First-Principles Study of CO, C2H2, and C2H4 Adsorption on Penta-Graphene for Transformer Oil Gas Sensing Applications
Abstract
1. Introduction
2. Computational Methods
3. Results and Discussions
4. Conclusions
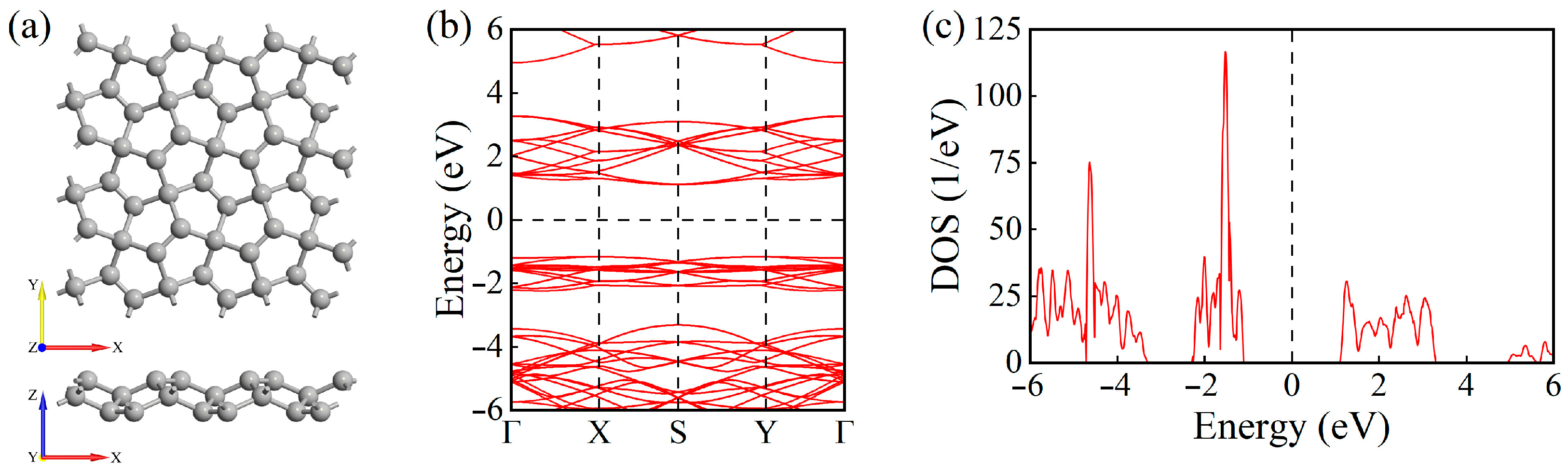
- Geometric and electronic properties: PG exhibits an indirect bandgap of 2.28 eV with a non-planar lattice structure. Its unique sp2-sp3 hybridized geometry provides reactive sites for molecular adsorption.
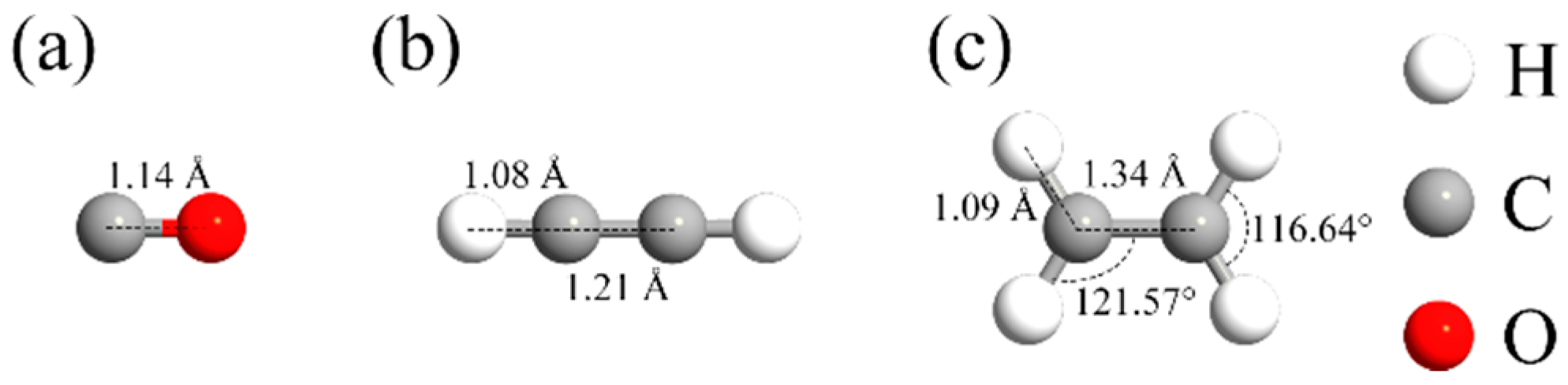
- Adsorption behavior: CO and C2H2 display moderate chemisorption on PG with adsorption energies of −1.32 eV and −2.99 eV, respectively, along with significant charge transfer and local electronic structure modifications. C2H4 shows a weaker adsorption energy of −0.09 eV, indicating a typical physisorption behavior.
