Use of Non-Saccharomyces Yeast to Enhance the Acidity of Wines Produced in a Warm Climate Region: Effect on Wine Composition
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Determination of Enological Parameters in Must and Wines and Fermentation Kinetics
2.3. Volatile Compounds Determination
2.4. Organoleptic Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Fermentation Kinetics
3.2. Oenological Parameters
3.3. Major Volatile Aroma Compounds and Polyols
3.4. Multiple Regression Analysis for Lactic Acid
3.5. Cluster and Principal Component Analysis
3.5.1. Cluster Analysis
3.5.2. Principal Component Analysis
3.6. Organoleptic Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giorgi, F.; Lionello, P. Climate Change Projections for the Mediterranean Region. Glob. Planet. Chang. 2008, 63, 90–104. [Google Scholar] [CrossRef]
- Ficklin, D.L.; Novick, K.A. Historic and Projected Changes in Vapor Pressure Deficit Suggest a Continental-scale Drying of the United States Atmosphere. J. Geophys. Res. Atmos. 2017, 122, 2061–2079. [Google Scholar] [CrossRef]
- Dusenge, M.E.; Duarte, A.G.; Way, D.A. Plant Carbon Metabolism and Climate Change: Elevated CO2 and Temperature Impacts on Photosynthesis, Photorespiration and Respiration. New Phytol. 2019, 221, 32–49. [Google Scholar] [CrossRef] [PubMed]
- van Leeuwen, C.; Destrac-Irvine, A.; Dubernet, M.; Duchêne, E.; Gowdy, M.; Marguerit, E.; Pieri, P.; Parker, A.; de Rességuier, L.; Ollat, N. An Update on the Impact of Climate Change in Viticulture and Potential Adaptations. Agronomy 2019, 9, 514. [Google Scholar] [CrossRef]
- Van Leeuwen, C.; Destrac-Irvine, A. Modified Grape Composition under Climate Change Conditions Requires Adaptations in the Vineyard. OENO One 2017, 51, 147–154. [Google Scholar] [CrossRef]
- Winefield, C.; Lee, T.-M.; Gambetta, J.M.; Friedel, M.; Holzapfel, B.P.; Stoll, M. Sunburn Grapes: A Review. Front. Plant Sci. 2021, 11, 604691. [Google Scholar] [CrossRef]
- Cataldo, E.; Eichmeier, A.; Mattii, G.B. Effects of Global Warming on Grapevine Berries Phenolic Compounds—A Review. Agronomy 2023, 13, 2192. [Google Scholar] [CrossRef]
