Structural Probing with MNase Tethered to Ribosome Assembly Factors Resolves Flexible RNA Regions within the Nascent Pre-Ribosomal RNA
Abstract
1. Introduction
2. Results
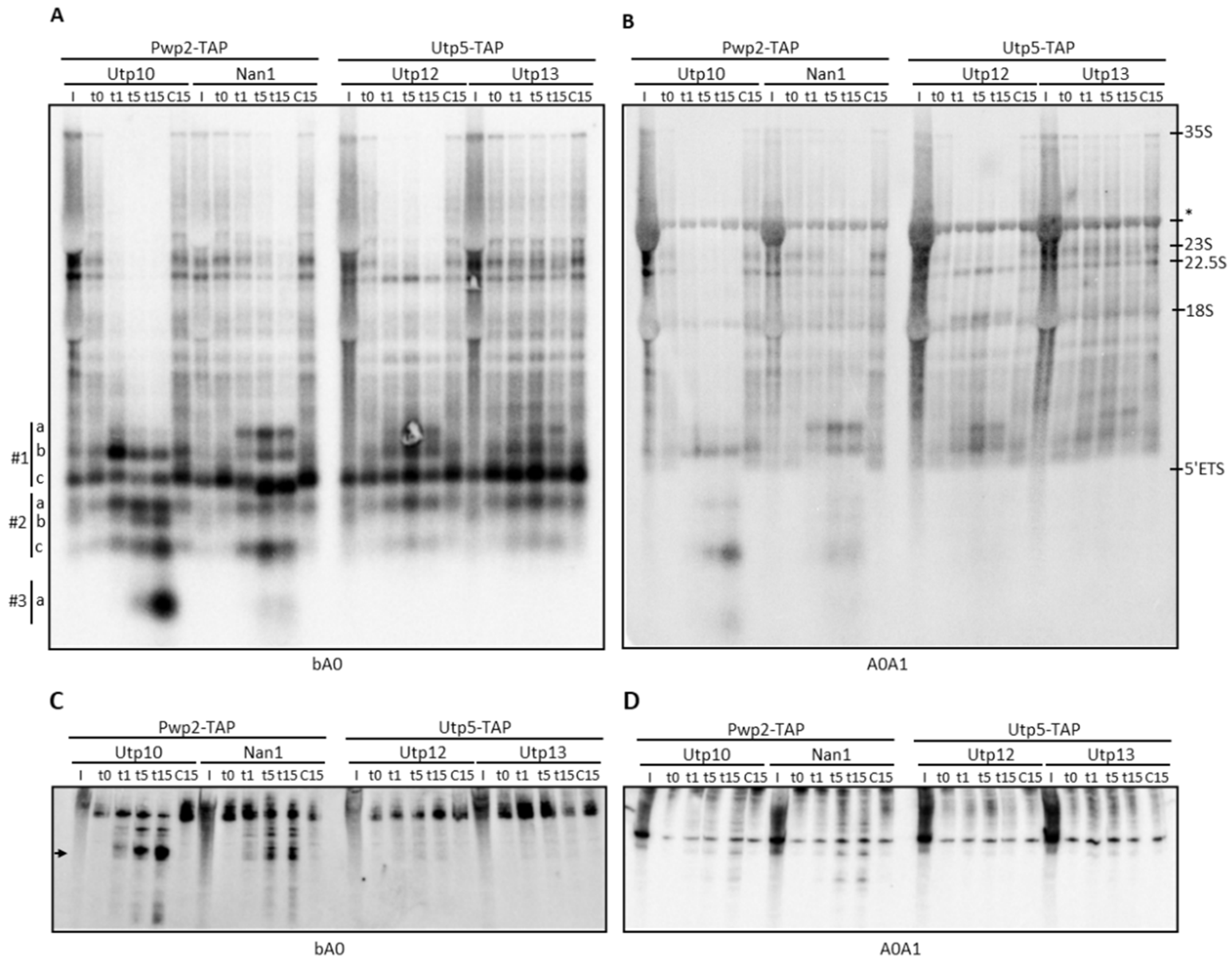
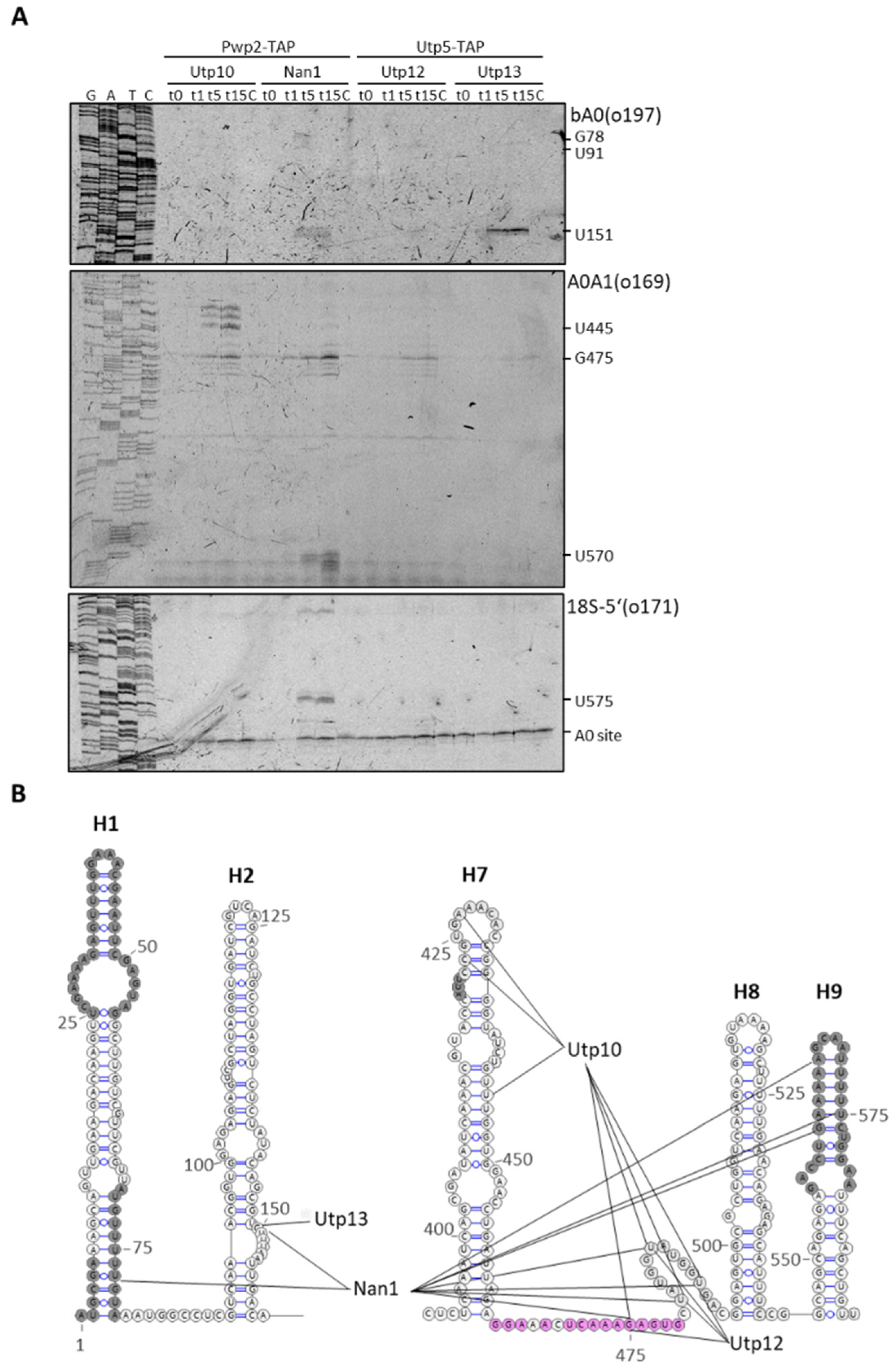
2.1. Structural Probing in the 5′ETS Region Using Tethered MNase to Utps
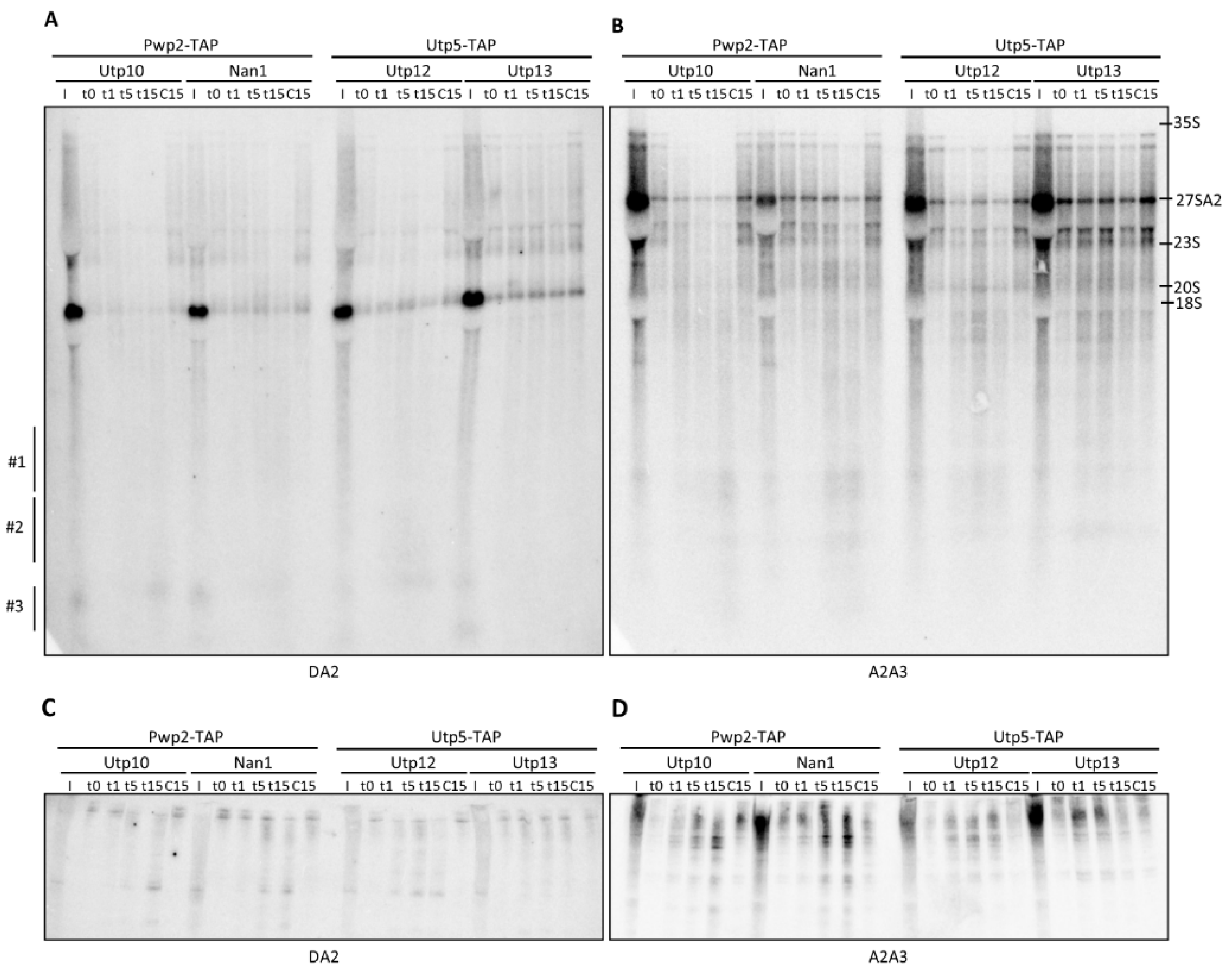
2.2. Structural Probing of the ITS1 Region
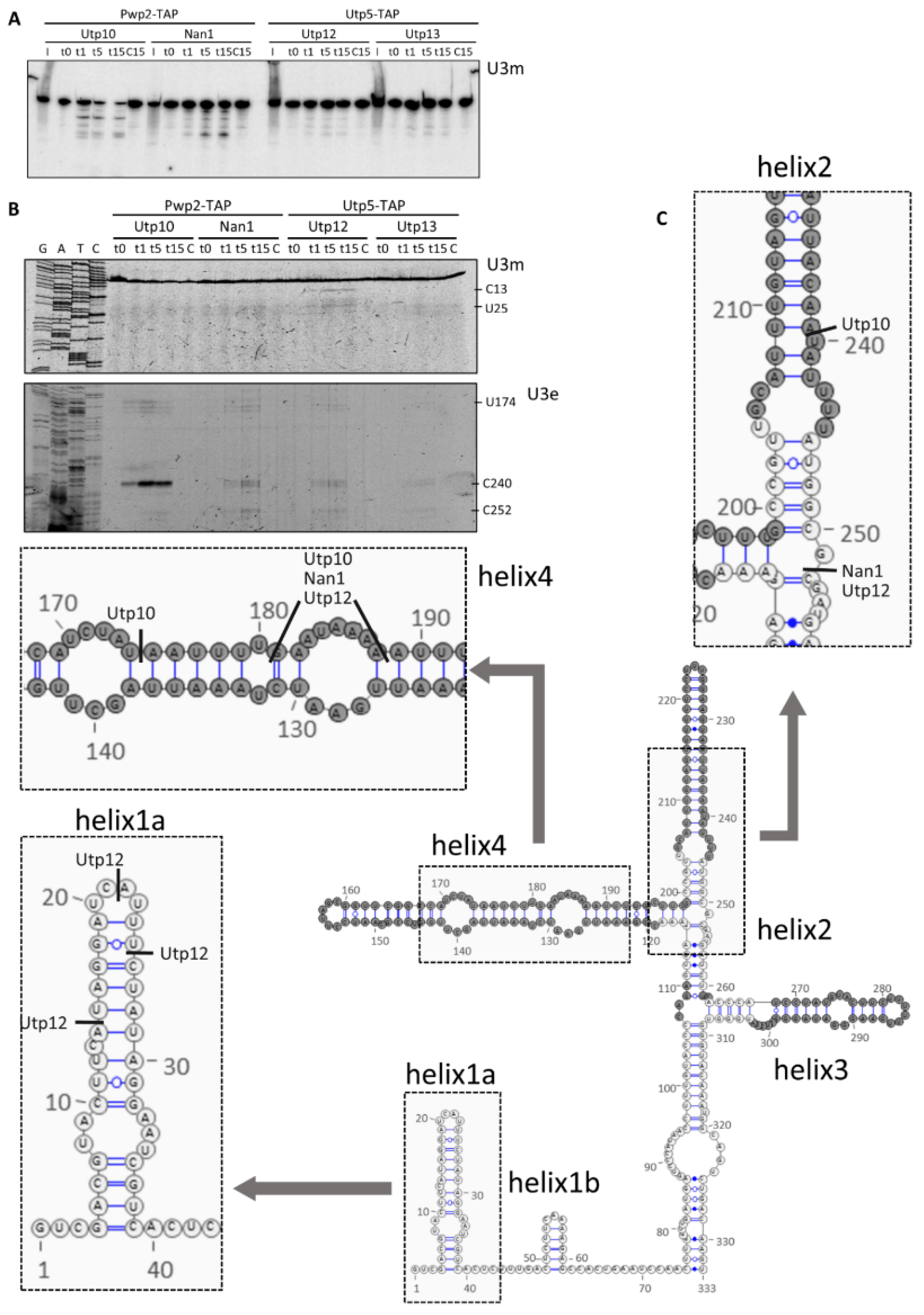
2.3. Structural Probing of the U3 snoRNA
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Osheim, Y.N.; French, S.L.; Keck, K.M.; Champion, E.A.; Spasov, K.; Dragon, F.; Baserga, S.J.; Beyer, A.L. Pre-18S ribosomal RNA is structurally compacted into the SSU processome prior to being cleaved from nascent transcripts in saccharomyces cerevisiae. Mol. Cell 2004, 16, 943–954. [Google Scholar] [CrossRef]
- Koš, M.; Tollervey, D. Yeast pre-RRNA processing and modification occur cotranscriptionally. Mol. cell 2010, 37, 809–820. [Google Scholar] [CrossRef]
- Hierlmeier, T.; Merl, J.; Sauert, M.; Perez-Fernandez, J.; Schultz, P.; Bruckmann, A.; Hamperl, S.; Ohmayer, U.; Rachel, R.; Jacob, A.; et al. Rrp5p, Noc1p and Noc2p form a protein module which is part of early large ribosomal subunit precursors in S. cerevisiae. Nucleic Acids Res. 2013, 41, 1191–1210. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Fernández, J.; Martín-Marcos, P.; Dosil, M. Elucidation of the assembly events required for the recruitment of Utp20, Imp4 and Bms1 onto nascent pre-ribosomes. Nucleic Acids Res. 2011, 39, 8105–8121. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Fernández, J.; Román, A.; Rivas, J.D.L.; Bustelo, X.R.; Dosil, M. The 90s preribosome is a multimodular structure that is assembled through a hierarchical mechanism. Mol. Cell. Biol. 2007, 27, 5414–5429. [Google Scholar] [CrossRef] [PubMed]
