Supercapacitor Using Polypyrrole and Carbon Nanotube Composite as Electrodes
Abstract
1. Introduction
2. Materials and Methods
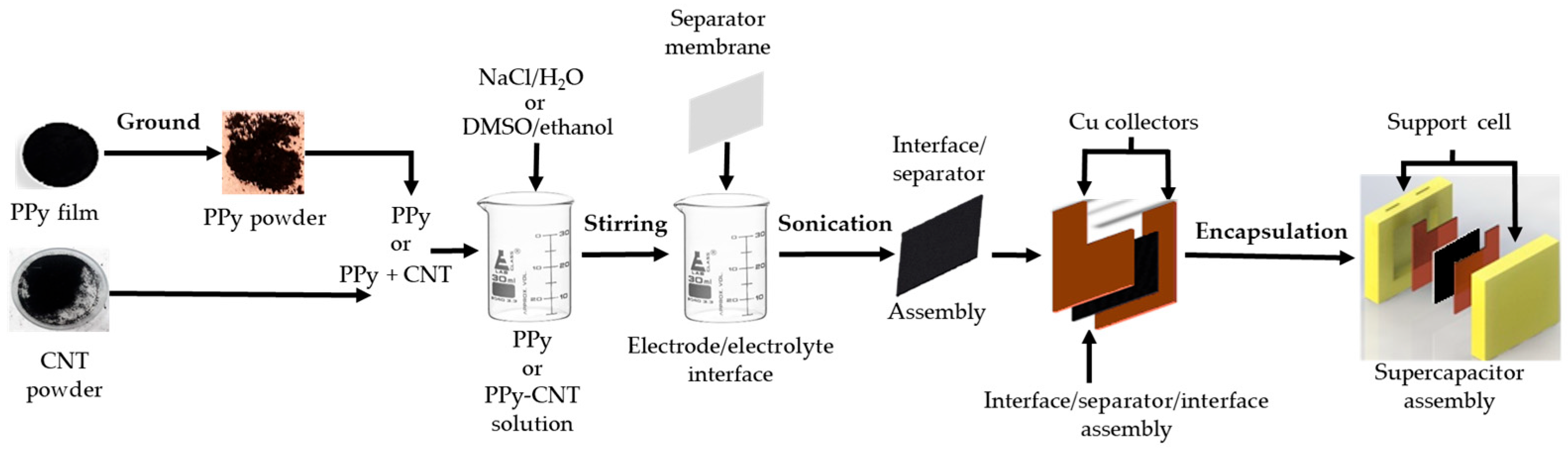
2.1. Construction of Supercapacitor
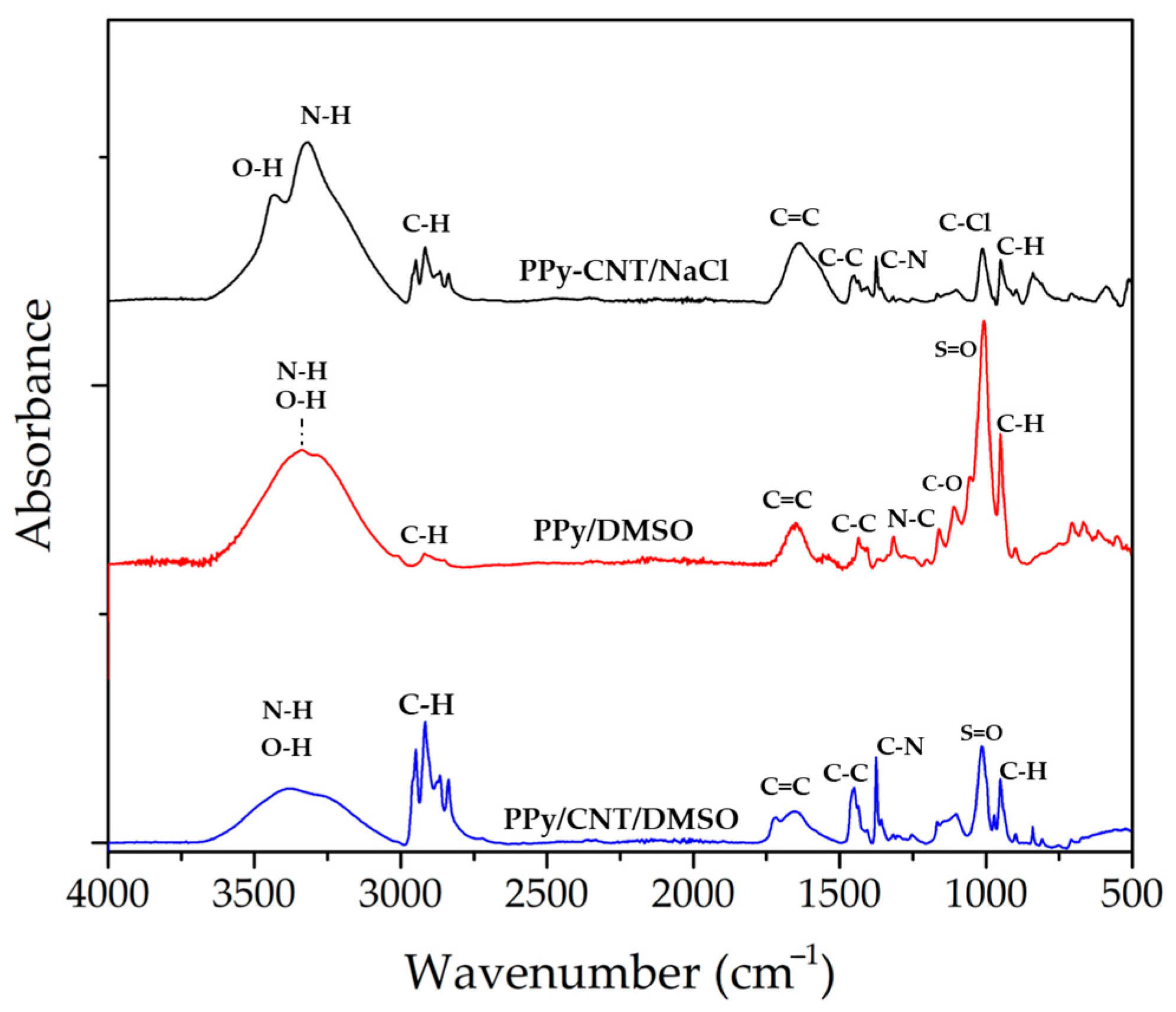
2.2. Characterization of Materials
2.3. Electrochemical Characterization
3. Results
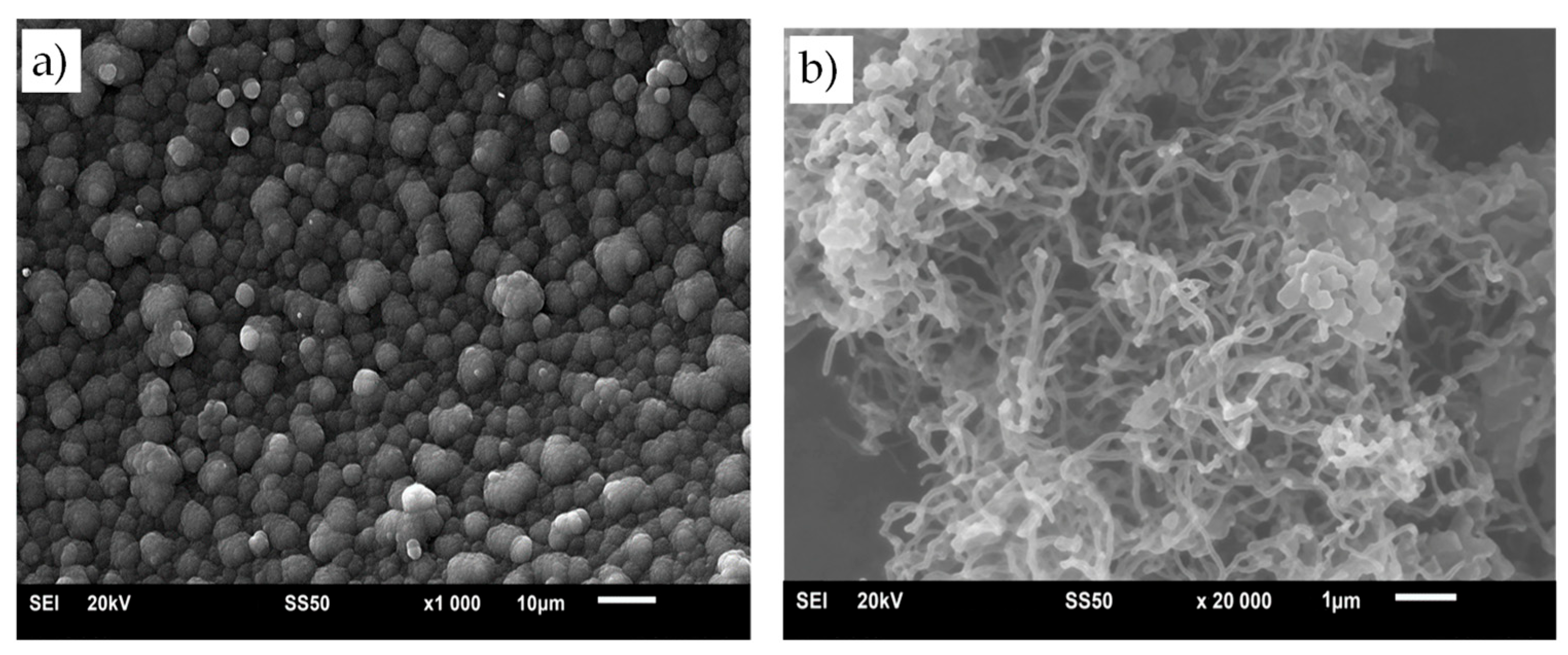
3.1. Structural Characterization
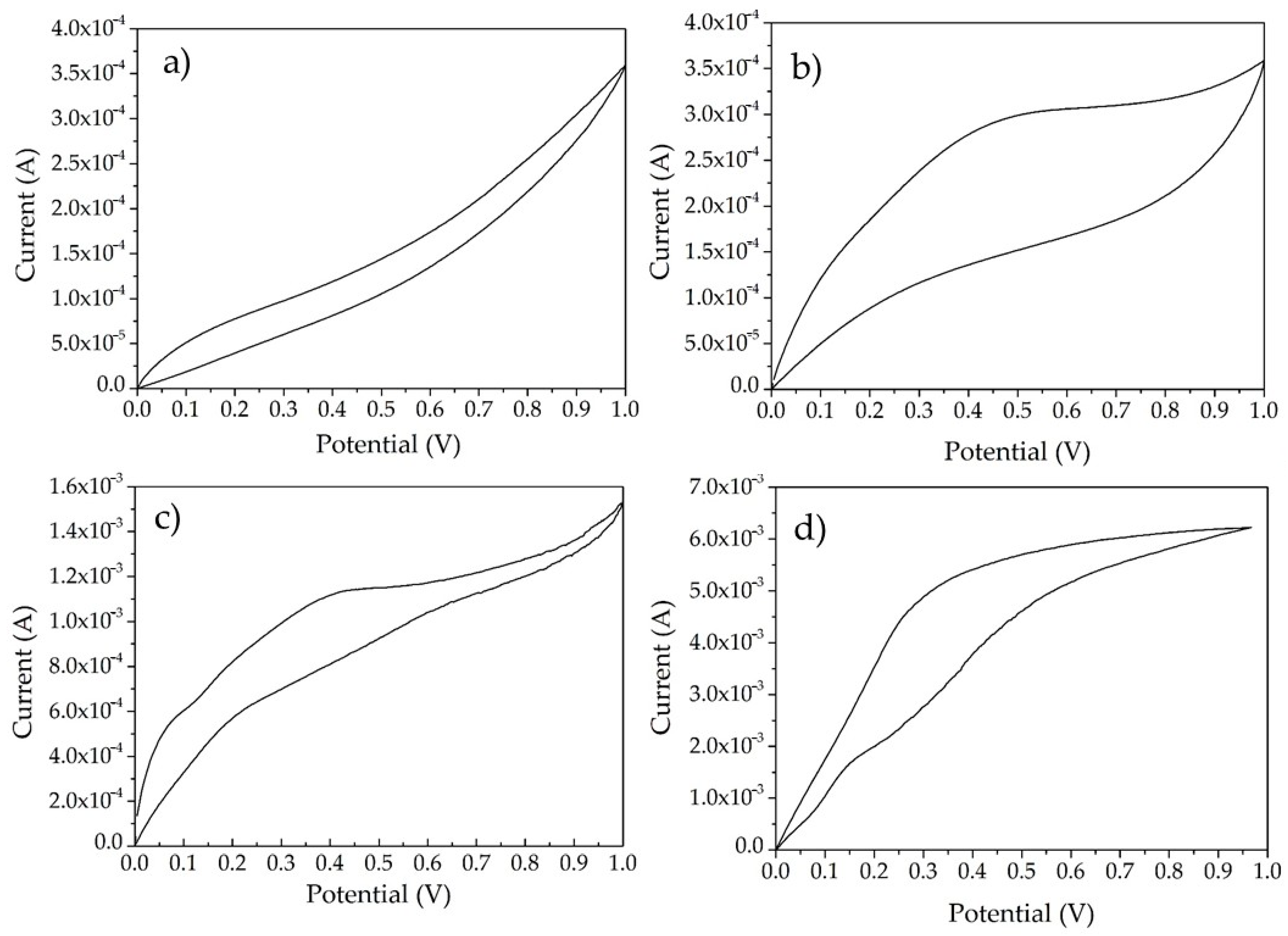
3.2. Electrochemical Characterization of Supercapacitor
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cherusseri, J.; Sharma, R.; Kar, K.K. Nanotechnology Advancements on Carbon Nanotube/Polypyrrole Composite Electrodes for Supercapacitors. In Handbook of Polymer Nanocomposites. Processing, Performance and Application. Volume B: Carbon Nanotube Based Polymer Composites, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 479–510. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y.; Dunn, B. Where Do Batteries End and Supercapacitors Begin? Mater. Sci. 2014, 343, 1210–1211. [Google Scholar] [CrossRef]
- Rosas Laverde, N.M.; Pruna, A.; Busquets Mataix, D. Improving Electrochemical Properties of Polypyrrole Coatings by Graphene Oxide and Carbon Nanotubes. Nanomaterials 2020, 10, 507. [Google Scholar] [CrossRef]
- Brzhezinskaya, M.; Zhivulin, V.E. Controlled modification of polyvinylidene fluoride as a way for carbyne synthesis. Polym. Degrad. Stab. 2022, 203, 110054. [Google Scholar] [CrossRef]
- Brzhezinskaya, M.; Kapitanova, O.O.; Kononenko, O.V.; Koveshnikov, S.; Korepanov, V.; Roshchupkin, D. Large-scalable graphene oxide films with resistive switching for non-volatile memory applications. J. Alloys Compd. 2020, 849, 156699. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Xu, C.; Li, Y. Flexible electrodes and supercapacitors for wearable energy storage: A review. J. Mater. Chem. A 2016, 4, 4659–4685. [Google Scholar] [CrossRef]