- Kliewer, W.M.; Torres, R.E. Effect of Controlled Day and Night Temperatures on Grape Coloration. Am. J. Enol. Vitic. 1972, 23, 71–77. [Google Scholar] [CrossRef]
- Reynolds, A.G.; Vanden Heuvel, J.E. Influence of Grapevine Training Systems on Vine Growth and Fruit Composition: A Review. Am. J. Enol. Vitic. 2009, 60, 251–268. [Google Scholar] [CrossRef]
- Gatti, M.; Frioni, T.; Garavani, A.; Biagioni, A.; Poni, S. Impact of Delayed Winter Pruning on Phenology and Ripening Kinetics of Pinot Noir Grapevines. BIO Web Conf. 2019, 13, 04002. [Google Scholar] [CrossRef]
- Zheng, W.; García, J.; Balda, P.; Martínez de Toda, F. Effects of Late Winter Pruning at Different Phenological Stages on Vine Yield Components and Berry Composition in La Rioja, North-Central Spain. OENO One 2017, 51, 363. [Google Scholar] [CrossRef]
- de Toda, F.; Sancha, J.; Zheng, W.; Balda, P. Leaf Area Reduction by Trimming, a Growing Technique to Restore the Anthocyanins: Sugars Ratio Decoupled by the Warming Climate. Vitis 2014, 53, 189–192. [Google Scholar]
- de Toda, F.; Balda, P. Delaying Berry Ripening through Manipulating Leaf Area to Fruit Ratio. Vitis J. Grapevine Res. 2013, 52, 171–176. [Google Scholar]
- Intrigliolo, D.S.; Lizama, V.; García-Esparza, M.J.; Abrisqueta, I.; Álvarez, I. Effects of Post-Veraison Irrigation Regime on Cabernet Sauvignon Grapevines in Valencia, Spain: Yield and Grape Composition. Agric. Water Manag. 2016, 170, 110–119. [Google Scholar] [CrossRef]
- Dinis, L.T.; Malheiro, A.C.; Luzio, A.; Fraga, H.; Ferreira, H.; Gonçalves, I.; Pinto, G.; Correia, C.M.; Moutinho-Pereira, J. Improvement of Grapevine Physiology and Yield under Summer Stress by Kaolin-Foliar Application: Water Relations, Photosynthesis and Oxidative Damage. Photosynthetica 2018, 56, 641–651. [Google Scholar] [CrossRef]
- Frioni, T.; Tombesi, S.; Luciani, E.; Sabbatini, P.; Berrios, J.G.; Palliotti, A. Kaolin Treatments on Pinot Noir Grapevines for the Control of Heat Stress Damages. BIO Web Conf. 2019, 13, 04004. [Google Scholar] [CrossRef]
