Obtaining Value from Wine Wastes: Paving the Way for Sustainable Development
Abstract
1. Introduction: An Overview of the Wine Industry and Its Wastes
2. Chemical Composition and Structure of Solid Winemaking Wastes
2.1. Composition and Structure of Grape Pomace Constituents
2.1.1. Grape Stalks
2.1.2. Grape Skins
2.1.3. Grape Seeds
2.2. Vine Shoots
2.3. Vine Leaves
2.4. Wine Lees
3. Products from Solid Winemaking Wastes
3.1. Chemicals
3.1.1. Tannins
3.1.2. Grape Seed and Leaves Oils
SFE of Grape Seed Oil
SFE of Grape Vine Leaves
3.2. Biofuels
3.3. Polymers
3.3.1. Polydroxyalkanoates
| Type of Grapes | Pretreatment | Bacteria | Carbon Source * | Process Configuration | PHA (g/L) | PHA (%cdw) | Prod (g/L.h) | Type of PHA | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| White | Enzymatic hydrolysis | Pseudomonas resinovorans (DSM 21078) | M | Batch in bioreactor | 21.3 | 23.3 | 0.05 | mcl-PHA | [142] |
| White | Water extraction | P. putida KT2400 (ATCC 47054) | M | Fed-batch pilot scale bioreactor | 21.8 | 77 | 0.10 | mcl-PHA | [143] |
| Red | Extraction with scCO2 + anaerobic digestion | Cupriavidus necator (DSM 545) | O | Fed-batch | n.d. | 68 | n.d. | P(3HB) | [140] |
| White | Enzymatic hydrolysis after phenolics extraction | C. necator H16 (CCM 3726) | M | Batch in bioreactor | 8.3 | 63.0 | 0.28 | P(3HB) | [141] |
| Halomonas halophila (CCM 3662) | Batch in shake flasks with NaCl 6.6% (w/w) | 1.8 | 57.0 | 0.025 | |||||
| Halomonas organivorans (CCM 7142) | Batch in shake flasks with NaCl 8.0% (w/w) | 2.1 | 55.4 | 0.029 | |||||
| White | Enzymatic hydrolysis after phenolics extraction | Tepidimonas taiwanensis (LMG 22826) | M | Batch in shake flasks at 50 °C | 2.09 | 47.9 | n.d. | P(3HB) | [144] |
| Red | 0.022 | 8.4 | n.d. | ||||||
| Rose | 0.236 | 12.3 | n.d. | ||||||
| Not reported | Solid state enzymatic hydrolysis | C. necator (DSMZ 428) | M | Solid-state fermentation in shake flasks | n.d. | 21.3 | n.d. | P(3HB) | [145] |
| Red | Acidogenic fermentation | P. putida (DSMZ 6125) | O | Fed-batch pH-stat mode | 10.4 | 61 | 0.21 | mcl-PHA | [10] |
| White | Hot water extraction at 100 °C, 2 bar | C. necator (DSM 545) | M | Batch in shake flasks | 5.48 | 86 | - | P(3HB) | This study |
| White | Hot water extraction at 100 °C, Patm | C. necator (DSM 531) | M | Batch in bioreactor | 2.19 | 85 | 0.03 | P(3HB) | This study |
3.3.2. Bacterial Cellulose
4. Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Organisation of Vine and Wine. State of the World Vitivinicultural Sector in 2022. 2023. Available online: https://www.oiv.int/what-we-do/statistics (accessed on 28 November 2023).
- Oliveira, M.; Duarte, E. Integrated approach to winery waste: Waste generation and data consolidation. Front. Environ. Sci. Eng. 2016, 10, 168–176. [Google Scholar] [CrossRef]
- Rodrigues, R.P.; Gando-Ferreira, L.M.; Quina, M.J. Increasing Value of Winery Residues through Integrated Biorefinery Processes: A Review. Molecules 2022, 27, 4709. [Google Scholar] [CrossRef] [PubMed]
- Bharathiraja, B.; Iyyappan, J.; Jayamuthunagai, J.; Kumar, R.P.; Sirohi, R.; Gnansounou, E.; Pandey, A. Critical review on bioconversion of winery wastes into value-added products. Ind. Crops Prod. 2020, 158, 112954. [Google Scholar] [CrossRef]
- Rivera, O.M.P.; Leos, M.D.S.; Solis, V.E.; Domínguez, J.M. Recent trends on the valorization of winemaking industry wastes. Curr. Opin. Green Sustain Chem. 2021, 27, 100415. [Google Scholar] [CrossRef]
- Christ, K.L.; Burritt, R.L. Critical environmental concerns in wine production: An integrative review. J. Clean. Prod. 2013, 53, 232–242. [Google Scholar] [CrossRef]
- Ferri, M.; Vannini, M.; Ehrnell, M.; Eliasson, L.; Xanthakis, E.; Monari, S.; Sisti, L.; Marchese, P.; Celli, A.; Tassoni, A. From winery waste to bioactive compounds and new polymeric biocomposites: A contribution to the circular economy concept. J. Adv. Res. 2020, 24, 1–11. [Google Scholar] [CrossRef] [PubMed]
- De Corato, U. Bioplastics from Winemaking By-products in the Buildings Sector: A Feasibility Study on the Main Opportunities, Barriers and Challenges. Circ. Econ. Sust. 2021, 1, 1313–1333. [Google Scholar] [CrossRef]
- Nanni, A.; Parisi, M.; Colonna, M. Wine By-Products as Raw Materials for the Production of Biopolymers and of Natural Reinforcing Fillers: A Critical Review. Polymers 2021, 13, 381. [Google Scholar] [CrossRef]
- Martinez, G.A.; Puccio, S.; Domingos, J.M.B.; Morselli, E.; Gioia, C.; Marchese, P.; Raspolli Galletti, A.M.; Celli, A.; Fava, F.; Bertin, L. Upgrading grape pomace contained ethanol into hexanoic acid, fuel additives and a sticky polyhydroxyalkanoate: An effective alternative to ethanol distillation. Green Chem. 2022, 24, 2882–2892. [Google Scholar] [CrossRef]
- Clarivate. Web of Science Platform. 2023. Available online: https://www.webofscience.com/wos (accessed on 28 November 2023).
- Fragoso, R.; Vieira, A.A.C. Efficiency analysis of the Portuguese wine industry using accounting and operational metrics. Results Eng. 2022, 14, 100389. [Google Scholar] [CrossRef]
- Faria, S.d.S.; Lourenço-Gomes, L.S.d.M.; de Gouveia, S.H.C.; Rebelo, J.F. Economic performance of the Portuguese wine industry: A microeconometric analysis. J. Wine Res. 2020, 31, 283–300. [Google Scholar] [CrossRef]
- Costa, J.M. Aproveitamento de subprodutos da vinificação. O bagaço como matéria-prima da indústria de óleos e grainha e de fabrico de rações para gado. In 1° Congresso Nacional das Indústrias Agro-Alimentares; Ministério da Agricultura Comércio e Pescas: Lisbon, Portugal, 1983. [Google Scholar]
- Yalcin, H.; Kavuncuoglu, H.; Ekici, L.; Sagdic, O. Determination of fatty acid composition, volatile components, physico-chemical and bioactive properties of grape (Vitis vinifera) seed and seed oil. J. Food Process. Preserv. 2017, 41, e12854. [Google Scholar] [CrossRef]
- Chowdhary, P.; Gupta, A.; Gnansounou, E.; Pandey, A.; Chaturvedi, P. Current trends and possibilities for exploitation of grape pomace as a potential source for value addition. Environ. Pollut. 2021, 278, 116796. [Google Scholar] [CrossRef] [PubMed]
- Spinei, M.; Oroian, M. The potential of grape pomace varieties as a dietary source of pectic substances. The potential of grape pomace varieties as a dietary source of pectic substances. Foods 2021, 10, 867. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; O’Hair, J.; Stewart, A.C.; O’Keefe, S.F.; Neilson, A.P.; Kim, Y.-T.; McGuire, M.; Lee, A.; Wilder, G.; Huang, H. Compositional characterization of different industrial white and red grape pomaces in Virginia and the potential valorization of the major components. Foods 2019, 8, 667. [Google Scholar] [CrossRef]
- Cruz, J.M.; Dominguez, H.; Parajo, J.C. Assessment of the production of antioxidants from winemaking waste solids. J. Agric. Food Chem. 2004, 52, 5612–5620. [Google Scholar] [CrossRef] [PubMed]
- Spigno, G.; Pizzorno, T.; De Faveri, D. Cellulose and hemicelluloses recovery from grape stalks. Bioresour. Technol. 2008, 99, 4329–4337. [Google Scholar] [CrossRef]
- Ping, L.; Brosse, N.; Sannigrahi, P.; Ragauskas, A. Evaluation of grape stalks as a bioresource. Ind. Crops Prod. 2011, 33, 200–204. [Google Scholar] [CrossRef]
- Ping, L.; Brosse, N.; Sannigrahi, P.; Ragauskas, A. Extraction of condensed tannins from grape pomace for use as wood adhesives. Ind. Crops Prod. 2011, 33, 253–257. [Google Scholar] [CrossRef]
- Prozil, S.O.; Evtuguin, D.V.; Cruz Lopes, L.P. Chemical composition of grape stalks of Vitis vinifera L. from red grape pomaces. Ind. Crops Prod. 2012, 35, 178–184. [Google Scholar] [CrossRef]
- Mangione, R.; Simões, R.; Pereira, H.; Catarino, S.; Ricardo-da-Silva, J.; Miranda, I.; Ferreira-Dias, S. Potential use of grape stems and pomaces from two red grapevine cultivars as source of oligosaccharides. Processes 2022, 10, 1896. [Google Scholar] [CrossRef]
