Abstract
There is currently quite a lot of scientific interest in carbon dioxide (CO2) capture and valorization with ionic liquids (ILs). In this manuscript, we analyze the influence of the potential applied, the nature of the cathode and the electrolyte using different organic mediators, such as nitro or cyano aromatic derivatives, to promote the electrochemical activation of CO2. An electrocatalytic process using a homogeneous catalysis is seen when nitroderivatives are used, yielding to oxalate in organic electrolytes and ILs. Turnover frequency (TOF) values and Farafay efficiencies were slightly higher in N,N’-dimethylformamide (DMF) than in ILs probably due to the viscosity of the electrolyte. The use of cyano derivatives allows to tune the electrochemical reactivity in function of the reduction potential value applied from electrocarboxylated products (via a nucleophile-electrophile reaction) to oxalate. These electrochemical reactions were also performed using three different cathodes, organic electrolytes and ionic liquids. The use of copper, as a cathode, and ionic liquids, as electrolytes, would be a cheaper and greener alternative for activating carbon dioxide.
1. Introduction
Nowadays methane, nitrous oxide and carbon dioxide (CO2) emissions represents approximately 98% of the total greenhouse gas (GHG) inventory worldwide [1,2,3], and their share are expected to increase this twenty-first century. CO2 represents the most important GHG, which represents approximately the 77% of the global GHG emissions (considering its global warming potential) worldwide. Moreover, the change in atmospheric CO2 concentration has been considered the most important driver of global warming. In 2019 carbon dioxide emissions from energy stagnated at 33 Gt, which are mainly due to anthropogenic activities. Besides, it is expected to increase to 40.3 Gt by 2030 and to 50 Gt by 2050, if proper measures are not taken [4,5,6,7].
In the last decade, new concepts have been developed as a suitable approaches for facing the challenges of the current global scenario; so biomimetic [8] and circular economy models have been formulated. All the approaches involve a first capture step for an efficient removal of CO2 from common points sources prior to the release of gases into atmosphere [9,10,11,12].
Carbon capture and storage (CCS) approaches include planning the confinement of CO2 into depleted oil and gas wells, deep oceans, and aquifers [13]. Capture approaches based on chemical absorption and desorption using an aqueous amine solution are also one of the most promising option for separating CO2 from fossil-fuel-derived flue gas due to its simple operation, high absorption efficiency, cost-effectiveness and maturity [14,15]. However, all these strategies only partially solve the problem, so approaches to recover valuable products from its conversion through a circular economy vision are highly desirable [16,17]. In this sense, some conversion routes are designed for the reuse of fuels, especially when inexpensive renewable energy processes are available.
New approaches that transform CO2 in fine chemicals or value-added products are also being deeply investigating. The remunerability of the product is much higher and can economically support the development of the technologies involved. However, the volume of the potential market of these fine chemicals can hardly match with the volume of CO2 emissions, needing the development of a network of parallel CCU technologies. Hence, different strategies, including biological methods, have been developed either for directly producing reduced products (i.e., carbonic anhydrase, hydrogenation of CO2 to formate, reduction of CO2 to methane, CO2 conversion into methanol by enzyme cascade) or to store CO2 in biomass (e.g., algae) [18,19,20]. The photocatalytic reduction approaches also allow to synthetize a wide spectrum of CO2 reduction products, such as HCOOH, HCHO, CH3OH, or CH4, and can be effectively used by using visible responsive materials [21,22,23,24,25,26,27]. Adsorption, CO2 activation and further reduction to produce value-added products are crucial steps for photocatalytic processes. Sharma et al. reported a combined theoretical and experimental study describing the selective reduction of CO2 into methane through a robust visible-light photocatalyst based on single-phase ternary sulfide (CTS) [28]. Zhou et al. proposed the use of aqueous suspensions of cubic ZnS nanocrystals for the photocatalytic reduction of CO2 into formate [29]; and the development of heterostructures, such as Cu2O/TiO2, for artificial photosynthesis [30].
Chemical catalytic strategies for CO2 reduction have been proposed mainly based on the Sabatier reaction [31]. Finally, it is worthy to highlight that in the last years, it has been also reported that the development of electrochemical technologies to capture and transform CO2 into high-added value products is a suitable and green way for activating CO2 compared to others Carbon Capture, Utilization and Storage (CCUS) strategies [32].
Direct carboxylation of carbon nucleophile using CO2 as an electrophile is a straightforward route to prepare carboxylic acids. The main drawback associated to this process is related to the use of toxics reagents and the production of a large amounts of waste [33]. For instance, the conventional route for synthesizing 6-aminonicotinic acid from the corresponding nitriles involves low yields, hazardous chemicals (such as cyanide and ammonia gas) and high temperatures [34,35,36]. Nevertheless, using organic electrochemistry techniques improve environmental conditions [37,38,39,40,41,42,43,44,45,46,47,48,49,50]. One of the most widely used approaches for valorizing CO2, is to use organic halides with electrochemical techniques, which makes it possible to activate the CO2 (Scheme 1, Equations (1)–(4)); In a first step, a one electron transfer process generates the organic radical anion, which later converts to an anion though a second reduction electron transfer and a halide anion. The key to this approach relies on the reduction potential value of the organic halides and the stability of the organic anion formed after the reduction process [51,52,53,54,55,56,57,58,59,60,61,62,63,64,65].
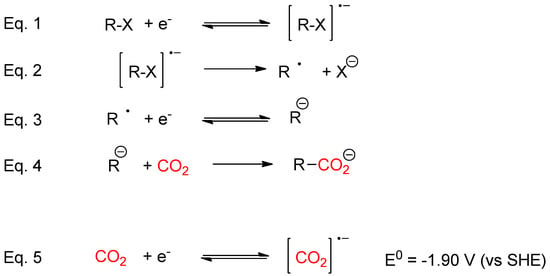
Scheme 1.
Electrochemical strategies to valorize CO2.
The development of selective electrocatalysis processes for the reduction of carbon dioxide (CO2) to yield C1 products (such as CO (2e−), or higher Cn products) would allow the use of CO2 as a carbon feedstock (Scheme 1). Nevertheless, it is necessary to overcome high-energy barriers for the direct reduction of CO2, which commonly implies reduction potential values significantly more negative than the corresponding thermodynamic reduction potential value of CO2. In this sense, different types of electrodes have been studied for direct reduction of CO2. These cathodes are classified based on the nature of the main product obtained in the electrochemical process in aqueous and non-aqueous supporting electrolytes [66]. Moreover, it has also been developed other kinds of modified or doped electrodes (with immobilized enzymes, nanoparticles or metallic oxides) to catalyze CO2 direct reduction [67,68,69]. Hence, the improvement of electrocatalytic processes could lower overpotential requirements while maintaining appropriate catalytic rates and selectivity [70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91].
One of the most common techniques to analyze electrocatalysis is cyclic voltammetry, which provides direct and rapid information regarding the relationship between driving force (i.e., overpotential) and catalytic turnover (i.e., current) compared to rather complicated alternative methods monitored by spectroscopy or other means. However, understanding detailed mechanistic schemes behind voltammograms is quite challenging due to complicated and intertwining processes, such as mass transport, electron transfer, chemical reaction and interfacial chemistry between electrode and reactants. In this sense, developing the foot of the wave analysis (FOWA) of cyclic voltammetry by Savéant and Costentin has provided a feasible method to benchmark molecular electrocatalysts by turnover frequency (TOF) and turnover number (TON) [92,93,94,95,96].
Molecular catalysts are an attractive option owing to the high degree of tunability of electronic and geometric parameters, which allows for systematic reactivity studies that can lead to new catalyst design guidelines. The well-defined structure of the catalytic sites also allows the establishment of a precise structural model for better analysis of the multiple proton-coupled electron transfer processes involved in CO2 reduction and better understanding of the CO2 reduction mechanism. For example, Leung et al. proposed a mechanism for CO production using Co-based porphyrins [97,98], where the [Co(P)–(CO2)]2− intermediate will be protonated to form [Co(P)–(COOH)]−. Later, it will decompose to provide CO. Koper and co-workers proposed a different mechanism [99], where the formation of the CO2−, anion intermediate, will be protonated yielding to [Co(P)–(COOH)]0 intermediate. Then, [Co(P)–(COOH)]0 will decompose to CO. Finally, Yao et al. proposed a pre-activation process to form a local proton source could facilitate CO2 reduction [100].
The electrocatalysts participates in an electron transfer reaction (on the electrode surface) and facilitates acceleration of a chemical reaction. Both electron transfer and chemical kinetics must be fast for an efficient electrocatalyst. In addition, an optimal electrocatalyst must display a good thermodynamic match between the redox potential (E0) for the electron transfer reaction and the chemical reaction that is being catalyzed (in this work; CO2 reduction). Chemical tuning of the electrocatalyst can optimize these factors. A general approach for an electrocatalytic system is given in Scheme 2.
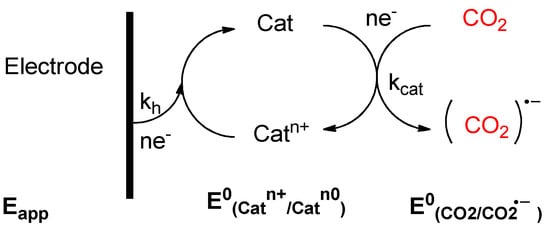
Scheme 2.
General approach for CO2 electrocatalytic system.