- Chaker-Margot, M.; Hunziker, M.; Barandun, J.; Dill, B.D.; Klinge, S. Stage-specific assembly events of the 6-MDa small-subunit processome initiate eukaryotic ribosome biogenesis. Nat. Struct. Mol. Biol. 2015, 22, 920–923. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, C.; Cai, G.; Chen, S.; Ye, K. Stepwise and dynamic assembly of the earliest precursors of small ribosomal subunits in yeast. Genes Dev. 2016, 30, 718–732. [Google Scholar] [CrossRef]
- Boissier, F.; Schmidt, C.M.; Linnemann, J.; Fribourg, S.; Perez-Fernandez, J. Pwp2 mediates UTP-B assembly via two structurally independent domains. Sci. Rep. 2017, 7, 3169. [Google Scholar] [CrossRef]
- Hunziker, M.; Barandun, J.; Buzovetsky, O.; Steckler, C.; Molina, H.; Klinge, S. Conformational switches control early maturation of the eukaryotic small ribosomal subunit. eLife 2019, 8, e45185. [Google Scholar] [CrossRef]
- Woese, C.R.; Magrum, L.J.; Gupta, R.; Siegel, R.B.; Stahl, D.A.; Kop, J.; Crawford, N.; Brosius, J.; Gutell, R.; Hogan, J.J.; et al. Secondary structure model for bacterial 16S ribosomal RNA: Phylogenetic, enzymatic and chemical evidence. Nucleic Acids Res. 1980, 8, 2275–2293. [Google Scholar] [CrossRef]
- Lempereur, L.; Nicoloso, M.; Riehl, N.; Ehresmann, C.; Ehresmann, B.; Bachellerie, J.P. Conformation of yeast 18S RRNA. Direct chemical probing of the 5′ domain in ribosomal subunits and in deproteinized RNA by reverse transcriptase mapping of dimethyl sulfate-accessible. Nucleic Acids Res. 1985, 13, 8339–8357. [Google Scholar] [CrossRef]
