Cloning Systems in Bacillus: Bioengineering of Metabolic Pathways for Valuable Recombinant Products
Abstract
1. Introduction
2. Cloning Systems in Bacillus spp.
2.1. Host Strains
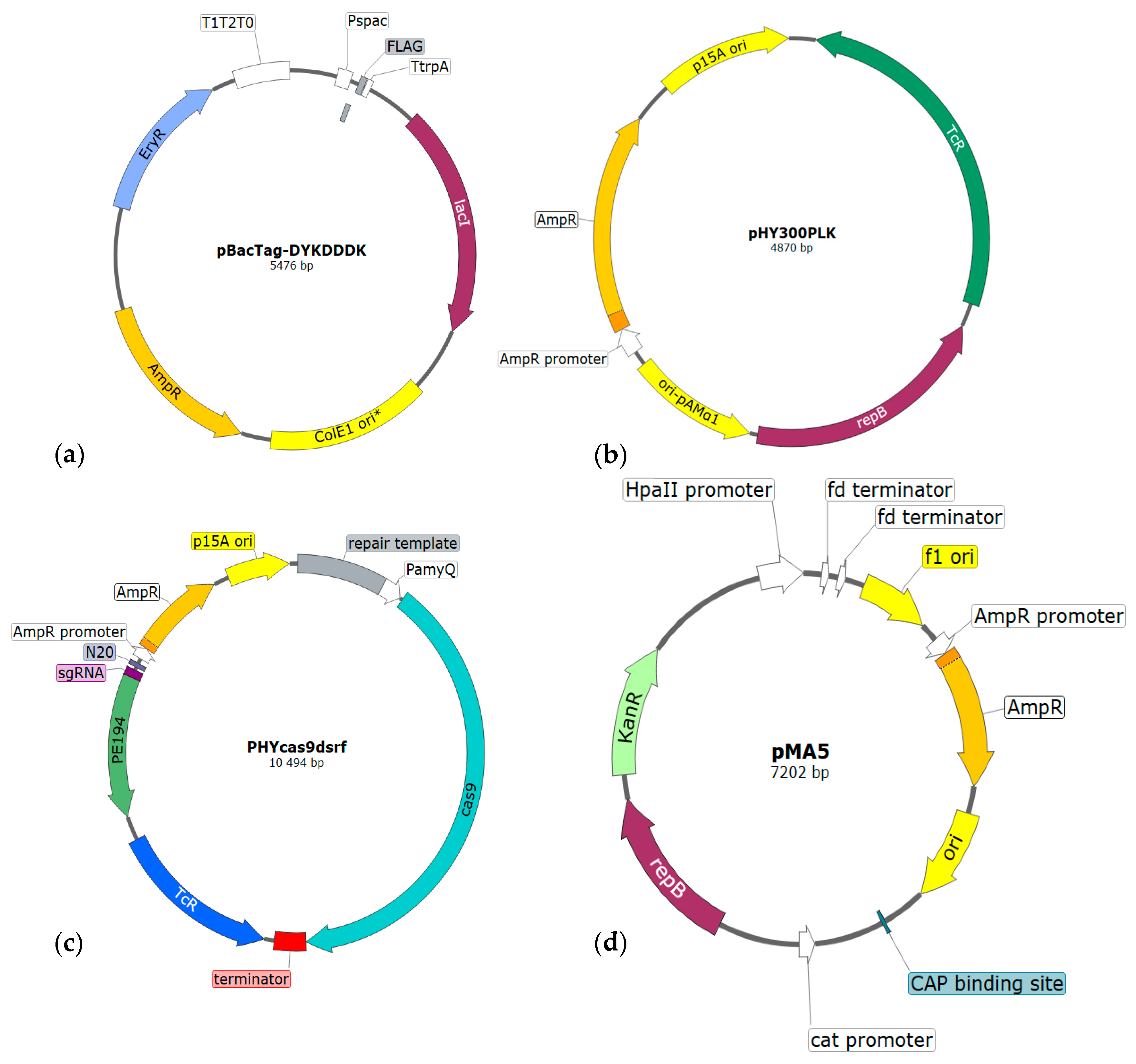
2.2. Vectors
2.2.1. Autonomously Replicating Vectors
2.2.2. Integrative Vectors
2.2.3. CRISPR/Cas9
| Vector | Function | Size, bp | Selection 1 | Features | Reference |
|---|---|---|---|---|---|
| pMA5 | Expression | 7202 | Kan, Amp | PhpaII, PAmpR, f1 ori, repB | [21] |
| pBE-S | Expression | 5938 | Kan, Amp | PaprE, SPaprE, colE1 ori, pUB ori, His tag | [23] |
| pHT43 | Expression | 8057 | Amp, Cm | Pgrac, SPamyQ, LacI, ColE1 | [44] |
| pHY300PLK | Expression | 4870 | Amp, Tet | ori-pAMα1, ori-177, repB | [31] |
| pHYAMC | Integration | 7513 | Amp, Tet | PApR, ori-pAMα1, ori-177, amyE’ | [32] |
| pBacTag | Integration | 5476 | Amp, Ery | Pspac, lacI, ColE1 ori, tag 2 | [45] |
| pHBintE | Integration | 5683 | Amp, Ery | PxylA, repF, E. coli ori, Bacillus ori | [46] |
| pAX01 | Integration | 7781 | Ery | PxylA, xylR | [29] |
| pJOE8999 | Editing | 7794 | Kan | Cas9, pUC ori, rep pE19ts, PmanP, PvanP | [38] |
| PHYcas9dsrf | Editing | 10,494 | Amp, Tet | Cas9, Pgrac, p15A ori, PamyQ | [41] |