The electrocatalytic activity can be analyzed in terms of in cyclic voltammetry (CV). In a CV under a dry inert atmosphere, an electrocatalyst should show a reversible redox couple. Upon addition of the reagent which is catalyzed (i.e., CO2), the diffusion limited current should increase significantly, while the potential shifts anodically, and the reversibility in the return oxidation wave is lost due to the chemical reaction between reagent and electrocatalyst. Electrocatalyst offer critical solutions to lowering the overpotentials, improving selectivity, and increasing the reaction kinetics of carbon dioxide conversion [101]. There are a lot of different catalysts reported in literature, although most of them involve the use of metal complexes, such as salen ones [102,103], offering highs values of TOF with orders of h−1. On the other side, ionic liquids have been developed as a new catalyst, with a high dependence between TOF and the nature of the cation and anion which form the ionic liquid [104]. The order of TOF in these cases are in order of s−1, allowing avoiding but the use of metals.
Up to now, the main disadvantage of electrochemical technologies is the use of organic solvents such as N,N’-dimethylformamide, which are well-known to be hazardous and flammable, as well as the use of large amount of supporting electrolyte [105]. For this reason, replacing organic aprotic solvents by ionic liquids (ILs), which are considered a greener option, would improve the process [106,107,108,109]. Ionic liquids are a family of solvents with unique properties that have led to their consideration as interesting alternatives, especially for electrochemical applications. Its wide electrochemical windows and good conductivities are obtained combining unsymmetrical bulky organic cations combined with hydrophobic anions [110,111,112,113,114,115,116,117,118,119,120,121,122,123]. For electrocarboxylation process, the use of ionic liquids offers the possibility of recycling the solvent as well as to improve the CO2 capture due to their high solubility and conductivities [124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141].
Therefore, this work shows different strategies to valorize CO2 using electrochemical technologies in ionic liquids. In one hand, with a series of nitro-compounds (Figure 1) using three different cathodes (carbon, copper and silver). One the other hand, the replacement of the nitro- group for a cyano- group, involves changes in the reaction mechanism and tune the CO2 reduction products.
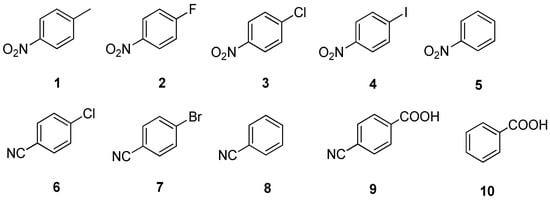
Figure 1.
Structures of discussed compounds.
2. Materials and Methods
2.1. Materials
Carburos Metálicos S.A. (Cornellà de Llobregat, Spain) provided the inert atmosphere gases (nitrogen, N2, or argon Ar) and carbon dioxide (CO2) with purity of 99.9999%. All nitro- and cyano-compounds were obtained from Sigma-Aldrich (Madrid, Spain) and used without further purification. Anhydrous N,N-dimethylformamide (DMF) solvent (99.8%) was purchased from SDS (Madrid, Spain). The ionic liquid 1-methyl-1-ethylimidazolium bis(trifluoro-methanesulfonyl)imide (EMIM TFSI, purity 99%, H2O ≤ 0.2%) was acquired from Solvionic (Toulouse, France) and was dried under vacuum using activated molecular sieves for 24 h to make sure that the amount of water was always less than H2O ≤ 0.001% [142].
2.2. Methods
2.2.1. Cyclic Voltammetry
All electrochemical experiments were performed in an electrochemical conical cell with a set-up of the three-electrode system. For CV experiments, the working electrode is a vitreous carbon disk (1 mm diameter), silver disk (3 mm diameter) and copper disk (3 mm electrode). The counter electrode is a Pt disk (<1 mm diameter). All electrodes are polished using a 1 mm diamond paste. All the electrochemical potentials were measured using a saturated calomel electrode, SCE (+0.2411 V vs. SHE) isolated from the working electrode compartment by a salt bridge (salt-solution of the reference calomel electrode is separated from the electrochemical solution by a salt-bridge ended with a ceramic material frit, allowing ionic conduction between the two solutions and avoiding appreciable contamination). Ideally, the electrolyte solution present in the bridge is the same as the one used for the electrochemical solution in order to minimize junction potentials. When such a bridge is used, the ions in the bridge are present in large excess at the junction and they carry almost the whole of the current across the boundary. In our case were used DMF/0.1M TBA BF4 (trying to weigh the same amount of support electrolyte that in the electrolyte solution) or EMIM TFSI, in both cases without electroactive substance. The error associated with the potential values is less than 5 mV. The ohmic drop can be one of the main sources of error when ILs are used as solvents, since they are more resistive media than aprotic polar solvents with 0.1 M concentration of supporting electrolyte. Before and after performing any electrochemical experiments, the solution is purged with inert gas (nitrogen or argon) for 20 min to avoid secondary reactions associated to OER and ORR due to dissolved oxygen in the solution [143,144].
The number of electrons involved in the first reduction process of nitro-compounds were determined by comparison with very well-known one-electron reduction of 9-fluorenone (redox probe), in the same medium using the same electrochemical set-up, by terms of cyclic voltammetry.
It is used 9-fluorenone and nitrobenzene as probe because both compounds have the same magnitude value of diffusion coefficient without further limitations (DMF (10−9 m2·s−1) nor ionic liquids (10−11 m2·s−1) [145,146]. The number of electrons involved in this first electron transfer was also confirmed by controlled-potential electrolysis.
2.2.2. Electrocarboxylation Processes
Cyano-compounds were electrolyzed at a negative potential of ~0.1 V more negative than the Epc potential value under nitrogen/argon or carbon dioxide saturated solutions. When the reaction is completed, the resulting solution in the electrolysis is extracted with ether. The organic layer is dried with Na2SO4 and evaporated to yield a residue that is analyzed by gas chromatography-mass spectrometry, and Proton Nuclear Magnetic Resonance (1H-NMR). Thus, all products obtained, and the commercial analogues were characterized by 1H-NMR. Measurements were made using a DPX360 (250 MHz) spectrometer (Bruker, Billerica, MA, USA) spectrometer. Proton chemical shifts were reported in ppm (d) (CDCl3, δ 7.26, or CD3CN, δ 1.94). The J values are reported in Hz.
Benzonitrile (8). Colorless liquid, 31–35% yield. 1H-NMR (CDCl3) δ (ppm): 7.70–7.55 (m, 3H), 7.47 (t, J = 7.4 Hz, 2H).
4-Cyanobenzoic acid (9). Yellow pale solid, 15–20% yield. 1H-NMR (CDCl3) δ (ppm): 8.19 (d, J = 8.5 Hz, 2H), 7.78 (d, J = 7.6 Hz, 2H).
Benzoic acid (10). Colorless solid, 7–12% yield. 1H-NMR (CDCl3) δ (ppm): 8.13 (d, J = 7.5 Hz, 2H), 7.62 (t, J = 6.8 Hz, 1H), 7.48 (t, J = 6.9 Hz, 2H).
When pure IL is used as electrolyte, the products of the electrolyzed solution are extracted with ether, allowing one to recover almost an 80% of the IL at the end of the experiment [147].
2.2.3. Determination of CO2 Concentration
A thermal mass flowmeter of modular construction with a ‘laboratory style’ pc-board housing (EL-FLOW® Mass Flow Meter/Controller) from Bronkhorst Hi-Tec (Ruurlo, Netherlands), was used to monitor the CO2 concentrations in the solution. Control valves were integrated to measure and control a gas flow from the lowest range of 0.2 up to 10 mL·min−1.
3. Results and Discussion
3.1. Electrochemical Behaviour of Nitro-Compounds under Inert Atmposphere
The electrochemical behavior of a sequence of four p-nitro-compounds was studied in different solvents, and electrodes under inert atmosphere.
Cyclic voltammograms (CVs) of a 3–10 mL solution of 1-4 in DMF using 0.10 M of tetrabutylammonium tetrafluoroborate (TBA BF4), and 1-methyl-1-ethylimidazolium bis(trifluoro-methanesulfonyl)imide (EMIM TFSI) were recorded at different scan rates (from 0.10 a 1.0 V s−1) using glassy carbon (C, blue lines), Ag (black lines) and copper, Cu (red lines) as working electrodes under a N2 atmosphere (Figure 2). Focusing in the first wave, the same general trend was observed in all electrodes and both solvents. Compounds 1-3 shows a monoelectronic fast reversible electron transfer, whereas compound 4 has an irreversible electron transfer (Table 1).
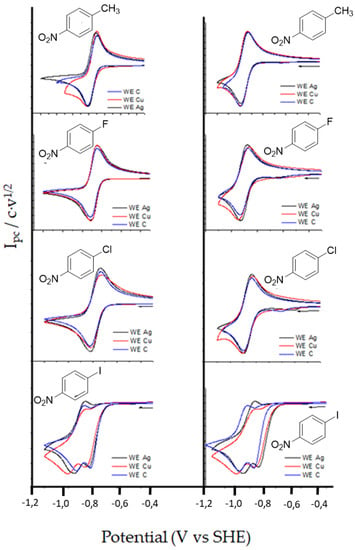
Figure 2.
Cyclic voltammetry of 10 mM p-nitro compounds 1-4 with Ag (black line), Cu (red line) and C (blue line), under nitrogen atmosphere. Scan rate: 0.5 V·s−1. Solution of DMF/0.1M TBA BF4 (left) and solution of 3 mL EMIM TFSI (right).

Table 1.
Standard potential (E°), cathodic peak potential (in V vs. SCE, Epc) and peak witdh (ΔEp (mV)) for 1-4 in different solvents using different working electrodes (WE) at 20 °C.
A closer look in CVs shows that of 4-iodonitrobenzene has irreversible wave at low scan rate, however it is possible to calculate the E0 and the value of the kinetic constant associated to the chemical reaction (k~2·104 s−1) by increasing the scan rate (Scheme 3).
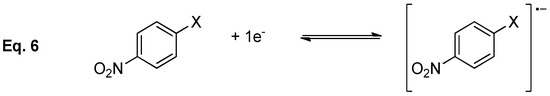
Scheme 3.