- Yeh, L.-C.C.; Lee, J.C. Structure Analysis of the 5′ external transcribed spacer of the precursor ribosomal RNA from Saccharomyces cerevisiae. J. Mol. Biol. 1992, 228, 827–839. [Google Scholar] [CrossRef]
- Yeh, L.C.; Thweatt, R.; Lee, J.C. Internal transcribed spacer 1 of the yeast precursor ribosomal RNA. Higher order structure and common structural motifs. Biochemistry 1990, 29, 5911–5918. [Google Scholar] [CrossRef]
- Méreau, A.; Fournier, R.; Grégoire, A.; Mougin, A.; Fabrizio, P.; Lührmann, R.; Branlant, C. An in vivo and in vitro structure-function analysis of the Saccharomyces cerevisiae U3A snoRNP: Protein-RNA contacts and base-pair interaction with the pre-ribosomal RNA. J. Mol. Biol. 1997, 273, 552–571. [Google Scholar] [CrossRef]
- Ségault, V.; Mougin, A.; Grégoire, A.; Banroques, J.; Branlant, C. An experimental study of Saccharomyces cerevisiae U3 SnRNA conformation in solution. Nucleic Acids Res. 1992, 20, 3443–3451. [Google Scholar] [CrossRef]
- Beltrame, M.; Tollervey, D. Identification and functional analysis of two U3 binding sites on yeast pre-ribosomal RNA. EMBO J. 1992, 11, 1531–1542. [Google Scholar] [CrossRef] [PubMed]
- Dutca, L.M.; Gallagher, J.E.; Baserga, S.J. The initial U3 SnoRNA:Pre-RRNA base pairing interaction required for pre-18S RRNA folding revealed by in vivo chemical probing. Nucleic Acids Res. 2011, 39, 5164–5180. [Google Scholar] [CrossRef]
- Barandun, J.; Chaker-Margot, M.; Hunziker, M.; Molloy, K.R.; Chait, B.T.; Klinge, S. The complete structure of the small-subunit processome. Nat. Struct. Mol. Biol. 2017, 24, 944–953. [Google Scholar] [CrossRef]