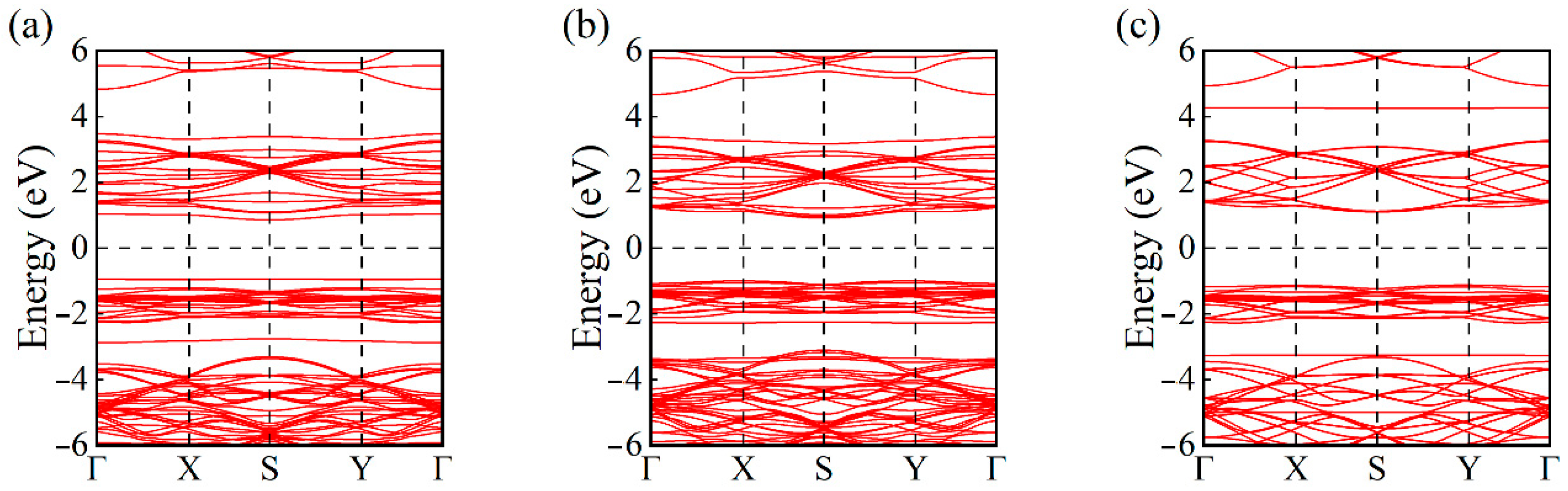
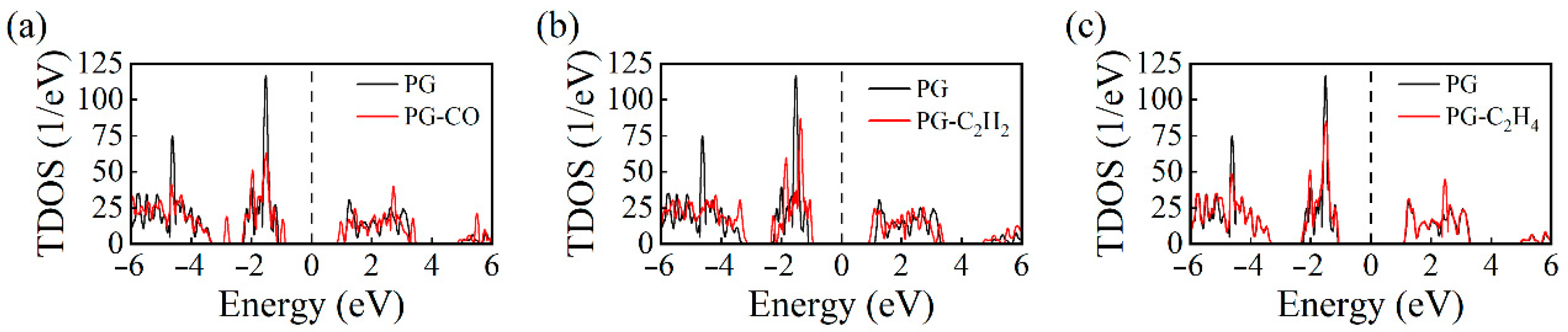
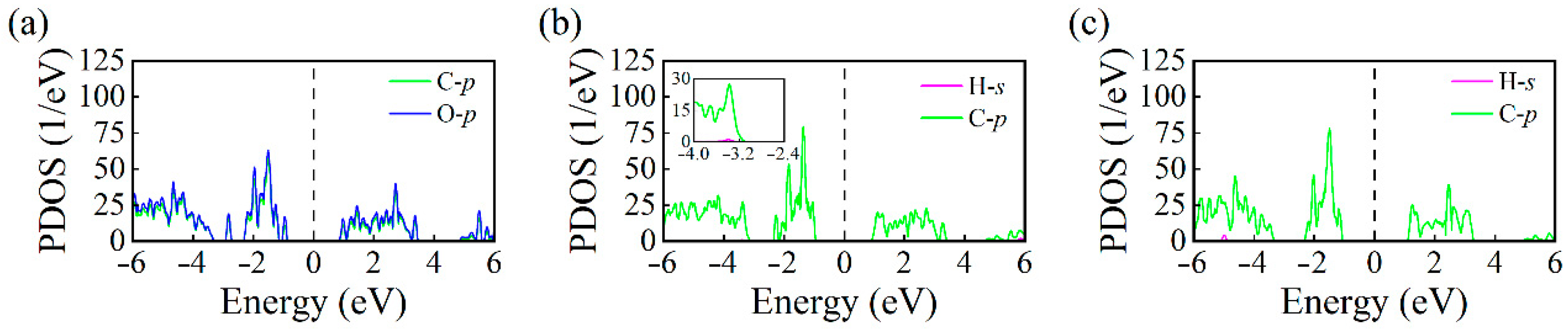
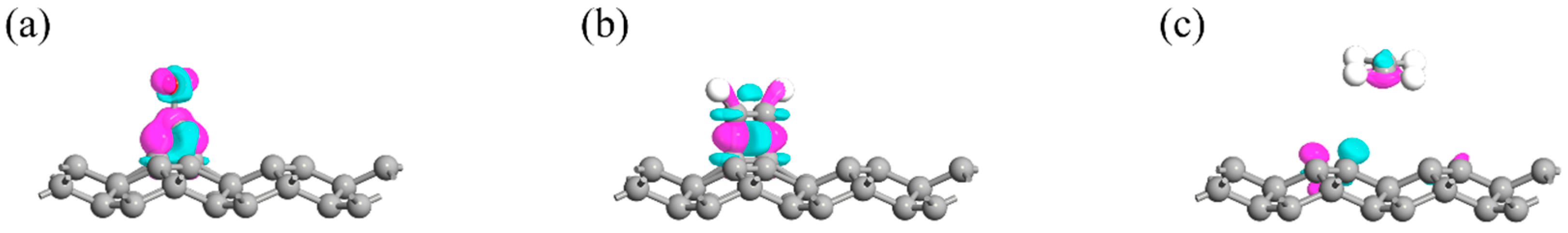
- Electronic Structure Analysis: Both TDOS and PDOS reveal that CO and C2H2 adsorption induce notable changes near the Fermi level, suggesting an enhanced sensing response. CDD plots support the presence of electron redistribution upon adsorption.