- Atatoprak, T.; Amorim, M.M.; Ribeiro, T.; Pintado, M.; Madureira, A.R. Grape stalk valorization for fermentation purposes. Food Chem. Mol. Sci. 2022, 4, 100067. [Google Scholar] [CrossRef] [PubMed]
- Prozil, S.O.; Costa, E.V.; Evtuguin, D.V.; Cruz Lopes, L.P.; Domingues, M.R.M. Structural characterization of polysaccharides isolated from grape stalks of Vitis vinifera L. Carbohydr. Res. 2012, 356, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Prozil, S.O.; Evtuguin, D.V.; Silva, A.M.S.; Cruz Lopes, L.P. Structural characterization of lignin from grape stalks (Vitis vinifera L.). J. Agric. Food Chem. 2012, 62, 5420–5428. [Google Scholar] [CrossRef] [PubMed]
- Souquet, J.-M.; Labarbe, B.; Le Guerneve, C.; Cheynier, V.; Moutounet, M. Phenolic Composition of Grape Stems. J. Agric. Food Chem. 2000, 62, 5420–5428. [Google Scholar] [CrossRef] [PubMed]
- Mendes, J.A.S.; Prozil, S.O.; Evtuguin, D.V.; Silva, A.M.S.; Cruz Lopes, L.P. Towards comprehensive utilization of winemaking residues: Characterization of grape skins from red grape pomaces of variety Touriga Nacional. Ind. Crops. Prod. 2013, 43, 25–32. [Google Scholar] [CrossRef]
- Mendes, J.A.S.; Xavier, A.M.R.B.; Evtuguin, D.V.; Cruz Lopes, L.P. Integrated utilization of grape skins from white grape pomaces. Ind. Crops Prod. 2013, 49, 286–291. [Google Scholar] [CrossRef]
- Deng, Q.; Penner, M.H.; Zhao, Y. Chemical composition of dietary fiber and polyphenols of five different varieties of wine grape pomace skins. Food Res. Intern. 2013, 44, 2712–2720. [Google Scholar] [CrossRef]
- Fernandes, A.; Oliveira, J.; Teixeira, N.; Mateus, N.; de Freitas, V. A review of the current knowledge of red wine colour. OENO One 2017, 51, 1604. [Google Scholar] [CrossRef]
- Mendes, J.A.S.; Lopes, S.; Prozil, S.O.; Evtuguin, D.V.; Cruz Lopes, L.P. Chemical characterization of white and red grape skins from typical variety castes of Dão region. Millenium 2014, 46, 19–32. [Google Scholar]
- Rondeau, P.; Gambier, F.; Jolibert, F.; Brosse, N. Compositions and chemical variability of grape pomaces from French vineyard. Ind. Crops Prod. 2013, 43, 251–254. [Google Scholar] [CrossRef]
- Ladania, M.S. Fruit biochemistry. In Citrus Fruit. Biology, Technology and Evaluation; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar] [CrossRef]
- Domínguez, E.; Heredia-Guerrero, J.A.; Heredia, A. The biophysical design of plant cuticles: An overview. New Phytol. 2011, 189, 938–949. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Wen, X.; Sun, H. Oleanolic acid derivatives for pharmaceutical use: A patent review. Expert Opin. Ther. Pat. 2016, 26, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Moriam, K.; Rissanen, M.; Sawada, D.; Altgen, M.; Johansson, L.-S.; Evtyugin, D.V.; Guizani, C.; Hummel, M.; Sixta, H. Hydrophobization of the man-made cellulosic fibers by incorporating plant-derived hydrophobic compounds. ACS Sustain. Chem. Eng. 2021, 9, 4915–4925. [Google Scholar] [CrossRef]
- Makris, D.P.; Boskou, G.; Andrikopoulos, N.K. Recovery of antioxidant phenolics from white vinification solid by-products employing water/ethanol mixtures. Bioresour. Technol. 2017, 98, 2963–2967. [Google Scholar] [CrossRef]
- Makris, D.P.; Boskou, G.; Andrikopoulos, N.K. Polyphenolic content and in vitro antioxidant characteristics of wine industry and other agrifood solid waste extracts. J. Food Compost. Anal. 2007, 20, 125–132. [Google Scholar] [CrossRef]
- Gagné, S.; Saucier, C.; Gény, L. Composition and cellular localization of tannins in cabernet sauvignon skins during growth. J. Agric. Food Chem. 2006, 54, 9465–9471. [Google Scholar] [CrossRef]
- Busse-Valverde, N.; Bautista-Ortín, A.B.; Gómez-Plaza, E.; Fernandez-Fernandez, J.I.; Gil-Munoz, R. Influence of skin maceration time on the proanthocyanidin content of red wines. Eur. Food Res. Technol. 2012, 235, 1117–1123. [Google Scholar] [CrossRef]
- Llobera, A.; Canellas, J. Dietary fibre content and antioxidant activity of Manto Negro red grape (Vitis vinifera): Pomace and stem. Food Chem. 2007, 101, 659–666. [Google Scholar] [CrossRef]
- Ovcharova, T.M.; Zlatanov, M.; Dimitrova, R. Chemical composition of seeds of four Bulgarian grape varieties. Ciência Téc Vitiv. 2016, 31, 31–40. [Google Scholar] [CrossRef]
- Adamovic, T.; Tarasov, D.; Demirkaya, E.; Balakshin, M.; Cocero, M.J. A feasibility study on green biorefinery of high lignin content agro-food industry waste through supercritical water treatment. J. Clean. Prod. 2021, 323, 129110. [Google Scholar] [CrossRef]
- Bellili, S.; Jazi, S.; ben Nasr, S.; Dhifi, W.; Neves, M.A.; Miguel, M.G.C.; Mnif, W. Grape seed oil: Chemical composition, biological properties and health benefits. In Seed Oil: Production, Uses and Benefits; Hong, N.K.D., Ed.; Nova Science Publishers Inc.: New York, NY, USA, 2018; pp. 145–174. [Google Scholar]
- Shinagawa, F.B.; Santana, F.C.; Torres, L.R.O.; Mancini-Filho, J. Grapeseed oil: A potential functional food? Food Sci. Technol. 2015, 35, 399–406. [Google Scholar] [CrossRef]
- Vostrejs, P.; Adamcova, D.; Vaverkova, M.D.; Enev, V.; Kalina, M.; Machovsky, M.; Sourkova, M.; Ivana Marova, I.; Kovalcik, A. Active biodegradable packaging films modified with grape seeds lignin. RSC Adv. 2020, 10, 29202. [Google Scholar] [CrossRef] [PubMed]
- Wua, Z.; Deng, W.; Luo, J.; Deng, D. Multifunctional nano-cellulose composite films with grape seed extracts and immobilized silver nanoparticles. Carbohydr. Polym. 2019, 205, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.; Ramalhosa, E.; Pires, P.; Verdial, J.; Valentão, P.; Andrade, P.; Albino, B.; Pereira, J.A. Vitis vinifera leaves towards bioactivity. Ind. Crops Prod. 2013, 43, 434–440. [Google Scholar] [CrossRef]
- Fernandes, L.; Casal, S.; Cruz, R.; Pereira, J.A.; Ramalhosa, E. Seed oil often traditional Portuguese grape varieties with interesting chemical and antioxidant properties. Food Res. Int. 2013, 50, 161–166. [Google Scholar] [CrossRef]
- Garavaglia, J.; Merkoski, M.M.; Oliveira, A.; Marcadenti, A. Grape seed oil compounds: Biological and chemical actions for health. Nutr. Metab. Insights 2016, 9, 59–64. [Google Scholar] [CrossRef]
- Bail, S.; Stuebiger, G.; Krist, S.; Unterweger, H.; Buchbauer, G. Characteristics of various grape seed oils by volatile compounds, triacylglycerol composition, total phenols and antioxidant capacity. Food Chem. 2008, 108, 1122–1132. [Google Scholar] [CrossRef]
- Pastrana-Bonilla, E.; Akoh, C.C.; Sellappan, S.; Krewer, G. Phenolic content and antioxidant capacity of Muscadine grapes. J. Agric. Food Chem. 2003, 51, 5497–5503. [Google Scholar] [CrossRef]
- Guendez, R.; Kallithraka, S.; Makris, D.P.; Kefalas, P. Determination of low molecular weight polyphenolic constituents in grape (Vitis vinifera sp.) seed extracts: Correlation with antiradical activity. Food Chem. 2005, 89, 1–9. [Google Scholar] [CrossRef]
- Matthäus, B. Virgin grape seed oil: Is it really a nutritional highlight? Eur. J. Lipid Sci. Technol. 2008, 110, 645–650. [Google Scholar] [CrossRef]
- Bustos, G.; de la Torre, N.; Moldes, A.B.; Cruz, J.M.; Domínguez, J.M. Revalorization of hemicellulosic trimming vine shoots hydrolyzates trough continuous production of lactic acid and biosurfactants by L. pentosus. J. Food Eng. 2007, 78, 405–412. [Google Scholar] [CrossRef]
- Bustos, G.; Moldes, A.B.; Cruz, J.M.; Domínguez, J.M. Influence of the metabolism pathway on lactic acid production from hemicellulosic trimming vine shoots hydrolyzates using Lactobacillus pentosus. Biotechnol. Prog. 2005, 21, 793–795. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Martín, M.; Valdés-Sánchez, E.; Alexandre-Franco, M.F.; Fernández-González, M.C.; de la Torre, M.V.; Cuerda-Correa, M.E.; Gómez-Serrano, V. Waste valorization in winemaking industry: Vine shoots as precursors to optimize sensory features in white wine. LWT 2022, 163, 113601. [Google Scholar] [CrossRef]