- Coniberti, A.; Ferrari, V.; Dellacassa, E.; Boido, E.; Carrau, F.; Gepp, V.; Disegna, E. Kaolin over Sun-Exposed Fruit Affects Berry Temperature, Must Composition and Wine Sensory Attributes of Sauvignon Blanc. Eur. J. Agron. 2013, 50, 75–81. [Google Scholar] [CrossRef]
- van Leeuwen, C.; Roby, J.-P.; Alonso-Villaverde, V.; Gindro, K. Impact of Clonal Variability in Vitis vinifera Cabernet Franc on Grape Composition, Wine Quality, Leaf Blade Stilbene Content, and Downy Mildew Resistance. J. Agric. Food Chem. 2013, 61, 19–24. [Google Scholar] [CrossRef]
- Salgado, C.M.; Fernández-Fernández, E.; Palacio, L.; Carmona, F.J.; Hernández, A.; Prádanos, P. Application of Pervaporation and Nanofiltration Membrane Processes for the Elaboration of Full Flavored Low Alcohol White Wines. Food Bioprod. Process. 2017, 101, 11–21. [Google Scholar] [CrossRef]
- Biyela, B.N.E.; du Toit, W.J.; Divol, B.; Malherbe, D.F.; van Rensburg, P. The production of reduced-alcohol wines using Gluzyme Mono® 10.000 BG-treated grape juice. South. Afr. J. Enol. Vitic. 2016, 30, 124–132. [Google Scholar] [CrossRef][Green Version]
- Liguori, L.; Albanese, D.; Crescitelli, A.; Di Matteo, M.; Russo, P. Impact of Dealcoholization on Quality Properties in White Wine at Various Alcohol Content Levels. J. Food Sci. Technol. 2019, 56, 3707–3720. [Google Scholar] [CrossRef] [PubMed]
- Contreras, A.; Hidalgo, C.; Henschke, P.A.; Chambers, P.J.; Curtin, C.; Varela, C. Evaluation of Non-Saccharomyces Yeasts for the Reduction of Alcohol Content in Wine. Appl. Environ. Microbiol. 2014, 80, 1670–1678. [Google Scholar] [CrossRef] [PubMed]
- Peinado, R.A.; Moreno, J.; Bueno, J.E.; Moreno, J.A.; Mauricio, J.C. Comparative Study of Aromatic Compounds in Two Young White Wines Subjected to Pre-Fermentative Cryomaceration. Food Chem. 2004, 84, 585–590. [Google Scholar] [CrossRef]
- Benito, S. The Impact of Torulaspora delbrueckii Yeast in Winemaking. Appl. Microbiol. Biotechnol. 2018, 102, 3081–3094. [Google Scholar] [CrossRef] [PubMed]