Electron transfer followed by compounds 1-4.
The cyclic voltammogram of 4 also shows a second monoelectronic reversible wave following the first irreversible one. To check the nature of the product obtained after this first electron transfer, a control potential electrolysis of 4 was performed, being nitrobenzene (5) the only product obtained. Considering cyclic voltammetry and control potential electrolysis experiments under inert atmosphere, it is possible to conclude that 4-iodonitrobenzene follows an electron transfer mechanism (E) followed by a chemical reaction (C). Hence, in a first electrochemical step (E) the radical anion, 4·-, was generated, which evolves to cleavage C-I bond, obtaining nitrobenzene radical which produces nitrobenzene and iodine with an EC mechanism (Scheme 4).
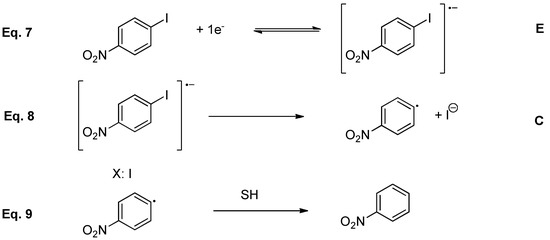
Scheme 4.
Proposal mechanism for 4-nitrobenzene electrochemical reduction under inert atmosphere.
3.2. Electrochemical Behaviour of Nitro-Compounds under CO2 Atmposphere
Compounds 1-4 shows a clearly different behaviour when the cyclic volammograms are recorded under CO2 atmosphere, although they show the same general trend in aprotic solvent and ionic liquid. Also the follow same tendency in C, Ag and Cu working electrodes; there was a rising in current value of the reduction peak and its reversibility was lost (Figure 3), which indicates that p-nitrocompounds 1-4 follow a demeanor of an organic mediator for CO2 indirect reduction through a homogeneous catalytic process (Scheme 5).
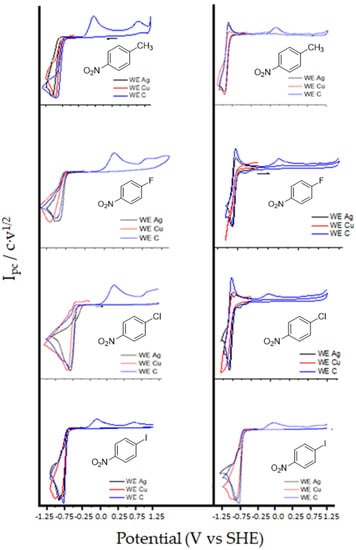
Figure 3.
Cyclic voltammetry of 10 mM p-nitro compounds 1-4 with Ag (black line), Cu (red line) and C (blue line), under CO2 atmosphere. Scan rate: 0.5 V·s−1. Solution of DMF/0.1M TBA BF4 (left) and solution of 3 mL EMIM TFSI (right).
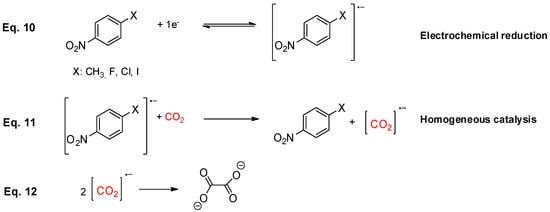
Scheme 5.
Proposal mechanism for general catalytic process between CO2 and p-nitrobenzene halides.
In the case of carbon cathode, C, it is possible to see an oxidation peak in the corresponding anodic counter scan (copper and silver electrodes would be oxidized under this experimental conditions) between +0.19 and +0.44 V (vs. SHE). In all cases this peak corresponds to the oxidation of oxalate anion [148], which is formed after reducing the nitroaromatic compound. It is important to remark that the oxidation potential value obtained for oxalate is strongly dependent of the amount of water present in the solvent (Figure 4). The same results are obtained for a sample of pure tetrabutylammonium oxalate, since different amount of water are the oxalate oxidation peak shifts to higher oxidation potentials. Moreover, by using a carbon cathode it is possible to determine the concentration of oxalate formed upon reduction using CV, since the anodic peak current value is related with the oxalate concentration. Hence, we built a calibration curve using pure tetrabutylamonium oxalate for determining the amount of oxalate obtained upon reduction for each p-nitro compound. Finally, the TOF values were obtained using through FOWA’s method. All the values were summarized in Table 2.
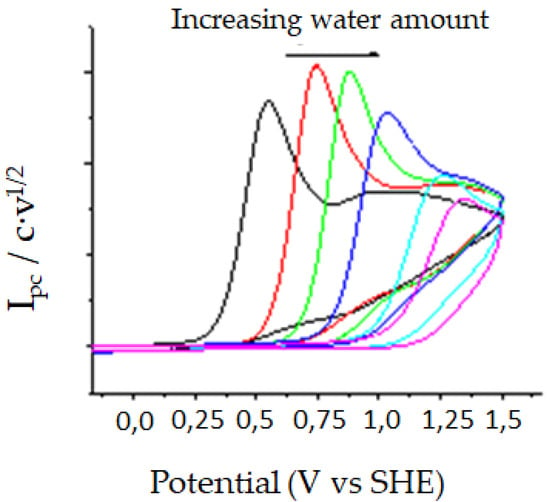
Figure 4.
Cyclic voltammetry of 5 mM TBA2C2O4 with glassy carbon under N2 atmosphere. Scan rate: 0.5 V·s−1.

Table 2.
Catalytic parameters in homogeneous catalysis of CO2.
Knowing that with carbon cathode it is possible to determine oxalate’s peak with CV, with a calibration curve is determined the amount of oxalate obtained with each p-nitro compound. Also, it is possible to obtain TOF values through Fowa’s method [92,93,94,95,96]. All the values were summarized in Table 2. Note that changing a methyl group by halides, the standard potential is lowered between 80–200 mV depending on electrode and halide. TOF values and oxalate amount obtained in DMF were higher than in EMIM TFSI. Faraday efficiencies were slightly higher in DMF solutions (from 30% to 88%) than in EMIM TFSI (from 10 to 51%) depending on the organic mediator used. These results can be explained due to low viscosity of the ionic liquid and coefficient diffusion value of CO2 (Table 3).

Table 3.
Viscosities and coefficient diffusion values of DMF and EMIM TFSI.
Note, that it is possible to see an improvement in overpotential values with the use of ionic liquid instead DMF when p-nitrocompound has a halide group in its structure. Finally, note that it is possible to use small organic compound and ionic liquids to obtaining similar TOF values (in the order of s−1) to previously published one for organometallic and more sophisticated organic compounds [89,138,149,150]. On the other hand, we observed that for compound 4 the anion radical generated after electron transfer evolves to nitrobenzene radical at the same time that the CO2 reduction is electrocatalyzed. To check if it is possible to obtain electrocarboxylation product of 5 upon reaction of its radical with CO2, a control potential electrolysis at −0.96 V (vs. SHE) was performed. After the passage of 1F, no electrocarboxylated aromatic compounds were formed, being oxalate the only electroreduced product of CO2 formed. These results seem to indicate that the nitrobenzene radical formed upon reduction of 4 (Scheme 4) show a low nucleophility, being not able to attack and capture CO2.
3.3. Electrocarboxylation of Cyano-Compounds 6 and 7 under CO2 Atmposphere
According to previous results obtained in our research group [151], 4-iodobenzonitrile follows an ECE reduction mechanism in ionic liquids (Scheme 6). In the presence of CO2 the electrogenerated anion intermediate leads to the corresponding carboxylation process. Hence, with the aim to obtain a stronger electrogenerated nucleophile, R− (Scheme 6), we decided to move from nitro to cyano aromatic compounds. Thus, two new different p-cyanobenzene halides (4-chlorobenzonitrile (6) and 4-bromobenzonitrile (7)) were explored in this section.
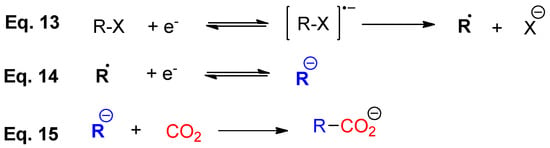
Scheme 6.
General overview for an ECE electrocarboxylation mechanism.
Figure 5 shows CVs at DMF/0.1M TBA BF4 and one example in EMIM TFSI obtained under inert atmosphere and CO2 atmosphere. All electrochemical parameters are summarized in Table 4. The same general trend was observed in aprotic solvents and ionic liquids. Moreover, not significate differences were detected when the nature of the working electrode was changed.
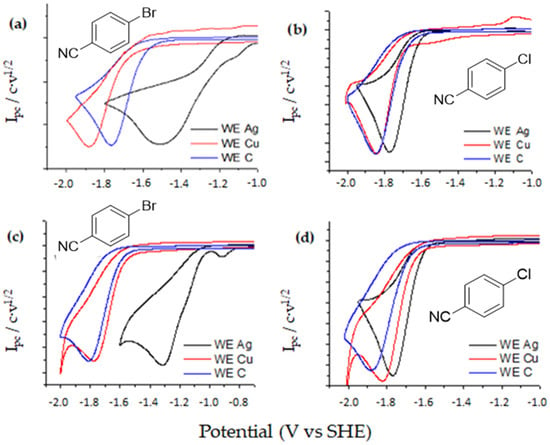
Figure 5.
Cyclic voltammetry of 10 mM p-cyano compounds 6 and 7 in DMF/0.1M TBA BF4 with Ag (black line), Cu (red line) and C (blue line), under inert atmosphere (left) and CO2 atmosphere (right). Scan rate: 0.5 V·s−1.

Table 4.
Electrochemical parameters for electroreduction of compounds 6 and 7 in DMF/0.1M TBA BF4 and EMIM TFSI.