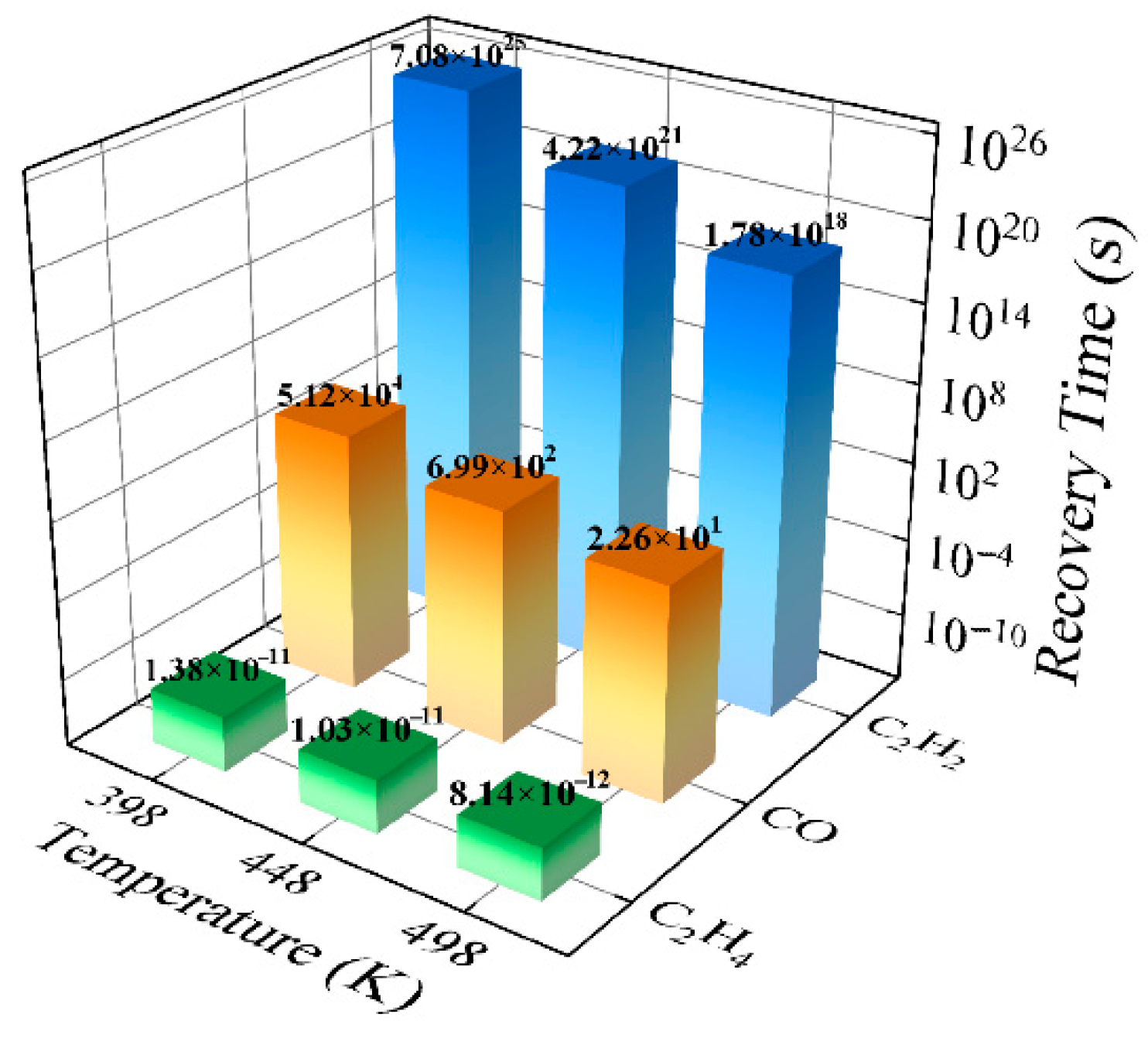
- Desorption dynamics: Calculated desorption times indicate that CO and C2H2 molecules are more strongly bound to the surface, requiring thermal activation for rapid desorption, whereas C2H4 can be readily desorbed at ambient temperatures.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gupta, A.; Sakthivel, T.; Seal, S. Recent development in 2D materials beyond graphene. Prog. Mater. Sci. 2015, 73, 44–126. [Google Scholar]
- Li, H.; Li, C.; Tao, B.; Gu, S.; Xie, Y.; Wu, H.; Zhang, G.; Wang, G.; Zhang, W.; Chang, H. Two-Dimensional Metal Telluride Atomic Crystals: Preparation, Physical Properties, and Applications. Adv. Funct. Mater. 2021, 31, 2010901. [Google Scholar]
- Wei, Z.; Li, B.; Xia, C.; Cui, Y.; He, J.; Xia, J.-B.; Li, J. Various Structures of 2D Transition-Metal Dichalcogenides and Their Applications. Small Methods 2018, 2, 1800094. [Google Scholar]
- Zhao, B.; Shen, D.; Zhang, Z.; Lu, P.; Hossain, M.; Li, J.; Li, B.; Duan, X.; Dichalcogenides, D.M.T.-M. Synthesis; Properties; Applications. Adv. Funct. Mater. 2021, 31, 2105132. [Google Scholar]
- Liu, J.; Bao, S.; Wang, X. Applications of Graphene-Based Materials in Sensors: A Review. Micromachines 2022, 13, 184. [Google Scholar] [CrossRef]
- Verma, M.L.; Sukriti; Dhanya, B.S.; Saini, R.; Das, A.; Varma, R.S. Synthesis and application of graphene-based sensors in biology: A review. Environ. Chem. Lett. 2022, 20, 2189–2212. [Google Scholar]
- Zhang, Z.; Liu, Q.; Ma, H.; Ke, N.; Ding, J.; Zhang, W.; Fan, X. Recent Advances in Graphene-Based Pressure Sensors: A Review. IEEE Sens. J. 2024, 24, 25227–25248. [Google Scholar]
- Novoselov, K.S.; Fal, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar]
- Marconcini, P.; Macucci, M. Transport Simulation of Graphene Devices with a Generic Potential in the Presence of an Orthogonal Magnetic Field. Nanomaterials 2022, 12, 1087. [Google Scholar] [CrossRef]
- Donarelli, M.; Ottaviano, L. 2D Materials for Gas Sensing Applications: A Review on Graphene Oxide, MoS2, WS2 and Phosphorene. Sensors 2018, 18, 3638. [Google Scholar] [CrossRef]
- Ruan, H.; Guo, J.; Zhang, S.; Gao, Y.; Shang, W.; Liu, Y.; Su, M.; Liu, Y.; Wang, H.; Xie, T.; et al. In Situ Local Band Engineering of Monolayer Graphene Using Triboelectric Plasma. Small 2024, 20, 2309318. [Google Scholar]
