson, A.J.; Cartmell, A.; et al. Human gut Bacteroidetes can utilize yeast mannan through a selfish mechanism. Nature 2015, 517, 165–169. [Google Scholar] [CrossRef]
- Oba, S.; Sunagawa, T.; Tanihiro, R.; Awashima, K.; Sugiyama, H.; Odani, T.; Nakamura, Y.; Kondo, A.; Sasaki, D.; Sasaki, K. Prebiotic effects of yeast mannan, which selectively promotes Bacteroides thetaiotaomicron and Bacteroides ovatus in a human colonic microbiota model. Sci. Rep. 2020, 10, 17351. [Google Scholar] [CrossRef]
- Scott, B.M.; Gutiérrez-Vázquez, C.; Sanmarco, L.M.; da Silva Pereira, J.A.; Li, Z.; Plasencia, A.; Hewson, P.; Cox, L.M.; O’Brien, M.; Chen, S.K.; et al. Self-tunable engineered yeast probiotics for the treatment of inflammatory bowel disease. Nat. Med. 2021, 27, 1212–1222. [Google Scholar] [CrossRef]
- Durmusoglu, D.; Al’Abri, I.S.; Collins, S.P.; Cheng, J.; Eroglu, A.; Beisel, C.L.; Crook, N. In Situ Biomanufacturing of Small Molecules in the Mammalian Gut by Probiotic Saccharomyces boulardii. ACS Synth. Biol. 2021, 10, 1039–1052. [Google Scholar] [CrossRef]
- Zhang, G.-C.; Kong, I.I.; Kim, H.; Liu, J.-J.; Cate, J.H.D.; Jin, Y.-S. Construction of a quadruple auxotrophic mutant of an industrial polyploid Saccharomyces cerevisiae strain by using RNA-guided Cas9 nuclease. Appl. Environ. Microbiol. 2014, 80, 7694–7701. [Google Scholar] [CrossRef]
- Chen, K.; Zhu, Y.; Zhang, Y.; Hamza, T.; Yu, H.; Fleur, A.S.; Galen, J.; Yang, Z.; Feng, H. A probiotic yeast-based immunotherapy against Clostridioides difficile infection. Sci. Transl. Med. 2020, 12, eaax4905. [Google Scholar] [CrossRef]
- Mugwanda, K.; Hamese, S.; Van Zyl, W.F.; Prinsloo, E.; Plessis, M.D.; Dicks, L.M.T.; Thimiri Govinda Raj, D.B. Recent advances in genetic tools for engineering probiotic lactic acid bacteria. Biosci. Rep. 2023, 43, BSR20211299. [Google Scholar] [CrossRef]
- Kim, J.; Atkinson, C.; Miller, M.J.; Kim, K.H.; Jin, Y.-S. Microbiome Engineering Using Probiotic Yeast: Saccharomyces boulardii and the Secreted Human Lysozyme Lead to Changes in the Gut Microbiome and Metabolome of Mice. Microbiol. Spectr. 2023, 11, e0078023. [Google Scholar] [CrossRef]
- Liu, C.-H.; Chang, J.-H.; Chang, Y.-C.; Mou, K.Y. Treatment of murine colitis by Saccharomyces boulardii secreting atrial natriuretic peptide. J. Mol. Med. 2020, 98, 1675–1687. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Cheong, Y.E.; Yu, S.; Jin, Y.-S.; Kim, K.H. Strain engineering and metabolic flux analysis of a probiotic yeast Saccharomyces boulardii for metabolizing L-fucose, a mammalian mucin component. Microb. Cell Fact. 2022, 21, 204. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Falcón, M.S.; Buitrago-Arias, C.; Avila-Reyes, S.V.; Solorza-Feria, J.; Arenas-Ocampo, M.L.; Camacho-Díaz, B.H.; Jiménez-Aparicio, A.R. Kinetics and Mechanisms of Saccharomyces boulardii Release from Optimized Whey Protein-Agavin-Alginate Beads under Simulated Gastrointestinal Conditions. Bioengineering 2022, 9, 460. [Google Scholar] [CrossRef]
- Hedin, K.A.; Kruse, V.; Vazquez-Uribe, R.; Sommer, M.O.A. Biocontainment strategies for in vivo applications of Saccharomyces boulardii. Front. Bioeng. Biotechnol. 2023, 11, 1136095. [Google Scholar] [CrossRef] [PubMed]