- Jyothibasu, J.P.; Chen, M.Z.; Lee, R.H. Polypyrrole/Carbon Nanotube Freestanding Electrode with Excellent Electrochemical Properties for High-Performance All-Solid-State Supercapacitors. ACS Omega 2020, 5, 6441–6451. [Google Scholar] [CrossRef]
- Zhou, H.; Han, G. One-step fabrication of heterogeneous conducting polymers-coated graphene oxide/carbon nanotubes composite films for high-performance supercapacitors. Electrochim. Acta 2016, 192, 448–455. [Google Scholar] [CrossRef]
- Yalovega, G.E.; Brzhezinskaya, M.; Dmitriev, V.O.; Shmatko, V.A.; Ershov, I.V.; Ulyankina, A.A.; Chernysheva, D.V.; Smirnova, N.V. Interfacial Interaction in MeOx/MWNTs (Me–Cu, Ni) Nanostructures as Efficient Electrode Materials for High-Performance Supercapacitors. Nanomaterials 2024, 14, 947. [Google Scholar] [CrossRef]
- Askari, M.B.; Salarizadeh, P.; Moghadam, M.T.T.; Azizi, S. Binary transition metal oxide/carbon compounds-based electrode materials for supercapacitor application: A comprehensive review. J. Alloys Compd. 2025, 1027, 180573. [Google Scholar] [CrossRef]
- Oumer, A.; Adem, J.K.; Kim, G.M.; Choi, Y.C.; Gwon, S. Advanced energy storage systems in construction materials: A comprehensive review of cementitious-based batteries and supercapacitors. J. Bridge Eng. 2025, 106, 112553. [Google Scholar] [CrossRef]
- De Oliveira, A.H.P.; Nascimento, M.L.F.; De Oliveira, H.P. Carbon Nanotube@ MnO2@ Polypyrrole Composites: Chemical Synthesis, Characterization and Application in Supercapacitors. Mater. Res. 2016, 19, 1080–1087. [Google Scholar] [CrossRef]
- Jalal, N.I.; Ibrahim, R.I.; Oudah, M.K. A review on Supercapacitors: Types and components. J. Phys. Conf. Ser. 2021, 1973, 012015. [Google Scholar] [CrossRef]
- Yu, Z.; Tetard, L.; Zhai, L.; Thomas, J. Supercapacitor electrode materials: Nanostructures from 0 to 3 dimensions. Energy Environ. Sci. 2015, 8, 702–730. [Google Scholar] [CrossRef]
