The Impact of Population Variation in the Analysis of microRNA Target Sites
Abstract
1. Introduction
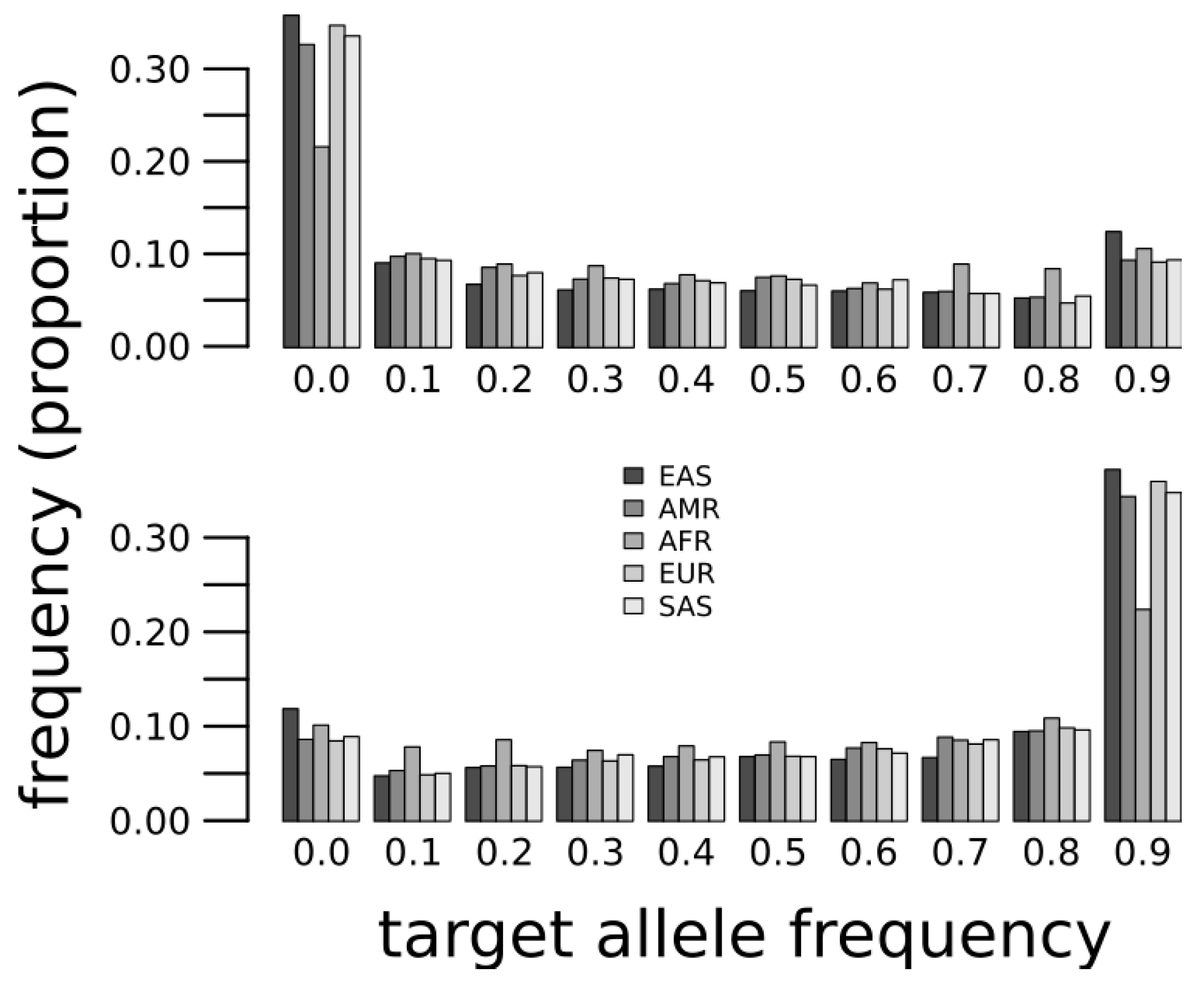
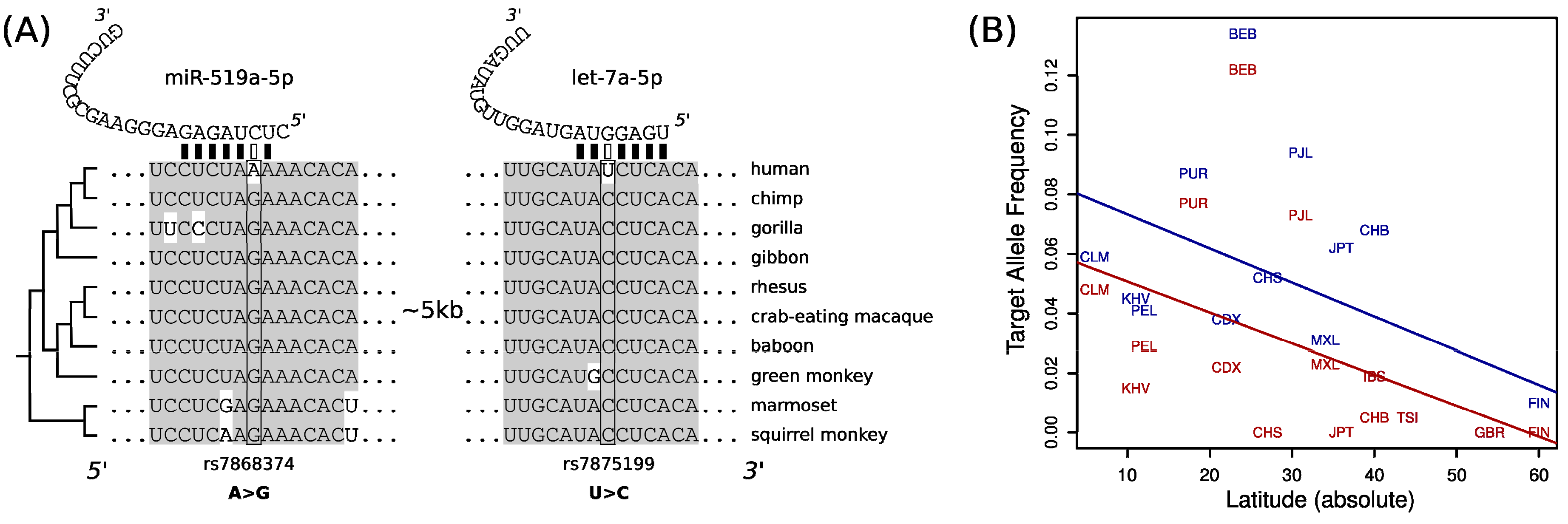
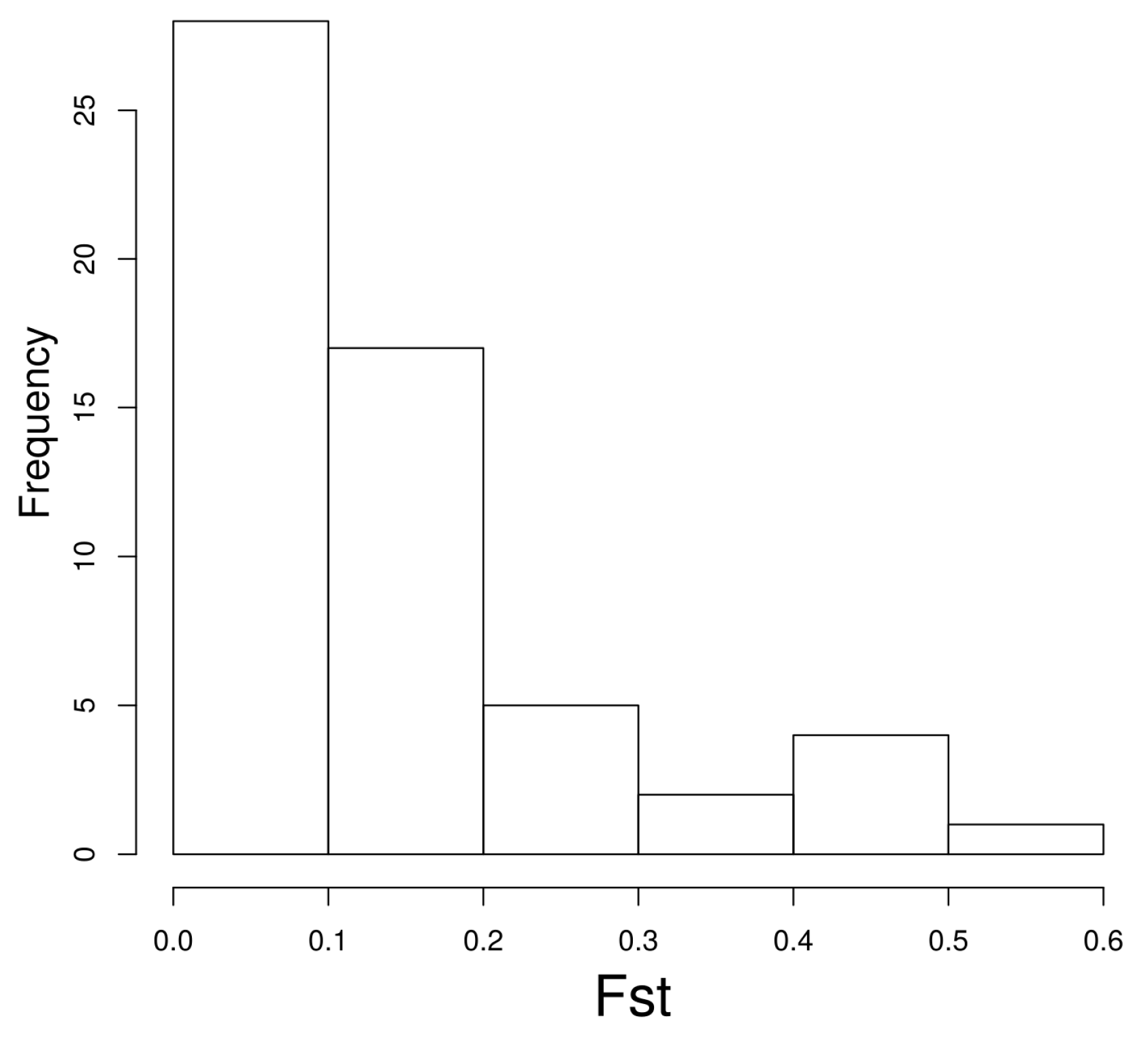
2. Results and Discussion
3. Methods
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bartel, D.P. MicroRNAs: Target Recognition and Regulatory Functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Axtell, M.J.; Westholm, J.O.; Lai, E.C. Vive la différence: Biogenesis and evolution of microRNAs in plants and animals. Genome Biol. 2011, 12, 221. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Wang, Y.; Hao, Y.; Juan, L.; Teng, M.; Zhang, X.; Li, M.; Wang, G.; Liu, Y. miR2Disease: A manually curated database for microRNA deregulation in human disease. Nucleic Acids Res. 2009, 37, D98–D104. [Google Scholar] [CrossRef] [PubMed]
- Di Leva, G.; Garofalo, M.; Croce, C.M. microRNAs in cancer. Annu. Rev. Pathol. 2014, 9, 287–314. [Google Scholar] [CrossRef] [PubMed]
- Croce, C.M. Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet 2009, 10, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Ghildiyal, M.; Zamore, P.D. Small silencing RNAs: An expanding universe. Nat. Rev. Genet. 2009, 10, 94–108. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Ziebarth, J.D.; Cui, Y. PolymiRTS Database 3.0: Linking polymorphisms in microRNAs and their target sites with human diseases and biological pathways. Nucleic Acids Res. 2014, 42, D86–D91. [Google Scholar] [CrossRef] [PubMed]
- Hiard, S.; Charlier, C.; Coppieters, W.; Georges, M.; Baurain, D. Patrocles: A database of polymorphic miRNA-mediated gene regulation in vertebrates. Nucleic Acids Res. 2010, 38, D640–D651. [Google Scholar] [CrossRef]