- Alexander, L.M.; van Pijkeren, J.-P. Modes of therapeutic delivery in synthetic microbiology. Trends Microbiol. 2023, 31, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J. Production of biopharmaceutical proteins by yeast: Advances through metabolic engineering. Bioengineered 2023, 4, 207–211. [Google Scholar] [CrossRef]
- Yang, S.; Song, L.; Wang, J.; Zhao, J.; Tang, H.; Bao, X. Engineering Saccharomyces cerevisiae for efficient production of recombinant proteins. Eng. Microbiol. 2023, 4, 100122. [Google Scholar] [CrossRef]
- Jin, Y.; Yu, S.; Liu, J.-J.; Yun, E.J.; Lee, J.W.; Jin, Y.-S.; Kim, K.H. Production of neoagarooligosaccharides by probiotic yeast Saccharomyces cerevisiae var. boulardii engineered as a microbial cell factory. Microb. Cell Factories 2021, 20, 160. [Google Scholar] [CrossRef] [PubMed]
- Kwak, S.; Kim, S.R.; Xu, H.; Zhang, G.-C.; Lane, S.; Kim, H.; Jin, Y.-S. Enhanced isoprenoid production from xylose by engineered Saccharomyces cerevisiae. Biotechnol. Bioeng. 2017, 114, 2581–2591. [Google Scholar] [CrossRef]
- Vedantam, G.; Clark, A.; Chu, M.; McQuade, R.; Mallozzi, M.; Viswanathan, V.K. Clostridium difficile infection: Toxins and non-toxin virulence factors, and their contributions to disease establishment and host response. Gut Microbes 2012, 3, 121–134. [Google Scholar] [CrossRef]
- Maury, J.; Germann, S.M.; Jacobsen, S.A.B.; Jensen, N.B.; Kildegaard, K.R.; Herrgård, M.J.; Schneider, K.; Koza, A.; Forster, J.; Nielsen, J.; et al. EasyCloneMulti: A Set of Vectors for Simultaneous and Multiple Genomic Integrations in Saccharomyces cerevisiae. PLoS ONE 2016, 11, e0150394. [Google Scholar] [CrossRef]
- Paramasivan, K.; Mutturi, S. Progress in terpene synthesis strategies through engineering of Saccharomyces cerevisiae. Crit. Rev. Biotechnol. 2017, 37, 974–989. [Google Scholar] [CrossRef]
- Bauer, M.A.; Kainz, K.; Carmona-Gutierrez, D.; Madeo, F. Microbial wars: Competition in ecological niches and within the microbiome. Microb. Cell 2018, 5, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Feng, X.; Luan, H.; Wang, J.; Ge, R.; Li, Z.; Bian, J. Current knowledge on the nucleotide agonists for the P2Y2 receptor. Bioorganic Med. Chem. 2018, 26, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Hagen, D.C.; McCaffrey, G.; Sprague, G.F. Pheromone response elements are necessary and sufficient for basal and pheromone-induced transcription of the FUS1 gene of Saccharomyces cerevisiae. Mol. Cell. Biol. 1991, 11, 2952–2961. [Google Scholar] [PubMed]
- Zalatan, J.G.; Lee, M.E.; Almeida, R.; Gilbert, L.A.; Whitehead, E.H.; La Russa, M.; Tsai, J.C.; Weissman, J.S.; Dueber, J.E.; Qi, L.S.; et al. Engineering complex synthetic transcriptional programs with CRISPR RNA scaffolds. Cell 2015, 160, 339–350. [Google Scholar] [CrossRef]
- Greger, I.H.; Aranda, A.; Proudfoot, N. Balancing transcriptional interference and initiation on the GAL7 promoter of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 2000, 97, 8415–8420. [Google Scholar] [CrossRef]
- Klein, S.M.; Elmer, G.W.; McFarland, L.V.; Surawicz, C.M.; Levy, R.H. Recovery and elimination of the biotherapeutic agent, Saccharomyces boulardii, in healthy human volunteers. Pharm. Res. 1993, 10, 1615–1619. [Google Scholar] [CrossRef]