- Bely, M.; Stoeckle, P.; Masneuf-Pomarède, I.; Dubourdieu, D. Impact of Mixed Torulaspora delbrueckii–Saccharomyces cerevisiae Culture on High-Sugar Fermentation. Int. J. Food Microbiol. 2008, 122, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Belda, I.; Navascués, E.; Marquina, D.; Santos, A.; Calderon, F.; Benito, S. Dynamic Analysis of Physiological Properties of Torulaspora delbrueckii in Wine Fermentations and Its Incidence on Wine Quality. Appl. Microbiol. Biotechnol. 2015, 99, 1911–1922. [Google Scholar] [CrossRef] [PubMed]
- Azzolini, M.; Tosi, E.; Lorenzini, M.; Finato, F.; Zapparoli, G. Contribution to the Aroma of White Wines by Controlled Torulaspora delbrueckii Cultures in Association with Saccharomyces cerevisiae. World J. Microbiol. Biotechnol. 2015, 31, 277–293. [Google Scholar] [CrossRef]
- Belda, I.; Ruiz, J.; Beisert, B.; Navascués, E.; Marquina, D.; Calderón, F.; Rauhut, D.; Benito, S.; Santos, A. Influence of Torulaspora delbrueckii in Varietal Thiol (3-SH and 4-MSP) Release in Wine Sequential Fermentations. Int. J. Food Microbiol. 2017, 257, 183–191. [Google Scholar] [CrossRef]
- Renault, P.; Coulon, J.; Moine, V.; Thibon, C.; Bely, M. Enhanced 3-Sulfanylhexan-1-Ol Production in Sequential Mixed Fermentation with Torulaspora delbrueckii/Saccharomyces cerevisiae Reveals a Situation of Synergistic Interaction between Two Industrial Strains. Front. Microbiol. 2016, 7, 293. [Google Scholar] [CrossRef]
- Ruiz, J.; Belda, I.; Beisert, B.; Navascués, E.; Marquina, D.; Calderón, F.; Rauhut, D.; Santos, A.; Benito, S. Analytical Impact of Metschnikowia pulcherrima in the Volatile Profile of Verdejo White Wines. Appl. Microbiol. Biotechnol. 2018, 102, 8501–8509. [Google Scholar] [CrossRef]
- Morata, A.; Bañuelos, M.A.; Vaquero, C.; Loira, I.; Cuerda, R.; Palomero, F.; González, C.; Suárez-Lepe, J.A.; Wang, J.; Han, S.; et al. Lachancea thermotolerans as a Tool to Improve PH in Red Wines from Warm Regions. Eur. Food Res. Technol. 2019, 245, 885–894. [Google Scholar] [CrossRef]