2.3. Methods for Vector Delivery
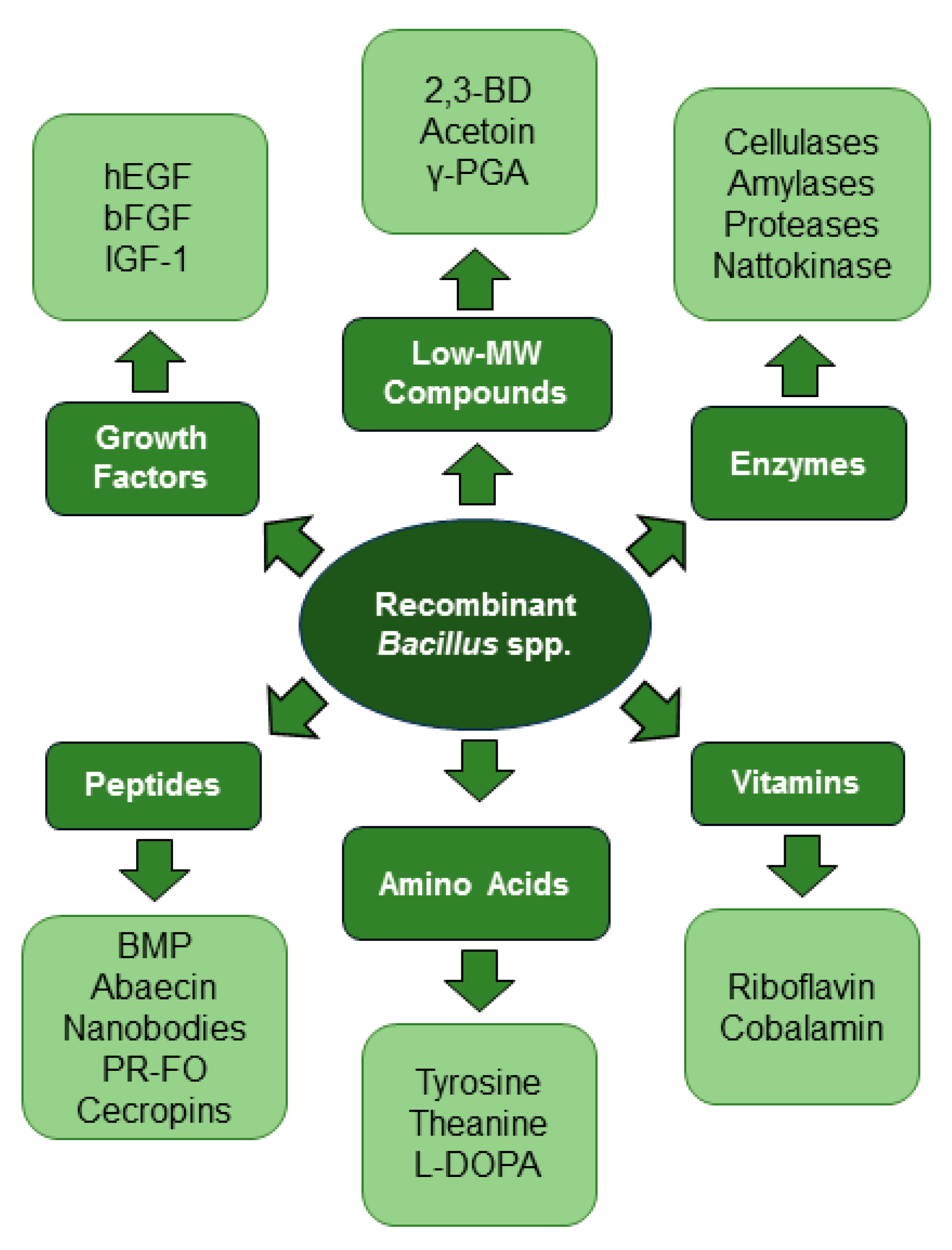
3. Biotechnological Versatility of Bacillus spp.
3.1. Enzymes
| Strain | Vector | Compound | Genetic Source | Yield | Reference |
|---|---|---|---|---|---|
| B. subtilis WHS11YSA | pHYYamySA | α-amylase | B. stearothermophilus | 9201.1 U/mL | [55] |
| Brevibacillus choshinensis (B. brevis) BCPPSQ | pNCamyS-prsQ | α-amylase | B. stearothermophilus | 17,925.6 U/mL | [56] |
| B. subtilis WHS9GSAB | pHYGamySAsecYEG | α-amylase | B. stearothermophilus | 35,779.5 U/mL | [57] |
| Br. choshinensis (B. brevis) | pNCMO2 | β-amylase | B. aryabhattai CCTCC M2017320 | 5371.8 U/mL | [58] |
| B. subtilis WS9PUL | pHYcas9 | pullulanase | B. deramificans | 5951.8 U/mL | [59] |
| B. subtilis WB600 | pMA5 | lipase A | B. subtilis | 1164.9 U/mL | [60] |
| B. subtilis DB10 | pSKE194 | xylanase | B. subtilis | 1296 U/mg | [61] |
| B. licheniformis MW3 | pKVM1 | 2,3-butanediol | B. licheniformis | 123.7 g/L | [62] |
| B. amyloliquefaciens B 10-127 | pMA5 | 2,3-butanediol | B. amyloliquefaciens | 132.9 g/L | [63] |
| B. subtilis 168 | pMA5 | acetoin | B. subtilis | 91.8 g/L | [64] |
| B. subtilis KH2 | pKVM1 pMA5 | poly-γ-glutamic acid | B. subtilis, B. licheniformis | 23.28 g/L | [65] |
| B. subtilis G600 | T7-BOOST * | GABA † | B. subtilis | 109.8 g/L | [66] |
3.2. Growth Factors, Vitamins, and Amino Acids
3.3. Antimicrobial and Immunization Peptides
3.4. Low-MW Compounds
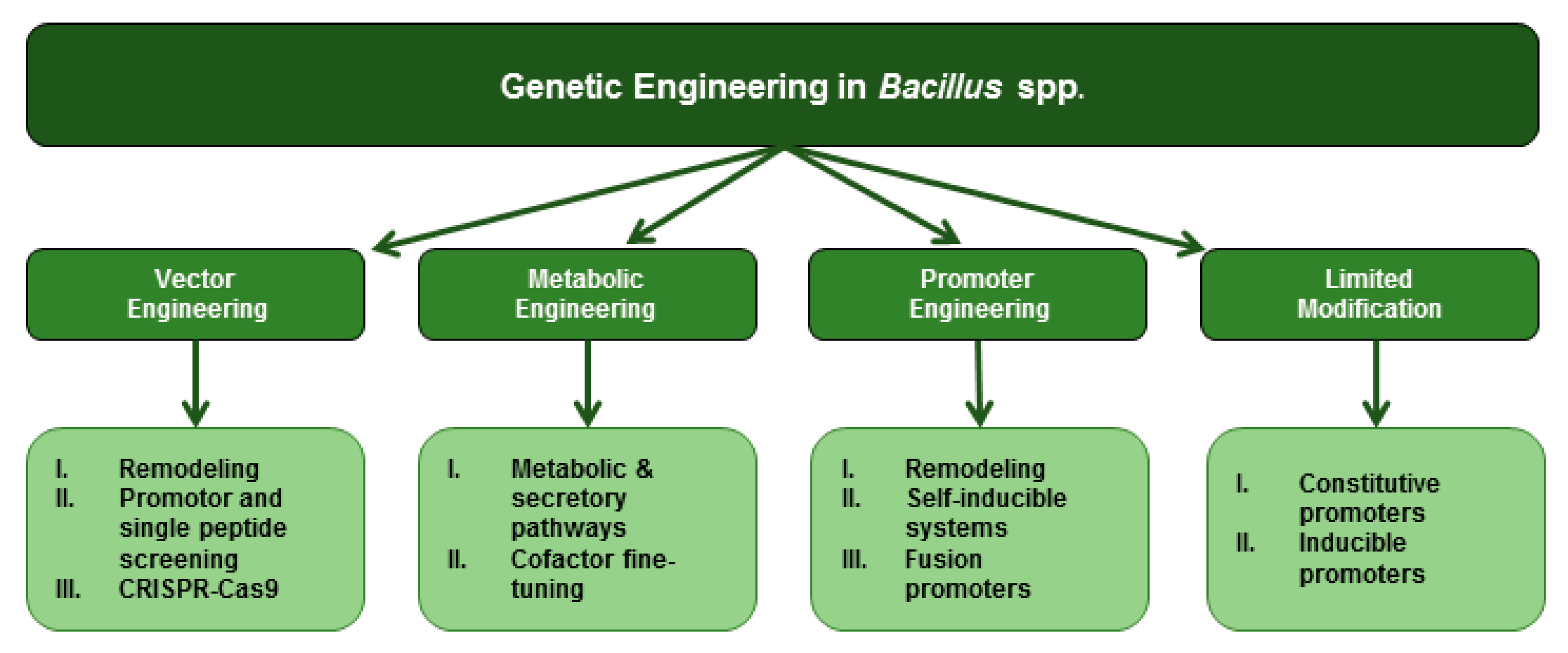
4. Genetic Engineering in Bacillus spp.
4.1. Heterologous Expression with Limited Modification
4.1.1. Constitutive Promoters
4.1.2. Inducible Promoters
| Promoter | Signal Peptide | Vector | Genetic Modifications 1 | Target Compound 2 | Source 3 | Host 3 | Effect 4 | Reference |
|---|---|---|---|---|---|---|---|---|
| P43 | SPamyE | pP43NMK | cloning of ASN (BcA) | L-asparaginase | B. cereus BDRD-ST26 | Bs WB600 | 20-fold higher BcA activity; 72% decrease of acrylamide in pretreated potato strips | [99] |
| P43 | SPsacB | pWB980 | cloning of GM2938 | trypsin | Streptomyces populi A249 | Bs SCK6 | 1622 U/mL esterase activity and 34 U/mL amidase activity for purified GM2938 | [98] |
| PaprE | - | pBE-S | cloning of cel8A and cel48S | 2 cellulases | Acetivibrio thermocellus | Bl 24 Bv 5RB | 7-fold higher EA for Cel8a in Bl 24 and Cel48S in Bv 5RB | [49] |
| PaprE | SPlipA | pBE-S | SP exchange | β-agarase | Ps. hodoensis | Bs RIK1285 | 44% higher secretion than SPaprE | [100] |
| PhpaII | - | pBSMuL3 | host exchange | sucrose phosphorylase | Bifidobacterium adolescentis | Bs CCTCC M 2016536 | 3.5-fold higher extracellular EA than cloning in E. coli | [103] |
| P43 | - | pMA0911 | PhpalI exchanged for P43 | pullulanase | B. naganoensis JNB-1 | Bs WB600 | 6-fold higher EA than the same vector with PhpalI in Bs WB800 | [104] |
| PhpaII | - | pMA5 | poly(A/T) tail added to 3′-end of ggt | L-theanine | B. pumilus ML413 | Bs 168 | Poly(A/T) increased mRNA stability by 58% and GGT activity by 60%; 53 g/L after 16 h | [105] |
| Pveg | - | pJOE-8739 | deletion of sporulation genes; promoter change | γ-PGA | Bs 168 | Bs IIG-Bs2 | 129% higher carbon yield with glucose as a source | [115] |
| T7 | SPxynD (lypo type) | pDMT pDBT | 2 copies of hEGF cassette; ΔnprB; Δmpr | human epidermal growth factor (hEGF) | Homo sapiens | Bs PT5, PT6, PT7 | Almost a 2-fold increase due to SP; 12% more with 2 copies of hEGF | [102] |
| PsacB | SPlipA | pMA0911 | enhancers DegQ, DegS, DegU | pullulanase | B. naganoensis | Bs WB800 | 5.9-fold higher activity with DegQ | [114] |
| Pglv | SPlipA | pMA5 | PhpaII discarded | creatinase | - | Bs 1A751 | 5-fold higher EA than PhpaII | [105] |
| Pglv | - | pGJ148 | 6xHis-SUMO tag | T9W | synthetic | Bs WB800N | 2.3 mg/L purified T9W | [106] |
| Pglv | SPsacB | pGJ148 | - | cecropin AD (CAD) | synthetic | Bs WB800N | 24.6 mg/L CAD, 93% purity, similar antimicrobial activity to synthetic CAD | [107] |
| Pglv | SPsacB SPamyQ | pGJ148 | - | PR-FO | synthetic | Bs WB800N | 3–4 mg/L purified PR-FO | [99] |
| Pgrac | SPyoaW | pJHS | SPyoaW fused with StrepII-SUMO | alkaline phosphatase (r) | - | Bs WB800N Bs KO7A | 5–6 times higher activity than with SPamyQ | [107] |
| Pgrac01 | - | pHT43 pTz57R/BMP2 | - | human bone morphogenetic protein-2 (rhBMP2) | Homo sapiens | Bs SCK6 Bs WB600 | 5–9 mg/L | [111] |
| Pgrac212 | - | pHT212 | solubility tag at the N-terminus | HRV3C (r) | Homo sapiens | Bs 1012 | 8065 U/mg for purified protease | [112] |