- Cheng, J.; Kellner, N.; Berninghausen, O.; Hurt, E.; Beckmann, R. 3.2-Å-resolution structure of the 90S preribosome before A1 pre-rRNA cleavage. Nat. Struct. Mol. Biol. 2017, 24, 954–964. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Lau, B.; Venuta, G.L.; Ameismeier, M.; Berninghausen, O.; Hurt, E.; Beckmann, R. 90S pre-ribosome transformation into the primordial 40S subunit. Science 2020, 369, 1470–1476. [Google Scholar] [CrossRef]
- Du, Y.; An, W.; Zhu, X.; Sun, Q.; Qi, J.; Ye, K. Cryo-EM structure of 90S small ribosomal subunit precursors in transition states. Science 2020, 369, 1477–1481. [Google Scholar] [CrossRef]
- Sun, Q.; Zhu, X.; Qi, J.; An, W.; Lan, P.; Tan, D.; Chen, R.; Wang, B.; Zheng, S.; Zhang, C.; et al. Molecular architecture of the 90S small subunit pre-ribosome. eLife 2017, 6, e22086. [Google Scholar] [CrossRef]
- Ohmayer, U.; Perez-Fernandez, J.; Hierlmeier, T.; Pöll, G.; Williams, L.; Griesenbeck, J.; Tschochner, H.; Milkereit, P. Local tertiary structure probing of ribonucleoprotein particles by nuclease fusion proteins. PLoS ONE 2012, 7, e42449. [Google Scholar] [CrossRef]
- Pöll, G.; Li, S.; Ohmayer, U.; Hierlmeier, T.; Milkereit, P.; Perez-Fernandez, J. In vitro reconstitution of yeast TUTP/UTP A and UTP B subcomplexes provides new insights into their modular architecture. PLoS ONE 2014, 9, e114898. [Google Scholar] [CrossRef]
- Krogan, N.J.; Peng, W.-T.T.; Cagney, G.; Robinson, M.D.; Haw, R.; Zhong, G.; Guo, X.; Zhang, X.; Canadien, V.; Richards, D.P.; et al. High-definition macromolecular composition of yeast RNA-processing complexes. Mol. Cell 2004, 13, 225–239. [Google Scholar] [CrossRef]