- Pensec, F.; Szakiel, A.; Pączkowski, C.; Woźniak, A.; Grabarczyk, M.; Bertsch, C.; Fischer, M.J.C.; Chong, J. Characterization of triterpenoid profiles and triterpene synthase expression in the leaves of eight Vitis vinifera cultivars grown in the Upper Rhine Valley. J. Plant Res. 2016, 129, 499–512. [Google Scholar] [CrossRef]
- Pérez-Serradilla, J.A.; Luque de Castro, M.D. Microwave-assisted extraction of phenolic compounds from wine lees and spray-drying of the extract. Food Chem. 2011, 124, 1652–1659. [Google Scholar] [CrossRef]
- Dimou, C.; Kopsahelis, N.; Papadaki, A.; Papanikolaou, S.; Kookos, I.K.; Mandala, I.; Koutinas, A.A. Wine lees valorization: Biorefinery development including production of a generic fermentation feedstock employed for poly (3-hydroxybutyrate) synthesis. Food Res. Int. 2015, 73, 81–87. [Google Scholar] [CrossRef]
- Maicas, S.; Mateo, J.J. Sustainability of Wine Production. Sustainability 2020, 12, 559. [Google Scholar] [CrossRef]
- Ping, L.; Pizzi, A.; Zhou, D.G.; Brosse, N. Condensed tannins from grape pomace: Characterization by FTIR and MALDI TOF and production of environment friendly wood adhesive. Ind. Crops Prod. 2012, 40, 13–20. [Google Scholar] [CrossRef]
- Dhawale, P.V.; Vineeth, S.K.; Gadhave, R.V.; Jabeen Fatima, M.J.; Supekar, M.V.; Thakur, V.K.; Raghavan, P. Tannin as a renewable raw material for adhesive applications: A review. Mater. Adv. 2022, 3, 3365–3388. [Google Scholar] [CrossRef]
- Ping, L.; Gambier, F.; Pizzi, A.P.; Guo, Z.; Brosse, N. Wood adhesives from agricultural by-products: Lignins and tannins for the elaboration of particleboards. Cellul. Chem. Technol. 2012, 46, 457–462. [Google Scholar]
- Cano, A.; Contreras, C.; Chiralt, A.; Gonzalez-Martínez, C. Using tannins as active compounds to develop antioxidant and antimicrobial chitosan and cellulose based films. Carbohydr. Polym. Technol. Appl. 2021, 2, 100156. [Google Scholar] [CrossRef]
- Saratale, R.G.; Saratale, G.D.; Ahn, S.; Shin, H.-S. Grape Pomace Extracted Tannin for Green Synthesis of Silver Nanoparticles: Assessment of Their Antidiabetic, Antioxidant Potential and Antimicrobial Activity. Polymers 2021, 13, 4355. [Google Scholar] [CrossRef] [PubMed]
- Ruberto, G.; Renda, A.; Daquino, C.; Amico, V.; Spatafora, C.; Tringali, C.; De Tommasi, N. Polyphenol constituents and antioxidant activity of grape pomace extracts from five Sicilian red grape cultivars. Food Chem. 2007, 100, 203–210. [Google Scholar] [CrossRef]
- Joshi, V.K.; Devi, M.P. Resveratrol: Importance, role, contents in wine and factors influencing its production. Proc. Natl. Acad. Sci. India B 2009, 79, 212–226. [Google Scholar]
- Beres, C.; Costa, G.N.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.; Cruz, A.P.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.; Freitas, S.P. Towards integral utilization of grape pomace from winemaking process: A review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.; Igrejas, G.; Falcoe, V.; Santose, T.P.; Torres, C.; Oliveira, A.M.P.; Pereira, J.E.; Amarali, J.S.; Poeta, P. Chemical composition, antioxidant and antimicrobial activity of phenolic compounds extracted from wine industry by-products. Food Control 2018, 92, 516–522. [Google Scholar] [CrossRef]
- Olejar, K.J.; Arianna Ricci, A.; Simon Swift, S.; Zujovic, Z.; Gordon, K.C.; Fedrizzi, B.; Versari, A.; Kilmartin, P.A. Characterization of an antioxidant and antimicrobial extract from cool climate, white grape marc. Antioxidants 2019, 8, 232. [Google Scholar] [CrossRef]
- Ghendov-Mosanu, A.; Cojocari, D.; Balan, G.; Patras, A.; Lung, I.; Soran, M.-L.; Opris, O.; Cristea, E.; Sturza, R. Chemometric optimization of biologically active compounds extraction from grape marc: Composition and antimicrobial activity. Molecules 2022, 27, 1610. [Google Scholar] [CrossRef]
- Sneha, V.; Sumathy, V.J.H. Comparative Analysis of Antioxidant and Antimicrobial Activity of Grape Seed and Grape Skin. Int. J. Curr. Trends Pharm. Res. 2016, 4, 338–344. [Google Scholar]
- Xia, E.-Q.; Deng, G.-F.; Guo, Y.-J.; Li, H.-B. Biological activities of polyphenols from grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Martinez, I.; Arreaza-Gil, V.; Muguerza, B.; Arola-Arnal, A.; Bravo, F.I.; Torres-Fuentes, C.; Suárez, M. Administration time significantly affects plasma bioavailability of grape seed proanthocyanidins extract in healthy and obese Fischer 344 rats. Mol. Nutr. Food Res. 2022, 66, 2100552. [Google Scholar] [CrossRef] [PubMed]
- Crews, C.; Hough, P.; Godward, J.; Brereton, P.; Lees, M.; Guiet, S.; Winkelmann, W. Quantitation of the main constituents of some authentic grape-seed oils of different origin. J. Agric. Food Chem. 2006, 54, 6261–6265. [Google Scholar] [CrossRef] [PubMed]
- De Melo, M.M.R.; Carius, B.; Simões, M.M.Q.; Portugal, I.; Saraiva, J.; Silva, C.M. Supercritical CO2 extraction of V. vinifera leaves: Influence of cosolvents and particle size on removal kinetics and selectivity to target compounds. J. Supercrit. Fluids 2020, 165, 104959. [Google Scholar] [CrossRef]
- De Melo, M.M.R.; Portugal, I.; Silvestre, A.J.D.; Silva, C.M. Environmentally Benign Supercritical Fluid Extraction. In The Application of Green Solvents in Separation Processes; Elsevier: Amsterdam, The Netherlands, 2017; pp. 325–348. [Google Scholar] [CrossRef]
- Marchi, M.; Neri, E.; Pulselli, F.M.; Bastianoni, S. CO2 recovery from wine production: Possible implications on the carbon balance at territorial level. J. CO2 Util. 2018, 28, 137–144. [Google Scholar] [CrossRef]
- Crampon, C.; Mouahid, A.; Toudji, S.A.A.; Leṕine, O.; Badens, E. Influence of pretreatment on supercritical CO2 extraction from Nannochloropsis oculata. J. Supercrit. Fluids 2013, 79, 337–344. [Google Scholar] [CrossRef]
- De Melo, M.M.R.; Silvestre, A.J.D.; Silva, C.M. Supercritical Fluid Extraction of Oil and Bioactive Compounds from Grape Residues: Experimental Optimization, Modeling and Economic Evaluation. In Grapes: Production, Phenolic Composition and Potential Biomedical Effects; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2014. [Google Scholar]
- de Melo, M.M.R.; Silvestre, A.J.D.; Silva, C.M. Supercritical fluid extraction of vegetable matrices: Applications, trends and future perspectives of a convincing green technology. J. Supercrit. Fluids 2014, 92, 115–176. [Google Scholar] [CrossRef]
- Sovová, H. Rate of the vegetable oil extraction with supercritical CO2—I. Modelling of extraction curves. Chem. Eng. Sci. 1994, 49, 409–414. [Google Scholar] [CrossRef]
- Silva, C.M.; Passos, C.P.; Coimbra, M.A.; Da Silva, F.F.A. Numerical simulation of supercritical extraction processes. Chem. Prod. Process Model. 2009, 4. [Google Scholar] [CrossRef]
- Sovová, H.; Kučera, J.; Jež, J. Rate of the vegetable oil extraction with supercritical CO2-II. Extraction of grape oil. Chem. Eng. Sci. 1994, 49, 415–420. [Google Scholar] [CrossRef]
- Pascual-Martí, M.C.; Salvador, A.; Chafer, A.; Berna, A. Supercritical fluid extraction of resveratrol from grape skin of Vitis vinifera and determination by HPLC. Talanta 2001, 54, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Bravi, E.; Perretti, G.; Montanari, L.; Favati, F.; Fantozzi, P. Supercritical fluid extraction for quality control in beer industry. J. Supercrit. Fluids 2007, 42, 342–346. [Google Scholar] [CrossRef]
- Passos, C.P.; Silva, R.M.; Da Silva, F.A.; Coimbra, M.A.; Silva, C.M. Supercritical fluid extraction of grape seed (Vitis vinifera L.) oil. Effect of the operating conditions upon oil composition and antioxidant capacity. Chem. Eng. J. 2010, 160, 634–640. [Google Scholar] [CrossRef]
- Fiori, L. Supercritical extraction of grape seed oil at industrial-scale: Plant and process design, modeling, economic feasibility. Chem. Eng. Process. Process Intensif. 2010, 49, 866–872. [Google Scholar] [CrossRef]