4.2. Promoter Engineering in Bacillus spp.
4.2.1. Self-Inducible Systems
4.2.2. Promoter Remodeling
| Promoter | Signal Peptide | Vector | Genetic Modifications 1 | Target 2 | Source 3 | Host | Effect 4 | Reference |
|---|---|---|---|---|---|---|---|---|
| PsrfA | - | pMA09 | 8BMP (multi-copy BMP) autoinduced | BMP | - | Bs 168 | successful expression and purification with industrial promise | [118] |
| mutPsrfA | SPAP | pBSG01 pMA05 | (−10) and (−35) core sequences substituted with consensus sequences | aminopeptidase (AP) | Bs Zj016 | Bs 168 | 1.7-fold AP overexpression compared to the PhpaII promoter; confirmed on protein level | [116] |
|
P23 (PsrfA–PhpaII) | - | pAX-01 pBSG03 | library of PsrfA derivatives; chromosome integration; 12 dual promoters tested | GFP | Bs Zj016 Bs natto | Bs BSG1682 | 2.5-fold stronger promoter activity than PsrfA | [117] |
|
Pgrac01 Pgrac100 | - | pHT1655 | lacI removal | β-galactosidase (r) inducer-free | - | Bs 1012 | Expression levels are similar to those with induction | [119] |
| Pgrac212 | - | pHT2080 | genome integration at lacA or amyE locus | β-galactosidase (r) inducer-free | - | Bs 1012 | 53.4% higher expression after integration into the chromosome | [120] |
|
PluxR PluxI | - | pBS3Clux | expression system based on luxR and luxI; (−40) and (−10) regions optimized | riboflavin | Aliivibrio fischeri Bs 168 | Bs K07 | 2.5 to 3.2 times stronger promoter responses than PsrfA and Pveg | [122] |
| Pylb | - SPamy | pUBC19 | 11 promoters tested: α-amylase SP from B. amyloliquefaciens | pullulanase organophosphorus hydrolase | B. naganoensis Ps. pseudoalcaligenes | Bs WB600 | 7.4 times higher activity than P43 2.3 times higher activity than P43 no inducer in both cases | [121] |
| Pv1 | - | pBSG03 | randomized mutations adjacent to the (−10) region | aspartase (r) | - | Bs 168 | 1.6-fold higher transcriptional activity than PsrfA after 12 h | [123] |
| P04 | SPwapA | pMA0911 | mutations in −35 and −10 regions of PsrfA; Cis-acting CodY at 5′-UTR | nattokinase | - | Bs WB600 Bs WB800 | ~30% higher EA with SPwapA than SPepr; further ~30% increase with best of 5 synthetic promoters | [124] |
| PBH4 | - | pAX01 pBSG03 | synthetic promoter library | β-glucuronidase nattokinase | - | Bs WB600 | 3 times greater promoter strength than PsrfA | [125] |
| Pgrac100 | - | pHT100 | UP of Pgrac01 element optimized | β-galactosidase (r) | - | Bs 1012 | 9.2 times higher expression compared to Pgrac01 | [126] |
| P43′–riboE1 | - | pBSG03 | P43 combined with theophylline riboswitch; 9-bp spacer SD; and start codon | β-glucuronidase (r) | - | Bs 168 | switch from constitutive to inducible expression | [127] |
| PgroE | SPamyQ | pHT43 | lac operator from E. coli added | nanobodies | Camelidae | Bs WB800N | successful IPTG-induced production | [128] |
| PhpalI–Pylb | - | pP43NMK | RBS site modification | pullulanase | Bs 168 | Bs WB800 Bs RBS7 | 136.8 times higher activity than the wild type | [129] |
| PamyE-cdd | SPpac | pP43NMK | 33 promoters screened | amidase | B. megaterium | Bs WB800 | 3.58-fold greater activity than control (pBSH1) | [130] |
| P43–Plaps | - | pBE980a | OE due to dual promoter | 2,3-BD, TTMP, acetoin | Bs BS2 | Bs BS2 | 36.4% more BD, 36.7% more acetoin, and 95.5% more TTMP vs. single Plaps/P43 | [85] |
| PhpaII–PamyQ | SPamyQ | pHYCGT1 | multiple deletions (srfC, spoIIAC, nprE, aprE, amyE) | β-CGTase (r) | - | Bs CCTCC M 2016536 | 20% higher expression than PamyQ′(>2.4-fold increase compared to 7 other promoters) | [131] |
| PgsiB–PhpaII | SPYncM | pBSG11 (pMA5-BSAP) | 6 fusion promoters compared SP library screening | aminopeptidase (r) | - | Bs WB600 | >2-fold higher EA than the single promoters; <20% increase with SPYncM | [132] |
| P43–PhpaII | - | pUB110 | dal KO in Bs chromosome via cre/Lox recombination | D-psicose 3-epimerase | Clostridium scindens 35704 | Bs 1A751 | 20–30% higher EA than the PhpaII | [133] |
4.2.3. Fusion Promoters
4.3. Vector Engineering in Bacillus spp.
4.3.1. Vector Remodeling
4.3.2. Promoter and Signal Peptide Screening
| Promoter | Signal Peptide | Vector | Genetic Modifications 1 | Target 2 | Source 3 | Host 3 | Effect 4 | Reference |
|---|---|---|---|---|---|---|---|---|
| PopuAA | SPsubE | pSaltExSePR5 | new vector with a salt-inducible promoter | protease | Hallobacillus sp. SR5-3 | Bs WB800 | 70-fold higher protease activity with 4 M NaCl than the non-induced culture | [135] |
| P43 | - | pUC980 | pUC19 ori inserted into pWB980, bleoR deletion | alkaline protease; pectate lyase | Bacillus sp. 221, Paenibacillus sp. 0602, Anoxybacillus sp. LM18-11 | Bs WB600 | 2.5–3 times higher activity than pWB980 constructs for pelN1 and spro1 | [137] |
| P43 | SPYwbN | pHT01 pIEFBPR | Pgrac discarded; 6 genes KO (xpF, skfA, lytC, sdpC, malP, amyE); SPPhoD exchanged for SPYwbN | trehalose synthase | - | Bs WB800N | about 10-fold increased activity overall | [136] |
| Pmglv | SPlipA | pMA5 | 6 SP and 4 promoters were cloned and tested | β-mannanase | B. licheniformis DSM13 | Bs 1A751 | 2-fold higher EA than least efficient (SPnprB); 3-fold higher EA than PhpaII | [140] |
| PhpaII | SPwapA SPamyQ | pHT43 pMA5 | Inducible Plac used for SPamyQ | MTG | Str. mobaraensis CGMCC 4.5591 | Bs 168 Bs WB600 | 63 mg/L MTG with SPwapA; 10–15% less with SPamyQ; almost no difference in enzymatic activity | [138] |
| PaprE | SPnprE | pMA5 pDL | PrsA lipoprotein OE; 6 SP tested | amylase | B. licheniformis CICC 10181 | Bs 1A751 | 2.5-fold overall increase | [141] |
| PyvyD | SPsacB | pWB980 | pro-peptide from S. hygroscopicus | MTG | Str. mobaraensis | Bs WB600 | >20% higher EA compared to P43 | [142] |
| T7 | SPxynD (lypo type) | pDMT pDBT | 24 SP tested; nprB and mpr KO; hEGF cassette integrated into nprB | hEGF | Homo sapiens | Bs PT5 Bs PT6 Bs PT7 | almost 2-fold increase SPxynD; only 6 of 24 SP guided hEGF into extracellular space | [93] |
| PBsamy–PBaamy | SPDacB | pWBPRO1 | 72 SP, 9 dual, and 5 triple promoters were screened | alkaline serine protease (r) | B. clausii | Bs WB600 | 3.7-fold increase with SPDacBand PBsamy-PBaamy | [134] |
| PgroES | SPamyE | pLIKE | trpA-terminator to the 3′ end and lacO-stem-loop to the 5′ end of the reporter gene | MAK33-VL | - | Bs K7 Bs PG10 | 10-fold increased expression with GFP; verified with MAK33-L | [139] |
| P43 | - | pHY300 T2(2)-ori | Δepr, ΔwprA, Δmpr, ΔaprE, Δvpr, ΔbprA, ΔbacABC; aprN inserted | nattokinase | Bs 168 | Bl DW2 | 25.7% higher EA in the strain with 7 deletions | [143] |
| P43 | - | pBSCas9 pHP13 | multiplex genome editing; ribA, ribB, and ribH engineered for improved riboflavin production | riboflavin | - | Bs BS89 | 80% success in 1–8 kb deletions >90% success in 1–2 kb insertions 100% site-directed mutagenesis | [144] |
| Pgrac PhpaII | SPnpr | pHT01 pDR-sgRNA | KO epsA-O, cwlO, sacB; OE CscA; SacC, OsC introduced | γ-PGA | Ps. mucidolens Bl 14580 | Ba NB | 32% more γ-PGA | [145] |
4.3.3. CRISPR/Cas9 Genome Editing
5. Metabolic Engineering in Bacillus spp.
5.1. Manipulation of Metabolic and Secretory Pathways
5.2. Cofactors Fine-Tuning
| Promoter | Vector | Genetic Modifications 1 | Target 2 | Source 3 | Host 3 | Effect 4 | Reference |
|---|---|---|---|---|---|---|---|
| P43 | T2(2)-Ori | OE 1 pdhABCD and citA; ΔpflB; repression of aceA | γ-PGA | Bs 168 Bl WX-02 | Bl WX-02 | 69% higher yield | [147] |
| PbdhA | pMA5-PA | ΔbdhA; moderate expression of yodC; PHpaII exchanged for PbdhA | acetoin | Bs 168 | Bs JNA 3-10 | 35.3% more acetoin; 92.3%, 70.1%, and 75.0% less BD, LA, and EtOH, respectively | [146] |
| P43 | T2(2)-ori pHY300PLK | OE glcP; gabT1 and gutB1 integrated; amyL terminator from Bl DW2 | 1-DNJ | - | Ba HZ-12 | 33% increased production 36.7% less acetoin by-product | [148] |
| P43 | T2(2)-ori pHY300PLK | ptsG weakened; ΔiolR; promoter change; and 5′-UTR optimizations | 1-DNJ | - | Ba HZ-12 | 10.2-fold higher amount overall | [149] |
| P43 Pspac | pP43NMK PDG148 | ΔyyzE, ΔypqE, ΔptsG; glcP and glcK OE; pathway repression with codon-optimizing strategies | GlcNAc | S. cerevisiae B. cereus Bs 168 | Bs BN0-GNA1 | 2-fold higher titer than the original strain in flasks; 1.72-fold more in a 3 L fed-batch bioreactor | [150] |
| P43 | pHY300PLK | TamyL terminator Bl WX-02; synthetic 5′-UTR; 15 genes for prephenate dehydrogenase screened | L-tyrosine | Ba HZ-12 | Ba HZ-12 | 42% higher yield than the control strain | [151] |
| PhpaII | pMA5 | OE of 23 genes involved in the Sec pathway, PrsA lipoprotein, partial dnaK operon; SPamyL, SPamyS; | 2 amylases AmyL AmyS | Bl CICC 10181 Gs ATCC 31195 | Bs 1A751 | 3.2-fold higher expression for AmyL; 5.5-fold for AmyS; 60 and 73% higher EA | [152] |
| PAE | pHP13 | OE of 4 Sec pathway components (secA-prfB, secDF, secYEG, prsA); promoter change | lipase | Bs 168 | Bs BNA | 14-fold increase in EA compared to P43; further 59% higher with secDF and prsA OE | [153] |
