Investigating Pervaporation as a Process Method for Concentrating Formic Acid Produced from Carbon Dioxide
Abstract
1. Introduction
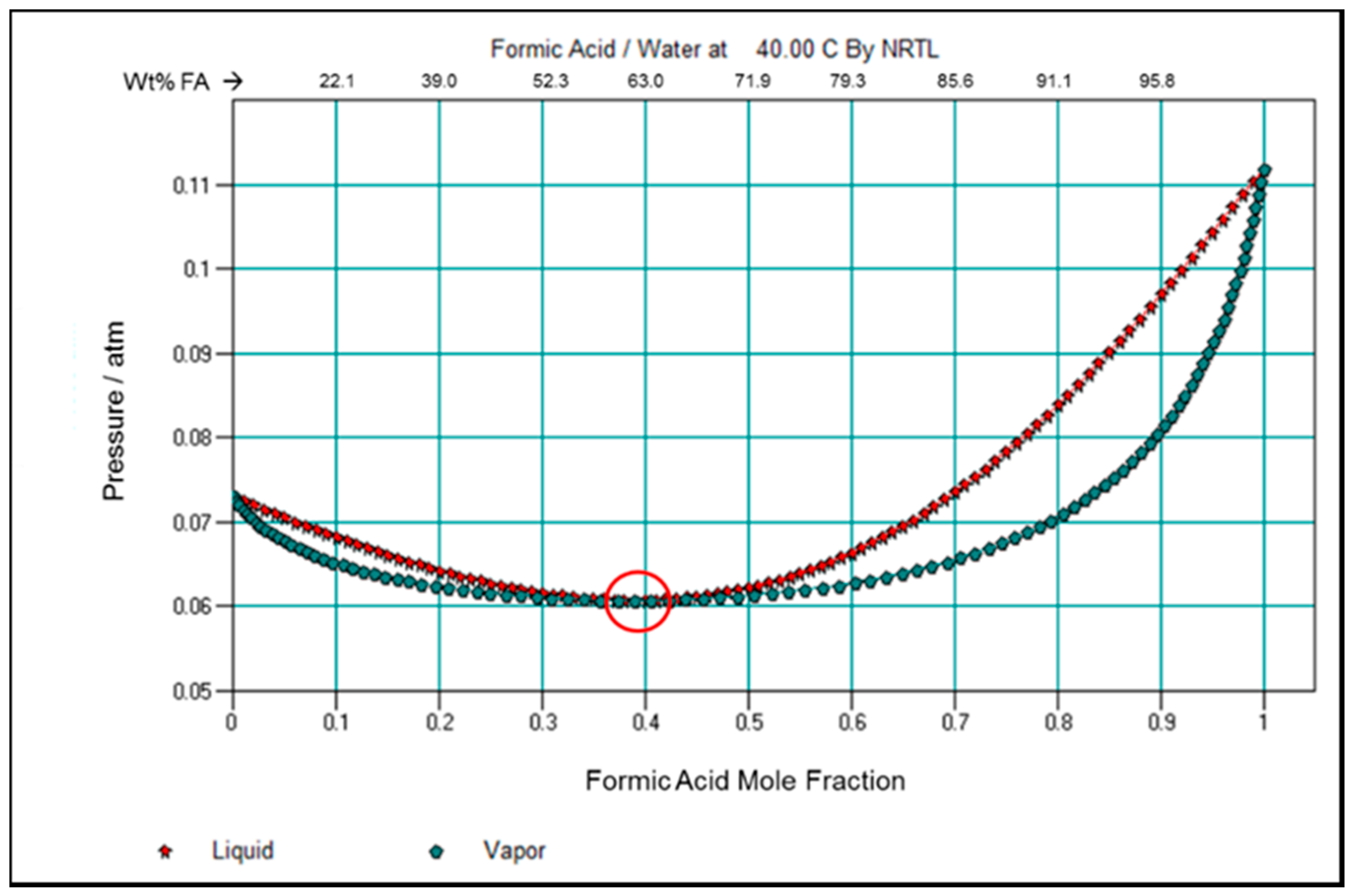
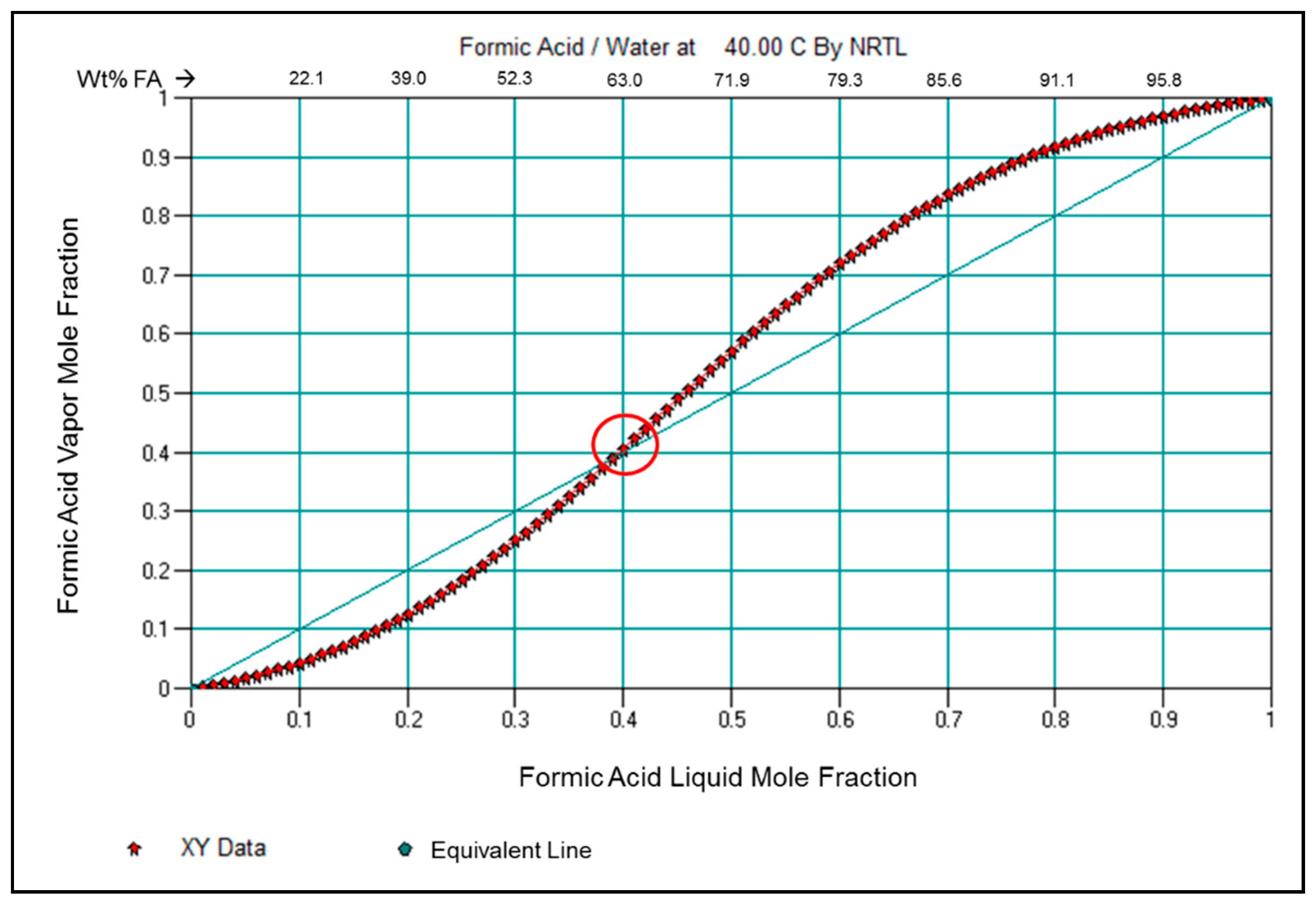
1.1. Formic Acid—Water Azeotrope
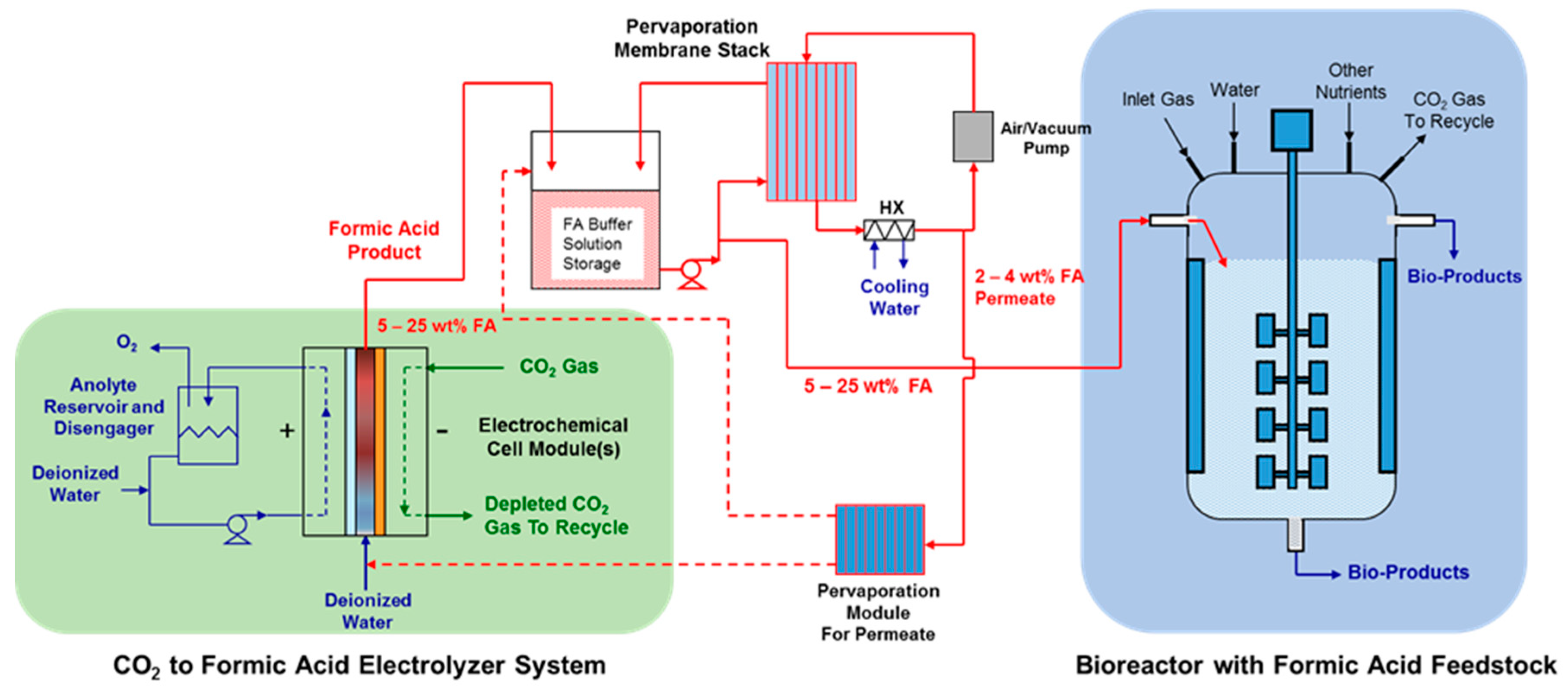
1.2. Formate/Formic Acid Use in Bioreactors
1.3. Pervaporation Performance Factors
2. Materials and Methods
2.1. Experimental Pervaporation System
2.2. Equipment and Chemicals
3. Results and Discussion
3.1. Membrane Permeation Test Results
3.2. FA Extended Time Batch Pervaporation Experimental Run Results
3.3. FA Pervaporation Batch Calculations
- At FA feed concentrations of 10 to 25 wt%, the water permeation mass flux rate was found to be relatively constant for most of the membrane separator materials, around 0.78 kg/m2 h for H2O and a much lower mass permeation flux rate for FA at 0.026 kg/m2 h for temperatures in the 22 to 60 °C range.
- At 30 to 60 wt% FA feed concentrations, the water permeation mass flux rate is lower, and the FA permeation mass flux rate increases to about 0.10 kg/m2 h for FA feed concentrations above 30 wt%.