As it was expected compounds 6 and 7 exhibit an irreversible bielectronic wave under nitrogen atmosphere for any of the working electrodes investigated (Figure 5a,b). The same electrochemical behavior is observed when DMF is replaced by EMIM TFSI. A closer look to cyclic voltammograms reveals the electrocatalytic properties of silver as a working electrode, since the peak potential value of the reduction peak is shifted to less negative potentials. In order to determine the nature of the product formed upon reduction, a controlled potential electrolysis was performed. In all the cases benzonitrile, 8, was the only product obtained after the passage of 2F. Taking into account CVs and the electrochemical data obtained from the electrolysis, it is possible to conclude that compound 6 and 7 follows an ECE mechanism. In a first electrochemical step, the radical anion of benzonitrile halide was generated. After that, a chemical reaction coupled to this first electron transfer led to a benzonitrile radical and the corresponding halide anion through a C-X cleavage reaction. Later, benzonitrile radical was reduced to its corresponding anion at the electrode surface (Scheme 7). It is important to remark that when cyano derivatives are used, it is possible to electrogenerate a strong nucleophile (benzonitrile anion), which is stable enough under our experimental conditions for reacting with CO2 leading to the corresponding electrocarboxylated product.
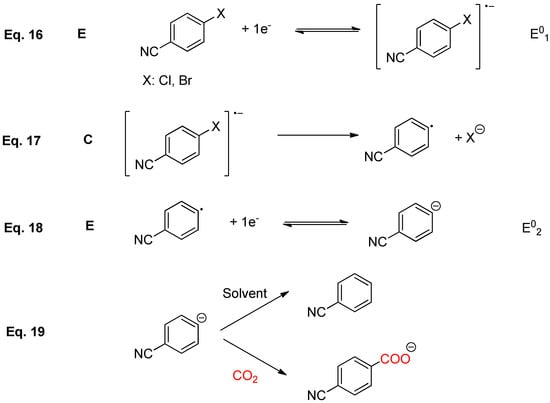
Scheme 7.
ECE proposal mechanism for the electrochemical reduction of 6 and 7.
Figure 5c,d show that halobenzonitriles showed the same electrochemical behavior, a first two-electron reduction wave is observed for halobenzonitriles (Figure 5a,b) in nitrogen and carbon dioxide atmosphere. In none of the cases the presence of oxalate anion was detected.
Once the electrochemical reduction mechanism of halobenzonitriles under CO2 atmosphere was disclosed, electrocarboxylation process were performed using carbon graphite rod. For all the compounds the applied potential was 0.1 V more negative than potential of the first reduction wave. The results of carboxylation reactions were summarized in Table 5. It could observe that p-cyanobenzoic acid (9) and benzoic acid (10) were obtained in moderated yields after the passage of 2F. Benzonitrile (8) is also obtained, showing that there was a competition between carboxylation and protonation process. Benzoic acid obtention would be explained because at the potential of electrolysis, p-cyanobenzoic acid is also reduced, since its reduction potential is c. a. −1.36 V (vs. SHE).

Table 5.
Results of the electrocarboxylation of 6 and 7 at 2F.
Electrocatalytic Behaviour of Cyano-Compounds 6 and 7 under CO2 Atmposphere
Figure 6 shows several cyclic voltammograms of 6 and 7 under nitrogen and CO2 atmosphere using silver and glassy carbon (GC) as a cathode. The use of DMF as a solvent and GC as a cathode allows to see a second monoelectronic fast reversible wave after first one (Figure 5). This second wave corresponds to the electrochemical reduction of benzonitrile, which is the only product obtained after the reduction of compounds 6 and 7. It is noticeable that benzonitrile acts as a redox mediator for CO2 reduction when reaction is performed under CO2 atmosphere, since there is an increase of the peak current value and a loss of reversibility of this second reduction wave (homogeneous indirect CO2 reduction catalytic process (Figure 6a,c)).
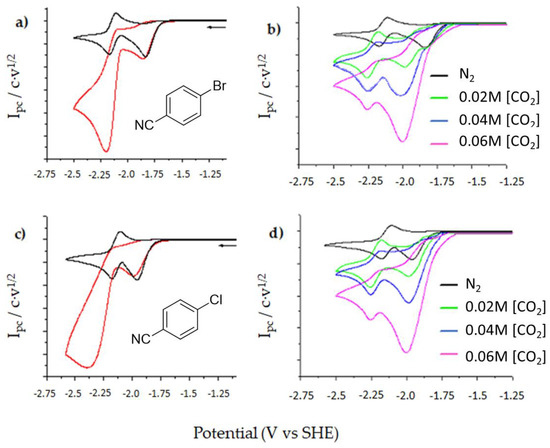
Figure 6.
Cyclic voltammograms in DMF/0.1M under nitrogen atmosphere and 20–60 mM [CO2] atmosphere using a solution of 10 mM of 6 (a) with GC (b) Ag. A solution of 10 mM of 7 with (c) GC and (d) Ag. Scan rate 0.5 V s−1.
The use of silver electrode allows to tune the CO2 reduction mechanism. In this case the current value of the first reduction is increasing as well as the peak potential value is shifting when the concentration of CO2 is rising. These results can be explained taking into account that under these experimental conditions two electrochemical processes are happening at the electrode surface at same potential; the reduction of the cyanoaromatic halide and the direct reduction of CO2 (Figure 6b,d). The same trend is observed with the use of copper cathode and a ionic liquid with wide electrochemical window, such as 1-methyl-1-propylpiperidinium bis(trifluoromethylsulphonyl)-imide ([PP13] TFSI). Note that in this cases EMIM TFSI cannot be used as a IL, since the reduction of the EMIM cation takes place c.a. −2.06 V vs. SHE. [151].
4. Conclusions
In conclusion, we describe an efficient approach for producing high value products using CO2 as building blocks. The methodology employed is based on electrochemical techniques and ILs, which provide eco-friendly chemistry solutions. These can be employed to offer a potential long-term strategy for using CO2 feedstocks. There are two different strategies to obtain a CO2 valorization product depending on the functional group, nitro or cyano, of the aromatic halide. An electrocatalytic process using a homogeneous catalysis, which provides an easy way of obtaining oxalate is seen when nitro derivatives are used, removing metal complexes as a catalyst. The use of cyano derivatives allows to tune the reactivity in function of the reduction potential value applied from electrocarboxylated products (via a nucleophile-electrophile reaction) to oxalate. These electrochemical reactions were performed with three different electrodes and in aprotic solvents and ionic liquids, which all showed the same trend. This opens the possibility of using a cooper electrode and ionic liquids to valorize CO2, which would be a cheaper and greener alternative.
Author Contributions
G.G. conceptualized the research topic, conceived and designed the experiments; S.M. perform the experiments, G.G and S.M. analyzed and interpreted the results. S.M. and G.G. prepared the manuscript. All authors corrected the draft. G.G. obt ained the funds for the research. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by MINECO/FEDER (project CTQ2015-65439-R).