- Pacheco, A.R.; Curtis, M.M.; Ritchie, J.M.; Munera, D.; Waldor, M.K.; Moreira, C.G.; Sperandio, V. Fucose sensing regulates bacterial intestinal colonization. Nature 2012, 492, 113–117. [Google Scholar] [CrossRef]
- Sicard, J.-F.; Le Bihan, G.; Vogeleer, P.; Jacques, M.; Harel, J. Interactions of Intestinal Bacteria with Components of the Intestinal Mucus. Front. Cell. Infect. Microbiol. 2017, 7, 387. [Google Scholar] [CrossRef]
- Jiang, Y.; Proteau, P.; Poulter, D.; Ferro-Novick, S. BTS1 encodes a geranylgeranyl diphosphate synthase in Saccharomyces cerevisiae. J. Biol. Chem. 1995, 270, 21793–21799. [Google Scholar] [CrossRef]
- Orlean, P. Architecture and biosynthesis of the Saccharomyces cerevisiae cell wall. Genetics 2012, 192, 775–818. [Google Scholar] [CrossRef]
- Pfeiffer, T.; Morley, A. An evolutionary perspective on the Crabtree effect. Front. Mol. Biosci. 2014, 1, 17. [Google Scholar] [CrossRef] [PubMed]
- Boles, E.; Göhlmann, H.W.; Zimmermann, F.K. Cloning of a second gene encoding 6-phosphofructo-2-kinase in yeast, and characterization of mutant strains without fructose-2,6-bisphosphate. Mol. Microbiol. 1996, 20, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Kwak, S.; Yun, E.J.; Lane, S.; Oh, E.J.; Kim, K.H.; Jin, Y.-S. Redirection of the glycolytic flux enhances isoprenoid production in Saccharomyces cerevisiae. Biotechnol. J. 2019, 15, e1900173. [Google Scholar] [CrossRef] [PubMed]
- Swinnen, S.; Goovaerts, A.; Schaerlaekens, K.; Dumortier, F.; Verdyck, P.; Souvereyns, K.; Van Zeebroeck, G.; Foulquié-Moreno, M.R.; Thevelein, J.M. Auxotrophic Mutations Reduce Tolerance of Saccharomyces cerevisiae to Very High Levels of Ethanol Stress. Eukaryot. Cell 2015, 14, 884–897. [Google Scholar] [CrossRef] [PubMed]
- O’Doherty, P.J.; Lyons, V.; Tun, N.M.; Rogers, P.J.; Bailey, T.D.; Wu, M.J. Transcriptomic and biochemical evidence for the role of lysine biosynthesis against linoleic acid hydroperoxide-induced stress in Saccharomyces cerevisiae. Free Radic. Res. 2014, 48, 1454–1461. [Google Scholar] [CrossRef] [PubMed]
- Petti, A.A.; Crutchfield, C.A.; Rabinowitz, J.D.; Botstein, D. Survival of starving yeast is correlated with oxidative stress response and nonrespiratory mitochondrial function. Proc. Natl. Acad. Sci. USA 2011, 108, E1089–E1098. [Google Scholar] [CrossRef] [PubMed]
- Bauer, B.E.; Rossington, D.; Mollapour, M.; Mamnun, Y.; Kuchler, K.; Piper, P.W. Weak organic acid stress inhibits aromatic amino acid uptake by yeast, causing a strong influence of amino acid auxotrophies on the phenotypes of membrane transporter mutants. Eur. J. Biochem. 2003, 270, 3189–3195. [Google Scholar] [CrossRef]
- Stanhill, A.; Schick, N.; Engelberg, D. The yeast ras/cyclic AMP pathway induces invasive growth by suppressing the cellular stress response. Mol. Cell. Biol. 1999, 19, 7529–7538. [Google Scholar] [CrossRef][Green Version]
- Cassone, M.; Serra, P.; Mondello, F.; Girolamo, A.; Scafetti, S.; Pistella, E.; Venditti, M. Outbreak of Saccharomyces cerevisiae subtype boulardii fungemia in patients neighboring those treated with a probiotic preparation of the organism. J. Clin. Microbiol. 2003, 41, 5340–5343. [Google Scholar] [CrossRef]
- Thygesen, J.B.; Glerup, H.; Tarp, B. Saccharomyces boulardii fungemia caused by treatment with a probioticum. BMJ Case Rep. 2012, 2012, bcr0620114412. [Google Scholar] [CrossRef]