- Casas, L.; Mantell, C.; Rodríguez, M.; Ossa, E.J.M.d.l.; Roldán, A.; Ory, I.; De Caro, I.; Blandino, A. Extraction of resveratrol from the pomace of Palomino fino grapes by supercritical carbon dioxide. J. Food Eng. 2010, 96, 304–308. [Google Scholar] [CrossRef]
- Jokić, S.; Bijuk, M.; Aladić, K.; Bilić, M.; Molnar, M. Optimisation of supercritical CO2 extraction of grape seed oil using response surface methodology. Int. J. Food Sci. Technol. 2016, 51, 403–410. [Google Scholar] [CrossRef]
- Todd, R.; Baroutian, S. A techno-economic comparison of subcritical water, supercritical CO2 and organic solvent extraction of bioactives from grape marc. J. Clean. Prod. 2017, 158, 349–358. [Google Scholar] [CrossRef]
- Cao, X.; Ito, Y. Supercritical fluid extraction of grape seed oil and subsequent separation of free fatty acids by high-speed counter-current chromatography. J. Chromatogr. A 2003, 1021, 117–124. [Google Scholar] [CrossRef]
- Prado, J.M.; Dalmolin, I.; Carareto, N.D.D.; Basso, R.C.; Meirelles, A.J.A.; Oliveira, J.V.; Batista, E.A.C.; Meireles, M.A.A. Supercritical fluid extraction of grape seed: Process scale-up, extract chemical composition and economic evaluation. J. Food Eng. 2012, 109, 249–257. [Google Scholar] [CrossRef]
- Murga, R.; Ruiz, R.; Beltran, S.; Cabezas, J.L. Extraction of natural complex phenols and tannins from grape seeds by using supercritical mixtures of carbon dioxide and alcohol. J. Agric. Food Chem. 2000, 48, 3408–3412. [Google Scholar] [CrossRef]
- Chafer, A.; Pascual-Martí, M.C.; Salvador, A.; Berna, A. Supercritical fluid extraction and HPLC determination of relevant polyphenolic compounds in grape skin. J. Sep. Sci. 2005, 28, 2050–2056. [Google Scholar] [CrossRef] [PubMed]
- Pinelo, M.; Ruiz-Rodríguez, A.; Sineiro, J.; Señoráns, F.J.; Reglero, G.; Núñez, M.J. Supercritical fluid and solid-liquid extraction of phenolic antioxidants from grape pomace: A comparative study. Eur. Food Res. Technol. 2007, 226, 199–205. [Google Scholar] [CrossRef]
- Passos, C.P.; Silva, R.M.; Da Silva, F.A.; Coimbra, M.A.; Silva, C.M. Enhancement of the supercritical fluid extraction of grape seed oil by using enzymatically pre-treated seed. J. Supercrit. Fluids 2009, 48, 225–229. [Google Scholar] [CrossRef]
- Floris, T.; Filippino, G.; Scrugli, S.; Pinna, M.B.; Argiolas, F.; Argiolas, A.; Murru, M.; Reverchon, E. Antioxidant compounds recovery from grape residues by a supercritical antisolvent assisted process. J. Supercrit. Fluids 2010, 54, 165–170. [Google Scholar] [CrossRef]
- Passos, C.P.; Coimbra, M.A.; Da Silva, F.A.; Silva, C.M. Modelling the supercritical fluid extraction of edible oils and analysis of the effect of enzymatic pre-treatments of seed upon model parameters. Chem. Eng. Res. Des. 2011, 89, 1118–1125. [Google Scholar] [CrossRef]
- Farías-Campomanes, A.M.; Rostagno, M.A.; Meireles, M.A.A. Production of polyphenol extracts from grape bagasse using supercritical fluids: Yield, extract composition and economic evaluation. J. Supercrit. Fluids 2013, 77, 70–78. [Google Scholar] [CrossRef]
- Da Porto, C.; Decorti, D.; Natolino, A. Water and ethanol as co-solvent in supercritical fluid extraction of proanthocyanidins from grape marc: A comparison and a proposal. J. Supercrit. Fluids 2014, 87, 1–8. [Google Scholar] [CrossRef]
- Da Porto, C.; Natolino, A.; Decorti, D. The combined extraction of polyphenols from grape marc: Ultrasound assisted extraction followed by supercritical CO2 extraction of ultrasound-raffinate. LWT Food Sci. Technol. 2015, 61, 98–104. [Google Scholar] [CrossRef]
- Ben Mohamed, H.; Duba, K.S.; Fiori, L.; Abdelgawed, H.; Tlili, I.; Tounekti, T.; Zrig, A. Bioactive compounds and antioxidant activities of different grape (Vitis vinifera L.) seed oils extracted by supercritical CO2 and organic solvent. LWT 2016, 74, 557–562. [Google Scholar] [CrossRef]
- Duba, K.; Fiori, L. Supercritical CO2 extraction of grape seeds oil: Scale-up and economic analysis. Int. J. Food Sci. Technol. 2019, 54, 1306–1312. [Google Scholar] [CrossRef]
- Hayrapetyan, G.; Trchounian, K.; Buon, L.; Noret, L.; Pinel, B.; Lagrued, J.; Assifaoui, A. Sequential extraction of high-value added molecules from grape pomaces using supercritical fluids with water as a co-solvent. RSC Sustain. 2023, 1, 2014–2023. [Google Scholar] [CrossRef]
- Salgado-Ramos, M.; Martí-Quijal, F.J.; Huertas-Alonso, A.J.; Sánchez-Verdú, M.P.; Moreno, A.; Barba, F.J. A preliminary multistep combination of pulsed electric fields and supercritical fluid extraction to recover bioactive glycosylated and lipidic compounds from exhausted grape marc. LWT 2023, 180, 114725. [Google Scholar] [CrossRef]
- Ćurko, N.; Lukić, K.; Tušek, A.J.; Balbino, S.; Pavičić, T.V.; Tomašević, M.; Redovniković, I.V.; Ganić, K.K. Effect of cold pressing and supercritical CO2 extraction assisted with pulsed electric fields pretreatment on grape seed oil yield, composition and antioxidant characteristics. LWT 2023, 184, 114974. [Google Scholar] [CrossRef]
- Passos, C.P.; Yilmaz, S.; Silva, C.M.; Coimbra, M.A. Enhancement of grape seed oil extraction using a cell wall degrading enzyme cocktail. Food Chem. 2009, 115, 48–53. [Google Scholar] [CrossRef]
- Branco, R.H.R.; Serafim, L.S.; Xavier, A.M.R.B. Second generation bioethanol production: On the use of pulp and paper industry wastes as feedstock. Fermentation 2019, 5, 4. [Google Scholar] [CrossRef]
- Amândio, M.S.T.; Rocha, J.M.S.; Serafim, L.S.; Xavier, A.M.R.B. Bioethanol from Wastes for Mobility: Europe on the Road to Sustainability. In Clean Fuels for Mobility. Energy, Environment, and Sustainability; Di Blasio, G., Agarwal, A.K., Belgiorno, G., Shukla, P.C., Eds.; Springer: Singapore, 2022; pp. 97–123. [Google Scholar] [CrossRef]
- Branco, R.H.R.; Amândio, M.S.T.; Serafim, L.S.; Xavier, A.M.R.B. Ethanol production from hydrolyzed kraft pulp by mono- and co-cultures of yeasts: The challenge of C6 and C5 sugars consumption. Energies 2020, 13, 744. [Google Scholar] [CrossRef]
- Fernandes, D.L.A.; Pereira, S.R.; Serafim, L.S.; Evtuguin, D.; Xavier, A.M.R.B. Second generation bioethanol from lignocellulosics: Processing of hardwood sulphite spent liquor. In Bioethanol; Lima, M.A.P., Ed.; Intech: Rijeka, Croatia, 2011. [Google Scholar] [CrossRef]
- François, E.; Dumas, C.; Gougeon, R.D.; Alexandre, H.; Vuilleumier, S.; Ernst, B. Unexpected high production of biohydrogen from the endogenous fermentation of grape must deposits. Bioresour. Technol. 2021, 320, 124334. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, Q.; Zhang, Z.; Jing, Y.; Hu, J.; He, C.; Lu, C. A review on biological recycling in agricultural waste-based biohydrogen production: Recent developments. Bioresour. Technol. 2022, 347, 126595. [Google Scholar] [CrossRef]
- Ferdeș, M.; Zăbavă, B.Ú.; Paraschiv, G.; Ionescu, M.; Dincă, M.N.; Moiceanu, G. Food Waste Management for Biogas Production in the Context of Sustainable Development. Energies 2022, 15, 6268. [Google Scholar] [CrossRef]
- Achkar, J.H.; El Lendormi, T.; Hobaika, Z.; Salameh, D.; Louka, N.; Maroun, R.G.; Lanoisellé, J.-L. Anaerobic digestion of grape pomace: Biochemical characterization of the fractions and methane production in batch and continuous digesters. Waste Manag. 2016, 50, 275–282. [Google Scholar] [CrossRef]
- Olczak, M.; Piebalgs, A. Energy Security Meets the Circular Economy: A Stronger Case for Sustainable Biomethane Production in the EU; European University Institute, Publications Office of the European Union: Fiesole, Italy, 2022. [Google Scholar] [CrossRef]
- BIP Europe. Insights into the Current Cost of Biomethane Production from Real Industry Data; Executive Summary Task Force 4. 2023. Available online: https://www.oiv.int/what-we-do/statistics (accessed on 7 November 2023).