| P43 | pUCL92 | OE purF, purM, purN, purH, purD; promoter exchange | riboflavin | - | Bs PK | 31% higher titer, 25% higher yield | [154] |
| - Pspac P43 | pSS pMUNTIN4 pMX45 | mutations RibC (G199D), ribD+ (G+39A) and YvrH (R222Q) | riboflavin | - | Bs 24/pMX45 | 3.4-fold higher titer than the initial strain; 23.4% increase due to the YvrH mutation | [155] |
| PvegI | pHP13 | KO apt, xpt, adeC, nrdE, nrdF | riboflavin | - | Bs 168 | 41.50% higher production in ΔadeC mutants; 13.12% increase with RNR repressed | [156] |
| PbdhA | pMA5 | OE dhaD, gldA, acr introduction of ALsR | 2,3-BD | K. pneumoniae ATCC 25955 | Ba B10-127 | 102.3 g/L; 1.16 g/L/h | [92] |
| P43 | T2(2)-Ori | OE zwf, pyk, argA; ΔargF, ΔahrC; TamyL terminator Bl WX-02 | putrescine | E. coli | Ba HZ-12 | 5.51 g/L, 0.11 g/L/h, and 0.14 g/g, with xylose as substrate | [157] |
| P43 | pP43NMK PDG148 | KO pyk, kdgA, ywkA, pckA, ytsJ melA, malS; OE pycA, pfkA, fbaA | GlcNAc | S. cerevisiae Bs 168 A. flocculosa | Bs BN[0…6]-GNA1 Bs BP[6…18]-afGNA1 | 3.7-fold higher titer, 4-fold higher yield, and 1.6-fold higher productivity than the initial strain | [158] |
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Qian, J.; Wang, Y.; Hu, Z.; Shi, T.; Wang, Y.; Ye, C.; Huang, H. Bacillus sp. as a microbial cell factory: Advancements and future prospects. Biotechnol. Adv. 2023, 69, 108278. [Google Scholar] [CrossRef] [PubMed]
- Leal, C.; Fontaine, F.; Aziz, A.; Egas, C.; Clément, C.; Trotel-Aziz, P. Genome sequence analysis of the beneficial Bacillus subtilis PTA-271 isolated from a Vitis vinifera (cv. Chardonnay) rhizospheric soil: Assets for sustainable biocontrol. Environ. Microbiome 2021, 16, 3. [Google Scholar] [CrossRef] [PubMed]
- Arsov, A.; Gerginova, M.; Paunova-Krasteva, T.; Petrov, K.; Petrova, P. Multiple cry Genes in Bacillus thuringiensis Strain BTG Suggest a Broad-Spectrum Insecticidal Activity. Int. J. Mol. Sci. 2023, 24, 11137. [Google Scholar] [CrossRef] [PubMed]
- Petrova, P.; Velikova, P.; Petrov, K. Genome Sequence of Bacillus velezensis 5RB, an Overproducer of 2,3-Butanediol. Microbiol. Resour. Announc. 2019, 8, e01475-18. [Google Scholar] [CrossRef] [PubMed]
- Petrova, P.; Gerginova, M.; Arsov, A.; Armenova, N.; Tsigoriyna, L.; Gergov, E.; Petrov, K. Whole-genome sequence of Bacillus velezensis strain R22 isolated from Oryza sativa rhizosphere in Bulgaria. Microbiol. Resour. Announc. 2023, 12, e0069323. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Han, L.; Suo, F.; Liu, Z.; Zhou, Z. Exploitation of Bacillus subtilis as a robust workhorse for production of heterologous proteins and beyond. World J. Microbiol. Biotechnol. 2018, 34, 145. [Google Scholar] [CrossRef]
- Lakowitz, A.; Godard, T.; Biedendieck, R.; Krull, R. Mini review: Recombinant production of tailored bio-pharmaceuticals in different Bacillus strains and future perspectives. Eur. J. Pharm. Biopharm. 2018, 126, 27–39. [Google Scholar] [CrossRef]
- Cai, D.; Rao, Y.; Zhan, Y.; Wang, Q.; Chen, S. Engineering Bacillus for efficient production of heterologous protein: Current progress, challenge and prospect. J. Appl. Microbiol. 2019, 126, 1632–1642. [Google Scholar] [CrossRef]
- Stülke, J.; Grüppen, A.; Bramkamp, M.; Pelzer, S. Bacillus subtilis, a Swiss Army Knife in Science and Biotechnology. J. Bacteriol. 2023, 25, e0010223. [Google Scholar] [CrossRef]
- Su, Y.; Liu, C.; Fang, H.; Zhang, D. Bacillus subtilis: A universal cell factory for industry, agriculture, biomaterials and medicine. Microb. Cell Fact. 2020, 19, 173. [Google Scholar] [CrossRef]
- Zhang, K.; Su, L.; Wu, J. Recent Advances in Recombinant Protein Production by Bacillus subtilis. Annu. Rev. Food Sci. Technol. 2020, 25, 295–318. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Jeong, D.E.; Park, S.H.; Kim, S.J.; Choi, S.K. Complete Genome Sequence of Bacillus subtilis Strain WB800N, an Extracellular Protease-Deficient Derivative of Strain 168. Microbiol. Resour. Announc. 2018, 7, e01380-18. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Yan, Y.; Du, S.; Zhu, Y.; Pan, F.; Wang, R.; Xu, Z.; Xu, Z.; Li, S.; Xu, H. Recent advances and prospects of Bacillus amyloliquefaciens as microbial cell factories: From rational design to industrial applications. Crit. Rev. Biotechnol. 2023, 43, 1073–1091. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Hu, Y.; Yan, Y.; Du, S.; Pan, F.; Li, S.; Xu, H.; Luo, Z. Metabolic Engineering of Bacillus amyloliquefaciens to Efficiently Synthesize L-Ornithine From Inulin. Front. Bioeng. Biotechnol. 2022, 10, 905110. [Google Scholar] [CrossRef] [PubMed]
- Petrov, K.; Petrova, P. Current Advances in Microbial Production of Acetoin and 2,3-Butanediol by Bacillus spp. Fermentation 2021, 7, 307. [Google Scholar] [CrossRef]
- Song, C.W.; Chelladurai, R.; Park, J.M.; Song, H. Engineering a newly isolated Bacillus licheniformis strain for the production of (2R,3R)-butanediol. J. Ind. Microbiol. 2020, 47, 97–108. [Google Scholar] [CrossRef]
- Bunk, B.; Schulz, A.; Stammen, S.; Münch, R.; Warren, M.J.; Rohde, M.; Jahn, D.; Biedendieck, R. A short story about a big magic bug. Bioeng. Bugs. 2010, 1, 85–91. [Google Scholar] [CrossRef]
- Degering, C.; Eggert, T.; Puls, M.; Bongaerts, J.; Evers, S.; Maurer, K.H.; Jaeger, K.E. Optimization of protease secretion in Bacillus subtilis and Bacillus licheniformis by screening of homologous and heterologous signal peptides. Appl. Environ. Microbiol. 2010, 76, 6370–6376. [Google Scholar] [CrossRef]
- Adimpong, D.B.; Sørensen, K.I.; Thorsen, L.; Stuer-Lauridsen, B.; Abdelgadir, W.S.; Nielsen, D.S.; Derkx, P.M.; Jespersen, L. Antimicrobial susceptibility of Bacillus strains isolated from primary starters for African traditional bread production and characterization of the bacitracin operon and bacitracin biosynthesis. Appl. Environ. Microbiol. 2012, 78, 7903–7914. [Google Scholar] [CrossRef]
- Yenikeyev, R.R.; Tatarinova, N.Y.; Zakharchuk, L.M.; Vinogradova, E.N. Mechanisms of Resistance to Clinically Significant Antibiotics in Bacillus Strains Isolated from Samples Obtained from a Medical Institution. Mosc. Univ. Biol.Sci. Bull. 2022, 77, 84–91. [Google Scholar] [CrossRef]
- Westers, L.; Dijkstra, D.S.; Westers, H.; van Dijl, J.M.; Quax, W.J. Secretion of functional human interleukin-3 from Bacillus subtilis. J. Biotechnol. 2006, 17, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Fu, G.; Tu, R.; Jin, Z.; Zhang, D. High-efficiency expression and secretion of human FGF21 in Bacillus subtilis by intercalation of a mini-cistron cassette and combinatorial optimization of cell regulatory components. Microb. Cell. Fact. 2019, 28, 17. [Google Scholar] [CrossRef] [PubMed]
- Khadye, V.S.; Sawant, S.; Shaikh, K.; Srivastava, R.; Chandrayan, S.; Odaneth, A.A. Optimal secretion of thermostable Beta-glucosidase in Bacillus subtilis by signal peptide optimization. Protein. Expr. Purif. 2021, 182, 105843. [Google Scholar] [CrossRef] [PubMed]
- Titok, M.A.; Chapuis, J.; Selezneva, Y.V.; Lagodich, A.V.; Prokulevich, V.A.; Ehrlich, S.D.; Jannière, L. Bacillus subtilis soil isolates: Plasmid replicon analysis and construction of a new theta-replicating vector. Plasmid 2003, 49, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.D.; Nguyen, Q.A.; Ferreira, R.C.; Ferreira, L.C.; Tran, L.T.; Schumann, W. Construction of plasmid-based expression vectors for Bacillus subtilis exhibiting full structural stability. Plasmid 2005, 54, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Zarzosa, A.; López-Meza, J.E. Shuttle Vectors of Bacillus thuringiensis. In Bacillus thuringiensis Biotechnology; Sansinenea, E., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 175–184. [Google Scholar] [CrossRef]
- Phan, T.T.; Nguyen, H.D.; Schumann, W. Novel plasmid-based expression vectors for intra- and extracellular production of recombinant proteins in Bacillus subtilis. Protein Expr. Purif. 2006, 46, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, K.J.; Simmons, L.A. Genome Editing Methods for Bacillus subtilis. In Recombineering Methods in Molecular Biology; Reisch, C.R., Ed.; Humana Press: New York, NY, USA, 2022; Volume 2479. [Google Scholar] [CrossRef]
- Härtl, B.; Wehrl, W.; Wiegert, T.; Homuth, G.; Schumann, W. Development of a new integration site within the Bacillus subtilis chromosome and construction of compatible expression cassettes. J. Bacteriol. 2001, 183, 2696–2699, Erratum in: J. Bacteriol. 2001, 183, 4393. [Google Scholar] [CrossRef]
- Zeigler, D. Bacillus Genetic Stock Center Catalog of Strains, Seventh Edition, Volume 4, Integration Vectors for Gram-Positive Organisms. 2002, pp. 5–10. Available online: https://bgsc.org/_catalogs/Catpart4.pdf (accessed on 12 December 2023).