- Nazir, M.A.; Hassan, A.; Shen, Y.; Wang, Q. Research progress on penta-graphene and its related materials: Properties and applications. Nano Today 2022, 44, 101501. [Google Scholar]
- Zhang, S.; Zhou, J.; Wang, Q.; Chen, X.; Kawazoe, Y.; Jena, P. Penta-graphene: A new carbon allotrope. Proc. Natl. Acad. Sci. USA 2015, 112, 2372–2377. [Google Scholar] [PubMed]
- Berdiyorov, G.R.; Dixit, G.; Madjet, M.E. Band gap engineering in penta-graphene by substitutional doping: First-principles calculations. J. Phys. Condens. Matter 2016, 28, 475001. [Google Scholar]
- Morales-Ferreiro, J.O.; Silva-Oelker, G.; Kumar, C.; Zambra, C.; Liu, Z.; Diaz-Droguett, D.E.; Celentano, D. Tuning the Electronic Bandgap of Penta-Graphene from Insulator to Metal Through Functionalization: A First-Principles Calculation. Nanomaterials 2024, 14, 1751. [Google Scholar]
- Yu, Z.G.; Zhang, Y.-W. A comparative density functional study on electrical properties of layered penta-graphene. J. Appl. Phys. 2015, 118, 165706. [Google Scholar]
- Deb, J.; Seriani, N.; Sarkar, U. Ultrahigh carrier mobility of penta-graphene: A first-principle study. Phys. E Low-Dimens. Syst. Nanostructures 2021, 127, 114507. [Google Scholar]
- Guo, Y.; Wang, F.Q.; Wang, Q. An all-carbon vdW heterojunction composed of penta-graphene and graphene: Tuning the Schottky barrier by electrostatic gating or nitrogen doping. Appl. Phys. Lett. 2017, 111, 073503. [Google Scholar]
- He, C.; Wang, X.F.; Zhang, W.X. Coupling effects of the electric field and bending on the electronic and magnetic properties of penta-graphene nanoribbons. Phys. Chem. Chem. Phys. 2017, 19, 18426–18433. [Google Scholar]
- Peng, R.; Zeng, W.; Zhou, Q. Adsorption and gas sensing of dissolved gases in transformer oil onto Ru3-modified SnS2: A DFT study. Appl. Surf. Sci. 2023, 615, 156445. [Google Scholar]
- Wang, C.-B.; Tian, Y.-P.; Duan, J.-X.; Zhang, B.-Y.; Gong, W.-J. Enhanced organic gas sensing performance of Borophene/Graphene van der Waals heterostructures via transition metal doping. Surf. Interfaces 2023, 43, 103544. [Google Scholar]
- Zhang, X.; Fang, R.; Chen, D.; Zhang, G. Using Pd-Doped γ-Graphyne to Detect Dissolved Gases in Transformer Oil: A Density Functional Theory Investigation. Nanomaterials 2019, 9, 1490. [Google Scholar] [PubMed]
- Ghoneim, S.S.M.; Taha, I.B.M. A new approach of DGA interpretation technique for transformer fault diagnosis. Int. J. Electr. Power Energy Syst. 2016, 81, 265–274. [Google Scholar]