- Xavier, A.M.R.B.; Correia, M.F.; Pereira, S.R.; Evtuguin, D.V. Second-generation bioethanol from eucalypt sulphite spent liquor. Bioresour. Technol. 2010, 101, 2755–2761. [Google Scholar] [CrossRef] [PubMed]
- Amândio, M.S.T.; Rocha, J.M.S.; Serafim, L.S.; Xavier, A.M.R.B. Cellulosic bioethanol from industrial Eucalyptus globulus bark residues using kraft pulping as a pretreatment. Energies 2021, 14, 2185. [Google Scholar] [CrossRef]
- Rodríguez, L.A.; Toro, M.E.; Vazquez, F.; Correa-Daneri, M.L.; Gouiric, S.C.; Vallejo, M.D. Bioethanol production from grape and sugar beet pomaces by solid-state fermentation. Int. J. Hydrogen Energy 2010, 35, 5914–5917. [Google Scholar] [CrossRef]
- Corbin, K.R.; Hsieh, Y.S.Y.; Betts, N.S.; Byrt, C.S.; Henderson, M.; Stork, J.; DeBolt, S.; Fincher, G.B.; Burton, R.A. Grape marc as a source of carbohydrates for bioethanol: Chemical composition, pre-treatment and saccharification. Bioresour Technol. 2015, 193, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Egüés, I.; Serrano, L.; Amendola, D.; De Faveri, D.M.; Spigno, G.; Labidi, J. Fermentable sugars recovery from grape stalks for bioethanol production. Renew. Energ. 2013, 60, 553–558. [Google Scholar] [CrossRef]
- Senila, L.; Kovacs, E.; Scurtu, D.A.; Cadar, O.; Becze, A.; Senila, M.; Levei, E.A.; Dumitras, D.E.; Tenu, I.; Roman, C. Bioethanol production from vineyard waste by autohydrolysis pretreatment and chlorite delignification via simultaneous saccharification and fermentation. Molecules 2020, 25, 2606. [Google Scholar] [CrossRef] [PubMed]
- Jesus, M.S.; Romaní, A.; Genisheva, Z.; Teixeira, J.A.; Domingues, L. Integral valorization of vine pruning residue by sequential autohydrolysis stages. J. Clean. Prod. 2017, 168, 74–86. [Google Scholar] [CrossRef]
- Prozil, S.O.; Evtuguin, D.V.; Lopes, S.M.; Lopes, L.P.C.; Arshanitsa, A.S.; Solodovnik, V.P.; Telysheva, G.M. Evaluation of grape stalks as a feedstock for pellets production. In Proceedings of the European Worpshop on Lignocellulosics and Pulp, Seville, Spain, 24–27 June 2014; pp. 683–686. [Google Scholar]
- Ferreira, J.; Esteves, B.; Cruz-Lopes, L.; Evtuguin, D.V.; Domingos, I. Environmental advantages to produce energy from grape stalk pellets instead wood pellets and other sources. Int. J. Environ. Stud. 2018, 75, 812–826. [Google Scholar] [CrossRef]
- Senila, L.; Tenu, I.; Carlescu, P.; Corduneanu, O.R.; Dumitrachi, E.P.; Kovacs, E.; Scurtu, D.A.; Cadar, O.; Becze, A.; Senila, M.; et al. Sustainable biomass pellets production using vineyard wastes. Agriculture 2020, 10, 501. [Google Scholar] [CrossRef]
- Blanco, F.G.; Hernández, N.; Rivero-Buceta, V.; Maestro, B.; Sanz, J.M.; Mato, A.; Hernández-Arriaga, A.M.; Prieto, M.A. From residues to added-value bacterial biopolymers as nanomaterials for biomedical applications. Nanomaterials 2021, 11, 1492. [Google Scholar] [CrossRef]
- Steinbüchel, A.; Hein, S. Biochemical and molecular basis of microbial synthesis of polyhydroxyalkanoates in microorganisms. Adv. Biochem. Eng. Biotechnol. 2001, 71, 81–123. [Google Scholar] [CrossRef] [PubMed]
- Volova, T.G. Polyhydroxyalkanoates—Plastic Materials of the 21st Century: Production, Properties, Applications; Nova Science Publishers, Inc.: New York, NY, USA, 1994. [Google Scholar]
- Serafim, L.S.; Queirós, D.; Rossetti, S.; Lemos, P.C. Biopolymer production by mixed-microbial cultures: Integrating remediation with valorization. In Recent Advances in Biotechnology—Volume 1—Microbial Polyester Production, Performance and Processing, Microbiology, Feedstocks, and Metabolism, 1st ed.; Bentham Science Publishers: Sharjah, India, 2016; pp. 226–264. [Google Scholar] [CrossRef][Green Version]
- Laycock, B.; Halley, P.; Pratt, S.; Werker, A.; Lant, P. The chemomechanical properties of microbial polyhydroxyalkanoates. Prog. Polym. Sci. 2013, 38, 536–583. [Google Scholar] [CrossRef]
- Bugnicourt, E.; Cinelli, P.; Lazzeri, P.; Alvarez, V. Polyhydroxyalkanoate (PHA): Review of synthesis, characteristics, processing and potential applications in packaging. Express Polym. Lett. 2014, 8, 791–808. [Google Scholar] [CrossRef]
- Serafim, L.S.; Xavier, A.M.R.B.; Lemos, P.C. Storage of Hydrophobic Polymers in Bacteria. In Biogenesis of Fatty Acids, Lipids and Membranes: Handbook of Hydrocarbon and Lipid Microbiology; Geiger, O., Ed.; Springer: Berlin/Heidelberg, Germany, 2018; ISBN 978-3-319-43676-0. [Google Scholar] [CrossRef]