3.4. Energy Steam Cost Estimates for Concentrating FA in an Azeotropic Distillation Tower
3.5. Examination of Formic Acid and Water Vapor Pressure and VLE Properties in View of the Membrane Pervaporation Results
3.6. Conceptual Systems Employing Pervaporation in Concentrating FA
4. Summary
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Aresta, M.; Dibenedetto, A. Utilisation of CO2 as a chemical feedstock: Opportunities and challenges. Dalton Trans. 2007, 28, 2975–2992. [Google Scholar] [CrossRef] [PubMed]
- Bocchini, S.; Castro, C.; Cocuzza, M.; Ferrero, S.; Latini, G.; Martis, A.; Pirri, F.; Scaltrito, L.; Rocca, V.; Verga, F.; et al. The virtuous CO2 circle or the three Cs: Capture, cache, and convert. J. Nanomater. 2017, 2017, 6594151. [Google Scholar] [CrossRef]
- Quadrelli, E.A.; Centi, G.; Duplan, J.-L.; Perathoner, S. Carbon Dioxide Recycling: Emerging Large-Scale Technologies with Industrial Potential. ChemSusChem 2011, 4, 1194–1215. [Google Scholar] [CrossRef] [PubMed]
- Masel, R.; Ni, R.; Liu, Z.; Chen, Q.; Kutz, R.; Nereng, L.; Lutz, D.; Lewinski, K. Unlocking the Potential of CO2 Conversion to Fuels and Chemicals as an Economically Viable Route to CCR. Energy Procedia 2014, 63, 7959–7962. [Google Scholar] [CrossRef][Green Version]
- Masel, R.; Liu, Z.; Zhao, D.; Chen, Q.; Lutz, D.; Nereng, L. CO2 Conversion to Chemicals with Emphasis on using Renewable Energy/Resources to Drive the Conversion. In Commercializing Biobased Products: Opportunities, Challenges, Benefits, and Risks; The Royal Society of Chemistry: London, UK, 2016; Chapter 10; pp. 215–257. [Google Scholar]
- Jhong, H.-R.M.; Ma, S.; Kenis, P.J.A. Electrochemical conversion of CO2 to useful chemicals: Current status, remaining challenges, and future opportunities. Curr. Opin. Chem. Eng. 2013, 2, 191–199. [Google Scholar] [CrossRef]
- Hu, B.; Guild, C.; Suib, S.L. Thermal, electrochemical, and photochemical conversion of CO2 to fuels and value-added products. J. CO2 Util. 2013, 1, 18–27. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A.; Quaranta, E. State of the art and perspectives in catalytic processes for CO2 conversion into chemicals and fuels: The distinctive contribution of chemical catalysis and biotechnology. J. Catal. 2016, 343, 2–45. [Google Scholar] [CrossRef]
- Peters, M.; Köhler, B.; Kuckshinrichs, W.; Leitner, W.; Markewitz, P.; Müller, T.E. Chemical Technologies for Exploiting and Recycling Carbon Dioxide into the Value Chain. ChemSusChem 2011, 4, 1216–1240. [Google Scholar] [CrossRef]
