Abstract
Background/Objectives: Competing endogenous RNAs (ceRNA) are molecules that compete for the binding to a microRNA (miR). Usually, there are two ceRNA, one of which is a protein-coding RNA (mRNA), with the other being a long non-coding RNA (lncRNA). The miR role is to inhibit mRNA expression, either promoting its degradation or impairing its translation. The lncRNA can “sponge” the miR, thus impeding its inhibitory action on the mRNA. In their easier configuration, these three molecules constitute a regulatory axis for protein expression. However, each RNA can interact with multiple targets, creating branched and intersected axes that, all together, constitute what is known as a competing endogenous RNA network (ceRNET). Methods: In this systematic review, we collected all available data from PubMed about experimentally verified (by luciferase assay) regulatory axes in endometrial cancer (EC), excluding works not using this test; Results: This search allowed the selection of 172 bibliographic sources, and manually building a series of ceRNETs of variable complexity showed the known axes and the deduced intersections. The main limitation of this search is the highly stringent selection criteria, possibly leading to an underestimation of the complexity of the networks identified. However, this work allows us not only to hypothesize possible gap fillings but also to set the basis to instruct artificial intelligence, using adequate prompts, to expand the EC ceRNET by comparing it with ceRNETs of other cancers. Moreover, these networks can be used to inform and guide research toward specific, though still unidentified, axes in EC, to complete parts of the network that are only partially described, or even to integrate low complexity subnetworks into larger more complex ones. Filling the gaps among the existing EC ceRNET will allow physicians to hypothesize new therapeutic strategies that may either potentiate or substitute existing ones. Conclusions: These ceRNETs allow us to easily visualize long-distance interactions, thus helping to select the best treatment, depending on the molecular profile of each patient, for personalized medicine. This would yield higher efficiency rates and lower toxicity levels, both of which are extremely relevant factors not only for patients’ wellbeing, but also for the legal, regulatory, and ethical aspects of miR-based innovative treatments and personalized medicine as a whole. This systematic review has been registered in PROSPERO (ID: PROSPERO 2025 CRD420251035222).
1. Introduction
Endometrial cancer (EC) is the most frequent gynecological malignancy in developed nations, with the highest rates in North America, Europe, Micronesia/Polynesia, and Australia/New Zealand; it is the sixth most diagnosed cancer in women, with 417,000 new cases and 97,000 deaths in 2020 [1]. Risk factors for EC include metabolic alterations (obesity, metabolic syndrome, insulin resistance) [2,3], hormonal imbalance [4], age at menopause [5], reproductive factors [6], and inherited conditions, such as Lynch Syndrome [7]. Other genetic factors have been identified and accounted for, thus allowing for the classification of EC into four classes with distinct clinical, pathologic, and molecular features [8], namely POLE (polymerase epsilon)/ultramutated (7% of cases), microsatellite instability (MSI)/hypermutated (28%), copy-number low/endometrioid (39%), and copy-number high/serous-like (26%). In addition to the mutation of genes specific for the above-mentioned conditions (POLE, MLH1, TP53), many other mutated genes had been identified in EC patients, including PTEN, PIK3CA, PIK3R1, CTNNB1, ARID1A, PPP2R1A, and FBXW7, all present at a high frequency in EC patients [9]. Additional genetic causes of EC etiopathogenesis include single-nucleotide polymorphisms in specific genes [10], altered telomere length [11], and epigenetic factors, such as DNA hypermethylation of target gene promoters (e.g., MLH1) [12,13].
Within living organisms, the complexity of genetics is not solely determined by the quantity of DNA in a genome. There are organisms deemed less “complex” despite having a higher amount of DNA in their cells, a phenomenon known as the C-value paradox. This also extends to the G-value paradox [14,15], which refers to the number of genes in their genomes. While both values typically correlate with organismal complexity [15], this correlation is notably disrupted in evolutionarily related species that significantly vary in either or both parameters. Complexity, rather than being quantified by DNA content, is better understood as “the extent of interactions, whether orderly or disorderly, among the components of a system”. Within living systems, complexity can be defined as “the total number of gene-gene/molecular interactions accumulated throughout an organism’s lifespan in its natural habitat” [16]. In recent decades, a growing body of the scientific literature has demonstrated that only a small fraction of the genome contains protein-coding genes, with the majority of the transcriptome being non-coding in nature. These non-coding RNAs (ncRNAs) encompass a larger proportion (both in mass and sheer number of molecules) due to the contributions of tRNA and rRNA [17]. However, a notable albeit smaller fraction of ncRNAs participates in regulating gene expression. The intricate web of interactions among molecules constitutes a significant portion of an organism’s complexity, with the control of gene expression playing a pivotal role in both physiological well-being and pathological conditions.
Overall, ncRNAs are divided into long and short—lncRNA and sncRNA, respectively—based on their length, with the former exceeding 200 nucleotides (nt) and the latter being below this threshold and usually in the 20–30 nt range. In the context of post-transcriptional gene expression control, microRNAs (abbreviated as miR or miRNA) are the most broadly researched kind of sncRNA. By annealing to their targets, such molecules can inhibit mRNA expression by either promoting its degradation or by impairing its translation [18]. The high number of miR inside human cells (>2500) and their imprecise annealing (partial mismatch is permitted, within certain limits) allows them to target multiple mRNAs at the same time, making them capable of regulating over 60% of human genes [19]. Among their many effects, miR directly regulate several genes involved in cell cycle control and cell survival, making them key players in carcinogenesis [20]. In turn, these molecules may be inhibited through their binding to lncRNA, which act as sponges to lower miR intracellular availability; in this case, lncRNA behave as miR inhibitors and, consequently, they are implicated in carcinogenesis as well [21]. Many currently available research findings point to this kind of interaction in tumors, allowing the identification of inhibitory axes made up of a lncRNA, a sponged miR, and a target mRNA. Since lncRNA and mRNA are both able to bind one or more miR, they are called competing endogenous RNAs (ceRNA) and the intertwined axes sharing one or two members of each axis create a network of cross-interactions collectively called a ceRNA network, or ceRNET [22]. In this perspective, EC is not an exception. Numerous scientific papers have been published, which elaborate on ceRNA interactions in this cancer, and we already summarized the current knowledge in the past [23,24,25,26,27,28,29]; however, a comprehensive and detailed analysis of this topic in recent years and the analysis of EC ceRNET in its entirety is still lacking.
For the purpose of this systematic review, we collected the most up-to-date knowledge on EC-related ceRNETs, revised previous research findings, and used such data to build a hand-curated ceRNET for EC. Our work aims to shed a light on the complexities of ceRNA interactions in this tumor and to aid making accurate predictions for both long-distance interactions and possible future discoveries, eventually making use in the future of adequate bioinformatic tools to speed up discoveries and compare ceRNET from different tumors. Also, we trust that this kind of data will be useful in helping physicians to select appropriate therapies, on a single-patient base, to improve the efficacy of personalized medicine.
2. Results
2.1. Data Collection
By applying the inclusion and exclusion criteria described in Section 4 (Materials and Methods), we were able to collect data from 172 different bibliographic sources, for a total of 201 ceRNET axes, including partial axes. These results are summarized in Table 1.

Table 1.
List of regulatory axes in EC. Each axis (a row in the table) is defined as a group of three elements, a lncRNA (column 2), and a mRNA (column 3) competing for the binding of the same microRNA (column 1). Data are ordered according to column 1. Undefined means that in the relevant publication (column 4), only two members of the axis have been described (partial axes). Column 5 indicates where the axis is illustrated in figures; it is reported in figures only if its ceRNET reaches at least four elements and two interconnected axes; if not, we report “no” in column 5. Asterisks indicate a putative additional connection in Figure 1 (see Section 3.2).
Due to their nature and biosynthesis [202] and their official nomenclature (summarized in [203]) and in absence of more specific data, we classified those with a general name and those with the specific 3p or 5p signature as the same miR; for example, we considered miR-101 and miR-101-3p to be the same molecule, as they both derive from the same precursor pre-miR and there is no evidence of sequence differences in the analyzed literature. Instead, miR with both 5p and 3p forms expressed in EC and, thus, potentially those with different targets (they have complementary sequences), were considered as different molecules, for example, miR-129-2-3p and miR-129-5p. Finally, miR, identified by additional letters in their ID, were considered as different miR, as they have slightly different sequences and the research data herein analyzed do not show that they target the same ceRNA; for example, miR-106a and miR-106b were considered different molecules. Consequently, according to these criteria, we considered miR-181a and miR-181a-5p the same molecule but different from miR-181c, so in total, there are three miR forms but only two molecules involved; similar can be said for miR-200 (four forms, three molecules) and miR-27 (four forms, two molecules).
In conclusion, and according to the above-mentioned criteria, this screening allowed us to identify 79 lncRNA, 127 miR, and 140 mRNA targets in EC ceRNET (Table 1).
2.2. Data Assembly
We analyzed the axes reported in Table 1 (one ceRNET axis per table row) in search of common elements and joined together the axes using the shared element(s). This allowed us to group these axes in a series of sub-networks of different complexity, that we arbitrarily classified as low (5 subnetworks), medium (7 subnetworks), and high (1 subnetwork) complexity ceRNET. We defined low complexity subnetworks as all those networks with 4 to 5 elements (cells in Table 1) and connecting only 2–3 ceRNET axes (rows in Table 1). All axes with only 2–3 elements (i.e., depicting only 1 axis or 2 axes with a single shared element) are not illustrated. Then, we defined medium complexity subnetworks as all those networks with a number of elements between 6 and 50 and connecting more than 3 axes. Hence, the high complexity subnetwork we identified is defined as the one exceeding medium complexity ceRNET thresholds.
The single high complexity ceRNET we identified (Figure 1) contains a total of 125 elements, and it includes 41 miR (white circles), 35 lncRNA (green rectangles), and 49 mRNA targets (blue flags). These elements were drawn according to a grid so that every element can be easily found by a couple of coordinates, left to right (1–10) and top to bottom (a–o), to allow their easy localization according to such coordinates; numbers and letters surrounding the scheme represent the coordinates. Arrows indicate the direction of the inhibition, with lncRNA inhibiting miR action via sponging and miR inhibiting target mRNA expression via (mostly) 3′ UTR binding. For graphical reasons, the names of some elements in all figures are shrunk to a minimal unambiguous size; thus, for example, “101” (coordinates: b1) indicates miR-101 and circ1610 (coordinates: c5) indicates circ0001610. Full names of the molecules can be found in Table 1.
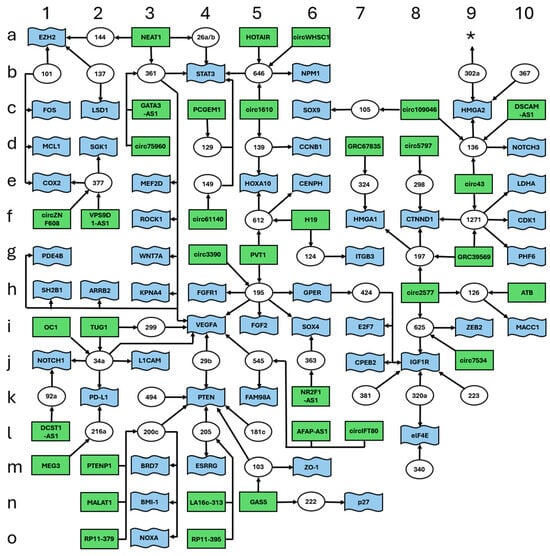
Figure 1.
High complexity ceRNET for EC. Data retrieved from Table 1. The figure shows the interconnections of 125 elements, namely 41 miR (white circles), 35 lncRNA (green rectangles), and 49 mRNA targets (blue flags). Arrows indicate the direction of the inhibition, with lncRNA inhibiting miR action via sponging and miR inhibiting target mRNA translation via (mostly) 3′ UTR binding. Letters and numbers surrounding the scheme help find single elements inside the scheme. The asterisk (coordinates: a9) indicates a possible connection with another peripheral branch; see Section 3 for further explanations.
In addition to the high complexity ceRNET, we found a series of smaller medium complexity unconnected subnetworks (Figure 2). These ceRNETs include a number of elements between 15 (Figure 2A) and 6 (Figure 2G).