- Hranilovic, A.; Gambetta, J.M.; Schmidtke, L.; Boss, P.K.; Grbin, P.R.; Masneuf-Pomarede, I.; Bely, M.; Albertin, W.; Jiranek, V. Oenological Traits of Lachancea thermotolerans Show Signs of Domestication and Allopatric Differentiation. Sci. Rep. 2018, 8, 14812. [Google Scholar] [CrossRef] [PubMed]
- Benito, S. The Impacts of Lachancea thermotolerans Yeast Strains on Winemaking. Appl. Microbiol. Biotechnol. 2018, 102, 6775–6790. [Google Scholar] [CrossRef] [PubMed]
- Balikci, E.K.; Tanguler, H.; Jolly, N.P.; Erten, H. Influence of Lachancea thermotolerans on Cv. Emir. Wine Fermentation. Yeast 2016, 33, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Benito, Á.; Calderón, F.; Palomero, F.; Benito, S. Quality and Composition of Airen Wines Fermented by Sequential Inoculation of Lachancea thermotolerans and Saccharomyces cerevisiae. Food Technol. Biotechnol. 2016, 54, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Vicente, J.; Kelanne, N.; Navascués, E.; Calderón, F.; Santos, A.; Marquina, D.; Yang, B.; Benito, S. Combined Use of Schizosaccharomyces pombe and a Lachancea thermotolerans Strain with a High Malic Acid Consumption Ability for Wine Production. Fermentation 2023, 9, 165. [Google Scholar] [CrossRef]
- Benito, Á.; Calderón, F.; Palomero, F.; Benito, S. Combine Use of Selected Schizosaccharomyces Pombe and Lachancea thermotolerans Yeast Strains as an Alternative to the Traditional Malolactic Fermentation in Red Wine Production. Molecules 2015, 20, 9510–9523. [Google Scholar] [CrossRef]
- Gobbi, M.; Comitini, F.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Lachancea thermotolerans and Saccharomyces cerevisiae in Simultaneous and Sequential Co-Fermentation: A Strategy to Enhance Acidity and Improve the Overall Quality of Wine. Food Microbiol. 2013, 33, 271–281. [Google Scholar] [CrossRef]
- International Organisation of Vine and Wine. Compendium of International Methods of Wine and Must Analysis; International Organisation of Vine and Wine: Dijon, France, 2021; ISBN 9782850380686. [Google Scholar]
- AENOR. AENOR. Análisis Sensorial. Tomo I. Alimentación; AENOR: Madrid, Spain, 1997. [Google Scholar]