- Halmann, M.M. Chemical Fixation of Carbon Dioxide: Methods for Recycling CO2 Into Useful Products; CRC Press: London, UK, 1993; pp. 67–127. [Google Scholar]
- Yang, H.; Kaczur, J.J.; Sajjad, S.D.; Masel, R.I. Electrochemical conversion of CO2 to formic acid utilizing Sustainion™ membranes. J. CO2 Util. 2017, 20, 208–217. [Google Scholar] [CrossRef]
- Yang, H.; Kaczur, J.J.; Sajjad, S.D.; Masel, R.I. CO2 Conversion to Formic Acid in a Three Compartment Cell with Sustainion™ Membranes. ECS Trans. 2017, 77, 1425–1431. [Google Scholar] [CrossRef]
- Kaczur, J.J.; Yang, H.; Liu, Z.; Sajjad, S.D.; Masel, R.I. Carbon dioxide and water electrolysis using new alkaline stable anion membranes. Front. Chem. 2018, 6, 263. [Google Scholar] [CrossRef] [PubMed]
- Kaczur, J.J.; Yang, H.; Sajjad, S.D.; Masel, R.I. Method and System for Electrochemical Production of Formic Acid from Carbon Dioxide. U.S. Patent 10,047,446, 14 August 2018. [Google Scholar]
- Kutz, R.B.; Chen, Q.; Yang, H.; Sajjad, S.D.; Liu, Z.; Masel, I.R. Sustainion Imidazolium-Functionalized Polymers for Carbon Dioxide Electrolysis. Energy Technol. 2017, 5, 929–936. [Google Scholar] [CrossRef]
- Pérez-Fortes, M.; Schöneberger, J.C.; Boulamanti, A.; Harrison, G.; Tzimas, E. Formic acid synthesis using CO2 as raw material: Techno-economic and environmental evaluation and market potential. Int. J. Hydrog. Energy 2016, 41, 16444–16462. [Google Scholar] [CrossRef]
- Hietala, J.; Vuori, A.; Johnsson, P.; Pollari, I.; Reutemann, W.; Kieczka, H. Formic Acid. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2016; pp. 1–22. [Google Scholar]
- Feng, X.; Huang, R.Y.M. Liquid separation by membrane pervaporation: A review. Ind. Eng. Chem. Res. 1997, 36, 1048–1066. [Google Scholar] [CrossRef]
- Chopade, S.P.; Mahajani, S.M. Pervaporation: Membrane Separations. In Encyclopedia of Separation Science; Wilson, I.D., Ed.; Academic Press: Oxford, UK, 2000; pp. 3636–3641. [Google Scholar]
- Kujawski, W. Application of pervaporation and vapor permeation in the environmental protection. Pol. J. Environ. Stud. 2000, 9, 13–26. [Google Scholar]
- Jyoti, G.; Keshav, A.; Anandkumar, J. Review on Pervaporation: Theory, Membrane Performance, and Application to Intensification of Esterification Reaction. J. Eng. 2015, 2015, 927068. [Google Scholar] [CrossRef]
- Hilmen, E.-K. Separation of Azeotropic Mixtures: Tools for Analysis and Studies on Batch Distillation Operation. Ph.D. Thesis, Norwegian University of Science and Technology, Department of Chemical Engineering , Trondheim, Norway, 2000. [Google Scholar]
- Shao, P.; Huang, R.Y.M. Polymeric membrane pervaporation. J. Membr. Sci. 2007, 287, 162–179. [Google Scholar] [CrossRef]