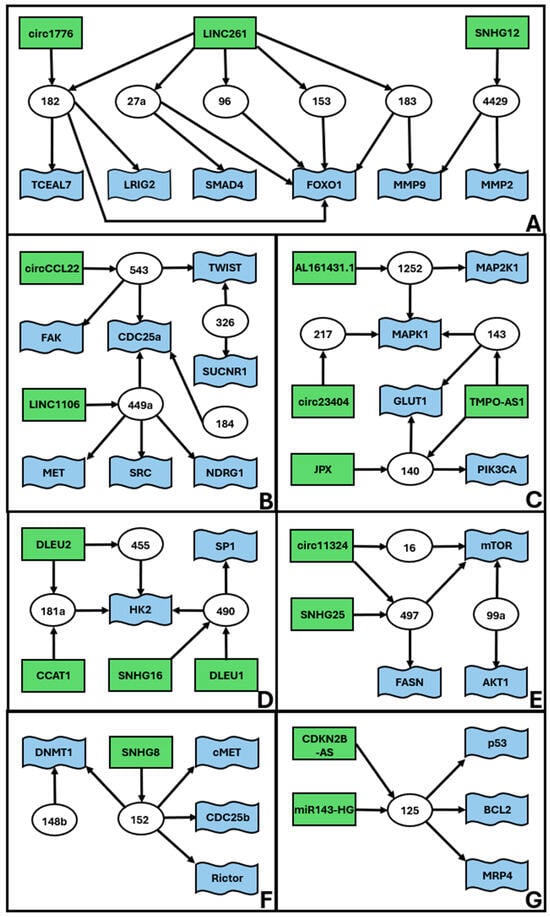
Figure 2.
Medium complexity ceRNETs for EC. Data retrieved from Table 1. The figure shows the interconnections of groups of elements, namely miR (white circles), lncRNA (green rectangles), and mRNA targets (blue flags). Arrows indicate the direction of the inhibition, with lncRNA inhibiting miR action via sponging and miR inhibiting target mRNA translation via (mostly) 3′ UTR binding. We define medium complexity ceRNET as all those networks (apparently) unrelated to the main ceRNET (Figure 1) and containing a number of elements between 5 and 50. The identified 7 subnetworks (A–G) are listed according to their complexity, i.e., number of elements in the ceRNET.
Finally, our search allowed us to identify a series of smaller low complexity unconnected ceRNET (Figure 3), including 4 to 5 elements, apparently not connected with those previously described.
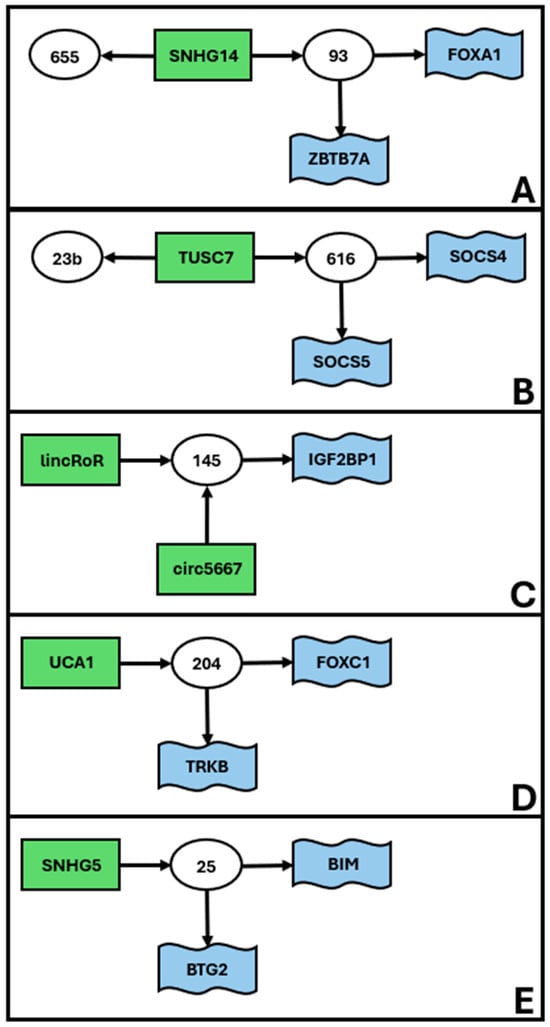
Figure 3.
Low complexity subnetworks for EC. Data retrieved from Table 1. The figure shows the interconnections of groups of elements, namely miR (white circles), lncRNA (green rectangles), and mRNA targets (blue flags). Arrows indicate the direction of the inhibition, with lncRNA inhibiting miR action via sponging and miR inhibiting target mRNA translation via (mostly) 3′ UTR binding. We define low complexity ceRNET as all those subnetworks (apparently) unrelated to the main ceRNET (Figure 1) and having 4 to 5 elements, as they connect only 2–3 ceRNET axes. The idenfified 5 subnetworks (A–E) are listed according to their complexity, i.e., the number of elements in the network. All axes have only 2–3 elements (i.e., depicting only 1–2 axes) and are not reported in figures but can be found in Table 1.
3. Discussion
3.1. General Considerations
To the best of our knowledge, the present work is the first graphical representation of the functional and experimentally verified networks of EC epigenetic gene expression control. Although far from complete, these schemes provide a good idea as to the complexity of cross-interactions acting at the same time inside an endometrial cell, to maintain its homeostasis. To build these schemes, we manually reviewed the available literature, with stringent selection criteria (see Section 4) to lower the presence of false positives as much as possible. Indeed, especially for medium and high complexity networks, the identified connections provide additional indirect evidence of the robustness of our approach (see also the next paragraph). However, in many cases, these axes had been investigated only in one or two papers, so we encourage other scientists to further validate the connections, especially those which were only inferred, to improve the reliability of our predictions, to expand the general EC ceRNET, and/or to connect the subnetworks we illustrated in Figure 1, Figure 2 and Figure 3.
3.2. Using Our ceRNET for Investigating Data Robustness
The very essence of scientific data is well known to reside in its replication. If an experiment fails to be replicated in an independent set-up by other scientists, the most common explanation is that the data are not thoroughly reliable or, in the worst-case scenario, they are either mistaken or misinterpreted. Functional data replication can be obtained in two ways: by replicating the experiment as it is or by finding (hopefully numerous) indirect supporting data. In this report, the use of a luciferase assay has been used as a mandatory selection criterion to verify the physical interaction of any miRNA with a ceRNA (see Section 4). Nonetheless, additional and diverse experimental approaches were used in the research sources we have drawn upon to further validate these findings, such as the use of miR-mimics, or analysis of cells and tissue samples with RT-qPCR and Western blotting. However, other approaches might also be used, such as RNA pull-down or CRISPR screens or the application of -omics data to refine the ceRNET, which would equally substantiate the validity of the luciferase assay. At present, we do not believe that one specific test would suffice to corroborate the luciferase assay result, but a combined use of more approaches would certainly be highly recommended. This would clearly have a positive effect not just on the ceRNET validity but also on the clinical applications derived from its analysis during patients’ diagnosis and prognosis.
If we examine the intricate EC ceRNET depicted in Figure 1, we observe that the majority of its components have been identified in various studies. However, these studies often do not align in their axis descriptions, sharing only individual elements. Within this framework, the presence of miR-195 (coordinates: h5) is highly improbable to be an error or a misinterpretation. The robustness of miR-195 as a central element in EC etiopathogenesis is evident, given its involvement in eight distinct connections sourced from four different literature references (Table 1). Consequently, the study elucidating the interaction between circ_0003390 (coordinates: g4) and miR-195 stands on a solid foundation, even though it is backed by a single bibliographic citation. This long non-coding RNA is in direct association with a sturdy element of the ceRNET, emphasizing its significance. In this context, the relative strength of each component within the complex ceRNET illustrated in Figure 1 can be promptly and effortlessly evaluated based on its interactions with neighboring elements and the extent of research exploring its functions. Thus, any new epigenetic player that will be discovered in the future may be compared to the EC ceRNET reported here to evaluate its affordability on the basis of its possible interactions with known elements of this network. As a mere philosophical exercise, we mined PubMed by using the search string “endometrial cancer AND miR AND retracted” for unverified data on this topic. Our search provided 30 eligible publications (see Section 2—Results, and Section 4—Materials and Methods), describing a total of 27 different miR; of them, 16/27 (ca. 60%) miR are absent in our ceRNETs and 11/30 (ca. 37%) publications did not show a single ceRNET element in common with our reported data (Supplemental Table S1). Of course, these numbers are not compatible with a statistical validation of new ceRNET axes to be discovered in the future, nor is our high complexity network comprehensive enough to make such assumptions; nonetheless, they might provide a hint about those axes that need to be verified with higher-than-average accuracy.
Our work is far from being complete; additional experimental validation is required for those molecules that have only once been identified or only have a limited number of connections. This would also help in expanding the ceRNET through already existing data. In this context, a special case is represented by miR-302a-3p (not included in Figure 1) and miR-302a-5p (coordinates: b9) (Figure 1) (Table 1). As illustrated before, we considered these miRNAs as two different molecules because they have complementary sequences. However, they both come from the same pre-miRNA, and they both are expressed in EC, suggesting a strong functional, spatial, and temporal correlation. In this case, it would be worth exploring the possibility of analyzing if the forked axis NFYA (mRNA)/LINC01016 (lncRNA)/miR-302a-3p/miR-3130-3p could be attached to our high complexity ceRNET at coordinates a9 (asterisk in Figure 1). Finding new EC axes sharing one or more elements between miR-302a-3p and the high complexity ceRNET would solve the problem and would also contribute to further validating our EC networks.
Finally, it is worth pointing out that our methodology is meant to enable the visualization of unexplored axes where all components are already in situ. For instance, to the best of our knowledge, no scholarly reference delineating the lncRNA TUG1 (coordinates: i2) (Figure 1) within the same EC axis as the genes L1CAM (j3), NOTCH1 (j1), or PD-L1 (k2) exists. Nonetheless, TUG1 is well-known to interact with miR-34a, a microRNA that also regulates the aforementioned trio of genes (Table 1). It is to be expected that analyzing EC samples with impaired TUG1 expression will also result in the deregulation of at least some of these genes. Indeed, connections between TUG1 and either NOTCH1 or PD-L1 have already been described in the literature for glioma [204] and hepatocellular carcinoma [205], respectively. Exploring these yet unverified axes will eventually confirm the robustness of our identified ceRNET and inferred connections.
3.3. Using Our ceRNET for Investigating Metabolic Changes in EC
In its most basic form, a ceRNET axis allows controlling one target mRNA and thus alters the protein function it encodes by quantitative regulation. In case the target is, for example, an enzyme converting substance A into substance B, an impairment of the enzyme will cause the intracellular depletion of B and a consequent increase in A, a fact known since the experiments made in 1941 by Beadle and Tatum [206]. In those same years, Roger Williams introduced the concept of “metabolic fingerprint” by employing over 200,000 paper chromatograms to demonstrate the high variability of taste thresholds and excretion patterns of various substances among individuals, while remaining constant within individual subjects [207]. Presently, the metabolome is defined as the assemblage of small (<1500 Da) molecule metabolites that delineate a cell, an organ, or an organism, offering a snapshot of the physiological state of a living being [208]. The Human Metabolome DataBase v. 5.0 (https://www.hmdb.ca/, accessed on 25 March 2025) presently encompasses 220,945 metabolite entries comprising both water- and lipid-soluble metabolites, in addition to 8610 protein sequences (comprising enzymes and transporters) associated with these entries. The metabolome is invaluable in discerning diverse conditions impacting the same organ; for instance, in this scenario, to emphasize the distinctions among a healthy endometrium, endometriosis [209], and EC [210,211], as well as aiding in the differential diagnosis of EC [211]. In particular, a systematic Review identified 55 metabolites that hold diagnostic, prognostic, or histotype identification, metastasis, or recurrence risk significance [210]. This underscores the critical role of lipidic metabolites, such as phospholipids, essential for membrane growth during cell division, and those associated with glycolysis. In cancer, glycolysis often supplants oxidative phosphorylation even in aerobic conditions, a phenomenon known as the “Warburg effect” [212]. Another study revealed 111 significantly elevated and 148 reduced metabolites in EC [213]. Numerous findings have pinpointed phosphocholines, acylcholines, carnitines, and other lipid by-products as promising diagnostic biomarkers, alongside various amino acids (as reviewed in [211]).