- Villanueva Castañeda, M.; Hernandez Tenorio, C.; Moreno Saavedra, H.; Olayo, M.; Cruz, G.J. Liquid Plasma Iodine Doping of Electrochemically Synthesized Polypyrrole to Enhance the Electromagnetic Absorption. J. Inorg. Organomet. Polym. 2020, 30, 2098–2104. [Google Scholar] [CrossRef]
- Rajesh, M.; Raj, C.J.; Kim, B.C.; Cho, B.B.; Ko, J.M.; Yu, K.H. Supercapacitive studies on electropolymerized natural organic phosphate doped polypyrrole thin films. Electrochim. Acta 2016, 220, 373–383. [Google Scholar] [CrossRef]
- Yang, Q.; Hou, Z.; Huang, T. Self-assembled polypyrrole film by interfacial polymerization for supercapacitor applications. J. Appl. Polym. Sci. 2015, 132, 41615. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, J.; Li, B.; Du, W.; Huang, Q.; Zheng, M.; Xue, H.; Pang, H. Facile synthesis of polypyrrole nanowires for high-performance supercapacitor electrode materials. Prog. Nat. Sci. Mater. Int. 2016, 26, 237–242. [Google Scholar] [CrossRef]
- Song, Y.; Dong, Y.; Li, W.; Tan, Z.; Ma, P.; Wang, G.; Li, X. An In Situ Oxidative Polymerization Method to Synthesize Mesoporous Polypyrrole/MnO2 Composites for Supercapacitors. Molecules 2025, 30, 45. [Google Scholar] [CrossRef]
- Lota, K.; Acznik, I.; Sierczynska, A.; Lota, G. Enhancing the performance of polypyrrole composites as electrode materials for supercapacitors by carbon nanotubes additives. J. Appl. Polym. Sci. 2019, 137, 48867. [Google Scholar] [CrossRef]
- Yang, Z.; Tian, J.; Yin, Z.; Cui, C.; Qian, W.; Wei, F. Carbon nanotube-and graphene-based nanomaterials and applications in high-voltage supercapacitor: A review. Carbon. 2019, 141, 467–480. [Google Scholar] [CrossRef]
- González, A.; Goikolea, E.; Barren, J.A. Review on supercapacitors: Technologies and materials. Renew. Sust. Energ. Rev. 2016, 58, 1189–1206. [Google Scholar] [CrossRef]