- Agarwal, V.; Bell, G.W.; Nam, J.-W.; Bartel, D.P. Predicting effective microRNA target sites in mammalian mRNAs. eLife 2015, 4, e05005. [Google Scholar] [CrossRef]
- Paraskevopoulou, M.D.; Georgakilas, G.; Kostoulas, N.; Vlachos, I.S.; Vergoulis, T.; Reczko, M.; Filippidis, C.; Dalamagas, T.; Hatzigeorgiou, A.G. DIANA-microT web server v5.0: Service integration into miRNA functional analysis workflows. Nucleic Acids Res. 2013, 41, W169–W173. [Google Scholar] [CrossRef]
- Marco, A. SeedVicious: Analysis of microRNA target and near-target sites. PLoS ONE 2018, 13, e0195532. [Google Scholar] [CrossRef] [PubMed]
- Alexiou, P.; Maragkakis, M.; Papadopoulos, G.L.; Reczko, M.; Hatzigeorgiou, A.G. Lost in translation: An assessment and perspective for computational microRNA target identification. Bioinformatics 2009, 25, 3049–3055. [Google Scholar] [CrossRef] [PubMed]
- Pinzón, N.; Li, B.; Martinez, L.; Sergeeva, A.; Presumey, J.; Apparailly, F.; Seitz, H. microRNA target prediction programs predict many false positives. Genome Res. 2017, 27, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, D.E.; Martin, M.M.; Feldman, D.S.; Terry, A.V.; Nuovo, G.J.; Elton, T.S. Experimental validation of miRNA targets. Methods 2008, 44, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Barbujani, G.; Colonna, V. Human genome diversity: Frequently asked questions. Trends Genet. Tig. 2010, 26, 285–295. [Google Scholar] [CrossRef] [PubMed]
- 1000 Genomes Project Consortium; Abecasis, G.R.; Altshuler, D.; Auton, A.; Brooks, L.D.; Durbin, R.M.; Gibbs, R.A.; Hurles, M.E.; McVean, G.A. A map of human genome variation from population-scale sequencing. Nature 2010, 467, 1061–1073. [Google Scholar] [CrossRef] [PubMed]