EC ceRNET facilitates the identification of proteins involved in cancer-altered metabolic pathways and enables the detection of inferred connections between ceRNAs. This capability allows for a more profound analysis of the genetic, molecular, and metabolic status of the patient. Similarly, it offers insights into potential chemotherapy responses, thereby influencing the therapeutic approach. To validate this, we utilized the miRNet 2.0 tool (available at URL: https://www.mirnet.ca/ and accessed on 25 March 2025) [214] with default settings to conduct an enrichment analysis of the ceRNET for EC elements (miRNA, lncRNA, and mRNA targets) listed in Table 1. We applied the minimum network option and conducted gene ontology (GO) analysis, covering the biological process (BP), as well as KEGG pathway enrichment analysis, using the KEGG and GO BP databases within miRNet. The GO and KEGG categories, considered statistical significance at p < 0.05, are listed in Supplemental Table S2 in decreasing order of the adj.Pval value, that is, the pValue obtained by standard enrichment analysis based on the hypergeometric tests after adjustment for false discovery rate. As anticipated, the KEGG analysis unveiled eight different malignancies, encompassing chronic myeloid leukemia, prostate cancer, renal cell carcinoma, acute myeloid leukemia, glioma, small cell lung cancer, pancreatic cancer, and non-small cell lung cancer, in the top 20 rankings, in addition to pathways intricately linked to carcinogenesis such as ErbB [215], Wnt [216], and insulin [217], all of which are well-recognized contributors to EC. The inclusion of WNT7a within our principal ceRNET is notable (coordinates: g3). The GO analysis aligns seamlessly with our expectations; as previously mentioned, metabolites exhibit a close association with enzymes and transporters. Among the top 20 outcomes of the GO analysis, 11 pathways are dedicated to the movement of molecules, and notably, “response to ionizing radiation” is also featured, underscoring its relevance to the utilization of radiotherapy in patients. Collectively, these findings underscore the value of EC ceRNET analysis in assessing the metabolic profile of patients, thereby impacting diagnostic, prognostic, and therapeutic decisions.
3.4. Using External Data to Expand EC ceRNET
As described in the Results, our work allowed us to identify several minor subnetworks of lower complexity, compared to the one reported in Figure 1. At the moment, these ceRNETs (Figure 2 and Figure 3) do not share elements with each other and should be considered as independent players in EC pathogenesis. However, it is widely recognized that many of the molecules found in EC ceRNET are also deregulated in various other types of cancers. Consequently, by exploring ceRNET axes existing in different cancers yet sharing only partial components with the EC ceRNET, it becomes feasible to integrate smaller subnetworks into larger ones. For instance, miR-143 is a constituent of the moderately intricate EC ceRNET depicted in Figure 2C. In 2017, Wang and collaborators showed that, in bladder cancer (BC), miR-143 is a functional repressor of IGF-1R [218] that, in our EC ceRNET, is present in Figure 1 (coordinates: j8) and exhibits the above-mentioned characteristics of robustness. Li and coworkers described the same miR in the same tumor (BC) in 2020 [219]; they report its physical interaction with COX2 mRNA, another element of our high complexity EC ceRNET (coordinates: e1) (Figure 1). In addition, miR-143-3p interacts, in BC, also with EZH2 mRNA [220], which in our EC ceRNET is at coordinates a1 (Figure 1) and under the same miR-101 (coordinates: b1) control as COX2. Finally, again in BC, miR-143 also interacts with lncRNA UCA1 [221], reported in one of our low-complexity EC subnetworks (Figure 3D). Given that these data are from the same tumor (BC), it is likely that a deeper analysis of BC ceRNET will bring together all these elements, building a ceRNA network of at least medium complexity. This means that, using suitable approaches, i.e., by selecting appropriate elements connected to miR-143, the high complexity EC ceRNET of Figure 1 might be expanded to include the medium complexity one of Figure 2C and, maybe, also the low complexity one reported in Figure 3D.
By harnessing similar tactics, this approach will also enable the direct expansion of our EC ceRNET or increase data robustness by studying its peripheral elements, that is, those with only 1–2 connections, for which their physical interactors are known in other cancers.
3.5. Automation of ceRNET Construction Through Artificial Intelligence (AI) and Construction of a Multi-Cancer ceRNET
We are fairly confident that our selection criteria are rigorous enough to construct an economical ceRNET for any known cancer, provided that an ample amount of information (i.e., validated references) is accessible in bibliographic repositories. However, the growing understanding of ceRNET and the emergence of cutting-edge technologies to discover new epigenetic participants will soon render manual ceRNET construction an inconceivable undertaking. In this scenario, AI-driven methodologies could be employed for both the expansion and comparison of ceRNETs. To expand, appropriate cues, aligned with our selection criteria, should empower the AI to recommend to researchers which unexplored axes should be investigated, thereby optimizing the likelihood of yielding a favorable outcome. Furthermore, on a purely theoretical premise at present, we anticipate the future establishment of a “universal tumor ceRNET” where each individual tumor ceRNET could serve as a subnetwork. This would enable the research community to shed a light on the differences among tumors and possibly also the molecular differences of the same tumoral histotype. This will be central to gaining a deeper understanding of the genetic origin of the tumor and allow physicians to select appropriate therapies, on a single-patient basis, to maximize efficacy and minimize side effects, by targeting overlapping or non-overlapping parts of the ceRNET, according to the molecular characterization of the patient’s disease. We also expect that our data, adequately validated and expanded, will also help in deciding the most probable patient’s response to a specific treatment, thus inferring a more accurate prognosis. In this case, AI-based tools will be highly effective in highlighting similarities and differences among such subnetworks and assist physicians in decision-making.
Considering what we described above (Section 3.4) about BC and miR-143 interactors, it is quite straightforward to foresee possible algorithms for AI-tools to infer the existence of new EC ceRNET axes or at least to expand partial/peripheral portions of the network (Figure 4), eventually leading to additional in vitro and in vivo tests. For example, AI tools could be trained to identify the available literature using the selection criteria described in Section 4, but for a different tumor (for example, BC, but it would work with any cancer). Then, the researcher might prompt the AI to rank those ncRNAs with the higher robustness in BC (on the basis of the number of interactors and on the number of publications in which they appear) and weigh such a list against the one with EC ceRNET elements illustrated in Section 3.2 and reported in Figure 1. This would likely result in two groups of data: (i) a group of BC ncRNAs that are shared and integrated, using the same criteria, with our ceRNET EC (Figure 1) and (ii) BC-specific ncRNAs. In the first case, AI might serve as a means of comparison of shared ncRNA interactors in the two sets (EC and BC) to identify the differences and to verify the robustness of the non-shared elements in BC. Finding a robust interactor in BC that is not present in EC but that targets molecules common to both cancers should represent a viable clue to be tested at the bench (in vitro) or to be sought in EC patients (in vivo), by investigating the possible deregulation of such an element. In the latter case, the investigation of a given robust ncRNA in BC may be used in EC cases, in terms of the presence of its interactors, and then, following a similar procedure, to verify its presence in EC and adjust the EC ceRNET accordingly.
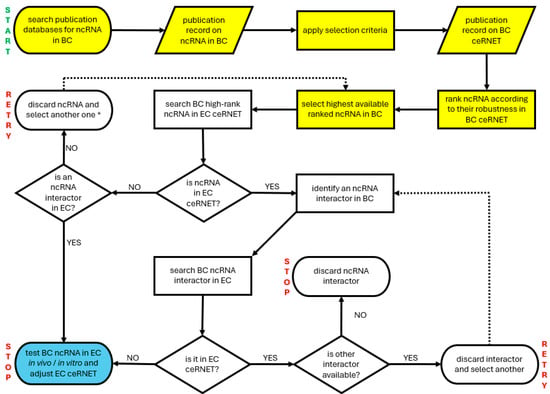
Figure 4.
A simplified flowchart illustrating a possible way to instruct an AI tool to expand an existing ceRNET. In this example, the intent is to expand the EC ceRNET using data coming from BC (bladder cancer) ceRNET. START and STOP indicate the start and end points of the algorithm; RETRY indicates where to start over searching. Yellow indicates the preparatory steps required to collect data to be compared (new ceRNET building) if not yet available. Blue indicates the step allowing us to expand/amend/adjust the EC ceRNET. Dotted arrows show where to restart in case of negative results, if possible. See the text for further explanations. Note that this algorithm is inefficient in the case of completely new ceRNET axes (the weak point is indicated by an asterisk in the retry box, second row) because it is based on the comparison of shared ncRNA; in those cases, a different strategy is required, such as starting over using a third tumor.
Such a methodological approach can serve as a mere illustration of guiding and training AI tools and does possess its constraints, the primary one being its reliance on the juxtaposition of various ceRNETs, thereby rendering it inadequate for delving into axes that are entirely non-existent within a specific ceRNET. In such instances, alternative approaches can be considered, e.g., acquiring data from a distinct tumor for comparative analysis, the underlying rationale being the improbability of a particular ceRNET axis, along with all its constituents, experiencing exclusive dysregulation in just one type of tumor.
3.6. Importance of EC ceRNET for Personalized Therapy: Implications for Precision Medicine
Current knowledge on any cancer-related ceRNET, including EC, is extremely fragmented because most published manuscripts either describe single ceRNET axes or make predictions on many ceRNAs based on bioinformatics only. Thus, it is extremely difficult for physicians to have a general yet thorough overview of the complexity of the interactions inside a tumor hence the difficulty in appropriately assessing a prognosis, choosing a therapy, and predicting, as much as possible, side effects. Our ceRNET representation, as well as any future expansion of it, will hopefully shed light on such aspects and foster informed decision-making. For example, PTEN (coordinates: k4) is known to be a frequent central player during sporadic EC formation and development; PTEN protein is involved in the PI3K–PTEN–AKT–mTOR pathway, which regulates cell growth, cell survival, protein synthesis, and metabolism [222]. In this scenario, having a patient that is positive for the alteration of PTEN function should prompt the doctors to also analyze its next interactors—PTEN expression is controlled in EC by at least six different miR (Figure 1), some of which also control the expression of other genes important for EC pathogenesis, such as VEGFA [223]. Finding an alteration in VEGFA (coordinates: i4) expression should prompt the analysis or its six miR controllers, which in turn regulate the expression of other central EC-related genes, such as NOTCH1 (j1), WNT7A (g3), STAT3 (b4), SOX4 (i6), FGFR1 (h4), or FGF2 (i5). This targeted molecular investigation is expected to significantly enhance not only the molecular profiling of the patient but also to have a more accurate prognosis and to knowingly decide on a more suitable therapy, not merely based on standard general protocols. For example, knowing that a patient has PTEN (Figure 1) or MAPK1 (Figure 2C) function impaired and knowing that these two genes are also implicated in chemotherapy resistance might drive the physician toward an alternative therapeutic approach.
Our data are derived in most cases from well-characterized patients with overt disease, and available information is not sufficient to assess whether it is theoretically possible to use EC ceRNET for early cancer diagnosis. We already discussed the possibility of using circulating miRNA as EC biomarkers [29]; there, we listed the 16 possible miRNAs potentially useful for this purpose. Quite interestingly, those miRNAs are underrepresented in this report. Only two of them (miR-205 at coordinates l4 and miR-223 at coordinates k9) appear in Figure 1, i.e., the high complexity major ceRNET. In addition, only miR-205 shows the robustness described above, with miR-223 being in a peripheral position (although connected with IGF1R, a robust element of the network). Overall, miR-205 seems a good candidate biomarker to detect EC using body fluids, while miR-223 needs further investigation. Of the remaining 14 miRNAs, 8 are completely absent from the present report. One possibility is that these miRNAs will join our ceRNET if specifically tested in suitable samples and with appropriate techniques (i.e., luciferase assay). Another possible explanation is that those eight miRNAs are not part of the EC ceRNET but might either fulfill other tasks or be a byproduct of general cellular metabolism deregulation, a typical cancer feature. The possible role of the remaining six miRNAs, namely miR-27a (Figure 2A), miR-449 (Figure 2B), miR-99a (Figure 2C), miR-204 (Figure 3D), miR-141, and miR-200a (no figure), is more puzzling. We suggest two possible explanations. First, the medium/low complexity EC ceRNETs containing them will eventually be joined to the main one, and in this case, their role in the EC major ceRNET will be clear. But another intriguing possibility arises if these minor ceRNET remain independent. In this case, it should be possible to hypothesize that more than one molecular mechanism could be involved in EC etiopathogenesis or that these minor networks describe temporally separated (early/transient markers?) events from the main pathway depicted in the remaining major ceRNET. Of course, in this phase of the research, this should be considered only a speculative plot, but from this perspective, it becomes crucial to investigate those minor networks to verify their independence from the main one.
We checked the website ClinicalTrials.gov (last accession: 15 January 2025), looking for any ncRNA listed in Table 1 and currently tested in therapy, either as a target or as a drug; we discarded those trials investigating them as mere biomarkers. None of the lncRNAs in Table 1 are present in the database. As for the miR, only two are present, namely miR-155 (NCT02580552, NCT03837457, NCT03713320) and miR-34a (NCT01829971, NCT02862145). To date, miR-155 seems to be a marginal player in EC (Table 1) while miR-34a is part of our major ceRNET (coordinates: j2) (Figure 1), where it shows interactions with two lncRNAs and four mRNAs. Functionally, this miR is also quite near to PTEN (k4) and directly connected to both VEGFA (i4) and NOTCH1 (j1) discussed above, making it an amenable EC therapy target. Unfortunately, both clinical trials involving a miR-34 mimic (MRX34) were aimed at testing its efficacy as a drug in various tumors including viral-related hepatocellular carcinoma, melanoma (non-cutaneous, excluding uveal), small cell lung cancer, triple-negative breast cancer, sarcoma, and bladder, renal, and ovarian cancers [224,225,226] but not EC. Beyond miR-34, our schemes point to additional molecules that could be tested in this perspective but for which no clinical trial is currently running or even planned.