- Obreja, V.V. Supercapacitors Based on Carbon Nanomaterials, In Carbon Nanomaterials for Advanced Energy Systems, 1st ed.; Lu, W., Baeng, J.B., Dai, L., Eds.; Wiley: Hoboken, NJ, USA, 2015; pp. 292–337. [Google Scholar]
- Argirusis, C.; Angelara, C.; Argirusis, N.; Karantonis, A.; Pandis, P.P.; Sourkouni, G. Preparation and Characterization of Supercapacitor Cells Using Modified CNTs and Bimetallic MOFs. Processes 2024, 12, 2778. [Google Scholar] [CrossRef]
- Chen, T.; Li, H.; Wang, J.; Jia, X. Flexible All-Carbon Nanoarchitecture Built from In Situ Formation of Nanoporous Graphene Within “Skeletal-Capillary” Carbon Nanotube Networks for Supercapacitors. Nanomaterials 2024, 14, 1683. [Google Scholar] [CrossRef]
- Snook, G.A.; Kao, P.; Best, A.S. Conducting-polymer-based supercapacitor devices and electrodes. J. Power Sources 2011, 196, 1–12. [Google Scholar] [CrossRef]
- Bae, K.L.; Kim, K. Flexible sodium-ion super based on polypyrrole/carbon electrode by use of harmless aqueous electrolyte for wearable devices. Int. J. Energ. Res. 2017, 41, 1335–1341. [Google Scholar] [CrossRef]
- Tripathi, S.K.; Kumar, A.; Hashmi, S.A. Electrochemical redox supercapacitors using PVdF-HFP based gel electrolytes and polypyrrole as conducting polymer electrode. Solid. State Ion. 2006, 177, 2979–2985. [Google Scholar] [CrossRef]
- Zhang, L.; Shuhua, Y.; Chang, J.; Zhao, D.; Wang, J.; Yang, C.; Cao, B. A Review of Redox Electrolytes for Supercapacitors. Front. Chem. 2020, 8, 413. [Google Scholar] [CrossRef]
- Dsoke, S.; Abbas, Q. Benefits of Organo-Aqueous Binary Solvents for Redox Supercapacitors Based on Polyoxometalates. Chem. Electro Chem. 2020, 7, 2466–2476. [Google Scholar] [CrossRef]
- Lu, X.; Vicent Luna, J.M.; Calero, S.; Madero Castro, R.M.; Gutiérrez, M.C.; Ferrer, M.L.; del Monte, F. EMIMBF 4 in ternary liquid mixtures of water, dimethyl sulfoxide and acetonitrile as “tri-solvent-in-salt” electrolytes for high-performance supercapacitors operating at −70 °C. Energy Storage Mater. 2021, 40, 368–385. [Google Scholar] [CrossRef]