- Gallagher, J.E.; Dunbar, D.A.; Granneman, S.; Mitchell, B.M.; Osheim, Y.; Beyer, A.L.; Baserga, S.J. RNA polymerase I transcription and pre-RRNA processing are linked by specific SSU processome components. Genes Dev. 2004, 18, 2506–2517. [Google Scholar] [CrossRef]
- Longtine, M.S.; Mckenzie, A., III; Demarini, D.J.; Shah, N.G.; Wach, A.; Brachat, A.; Philippsen, P.; Pringle, J.R. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 1998, 14, 953–961. [Google Scholar] [CrossRef]
- Janke, C.; Magiera, M.M.; Rathfelder, N.; Taxis, C.; Reber, S.; Maekawa, H.; Moreno-Borchart, A.; Doenges, G.; Schwob, E.; Schiebel, E.; et al. A versatile toolbox for PCR-based tagging of yeast genes: New fluorescent proteins, more markers and promoter substitution cassettes. Yeast 2004, 21, 947–962. [Google Scholar] [CrossRef] [PubMed]
- Knop, M.; Siegers, K.; Pereira, G.; Zachariae, W.; Winsor, B.; Nasmyth, K.; Schiebel, E. Epitope Tagging of Yeast Genes Using a PCR-based Strategy: More Tags and Improved Practical Routines. Yeast 1999, 15, 963–972. [Google Scholar] [CrossRef]
- Puig, O.; Caspary, F.; Rigaut, G.; Rutz, B.; Bouveret, E.; Bragado-Nilsson, E.; Wilm, M.; Séraphin, B. The tandem affinity purification (TAP) method: A general procedure of protein complex purification. Methods 2001, 24, 218–229. [Google Scholar] [CrossRef]
- Grandi, P.; Rybin, V.; Baßler, J.; Petfalski, E.; Strauß, D.; Marzioch, M.; Schäfer, T.; Kuster, B.; Tschochner, H.; Tollervey, D.; et al. 90S pre-ribosomes include the 35S Pre-RRNA, the U3 SnoRNP, and 40S subunit processing factors but predominantly lack 60S synthesis factors. Mol. Cell 2002, 10, 105–115. [Google Scholar] [CrossRef]