- Ishiwa, H.; Shibahara, H. New shuttle vectors for Escherichia coli and Bacillus subtilis. Jpn. J. Genet. 1985, 60, 235–243. [Google Scholar] [CrossRef]
- Mahipant, G.; Kato, J.; Kataoka, N.; Vangnai, A.S. An alternative genome-integrated method for undomesticated Bacillus subtilis and related species. J. Gen. Appl. Microbiol. 2019, 21, 96–105. [Google Scholar] [CrossRef]
- Radeck, J.; Kraft, K.; Bartels, J.; Cikovic, T.; Dürr, F.; Emenegger, J.; Kelterborn, S.; Sauer, C.; Fritz, G.; Gebhard, S.; et al. The Bacillus BioBrick Box: Generation and evaluation of essential genetic building blocks for standardized work with Bacillus subtilis. J. Biol. Eng. 2013, 7, 29. [Google Scholar] [CrossRef]
- Krüger, A.; Welsch, N.; Dürwald, A.; Brundiek, H.; Wardenga, R.; Piascheck, H.; Mengers, H.G.; Krabbe, J.; Beyer, S.; Kabisch, J.F.; et al. A host-vector toolbox for improved secretory protein overproduction in Bacillus subtilis. Appl. Microbiol. Biotechnol. 2022, 106, 5137–5151. [Google Scholar] [CrossRef] [PubMed]
- Koo, B.M.; Kritikos, G.; Farelli, J.D.; Todor, H.; Tong, K.; Kimsey, H.; Wapinski, I.; Galardini, M.; Cabal, A.; Peters, J.M.; et al. Construction and Analysis of Two Genome-Scale Deletion Libraries for Bacillus subtilis. Cell Syst. 2017, 22, 291–305.e7. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; He, S.; Jopkiewicz, A.; Setroikromo, R.; van Merkerk, R.; Quax, W.J. Development and application of CRISPR-based genetic tools in Bacillus species and Bacillus phages. J. Appl. Microbiol. 2022, 133, 2280–2298. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.-Q.; Liu, D.-Y.; Chen, T.; Wang, Z.-W. Recent advances in CRISPR/Cas9 mediated genome editing in Bacillus subtilis. World. J. Microbiol. Biotechnol. 2018, 34, 153. [Google Scholar] [CrossRef]
- Altenbuchner, J. Editing of the Bacillus subtilis Genome by the CRISPR-Cas9 System. Appl. Environ. Microbiol. 2016, 82, 5421–5427. [Google Scholar] [CrossRef]
- Wang, X.; Lyu, Y.; Wang, S.; Zheng, Q.; Feng, E.; Zhu, L.; Pan, C.; Wang, S.; Wang, D.; Liu, X.; et al. Application of CRISPR/Cas9 System for Plasmid Elimination and Bacterial Killing of Bacillus cereus Group Strains. Front. Microbiol. 2021, 12, 536357. [Google Scholar] [CrossRef]
- Hartz, P.; Gehl, M.; König, L.; Bernhardt, R.; Hannemann, F. Development and application of a highly efficient CRISPR-Cas9 system for genome engineering in Bacillus megaterium. J. Biotechnol. 2021, 10, 170–179. [Google Scholar] [CrossRef]
- Zhang, K.; Duan, X.; Wu, J. Multigene disruption in undomesticated Bacillus subtilis ATCC 6051a using the CRISPR/Cas9 system. Sci. Rep. 2016, 16, 27943. [Google Scholar] [CrossRef]
- Kim, M.S.; Jeong, D.E.; Choi, S.K. Bacillus integrative plasmid system combining a synthetic gene circuit for efficient genetic modifications of undomesticated Bacillus strains. Microb. Cell Fact. 2022, 21, 259. [Google Scholar] [CrossRef]
- Peters, J.M.; Colavin, A.; Shi, H.; Czarny, T.L.; Larson, M.H.; Wong, S.; Hawkins, J.S.; Lu, C.H.S.; Koo, B.M.; Marta, E.; et al. A Comprehensive, CRISPR-based Functional Analysis of Essential Genes in Bacteria. Cell 2016, 165, 1493–1506. [Google Scholar] [CrossRef]
- Jung, J.; Yu, K.O.; Ramzi, A.B.; Choe, S.H.; Kim, S.W.; Han, S.O. Improvement of surfactin production in Bacillus subtilis using synthetic wastewater by overexpression of specific extracellular signaling peptides, comX and phrC. Biotechnol. Bioeng. 2012, 109, 2349–2356. [Google Scholar] [CrossRef] [PubMed]
- Bacillus subtilis pBacTag Taggin Vectors, GmbH. 2014, pp. 10–15. Available online: https://www.mobitec.com/media/datasheets/mobitecgmbh/pBACTag-Handbook.pdf (accessed on 12 December 2023).
- Barg, H.; Malten, M.; Jahn, M.; Jahn, D. Protein and Vitamin Production in Bacillus megaterium. In Microbial Processes and Products. Methods in Biotechnology; Barredo, J.L., Ed.; Humana Press: New York, NY, USA, 2005; Volume 18, pp. 205–223. [Google Scholar] [CrossRef]
- Deng, L.; Wang, C.; Zhang, X.; Yang, W.; Tang, H.; Chen, X.; Du, S.; Chen, X. Cell-to-cell natural transformation in Bacillus subtilis facilitates large scale of genomic exchanges and the transfer of long continuous DNA regions. Nucleic Acids Res. 2023, 51, 3820–3835. [Google Scholar] [CrossRef] [PubMed]
- Xue, G.P.; Johnson, J.S.; Dalrymple, B.P. High osmolarity improves the electro-transformation efficiency of the gram-positive bacteria Bacillus subtilis and Bacillus licheniformis. J. Microbiol. Methods. 1999, 34, 183–191. [Google Scholar] [CrossRef]
- Arsov, A.; Petrov, K.; Petrova, P. Enhanced Activity by Genetic Complementarity: Heterologous Secretion of Clostridial Cellulases by Bacillus licheniformis and Bacillus velezensis. Molecules 2021, 26, 5625. [Google Scholar] [CrossRef] [PubMed]
- Zegeye, E.D.; Aspholm, M. Efficient electrotransformation of Bacillus thuringiensis for gene manipulation and expression. Curr. Protoc. 2022, 2, e588. [Google Scholar] [CrossRef]
- Yi, Y.; Kuipers, O.P. Development of an efficient electroporation method for rhizobacterial Bacillus mycoides strains. J. Microbiol. Methods. 2017, 133, 82–86. [Google Scholar] [CrossRef]
- Zhang, Z.; Ding, Z.T.; Shu, D.; Luo, D.; Tan, H. Development of an efficient electroporation method for iturin A-producing Bacillus subtilis ZK. Int. J. Mol. Sci. 2015, 16, 7334–7351. [Google Scholar] [CrossRef]
- Zhang, X.Z.; Zhang, Y. Simple, fast and high-efficiency transformation system for directed evolution of cellulase in Bacillus subtilis. Microb. Biotechnol. 2011, 4, 98–105. [Google Scholar] [CrossRef]
- Jeong, D.E.; Kim, M.S.; Kim, H.R.; Choi, S.K. Cell Factory Engineering of Undomesticated Bacillus Strains Using a Modified Integrative and Conjugative Element for Efficient Plasmid Delivery. Front. Microbiol. 2022, 26, 802040. [Google Scholar] [CrossRef]
- Yao, D.; Su, L.; Li, N.; Wu, J. Enhanced extracellular expression of Bacillus stearothermophilus α-amylase in Bacillus subtilis through signal peptide optimization, chaperone overexpression and α-amylase mutant selection. Microb. Cell Fact. 2019, 18, 69. [Google Scholar] [CrossRef]
- Yao, D.; Zhang, K.; Zhu, X.; Su, L.; Wu, J. Enhanced extracellular α-amylase production in Brevibacillus choshinensis by optimizing extracellular degradation and folding environment, J. Ind. Microbiol. Biotechnol. 2022, 49, kuab061. [Google Scholar] [CrossRef]
- Yao, D.; Zhang, K.; Su, L.; Liu, Z.; Wu, J. Enhanced extracellular Bacillus stearothermophilus α-amylase production in Bacillus subtilis by balancing the entire secretion process in an optimal strain. Biochem. Eng. J. 2021, 168, 107948. [Google Scholar] [CrossRef]
- Duan, X.; Shen, Z.; Zhang, X.; Wang, Y.; Huang, Y. Production of recombinant beta-amylase of Bacillus aryabhattai. Prep. Biochem. Biotechnol. 2019, 49, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Su, L.; Wu, J. Enhanced extracellular pullulanase production in Bacillus subtilis using protease-deficient strains and optimal feeding. Appl. Microbiol. Biotechnol. 2018, 102, 5089–5103. [Google Scholar] [CrossRef]
- Wu, F.; Ma, J.; Cha, Y.; Lu, D.; Li, Z.; Zhuo, M.; Luo, X.; Li, S.; Zhu, M. Using inexpensive substrate to achieve high-level lipase A secretion by Bacillus subtilis through signal peptide and promoter screening. Process Biochem. 2020, 99, 202–210. [Google Scholar] [CrossRef]
- Helianti, I.; Ulfah, M.; Nurhayati, N.; Suhendar, D.; Finalissari, A.K.; Wardani, A.K. Production of Xylanase by Recombinant Bacillus subtilis DB104 Cultivated in Agroindustrial Waste Medium. HAYATI J. Biosci. 2017, 23, 125. [Google Scholar] [CrossRef]
- Ge, Y.; Li, K.; Li, L.; Gao, C.; Zhang, L.; Ma, C.; Xu, P. Contracted but effective production of enantiopure 2,3-butanediol by thermophilic and GRAS Bacillus licheniformis. Green Chem. 2016, 18, 4693–4703. [Google Scholar] [CrossRef]
- Yang, T.; Rao, Z.; Zhang, X.; Xu, M.; Xu, Z.; Yang, S.T. Improved production of 2,3-butanediol in Bacillus amyloliquefaciens by over-expression of glyceraldehyde-3-phosphate dehydrogenase and 2,3-butanediol dehydrogenase. PLoS ONE 2013, 8, e76149. [Google Scholar] [CrossRef]
- Bao, T.; Zhang, X.; Rao, Z.; Zhao, X.; Zhang, R.; Yang, T.; Xu, Z.; Yang, S. Efficient Whole-Cell Biocatalyst for Acetoin Production with NAD+ Regeneration System through Homologous Co-Expression of 2,3-Butanediol Dehydrogenase and NADH Oxidase in Engineered Bacillus subtilis. PLoS ONE 2019, 9, e102951. [Google Scholar] [CrossRef]