- Cai, J.; Wang, Y.; Li, Q.; Cui, L.; Wu, J.; Liu, Z.; Liu, J. Dmso-Doped Polymer Hydrogel Electrolyte-Based Freeze-Resistant Flexible Supercapacitors with High Capacity Retention at Low Temperatures. Electrochim. Acta 2025, 530, 146136. [Google Scholar] [CrossRef]
- Choudhary, R.B.; Ansari, S.; Purty, B. Robust electrochemical performance of polypyrrole (PPy) and polyindole (PIn) based hybrid electrode materials for supercapacitor application: A Review. J. Energy Storage 2020, 29, 101302. [Google Scholar] [CrossRef]
- Mohd Abdah, M.A.A.; Mohd Razalia, N.S.; Lima, P.T.; Kulandaivalua, S.; Sulaimana, Y. One-step potentiostatic electrodeposition of polypyrrole/graphene oxide/multi-walled carbon nanotubes ternary nanocomposite for supercapacitor. Mater. Chem. Phys. 2018, 219, 120–128. [Google Scholar] [CrossRef]
- Farbod, M.; Elahi Asl, E.; Shojaeenezhad, S.S. Polypyrrole/multi-walled carbon nanotube nanocomposite as a high-performance material for supercapacitors’ electrodes. J. Appl. Electrochem. 2023, 53, 1623–1630. [Google Scholar] [CrossRef]
- Saini, R.; Singh, P.; Kumar, R.; Kulriya, P.; Kumar, S. Facile Synthesis of Polypyrrole/Reduced Graphene Oxide Composites for High-Performance Supercapacitor Applications. J. Electron. Mater. 2024, 53, 5971–5980. [Google Scholar] [CrossRef]
- Hernández Cortés, G.; Acevedo Peña, P.; Díaz Góngora, J.A.I.; Leyva-Porras, C.; Reguera, E. Self-standing multiwalled carbon nanotubes/polypyrrole foams for aqueous symmetric supercapacitors. Synth. Met. 2023, 299, 117479. [Google Scholar] [CrossRef]
- Sharma, S.; Chand, P. Supercapacitor and electrochemical techniques: A brief review. Results Chem. 2023, 5, 100885. [Google Scholar] [CrossRef]