3.7. Legal and Ethical Aspects of Using ceRNET in Cancer Therapy
As research illuminates EC ceRNET, researchers and doctors will better outline innovative therapeutic strategies to strengthen, optimize, or replace current approaches. ceRNETs help clarify long-term interactions for personalized medicine [227,228]. Personalized medicine is based on individual patient characteristics for effective treatment. Novel care approaches promise improved effectiveness and reduced toxicity. Legal and ethical implications of personalized medicine must be addressed [229,230,231]. Governance of personalized medicine protocols should prioritize privacy, fairness, and non-discrimination. Techniques of personalized medicine will expand with ethical guidelines. Multidisciplinary efforts are needed for the development of personalized medicine, which will evolve alongside big data analysis and artificial intelligence. Management of personalized medicine requires a diverse skill set beyond traditional medical professionals [27,232]. Such novel avenues will develop alongside other high-potential technologies such as big data analysis and artificial intelligence [27]. Such a highly complex and multi-faceted form of management and implementation cannot therefore rely solely on medical professionals or lab technicians in their traditional form and conventional skill-sets and training [232]. The tremendous still partly untapped potential of personalized approaches to cancer care had already been highlighted in 2014 by the European Society for Medical Oncology (ESMO) [229] in a guide characterizing personalized medicine as the future of cancer medicine. Yet, with great power and potential comes great responsibility: implications from novel approaches must be addressed to establish well-defined criteria based on fairness and equal opportunities while preventing discrimination [230]. While a global review of personalized medicine projects and initiatives is too extensive for this article, it is worth mentioning ongoing EU undertakings [233,234,235] taking into account future cancer diagnostics or therapeutics, translational research, the transition from an “organ-centric” notion of therapeutic and diagnostic pathways toward thorough molecular analysis [236], i.e., the ultimate driver of personalized approaches, in spite of the still relatively high degree of uncertainty in terms of outcomes [237]. European institutions recognize the breakthrough in future cancer diagnostics, translational research, and personalized approaches [238]. The key to personalized medicine lies in the synergy between health data and new technologies, leading to effective treatment strategies. This approach can have wide-ranging positive impacts on public health and healthcare systems. In 2022, the EU introduced measures to leverage innovative technologies like AI, big data, and genomics. Ongoing projects like PCM4EU and EUCAIM [239,240,241] aim to advance personalized medicine using novel tools. The EUCAIM platform is set to be fully released by December 2025. The EU aims to establish a federated European cancer images data repository by 2026. The Partnership on Personalized Medicine, launched in 2023 [242], focuses on involving public and private stakeholders and relying partly on funding allocated by Horizon Europe, i.e., the EU’s chief funding program aimed at furthering research and development. Harmonizing personalized medicine strategies and policies is, in fact, crucial for equitable access and to prevent “healthcare travels”.
Medicolegal Tenability Needs Updated Frames of Reference
The framing and acknowledgement of international standards are crucial in medicolegal aspects. Personalized medicine’s evolving nature may lead to uncertainty and malpractice risks [243]. Genetic malpractice is a relatively recent notion that entails the doctors’ failure or negligence to implement genetic testing or failing to adequately draw upon and interpret its findings. There is, in fact, no sufficient degree of uniformity in terms of assessing the values, scope, and timing of genetic testing or even on whether such testing ought to be executed at all, and under what conditions [243,244,245,246]. Data on such novel litigation profiles are still inconclusive, and it is still unclear how they may impact healthcare delivery and management [247]. Compliance with legislative standards and evidence-based guidelines is essential for genetic testing guidelines and needs to be thoroughly documented throughout the procedures, as the onus to prove adherence to guidelines and best practices rests on doctors and healthcare professionals, particularly under civil law statutes [248,249]. A high level of objectivity, rooted in validated scientific and clinical elements, will therefore be key to shaping legal and judicial trends and court rulings, thus shielding physicians and facilities from malpractice lawsuits, which are often frivolous and can unduly burden healthcare systems, much to the detriment of public health and sustainability.
4. Materials and Methods
We searched the PubMed database (URL: https://pubmed.ncbi.nlm.nih.gov/ accessed on 1 October 2024) using, as keywords, the terms “endometrial cancer” in combination with the following terms (Boolean operator: AND): (a) non-coding RNA; (b) ncRNA; (c) miRNA; (d) miR; (e) lncRNA; (f) lincRNA; (g) circRNA; (h) ceRNA; and (i) ceRNET. The literature collection was performed between 1 and 31 October 2024, with no time limit. Every article was analyzed manually by at least two researchers; no AI-based tool was used during the process, for either data selection or analysis.
The exclusion criteria were the following: (a) articles dealing with bioinformatics predictions but not showing bench data on the physical interaction between any given miR and at least one ceRNA; (b) studies involving only animal models (mostly, M. musculus); (c) indirect interactions or negative correlations not supported by data showing physical interactions between a miR and at least one ceRNA; (d) partial axes involving only a correlation between the expression of an lncRNA and a mRNA. Specifically, an exclusion criterium (d) was adopted because it has been repeatedly shown in the literature that lncRNA may alter target gene expression not only through a ceRNET but also through many additional and independent mechanisms, including chromatin remodeling, transcriptional control, and post-transcriptional processing [250], the formation of molecular scaffolds, bringing together different proteins to assemble functional complexes [251], the influence on mRNA stability and translation by binding to complementary sequences or interaction with RNA-binding proteins [251,252,253], and the formation of nuclear bodies and the maintenance of nuclear architecture [254]. In addition, the manuscripts we excluded were excluded by applying criterion (d) in most cases, which did not report an explanation about the described correlation.
The inclusion criteria were the following: (a) use of human specimens, either bioptic materials and/or EC cell lines; (b) use of the luciferase assay (mandatory criterium) and, possibly, other molecular biology approaches (including, but not limited to, miR-mimics, RT-qPCR, Western blotting; we considered these as optional criteria, meaning that the absence of these experimental techniques did not impede us to include the manuscript in our selected list in Table 1) supporting the functional correlation and the physical interaction between any given miR and at least one ceRNA; (c) complete axes description, involving the physical interaction between miR and lncRNA on the one side, and miR and mRNA on the other side; and (d) partial axes involving at least one miR and at least one ceRNA, provided that they satisfy both criteria (a) and (b).
A summary of the selection process is illustrated in the PRISMA flow diagram below (Figure 5).
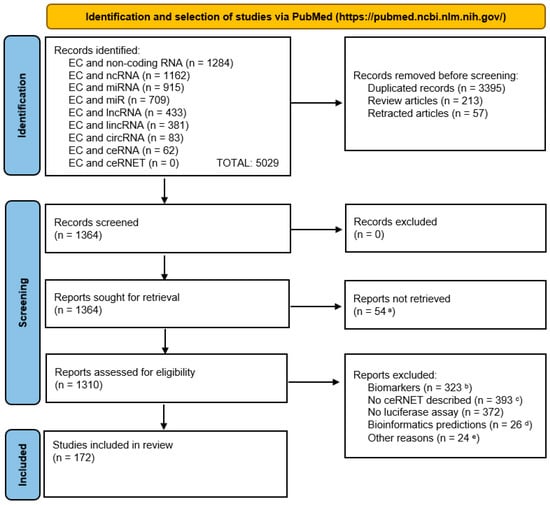
Figure 5.
PRISMA flow diagram showing the bibliographic selection criteria applied in this report. For graphical reasons, we abbreviated “endometrial cancer” as “EC”. Notes—a: manuscript not available or manuscript in non-English language; b: manuscripts dealing with the use of ncRNA only as cancer biomarkers; c: no ceRNET axis described (manuscript only focusing on lncRNA) or description of only lncRNA plus mRNA (no miRNA); d: works based only or mostly on bioinformatics predictions with no physical interaction investigated; e: numerically minor reasons, such as the use of non-human samples, unclear methodology, not peer-reviewed papers (e.g., those in the bioRxiv repository). We built the diagram using the PRISMA 2020 flow diagram template distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt, and build upon this work, for commercial use, provided the original work is properly cited; the website used as a source of the template is accessible at URL https://www.prisma-statement.org/prisma-2020-flow-diagram (accessed on: 18 March 2025).
This systematic review has been registered in PROSPERO (ID: PROSPERO 2025 CRD420251035222).
5. Conclusions
We used the available literature (Figure 5) to build a hand-curated ceRNET for EC. We identified a total of 217 elements (71 miR, 59 lncRNA and 87 mRNA) grouped in 13 ceRNET, of which 1 is high complexity (Figure 1), 7 is medium complexity (Figure 2), and 5 is low complexity (Figure 3), plus 8 miR, 20 lncRNA, and 53 mRNA, which belong to single axes and are not included in the above-mentioned ceRNET (Table 1). All interactions reported in our schemes represent physical interactions among coding and non-coding RNAs and have been validated by luciferase assay (mandatory) plus additional molecular experiments (optional). These data allowed us to identify 13 subnetworks that, potentially, could be joined into a major EC ceRNET. On the same basis, we foresee that similar work could be conducted for other types of cancer—possibly all, depending on data availability—and that all these ceRNET might, in future, be part of one very large and complex “general tumor ceRNET” that might be used as a reference for finding new cancer players for a more accurate prognosis and therapeutic strategies for cancer patients. We conclude this report by emphasizing that our data represent the starting, not the ending, point of EC ceRNET identification and characterization; although we used stringent selection criteria to build them, our networks need additional experimental testing for both their validation and expansion.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ncrna11030034/s1: Table S1: Retracted articles according to PubMed search: “endometrial cancer AND miR AND retracted” performed on 17 January 2025; Table S2: KEGG and GO analysis for the metabolic changes in EC linked to the data reported in Table 1.