- Gómez, N.A.; Wilhelm, H.M.; Santos, C.C.; Stocco, G.B. Dissolved gas analysis (DGA) of natural ester insulating fluids with different chemical compositions. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 1071–1078. [Google Scholar]
- Fan, J.; Wang, F.; Sun, Q.; Bin, F.; Ding, J.; Ye, H. SOFC detector for portable gas chromatography: High-sensitivity detection of dissolved gases in transformer oil. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 2854–2863. [Google Scholar]
- Li, Z.; Zhang, Q.; Wang, Z.; Dai, J. A highly sensitive low-pressure TDLAS sensor for detecting dissolved CO and CO2 in transformer insulating oil. Opt. Laser Technol. 2024, 174, 110622. [Google Scholar]
- Darwish, M.M.F.; Hassan, M.H.A.; Abdel-Gawad, N.M.K.; Lehtonen, M.; Mansour, D.-E.A. A new technique for fault diagnosis in transformer insulating oil based on infrared spectroscopy measurements. High Volt. 2024, 9, 319–335. [Google Scholar]
- Aldulaijan, S.; Ajeebi, A.M.; Jedidi, A.; Messaoudi, S.; Raouafi, N.; Dhouib, A. Surface modification of graphene with functionalized carbenes and their applications in the sensing of toxic gases: A DFT study. RSC Adv. 2023, 13, 19607–19616. [Google Scholar]
- Kim, T.; Lee, T.H.; Park, S.Y.; Eom, T.H.; Cho, I.; Kim, Y.; Kim, C.; Lee, S.A.; Choi, M.-J.; Suh, J.M.; et al. Drastic Gas Sensing Selectivity in 2-Dimensional MoS2 Nanoflakes by Noble Metal Decoration. ACS Nano 2023, 17, 4404–4413. [Google Scholar]
- Li, J.-H.; Wu, J.; Yu, Y.-X. DFT exploration of sensor performances of two-dimensional WO3 to ten small gases in terms of work function and band gap changes and I-V responses. Appl. Surf. Sci. 2021, 546, 149104. [Google Scholar]
- Viveka, N.; Poornimadevi, C.; Kala, C.P.; Thiruvadigal, D.J. DFT insights into the gas sensing properties of light platinum group metal (Ru, Rh & Pd) doped MoSe2 monolayers. Surf. Interfaces 2025, 66, 106579. [Google Scholar]
- Zhu, M.-Q.; Wang, X.-F.; Vasilopoulos, P. Transition metal-doped ZrS2 monolayer as potential gas sensor for CO2, SO2, and NO2: Density functional theory and non-equilibrium Green’s functions’ analysis. J. Phys. D Appl. Phys. 2025, 58, 135306. [Google Scholar]
- Cheng, M.-Q.; Chen, Q.; Yang, K.; Huang, W.-Q.; Hu, W.-Y.; Huang, G.-F. Penta-Graphene as a Potential Gas Sensor for NOx Detection. Nanoscale Res. Lett. 2019, 14, 306. [Google Scholar] [PubMed]