- Pacheco, M.; Mendoza, D.; Valdivia, R.; Santana, A.; Pacheco, J.; Alarcón, E.; Tu, X. Multilayer graphene growth assisted by sulfur using the arc discharge method at ambient conditions. IEEE T Plasma Sci. 2018, 46, 2407–2412. [Google Scholar] [CrossRef]
- Zhong, C.; Deng, Y.; Hu, W.; Qiao, J.; Zhang, L.; Zhang, J. A review of electrolyte materials and compositions for electrochemical supercapacitors. Chem. Soc. Rev. 2015, 44, 7484–7539. [Google Scholar] [CrossRef]
- Wu, Z.; Li, L.; Yan, J.M.; Zhang, X.B. Materials design and system construction for conventional and new-concept supercapacitors. Adv. Sci. 2017, 4, 1600382. [Google Scholar] [CrossRef]
- Azam, M.A.; Radzi, M.I.; Mupit, M.; Osman, H.; Munawari, R.F.; Samat, K.F.; Suan, M.S.M.; Isomura, K.; Islam, M.R. Cyclic Voltammetry and Galvanostatic Charge-Discharge Analyses of Polyaniline/Graphene Oxide Nanocomposite based Supercapacitor. Malays. J. Comp. Sci. Manuf. 2020, 3, 14–26. [Google Scholar] [CrossRef]
- Liu, J.; Xuan, D.; Lu, Z.; Wang, Z.; Liu, Q.; Li, S.; Zheng, Z. A novel perspective on interfacial interactions between polypyrrole and carbon materials for improving performance of supercapacitors. Appl. Surf. Sci. 2022, 573, 151626. [Google Scholar] [CrossRef]
- Smiechowski, M. The influence of intermolecular correlations on the infrared spectrum of liquid dimethyl sulfoxide. Spectrochim. Acta Part. A 2021, 260, 119869. [Google Scholar] [CrossRef] [PubMed]
- Caiying, L.; Pei, L.; Shuhuan, F.; Tengfei, X.; Liubing, D.; Jianhao, Z.; Jianhua, R. Phosphomolybdic acid-doped polypyrrole as electrode for integrated hydrogel supercapacitors. J. Mater. Sci: Mater. Electron. 2025, 36, 201. [Google Scholar] [CrossRef]
- Ren, Y.; Liu, Y.; Wang, S.; Wang, Q.; Li, S.; Wang, W.; Dong, X. Stretchable supercapacitor based on a hierarchical PPy/CNT electrode and hybrid hydrogel electrolyte with a wide operating temperature. Carbon. Energy 2022, 4, 527–538. [Google Scholar] [CrossRef]