Author Contributions
R.P. designed and supervised the study; S.S. and R.P. collected ceRNET data; all authors revised ceRNET manuscripts reported in Table 1; R.P. drew ceRNET drafts reported in Figure 1, Figure 2 and Figure 3; S.S. mined data in the ClinicalTrial.gov website; R.P. mined data in PubMed for retracted articles, reported in Table S1; R.P., S.Z. and E.M. drafted the manuscript and supervised its structuring; S.Z., L.D.P. and E.M. reviewed the legal, regulatory, and policy-making approaches and guidelines. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Belladelli, F.; Montorsi, F.; Martini, A. Metabolic Syndrome, Obesity and Cancer Risk. Curr. Opin. Urol. 2022, 32, 594–597. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-S.; Scherer, P.E. Obesity, Diabetes, and Increased Cancer Progression. Diabetes Metab. J. 2021, 45, 799–812. [Google Scholar] [CrossRef] [PubMed]
- Ignatov, A.; Ortmann, O. Endocrine Risk Factors of Endometrial Cancer: Polycystic Ovary Syndrome, Oral Contraceptives, Infertility, Tamoxifen. Cancers 2020, 12, 1766. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, W.; Liu, H.; Zhang, D. Age at Menopause and Risk of Developing Endometrial Cancer: A Meta-Analysis. Biomed. Res. Int. 2019, 2019, 8584130. [Google Scholar] [CrossRef]
- Ali, A.T. Reproductive Factors and the Risk of Endometrial Cancer. Int. J. Gynecol. Cancer 2014, 24, 384–393. [Google Scholar] [CrossRef]
- Idos, G.; Valle, L. Lynch Syndrome. In GeneReviews® [Internet]; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 2004; 1993–2025. [Google Scholar] [PubMed]
- Levine, D.A. The Cancer Genome Atlas Research Network Integrated Genomic Characterization of Endometrial Carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef]
- Bianco, B.; Barbosa, C.P.; Trevisan, C.M.; Laganà, A.S.; Montagna, E. Endometrial Cancer: A Genetic Point of View. Transl. Cancer Res. 2020, 9, 7706–7715. [Google Scholar] [CrossRef]
- Bafligil, C.; Thompson, D.J.; Lophatananon, A.; Smith, M.J.; Ryan, N.A.; Naqvi, A.; Evans, D.G.; Crosbie, E.J. Association between Genetic Polymorphisms and Endometrial Cancer Risk: A Systematic Review. J. Med. Genet. 2020, 57, 591–600. [Google Scholar] [CrossRef]
- Benati, M.; Montagnana, M.; Danese, E.; Mazzon, M.; Paviati, E.; Garzon, S.; Laganà, A.S.; Casarin, J.; Giudici, S.; Raffaelli, R.; et al. Aberrant Telomere Length in Circulating Cell-Free DNA as Possible Blood Biomarker with High Diagnostic Performance in Endometrial Cancer. Pathol. Oncol. Res. 2020, 26, 2281–2289. [Google Scholar] [CrossRef]
- Kaneko, E.; Sato, N.; Sugawara, T.; Noto, A.; Takahashi, K.; Makino, K.; Terada, Y. MLH1 Promoter Hypermethylation Predicts Poorer Prognosis in Mismatch Repair Deficiency Endometrial Carcinomas. J. Gynecol. Oncol. 2021, 32, e79. [Google Scholar] [CrossRef] [PubMed]
- Loukovaara, M.; Pasanen, A.; Bützow, R. Mismatch Repair Protein and MLH1 Methylation Status as Predictors of Response to Adjuvant Therapy in Endometrial Cancer. Cancer Med. 2021, 10, 1034–1042. [Google Scholar] [CrossRef]
- Lakhotia, S.C. C-Value Paradox: Genesis in Misconception That Natural Selection Follows Anthropocentric Parameters of ‘Economy’ and ‘Optimum’. BBA Adv. 2023, 4, 100107. [Google Scholar] [CrossRef]
- Choi, I.-Y.; Kwon, E.-C.; Kim, N.-S. The C- and G-Value Paradox with Polyploidy, Repeatomes, Introns, Phenomes and Cell Economy. Genes Genom. 2020, 42, 699–714. [Google Scholar] [CrossRef]
- Singh, R.S.; Gupta, B.P. Genes and Genomes and Unnecessary Complexity in Precision Medicine. NPJ Genom. Med. 2020, 5, 21. [Google Scholar] [CrossRef]
- Palazzo, A.F.; Lee, E.S. Non-Coding RNA: What Is Functional and What Is Junk? Front. Genet. 2015, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Naeli, P.; Winter, T.; Hackett, A.P.; Alboushi, L.; Jafarnejad, S.M. The Intricate Balance between MicroRNA-Induced MRNA Decay and Translational Repression. FEBS J. 2023, 290, 2508–2524. [Google Scholar] [CrossRef] [PubMed]
- Friedman, R.C.; Farh, K.K.H.; Burge, C.B.; Bartel, D.P. Most Mammalian MRNAs Are Conserved Targets of MicroRNAs. Genome Res. 2009, 19, 92. [Google Scholar] [CrossRef]
- Shu, J.; Resende E Silva, B.V.; Gao, T.; Xu, Z.; Cui, J. Dynamic and Modularized MicroRNA Regulation and Its Implication in Human Cancers. Sci. Rep. 2017, 7, 13356. [Google Scholar] [CrossRef]
- Kumar, S.; Gonzalez, E.A.; Rameshwar, P.; Etchegaray, J.P. Non-Coding RNAs as Mediators of Epigenetic Changes in Malignancies. Cancers 2020, 12, 3657. [Google Scholar] [CrossRef]
- Yang, N.; Liu, K.; Yang, M.; Gao, X. CeRNAs in Cancer: Mechanism and Functions in a Comprehensive Regulatory Network. J. Oncol. 2021, 2021, 1–12. [Google Scholar] [CrossRef]
- Vallone, C.; Rigon, G.; Gulia, C.; Baffa, A.; Votino, R.; Morosetti, G.; Zaami, S.; Briganti, V.; Catania, F.; Gaffi, M.; et al. Non-Coding RNAs and Endometrial Cancer. Genes 2018, 9, 187. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Wang, Y.; Meng, Q.; Su, X.; Shen, J.; Wang, J.; He, H.; Wen, B.; Zhang, C.; Xu, M. Integrated Bioinformatic Analysis of a Competing Endogenous RNA Network Reveals a Prognostic Signature in Endometrial Cancer. Front. Oncol. 2019, 9, 448. [Google Scholar] [CrossRef] [PubMed]
- Piergentili, R.; Zaami, S.; Cavaliere, A.F.; Signore, F.; Scambia, G.; Mattei, A.; Marinelli, E.; Gulia, C.; Perelli, F. Non-Coding Rnas as Prognostic Markers for Endometrial Cancer. Int. J. Mol. Sci. 2021, 22, 3151. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Ren, C.; Yao, Y.; Wang, Q.; Li, F.; Li, Y.; Jiang, A.; Wang, G. Identifying Prognostic Biomarkers in Endometrial Carcinoma Based on ceRNA Network. J. Cell. Biochem. 2020, 121, 2437–2446. [Google Scholar] [CrossRef]
- Cavaliere, A.F.; Perelli, F.; Zaami, S.; Piergentili, R.; Mattei, A.; Vizzielli, G.; Scambia, G.; Straface, G.; Restaino, S.; Signore, F. Towards Personalized Medicine: Non-Coding Rnas and Endometrial Cancer. Healthcare 2021, 9, 965. [Google Scholar] [CrossRef]
- Ravegnini, G.; Gorini, F.; De Crescenzo, E.; De Leo, A.; De Biase, D.; Di Stanislao, M.; Hrelia, P.; Angelini, S.; De Iaco, P.; Perrone, A.M. Can miRNAs Be Useful Biomarkers in Improving Prognostic Stratification in Endometrial Cancer Patients? An Update Review. Int. J. Cancer 2022, 150, 1077–1090. [Google Scholar] [CrossRef]
- Piergentili, R.; Gullo, G.; Basile, G.; Gulia, C.; Porrello, A.; Cucinella, G.; Marinelli, E.; Zaami, S. Circulating MiRNAs as a Tool for Early Diagnosis of Endometrial Cancer—Implications for the Fertility-Sparing Process: Clinical, Biological, and Legal Aspects. Int. J. Mol. Sci. 2023, 24, 11356. [Google Scholar] [CrossRef]
- Liu, Y.; Li, H.; Zhao, C.; Jia, H. MicroRNA-101 Inhibits Angiogenesis via COX-2 in Endometrial Carcinoma. Mol. Cell. Biochem. 2018, 448, 61–69. [Google Scholar] [CrossRef]
- Konno, Y.; Dong, P.; Xiong, Y.; Suzuki, F.; Lu, J.; Cai, M.; Watari, H.; Mitamura, T.; Hosaka, M.; Hanley, S.J.B.; et al. MicroRNA-101 Targets EZH2, MCL-1 and FOS to Suppress Proliferation, Invasion and Stem Cell-like Phenotype of Aggressive Endometrial Cancer Cells. Oncotarget 2014, 5, 6049–6062. [Google Scholar] [CrossRef]
- Wang, C.; Liu, B. MiR-101-3p Induces Autophagy in Endometrial Carcinoma Cells by Targeting EZH2. Arch. Gynecol. Obstet. 2018, 297, 1539–1548. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Song, W.Q.; Sun, P.; Jin, L.; Dai, H.Y. LncRNA-GAS5 Induces PTEN Expression through Inhibiting MiR-103 in Endometrial Cancer Cells. J. Biomed. Sci. 2015, 22, 100. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Zhang, F.; Zhang, L.; Jia, Y.; Chen, H. MicroRNA-103 Regulates the Progression in Endometrial Carcinoma through ZO-1. Int. J. Immunopathol. Pharmacol. 2019, 33. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Piao, J.; Ou, J.; Zhu, X. Circ_0109046 promotes the malignancy of endometrial carcinoma cells through the microRNA-105/SOX9/Wnt/β-catenin axis. IUBMB Life 2021, 73, 159–176. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Li, J.; Liu, H.; Zhou, F.; Liu, M. MiR-106a Promotes Tumor Growth, Migration, and Invasion by Targeting BCL2L11 in Human Endometrial Adenocarcinoma. Am. J. Transl. Res. 2017, 9, 4984–4993. [Google Scholar]
- Zhao, Z.-N.; Bai, J.-X.; Zhou, Q.; Yan, B.; Qin, W.-W.; Jia, L.-T.; Meng, Y.-L.; Jin, B.-Q.; Yao, L.-B.; Wang, T.; et al. TSA Suppresses MiR-106b-93-25 Cluster Expression through Downregulation of MYC and Inhibits Proliferation and Induces Apoptosis in Human EMC. PLoS ONE 2012, 7, e45133. [Google Scholar] [CrossRef]
- Bao, W.; Zhang, Y.; Li, S.; Fan, Q.; Qiu, M.; Wang, Y.; Li, Y.; Ji, X.; Yang, Y.; Sang, Z.; et al. MiR-107-5p Promotes Tumor Proliferation and Invasion by Targeting Estrogen Receptor-α in Endometrial Carcinoma. Oncol. Rep. 2018, 41, 1575–1585. [Google Scholar] [CrossRef]
- Chen, H.; Fan, Y.; Xu, W.; Chen, J.; Xu, C.; Wei, X.; Fang, D.; Feng, Y. MiR-10b Inhibits Apoptosis and Promotes Proliferation and Invasion of Endometrial Cancer Cells via Targeting HOXB3. Cancer Biother. Radiopharm. 2016, 31, 225–231. [Google Scholar] [CrossRef]
- Liu, Y.; Chang, Y.; Cai, Y. CircTNFRSF21, a Newly Identified Circular RNA Promotes Endometrial Carcinoma Pathogenesis through Regulating MiR-1227-MAPK13/ATF2 Axis. Aging 2020, 12, 6774–6792. [Google Scholar] [CrossRef]
- Liu, S.; Qiu, J.; Tang, X.; Cui, H.; Zhang, Q.; Yang, Q. LncRNA-H19 Regulates Cell Proliferation and Invasion of Ectopic Endometrium by Targeting ITGB3 via Modulating MiR-124-3p. Exp. Cell. Res. 2019, 381, 215–222. [Google Scholar] [CrossRef]
- Gu, Z.-R.; Liu, W. The LncRNA AL161431.1 Targets MiR-1252-5p and Facilitates Cellular Proliferation and Migration via MAPK Signaling in Endometrial Carcinoma. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 2294–2302. [Google Scholar] [PubMed]
- Shi, F.; Wang, T.; Liu, Z.; Zhang, Y.; Wang, J.; Zhang, K.; Su, J. LncRNA miR143HG Up-Regulates p53 In Endometrial Carcinoma By Sponging miR-125a. Cancer Manag. Res. 2019, 11, 10117–10123. [Google Scholar] [CrossRef]