- Kornprobst, M.; Turk, M.; Kellner, N.; Cheng, J.; Flemming, D.; Koš-Braun, I.; Koš, M.; Thoms, M.; Berninghausen, O.; Beckmann, R.; et al. Architecture of the 90S pre-ribosome: A structural view on the birth of the eukaryotic ribosome. Cell 2016, 166, 380–393. [Google Scholar] [CrossRef] [PubMed]
- Granneman, S.; Kudla, G.; Petfalski, E.; Tollervey, D. Identification of protein binding sites on U3 snoRNA and pre-RRNA by UV cross-linking and high-throughput analysis of CDNAs. In Proceedings of the National Academy of Sciences of the United States of America, San Francisco, CA, USA, 17 April 2009; 2009; Volume 106, pp. 9613–9618. [Google Scholar] [CrossRef]
- Hafner, M.; Katsantoni, M.; Köster, T.; Marks, J.; Mukherjee, J.; Staiger, D.; Ule, J.; Zavolan, M. CLIP and complementary methods. Nat. Rev. Methods Primers 2021, 1, 20. [Google Scholar] [CrossRef]
- Hunziker, M.; Barandun, J.; Petfalski, E.; Tan, D.; Delan-Forino, C.; Molloy, K.R.; Kim, K.H.; Dunn-Davies, H.; Shi, Y.; Chaker-Margot, M.; et al. UtpA and UtpB chaperone nascent pre-ribosomal RNA and U3 snoRNA to initiate eukaryotic ribosome assembly. Nat. Commun. 2016, 7, 12090. [Google Scholar] [CrossRef] [PubMed]
- Barandun, J.; Hunziker, M.; Klinge, S. Assembly and structure of the SSU processome—a nucleolar precursor of the small ribosomal subunit. Curr. Opin. Struct. Biol. 2018, 49, 85–93. [Google Scholar] [CrossRef]
- Lau, B.; Cheng, J.; Flemming, D.; La Venuta, G.; Berninghausen, O.; Beckmann, R.; Hurt, E. Structure of the maturing 90S pre-ribosome in association with the RNA exosome. Mol. Cell 2021, 81, 293–303. [Google Scholar] [CrossRef]