- Serafim, L.S.; Pereira, J.; Lemos, P.C. Polyhydroxyalkanoates by mixed microbial cultures: The journey so far and challenges ahead. In The Handbook of Polyhydroxyalkanoates; Koller, M., Ed.; CRC Press: Boca Raton, FL, USA, 2020; pp. 273–308. [Google Scholar]
- Martinez, G.A.; Rebecchi, S.; Decorti, D.; Domingos, J.M.; Natolino, A.; Del Rio, D.; Bertin, L.; Da Porto, C.; Fava, F. Towards multi-purpose biorefinery platforms for the valorisation of red grape pomace: Production of polyphenols, volatile fatty acids, polyhydroxyalkanoates and biogas. Green Chem. 2016, 18, 261–270. [Google Scholar] [CrossRef]
- Kovalcik, A.; Pernicova, I.; Obruca, S.; Szotkowski, M.; Enev, V.; Kalina, M.; Marova, I. Grape winery waste as a promising feedstock for the production of polyhydroxyalkanoates and other value-added products. Food Bioprod. Process. 2020, 124, 1–10. [Google Scholar] [CrossRef]
- Follonier, S.; Goyder, M.S.; Silvestri, A.-C.; Crelier, S.; Kalman, F.; Riesen, R.; Zinn, M. Fruit pomace and waste frying oil as sustainable resources for the bioproduction of medium-chain-length polyhydroxyalkanoates. Int. J. Biol. Macromol. 2014, 71, 42–52. [Google Scholar] [CrossRef]
- Follonier, S.; Riesen, R.; Zinn, M. Pilot-scale production of functionalized mcl-PHA from grape pomace supplemented with fatty acids. Chem. Biochem. Eng. Q. 2015, 29, 113–121. [Google Scholar] [CrossRef]
- Kourilova, X.; Pernicova, I.; Vidlakova, M.; Krejcirik, R.; Mrazova, K.; Hrubanova, K.; Krzyzanek, V.; Nebesarova, J.; Obruca, S. Biotechnological conversion of grape pomace to poly(3-hydroxybutyrate) by moderately thermophilic bacterium Tepidimonas taiwanensis. Bioengineering 2021, 8, 141. [Google Scholar] [CrossRef]
- Martínez-Avila, O.; Llimós, J.; Ponsá, S. Integrated solid-state enzymatic hydrolysis and solid-state fermentation for producing sustainable polyhydroxyalkanoates from low-cost agro-industrial residues. Food Bioprod. Process. 2021, 126, 334–344. [Google Scholar] [CrossRef]
- Montiel-Corona, V.; Buitrón, G. Polyhydroxyalkanoates and 5-aminolevulinic acid production by a mixed phototrophic culture using medium-chain carboxylic acids from winery effluents. Biores. Technol. 2023, 373, 128704. [Google Scholar] [CrossRef]
- Cerrutti, P.; Roldán, P.; García, R.M.; Galvagno, M.A.; Vázquez, A.; Foresti, M.L. Production of bacterial nanocellulose from wine industry residues: Importance of fermentation time on pellicle characteristics. J. Appl. Polym. Sci. 2016, 133, 43109. [Google Scholar] [CrossRef]
- Revin, V.; Liyaskina, E.; Nazarkina, M.; Bogatyreva, A.; Shchankin, M. Cost-effective production of bacterial cellulose using acidic food industry by-products. Braz. J. Microbiol. 2018, 49, 151–159. [Google Scholar] [CrossRef] [PubMed]
- El-Gendi, H.; Taha, T.H.; Ray, J.B.; Saleh, A.K. Recent advances in bacterial cellulose: A low-cost effective production media, optimization strategies and applications. Cellulose 2022, 29, 7495–7533. [Google Scholar] [CrossRef]
- Carreira, P.; Mendes, J.A.S.; Trovatti, E.; Serafim, L.S.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P. Utilization of residues from agro-forest industries in the production of high value bacterial cellulose. Biores. Technol. 2011, 102, 7354–7360. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, A.; Foresti, M.L.; Cerrutti, P.; Galvagno, M. Bacterial Cellulose from Simple and Low Cost Production Media by Gluconacetobacter xylinus. J. Polym. Environ. 2013, 21, 545–554. [Google Scholar] [CrossRef]
- Ogrizek, L.; Lamovšek, J.; Ćuš, F.; Leskovšek, M.; Gorjanc, M. Properties of bacterial cellulose produced using white and red grape bagasse as a nutrient source. Processes 2021, 9, 1088. [Google Scholar] [CrossRef]
- Filippi, K.; Papapostolou, H.; Alexandri, M.; Vlysidis, A.; Myrtsi, E.D.; Ladakis, D.; Pateraki, C.; Haroutounian, S.A.; Koutinas, A. Integrated biorefinery development using winery waste streams for the production of bacterial cellulose, succinic acid and value-added fractions. Biores. Technol. 2021, 343, 125989. [Google Scholar] [CrossRef] [PubMed]