- Wang, Y.; Widjojo, N.; Sukitpaneenit, P.; Chung, T.-S. Membrane Pervaporation. In Separation and Purification Technologies in Biorefineries; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 259–299. [Google Scholar]
- Qin, Y.; Sheth, J.P. Pervaporation membranes that are highly selective for acetic acid over water. Ind. Eng. Chem. Res. 2003, 42, 582–595. [Google Scholar] [CrossRef]
- Li, G.; Kikuchi, E.; Matsukata, M. A study on the pervaporation of water–acetic acid mixtures through ZSM-5 zeolite membranes. J. Membr. Sci. 2003, 218, 185–194. [Google Scholar] [CrossRef]
- Işıklan, N.; Şanlı, O. Separation characteristics of acetic acid–water mixtures by pervaporation using poly(vinyl alcohol) membranes modified with malic acid. Chem. Eng. Process. Process. Intensif. 2005, 44, 1019–1027. [Google Scholar] [CrossRef]
- Dave, H.K.; Nath, K. Effect of Temperature on Pervaporation Dehydration of Water-Acetic Acid Binary Mixture; NISCAIR-CSIR: Delhi, India, 2017. [Google Scholar]
- Dibenedetto, A.; Aresta, M.; Angelini, A.; Ethiraj, J.; Aresta, B.M. Synthesis, Characterization, and Use of NbV/CeIV-Mixed Oxides in the Direct Carboxylation of Ethanol by using Pervaporation Membranes for Water Removal. Chem. Eur. J. 2012, 18, 10324–10334. [Google Scholar] [CrossRef] [PubMed]
- Mulder, M.H.V.; Hendrickman, J.O.; Hegeman, H.; Smolders, C.A. Ethanol—Water separation by pervaporation. J. Membr. Sci. 1983, 16, 269–284. [Google Scholar] [CrossRef]
- Fouad, E.A.; Feng, X. Use of pervaporation to separate butanol from dilute aqueous solutions: Effects of operating conditions and concentration polarization. J. Membr. Sci. 2008, 323, 428–435. [Google Scholar] [CrossRef]
- Huang, S.-C.; Ball, I.J.; Kaner, R.B. Polyaniline membranes for pervaporation of carboxylic acids and water. Macromolecules 1998, 31, 5456–5464. [Google Scholar] [CrossRef]
- Akbar, S.; Punnathanam, S.; Pulkala, M. Adsorption of Aqueous Solutions of Carboxylic Acids on Montmorillonite, Silicalite, H-ZSM-5 and their Na+ - and Li+ - Exchanged Forms. J. Chem. Soc. Pak. 2008, 30, 664–673. [Google Scholar]
- Huang, R.Y.M. Novel Membrane Method. U.S. Patent 4,892,661, 9 January 1990. [Google Scholar]
- Yishai, O.; Lindner, S.N.; Gonzalez de la Cruz, J.; Tenenboim, H.; Bar-Even, A. The formate bio-economy. Curr. Opin. Chem. Biol. 2016, 35, 1–9. [Google Scholar] [CrossRef]
- Yishai, O.; Goldbach, L.; Tenenboim, H.; Lindner, S.N.; Bar-Even, A. Engineered Assimilation of Exogenous and Endogenous Formate in Escherichia coli. ACS Synth. Biol. 2017, 6, 1722–1731. [Google Scholar] [CrossRef]