- Allmang, C.; Henry, Y.; Wood, H.; Morrissey, J.P.; Petfalski, E.; Tollervey, D. Recognition of cleavage site A(2) in the yeast pre-rRNA. RNA 1996, 2, 51–62. [Google Scholar]
- van Nues, R.W.; Rientjes, J.M.; van der Sande, C.A.; Zerp, S.F.; Sluiter, C.; Venema, J.; Planta, R.J.; Raué, H.A. Separate structural elements within internal transcribed spacer 1 of Saccharomyces cerevisiae precursor ribosomal RNA di-rect the formation of 17S and 26S rRNA. Nucleic Acids Res. 1994, 22, 912–919. [Google Scholar] [CrossRef]
- Wells, G.R.; Weichmann, F.; Colvin, D.; Sloan, K.E.; Kudla, G.; Tollervey, D.; Watkins, N.J.; Schneider, C. The PIN domain endonuclease Utp24 cleaves pre-ribosomal RNA at two coupled sites in yeast and humans. Nucleic Acids Res. 2016, 44, 5399–5409. [Google Scholar] [CrossRef]
- An, W.; Du, Y.; Ye, K. Structural and functional analysis of Utp24, an endonuclease for processing 18S ribosomal RNA. PLoS ONE 2018, 13, e0195723. [Google Scholar] [CrossRef]
- Merz, K.; Hondele, M.; Goetze, H.; Gmelch, K.; Stoeckl, U.; Griesenbeck, J. Actively transcribed rRNA genes in S. cerevisiae are organized in a specialized chromatin associated with the high-mobility group protein Hmo1 and are largely devoid of histone molecules. Genes Dev. 2008, 22, 1190–1204. [Google Scholar] [CrossRef]
- Sambrook, J.; Rusell, D.W. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2000; Volume 3. [Google Scholar]
- Pöll, G.; Braun, T.; Jakovljevic, J.; Neueder, A.; Jakob, S.; Woolford, J.L., Jr.; Tschochner, H.; Milkereit, P. rRNA maturation in yeast cells depleted of large ribosomal subunit proteins. PLoS ONE 2009, 4, e8249. [Google Scholar] [CrossRef] [PubMed]