- Dai, Z.; Peng, C.; Chae, J.H. Cell voltage versus electrode potential range in aqueous supercapacitors. Sci. Rep. 2015, 5, 9854. [Google Scholar] [CrossRef]
- Dubal, D.P.; Lee, S.H.; Kim, J.G. Porous polypyrrole clusters prepared by electropolymerization for a high-performance supercapacitor. J. Mater. Chem. 2012, 22, 3044–3052. [Google Scholar] [CrossRef]
- Shinde, S.S.; Gund, G.S.; Dubal, D.P. Morphological modulation of polypyrrole thin films through oxidizing agents and their concurrent effect on supercapacitor performance. Electrochim. Acta 2014, 119, 1–10. [Google Scholar] [CrossRef]
- Thillaikkarasi, D.; Karthikeyan, S.; Ramesh, R.; Sengodan, P.; Malarvizhi, M.; Kavitha, D.; Nirmala, V. Facile synthesis of activated carbon and multiwalled carbon nanotubes and comparative performance of various AC-MWCNTs supercapacitor electrodes. J. Mater. Sci. Mater. Electron. 2023, 34, 353. [Google Scholar] [CrossRef]
- Yang, J.; Cao, J.; Peng, Y.; Bissett, M.; Kinloch, I.A.; Dryfe., R.A. Unlocking the energy storage potential of polypyrrole via electrochemical graphene oxide for high performance zinc-ion hybrid supercapacitors. J. Power Sources 2021, 516, 230663. [Google Scholar] [CrossRef]
- Raza, W.; Ali, F.; Raza, N. Recent advancements in supercapacitor technology. Nano Energy 2018, 52, 441–473. [Google Scholar] [CrossRef]
- Chaluvachar, P.; Mahesha, G.T.; Sudhakar, Y.N.; Nair, V.; Pai, D. A Review on Graphitic Carbon Nitride and Conducting Polymer Nanocomposite Electrodes for Supercapacitors. Eng. Proc. 2023, 59, 154. [Google Scholar] [CrossRef]
- Pour, G.B.; Ashourifar, H.; Aval, L.F.; Solaymani, S. CNTs-Supercapacitors: A Review of Electrode Nanocomposites Based on CNTs, Graphene, Metals, and Polymers. Symmetry 2023, 15, 1179. [Google Scholar] [CrossRef]