Acknowledgments
This work was supported by project CTQ2015-65439-R from the MINECO/FEDER. S.M. thanks the Universitat Autònoma de Barcelona for a predoctoral PIF fellowship.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Holtsmark, B. Quantifying the global warming potential of CO2 emissions from wood fuels. GCB Bioenergy 2015, 7, 195–206. [Google Scholar] [CrossRef]
- Hu, L.; Song, Y.; Jiao, S.; Liu, Y.; Ge, J.; Jiao, H.; Zhu, J.; Wang, J.; Zhu, H.; Fray, D.J. Direct Conversion of Greenhouse Gas CO2 into Graphene via Molten Salts Electrolysis. ChemSusChem 2016, 9, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Kramm, G.; Dlugi, R. Scrutinizing the atmospheric greenhouse effect and its climatic impact. Nat. Sci. 2011, 3, 971–998. [Google Scholar] [CrossRef][Green Version]
- Mohan, S.V.; Modestra, J.A.; Amulya, K.; Butti, S.K.; Velvizhi, G. A circular bioeconomy with biobased products from co2 sequestration. Trends Biotechnol. 2016, 34, 506–519. [Google Scholar] [CrossRef] [PubMed]
- Schleussner, C.F.; Lissner, T.K.; Fischer, E.M.; Wohland, J.; Perrette, M.; Golly, A.; Rogelj, J.; Childers, K.; Schewe, J.; Frieler, K.; et al. Differential climate impacts for policy-relevant limits to global warming: The case of 1.5 °C and 2 °C. Earth Syst. Dyn. 2016, 7, 327–351. [Google Scholar] [CrossRef]
- Szulejko, J.E.; Kumar, P.; Deep, A.; Kim, K.H. Global warming projections to 2100 using simple CO2 greenhouse gas modeling and comments on CO2 climate sensitivity factor. Atmos. Pollut. Res. 2017, 8, 136–140. [Google Scholar] [CrossRef]
- Song, C. Global challenges and strategies for control, conversion and utilization of CO2 for sustainable development involving energy, catalysis, adsorption and chemical processing. Catal. Today 2006, 115, 2–32. [Google Scholar] [CrossRef]
- Cuce, E.; Nachan, Z.; Cuce, P.M.; Sher, F.; Neighbour, G.B. Strategies for ideal indoor environments towards low/zero carbon buildings through a biomimetic approach. Int. J. Ambient Energy 2019, 40, 86–95. [Google Scholar] [CrossRef]
- De Guido, G.; Compagnoni, M.; Pellegrini, L.A.; Rossetti, I. Mature versus emerging technologies for CO2 capture in power plants: Key open issues in post-combustion amine scrubbing and in chemical looping combustion. Front. Chem. Sci. Eng. 2018, 12, 315–325. [Google Scholar] [CrossRef]
- Borhani, T.N.; Wang, M. Role of solvents in CO2 capture processes: The review of selection and design methods. Renew. Sustain. Energy Rev. 2019, 114, 109299. [Google Scholar] [CrossRef]
- Asif, M.; Suleman, M.; Haq, I.; Jamal, S.A. Post-combustion CO2 capture with chemical absorption and hybrid system: Current status and challenges. Greenh. Gases Sci. Technol. 2018, 8, 998–1031. [Google Scholar] [CrossRef]
- Zhu, X.; Li, S.; Shi, Y.; Cai, N. Recent advances in elevated-temperature pressure swing adsorption for carbon capture and hydrogen production. Prog. Energy Combust. Sci. 2019, 75, 100784. [Google Scholar] [CrossRef]
- Sambo, C.; Iferobi, C.C.; Babasafari, A.A.; Rezaei, S.; Akanni, O.A. The role of 4d time lapse seismic technology as reservoir monitoring and surveillance tool: A comprehensive review. J. Nat. Gas Sci. Eng. 2020, 103312. [Google Scholar] [CrossRef]
- Thompson, J.G.; Combs, M.; Abad, K.; Bhatnagar, S.; Pelgen, J.; Beaudry, M.; Rochelle, G.; Hume, S.; Link, D.; Figueroa, J.; et al. Pilot testing of a heat integrated 0.7 MWe CO2 capture system with two-stage air-stripping: Emission. Int. J. Greenh. Gas Control 2017, 64, 267–275. [Google Scholar] [CrossRef]
- Thompson, J.G.; Bhatnagar, S.; Combs, M.; Abad, K.; Onneweer, F.; Pelgen, J.; Link, D.; Figueroa, J.; Nikolic, H.; Liu, K. Pilot testing of a heat integrated 0.7 MWe CO2 capture system with two-stage air-stripping: Amine degradation and metal accumulation. Int. J. Greenh. Gas Control 2017, 64, 23–33. [Google Scholar] [CrossRef]
- Rafiee, A.; Khalilpour, K.R.; Milani, D.; Panahi, M. Trends in CO2 conversion and utilization: A review from process systems perspective. J. Environ. Chem. Eng. 2018, 6, 5771–5794. [Google Scholar] [CrossRef]
- Hassan, M.H.A.; Sher, F.; Zarren, G.; Suleiman, N.; Tahir, A.A.; Snape, C.E. Kinetic and thermodynamic evaluation of effective combined promoters for CO2 hydrate formation. J. Nat. Gas Sci. Eng. 2020, 78, 103313. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Bhatia, R.K.; Jeon, J.-M.; Kumar, G.; Yang, Y.-H. Carbon dioxide capture and bioenergy production using biological system—A review. Renew. Sustain. Energy Rev. 2019, 110, 143–158. [Google Scholar] [CrossRef]
- Cheng, J.; Zhu, Y.; Zhang, Z.; Yang, W. Modification and improvement of microalgae strains for strengthening CO2 fixation from coal-fired flue gas in power plants. Bioresour. Technol. 2019, 291, 121850. [Google Scholar] [CrossRef]
- Cheng, F.; Porter, M.D.; Colosi, L.M. Is hydrothermal treatment coupled with carbon capture and storage an energy-producing negative emissions technology? Energy Convers. Manag. 2020, 203, 112252. [Google Scholar] [CrossRef]
- Kim, J.; Kwon, E.E. Photoconversion of carbon dioxide into fuels using semiconductors. J. CO2 Util. 2019, 33, 72–82. [Google Scholar] [CrossRef]
- Galli, F.; Compagnoni, M.; Vitali, D.; Pirola, C.; Bianchi, C.L.; Villa, A.; Prati, L.; Rossetti, I. CO2 photoreduction at high pressure to both gas and liquid products over titanium dioxide. Appl. Catal. B Environ. 2017, 200, 386–391. [Google Scholar] [CrossRef]
- Bahadori, E.; Tripodi, A.; Villa, A.; Pirola, C.; Prati, L.; Ramis, G.; Rossetti, I. High pressure photoreduction of CO2: Effect of catalyst formulation, hole scavenger addition and operating conditions. Catalysts 2018, 8, 430. [Google Scholar] [CrossRef]
- Compagnoni, M.; Ramis, G.; Freyria, F.S.; Armandi, M.; Bonelli, B.; Rossetti, I. Innovative photoreactors for unconventional photocatalytic processes: The photoreduction of CO2 and the photo-oxidation of ammonia. Rend. Lincei 2017, 28, 151–158. [Google Scholar] [CrossRef]
- Rossetti, I.; Bahadori, E.; Tripodi, A.; Villa, A.; Prati, L.; Ramis, G. Conceptual design and feasibility assessment of photoreactors for solar energy storage. Sol. Energy 2018, 172, 225–231. [Google Scholar] [CrossRef]
- Bahadori, E.; Tripodi, A.; Villa, A.; Pirola, C.; Prati, L.; Ramis, G.; Dimitratos, N.; Wang, D.; Rossetti, I. High pressure CO2 photoreduction using Au/TiO2: Unravelling the effect of co-catalysts and of titania polymorphs. Catal. Sci. Technol. 2019, 9, 2253–2265. [Google Scholar] [CrossRef]
- Rossetti, I.; Villa, A.; Pirola, C.; Prati, L.; Ramis, G. A novel high-pressure photoreactor for CO2 photoconversion to fuels. RSC Adv. 2014, 4, 28883–28885. [Google Scholar] [CrossRef]
- Sharma, N.; Das, T.; Kumar, S.; Bhosale, R.; Kabir, M.; Ogale, S. Photocatalytic Activation and Reduction of CO2 to CH4 over Single Phase Nano Cu3SnS4: A Combined Experimental and Theoretical Study. ACS Appl. Energy Mater. 2019, 2, 5677–5685. [Google Scholar] [CrossRef]
- Zhou, R.; Guzman, M.I. CO2 reduction under periodic illumination of ZnS. J. Phys. Chem. C 2014, 118, 11649–11656. [Google Scholar] [CrossRef]
- Aguirre, M.E.; Zhou, R.; Eugene, A.J.; Guzman, M.I.; Grela, M.A. Cu2O/TiO2 heterostructures for CO2 reduction through a direct Z-scheme: Protecting Cu2O from photocorrosion. Appl. Catal. B Environ. 2017, 217, 485–493. [Google Scholar] [CrossRef]
- Navarro, J.C.; Centeno, M.A.; Laguna, O.H.; Odriozola, J.A. Policies and motivations for the CO2 valorization through the sabatier reaction using structured catalysts. A review of the most recent advances. Catalysts 2018, 8, 578. [Google Scholar] [CrossRef]
- Al-Shara, N.K.; Sher, F.; Yaqoob, A.; Chen, G.Z. Electrochemical investigation of novel reference electrode Ni/Ni(OH)2 in comparison with silver and platinum inert quasi-reference electrodes for electrolysis in eutectic molten hydroxide. Int. J. Hydrogen Energy 2019, 44, 27224–27236. [Google Scholar] [CrossRef]
- Hudlicky, T. Benefits of Unconventional Methods in the Total Synthesis of Natural Products. ACS Omega 2018, 3, 17326–17340. [Google Scholar] [CrossRef]
- Dummel, R.J.; Mosher, H.S. Some Nitropyridine Derivatives. J. Org. Chem. 1959, 24, 1007–1009. [Google Scholar] [CrossRef]
- Gennaro, A.; Sánchez-Sánchez, C.M.; Isse, A.A.; Montiel, V. Electrocatalytic synthesis of 6-aminonicotinic acid at silver cathodes under mild conditions. Electrochem. Commun. 2004, 6, 627–631. [Google Scholar] [CrossRef]