- Llauradó, J.M.; Rozès, N.; Constantí, M.; Mas, A. Study of Some Saccharomyces cerevisiae Strains for Winemaking after Preadaptation at Low Temperatures. J. Agric. Food Chem. 2005, 53, 1003–1011. [Google Scholar] [CrossRef]
- Kapsopoulou, K.; Mourtzini, A.; Anthoulas, M.; Nerantzis, E. Biological Acidification during Grape Must Fermentation Using Mixed Cultures of Kluyveromyces thermotolerans and Saccharomyces cerevisiae. World J. Microbiol. Biotechnol. 2007, 23, 735–739. [Google Scholar] [CrossRef]
- Benito, S. Combined Use of S. pombe and L. thermotolerans in Winemaking. Beneficial Effects Determined Through the Study of Wines’ Analytical Characteristics. Molecules 2016, 21, 1744. [Google Scholar] [CrossRef]
- Escribano, R.; González-Arenzana, L.; Portu, J.; Garijo, P.; López-Alfaro, I.; López, R.; Santamaría, P.; Gutiérrez, A.R. Wine Aromatic Compound Production and Fermentative Behaviour within Different Non- Saccharomyces Species and Clones. J. Appl. Microbiol. 2018, 124, 1521–1531. [Google Scholar] [CrossRef]
- Puertas, B.; Jiménez, M.J.; Cantos-Villar, E.; Cantoral, J.M.; Rodríguez, M.E. Use of Torulaspora delbrueckii and Saccharomyces cerevisiae in Semi-Industrial Sequential Inoculation to Improve Quality of Palomino and Chardonnay Wines in Warm Climates. J. Appl. Microbiol. 2017, 122, 733–746. [Google Scholar] [CrossRef]
- Moreno, J.J.; Peinado, R.A. Enological Chemistry; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Everitt, B.S.; Landau, S.; Leese, M.; Stahl, D. Cluster Analysis; Wiley: Hoboken, NJ, USA, 2011; ISBN 9780470749913. [Google Scholar]
- Vaquero, C.; Izquierdo-Cañas, P.M.; Mena-Morales, A.; Marchante-Cuevas, L.; Heras, J.M.; Morata, A. Use of Lachancea thermotolerans for Biological vs. Chemical Acidification at Pilot-Scale in White Wines from Warm Areas. Fermentation 2021, 7, 193. [Google Scholar] [CrossRef]
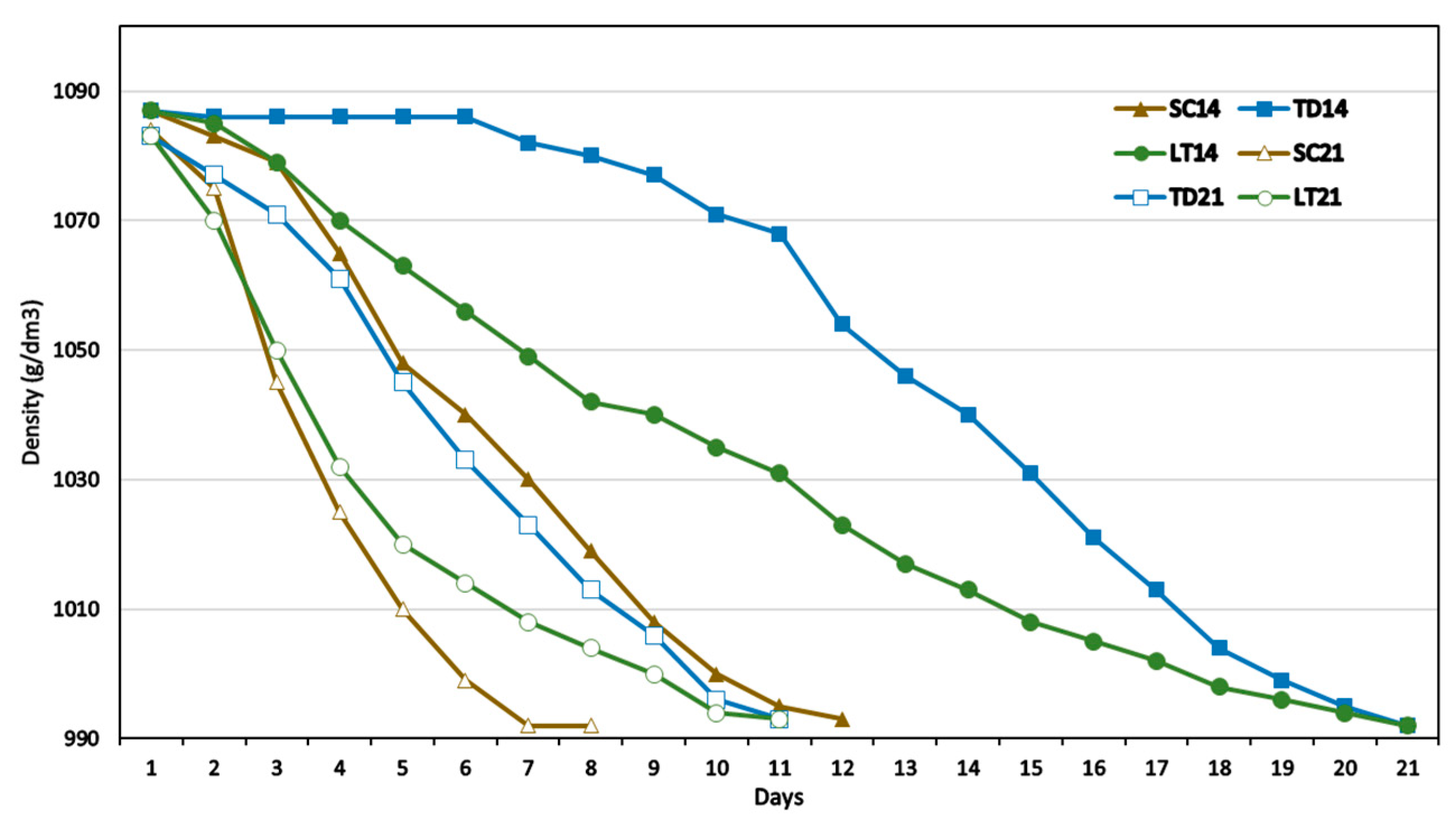
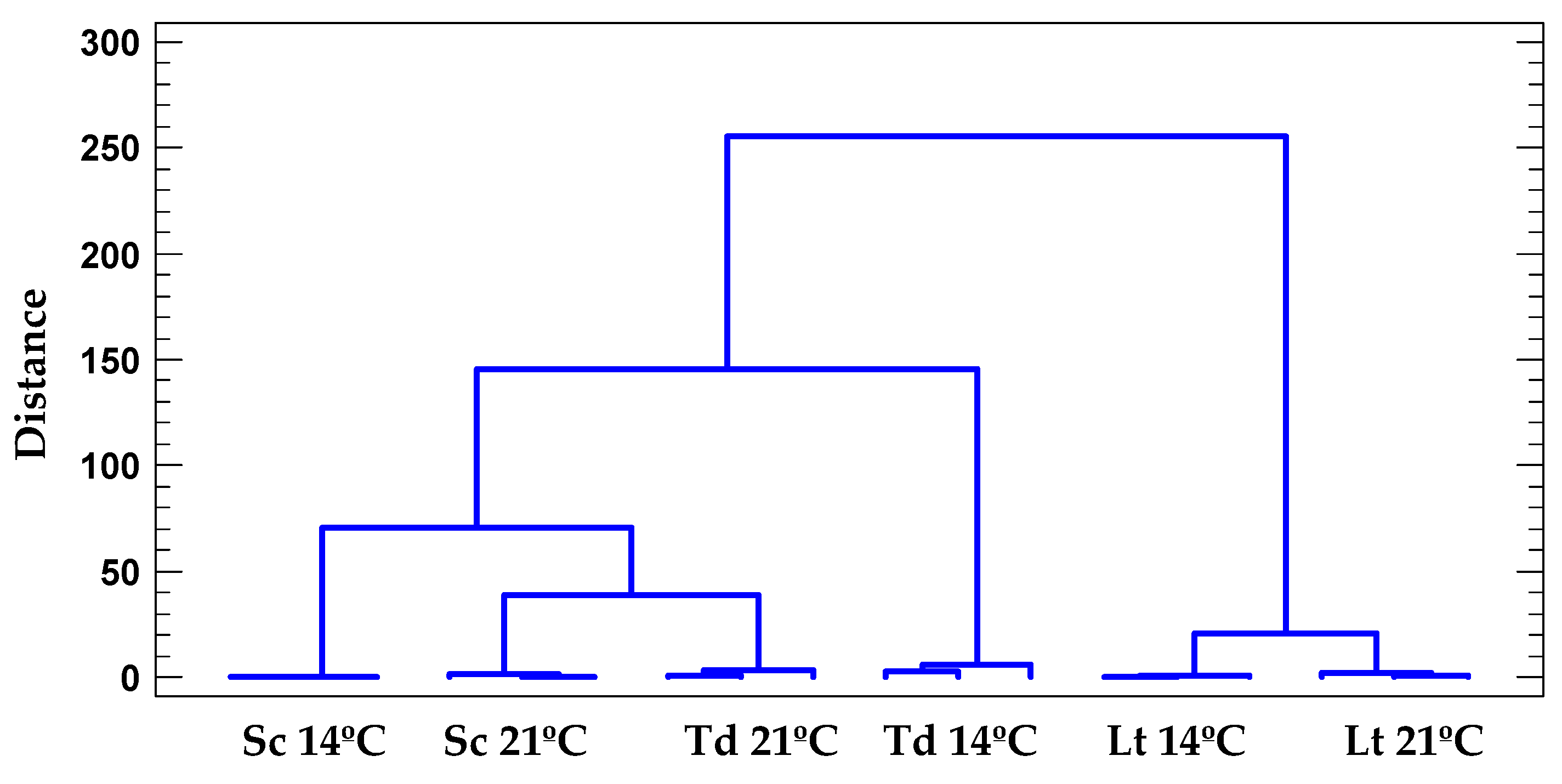
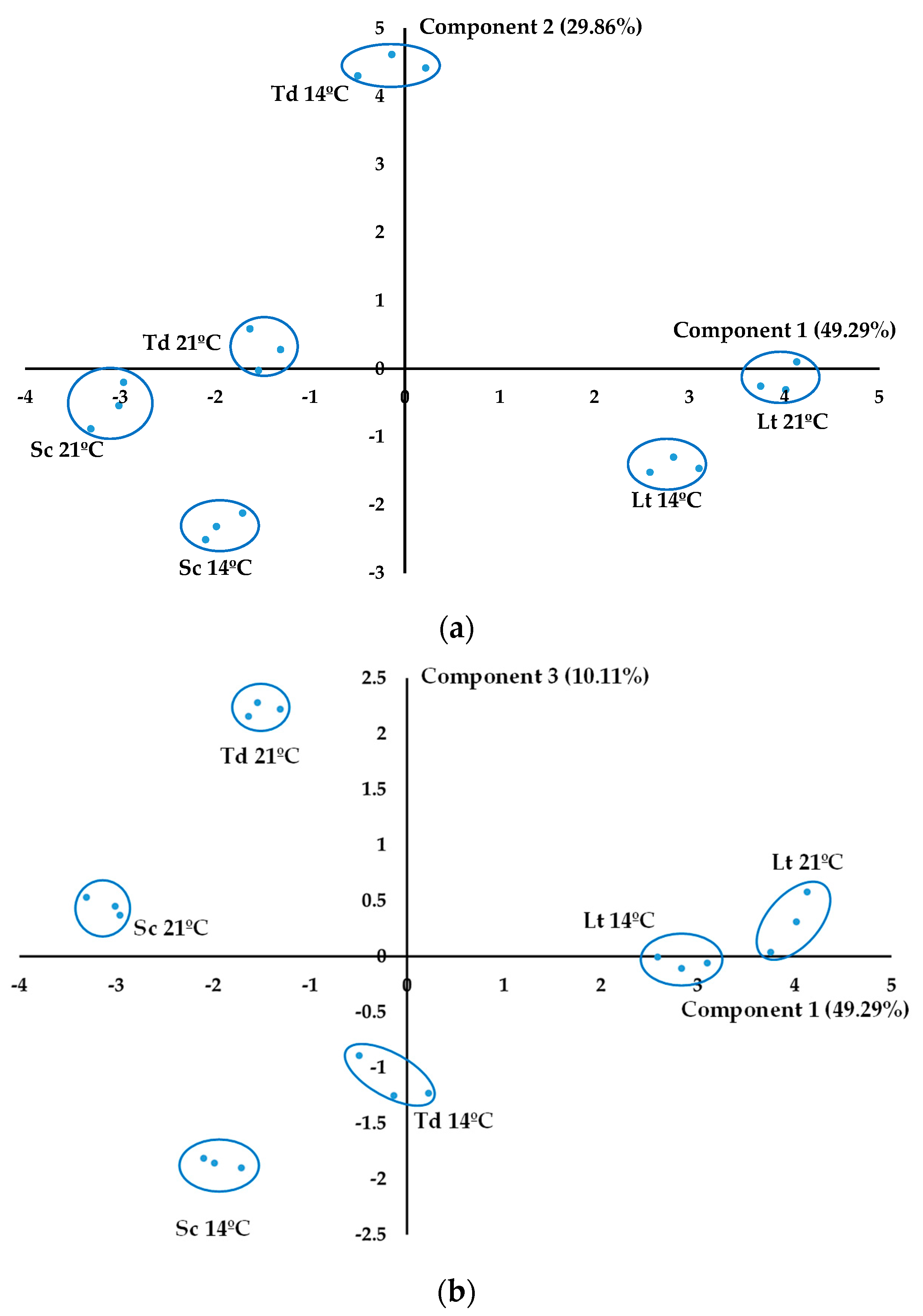


| Parameter | 14 °C | 21 °C | MANOVA | |||||
|---|---|---|---|---|---|---|---|---|
| Sc | Td | Lt | Sc | Td | Lt | T | Y | |