| Electrode | Electrolyte | (F) | (mg) | (F/g) | (W·h/kg) | (W/kg) |
|---|---|---|---|---|---|---|
| PPy | DMSO | 0.0196 | 1.83 | 1.1 | 9.8 | 0.2 |
| PPy50-CNT50 | DMSO | 0.0180 | 1.08 | 1.7 | 9.0 | 1.8 |
| CNT | NaCl | 0.0649 | 1.4 | 46.4 | 32.5 | 357.1 |
| PPy25-CNT75 | NaCl | 0.0583 | 1.5 | 40.2 | 29.2 | 229.9 |
| PPy50-CNT50 | NaCl | 0.0876 | 1.5 | 58.4 | 43.8 | 166.7 |
| PPy75-CNT25 | NaCl | 0.0456 | 1.6 | 39.2 | 22.8 | 129 |
| PPy | NaCl | 0.0318 | 1.6 | 19.9 | 15.9 | 78.1 |
| Electrode | Electrolyte | (F) | (F/g) | (F/m2) | (W·h/kg) | (W/kg) |
|---|---|---|---|---|---|---|
| CNT | NaCl | 0.120 | 86 | 1200 | 60 | 357.1 |
| PPy25-CNT75 | NaCl | 0.230 | 159 | 2300 | 115 | 229.9 |
| PPy50-CNT50 | NaCl | 0.310 | 207 | 3100 | 155 | 166.7 |
| PPy75-CNT25 | NaCl | 0.420 | 271 | 4200 | 210 | 129 |
| PPy | NaCl | 0.515 | 322 | 5150 | 258 | 78.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tepale-Cortés, A.; Moreno-Saavedra, H.; Pacheco, M.J.; Pacheco, J.O.; Hernández-Tenorio, C.; Valdivia, R. Supercapacitor Using Polypyrrole and Carbon Nanotube Composite as Electrodes. C 2025, 11, 80. https://doi.org/10.3390/c11040080
Tepale-Cortés A, Moreno-Saavedra H, Pacheco MJ, Pacheco JO, Hernández-Tenorio C, Valdivia R. Supercapacitor Using Polypyrrole and Carbon Nanotube Composite as Electrodes. C. 2025; 11(4):80. https://doi.org/10.3390/c11040080
Chicago/Turabian StyleTepale-Cortés, Arturo, Hilda Moreno-Saavedra, Marquidia J. Pacheco, Joel O. Pacheco, Celso Hernández-Tenorio, and Ricardo Valdivia. 2025. "Supercapacitor Using Polypyrrole and Carbon Nanotube Composite as Electrodes" C 11, no. 4: 80. https://doi.org/10.3390/c11040080
APA StyleTepale-Cortés, A., Moreno-Saavedra, H., Pacheco, M. J., Pacheco, J. O., Hernández-Tenorio, C., & Valdivia, R. (2025). Supercapacitor Using Polypyrrole and Carbon Nanotube Composite as Electrodes. C, 11(4), 80. https://doi.org/10.3390/c11040080