- Ramesh Raju, R.; Krishna Mohan, S.; Jayarama Reddy, S. Electroorganic synthesis of 6-aminonicotinic acid from 2-amino-5-chloropyridine. Tetrahedron Lett. 2003, 44, 4133–4135. [Google Scholar] [CrossRef]
- Amatore, C.; Savéant, J.M. Mechanism and Kinetic Characteristics of the Electrochemical Reduction of Carbon Dioxide in Media of Low Proton Availability. J. Am. Chem. Soc. 1981, 103, 5021–5023. [Google Scholar] [CrossRef]
- Frontana-Uribe, B.A.; Little, R.D.; Ibanez, J.G.; Palma, A.; Vasquez-Medrano, R. Organic electrosynthesis: A promising green methodology in organic chemistry. Green Chem. 2010, 12, 2099. [Google Scholar] [CrossRef]
- Gennaro, A.; Isse, A.A.; Severin, M.-G.; Vianello, E.; Bhugun, I.; Savéant, J.-M. Mechanism of the electrochemical reduction of carbon dioxide at inert electrodes in media of low proton availability. J. Chem. Soc. Faraday Trans. 1996, 92, 3963–3968. [Google Scholar] [CrossRef]
- Isse, A.A.; Galia, A.; Belfiore, C.; Silvestri, G.; Gennaro, A. Electrochemical reduction and carboxylation of halobenzophenones. J. Electroanal. Chem. 2002, 526, 41–52. [Google Scholar] [CrossRef]
- Machado, A.S.R.; Nunes, A.V.M.; da Ponte, M.N. Carbon dioxide utilization-Electrochemical reduction to fuels and synthesis of polycarbonates. J. Supercrit. Fluids 2018, 134, 150–156. [Google Scholar] [CrossRef]
- Mateos, R.; Escapa, A.; Vanbroekhoven, K.; Patil, S.A.; Moran, A.; Pant, D. Microbial Electrochemical Technologies for CO2 and Its Derived Products Valorization. In Microbial Electrochemical Technology; Mohan, S.V., Varjani, S., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 777–796. ISBN 9780444640529. [Google Scholar]
- Matthessen, R.; Fransaer, J.; Binnemans, K.; De Vos, D.E. Electrocarboxylation: Towards sustainable and efficient synthesis of valuable carboxylic acids. Beilstein J. Org. Chem. 2014, 10, 2484–2500. [Google Scholar] [CrossRef]
- Senboku, H.; Katayama, A. Electrochemical carboxylation with carbon dioxide. Curr. Opin. Green Sustain. Chem. 2017, 3, 50–54. [Google Scholar] [CrossRef]
- Tokuda, M. Efficient fixation of carbon dioxide by electrolysis—Facile synthesis of useful carboxylic acids—. J. Nat. Gas. Chem. 2006, 15, 275–281. [Google Scholar] [CrossRef]
- Whipple, D.T.; Kenis, P.J.A. Prospects of CO2 utilization via direct heterogeneous electrochemical reduction. J. Phys. Chem. Lett. 2010, 1, 3451–3458. [Google Scholar] [CrossRef]
- Windle, C.D.; Perutz, R.N. Advances in molecular photocatalytic and electrocatalytic CO2 reduction. Coord. Chem. Rev. 2012, 256, 2562–2570. [Google Scholar] [CrossRef]
- Yuan, X.; Lu, B.; Liu, J.; You, X.; Zhao, J.; Cai, Q. Electrochemical conversion of methanol and carbon dioxide to dimethyl carbonate at graphite-Pt electrode system. J. Electrochem. Soc. 2012, 159, 183–186. [Google Scholar] [CrossRef]
- Zhang, W.; Lü, X. Synthesis of carboxylic acids and derivatives using CO2 as carboxylative reagent. Chin. J. Catal. 2012, 33, 745–756. [Google Scholar] [CrossRef]
- Damodar, J.; Krishna Mohan, S.; Khaja Lateef, S.K.; Jayarama Reddy, S. Electrosynthesis of 2-arylpropionic acids from α-methylbenzyl chlorides and carbon dioxide by [Co(Salen)]. Synth. Commun. 2005, 35, 1143–1150. [Google Scholar] [CrossRef]
- Correa, A.; Martin, R. ChemInform abstract: Palladium-catalyzed direct carboxylation of aryl bromides with carbon dioxide. ChemInform 2010, 41, 15974–15975. [Google Scholar] [CrossRef]
- Damodar, J.; Krishna Mohan, S.R.; Jayarama Reddy, S.R. Synthesis of 2-arylpropionic acids by electrocarboxylation of benzylchlorides catalysed by PdCl2(PPh3)2. Electrochem. Commun. 2001, 3, 762–766. [Google Scholar] [CrossRef]
- Durante, C.; Isse, A.A.; Todesco, F.; Gennaro, A. Electrocatalytic activation of aromatic carbon-bromine bonds toward carboxylation at silver and copper cathodes. J. Electrochem. Soc. 2013, 160, G3073–G3079. [Google Scholar] [CrossRef]
- Durante, C.; Isse, A.A.; Sandonà, G.; Gennaro, A. Electrochemical hydrodehalogenation of polychloromethanes at silver and carbon electrodes. Appl. Catal. B Environ. 2009, 88, 479–489. [Google Scholar] [CrossRef]
- Isse, A.A.; De Giusti, A.; Gennaro, A.; Falciola, L.; Mussini, P.R. Electrochemical reduction of benzyl halides at a silver electrode. Electrochim. Acta 2006, 51, 4956–4964. [Google Scholar] [CrossRef]
- Isse, A.A.; Durante, C.; Gennaro, A. One-pot synthesis of benzoic acid by electrocatalytic reduction of bromobenzene in the presence of CO2. Electrochem. Commun. 2011, 13, 810–813. [Google Scholar] [CrossRef]
- Isse, A.A.; Falciola, L.; Mussini, P.R.; Gennaro, A. Relevance of electron transfer mechanism in electrocatalysis: The reduction of organic halides at silver electrodes. Chem. Commun. 2006, 1, 344–346. [Google Scholar] [CrossRef]
- Isse, A.A.; Gennaro, A. Electrochemical synthesis of cyanoacetic acid from chloroacetonitrile and carbon dioxide. J. Electrochem. Soc. 2002, 149, D113. [Google Scholar] [CrossRef]
- Isse, A.A.; Gottardello, S.; Durante, C.; Gennaro, A. Dissociative electron transfer to organic chlorides: Electrocatalysis at metal cathodes. Phys. Chem. Chem. Phys. 2008, 10, 2409–2416. [Google Scholar] [CrossRef]
- Isse, A.A.; Ferlin, M.G.; Gennaro, A. Electrocatalytic reduction of arylethyl chlorides at silver cathodes in the presence of carbon dioxide: Synthesis of 2-arylpropanoic acids. J. Electroanal. Chem. 2005, 581, 38–45. [Google Scholar] [CrossRef]
- Korsager, S.; Taaning, R.H.; Skrydstrup, T. Effective palladium-catalyzed hydroxycarbonylation of aryl halides with substoichiometric carbon monoxide. J. Am. Chem. Soc. 2013, 135, 2891–2894. [Google Scholar] [CrossRef]
- Lugaresi, O.; Minguzzi, A.; Locatelli, C.; Vertova, A.; Rondinini, S.; Amatore, C. Benzyl chloride electroreduction on Ag cathodes in CH3CN in the presence of small amounts of water: Evidences of quantitative effects on reaction rates and mechanism. Electrocatalysis 2013, 4, 353–357. [Google Scholar] [CrossRef]
- Scialdone, O.; Galia, A.; Filardo, G.; Isse, A.A.; Gennaro, A. Electrocatalytic carboxylation of chloroacetonitrile at a silver cathode for the synthesis of cyanoacetic acid. Electrochim. Acta 2008, 54, 634–642. [Google Scholar] [CrossRef]
- Yoo, W.J.; Kondo, J.; Rodríguez-Santamaría, J.A.; Nguyen, T.V.Q.; Kobayashi, S. Efficient synthesis of α-trifluoromethyl carboxylic acids and esters through fluorocarboxylation of gem-difluoroalkenes. Angew. Chem. Int. Ed. 2019, 58, 6772–6775. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Hara, S.; Senboku, H. Synthesis of 2-aryl-3,3,3-trifluoropropanoic acids using electrochemical carboxylation of (1-bromo-2,2,2-trifluoroethyl)arenes and its application to the synthesis of β,β,β-trifluorinated non-steroidal anti-inflammatory drugs. Tetrahedron 2010, 66, 473–479. [Google Scholar] [CrossRef]
- Scibioh, M.A.; Viswanathan, B. Electrochemical reduction of carbon dioxide: A status report. Proc. Indian Natl. Sci. Acad. 2004, 70, 407–462. [Google Scholar]
- Schlager, S.; Dumitru, L.M.; Haberbauer, M.; Fuchsbauer, A.; Neugebauer, H.; Hiemetsberger, D.; Wagner, A.; Portenkirchner, E.; Sariciftci, N.S. Electrochemical reduction of carbon dioxide to methanol by direct injection of electrons into immobilized enzymes on a modified electrode. ChemSusChem 2016, 9, 631–635. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Alinajafi, H.A.; Rezaei, B. Pt-modified nitrogen doped reduced graphene oxide: A powerful electrocatalyst for direct CO2 reduction to methanol. J. Electroanal. Chem. 2016, 783, 82–89. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Q.; Luo, J.-L. In-situ exsolved alloy nanoparticles on perovskite for direct CO2 reduction. ECS Trans. 2017, 75, 1–6. [Google Scholar] [CrossRef]
- Savéant, J.M. Molecular catalysis of electrochemical reactions. Mechanistic aspects. Chem. Rev. 2008, 108, 2348–2378. [Google Scholar] [CrossRef]
- Feng, D.M.; Zhu, Y.P.; Chen, P.; Ma, T.Y. Recent advances in transition-metal-mediated electrocatalytic CO2 reduction: From homogeneous to heterogeneous systems. Catalysts 2017, 7, 373. [Google Scholar] [CrossRef]
- Kang, P.; Chen, Z.; Brookhart, M.; Meyer, T.J. Electrocatalytic Reduction of Carbon Dioxide: Let the molecules do the work. Top. Catal. 2015, 58, 30–45. [Google Scholar] [CrossRef]