- Trovatti, E.; Serafim, L.S.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.N. Gluconacetobacter sacchari: An efficient bacterial cellulose cell-factory. Carbohydr. Polym. 2011, 86, 1417–1420. [Google Scholar] [CrossRef]
- de Amorim, J.D.P.; da Silva Junior, C.J.G.; de Medeiros, A.D.M.; do Nascimento, H.A.; Sarubbo, M.; de Medeiros, T.P.M.; Costa, A.F.d.S.; Sarubbo, L.A. Bacterial cellulose as a versatile biomaterial for wound dressing application. Molecules 2022, 27, 5580. [Google Scholar] [CrossRef]
- Navrátilová, M.; Beranová, M.; Severová, L.; Šrédl, K.; Svoboda, R.; Abrhám, J. The Impact of Climate Change on the Sugar Content of Grapes and the Sustainability of their Production in the Czech Republic. Sustainability 2021, 13, 222. [Google Scholar] [CrossRef]
- Ramos, M.; Martínez de Toda, F. Influence of weather conditions and projected climate change scenarios on the suitability of Vitis vinifera cv. Carignan in Rioja DOCa, Spain. Int. J. Biometeorol. 2022, 66, 1067–1078. [Google Scholar] [CrossRef]
- Casolani, N.; D’Eusanio, M.; Liberatore, L.; Raggi, A.; Petti, L. Life cycle assessment in the wine sector: A review on inventory phase. J. Clean. Prod. 2022, 379, 134404. [Google Scholar] [CrossRef]
- Pinto da Silva, L.; Esteves da Silva, J.C.G. Evaluation of the carbon footprint of the life cycle of wine production: A review. Clean. Cric. Bioecon. 2022, 2, 100021. [Google Scholar] [CrossRef]
- Ncube, A.; Fiorentino, G.; Colella, M.; Ulgiati, S. Upgrading wineries to biorefineries within a circular economy perspective: An Italian case study. Sci. Total Environ. 2021, 775, 145809. [Google Scholar] [CrossRef] [PubMed]
- Lucarini, M.; Durazzo, A.; Romani, A.; Campo, M.; Lombardi-Boccia, G.; Cecchini, F. Bio-based compounds from grape seeds: A biorefinery approach. Molecules 2018, 23, 1888. [Google Scholar] [CrossRef]
- Donia, E.; Marcello Mineo, A.; Sgroi, F. A methodological approach for assessing business investments in renewable resources from a circular economy perspective. Land Use Policy 2018, 76, 823–827. [Google Scholar] [CrossRef]
- Tsegaye, B.; Jaiswal, S.; Jaiswal, A.K. Food waste biorefinery: Pathway towards circular bioeconomy. Foods 2021, 10, 1174. [Google Scholar] [CrossRef]


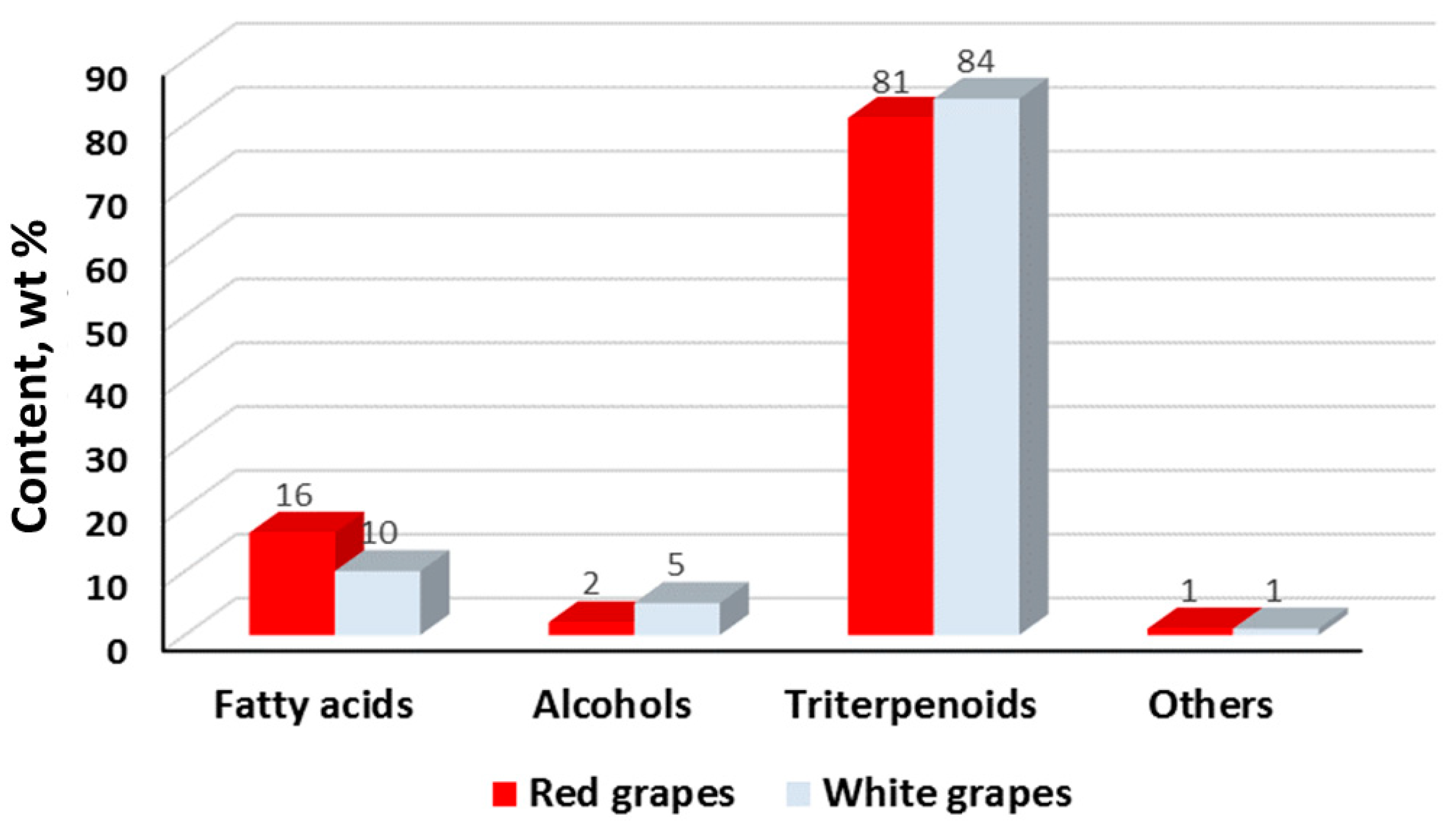

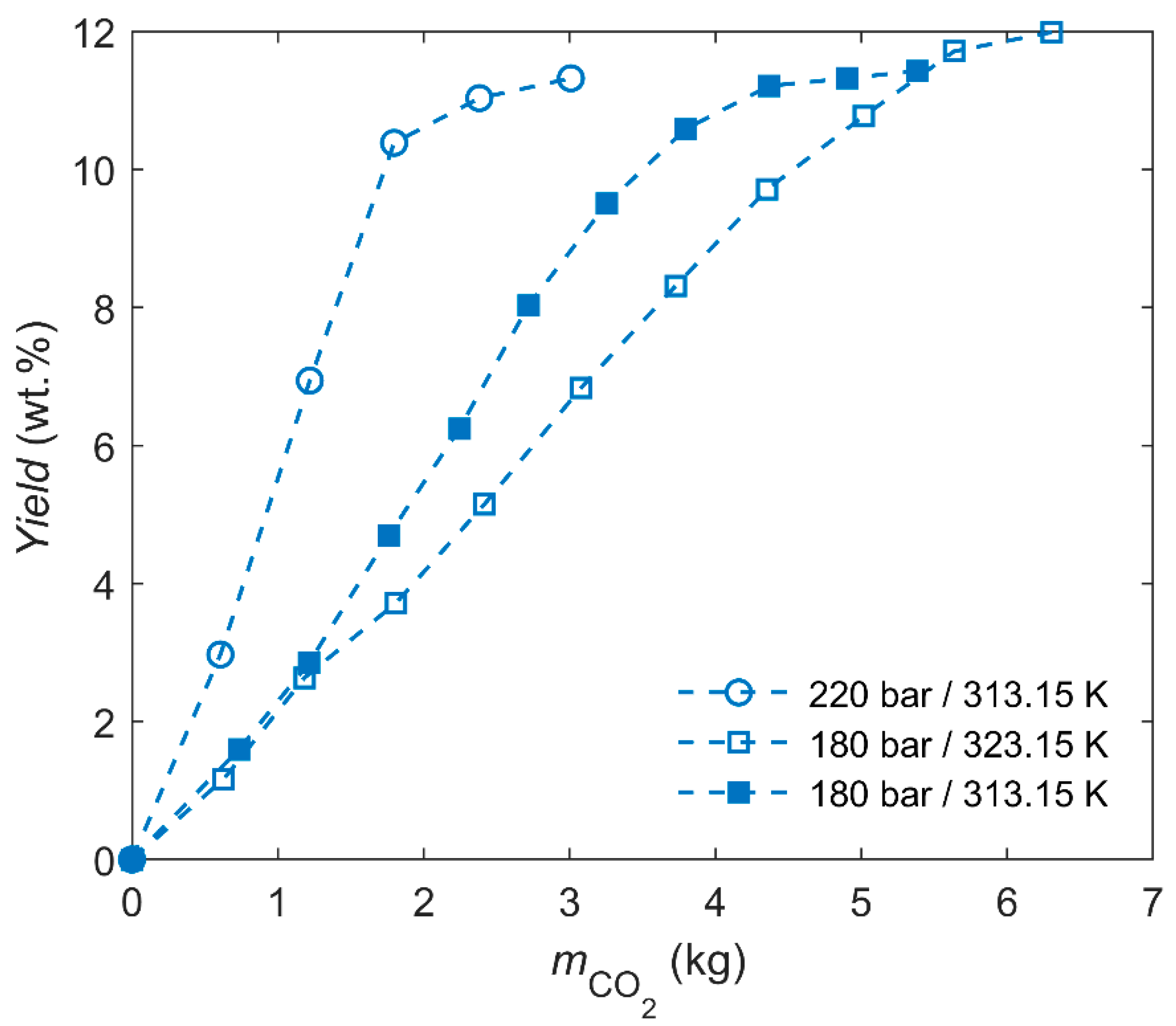
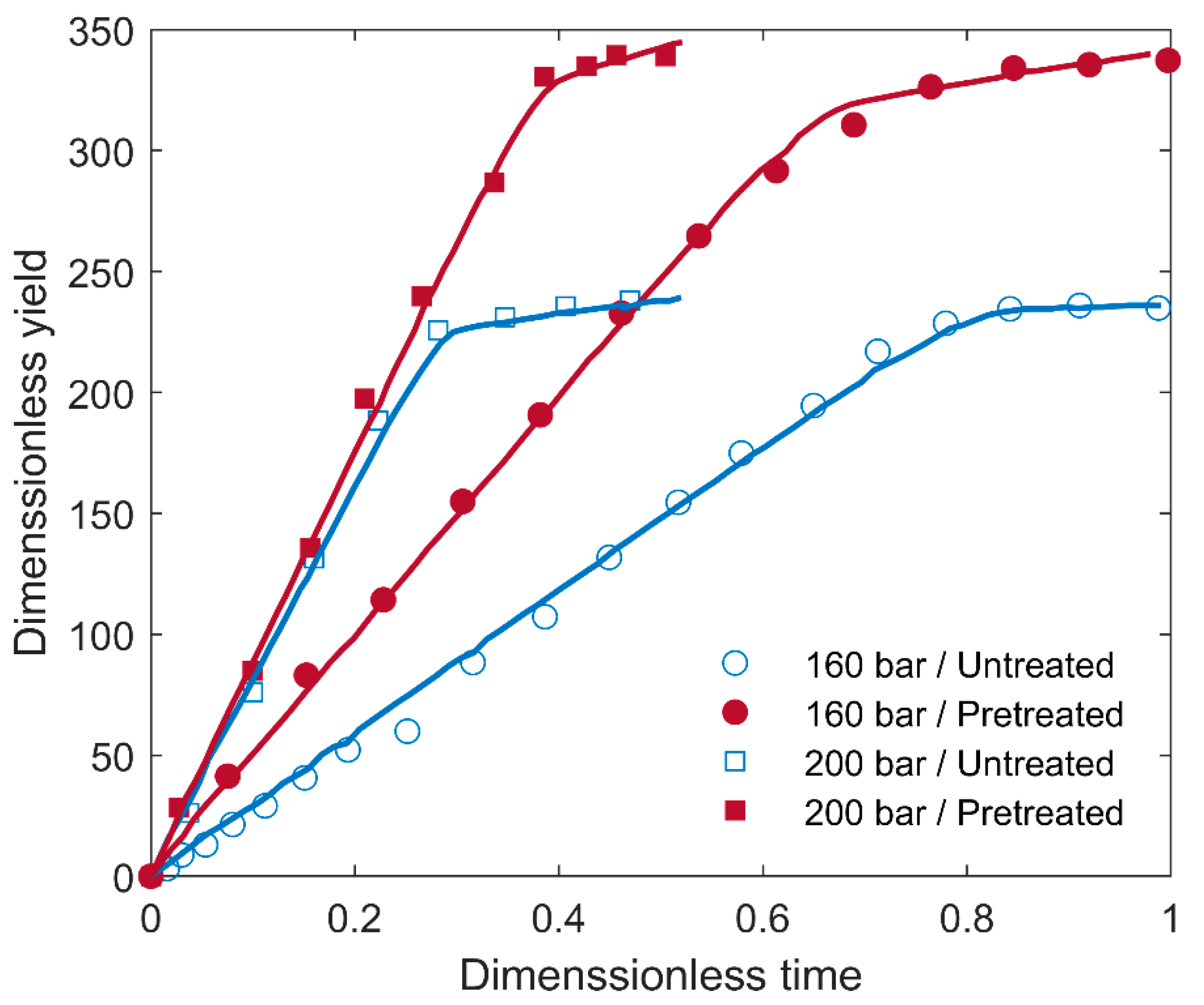
| Composition | Content (wt.%) | |
|---|---|---|
| Red Grape Stalks [23] | White Grape Stalks * | |
| Ash | 7.0 | 5.2 |
| Cellulose | 30.3 | 33.1 |
| Proteins | 6.1 | 10.8 |
| Tannins | 15.9 | 8.6 |
| Lignin (Klason) | 17.4 | 17.0 |
| Hemicelluloses | 21.0 | 22.4 |
| Extractives obtained with | ||
| Acetone | 2.3 | 2.9 |
| Dichloromethane | 1.0 | 1.9 |
| Hot water | 23.7 | - |
| Composition | Abundance (wt.%) | |
|---|---|---|
| Red Grape Skins | White Grape Skins | |
| Ash | 7.8 | 18.3 |
| Cellulose | 20.8 | 18.5 |
| Proteins | 18.8 | 6.7 |
| Tannins | 13.8 | 3.4 |
| Hemicelluloses | 12.5 | 9.0 |
| Extractives obtained with | ||
| Hexane | - | 1.3 |
| Dichloromethane | 2.9 | 2.4 |
| Hot water | 24.6 | 48.0 |
| Composition | Content (wt.%) |
|---|---|
| Ash | 2–4 |
| Cellulose | 10–30 |
| Lignin | 10–20 |
| Proteins | 4–9 |
| Tannins | 4–6 |
| Hemicelluloses | 3–6 |
| Extractives obtained with | |
| Hexane | 8–20 |
| Hot water | 5–10 |
| Properties/Characteristics | Fibreboards 1 | Particleboards 2 |
|---|---|---|
| Grape stalk:pine (wt.%) | 20:80 | 40:60 |
| Bulk density (kg/m3) | 753 | 710 |
| Thickness (mm) | 10 | 10 |
| Urea–formaldehyde resin (wt.%) | 8.0 | 10.0 |
| Paraffin (wt.%) | 1.5 | - |
| Bending strength, MOR (MPa) | 34.0 | 55.4 |
| Elongation (%) | 8.5 | 3.3 |
| Internal bond (MPa) | 0.54 | 0.59 |
| Water resistance, ΔW (%) | 31.0 | 25.2 |
| Formaldehyde (mg/100 g) | 2.5 | 4.0 |
| Year | Grape Part | Extraction Fluid | Target Compounds | Main Features | Ref. |
|---|---|---|---|---|---|
| 2000 | Seed | CO2 with ethanol or methanol | Phenolics | Solubility study | [97] |
| 2001 | Pomace (skin) | CO2 with ethanol | Resveratrol | Comparison of grape varieties | [88] |
| 2003 | Seed | CO2 with ethanol | Oil | DoE and scale-up study | [95] |
| 2005 | Pomace (skin) | CO2 with ethanol | Catechin, epicatechim, quercetin, rutin | Effect of pressure and cosolvent | [98] |
| 2007 | Pomace | CO2 with ethanol | Phenolics | Comparison of SFE with SLE | [99] |