| pH | 3.20 ± 0.02 | 3.23 ± 0.02 | 3.25 ± 0.01 | 3.31 ± 0.02 | 3.34 ± 0.02 | 3.22 ± 0.02 | *** | ** |
| Buffer capacity (meq/L) | 31 ± 1 | 34 ± 2 | 47 ± 2 | 30.0 ± 0.9 | 28.5 ± 0.7 | 49 ± 1 | ns | *** |
| Titratable acidity Tartaric acid (g/L) | 5.95 ± 0.08 | 5.7 ± 0.1 | 7.9 ± 0.1 | 4.9 ± 0.1 | 4.85 ± 0.09 | 7.65 ± 0.06 | *** | *** |
| Lactic acid (g/L) | 0.50 ± 0.02 | 0.48 ± 0.01 | 3.7 ± 0.1 | 0.15 ± 0.07 | 0.33 ± 0.04 | 4.8 ± 0.2 | ** | *** |
| Volatile acidity Acetic acid (g/L) | 0.5 ± 0.1 | 0.31 ± 0.02 | 0.74 ± 0.05 | 0.22 ± 0.05 | 0.52 ± 0.03 | 0.63 ± 0.01 | *** | *** |
| Ethanol (% v/v) | 11.5 ± 0.1 | 11.8 ± 0.1 | 11.7 ± 0.2 | 11.6 ± 0.1 | 11.2 ± 0.2 | 11.2 ± 0.1 | ** | ns |
| Reducing sugars (g/L) | 1.8 ± 0.2 | 1.7 ± 0.3 | 1.9 ± 0.2 | 2.5 ± 0.4 | 2.3 ± 0.3 | 1.8 ± 0.2 | ns | ns |
| Compound | 14 °C | 21 °C | MANOVA | |||||
|---|---|---|---|---|---|---|---|---|
| Sc | Td | Lt | Sc | Td | Lt | T | Y | |
| Methanol | 31 ± 3 | 32 ± 5 | 26.5 ± 0.4 | 31 ± 2 | 25 ± 3 | 31 ± 1 | * | ns |
| Propanol | 33 ± 1 | 55.3 ± 0.7 | 55 ± 4 | 28 ± 1 | 33 ± 2 | 58.1 ± 0.1 | *** | *** |
| Isobutanol | 28.6 ± 0.8 | 68 ± 1 | 58.8 ± 0.1 | 38 ± 2 | 70 ± 2 | 106 ± 1 | *** | *** |
| Isoamyl alcohols | 195 ± 4 | 270 ± 6 | 245 ± 2 | 261 ± 8 | 258 ± 3 | 254 ± 3 | *** | *** |
| 2-Phenylethanol | 24.6 ± 0.4 | 44.7 ± 0.2 | 27 ± 2 | 45 ± 1 | 58 ± 2 | 29 ± 5 | *** | *** |
| Ethyl acetate | 32 ± 1 | 34 ± 2 | 158 ± 0 | 38 ± 1 | 53.1 ± 0.2 | 152 ± 1 | *** | *** |
| Ethyl lactate | nd | nd | 73 ± 3 | nd | nd | 91 ± 3 | *** | *** |
| Diethyl succinate | 8.5 ± 0.2 | 15 ± 1 | 11.6 ± 0.8 | 9.7 ± 0.4 | 12.8 ± 0.3 | 13.5 ± 0.2 | ns | *** |
| 2,3-Butanediol (levo) | 224 ± 2 | 256 ± 31 | 213.2 ± 0.1 | 249 ± 11 | 231 ± 19 | 210 ± 10 | ns | ns |
| 2,3-Butanediol (meso) | 71 ± 5 | 209 ± 23 | 68 ± 1 | 78 ± 5 | 179 ± 10 | 46 ± 4 | ns | *** |
| Glycerol (g/L) | 7.6 ± 0.1 | 11 ± 1 | 7.1 ± 0.2 | 6.8 ± 0.3 | 6.4 ± 0.5 | 9.0 ± 0.4 | * | * |
| Acetaldehyde | 59 ± 2 | 177 ± 2 | 165 ± 2 | 36 ± 4 | 72 ± 2 | 103 ± 13 | *** | *** |
| Acetoin | 4.4 ± 0.3 | 13.2 ± 0.9 | 11.4 ± 0.1 | 3.3 ± 0.3 | 6.3 ± 0.5 | 18.3 ± 0.6 | ns | *** |