- Shang, C.; Ao, C.N.; Cheong, C.C.; Meng, L. Long Non-Coding RNA CDKN2B Antisense RNA 1 Gene Contributes to Paclitaxel Resistance in Endometrial Carcinoma. Front. Oncol. 2019, 9, 27. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, M.; Song, Y.; Feng, C. Long Noncoding RNA-ATB Impairs the Function of Tumor Suppressor MiR-126-Mediated Signals in Endometrial Cancer for Tumor Growth and Metastasis. Cancer Biother. Radiopharm. 2019, 34, 47–55. [Google Scholar] [CrossRef]
- Wang, W.; Jin, W.; Liu, X.; Zheng, L. Circ_0002577/MiR-126-5p/MACC1 Axis Promotes Endometrial Carcinoma Progression by Regulation of Proliferation, Migration, Invasion, and Apoptosis of Endometrial Carcinoma Cells. Arch. Gynecol. Obstet. 2022, 306, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Qu, Y.; Li, Y. Over-Expression of MiR-1271 Inhibits Endometrial Cancer Cells Proliferation and Induces Cell Apoptosis by Targeting CDK1. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 2816–2822. [Google Scholar]
- Tian, Y.; Chen, Y.; Han, A. MiR-1271 Inhibits Cell Proliferation and Metastasis by Targeting LDHA in Endometrial Cancer. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 5648–5656. [Google Scholar]
- Wei, D.; Tian, M.; Fan, W.; Zhong, X.; Wang, S.; Chen, Y.; Zhang, S. Circular RNA Circ_0000043 Promotes Endometrial Carcinoma Progression by Regulating MiR-1271-5p/CTNND1 Axis. Arch. Gynecol. Obstet. 2021, 303, 1075–1087. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Z.; Hu, Y.; Wei, Q.; Shao, X. Circ_0039569 Contributes to the Paclitaxel Resistance of Endometrial Cancer via Targeting MiR-1271-5p/PHF6 Pathway. Anticancer Drugs 2022, 33, 883–892. [Google Scholar] [CrossRef]
- Chen, S.; Liang, Y.; Shen, Y.; Wang, X. LncRNA XIST/MiR-129-2-3p Axis Targets CCP110 to Regulate the Proliferation, Invasion and Migration of Endometrial Cancer Cells. Exp. Ther. Med. 2023, 25, 159. [Google Scholar] [CrossRef]
- Li, Q.; Shen, F.; Zhao, L. The Relationship between LncRNA PCGEM1 and STAT3 during the Occurrence and Development of Endometrial Carcinoma. Biomed. Pharmacother. 2018, 107, 918–928. [Google Scholar] [CrossRef]
- Dong, P.; Karaayvaz, M.; Jia, N.; Kaneuchi, M.; Hamada, J.; Watari, H.; Sudo, S.; Ju, J.; Sakuragi, N. Mutant P53 Gain-of-Function Induces Epithelial–Mesenchymal Transition through Modulation of the MiR-130b–ZEB1 Axis. Oncogene 2013, 32, 3286–3295. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.; Wang, Y.; Zhang, Y.; Liu, D.; Wang, J.; Shi, T.; Xu, X.; Li, L. MTFR2 Promotes Endometrial Carcinoma Cell Proliferation and Growth via the MiR-132-3p /PI3K/Akt Signaling Pathway. Front. Med. 2024, 11, 1505071. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Liu, T.; Huang, Y. MicroRNA-134 Suppresses Endometrial Cancer Stem Cells by Targeting POGLUT1 and Notch Pathway Proteins. FEBS Lett. 2015, 589, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Zong, Z.; Liu, Y.; Chen, S.; Zhao, Y. Circ_PUM1 Promotes the Development of Endometrial Cancer by Targeting the MiR-136/NOTCH3 Pathway. J. Cell. Mol. Med. 2020, 24, 4127–4135. [Google Scholar] [CrossRef]
- Shi, Y.; Jia, L.; Wen, H. Circ_0109046 Promotes the Progression of Endometrial Cancer via Regulating MiR-136/HMGA2 Axis. Cancer Manag. Res. 2020, 12, 10993–11003. [Google Scholar] [CrossRef]
- Li, L.; Chen, P.; Huang, B.; Cai, P. LncRNA DSCAM-AS1 Facilitates the Progression of Endometrial Cancer via MiR-136-5p. Oncol. Lett. 2021, 22, 825. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, J.-H.; Shan, T.; Aguilera-Barrantes, I.; Wang, L.-S.; Huang, T.H.-M.; Rader, J.S.; Sheng, X.; Huang, Y.-W. MiR-137 Is a Tumor Suppressor in Endometrial Cancer and Is Repressed by DNA Hypermethylation. Lab. Investig. 2018, 98, 1397–1407. [Google Scholar] [CrossRef]
- Gu, X.; Shi, Y.; Dong, M.; Jiang, L.; Yang, J.; Liu, Z. Exosomal Transfer of Tumor-Associated Macrophage-Derived Hsa_circ_0001610 Reduces Radiosensitivity in Endometrial Cancer. Cell. Death Dis. 2021, 12, 818. [Google Scholar] [CrossRef]
- Liu, J.; Li, C.; Jiang, Y.; Wan, Y.; Zhou, S.; Cheng, W. Tumor-Suppressor Role of MiR-139-5p in Endometrial Cancer. Cancer Cell. Int. 2018, 18, 51. [Google Scholar] [CrossRef]
- Dong, P.; Wang, F.; Taheri, M.; Xiong, Y.; Ihira, K.; Kobayashi, N.; Konno, Y.; Yue, J.; Watari, H. Long Non-Coding RNA TMPO-AS1 Promotes GLUT1-Mediated Glycolysis and Paclitaxel Resistance in Endometrial Cancer Cells by Interacting with MiR-140 and MiR-143. Front. Oncol. 2022, 12, 912935. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Zhang, W.; Xie, M.; Chen, R.; Chen, H.; Lin, Q. Long Non-Coding RNA JPX Promotes Endometrial Carcinoma Progression via Janus Kinase 2/Signal Transducer and Activator of Transcription 3. Front. Oncol. 2024, 14, 1340050. [Google Scholar] [CrossRef]
- Cui, Z.; An, X.; Li, J.; Liu, Q.; Liu, W. LncRNA MIR22HG Negatively Regulates MiR-141-3p to Enhance DAPK1 Expression and Inhibits Endometrial Carcinoma Cells Proliferation. Biomed. Pharmacother. 2018, 104, 223–228. [Google Scholar] [CrossRef]
- Chang, L.; Zhang, D.; Shi, H.; Bian, Y.; Guo, R. MiR-143 Inhibits Endometrial Cancer Cell Proliferation and Metastasis by Targeting MAPK1. Oncotarget 2017, 8, 84384–84395. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ge, L.; Xu, X.-J.; Yang, T.; Yuan, Y.; Ma, X.-L.; Zhang, X.-H. LncRNA NEAT1 Promotes Endometrial Cancer Cell Proliferation, Migration and Invasion by Regulating the MiR-144-3p/EZH2 Axis. Radiol. Oncol. 2019, 53, 434–442. [Google Scholar] [CrossRef]
- Zhou, X.; Gao, Q.; Wang, J.; Zhang, X.; Liu, K.; Duan, Z. Linc-RNA-RoR Acts as a “Sponge” against Mediation of the Differentiation of Endometrial Cancer Stem Cells by MicroRNA-145. Gynecol. Oncol. 2014, 133, 333–339. [Google Scholar] [CrossRef]
- Sun, G.; Tian, J.; Xiao, Y.; Zeng, Y. Circular RNA Circ_0005667 Promotes Cisplatin Resistance of Endometrial Carcinoma Cells by Regulating IGF2BP1 through MiR-145-5p. Anticancer Drugs. 2022, 34, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lu, W.; Qu, J.; Ye, L.; Du, G.; Wan, X. Loss of Exosomal MiR-148b from Cancer-associated Fibroblasts Promotes Endometrial Cancer Cell Invasion and Cancer Metastasis. J. Cell. Physiol. 2019, 234, 2943–2953. [Google Scholar] [CrossRef]
- Liu, Y.; Chang, Y.; Cai, Y. Hsa_circ_0061140 Promotes Endometrial Carcinoma Progression via Regulating MiR-149-5p/STAT3. Gene 2020, 745, 144625. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, X.; Zhou, L.; Wan, Y.; Song, L.; Gu, W.; Liu, R.; Ma, Y.; Meng, H.; Tian, Y.; et al. LncRNA SNHG8 Participates in the Development of Endometrial Carcinoma through Regulating C-MET Expression by MiR-152. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 1629–1637. [Google Scholar]
- Xie, D.; Liang, Y.; Su, Y.; An, Y.; Qu, P. MiR-152 Inhibits Proliferation of Human Endometrial Cancer Cells via Inducing G2/M Phase Arrest by Suppressing CDC25B Expression. Biomed. Pharmacother. 2018, 99, 299–305. [Google Scholar] [CrossRef]
- Tsuruta, T.; Kozaki, K.; Uesugi, A.; Furuta, M.; Hirasawa, A.; Imoto, I.; Susumu, N.; Aoki, D.; Inazawa, J. MiR-152 Is a Tumor Suppressor MicroRNA That Is Silenced by DNA Hypermethylation in Endometrial Cancer. Cancer Res. 2011, 71, 6450–6462. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Sang, L.; Du, S. Long Noncoding RNA LINC00261 Regulates Endometrial Carcinoma Progression by Modulating MiRNA/FOXO1 Expression. Cell. Biochem. Funct. 2018, 36, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.H.; Park, Y.-A.; Choi, J.-J.; Song, T.; Song, S.Y.; Lee, Y.-Y.; Lee, J.-W.; Kim, T.-J.; Kim, B.-G.; Bae, D.-S. Angiotensin II Type I Receptor and MiR-155 in Endometrial Cancers: Synergistic Antiproliferative Effects of Anti-MiR-155 and Losartan on Endometrial Cancer Cells. Gynecol. Oncol. 2012, 126, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-M.; Wan, X.-H.; Sang, G.-Y.; Zhao, J.-D.; Zhu, Q.-Y.; Wang, D.-M. MiR-15a-5p Suppresses Endometrial Cancer Cell Growth via Wnt/β-Catenin Signaling Pathway by Inhibiting WNT3A. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 4810–4818. [Google Scholar]
- Liu, D.; Bi, X.; Yang, Y. Circular RNA Hsa_circ_0011324 Is Involved in Endometrial Cancer Progression and the Evolution of Its Mechanism. Bioengineered 2022, 13, 7485–7499. [Google Scholar] [CrossRef]
- Dong, P.; Xiong, Y.; Konno, Y.; Ihira, K.; Kobayashi, N.; Yue, J.; Watari, H. Long Non-Coding RNA DLEU2 Drives EMT and Glycolysis in Endometrial Cancer through HK2 by Competitively Binding with MiR-455 and by Modulating the EZH2/MiR-181a Pathway. J. Exp. Clin. Cancer Res. 2021, 40, 216. [Google Scholar] [CrossRef]
- Yu, J.; Jiang, L.; Gao, Y.; Sun, Q.; Liu, B.; Hu, Y.; Han, X. LncRNA CCAT1 Negatively Regulates MiR-181a-5p to Promote Endometrial Carcinoma Cell Proliferation and Migration. Exp. Ther. Med. 2019, 17, 4259–4266. [Google Scholar] [CrossRef]
- Zhuang, L.; Qu, H.; Cong, J.; Dai, H.; Liu, X. MiR-181c Affects Estrogen-Dependent Endometrial Carcinoma Cell Growth by Targeting PTEN. Endocr. J. 2019, 66, 523–533. [Google Scholar] [CrossRef]
- Jia, Y.; Liu, M.; Wang, S. CircRNA Hsa_circRNA_0001776 Inhibits Proliferation and Promotes Apoptosis in Endometrial Cancer via Downregulating LRIG2 by Sponging MiR-182. Cancer Cell. Int. 2020, 20, 412. [Google Scholar] [CrossRef]
- Guo, Y.; Liao, Y.; Jia, C.; Ren, J.; Wang, J.; Li, T. MicroRNA-182 Promotes Tumor Cell Growth by Targeting Transcription Elongation Factor A-like 7 in Endometrial Carcinoma. Cell. Physiol. Biochem. 2013, 32, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Ruan, H.; Liang, X.; Zhao, W.; Ma, L.; Zhao, Y. The Effects of MicroRNA-183 Promots Cell Proliferation and Invasion by Targeting MMP-9 in Endometrial Cancer. Biomed. Pharmacother. 2017, 89, 812–818. [Google Scholar] [CrossRef]
- Chen, Z.; Zhu, Y.; Fan, X.; Liu, Y.; Feng, Q. Decreased Expression of MiR-184 Restrains the Growth and Invasion of Endometrial Carcinoma Cells through CDC25A-Dependent Notch Signaling Pathway. Am. J. Transl. Res. 2019, 11, 755–764. [Google Scholar] [PubMed]
- Yang, C.; Ota-Kurogi, N.; Ikeda, K.; Okumura, T.; Horie-Inoue, K.; Takeda, S.; Inoue, S. MicroRNA-191 Regulates Endometrial Cancer Cell Growth via TET1-Mediated Epigenetic Modulation of APC. J. Biochem. 2020, 168, 7–14. [Google Scholar] [CrossRef]