| 2007 | Seed | CO2 | α-tocopherol | Particle size study | [89] |
| 2009 | Seed | CO2 | Oil | Effect of enzymatic pretreatment on SFE | [100] |
| 2010 | Pomace | CO2 with methanol | Phenolic anthocyanins | Supercritical antisolvent extraction | [101] |
| 2010 | Pomace | CO2 with ethanol | Resveratrol | Comparison of SFE with SLE | [92] |
| 2010 | Seed | CO2 | Oil (triacylglycerides) | Effect of SFE temperature and pressure | [90] |
| 2010 | Seed | CO2 | Oil | Modelling and economic study | [91] |
| 2011 | Seed | CO2 | Oil | Modelling of extraction curves | [102] |
| 2012 | Seed | CO2 | Oil | Scale-up study and economic evaluation | [96] |
| 2013 | Pomace | CO2 with ethanol | Polyphenol | Effect of pressure and economic study | [103] |
| 2014 | Pomace | CO2 with ethanol and/or water | Phenolics (proanthocyanidins) | Sequential extractions with CO2/water and CO2/ethanol | [104] |
| 2015 | Pomace | CO2 | Polyphenols | UAE combined with SFE | [105] |
| 2015 | Seed | CO2 | Oil | Optimization using DoE | [93] |
| 2016 | Seed | CO2 | Oil | Comparison of grape cultivars oil content | [106] |
| 2017 | Pomace | CO2 | Phenolics | Techno-economic comparison of subcritical water extraction, SFE with CO2, and SLE | [94] |
| 2019 | Pomace | CO2 | Oil | Scale-up study and economic evaluation | [107] |
| 2020 | Vine leaves | CO2 with ethanol or ethyl acetate | LCAA, triterpenes, sitosterol, tocopherol | Effect of cosolvent, temperature and biomass particle size | [79] |
| 2023 | Pomace | CO2 with water | Phenolics, polysaccharides | Water as cosolvent | [108] |
| 2023 | Pomace | CO2 | Glycosylated and lipidic compounds | Combined pulsed electric field and SFE process | [109] |
| 2023 | Pomace | CO2 | Phenolics, sterols, fatty acids | Combined pulsed electric field and SFE process, comparison with cold pressing | [110] |
| Parameter | Softwood Pellets | Grape Stalk Pellets |
|---|---|---|
| Water content, wt.% | 8.10 | 12.6 |
| High heating value, MJ/kg | 18.2 | 16.7 |
| Low heating value, MJ/kg | 16.6 | 15.3 |
| Length, mm | 16.7 ± 2.5 | 17.4 ± 1.2 |
| Diameter, mm | 6.06 ± 0.04 | 5.89 ± 0.07 |
| Bulk density, kg/m3 | 660 ± 10 | 670 ± 2 |
| Particle density, kg/m3 | 1098 ± 47 | 1129 ± 47 |
| Energy density, MWh/m3 | 3.05 | 2.85 |
| Durability, % | 95.6 | 95.8 |
| Specific energy consumption for pelletising, kWh/kg | 0.137 | 0.110 |
| Type of Grapes | Pretreatment | Bacteria | Process Configuration | BC (g/L) | Ref. |
|---|---|---|---|---|---|
| White | Water extraction, at 100 °C | G. sacchari | Static incubation in Erlenmeyer | 0.63 | [150] |
| White | Water extraction, at room temperature | K. xylinus DSM 6513 | Static incubation in Erlenmeyer | 8.0 | [151] |
| White | Acidic extraction, at pH = 5 and at room temperature | K. xylinus DSM 6513 | Static incubation in Erlenmeyer | 6.56 | [147] |
| White | Water extraction, at 70 °C | K. xylinus DSM 6513 | Static incubation in plates | 0.67 | [152] |
| Red | Water extraction, at 70 °C | K. xylinus DSM 6513 | Static incubation in plates | 0.28 | [152] |
| Red and white | Water extraction, at 40 °C | K. sucrofermentans DSM 15973 | Static tray reactors | 9.0 | [153] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evtuguin, D.; Aniceto, J.P.S.; Marques, R.; Portugal, I.; Silva, C.M.; Serafim, L.S.; Xavier, A.M.R.B. Obtaining Value from Wine Wastes: Paving the Way for Sustainable Development. Fermentation 2024, 10, 24. https://doi.org/10.3390/fermentation10010024
Evtuguin D, Aniceto JPS, Marques R, Portugal I, Silva CM, Serafim LS, Xavier AMRB. Obtaining Value from Wine Wastes: Paving the Way for Sustainable Development. Fermentation. 2024; 10(1):24. https://doi.org/10.3390/fermentation10010024
Chicago/Turabian StyleEvtuguin, Dmitry, José P. S. Aniceto, Rita Marques, Inês Portugal, Carlos M. Silva, Luísa S. Serafim, and Ana M. R. B. Xavier. 2024. "Obtaining Value from Wine Wastes: Paving the Way for Sustainable Development" Fermentation 10, no. 1: 24. https://doi.org/10.3390/fermentation10010024
APA StyleEvtuguin, D., Aniceto, J. P. S., Marques, R., Portugal, I., Silva, C. M., Serafim, L. S., & Xavier, A. M. R. B. (2024). Obtaining Value from Wine Wastes: Paving the Way for Sustainable Development. Fermentation, 10(1), 24. https://doi.org/10.3390/fermentation10010024