- Garfield, D.; Haygood, R.; Nielsen, W.J.; Wray, G.A. Population genetics of cis-regulatory sequences that operate during embryonic development in the sea urchin Strongylocentrotus purpuratus. Evol. Dev. 2012, 14, 152–167. [Google Scholar] [CrossRef]
- Kasowski, M.; Grubert, F.; Heffelfinger, C.; Hariharan, M.; Asabere, A.; Waszak, S.M.; Habegger, L.; Rozowsky, J.; Shi, M.; Urban, A.E.; et al. Variation in transcription factor binding among humans. Science 2010, 328, 232–235. [Google Scholar] [CrossRef]
- Chen, K.; Rajewsky, N. Natural selection on human microRNA binding sites inferred from SNP data. Nat. Genet. 2006, 38, 1452–1456. [Google Scholar] [CrossRef]
- Saunders, M.A.; Liang, H.; Li, W.-H. Human polymorphism at microRNAs and microRNA target sites. Proc. Natl. Acad. Sci. USA 2007, 104, 3300–3305. [Google Scholar] [CrossRef]
- Marco, A. Selection Against Maternal microRNA Target Sites in Maternal Transcripts. G3 Genesgenomesgenetics 2015, 5, 2199–2207. [Google Scholar] [CrossRef] [PubMed]
- Hatlen, A.; Marco, A. Pervasive selection against microRNA target sites in human populations. bioRxiv 2018, 420646. [Google Scholar]
- Kvaskoff, M.; Whiteman, D.C.; Zhao, Z.Z.; Montgomery, G.W.; Martin, N.G.; Hayward, N.K.; Duffy, D.L. Polymorphisms in naevus-associated genes MTAP, PLA2G6, and IRF4 and the risk of invasive cutaneous melanoma. Twin Res. Hum. Genet. 2011, 14, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Bishop, D.T.; Demenais, F.; Iles, M.M.; Harland, M.; Taylor, J.C.; Corda, E.; Randerson-Moor, J.; Aitken, J.F.; Avril, M.-F.; Azizi, E.; et al. Genome-wide association study identifies three loci associated with melanoma risk. Nat. Genet. 2009, 41, 920–925. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, Y.; Xin, X.; Kim, T.S.; Cabeza, E.A.; Ren, J.; Nielsen, R.; Wrana, J.L.; Zhang, Z. Evidence for Positive Selection on a Number of MicroRNA Regulatory Interactions during Recent Human Evolution. PLoS Genet. 2012, 8, e1002578. [Google Scholar] [CrossRef]