- Venema, J.; Planta, R.J.; Raué, H.A. In vivo mutational analysis of ribosomal RNA in Saccharomyces cerevisiae. Methods Mol. Biol. 1998, 77, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Knüppel, R.; Fenk, M.; Jüttner, M.; Ferreira-Cerca, S. In vivo RNA chemical footprinting analysis in Archaea. Methods Mol. Biol. 2019, 2106, 193–208. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dielforder, T.; Braun, C.M.; Hölzgen, F.; Li, S.; Thiele, M.; Huber, M.; Ohmayer, U.; Perez-Fernandez, J. Structural Probing with MNase Tethered to Ribosome Assembly Factors Resolves Flexible RNA Regions within the Nascent Pre-Ribosomal RNA. Non-Coding RNA 2022, 8, 1. https://doi.org/10.3390/ncrna8010001
Dielforder T, Braun CM, Hölzgen F, Li S, Thiele M, Huber M, Ohmayer U, Perez-Fernandez J. Structural Probing with MNase Tethered to Ribosome Assembly Factors Resolves Flexible RNA Regions within the Nascent Pre-Ribosomal RNA. Non-Coding RNA. 2022; 8(1):1. https://doi.org/10.3390/ncrna8010001
Chicago/Turabian StyleDielforder, Tom, Christina Maria Braun, Fabian Hölzgen, Shuang Li, Mona Thiele, Marina Huber, Uli Ohmayer, and Jorge Perez-Fernandez. 2022. "Structural Probing with MNase Tethered to Ribosome Assembly Factors Resolves Flexible RNA Regions within the Nascent Pre-Ribosomal RNA" Non-Coding RNA 8, no. 1: 1. https://doi.org/10.3390/ncrna8010001
APA StyleDielforder, T., Braun, C. M., Hölzgen, F., Li, S., Thiele, M., Huber, M., Ohmayer, U., & Perez-Fernandez, J. (2022). Structural Probing with MNase Tethered to Ribosome Assembly Factors Resolves Flexible RNA Regions within the Nascent Pre-Ribosomal RNA. Non-Coding RNA, 8(1), 1. https://doi.org/10.3390/ncrna8010001