| Coefficient | p-Value | |
|---|---|---|
| Constant | 1.564 | >0.05 |
| Volatile Acidity | 1.379 | <0.05 |
| Glycerol | −0.371 | <0.05 |
| Isobutanol | 0.0436 | <0.05 |
| Isoamyl alcohol | 0.0323 | <0.05 |
| 2-Phenyletanol | −0.136 | <0.05 |
| R2 | 99.10% | |
| Durbin–Watson | 3.033 | >0.05 |
| Model | <0.05 |
| Classifying Variables | Pc1 (49.29%) | Pc2 (29.86%) | Pc3 (10.11%) |
|---|---|---|---|
| Lactic acid | 0.3613 | −0.1223 | 0.0818 |
| Acetic acid | 0.2829 | −0.2076 | 0.1287 |
| Propanol | 0.3405 | 0.1663 | −0.2235 |
| Isobutanol | 0.2922 | 0.1927 | 0.3037 |
| Isoamyl alcohols | 0.0635 | 0.3739 | 0.3934 |
| 2-Phenylethanol | −0.1877 | 0.3039 | 0.5004 |
| Ethyl acetate | 0.3473 | −0.1333 | 0.1873 |
| Ethyl lactate | 0.3579 | −0.1308 | 0.1069 |
| Diethyl succinate | 0.1890 | 0.4120 | 0.0905 |
| 2,3-Butanediol (levo) | −0.2341 | 0.2925 | −0.1563 |
| 2,3-Butanediol (meso) | −0.1276 | 0.4110 | 0.0149 |
| Glycerol (g/L) | 0.1376 | 0.3108 | −0.5006 |
| Acetaldehyde | 0.2350 | 0.2446 | −0.3080 |
| Acetoin | 0.3453 | 0.1716 | −0.0679 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Suárez, F.; Peinado, R.A. Use of Non-Saccharomyces Yeast to Enhance the Acidity of Wines Produced in a Warm Climate Region: Effect on Wine Composition. Fermentation 2024, 10, 17. https://doi.org/10.3390/fermentation10010017
Sánchez-Suárez F, Peinado RA. Use of Non-Saccharomyces Yeast to Enhance the Acidity of Wines Produced in a Warm Climate Region: Effect on Wine Composition. Fermentation. 2024; 10(1):17. https://doi.org/10.3390/fermentation10010017
Chicago/Turabian StyleSánchez-Suárez, Fernando, and Rafael A. Peinado. 2024. "Use of Non-Saccharomyces Yeast to Enhance the Acidity of Wines Produced in a Warm Climate Region: Effect on Wine Composition" Fermentation 10, no. 1: 17. https://doi.org/10.3390/fermentation10010017
APA StyleSánchez-Suárez, F., & Peinado, R. A. (2024). Use of Non-Saccharomyces Yeast to Enhance the Acidity of Wines Produced in a Warm Climate Region: Effect on Wine Composition. Fermentation, 10(1), 17. https://doi.org/10.3390/fermentation10010017