- Chang, C.L.; Cai, J.J.; Huang, S.Y.; Cheng, P.J.; Chueh, H.Y.; Hsu, S.Y.T. Adaptive Human CDKAL1 Variants Underlie Hormonal Response Variations at the Enteroinsular Axis. PLoS ONE 2014, 9, e105410. [Google Scholar] [CrossRef]
- Chang, C.L.; Cai, J.J.; Cheng, P.J.; Chueh, H.Y.; Hsu, S.Y.T. Identification of Metabolic Modifiers That Underlie Phenotypic Variations in Energy-Balance Regulation. Diabetes 2011, 60, 726–734. [Google Scholar] [CrossRef]
- Duan, Y.; Hu, L.; Liu, B.; Yu, B.; Li, J.; Yan, M.; Yu, Y.; Li, C.; Su, L.; Zhu, Z.; et al. Tumor suppressor miR-24 restrains gastric cancer progression by downregulating RegIV. Mol. Cancer 2014, 13, 127. [Google Scholar] [CrossRef]
- Ipe, J.; Collins, K.S.; Hao, Y.; Gao, H.; Bhatia, P.; Gaedigk, A.; Liu, Y.; Skaar, T.C. PASSPORT-seq: A Novel High-Throughput Bioassay to Functionally Test Polymorphisms in Micro-RNA Target Sites. Front. Genet. 2018, 9, 219. [Google Scholar] [CrossRef]
- Pybus, M.; Dall’Olio, G.M.; Luisi, P.; Uzkudun, M.; Carreño-Torres, A.; Pavlidis, P.; Laayouni, H.; Bertranpetit, J.; Engelken, J. 1000 Genomes Selection Browser 1.0: A genome browser dedicated to signatures of natural selection in modern humans. Nucleic Acids Res. 2014, 42, D903–D909. [Google Scholar] [CrossRef]
- Karolchik, D.; Hinrichs, A.S.; Furey, T.S.; Roskin, K.M.; Sugnet, C.W.; Haussler, D.; Kent, W.J. The UCSC Table Browser data retrieval tool. Nucleic Acids Res. 2004, 32, D493–D496. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.-H.; Shrestha, S.; Yang, C.-D.; Chang, N.-W.; Lin, Y.-L.; Liao, K.-W.; Huang, W.-C.; Sun, T.-H.; Tu, S.-J.; Lee, W.-H.; et al. miRTarBase update 2018: A resource for experimentally validated microRNA-target interactions. Nucleic Acids Res. 2018, 46, D296–D302. [Google Scholar] [CrossRef] [PubMed]