- Hammouche, M.; Lexa, D.; Savêant, J.M.; Momenteau, M. Chemical catalysis of electrochemical reactions. Homogeneous catalysis of the electrochemical reduction of carbon dioxide by iron (“0”) porphyrins. Role of the addition of magnesium cations. J. Am. Chem. Soc. 1991, 113, 8455–8466. [Google Scholar] [CrossRef]
- Laitar, D.S.; Müller, P.; Sadighi, J.P. Efficient homogeneous catalysis in the reduction of CO2 to CO. J. Am. Chem. Soc. 2005, 127, 17196–17197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Fan, Q.; Xia, R.; Meyer, T.J. CO2 reduction: From homogeneous to heterogeneous electrocatalysis. Acc. Chem. Res. 2020, 53, 255–264. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, J.; Wang, H.; Chen, L.; Bian, Z. Photocatalytic and electrocatalytic reduction of CO2 to methanol by the homogeneous pyridine-based systems. Appl. Catal. A Gen. 2016, 520, 1–6. [Google Scholar] [CrossRef]
- Theaker, N.; Strain, J.M.; Kumar, B.; Brian, J.P.; Kumari, S.; Spurgeon, J.M. Heterogeneously catalyzed two-step cascade electrochemical reduction of CO2 to ethanol. Electrochim. Acta 2018, 274, 1–8. [Google Scholar] [CrossRef]
- Geri, J.B.; Ciatti, J.L.; Szymczak, N.K. Charge effects regulate reversible CO2 reduction catalysis. Chem. Commun. 2018, 54, 7790–7793. [Google Scholar] [CrossRef]
- Benson, E.E.; Kubiak, C.P.; Sathrum, A.J.; Smieja, J.M. Electrocatalytic and homogeneous approaches to conversion of CO2 to liquid fuels. Chem. Soc. Rev. 2009, 38, 89–99. [Google Scholar] [CrossRef]
- Grills, D.C.; Ertem, M.Z.; McKinnon, M.; Ngo, K.T.; Rochford, J. Mechanistic aspects of CO2 reduction catalysis with manganese-based molecular catalysts. Coord. Chem. Rev. 2018, 374, 173–217. [Google Scholar] [CrossRef]
- Luan, Y.X.; Ye, M. Transition metal-mediated or catalyzed hydrocarboxylation of olefins with CO2. Tetrahedron Lett. 2018, 59, 853–861. [Google Scholar] [CrossRef]
- Xie, J.N.; Yu, B.; Zhou, Z.H.; Fu, H.C.; Wang, N.; He, L.N. Copper(I)-based ionic liquid-catalyzed carboxylation of terminal alkynes with CO2 at atmospheric pressure. Tetrahedron Lett. 2015, 56, 7059–7062. [Google Scholar] [CrossRef]
- Mizuno, H.; Takaya, J.; Iwasawa, N. Rhodium(I)-catalyzed direct carboxylation of arenes with CO2 via chelation-assisted C-H bond activation. J. Am. Chem. Soc. 2011, 133, 1251–1253. [Google Scholar] [CrossRef]
- Honda, M.; Tamura, M.; Nakagawa, Y.; Tomishige, K. Catalytic CO2 conversion to organic carbonates with alcohols in combination with dehydration system. Catal. Sci. Technol. 2014, 4, 2830–2845. [Google Scholar] [CrossRef]
- Tappe, N.A.; Reich, R.M.; D’Elia, V.; Kühn, F.E. Current advances in the catalytic conversion of carbon dioxide by molecular catalysts: An update. Dalt. Trans. 2018, 47, 13281–13313. [Google Scholar] [CrossRef]
- Kleij, A.W.; North, M.; Urakawa, A. CO2 Catalysis. ChemSusChem 2017, 10, 1036–1038. [Google Scholar] [CrossRef]
- Dey, G.R.; Belapurkar, A.D.; Kishore, K. Photo-catalytic reduction of carbon dioxide to methane using TiO2 as suspension in water. J. Photochem. Photobiol. A Chem. 2004, 163, 503–508. [Google Scholar] [CrossRef]
- Veselovskaya, J.V.; Parunin, P.D.; Netskina, O.V.; Kibis, L.S.; Lysikov, A.I.; Okunev, A.G. Catalytic methanation of carbon dioxide captured from ambient air. Energy 2018, 159, 766–773. [Google Scholar] [CrossRef]
- Zhao, G.; Huang, X.; Wang, X.; Wang, X. Progress in catalyst exploration for heterogeneous CO2 reduction and utilization: A critical review. J. Mater. Chem. A 2017, 5, 21625–21649. [Google Scholar] [CrossRef]
- Dokania, A.; Ramirez, A.; Bavykina, A.; Gascon, J. Heterogeneous Catalysis for the Valorization of CO2: Role of Bifunctional Processes in the Production of Chemicals. ACS Energy Lett. 2018, 4, 167–176. [Google Scholar] [CrossRef]
- Gennaro, A.; Isse, A.A.; Savéant, J.M.; Severin, M.G.; Vianello, E. Homogeneous electron transfer catalysis of the electrochemical reduction of carbon dioxide. Do aromatic anion radicals react in an outer-sphere manner? J. Am. Chem. Soc. 1996, 118, 7190–7196. [Google Scholar] [CrossRef]
- Costentin, C.; Savéant, J.-M. Multielectron, multistep molecular catalysis of electrochemical reactions: Benchmarking of homogeneous catalysts. ChemElectroChem 2014, 1, 1226–1236. [Google Scholar] [CrossRef]
- Costentin, C.; Savéant, J.M. Homogeneous catalysis of electrochemical reactions: The steady-state and nonsteady-state statuses of intermediates. ACS Catal. 2018, 8, 5286–5297. [Google Scholar] [CrossRef]
- Costentin, C.; Robert, M.; Savéant, J.M. Catalysis of the electrochemical reduction of carbon dioxide. Chem. Soc. Rev. 2013, 42, 2423–2436. [Google Scholar] [CrossRef]
- Costentin, C.; Savéant, J.M. Homogeneous molecular catalysis of electrochemical reactions: Catalyst benchmarking and optimization strategies. J. Am. Chem. Soc. 2017, 139, 8245–8250. [Google Scholar] [CrossRef]
- Zhang, B.A.; Ozel, T.; Elias, J.S.; Costentin, C.; Nocera, D.G. Interplay of homogeneous reactions, mass transport, and kinetics in determining selectivity of the reduction of CO2 on gold electrodes. ACS Cent. Sci. 2019, 5, 1097–1105. [Google Scholar] [CrossRef]
- Nielsen, I.M.B.; Leung, K. Cobalt-porphyrin catalyzed electrochemical reduction of carbon dioxide in water. 1. A density functional study of intermediates. J. Phys. Chem. A 2010, 114, 10166–10173. [Google Scholar] [CrossRef]
- Leung, K.; Nielsen, I.M.B.; Sai, N.; Medforth, C.; Shelnutt, J.A. Cobalt-porphyrin catalyzed electrochemical reduction of carbon dioxide in water. 2. Mechanism from first principles. J. Phys. Chem. A 2010, 114, 10174–10184. [Google Scholar] [CrossRef]
- Shen, J.; Kolb, M.J.; Göttle, A.J.; Koper, M.T.M. DFT Study on the mechanism of the electrochemical reduction of CO2 catalyzed by cobalt porphyrins. J. Phys. Chem. C 2016, 120, 15714–15721. [Google Scholar] [CrossRef]
- Yao, C.L.; Li, J.C.; Gao, W.; Jiang, Q. Cobalt-porphine catalyzed CO2 electro-reduction: A novel protonation mechanism. Phys. Chem. Chem. Phys. 2017, 19, 15067–15072. [Google Scholar] [CrossRef]
- Bard, J.A.; Faulkner, L.R. Fundamentals and Applications, 2nd ed.; Harris, D., Swain, E., Robey, C., Aiello, E., Eds.; Wiley: Austin, TX, USA, 1990; ISBN 0471043729. [Google Scholar]
- Darensbourg, D.J. Making Plastics from Carbon Dioxide: Salen Metal Complexes as Catalysts for the Production of Polycarbonates from Epoxides and CO2. Chem. Rev. 2007, 107, 2388–2410. [Google Scholar] [CrossRef]
- Sakakura, T.; Choi, J.C.; Yasuda, H. Transformation of carbon dioxide. Chem. Rev. 2007, 107, 2365–2387. [Google Scholar] [CrossRef]
- Feng, J.; Zeng, S.; Feng, J.; Dong, H.; Zhang, X. CO2 electroreduction in ionic liquids: A review. Chin. J. Chem. 2018, 36, 961–970. [Google Scholar] [CrossRef]
- Gallardo, I.; Soler, S. Electrochemically promoted arylation of iodoaromatics. J. Electroanal. Chem. 2017, 799, 9–16. [Google Scholar] [CrossRef]
- Das, R.N.; Roy, K. Development of classification and regression models for Vibrio fischeri toxicity of ionic liquids: Green solvents for the future. Toxicol. Res. 2012, 1, 186–195. [Google Scholar] [CrossRef]
- Armand, M.; Endres, F.; Mac Farlane, D.R.; Ohno, H.; Scrosati, B. Ionic-liquid materials for the electrochemical challenges of the future. Nat. Mater 2009, 8, 621–629. [Google Scholar]
- Allen, G.D.; Buzzeo, M.C.; Davies, I.G.; Villagrán, C.; Hardacre, C.; Compton, R.G. A comparative study on the reactivity of electrogenerated bromine with cyclohexene in acetonitrile and the room temperature ionic liquid, 1-Butyl-3-methylimidazolium bis[(trifluoromethyl)sulfonyl]imide. J. Phys. Chem. B 2004, 108, 16322–16327. [Google Scholar] [CrossRef]
- Maca, J.; Sedlarikova, M.; Libich, J.; Kazda, T.; Vondrak, J. Ionic Liquids as Electrolytes and Aging Process. ECS Trans. 2016, 74, 179–184. [Google Scholar] [CrossRef]
- Barrosse-Antle, L.E.; Bond, A.M.; Compton, R.G.; O’Mahony, A.M.; Rogers, E.I.; Silvester, D.S. Voltammetry in room temperature ionic liquids: Comparisons and contrasts with conventional electrochemical solvents. Chem. Asian J. 2010, 5, 202–230. [Google Scholar] [CrossRef]
- Bhatt, V.D.; Gohil, K. Ion exchange synthesis and thermal characteristics of some [N2222]+ based ionic liquids. Bull. Mater. Sci. 2013, 36, 1121–1125. [Google Scholar] [CrossRef][Green Version]