- Roy, U.; Jessani, L.G.; Rudramurthy, S.M.; Gopalakrishnan, R.; Dutta, S.; Chakravarty, C.; Jillwin, J.; Chakrabarti, A. Seven cases of Saccharomyces fungaemia related to use of probiotics. Mycoses 2017, 60, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Niault, M.; Thomas, F.; Prost, J.; Ansari, F.H.; Kalfon, P. Fungemia due to Saccharomyces species in a patient treated with enteral Saccharomyces boulardii. Clin. Infect. Dis. 1999, 28, 930. [Google Scholar] [CrossRef] [PubMed]
- Cherifi, S.; Robberecht, J.; Miendje, Y. Saccharomyces cerevisiae fungemia in an elderly patient with Clostridium difficile colitis. Acta Clin. Belg. 2004, 59, 223–224. [Google Scholar] [CrossRef] [PubMed]
- Lherm, T.; Monet, C.; Nougière, B.; Soulier, M.; Larbi, D.; Le Gall, C.; Caen, D. Malbrunot. Seven cases of fungemia with Saccharomyces boulardii in critically ill patients. Intensive Care Med. 2002, 28, 797–801. [Google Scholar] [CrossRef]
- Kapteyn, J.C.; Riet, B.T.; Vink, E.; Blad, S.; De Nobel, H.; Van Den Ende, H.; Klis, F.M. Low external pH induces HOG1-dependent changes in the organization of the Saccharomyces cerevisiae cell wall. Mol. Microbiol. 2001, 39, 469–479. [Google Scholar] [CrossRef]
| Health Benefit | Study Design and Methodology | Outcome | Ref. |
|---|---|---|---|
| Protection against C. difficile infection | Randomized placebo-controlled clinical trial, the combination of Sb and antibiotics | Lower relative risk of recurrent C. difficile infection in Sb recipients than placebo | [20,21] |
| In vivo (mice), Sb administration | Dose- and viability-dependent prophylactic effect of Sb decreasing lethality | [22] | |
| In vivo (rats), Sb administration | 54 kDa protease digested TcdA and inhibited its binding to rat ileal brush border | [23] | |
| In vitro (human colonic mucosa), functional validation of 54 kDa protease of Sb | Attenuation of toxin-induced electrophysiologic and cytotoxic effects | [24] | |
| Potential protection from anthrax | In vitro, biochemical assay of B. anthracis lethal toxin and Sb cells | Trapping and proteolysis of protective antigens of lethal toxin by Sb | [25] |
| Inactivation of E. coli endotoxin | Isolation of phosphatases from rat small intestines after Sb administration | Dephosphorylation and inhibition of E. coli O55:B5 LPS toxicity by 63 kDa protein | [26] |
| Protection against cholera pathogenesis | In vitro (rat small intestine epithelial and human colon cells), Sb or Sb product treatment | Modulation of cAMP levels by 120 kDa protein in Sb-conditioned medium | [27] |
| Recovery from proximal enterectomy | In vivo (60% proximal enterectomy rats), Sb administration | Improvement of functional adaptation of remnant ileum via polyamine metabolites | [28] |
| Activation of host immune system | In vivo (rats), Sb administration | Enhanced secretory IgA in the duodenal fluid of rats after Sb administration | [29] |
| In vitro (murine macrophage and fibroblast cells), Sc cell wall fraction treatment | Nonspecific immune stimulation (higher NO secretion and macrophage activity) | [30] | |
| Absorbing enteric pathogens | In vitro, binding assays of Sb and enteric pathogens | Adhesion and sedimentation with S. enterica Typhimurium and enterohemorrhagic E. coli | [31] |