- Bar-Even, A. Formate Assimilation: The Metabolic Architecture of Natural and Synthetic Pathways. Biochemistry 2016, 55, 3851–3863. [Google Scholar] [CrossRef]
- Bar-Even, A.; Noor, E.; Flamholz, A.; Milo, R. Design and analysis of metabolic pathways supporting formatotrophic growth for electricity-dependent cultivation of microbes. Biochim. Biophys. Acta (BBA) Bioenerg. 2013, 1827, 1039–1047. [Google Scholar] [CrossRef]
- Claassens, N.J.; Sánchez-Andrea, I.; Sousa, D.Z.; Bar-Even, A. Towards sustainable feedstocks: A guide to electron donors for microbial carbon fixation. Curr. Opin. Biotechnol. 2018, 50, 195–205. [Google Scholar] [CrossRef]
- Wexler, A. Vapor Pressure Formulation for Water in Range 0 to 100 °C. A Revision. J. Res. Natl. Bur. Stand. A Phys. Chem. 1976, 80, 775–785. [Google Scholar] [CrossRef] [PubMed]


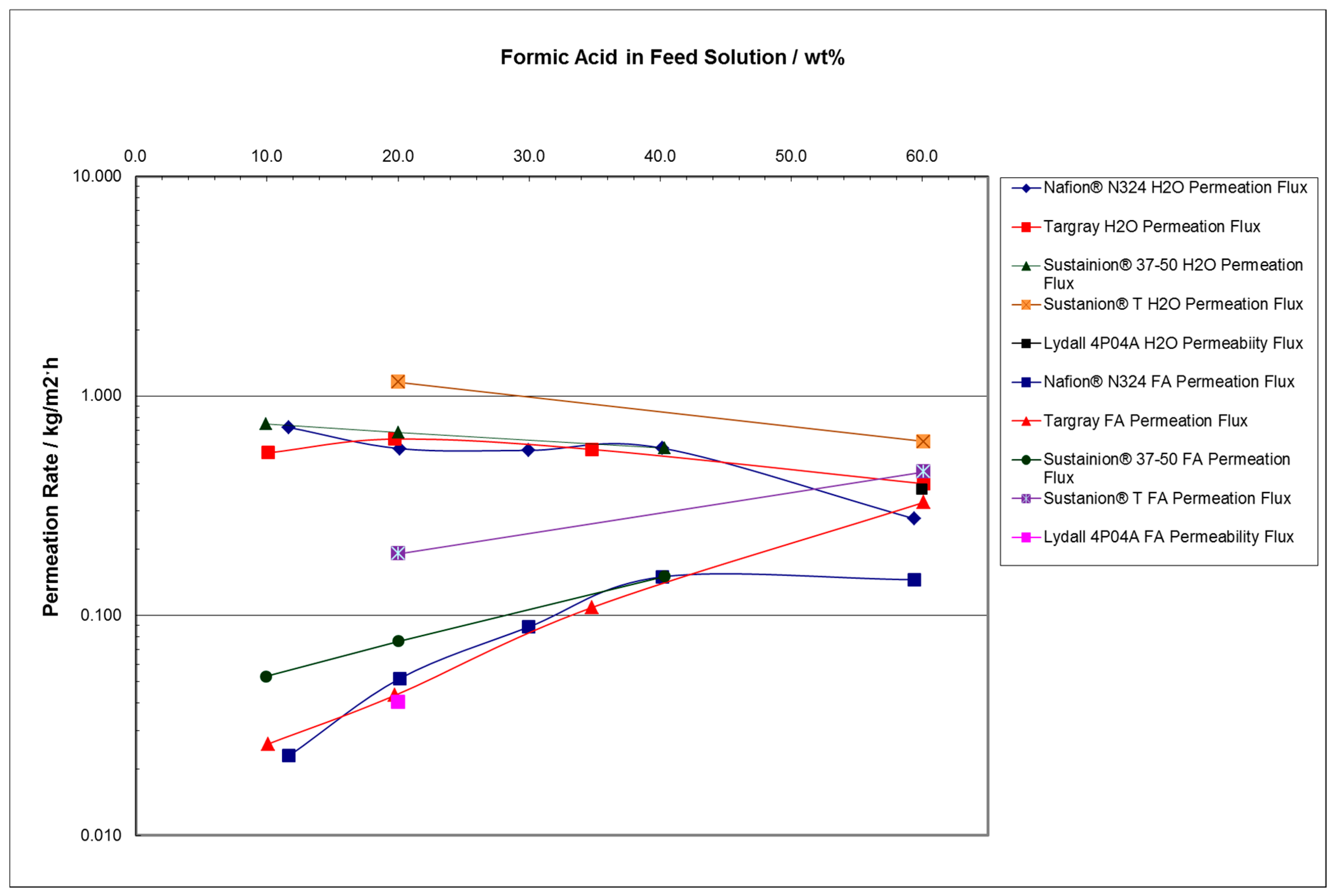

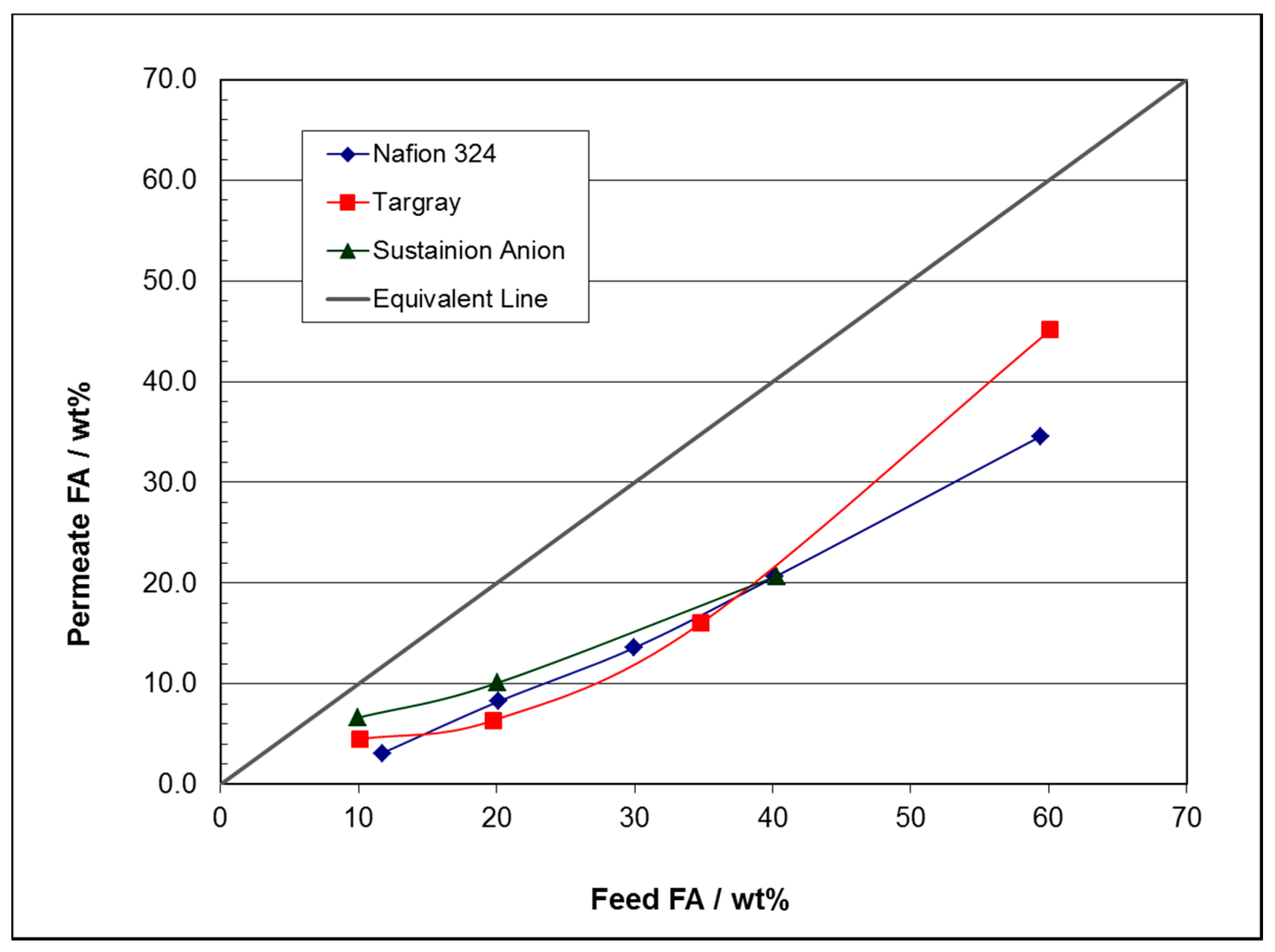
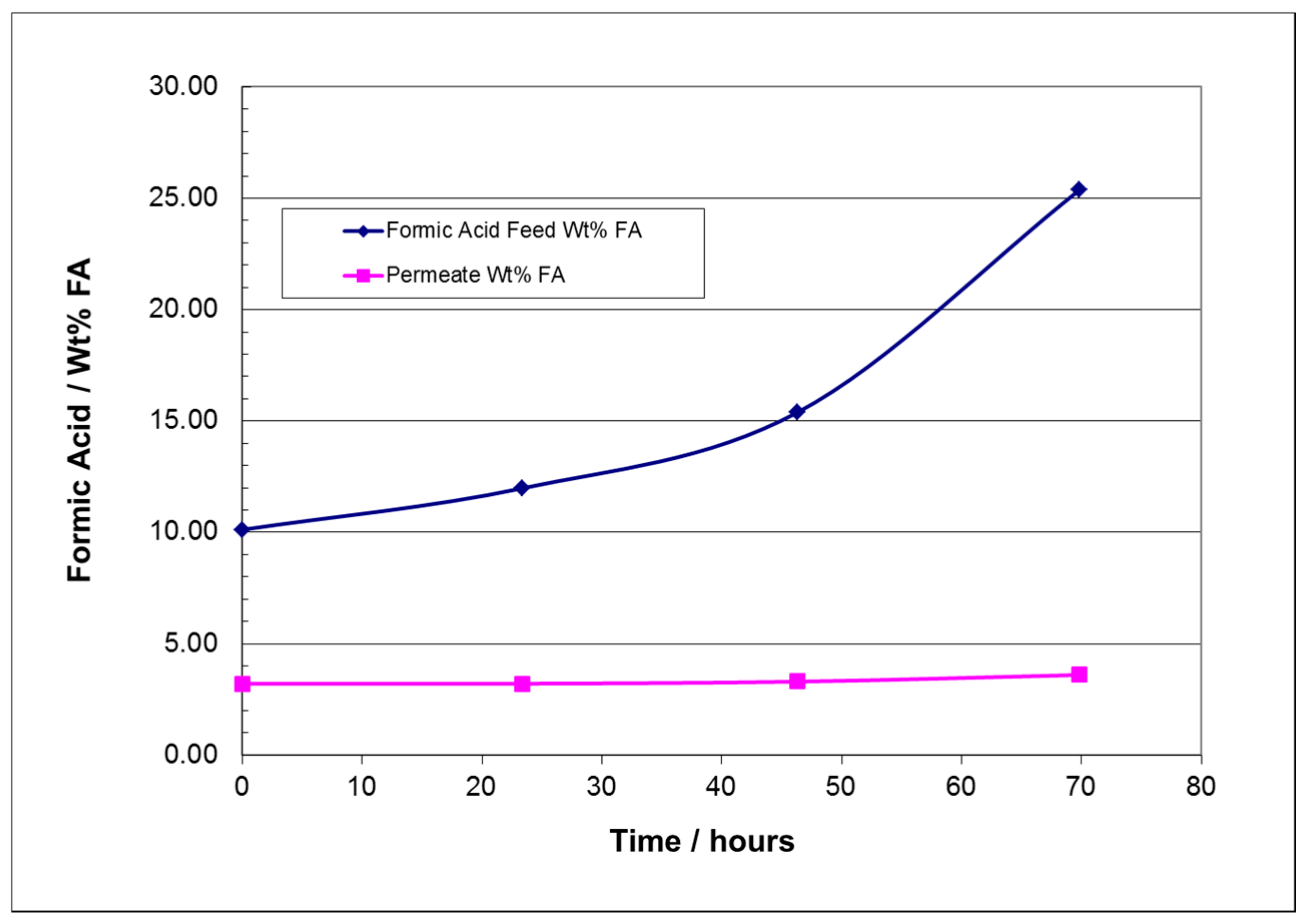

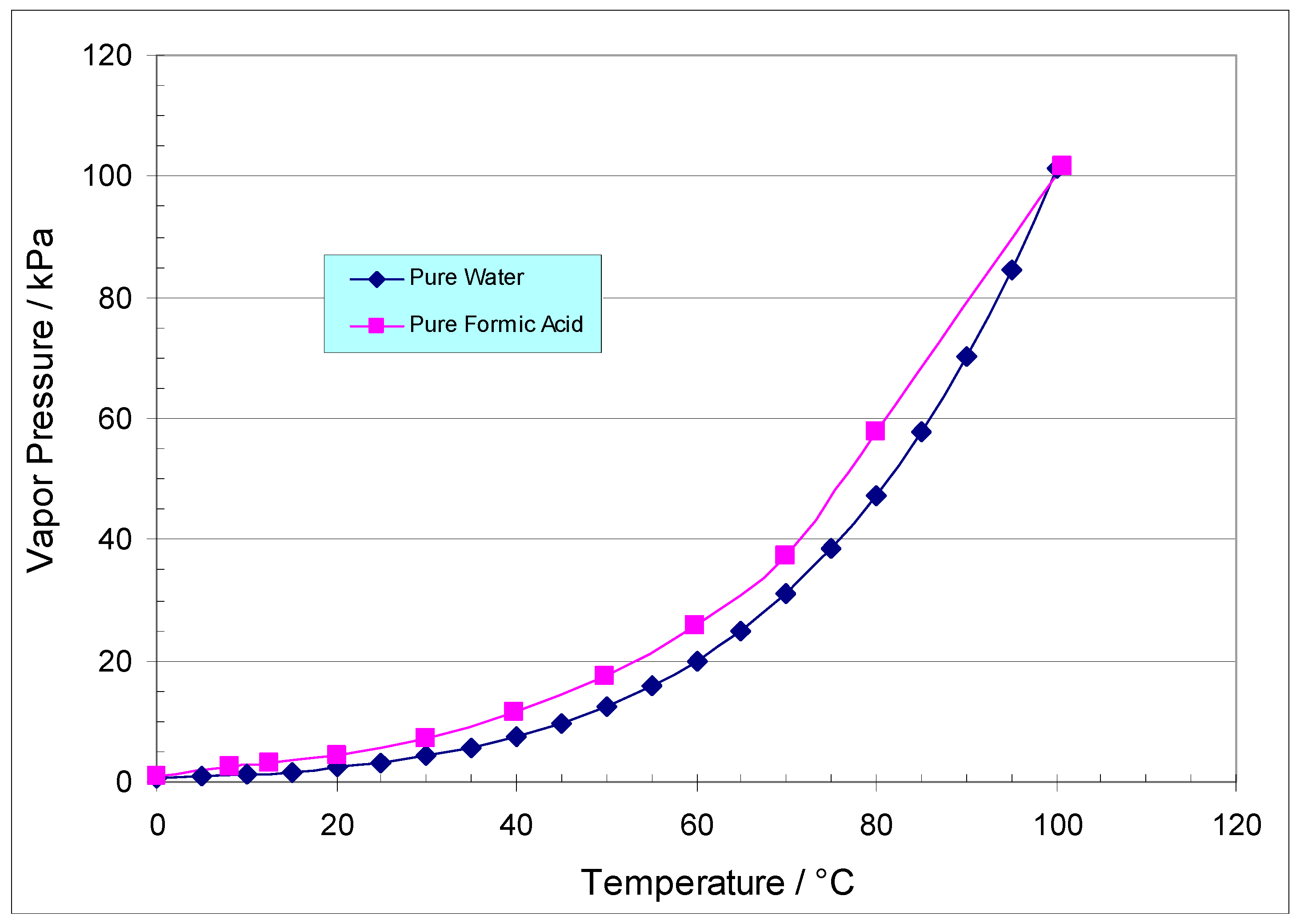
| Membrane | Source | Properties | Cost |
|---|---|---|---|
| Nafion® N324 | Ion Power (New Castle, DE USA) | Fluoropolymer sulfonic acid-based cation ion exchange membrane | Expensive per m2 ($900–$1200) |
| Targray SD425101 | Targray (Montreal, Quebec, Canada) | PE-based lithium battery separator, 45% open area | Inexpensive per m2 ($10–$30) |
| Sustainion® 37–50 | Dioxide Materials (Boca Raton, FL, USA) | Styrene backbone, imidazole functionalized anion ion exchange membrane | Moderately expensive per m2 (Est. $20–$200) when fully commercialized |
| Sustainion® 37-50 T | Dioxide Materials (Boca Raton, FL, USA) | Styrene backbone, imidazole functionalized anion ion exchange membrane with ePTFE support | Moderately expensive per m2 (Est. $30–$300) when fully commercialized |
| Lydall Solupor® 4P04A | Lydall Performance Materials Inc. (Rochester, NH, USA) | HDPE-based filtration membrane, 80% open area | Inexpensive per m2 ($10–$30) |
| Membrane | Temp | Feed Composition (wt%) | Permeate Composition (wt%) | Permeability Separation Factor | Permeation Flux or Rate of Water and FA through Membrane | Flux Ratio | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| °C | H2O (A) | FA (B) | H2O (A) | FA (B) | αab A/B * | kg/m2ˑh | kg/m2ˑh | H2O/FA Ratio | |||
| Nafion® 324 | 22 | 90.78 | 9.22 | 96.36 | 3.64 | 2.69 | H2O | 0.239 | FA | 0.009 | 26.5 |
| Nafion® 324 | 40 | 90.77 | 9.23 | 96.74 | 3.26 | 3.02 | H2O | 0.463 | FA | 0.016 | 29.7 |
| Nafion® 324 | 60 | 88.31 | 11.69 | 96.9 | 3.10 | 4.14 | H2O | 0.719 | FA | 0.023 | 31.3 |