- Chen, S.; Fu, J.; Yu, B.; Wang, L. Development of a Conjugation-Based Genome Editing System in an Undomesticated Bacillus subtilis Strain for Poly-γ-glutamic Acid Production with Diverse Molecular Masses. J. Agric. Food Chem. 2023, 71, 7734–7743. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Zhang, Y.; Liu, Y.; Li, J.; Du, G.; Lv, X.; Liu, L. Efficient Protein Expression and Biosynthetic Gene Cluster Regulation in Bacillus subtilis Driven by a T7-BOOST System. ACS Synth. Biol. 2023, 12, 3328–3339. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; He, W.; Yan, X.; Zhang, T.; Jiang, B.; Stressler, T.; Fischer, L.; Mu, W. Construction of an enzymatic route using a food-grade recombinant Bacillus subtilis for the production and purification of epilactose from lactose. J. Dairy Sci. 2018, 101, 1872–1882. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, K.; Zhu, X.; Lv, H.; Wu, J. High level food-grade expression of maltogenic amylase in Bacillus subtilis through dal gene auxotrophic selection marker. Int. J. Biol. Macromol. 2023, 254, 127372. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.; Shi, C.; Gong, W.; Liu, J.; Meng, X.; Liu, F.; Lu, F.; Zhang, H. Heterologous expression and characterization of an M4 family extracellular metalloprotease for detergent application. J. Gen. Appl. Microbiol. 2023, 69. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xuan, X.; Gao, R.; Xie, G. Increased Expression Levels of Thermophilic Serine Protease TTHA0724 through Signal Peptide Screening in Bacillus subtilis and Applications of the Enzyme. Int. J. Mol. Sci. 2023, 24, 15950. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-Z.; Zhang, Y.-H.P. One-step production of biocommodities from lignocellulosic biomass by recombinant cellulolytic Bacillus subtilis: Opportunities and challenges. Eng. Life Sci. 2010, 10, 398–406. [Google Scholar] [CrossRef]
- Sun, X.; Yang, J.; Fu, X.; Zhao, X.; Zhen, J.; Song, H.; Xu, J.; Zheng, H.; Bai, W. Trehalose Production Using Three Extracellular Enzymes Produced via One-Step Fermentation of an Engineered Bacillus subtilis Strain. Bioengineering 2023, 10, 977. [Google Scholar] [CrossRef] [PubMed]
- El Salamony, D.H.; Salah Eldin Hassouna, M.; Zaghloul, T.I.; Moustafa Abdallah, H. Valorization of chicken feather waste using recombinant Bacillus subtilis cells by solid-state fermentation for soluble proteins and serine alkaline protease production. Bioresour. Technol. 2023, 393, 130110. [Google Scholar] [CrossRef]
- Jun, J.-S.; Jeong, H.-E.; Hong, K.-W. Exploring and Engineering Novel Strong Promoters for High-Level Protein Expression in Bacillus subtilis DB104 through Transcriptome Analysis. Microorganisms 2023, 11, 2929. [Google Scholar] [CrossRef]
- Lam, K.H.; Chow, K.C.; Wong, W.K. Construction of an efficient Bacillus subtilis system for extracellular production of heterologous proteins. J. Biotechnol. 1998, 63, 167–177. [Google Scholar] [CrossRef]
- Ebisu, S.; Takagi, H.; Kadowaki, K.; Yamagata, H.; Udaka, S. Production of human epidermal growth factor by Bacillus brevis increased with use of a stable plasmid from B. brevis 481. Biosci. Biotechnol. Biochem. 1992, 56, 812–813. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yu, H.; Tian, S.; Yang, H.; Wang, J.; Zhu, W. Recombinant expression insulin-like growth factor 1 in Bacillus subtilis using a low-cost heat-purification technology. Process Biochem. 2017, 63, 49–54. [Google Scholar] [CrossRef]
- Hu, X.; Lai, C.Y.N.; Sivakumar, T.; Wang, H.; Ng, K.L.; Lam, C.C.; Wong, W.K.R. Novel strategy for expression of authentic and bioactive human basic fibroblast growth factor in Bacillus subtilis. Appl. Microbiol. Biotechnol. 2018, 102, 7061–7069. [Google Scholar] [CrossRef] [PubMed]
- Brey, R.N.; Banner, C.D.; Wolf, J.B. Cloning of multiple genes involved with cobalamin (Vitamin B12) biosynthesis in Bacillus megaterium. J. Bacteriol. 1986, 167, 623–630. [Google Scholar] [CrossRef]
- Biedendieck, R.; Malten, M.; Barg, H.; Bunk, B.; Martens, J.H.; Deery, E.; Leech, H.; Warren, M.J.; Jahn, D. Metabolic engineering of cobalamin (vitamin B12) production in Bacillus megaterium. Microb. Biotechnol. 2010, 3, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, C.; Ban, R. Improving riboflavin production by modifying related metabolic pathways in Bacillus subtilis. Lett. Appl. Microbiol. 2022, 74, 78–83. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Yang, C.; Pan, X.; Hu, M.; Du, Y.; Osire, T.; Yang, T.; Rao, Z. Metabolic engineering of Bacillus subtilis for enhancing riboflavin production by alleviating dissolved oxygen limitation. Bioresour. Technol. 2021, 333, 125228. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Y.; Wu, Z.; Lu, Y.; Tao, G.; Zhang, L.; Ding, Z.; Shi, G. Combining Precursor-Directed Engineering with Modular Designing: An Effective Strategy for De Novo Biosynthesis of l-DOPA in Bacillus licheniformis. ACS Synth. Biol. 2022, 11, 700–712. [Google Scholar] [CrossRef]
- Li, L.; Mu, L.; Wang, X.; Yu, J.; Hu, R.; Li, Z. A novel expression vector for the secretion of abaecin in Bacillus subtilis. Braz. J. Microbiol. 2017, 48, 809–814. [Google Scholar] [CrossRef]
- Xu, J.; Zhong, F.; Zhang, Y.; Zhang, J.; Huo, S.; Lin, H.; Wang, L.; Cui, D.; Li, X. Construction of Bacillus subtilis strain engineered for expression of porcine β-defensin-2/cecropin P1 fusion antimicrobial peptides and its growth-promoting effect and antimicrobial activity. Asian-Australas. J. Anim. Sci. 2017, 30, 576–584. [Google Scholar] [CrossRef]
- Chen, M.; Lin, N.; Liu, X.; Tang, X.; Wang, Z.; Zhang, D. A novel antimicrobial peptide screened by a Bacillus subtilis expression system, derived from Larimichthys crocea Ferritin H, exerting bactericidal and parasiticidal activities. Front. Immunol. 2023, 14, 1168517. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Y.; Peng, C.; Li, Z.; Wang, G.; Wang, H.; Yu, L.; Wang, F. Both recombinant Bacillus subtilis Expressing PCV2d Cap protein and PCV2d-VLPs can stimulate strong protective immune responses in mice. Heliyon 2023, 9, e22941. [Google Scholar] [CrossRef] [PubMed]
- Lv, P.; Zhang, X.; Song, M.; Hao, G.; Wang, F.; Sun, S. Oral administration of recombinant Bacillus subtilis expressing a multi-epitope protein induces strong immune responses against Salmonella Enteritidis. Vet. Microbiol. 2023, 276, 109632. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Rao, Z.; Zhang, X.; Xu, M.; Xu, Z.; Yang, S.-T. Metabolic engineering strategies for acetoin and 2,3-butanediol production: Advances and prospects. Crit. Rev. Biotechnol. 2017, 37, 990–1005. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhang, J.; Li, L.; Wen, Z.; Nomura, C.; Wu, S.; Chen, S. Engineering Bacillus licheniformis for the production of meso-2,3-butanediol. Biotechnol. Biofuels 2016, 9, 117. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Huo, G.; Feng, L.; Mao, Y.; Wang, Z.; Ma, H.; Chen, T.; Zhao, X. Metabolic engineering of Bacillus subtilis for chiral pure meso-2,3-butanediol production. Biotechnol. Biofuels 2016, 9, 90. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Rao, Z.; Zhang, X.; Xu, M.; Xu, Z.; Yang, S.T. Enhanced 2,3-butanediol production from biodiesel-derived glycerol by engineering of cofactor regeneration and manipulating carbon flux in Bacillus amyloliquefaciens. Microb. Cell Fact. 2015, 14, 122. [Google Scholar] [CrossRef]
- Tsigoriyna, L.; Arsov, A.; Petrova, P.; Gergov, E.; Petrov, K. Heterologous Expression of Inulinase Gene in Bacillus licheniformis 24 for 2,3-Butanediol Production from Inulin. Catalysts 2023, 13, 841. [Google Scholar] [CrossRef]
- Shi, L.; Lin, Y.; Song, J.; Li, H.; Gao, Y.; Lin, Y.; Huang, X.; Meng, W.; Qin, W. Engineered Bacillus subtilis for the Production of Tetramethylpyrazine,(R,R)-2,3-Butanediol and Acetoin. Fermentation 2023, 9, 488. [Google Scholar] [CrossRef]
- Lü, C.; Ge, Y.; Cao, M.; Guo, X.; Liu, P.; Gao, C.; Xu, P.; Ma, C. Metabolic Engineering of Bacillus licheniformis for Production of Acetoin. Front. Bioeng. Biotechnol. 2020, 8, 125. [Google Scholar] [CrossRef]
- Li, L.; Wei, X.; Yu, W.; Wen, Z.; Chen, S. Enhancement of acetoin production from Bacillus licheniformis by 2,3-butanediol conversion strategy: Metabolic engineering and fermentation control. Process Biochem. 2017, 57, 35–42. [Google Scholar] [CrossRef]
- Souza, C.C.; Guimarães, J.M.; Pereira, S.D.S.; Mariúba, L.A.M. The multifunctionality of expression systems in Bacillus subtilis: Emerging devices for the production of recombinant proteins. Exp. Biol. Med. 2021, 246, 2443–2453. [Google Scholar] [CrossRef]