- Nagarajan, V.; Bhuvaneswari, R.; Chandiramouli, R. Sensing studies of acenaphthene and acenaphthylene molecules using penta-graphene sheets—A DFT outlook. Comput. Theor. Chem. 2023, 1230, 114391. [Google Scholar]
- Lima, K.A.L.; Júnior, M.L.P.; Monteiro, F.F.; Roncaratti, L.F.; Júnior, L.A.R. O2 adsorption on defective Penta-Graphene lattices: A DFT study. Chem. Phys. Lett. 2021, 763, 138229. [Google Scholar]
- Lu, X.; Wang, M.; Luo, G.; Zhou, S.; Wang, J.; Xin, H.; Wang, Z.; Liu, S.; Wei, S. High-efficiency CO2 capture and separation over N2 in penta-graphene pores: Insights from GCMC and DFT simulations. J. Mater. Sci. 2020, 55, 16603–16611. [Google Scholar]
- Li, B.; Shao, Z.-G. Adsorption of DNA/RNA nucleobases and base pairs on penta-graphene from first principles. Appl. Surf. Sci. 2020, 512, 145635. [Google Scholar]
- Brandbyge, M.; Mozos, J.-L.; Ordejón, P.; Taylor, J.; Stokbro, K. Density-functional method for nonequilibrium electron transport. Phys. Rev. B 2002, 65, 165401. [Google Scholar]
- Taylor, J.; Guo, H.; Wang, J. Ab initio modeling of quantum transport properties of molecular electronic devices. Phys. Rev. B 2001, 63, 245407. [Google Scholar]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [PubMed]
- Perdew, J.P.; Chevary, J.A.; Vosko, S.H.; Jackson, K.A.; Pederson, M.R.; Singh, D.J.; Fiolhais, C. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B 1992, 46, 6671–6687. [Google Scholar]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [PubMed]
- Borlido, P.; Doumont, J.; Tran, F.; Marques, M.A.L.; Botti, S. Validation of Pseudopotential Calculations for the Electronic Band Gap of Solids. J. Chem. Theory Comput. 2020, 16, 3620–3627. [Google Scholar] [PubMed]
- van Setten, M.J.; Giantomassi, M.; Bousquet, E.; Verstraete, M.J.; Hamann, D.R.; Gonze, X.; Rignanese, G.M. The PseudoDojo: Training and grading a 85 element optimized norm-conserving pseudopotential table. Comput. Phys. Commun. 2018, 226, 39–54. [Google Scholar]
- Chadi, D.J. Special points for Brillouin-zone integrations. Phys. Rev. B 1977, 16, 1746–1747. [Google Scholar]
- Liu, D.C.; Nocedal, J. On the limited memory BFGS method for large scale optimization. Math. Program. 1989, 45, 503–528. [Google Scholar]
- Tarawneh, K.; Al-Khatatbeh, Y. Adsorption of 3d transition-metal atoms on two-dimensional penta-graphene: A first-principles study. J. Saudi Chem. Soc. 2023, 27, 101611. [Google Scholar]
- Rajbanshi, B.; Sarkar, S.; Mandal, B.; Sarkar, P. Energetic and electronic structure of penta-graphene nanoribbons. Carbon 2016, 100, 118–125. [Google Scholar]