| MicroRNA | Gene | SNP a | EAS b | AMR b | AFR b | EUR b | SAS b | Fst |
|---|---|---|---|---|---|---|---|---|
| miR-519a-5p 1 | MTAP | rs7868374 | 0.0079 | 0.0476 | 0.6838 | 0.0050 | 0.1176 | 0.8239 |
| let-7a-5p 2 | MTAP | rs7875199 | 0.0536 | 0.0576 | 0.7958 | 0.0070 | 0.1288 | 0.7997 |
| miR-337-3p 3 | ATP1A1 | rs1885802 | 0.0427 | 0.1268 | 0.8109 | 0.0338 | 0.0450 | 0.7914 |
| miR-185-3p 4 | SLC5A10 | rs1624825 | 0.9970 | 0.5159 | 0.9667 | 0.2028 | 0.7495 | 0.7859 |
| miR-1180-3p | MYEF2 | rs2470102 | 0.7530 | 0.3386 | 0.9251 | 0.0060 | 0.2720 | 0.7726 |
| miR-151a-5p 5 | SCN2B | rs624328 | 0.9405 | 0.8905 | 0.2519 | 0.9513 | 0.9673 | 0.7674 |
| miR-4732-5p | AP5M1 | rs1889720 | 0.9335 | 0.9107 | 0.1551 | 0.9433 | 0.9182 | 0.7637 |
| miR-584-5p | CYB5R4 | rs6903739 | 0.0000 | 0.0749 | 0.7738 | 0.0477 | 0.0061 | 0.7514 |
| miR-618 | CYB5R4 | rs9449733 | 1.0000 | 0.9251 | 0.2247 | 0.9523 | 0.9939 | 0.7397 |
| miR-18a-3p | ANKRD65 | rs904589 | 0.1131 | 0.1945 | 0.8222 | 0.0964 | 0.1881 | 0.7332 |
| miR-329-3p 6 | CEP162 | rs6901546 | 0.0139 | 0.1628 | 0.7670 | 0.0706 | 0.0900 | 0.7326 |
| miR-150-5p 7 | HM13 | rs6059873 | 1.0000 | 0.8156 | 0.1725 | 0.8429 | 0.9622 | 0.7298 |
| miR-513a-3p 8 | TCERG1 | rs3822506 | 0.7183 | 0.1167 | 0.0325 | 0.0915 | 0.2198 | 0.7224 |
| miR-128-1-5p 9 | BCL7C | rs11864054 | 0.9117 | 0.5447 | 0.0242 | 0.3817 | 0.1595 | 0.7144 |
| miR-192-5p 10 | C12orf65 | rs1533703 | 0.9980 | 0.7262 | 0.1490 | 0.7694 | 0.7945 | 0.7074 |
| MicroRNA | Gene | SNP a | EAS b | AMR b | AFR b | EUR b | SAS b | Fst |