- Wang, Z.; Li, X.; Tian, J.; Chu, Y.; Tian, Y. Cloning, heterologous expression and characterization of a novel streptomyces trypsin in Bacillus subtilis SCK6. Int. J. Biol. Macromol. 2020, 15, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Liu, S.; Jiao, Y.; Wang, Y.; Wang, M.; Du, G. Gene cloning and expression of the l-asparaginase from Bacillus cereus BDRD-ST26 in Bacillus subtilis WB600. J. Biosci. Bioeng. 2019, 127, 418–424. [Google Scholar] [CrossRef]
- Ramos, K.R.; Valdehuesa, K.N.; Cabulong, R.B.; Moron, L.S.; Nisola, G.M.; Hong, S.K.; Lee, W.K.; Chung, W.J. Overexpression and secretion of AgaA7 from Pseudoalteromonas hodoensis sp. nov in Bacillus subtilis for the depolymerization of agarose. Enzyme Microb. Technol. 2016, 90, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Su, H.-H.; Chen, J.-C.; Chen, P.-T. Production of recombinant human epidermal growth factor in Bacillus Subtilis. J. Taiwan Inst. Chem. Eng. 2020, 106, 86–91. [Google Scholar] [CrossRef]
- Yang, T.; Irene, K.; Liu, H.; Liu, S.; Zhang, X.; Xu, M.; Rao, Z. Enhanced extracellular gamma glutamyl transpeptidase production by overexpressing of PrsA lipoproteins and improving its mRNA stability in Bacillus subtilis and application in biosynthesis of L-theanine. J. Biotechnol. 2019, 20, 85–91. [Google Scholar] [CrossRef]
- Wang, M.; Wu, J.; Wu, D. Cloning and expression of the sucrose phosphorylase gene in Bacillus subtilis and synthesis of kojibiose using the recombinant enzyme. Microb. Cell Fact. 2018, 17, 23. [Google Scholar] [CrossRef]
- Song, W.; Nie, Y.; Mu, X.Q.; Xu, Y. Enhancement of extracellular expression of Bacillus naganoensis pullulanase from recombinant Bacillus subtilis: Effects of promoter and host. Protein Expr. Purif. 2016, 124, 23–31. [Google Scholar] [CrossRef]
- Tao, Z.; Fu, G.; Wang, S.; Jin, Z.; Wen, J.; Zhang, D. Hyper-secretion mechanism exploration of a heterologous creatinase in Bacillus subtilis. Biochem. Engin. J. 2020, 153, 107419. [Google Scholar] [CrossRef]
- Zhang, L.; Li, G.; Zhan, N.; Sun, T.; Cheng, B.; Li, Y.; Shan, A. Expression of a Pseudomonas aeruginosa-targeted antimicrobial peptide T9W in Bacillus subtilis using a maltose-inducible vector. Process Biochem. 2019, 81, 22–27. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, D.; Zhan, N.; Sun, T.; Shan, B.; Shan, A. Heterologous expression of the novel α-helical hybrid peptide PR-FO in Bacillus subtilis. Bioprocess Biosyst. Eng. 2020, 43, 1619–1627. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, X.; Zhan, N.; Sun, T.; Li, J.; Shan, A. Maltose Induced Expression of Cecropin AD by SUMO Technology in Bacillus subtilis WB800N. Protein J. 2020, 39, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.D.G.; Azzoni, A.R.; Freitas, S. Techno-economic analysis of the industrial production of a low-cost enzyme using E. coli: The case of recombinant β-glucosidase. Biotechnol. Biofuels 2018, 11, 81. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, V.M.; Campani, G.; Santos, M.P.; Silva, G.G.; Pires, M.C.; Gonçalves, V.M.; de C. Giordano, R.; Sargo, C.R.; Horta, A.C.L.; Zangirolami, T.C. Cost analysis based on bioreactor cultivation conditions: Production of a soluble recombinant protein using Escherichia coli BL21(DE3). Biotechnol. Rep. 2020, 22, e00441. [Google Scholar] [CrossRef] [PubMed]
- Hanif, M.U.; Gul, R.; Hanif, M.I.; Hashmi, A.A. Heterologous Secretory Expression and Characterization of Dimerized Bone Morphogenetic Protein 2 in Bacillus subtilis. Biomed Res. Int. 2017, 2017, 9350537. [Google Scholar] [CrossRef]
- Le, V.D.; Phan, T.T.P.; Nguyen, T.M.; Brunsveld, L.; Schumann, W.; Nguyen, H.D. Using the IPTG-Inducible Pgrac212 Promoter for Overexpression of Human Rhinovirus 3C Protease Fusions in the Cytoplasm of Bacillus subtilis Cells. Curr. Microbiol. 2019, 76, 1477–1486. [Google Scholar] [CrossRef]
- Heinrich, J.; Drewniok, C.; Neugebauer, E.; Kellner, H.; Wiegert, T. The YoaW signal peptide directs efficient secretion of different heterologous proteins fused to a StrepII-SUMO tag in Bacillus subtilis. Microb. Cell Fact. 2019, 18, 31. [Google Scholar] [CrossRef]
- Deng, Y.; Nie, Y.; Zhang, Y.; Wang, Y.; Xu, Y. Improved inducible expression of Bacillus naganoensis pullulanase from recombinant Bacillus subtilis by enhancer regulation. Protein Expr. Purif. 2018, 148, 9–15. [Google Scholar] [CrossRef]
- Halmschlag, B.; Putri, S.P.; Fukusaki, E.; Blank, L.M. Poly-γ-glutamic acid production by Bacillus subtilis 168 using glucose as the sole carbon source: A metabolomic analysis. J. Biosci. Bioeng. 2020, 130, 272–282. [Google Scholar] [CrossRef]
- Guan, C.; Cui, W.; Cheng, J.; Zhou, L.; Guo, J.; Hu, X.; Xiao, G.; Zhou, Z. Construction and development of an auto-regulatory gene expression system in Bacillus subtilis. Microb. Cell Fact. 2015, 14, 150. [Google Scholar] [CrossRef] [PubMed]
- Guan, C.; Cui, W.; Cheng, J.; Zhou, L.; Liu, Z.; Zhou, Z. Development of an efficient autoinducible expression system by promoter engineering in Bacillus subtilis. Microb. Cell Fact. 2016, 15, 66. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Wu, Y.; Ding, W.; Wang, L.; Wu, L.; Lin, L.; Che, Z.; Zhu, L.; Liu, Y.; Chen, X. An auto-inducible expression and high cell density fermentation of Beefy Meaty Peptide with Bacillus subtilis. Bioprocess Biosyst. Eng. 2020, 43, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.T.M.; Phan, T.T.P.; Huynh, T.K.; Dang, N.T.K.; Huynh, P.T.K.; Nguyen, T.M.; Truong, T.T.T.; Tran, T.L.; Schumann, W.; Nguyen, H.D. Development of inducer-free expression plasmids based on IPTG-inducible promoters for Bacillus subtilis. Microb. Cell Fact. 2017, 16, 130. [Google Scholar] [CrossRef]
- Tran, D.T.M.; Phan, T.T.P.; Doan, T.T.N.; Tran, T.L.; Schumann, W.; Nguyen, H.D. Integrative expression vectors with Pgrac promoters for inducer-free overproduction of recombinant proteins in Bacillus subtilis. Biotechnol. Rep. 2020, 28, e00540. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Xu, J.; Liu, X.; Chu, X.; Wang, P.; Tian, J.; Wu, N.; Fan, Y. Identification of a highly efficient stationary phase promoter in Bacillus subtilis. Sci. Rep. 2015, 17, 18405. [Google Scholar] [CrossRef]
- Corrêa, G.G.; Lins, M.R.D.C.R.; Silva, B.F.; de Paiva, G.B.; Zocca, V.F.B.; Ribeiro, N.V.; Picheli, F.P.; Mack, M.; Pedrolli, D.B. A modular autoinduction device for control of gene expression in Bacillus subtilis. Metab. Eng. 2020, 61, 326–334. [Google Scholar] [CrossRef]
- Han, L.; Suo, F.; Jiang, C.; Gu, J.; Li, N.; Zhang, N.; Cui, W.; Zhou, Z. Fabrication and characterization of a robust and strong bacterial promoter from a semi-rationally engineered promoter library in Bacillus subtilis. Process Biochem. 2017, 61, 56–62. [Google Scholar] [CrossRef]
- Cui, W.; Suo, F.; Cheng, J.; Han, L.; Hao, W.; Guo, J.; Zhou, Z. Stepwise modifications of genetic parts reinforce the secretory production of nattokinase in Bacillus subtilis. Microb. Biotechnol. 2018, 11, 930–942. [Google Scholar] [CrossRef]
- Han, L.; Cui, W.; Suo, F.; Miao, S.; Hao, W.; Chen, Q.; Guo, J.; Liu, Z.; Zhou, L.; Zhou, Z. Development of a novel strategy for robust synthetic bacterial promoters based on a stepwise evolution targeting the spacer region of the core promoter in Bacillus subtilis. Microb. Cell Fact. 2019, 18, 96. [Google Scholar] [CrossRef]
- Phan, T.T.; Tran, L.T.; Schumann, W.; Nguyen, H.D. Development of Pgrac100-based expression vectors allowing high protein production levels in Bacillus subtilis and relatively low basal expression in Escherichia coli. Microb. Cell Fact. 2015, 14, 72. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Han, L.; Cheng, J.; Liu, Z.; Zhou, L.; Guo, J.; Zhou, Z. Engineering an inducible gene expression system for Bacillus subtilis from a strong constitutive promoter and a theophylline-activated synthetic riboswitch. Microb. Cell Fact. 2016, 15, 199. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhu, G.; Korza, G.; Sun, X.; Setlow, P.; Li, J. Engineering Bacillus subtilis as a Versatile and Stable Platform for Production of Nanobodies. Appl. Environ. Microbiol. 2020, 86, e02938-19. [Google Scholar] [CrossRef] [PubMed]
- Pang, B.; Zhou, L.; Cui, W.; Liu, Z.; Zhou, Z. Production of a Thermostable Pullulanase in Bacillus subtilis by Optimization of the Expression Elements. Starch Stärke 2020, 72, 2000018. [Google Scholar] [CrossRef]