- Gui, Y.; Liu, Z.; Ji, C.; Xu, L.; Chen, X. Adsorption behavior of metal oxides (CuO, NiO, Ag2O) modified GeSe monolayer towards dissolved gases (CO, CH4, C2H2, C2H4) in transformer oil. J. Ind. Eng. Chem. 2022, 112, 134–145. [Google Scholar]
- Lin, G.; Xie, H.; Wu, H.; Tong, Z.; He, Y.; Wu, F.; Jiang, T. Dual-configuration Pd4 facilitates Janus SnSSe monolayer capture of electrical equipment fault gases (C2H2, C2H4, CO): A DFT study. Colloids Surf. A Physicochem. Eng. Asp. 2025, 708, 136015. [Google Scholar]
- Wang, K.; Li, J.; Zhao, Y.; Tan, S.; Bi, M.; Jiang, T. Adsorption behaviors and gas-sensing properties of Agn(n = 1–3)-MoSSe for gases (C2H2, C2H4, CO) in oil-filled electrical equipment. Chem. Phys. Lett. 2025, 858, 141746. [Google Scholar]
- Stowasser, R.; Hoffmann, R. What Do the Kohn−Sham Orbitals and Eigenvalues Mean? J. Am. Chem. Soc. 1999, 121, 3414–3420. [Google Scholar]
- Laidler, K.J. A glossary of terms used in chemical kinetics, including reaction dynamics (IUPAC Recommendations 1996). Pure Appl. Chem. 1996, 68, 149–192. [Google Scholar]
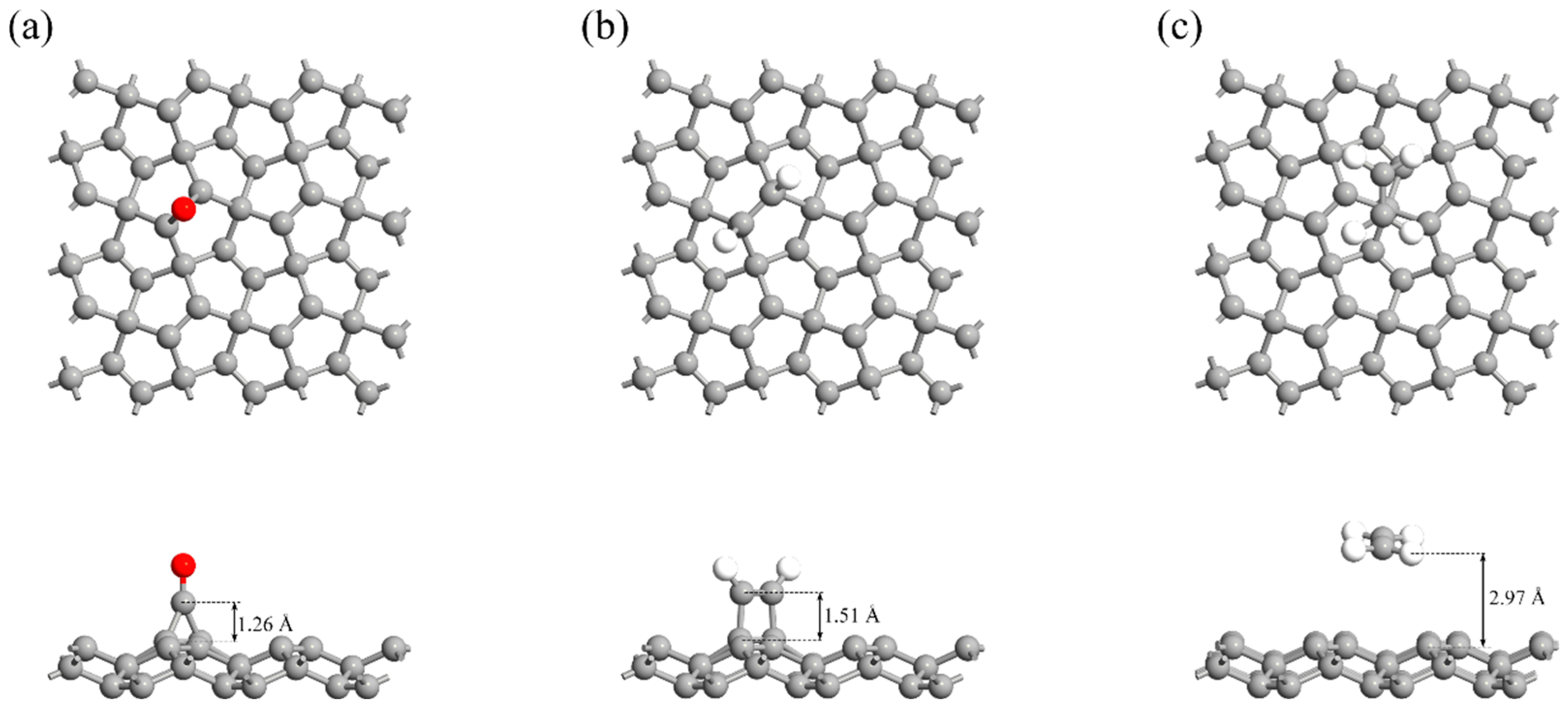
| System | d (Å) | Ead (eV) | Eg (eV) | Qt (e) | (s) |
|---|---|---|---|---|---|
| PG/CO | 1.26 | −1.32 | 1.80 | 0.18 | 5.12 × 104 (398 K) 6.99 × 102 (448 K) 2.26 × 101 (498 K) |
| PG/C2H2 | 1.51 | −2.99 | 1.91 | −0.23 | 7.08 × 1025 (398 K) 4.22 × 1021 (448 K) 1.78 × 1018 (498 K) |
| PG/C2H4 | 2.97 | −0.09 | 2.23 | −0.02 | 1.38 × 10−11 (398 K) 1.03 × 10−11 (448 K) 8.14 × 10−12 (498 K) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, M.-Q.; Wang, X.-F. First-Principles Study of CO, C2H2, and C2H4 Adsorption on Penta-Graphene for Transformer Oil Gas Sensing Applications. C 2025, 11, 49. https://doi.org/10.3390/c11030049
Zhu M-Q, Wang X-F. First-Principles Study of CO, C2H2, and C2H4 Adsorption on Penta-Graphene for Transformer Oil Gas Sensing Applications. C. 2025; 11(3):49. https://doi.org/10.3390/c11030049
Chicago/Turabian StyleZhu, Min-Qi, and Xue-Feng Wang. 2025. "First-Principles Study of CO, C2H2, and C2H4 Adsorption on Penta-Graphene for Transformer Oil Gas Sensing Applications" C 11, no. 3: 49. https://doi.org/10.3390/c11030049
APA StyleZhu, M.-Q., & Wang, X.-F. (2025). First-Principles Study of CO, C2H2, and C2H4 Adsorption on Penta-Graphene for Transformer Oil Gas Sensing Applications. C, 11(3), 49. https://doi.org/10.3390/c11030049