- Ni, J.; Tian, W.; Liang, S.; Wang, H.; Ren, Y. Promoter Methylation-Mediated Silencing of the MiR-192-5p Promotes Endometrial Cancer Progression by Targeting ALX1. Int. J. Med. Sci. 2021, 18, 2510–2520. [Google Scholar] [CrossRef]
- Deng, J.; Wang, W.; Yu, G.; Ma, X. MicroRNA-195 Inhibits Epithelial-mesenchymal Transition by Targeting G Protein-coupled Estrogen Receptor 1 in Endometrial Carcinoma. Mol. Med. Rep. 2019, 20, 4023–4032. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Dai, L.; Yue, Q.; Wang, H.; Wang, X.U.; Li, Y.; Chen, R. MiR-195 Inhibits Migration, Invasion and Epithelial-Mesenchymal Transition (EMT) of Endometrial Carcinoma Cells by Targeting SOX4. J. Biosci. 2019, 44, 146. [Google Scholar] [CrossRef]
- Ma, J.; Zhao, X.; Shi, L. Circ 003390/Eukaryotic Translation Initiation Factor 4A3 Promoted Cell Migration and Proliferation in Endometrial Cancer via Vascular Endothelial Growth Factor Signaling by MiR-195-5p. Bioengineered 2022, 13, 11958–11972. [Google Scholar] [CrossRef]
- Kong, F.; Ma, J.; Yang, H.; Yang, D.; Wang, C.; Ma, X. Long Non-Coding RNA PVT1 Promotes Malignancy in Human Endometrial Carcinoma Cells through Negative Regulation of MiR-195-5p. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2018, 1865, 1479–1490. [Google Scholar] [CrossRef]
- Xu, H.; Sun, Y.; Ma, Z.; Xu, X.; Qin, L.; Luo, B. LOC134466 Methylation Promotes Oncogenesis of Endometrial Carcinoma through LOC134466/Hsa-MiR-196a-5p/TAC1 Axis. Aging 2018, 10, 3353–3370. [Google Scholar] [CrossRef]
- Shen, Q.; He, T.; Yuan, H. Hsa_circ_0002577 Promotes Endometrial Carcinoma Progression via Regulating MiR-197/CTNND1 Axis and Activating Wnt/β-Catenin Pathway. Cell Cycle 2019, 18, 1229–1240. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Pan, A.; Zhang, Y.; Li, X. Hsa_circ_0039569 Facilitates the Progression of Endometrial Carcinoma by Targeting the MiR-197/High Mobility Group Protein A1 Axis. Bioengineered 2022, 13, 4212–4225. [Google Scholar] [CrossRef]
- Shi, W.; Wang, X.; Ruan, L.; Fu, J.; Liu, F.; Qu, J. MiR-200a Promotes Epithelial-Mesenchymal Transition of Endometrial Cancer Cells by Negatively Regulating FOXA2 Expression. Pharmazie 2017, 72, 694–699. [Google Scholar] [CrossRef]
- Dai, Y.; Xia, W.; Song, T.; Su, X.; Li, J.; Li, S.; Chen, Y.; Wang, W.; Ding, H.; Liu, X.; et al. MicroRNA-200b Is Overexpressed in Endometrial Adenocarcinomas and Enhances MMP2 Activity by Downregulating TIMP2 in Human Endometrial Cancer Cell Line HEC-1A Cells. Nucleic Acid Ther. 2013, 23, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, C.; Chen, R.; Xiong, H.; Qiu, F.; Liu, S.; Zhang, M.; Wang, F.; Wang, Y.; Zhou, X.; et al. Disrupting MALAT1/MiR-200c Sponge Decreases Invasion and Migration in Endometrioid Endometrial Carcinoma. Cancer Lett. 2016, 383, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zhang, M.; Liu, W.; Chen, H.; Cai, T.; Xiong, H.; Sheng, X.; Liu, S.; Peng, J.; Wang, F.; et al. Estrogen Affects the Negative Feedback Loop of PTENP1-MiR200c to Inhibit PTEN Expression in the Development of Endometrioid Endometrial Carcinoma. Cell. Death Dis. 2018, 10, 4. [Google Scholar] [CrossRef]
- Li, F.; Liang, A.; Lv, Y.; Liu, G.; Jiang, A.; Liu, P. MicroRNA-200c Inhibits Epithelial-Mesenchymal Transition by Targeting the BMI-1 Gene Through the Phospho-AKT Pathway in Endometrial Carcinoma Cells In Vitro. Med. Sci. Monit. 2017, 23, 5139–5149. [Google Scholar] [CrossRef]
- Park, Y.-A.; Lee, J.-W.; Choi, J.-J.; Jeon, H.-K.; Cho, Y.; Choi, C.; Kim, T.-J.; Lee, N.W.; Kim, B.-G.; Bae, D.-S. The Interactions between MicroRNA-200c and BRD7 in Endometrial Carcinoma. Gynecol. Oncol. 2012, 124, 125–133. [Google Scholar] [CrossRef]
- Xin, W.; Gao, X.; Zhao, P.; Wang, T.; Ding, X.; Wu, Q.; Hua, K. Long Non-Coding RNA RP11-379k17.4 Derived MicroRNA-200c-3p Modulates Human Endometrial Cancer by Targeting Noxa. J. Cancer 2021, 12, 2268–2274. [Google Scholar] [CrossRef]
- Chung, T.K.H.; Lau, T.S.; Cheung, T.H.; Yim, S.F.; Lo, K.W.K.; Siu, N.S.S.; Chan, L.K.Y.; Yu, M.Y.; Kwong, J.; Doran, G.; et al. Dysregulation of MicroRNA-204 Mediates Migration and Invasion of Endometrial Cancer by Regulating FOXC1. Int. J. Cancer. 2012, 130, 1036–1045. [Google Scholar] [CrossRef]
- Jing, S.; Feng, Y.; He, X.L.; Wang, Y. Effects of LncRNA-UCA1 Targeting MiR-204-5p on the Proliferation, Migration, Apoptosis and Immune Escape of Endometrial Carcinoma Cells. Zhonghua Zhong Liu Za Zhi 2023, 45, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Wang, H.-H.; Tian, F.-J.; He, X.-Y.; Qiu, M.-T.; Wang, J.-Y.; Zhang, H.-J.; Wang, L.-H.; Wan, X.-P. A TrkB–STAT3–MiR-204-5p Regulatory Circuitry Controls Proliferation and Invasion of Endometrial Carcinoma Cells. Mol. Cancer 2013, 12, 155. [Google Scholar] [CrossRef] [PubMed]
- Su, N.; Qiu, H.; Chen, Y.; Yang, T.; Yan, Q.; Wan, X. MiR-205 Promotes Tumor Proliferation and Invasion through Targeting ESRRG in Endometrial Carcinoma. Oncol. Rep. 2013, 29, 2297–2302. [Google Scholar] [CrossRef]
- Zhang, G.; Hou, X.; Li, Y.; Zhao, M. MiR-205 Inhibits Cell Apoptosis by Targeting Phosphatase and Tensin Homolog Deleted on Chromosome Ten in Endometrial Cancer Ishikawa Cells. BMC Cancer 2014, 14, 440. [Google Scholar] [CrossRef]
- Xin, W.; Zhao, S.; Han, X.; Zhao, P.; Yu, H.; Gao, X.; Li, P.; Wu, Q.; Ding, J.; Hua, K. LncRNA LA16c-313D11.11 Modulates the Development of Endometrial Cancer by Binding to and Inhibiting MicroRNA-205-5p Function and Indirectly Increasing PTEN Activity. Int. J. Oncol. 2020, 57, 355–363. [Google Scholar] [CrossRef]
- Xin, W.; Gao, X.; Zhao, S.; Zhao, P.; Yu, H.; Wu, Q.; Hua, K. LncRNA RP11-395G23.3 Suppresses the Endometrial Cancer Progression via Regulating MicroRNA-205-5p/PTEN Axis. Am. J. Transl. Res. 2020, 12, 4422–4433. [Google Scholar] [PubMed]
- Zheng, Y.; Yang, X.; Wang, C.; Zhang, S.; Wang, Z.; Li, M.; Wang, Y.; Wang, X.; Yang, X. HDAC6, Modulated by MiR-206, Promotes Endometrial Cancer Progression through the PTEN/AKT/MTOR Pathway. Sci. Rep. 2020, 10, 3576. [Google Scholar] [CrossRef]
- Dai, Z.; Luo, H.; Chen, J.; Li, L. MiR-210-3p Accelerates Tumor-Relevant Cell Functions of Endometrial Carcinoma by Repressing RUNX1T1. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2022, 825, 111793. [Google Scholar] [CrossRef]
- Sun, J.; Gao, S.; Lu, C. Knockdown of Differentiation Antagonizing Non-Protein Coding RNA Exerts Anti-Tumor Effect by up-Regulating MiR-214 in Endometrial Carcinoma. Mol. Cell. Biochem. 2019, 460, 9–15. [Google Scholar] [CrossRef]
- Wang, C.; Li, Q.; He, Y. MicroRNA-21-5p Promotes Epithelial to Mesenchymal Transition by Targeting SRY-box 17 in Endometrial Cancer. Oncol. Rep. 2020, 43, 1897–1905. [Google Scholar] [CrossRef]
- Xu, D.; Dong, P.; Xiong, Y.; Chen, R.; Konno, Y.; Ihira, K.; Yue, J.; Watari, H. PD-L1 Is a Tumor Suppressor in Aggressive Endometrial Cancer Cells and Its Expression Is Regulated by MiR-216a and LncRNA MEG3. Front. Cell. Dev. Biol. 2020, 8, 598205. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Huang, M.; You, J.; Lin, Y.; Huang, Q.; He, C. Circular RNA Hsa_circ_0023404 Promotes the Proliferation, Migration and Invasion in Endometrial Cancer Cells through Regulating MiR-217/MAPK1 Axis. Eur. J. Med. Res. 2022, 27, 242. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-C.; Hai, J.-J.; Tan, Y.-J.; Yue, Q.-F.; Liu, L.-J. MiR-218 Suppresses Metastasis and Invasion of Endometrial Cancer via Negatively Regulating ADD2. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1408–1417. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yu, Z.; Meng, X.; Zhou, S.; Xiao, S.; Li, X.; Liu, S.; Yu, P. Long Noncoding RNA GAS5 Impairs the Proliferation and Invasion of Endometrial Carcinoma Induced by High Glucose via Targeting MiR-222-3p/P27. Am. J. Transl. Res. 2019, 11, 2413–2421. [Google Scholar]
- Huang, K.; Dong, X.; Sui, C.; Hu, D.; Xiong, T.; Liao, S.; Zhang, H. MiR-223 Suppresses Endometrial Carcinoma Cells Proliferation by Targeting IGF-1R. Am. J. Transl. Res. 2014, 6, 841–849. [Google Scholar]
- Shang, C.; Lang, B.; Ao, C.N.; Meng, L. Long Non-Coding RNA Tumor Suppressor Candidate 7 Advances Chemotherapy Sensitivity of Endometrial Carcinoma through Targeted Silencing of MiR-23b. Tumor Biol. 2017, 39, 101042831770788. [Google Scholar] [CrossRef]
- Lu, M.; Ding, N.; Zhuang, S.; Li, Y. LINC01410/MiR-23c/CHD7 Functions as a CeRNA Network to Affect the Prognosis of Patients with Endometrial Cancer and Strengthen the Malignant Properties of Endometrial Cancer Cells. Mol. Cell. Biochem. 2020, 469, 9–19. [Google Scholar] [CrossRef]
- Li, S.; Shan, Y.; Li, X.; Zhang, C.; Wei, S.; Dai, S.; Zhao, R.; Zhao, X.; Zhao, L.; Shan, B. LncRNA SNHG5 Modulates Endometrial Cancer Progression via the MiR-25-3p/BTG2 Axis. J. Oncol. 2019, 2019, 7024675. [Google Scholar] [CrossRef]
- Fan, J.-T.; Zhou, Z.-Y.; Luo, Y.-L.; Luo, Q.; Chen, S.-B.; Zhao, J.-C.; Chen, Q.-R. Exosomal LncRNA NEAT1 from Cancer-Associated Fibroblasts Facilitates Endometrial Cancer Progression via MiR-26a/b-5p-Mediated STAT3/YKL-40 Signaling Pathway. Neoplasia 2021, 23, 692–703. [Google Scholar] [CrossRef]
- Che, X.; Jian, F.; Chen, C.; Liu, C.; Liu, G.; Feng, W. PCOS Serum-Derived Exosomal MiR-27a-5p Stimulates Endometrial Cancer Cells Migration and Invasion. J. Mol. Endocrinol. 2020, 64, 1–12. [Google Scholar] [CrossRef]
- Fan, L.; Wang, C.; Zhan, P.; Liu, Y. LncRNA RBAT1 Reduces Chemosensitivity of Cancer Cells to Carboplatin/Paclitaxel by Sponging MiR-27b in Endometrial Carcinoma. J. Ovarian Res. 2023, 16, 147. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Hu, J.; Yu, T.; You, S.; Zhang, Y.; Hu, L. MiR-27b-3p/MARCH7 Regulates Invasion and Metastasis of Endometrial Cancer Cells through Snail-Mediated Pathway. Acta Biochim. Biophys. Sin 2019, 51, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Xing, G.; Liu, S.; Li, B.; He, Y.; Wang, F. LncRNA LOXL1-AS1 Promotes Endometrial Cancer Progression by Sponging MiR-28-5p to Upregulate RAP1B Expression. Biomed. Pharmacother. 2020, 125, 109839. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, H.; He, T. Downregulated Circular RNA Hsa_circ_0005797 Inhibits Endometrial Cancer by Modulating MicroRNA-298/Catenin Delta 1 Signaling. Bioengineered 2022, 13, 4634–4645. [Google Scholar] [CrossRef]