| In vivo (gnotobiotic mice), evaluation of Sb–pathogen adhesion | Adhesion between Sb and S. enterica Typhimurium on intestinal epithelium | [32] | |
| Absorbing mycotoxins | In vivo (broiler chicks), Sc administration after aflatoxicosis | Positive protection effect of Sc administration on liver weight, histopathology, and growth | [33] |
| In vivo (rats), MOS, thermolyzed Sc, and dehydrated Sc treatment after aflatoxicosis | Attenuation of the toxicity and liver damage only by dehydrated Sc administration | [34] | |
| Obesity and type 2 diabetes | In vivo (obese and type 2 diabetic mice), Sb administration | Reduction of fat mass, hepatic steatosis, and inflammation with shift in host gut microbiome | [35] |
| Strategy | Purpose | Strain | Ref. |
|---|---|---|---|
| In situ delivery of therapeutic proteins | |||
| Secretion of human lysozyme | Reshaping the taxonomic architecture of the host gut microbiome | Sb | [68] |
| Secretion of the antibody fragment-neutralizing TcdA and TcdB | Performing yeast-based immunotherapy for C. difficile infection | Sb | [66] |
| Multi-copy genomic integration of atrial natriuretic peptide secretion cassettes | Alleviating colitis in the mammalian host gut | Sb | [69] |
| Secretion of apyrase degrading extracellular ATP | Controlling the inflammatory mechanism induced by extracellular ATP | Sc | [63] |
| In situ delivery of small molecules | |||
| Optimization and assembly of genetic elements for multiple gene expressions | In situ biomanufacturing and delivery of β-carotene and violacein | Sb | [64] |
| Biosensing and expression systems | |||
| Engineering human P2Y2 receptor | Achieving extracellular ATP-specific apyrase secretion system | Sc | [63] |
| dCas9-scRNA-based synthetic transactivation | Achieving nutrient-dependent synthetic signaling mechanisms | Sb | [8] |
| Control of the viability and activity | |||
| Introduction of heterogenous L-fucose assimilation pathway | Improving competence in the mammalian host gut | Sb | [70] |
| Whey protein–agavin–alginate encapsulation | Enhancing Sb viability after the gastrointestinal digestion | Sb | [71] |
| Knock-out of THI6 and BTS1 | Building multi-layered biocontainment via cold-sensitive thiamine auxotroph | Sb | [72] |
| Cell wall oligosaccharide engineering | |||
| Modulation of glycolysis and sugar nucleotide synthetic pathways | Enhancing cell wall oligosaccharide contents and related prebiotic and parabiotic effects | Sb, Sc | [9] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwak, S. Therapeutic Applications of Native and Engineered Saccharomyces Yeasts. Fermentation 2024, 10, 51. https://doi.org/10.3390/fermentation10010051
Kwak S. Therapeutic Applications of Native and Engineered Saccharomyces Yeasts. Fermentation. 2024; 10(1):51. https://doi.org/10.3390/fermentation10010051
Chicago/Turabian StyleKwak, Suryang. 2024. "Therapeutic Applications of Native and Engineered Saccharomyces Yeasts" Fermentation 10, no. 1: 51. https://doi.org/10.3390/fermentation10010051
APA StyleKwak, S. (2024). Therapeutic Applications of Native and Engineered Saccharomyces Yeasts. Fermentation, 10(1), 51. https://doi.org/10.3390/fermentation10010051