| Targray SD425101 | 22 | 89.07 | 10.93 | 95.86 | 4.14 | 2.84 | H2O | 0.215 | FA | 0.009 | 23.2 |
| Targray SD425101 | 40 | 89.73 | 10.27 | 96.14 | 3.86 | 2.85 | H2O | 0.801 | FA | 0.032 | 24.9 |
| Targray SD425101 | 60 | 89.90 | 10.10 | 95.49 | 4.51 | 2.38 | H2O | 0.552 | FA | 0.026 | 21.2 |
| Membrane | Temperature °C | H2O/FA Flux Permeation Ratio |
|---|---|---|
| Nafion® N324 | 22 | 26.5 |
| 40 | 29.7 | |
| 60 | 31.3 | |
| Targray SD425101 | 22 | 23.2 |
| 40 | 24.9 | |
| 60 | 21.2 |
| Membrane | Temp | Feed Composition (wt%) | Permeate Composition (wt%) | Perme-Ability Separation Factor | Permeation Flux or Rate of Water and FA through Membrane | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| °C | H2O (A) | FA (B) | H2O (A) | FA (B) | αab A/B * | kg/m2ˑh | kg/m2ˑh | H2O/FA Ratio | |||
| Nafion® 324 | 60 | 88.3 | 11.7 | 96.9 | 3.1 | 4.14 | H2O | 0.719 | FA | 0.023 | 31.3 |
| Nafion® 324 | 60 | 79.9 | 20.2 | 91.8 | 8.3 | 2.81 | H2O | 0.576 | FA | 0.052 | 11.1 |
| Nafion® 324 | 60 | 70.0 | 30.0 | 86.4 | 13.6 | 2.72 | H2O | 0.566 | FA | 0.089 | 6.4 |
| Nafion® 324 | 60 | 59.9 | 40.2 | 79.4 | 20.6 | 2.58 | H2O | 0.579 | FA | 0.150 | 3.9 |
| Nafion® 324 | 60 | 40.6 | 59.4 | 65.4 | 34.6 | 2.77 | H2O | 0.276 | FA | 0.146 | 1.9 |
| Targray SD425101 | 60 | 89.9 | 10.1 | 95.5 | 4.5 | 2.38 | H2O | 0.552 | FA | 0.026 | 21.2 |
| Targray SD425101 | 60 | 80.2 | 19.8 | 93.6 | 6.4 | 3.62 | H2O | 0.637 | FA | 0.043 | 14.7 |
| Targray SD425101 | 60 | 65.2 | 34.8 | 84.0 | 16.0 | 2.80 | H2O | 0.571 | FA | 0.109 | 5.2 |
| Targray SD425101 | 60 | 39.9 | 60.1 | 54.9 | 45.1 | 1.83 | H2O | 0.399 | FA | 0.328 | 1.2 |
| Sustainion® 37-50 | 60 | 90.1 | 9.9 | 93.4 | 6.6 | 1.55 | H2O | 0.745 | FA | 0.053 | 14.1 |
| Sustainion® 37-50 | 60 | 80.0 | 20.1 | 89.9 | 10.1 | 2.24 | H2O | 0.683 | FA | 0.077 | 8.9 |
| Sustainion® 37-50 | 60 | 59.7 | 40.3 | 79.3 | 20.7 | 2.59 | H2O | 0.578 | FA | 0.151 | 3.8 |
| Sustainion® 37-T ePTFE Reinforced | 60 | 80.0 | 20.1 | 85.8 | 14.2 | 1.51 | H2O | 1.155 | FA | 0.191 | 6.0 |
| Sustainion® 37-T ePTFE Reinforced | 60 | 39.9 | 60.1 | 57.9 | 42.1 | 2.07 | H2O | 0.620 | FA | 0.451 | 1.4 |
| Lydall Solupor® 4PO4A | 60 | 80.0 | 20.1 | 90.3 | 9.7 | 2.33 | H2O | 0.376 | FA | 0.040 | 9.3 |
| Selected Membrane | Temperature °C | Feed wt% FA | Permeate wt% FA | H2O/FA Flux Ratio |
|---|---|---|---|---|
| Nafion® 324 | 60 | 11.7 | 3.1 | 31.3 |
| Nafion® 324 | 60 | 20.2 | 8.3 | 11.1 |
| Nafion® 324 | 60 | 30.0 | 13.6 | 6.4 |
| Nafion® 324 | 60 | 40.2 | 20.6 | 3.9 |
| Nafion® 324 | 60 | 59.4 | 34.6 | 1.9 |