- Cruz, H.; Gallardo, I.; Guirado, G. Understanding specific effects on the standard potential shifts of electrogenerated species in 1-butyl-3-methylimidazolium ionic liquids. Electrochim. Acta 2008, 53, 5968–5976. [Google Scholar] [CrossRef]
- Deetlefs, M.; Seddon, K.R.; Shara, M. Predicting physical properties of ionic liquids. Phys. Chem. Chem. Phys. 2006, 8, 642–649. [Google Scholar] [CrossRef]
- Doherty, A.P.; Brooks, C.A. Organic Electrochemistry in Ionic Liquids; Rogers, R., Seddon, K.R., Eds.; American Chemical Society: Washington, DC, USA, 2003; pp. 410–420. ISBN 978084123856. [Google Scholar]
- Earle, M.J.; Esperança, J.M.S.S.; Gilea, M.A.; Lopes, J.N.C.; Rebelo, L.P.N.; Magee, J.W.; Seddon, K.R.; Widegren, J.A. The distillation and volatility of ionic liquids. Nature 2006, 439, 831–834. [Google Scholar] [CrossRef]
- Gutowski, K.E. Industrial uses and applications of ionic liquids 1. Phys. Sci. Rev. 2018, 3. [Google Scholar] [CrossRef]
- Hayyan, M.; Mjalli, F.S.; Hashim, M.A.; AlNashef, I.M.; Mei, T.X. Investigating the electrochemical windows of ionic liquids. J. Ind. Eng. Chem. 2013, 19, 106–112. [Google Scholar] [CrossRef]
- Jensen, M.P.; Neuefeind, J.; Beitz, J.V.; Skanthakumar, S.; Soderholm, L. Mechanisms of metal ion transfer into room-temperature ionic liquids: The role of anion exchange. J. Am. Chem. Soc. 2003, 125, 15466–15473. [Google Scholar] [CrossRef]
- Picquet, M.; Tkatchenko, I.; Tommasi, I.; Wasserscheid, P.; Zimmermann, J. Ionic Liquids, 3. Synthesis and utilisation of protic imidazolium salts in homogeneous catalysis. Adv. Synth. Catal. 2003, 345, 959–962. [Google Scholar] [CrossRef]
- Reche, I.; Gallardo, I.; Guirado, G. The role of cations in the reduction of 9-fluorenone in bis (trifluoromethylsulfonyl) imide room temperature ionic liquids. New J. Chem. 2014, 38, 5030–5036. [Google Scholar] [CrossRef]
- Seddon, K.R. Ionic Liquids for Clean Technolog. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 1997, 64, 351–356. [Google Scholar]
- Wasserscheid, P.; Welton, T. Ionic Liquids Ionic Liquids. Top. Curr. Chem. 1999, 1, 223–226. [Google Scholar]
- Welton, T. Ionic liquids: A brief history. Biophys. Rev. 2018, 10, 691–706. [Google Scholar] [CrossRef]
- Zhou, F.; Liu, S.; Yang, B.; Wang, P.; Alshammari, A.S.; Deng, Y. Electrochemistry communications highly selective and stable electro-catalytic system with ionic liquids for the reduction of carbon dioxide to carbon monoxide. Electrochem. Commun. 2015, 55, 43–46. [Google Scholar] [CrossRef]
- Tateno, H.; Nakabayashi, K.; Kashiwagi, T.; Senboku, H.; Atobe, M. Electrochemical fixation of CO2 to organohalides in room-temperature ionic liquids under supercritical CO2. Electrochim. Acta 2015, 161, 212–218. [Google Scholar] [CrossRef]
- Tanner, E.E.L.; Batchelor-McAuley, C.; Compton, R.G. Carbon dioxide reduction in room-temperature ionic liquids: The effect of the choice of electrode material, cation, and anion. J. Phys. Chem. C 2016, 120, 26442–26447. [Google Scholar] [CrossRef]
- Sung, S.; Kumar, D.; Gil-Sepulcre, M.; Nippe, M. Electrocatalytic CO2 reduction by imidazolium-functionalized molecular catalysts. J. Am. Chem. Soc. 2017, 139, 13993–13996. [Google Scholar] [CrossRef]
- Sun, L.; Ramesha, G.K.; Kamat, P.V.; Brennecke, J.F. Switching the reaction course of electrochemical CO2 reduction with ionic liquids. Langmuir 2014, 30, 6302–6308. [Google Scholar] [CrossRef]
- Rosen, B.A.; Salehi-Khojin, A.; Thorson, M.R.; Zhu, W.; Whipple, D.T.; Kenis, P.J.A.; Masel, R.I. Ionic liquid-mediated selective conversion of CO2 to CO at low overpotentials. Science 2011, 334, 643–644. [Google Scholar] [CrossRef]
- Reche, I.; Gallardo, I.; Guirado, G. Cyclic voltammetry using silver as cathode material: A simple method for determining electro and chemical features and solubility values of CO2 in ionic liquids. Phys. Chem. Chem. Phys. 2015, 17, 2339–2343. [Google Scholar] [CrossRef]
- Reche, I.; Gallardo, I.; Guirado, G. Electrochemical studies of CO2 in imidazolium ionic liquids using silver as a working electrode: A suitable approach for determining diffusion coefficients, solubility values, and electrocatalytic effects. RSC Adv. 2014, 4, 65176–65183. [Google Scholar] [CrossRef]
- Niu, D.; Zhang, J.; Zhang, K.; Xue, T.; Lu, J. Electrocatalytic carboxylation of benzyl chloride at silver cathode in ionic liquid BMIMBF4. Chin. J. Chem. 2009, 27, 1041–1044. [Google Scholar] [CrossRef]
- Mena, S.; Sanchez, J.; Guirado, G. Electrocarboxylation of 1-cholor-(4-isobutylphenyl)ethane with a silver cathode in ionic liquids: An environmentally benign and efficient way to synthesize Ibuprofen. RSC Adv. 2019, 9, 15115–15123. [Google Scholar] [CrossRef]
- Mei, K.; He, X.; Chen, K.; Zhou, X.; Li, H.; Wang, C. Highly efficient CO2 capture by imidazolium ionic liquids through a reduction in the formation of the carbene−CO2 complex. Ind. Eng. Chem. Res. 2017, 56, 8066–8072. [Google Scholar] [CrossRef]
- Marrucho, I.M.; Branco, L.C.; Rebelo, L.P.N. Ionic liquids in pharmaceutical applications. Annu. Rev. Chem. Biomol. Eng. 2014, 5, 527–546. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhang, P.; Zhang, S.; Yan, T.; Xin, J.; Zhang, X. Ionic liquids and supercritical carbon dioxide: Green and alternative reaction media for chemical processes. Rev. Chem. Eng. 2016, 32, 587–609. [Google Scholar] [CrossRef]
- Lim, H.K.; Kim, H. The mechanism of room-Temperature ionic-liquid-based electrochemical CO2 reduction: A review. Molecules 2017, 22, 536. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jia, D.; Guo, Z.; Liu, Y.; Lyu, Y.; Zhou, Y.; Wang, J. Imidazolinium based porous hypercrosslinked ionic polymers for efficient CO2 capture and fixation with epoxides. Green Chem. 2017, 19, 2675–2686. [Google Scholar] [CrossRef]
- Lau, G.P.S.; Schreier, M.; Vasilyev, D.; Scopelliti, R.; Gra, M.; Dyson, P.J. New Insights Into the Role of Imidazolium-Based Promoters for the Electroreduction of CO2 on a Silver Electrode. J. Am. Chem. Soc. 2016, 138, 7820–7823. [Google Scholar] [CrossRef]
- Faggion, D., Jr.; Gonçalves, W.D.G.; Dupont, J. CO2 Electroreduction in Ionic Liquids. Front. Chem. 2019, 7, 1–8. [Google Scholar] [CrossRef]
- Aghaie, M.; Rezaei, N.; Zendehboudi, S. A systematic review on CO2 capture with ionic liquids: Current status and future prospects. Renew. Sustain. Energy Rev. 2018, 96, 502–525. [Google Scholar] [CrossRef]
- Williams, D.B.G.; Lawton, M. Drying of organic solvents: Quantitative evaluation of the efficiency of several desiccants. J. Org. Chem. 2010, 75, 8351–8354. [Google Scholar] [CrossRef]
- Strmcnik, D. When small is big: The role of impurities in electrocatalysis. Top. Catal. 2015, 58, 1174–1180. [Google Scholar] [CrossRef]
- Taylor, R.J. Electrochemical studies on glassy carbon electrodes. III. Oxygen reduction in solutions of low pH (pH <10). J. Electroanal. Chem. 1975, 64, 85. [Google Scholar]
- Saveant, J.M.; Tessier, D. Potential dependence of the electrochemical transfer coefficient. Reduction of some nitro compounds in aprotic media. J. Phys. Chem. 1977, 81, 2192–2197. [Google Scholar] [CrossRef]
- Brooks, C.A. Electrochemistry in Ionic Liquids; Torriero, A.A.J., Ed.; Springer: London, UK, 2002; Volume 2002–19, ISBN 9783319151311. [Google Scholar]
- Mena, S.; Santiago, S.; Gallardo, I.; Guirado, G. Sustainable and efficient electrosynthesis of naproxen using carbon dioxide and ionic liquids. Chemosphere 2020, 245, 125557. [Google Scholar] [CrossRef]
- Kumar, B.; Llorente, M.; Froehlich, J.; Dang, T.; Sathrum, A.; Kubiak, C.P. Photochemical and photoelectrochemical reduction of CO2. Annu. Rev. Phys. Chem. 2012, 63, 541–569. [Google Scholar] [CrossRef]
- Al-Omari, A.A.; Yamani, Z.H.; Nguyen, H.L. Electrocatalytic CO2 reduction: From homogeneous catalysts to heterogeneous-based reticular chemistry. Molecules 2018, 23, 2835. [Google Scholar] [CrossRef]
- Yang, N.; Waldvogel, S.R.; Jiang, X. Electrochemistry of carbon dioxide on carbon electrodes. ACS Appl. Mater. Interfaces 2016, 8, 28357–28371. [Google Scholar] [CrossRef]
- Mena, S.; Gallardo, I.; Guirado, G. Electrocatalytic Processes for the Valorization of CO2: Synthesis of cyanobenzoic acid using eco-friendly strategies. Catalysts 2019, 9, 413. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).