- Kang, X.M.; Cai, X.; Huang, Z.H.; Liu, Z.Q.; Zheng, Y.G. Construction of a highly active secretory expression system in Bacillus subtilis of a recombinant amidase by promoter and signal peptide engineering. Int. J. Biol. Macromol. 2020, 143, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Su, L.; Duan, X.; Liu, L.; Wu, J. High-level extracellular protein production in Bacillus subtilis using an optimized dual-promoter expression system. Microb. Cell Fact. 2017, 16, 32. [Google Scholar] [CrossRef] [PubMed]
- Guan, C.; Cui, W.; Cheng, J.; Liu, R.; Liu, Z.; Zhou, L.; Zhou, Z. Construction of a highly active secretory expression system via an engineered dual promoter and a highly efficient signal peptide in Bacillus subtilis. N. Biotechnol. 2016, 33, 372–379. [Google Scholar] [CrossRef]
- He, W.; Mu, W.; Jiang, B.; Yan, X.; Zhang, T. Construction of a Food Grade Recombinant Bacillus subtilis Based on Replicative Plasmids with an Auxotrophic Marker for Biotransformation of d-Fructose to d-Allulose. J. Agric. Food Chem. 2016, 64, 3243–3250. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, C.; Li, D.; Chen, X.; Li, J.; Zhang, Y.; Yuan, H.; Li, Y.; Lu, F. Engineering a highly efficient expression system to produce BcaPRO protease in Bacillus subtilis by an optimized promoter and signal peptide. Int. J. Biol. Macromol. 2019, 138, 903–911. [Google Scholar] [CrossRef]
- Promchai, R.; Promdonkoy, B.; Tanapongpipat, S.; Visessanguan, W.; Eurwilaichitr, L.; Luxananil, P. A novel salt-inducible vector for efficient expression and secretion of heterologous proteins in Bacillus subtilis. J. Biotechnol. 2016, 222, 86–93. [Google Scholar] [CrossRef]
- Liu, H.; Wang, S.; Song, L.; Yuan, H.; Liu, K.; Meng, W.; Wang, T. Trehalose Production Using Recombinant Trehalose Synthase in Bacillus subtilis by Integrating Fermentation and Biocatalysis. J. Agric. Food Chem. 2019, 67, 9314–9324. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Xu, J.; Tan, M.; Zhen, J.; Shu, W.; Yang, S.; Ma, Y.; Zheng, H.; Song, H. High copy number and highly stable Escherichia coli-Bacillus subtilis shuttle plasmids based on pWB980. Microb. Cell Fact. 2020, 19, 25. [Google Scholar] [CrossRef] [PubMed]
- Mu, D.; Lu, J.; Qiao, M.; Kuipers, O.P.; Zhu, J.; Li, X.; Yang, P.; Zhao, Y.; Luo, S.; Wu, X.; et al. Heterologous signal peptides-directing secretion of Streptomyces mobaraensis transglutaminase by Bacillus subtilis. Appl. Microbiol. Biotechnol. 2018, 102, 5533–5543. [Google Scholar] [CrossRef] [PubMed]
- Scheidler, C.M.; Vrabel, M.; Schneider, S. Genetic Code Expansion, Protein Expression, and Protein Functionalization in Bacillus subtilis. ACS Synth. Biol. 2020, 9, 486–493. [Google Scholar] [CrossRef]
- Song, Y.; Fu, G.; Dong, H.; Li, J.; Du, Y.; Zhang, D. High-Efficiency Secretion of β-Mannanase in Bacillus subtilis through Protein Synthesis and Secretion Optimization. J. Agric. Food Chem. 2017, 65, 2540–2548. [Google Scholar] [CrossRef]
- Chen, J.; Gai, Y.; Fu, G.; Zhou, W.; Zhang, D.; Wen, J. Enhanced extracellular production of α-amylase in Bacillus subtilis by optimization of regulatory elements and over-expression of PrsA lipoprotein. Biotechnol. Lett. 2015, 37, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Wang, Y.; Ju, J.; Cheng, L.; Xu, Y.; Yu, B.; Wang, L. Extracellular production of active-form Streptomyces mobaraensis transglutaminase in Bacillus subtilis. Appl. Microbiol. Biotechnol. 2020, 104, 623–631. [Google Scholar] [CrossRef]
- Li, K.; Cai, D.; Wang, Z.; He, Z.; Chen, S. Development of an Efficient Genome Editing Tool in Bacillus licheniformis Using CRISPR-Cas9 Nickase. Appl. Environ. Microbiol. 2018, 84, e02608-17. [Google Scholar] [CrossRef]
- Liu, D.; Huang, C.; Guo, J.; Zhang, P.; Chen, T.; Wang, Z.; Zhao, X. Development and characterization of a CRISPR/Cas9n-based multiplex genome editing system for Bacillus subtilis. Biotechnol. Biofuels. 2019, 12, 197. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhu, Y.; Sha, Y.; Lei, P.; Luo, Z.; Feng, Z.; Li, S.; Xu, H. Development of a Robust Bacillus amyloliquefaciens Cell Factory for Efficient Poly(γ-glutamic acid) Production from Jerusalem Artichoke. ACS Sustain. Chem. Eng. 2020, 8, 9763–9774. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, R.; Bao, T.; Rao, Z.; Yang, T.; Xu, M.; Xu, Z.; Li, H.; Yang, S. The rebalanced pathway significantly enhances acetoin production by disruption of acetoin reductase gene and moderate-expression of a new water-forming NADH oxidase in Bacillus subtilis. Metab. Eng. 2014, 23, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Cai, D.; Chen, S. Metabolic Engineering of Central Carbon Metabolism of Bacillus licheniformis for Enhanced Production of Poly-γ-glutamic Acid. Appl. Biochem. Biotechnol. 2021, 193, 3540–3552. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Cheng, X.; Deng, H.; Chen, S.; Ji, Z. Improvement of 1-deoxynojirimycin production of Bacillus amyloliquefaciens by gene overexpression and medium optimization. LWT 2021, 149, 111812. [Google Scholar] [CrossRef]
- Li, X.; Zhang, M.; Lu, Y.; Wu, N.; Chen, J.; Ji, Z.; Zhan, Y.; Ma, X.; Chen, J.; Cai, D.; et al. Metabolic engineering of Bacillus amyloliquefaciens for efficient production of α-glucosidase inhibitor1-deoxynojirimycin. Synth. Syst. Biotechnol. 2023, 8, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Deng, J.; Liu, Y.; Li, J.; Shin, H.D.; Du, G.; Chen, J.; Liu, L. Rewiring the Glucose Transportation and Central Metabolic Pathways for Overproduction of N-Acetylglucosamine in Bacillus subtilis. Biotechnol. J. 2017, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Ji, A.; Bao, P.; Ma, A.; Wei, X. An Efficient Prephenate Dehydrogenase Gene for the Biosynthesis of L-tyrosine: Gene Mining, Sequence Analysis, and Expression Optimization. Foods 2023, 12, 3084. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Fu, G.; Gai, Y.; Zheng, P.; Zhang, D.; Wen, J. Combinatorial Sec pathway analysis for improved heterologous protein secretion in Bacillus subtilis: Identification of bottlenecks by systematic gene overexpression. Microb Cell. Fact. 2015, 14, 92. [Google Scholar] [CrossRef]
- Ma, R.J.; Wang, Y.H.; Liu, L.; Bai, L.L.; Ban, R. Production enhancement of the extracellular lipase LipA in Bacillus subtilis: Effects of expression system and Sec pathway components. Protein Expr. Purif. 2018, 142, 81–87. [Google Scholar] [CrossRef]
- Shi, S.; Shen, Z.; Chen, X.; Chen, T.; Zhao, X. Increased production of riboflavin by metabolic engineering of the purine pathway in Bacillus subtilis. Biochem. Eng. J. 2009, 46, 28–33. [Google Scholar] [CrossRef]
- Wang, G.; Shi, T.; Chen, T.; Wang, X.; Wang, Y.; Liu, D.; Guo, J.; Fu, J.; Feng, L.; Wang, Z.; et al. Integrated whole-genome and transcriptome sequence analysis reveals the genetic characteristics of a riboflavin-overproducing Bacillus subtilis. Metab. Eng. 2018, 48, 138–149. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, C.; Tang, W.; Zhang, D. Manipulation of Purine Metabolic Networks for Riboflavin Production in Bacillus subtilis. ACS Omega. 2020, 5, 29140–29146. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zou, D.; Ji, A.; He, Y.; Liu, Y.; Deng, Y.; Chen, S.; Wei, X. Multilevel Metabolic Engineering of Bacillus amyloliquefaciens for Production of the Platform Chemical Putrescine from Sustainable Biomass Hydrolysates. ACS Sustain. Chem. Eng. 2020, 8, 2147–2157. [Google Scholar] [CrossRef]
- Gu, Y.; Lv, X.; Liu, Y.; Li, J.; Du, G.; Chen, J.; Rodrigo, L.A.; Liu, L. Synthetic redesign of central carbon and redox metabolism for high yield production of N-acetylglucosamine in Bacillus subtilis. Metab. Eng. 2019, 51, 59–69. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arsov, A.; Armenova, N.; Gergov, E.; Petrov, K.; Petrova, P. Cloning Systems in Bacillus: Bioengineering of Metabolic Pathways for Valuable Recombinant Products. Fermentation 2024, 10, 50. https://doi.org/10.3390/fermentation10010050
Arsov A, Armenova N, Gergov E, Petrov K, Petrova P. Cloning Systems in Bacillus: Bioengineering of Metabolic Pathways for Valuable Recombinant Products. Fermentation. 2024; 10(1):50. https://doi.org/10.3390/fermentation10010050
Chicago/Turabian StyleArsov, Alexander, Nadya Armenova, Emanoel Gergov, Kaloyan Petrov, and Penka Petrova. 2024. "Cloning Systems in Bacillus: Bioengineering of Metabolic Pathways for Valuable Recombinant Products" Fermentation 10, no. 1: 50. https://doi.org/10.3390/fermentation10010050
APA StyleArsov, A., Armenova, N., Gergov, E., Petrov, K., & Petrova, P. (2024). Cloning Systems in Bacillus: Bioengineering of Metabolic Pathways for Valuable Recombinant Products. Fermentation, 10(1), 50. https://doi.org/10.3390/fermentation10010050