| Targray SD425101 | 60 | 10.1 | 4.5 | 21.2 |
| Targray SD425101 | 60 | 19.8 | 6.4 | 14.7 |
| Targray SD425101 | 60 | 34.8 | 16.0 | 5.2 |
| Targray SD425101 | 60 | 60.1 | 45.1 | 1.2 |
| Sustainion® 37-50 | 60 | 9.9 | 6.6 | 14.1 |
| Sustainion® 37-50 | 60 | 20.1 | 10.1 | 8.9 |
| Sustainion® 37-50 | 60 | 40.3 | 20.7 | 3.8 |
| Sustainion® 37-50 T ePTFE Reinforced | 60 | 20.1 | 14.2 | 6.0 |
| Sustainion® 37-50 T ePTFE Reinforced | 60 | 60.1 | 42.1 | 1.4 |
| Lydall Solupor® 4PO4A | 60 | 20.1 | 9.7 | 9.3 |
| Membrane | Temp | Run: Collection Time | Feed Composition (wt%) | Permeate Composition (wt%) | Permeation Flux or Rate of Water and FA through Membrane | Flux Ratio | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| °C | Hours | H2O (A) | FA (B) | H2O (A) | FA (B) | kg/m2 h | kg/m2 h | H2O/FA Ratio | |||
| Targray SD425101 | 40 | 22.3 | 89.9 | 10.12 | 96.8 | 3.21 | H2O | 0.771 | FA | 0.026 | 30.2 |
| Targray SD425101 | 40 | 45.3 | 88.0 | 11.98 | 96.7 | 3.30 | H2O | 0.394 | FA | 0.013 | 29.3 |
| Targray SD425101 | 40 | 69.8 | 84.6 | 15.40 | 96.4 | 3.60 | H2O | 0.265 | FA | 0.010 | 26.8 |
| Totals | 69.8 | 74.6 | 25.38 | 96.5 | 3.49 | H2O | 0.768 | FA | 0.027 | 28.6 | |
| Feed | FA/h | Btu/h | MM Btu/kg FA | MM Btu/Tonne FA | $10/MM Btu | $8/MM Btu |
|---|---|---|---|---|---|---|
| wt% FA | kg/h | MM Btu | Steam Cost | Steam Cost | ||
| 5 | 4.91 | 0.38 | 0.077 | 77.02 | $770 | $616 |
| 10 | 9.93 | 0.36 | 0.037 | 36.56 | $366 | $292 |
| 15 | 14.93 | 0.39 | 0.026 | 25.86 | $259 | $207 |
| 20 | 19.93 | 0.41 | 0.021 | 20.52 | $205 | $164 |
| 25 | 24.93 | 0.41 | 0.016 | 16.27 | $163 | $130 |
| 30 | 29.94 | 0.40 | 0.013 | 13.31 | $133 | $107 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaczur, J.J.; McGlaughlin, L.J.; Lakkaraju, P.S. Investigating Pervaporation as a Process Method for Concentrating Formic Acid Produced from Carbon Dioxide. C 2020, 6, 42. https://doi.org/10.3390/c6020042
Kaczur JJ, McGlaughlin LJ, Lakkaraju PS. Investigating Pervaporation as a Process Method for Concentrating Formic Acid Produced from Carbon Dioxide. C. 2020; 6(2):42. https://doi.org/10.3390/c6020042
Chicago/Turabian StyleKaczur, Jerry J., Liam J. McGlaughlin, and Prasad S. Lakkaraju. 2020. "Investigating Pervaporation as a Process Method for Concentrating Formic Acid Produced from Carbon Dioxide" C 6, no. 2: 42. https://doi.org/10.3390/c6020042
APA StyleKaczur, J. J., McGlaughlin, L. J., & Lakkaraju, P. S. (2020). Investigating Pervaporation as a Process Method for Concentrating Formic Acid Produced from Carbon Dioxide. C, 6(2), 42. https://doi.org/10.3390/c6020042