|---|---|---|---|---|---|---|---|---|
| miR-1307-3p | VSTM4 | rs4240499 | 0.2361 | 0.4971 | 0.9017 | 0.3976 | 0.5031 | 0.5352 |
| miR-512-3p | EIF2B2 | rs4556 | 0.8393 | 0.6441 | 0.1884 | 0.5388 | 0.5194 | 0.4327 |
| miR-197-3p | DBT | rs6701655 | 0.9296 | 0.8905 | 0.4168 | 0.8648 | 0.8978 | 0.4186 |
| miR-17-5p | MRPS10 | rs3199638 | 0.7937 | 0.6369 | 0.2148 | 0.6372 | 0.7679 | 0.3946 |
| miR-326 | SLC27A4 | rs7048106 | 0.3185 | 0.2176 | 0.7617 | 0.2386 | 0.3476 | 0.3782 |
| miR-24-3p | CASP10 | rs13432040 | 0.000 | 0.0259 | 0.2587 | 0.000 | 0.000 | 0.2943 |
| miR-342-3p | LRPAP1 | rs3468 | 0.5089 | 0.2507 | 0.1611 | 0.3489 | 0.2669 | 0.238 |
| TargetScan Dataset | Sample Size (pairs) | p | Shift Median 1 | Shift 95% CI2 |
|---|---|---|---|---|
| conserved | 9245 | <2.2 × 10−16 | 7.976 × 10−4 | (7.833–7.878) 10−4 |
| nonconserved | 1041868 | <2.2 × 10−16 | 1.174 × 10−3 | (1.151–1.210) 10−3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Helmy, M.; Hatlen, A.; Marco, A. The Impact of Population Variation in the Analysis of microRNA Target Sites. Non-Coding RNA 2019, 5, 42. https://doi.org/10.3390/ncrna5020042
Helmy M, Hatlen A, Marco A. The Impact of Population Variation in the Analysis of microRNA Target Sites. Non-Coding RNA. 2019; 5(2):42. https://doi.org/10.3390/ncrna5020042
Chicago/Turabian StyleHelmy, Mohab, Andrea Hatlen, and Antonio Marco. 2019. "The Impact of Population Variation in the Analysis of microRNA Target Sites" Non-Coding RNA 5, no. 2: 42. https://doi.org/10.3390/ncrna5020042
APA StyleHelmy, M., Hatlen, A., & Marco, A. (2019). The Impact of Population Variation in the Analysis of microRNA Target Sites. Non-Coding RNA, 5(2), 42. https://doi.org/10.3390/ncrna5020042