- Liu, L.; Chen, X.; Zhang, Y.; Hu, Y.; Shen, X.; Zhu, W. Long Non-Coding RNA TUG1 Promotes Endometrial Cancer Development via Inhibiting MiR-299 and MiR-34a-5p. Oncotarget 2017, 8, 31386–31394. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Xiao, J.; Ma, C. Circular RNA FOXO3 Regulates Endometrial Carcinoma Progression through the MicroRNA-29a-3p/HDAC4 Axis. Am. J. Transl. Res. 2022, 14, 8572–8587. [Google Scholar] [PubMed]
- Kong, J.; He, X.; Wang, Y.; Li, J. Effect of MicroRNA-29b on Proliferation, Migration, and Invasion of Endometrial Cancer Cells. J. Int. Med. Res. 2019, 47, 3803–3817. [Google Scholar] [CrossRef]
- Chen, H.-X.; Xu, X.-X.; Tan, B.-Z.; Zhang, Z.; Zhou, X.-D. MicroRNA-29b Inhibits Angiogenesis by Targeting VEGFA through the MAPK/ERK and PI3K/Akt Signaling Pathways in Endometrial Carcinoma. Cell. Physiol. Biochem. 2017, 41, 933–946. [Google Scholar] [CrossRef]
- Pan, X.; Li, D.; Huo, J.; Kong, F.; Yang, H.; Ma, X. LINC01016 Promotes the Malignant Phenotype of Endometrial Cancer Cells by Regulating the MiR-302a-3p/MiR-3130-3p/NFYA/SATB1 Axis. Cell. Death Dis. 2018, 9, 303. [Google Scholar] [CrossRef]
- Ma, J.; Li, D.; Kong, F.-F.; Yang, D.; Yang, H.; Ma, X.-X. MiR-302a-5p/367-3p-HMGA2 Axis Regulates Malignant Processes during Endometrial Cancer Development. J. Exp. Clin. Cancer Res. 2018, 37, 19. [Google Scholar] [CrossRef]
- Hu, Y. MicroRNA-30c Negatively Regulates Endometrial Cancer Cells by Targeting Metastasis-Associated Gene-1. Oncol. Rep. 2011, 27, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Shu, S.; Liu, X.; Xu, M.; Gao, X.; Chen, S.; Zhang, L.; Li, R. MicroRNA-320a Acts as a Tumor Suppressor in Endometrial Carcinoma by Targeting IGF-1R. Int. J. Mol. Med. 2019, 43, 1505–1512. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, R.; Li, Y.; Yu, X.; Sun, Q.; Li, A.; Kong, Y. EIF4E-related MiR-320a and MiR-340-5p Inhibit Endometrial Carcinoma Cell Metastatic Capability by Preventing TGF-β1-induced Epithelial-mesenchymal Transition. Oncol. Rep. 2019, 43, 447–460. [Google Scholar] [CrossRef]
- Liu, Y.; Chang, Y.; Cai, Y. Circ_0067835 Sponges MiR-324-5p to Induce HMGA1 Expression in Endometrial Carcinoma Cells. J. Cell. Mol. Med. 2020, 24, 13927–13937. [Google Scholar] [CrossRef]
- Gao, Y.; Qian, H.; Tang, X.; Du, X.; Wang, G.; Zhang, H.; Ye, F.; Liu, T. Superparamagnetic Iron Oxide Nanoparticle-Mediated Expression of miR-326 Inhibits Human Endometrial Carcinoma Stem Cell Growth. Int. J. Nanomed. 2019, 14, 2719–2731. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, B.; Xu, N.; Wang, M.-J.; Liu, Q. MiR-326 Regulates EMT and Metastasis of Endometrial Cancer through Targeting TWIST1. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 3787–3793. [Google Scholar]
- Wang, R.; Zhang, C.; Guan, W.; Yang, Q. MiRNA-329-3p Suppresses Proliferation and Metastasis of Endometrial Carcinoma through Downregulating E2F1. Neoplasma 2023, 70, 566–579. [Google Scholar] [CrossRef]
- Dou, X.; Chen, X.; Zhou, Q.; Wen, M.; Zhang, S.; Zhang, S. MiR-335 Modulates Numb Alternative Splicing via Targeting RBM10 in Endometrial Cancer. Kaohsiung J. Med. Sci. 2020, 36, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chang, Y.; Cai, Y. Inhibition of Lnc-OC1 Induced Cell Apoptosis and Decreased Cell Viability by Releasing MiR-34a and Inhibiting PD-L1 in Endometrial Carcinoma. Reprod. Sci. 2020, 27, 1848–1856. [Google Scholar] [CrossRef]
- Schirmer, U.; Doberstein, K.; Rupp, A.-K.; Bretz, N.P.; Wuttig, D.; Kiefel, H.; Breunig, C.; Fiegl, H.; Müller-Holzner, E.; Zeillinger, R.; et al. Role of MiR-34a as a Suppressor of L1CAM in Endometrial Carcinoma. Oncotarget 2014, 5, 462–472. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, W.; Huang, K.; Wang, Y.; Li, J.; Yang, X. MicroRNA-34a Inhibits Cells Proliferation and Invasion by Downregulating Notch1 in Endometrial Cancer. Oncotarget 2017, 8, 111258–111270. [Google Scholar] [CrossRef]
- Li, F.; Chen, H.; Huang, Y.; Zhang, Q.; Xue, J.; Liu, Z.; Zheng, F. MiR-34c Plays a Role of Tumor Suppressor in HEC-1-B Cells by Targeting E2F3 Protein. Oncol. Rep. 2015, 33, 3069–3074. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Meng, W.; Zeng, J.; Hu, H.; Lu, L. MiR-34c Oligonucleotide Enhances Chemosensitivity of Ishikawa Cell to Cisplatin by Inducing Apoptosis. Cell. Biol. Int. 2013, 37, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yuan, S.; Liu, X.; Huang, Y.; Qiu, P.; Gao, J.; Deng, G. LncRNA GATA3-AS1 Promoted Invasion and Migration in Human Endometrial Carcinoma by Regulating the MiR-361/ARRB2 Axis. J. Mol. Med. 2022, 100, 1271–1286. [Google Scholar] [CrossRef]
- Dong, P.; Xiong, Y.; Yue, J.; Xu, D.; Ihira, K.; Konno, Y.; Kobayashi, N.; Todo, Y.; Watari, H. Long Noncoding RNA NEAT1 Drives Aggressive Endometrial Cancer Progression via MiR-361-Regulated Networks Involving STAT3 and Tumor Microenvironment-Related Genes. J. Exp. Clin. Cancer Res. 2019, 38, 295. [Google Scholar] [CrossRef]
- Wu, B.; Ren, A.; Tian, Y.; Huang, R. Hsa_circ_0075960 Serves as a Sponge for MiR-361-3p/SH2B1 in Endometrial Carcinoma. Technol. Cancer Res. Treat. 2020, 19, 1533033820983079. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, S.; Mingxin, Y.U. LncRNA NR2F1-AS1 Is Involved in the Progression of Endometrial Cancer by Sponging MiR-363 to Target SOX4. Pharmazie 2019, 74, 295–300. [Google Scholar] [CrossRef]
- Liu, B.-L.; Sun, K.-X.; Zong, Z.-H.; Chen, S.; Zhao, Y. MicroRNA-372 Inhibits Endometrial Carcinoma Development by Targeting the Expression of the Ras Homolog Gene Family Member C (RhoC). Oncotarget 2016, 7, 6649–6664. [Google Scholar] [CrossRef]
- Liang, H.; Liu, Y.; Fu, L.; Li, L.; Gong, N. Berberine Inhibits the Development of Endometrial Cancer through Circ_ZNF608/MiR-377-3p/COX2 Axis. Autoimmunity 2022, 55, 485–495. [Google Scholar] [CrossRef]
- Peng, T.; Zhou, Y.; Zhou, J.; Zhou, Y.; Li, X.; Ouyang, Q. Long Non-coding RNA VPS9D1-AS1Enhances Proliferation, Invasion, and Epithelial-mesenchymal Transition in Endometrial Cancer via miR-377-3p/ SGK1. Kaohsiung J. Med. Sci. 2022, 38, 1048–1059. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, R.; Li, M.; Yang, Q. Long Non-coding RNA BLACAT2/MiR-378a-3p/YY1 Feedback Loop Promotes the Proliferation, Migration and Invasion of Uterine Corpus Endometrial Carcinoma. Oncol. Rep. 2023, 49, 108. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.; Wang, F.; Wan, J. MicroRNA-381 Inhibits Cell Proliferation and Invasion in Endometrial Carcinoma by Targeting the IGF-1R. Mol. Med. Rep. 2017, 17, 4090–4098. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, B.; Wu, L. MicroRNA-409 May Function as a Tumor Suppressor in Endometrial Carcinoma Cells by Targeting Smad2. Mol. Med. Rep. 2018, 19, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Lin, J.; Jiang, J. Osthole Inhibited Cell Proliferation and Induced Cell Apoptosis through Decreasing CPEB2 Expression via Up-Regulating MiR-424 in Endometrial Carcinoma. J. Recept. Signal Transduct. 2020, 40, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Qiu, X.-M.; Li, Q.-H.; Wang, X.-Y.; Li, L.; Xu, M.; Dong, M.; Xiao, Y.-B. MicroRNA-424 May Function as a Tumor Suppressor in Endometrial Carcinoma Cells by Targeting E2F7. Oncol. Rep. 2015, 33, 2354–2360. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Chen, Z.; Wang, W. MicroRNA-424 Suppresses Estradiol-Induced Cell Proliferation via Targeting GPER in Endometrial Cancer Cells. Cell. Mol. Biol. 2015, 61, 96–101. [Google Scholar] [PubMed]
- Shu, S.; Liu, X.; Xu, M.; Gao, X.; Fan, J.; Liu, H.; Li, R. MicroRNA-424 Regulates Epithelial-mesenchymal Transition of Endometrial Carcinoma by Directly Targeting Insulin-like Growth Factor 1 Receptor. J. Cell. Biochem. 2019, 120, 2171–2179. [Google Scholar] [CrossRef]
- Cai, P.; Wu, M.; Zhang, B.; Wu, S.; Wei, H.; Wei, L. Long Non-coding RNA SNHG12 Regulates Cell Proliferation, Invasion and Migration in Endometrial Cancer by Targeting MiR-4429. Mol. Med. Rep. 2020, 22, 2842–2850. [Google Scholar] [CrossRef]
- Gao, X.; Yu, L.; Zhang, J.; Xue, P. Silencing of Long Non-Coding RNA LINC01106 Suppresses the Proliferation, Migration and Invasion of Endometrial Cancer Cells Through Regulating the MiR-449a/MET Axis. Onco. Targets Ther. 2020, 13, 9643–9655. [Google Scholar] [CrossRef]
- Ye, W.; Xue, J.; Zhang, Q.; Li, F.; Zhang, W.; Chen, H.; Huang, Y.; Zheng, F. MiR-449a Functions as a Tumor Suppressor in Endometrial Cancer by Targeting CDC25A. Oncol. Rep. 2014, 32, 1193–1199. [Google Scholar] [CrossRef]
- Wu, A.-Y.; Hu, Y.; Cang, W.; Li, D.; Wang, W.-J.; Tian, Q.; Gu, L.-Y.; Zhang, N.; Ji, F.; Qiu, L.-H. Suppressive Effect of MicroRNA-449a on the NDRG1/PTEN/AKT Axis Regulates Endometrial Cancer Growth and Metastasis. Exp. Cell. Res. 2019, 382, 111468. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wu, A.-Y.; Xu, C.; Song, K.-Q.; Wang, W.-J.; Yin, X.; Di, W.; Hong, Z.-B.; Qiu, L.-H. MicroRNA-449a Inhibits Tumor Metastasis through AKT/ERK1/2 Inactivation by Targeting Steroid Receptor Coactivator (SRC) in Endometrial Cancer. J. Cancer 2019, 10, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.; Li, W.; Yan, X.; Ma, T.; Ren, Y.; Hua, M.; Yang, H.; Wu, H.; Zhu, H. Long Non-coding RNA LINC01224 Promotes Cell Proliferation and Inhibits Apoptosis by Regulating AKT3 Expression via Targeting MiR-485-5p in Endometrial Carcinoma. Oncol. Rep. 2021, 46, 186. [Google Scholar] [CrossRef]
- Zheng, X.; Xu, K.; Zhu, L.; Mao, M.; Zhang, F.; Cui, L. MiR-486-5p Act as a Biomarker in Endometrial Carcinoma: Promotes Cell Proliferation, Migration, Invasion by Targeting MARK1. Onco. Targets Ther. 2020, 13, 4843–4853. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; Li, Y.; Chen, F.; Jia, H.; Jia, J.; Fu, Y. Long Non-Coding RNA DLEU1 Contributes to the Development of Endometrial Cancer by Sponging MiR-490 to Regulate SP1 Expression. Pharmazie 2018, 73, 379–385. [Google Scholar] [CrossRef]
- Zhang, G.; Ma, A.; Jin, Y.; Pan, G.; Wang, C. LncRNA SNHG16 Induced by TFAP2A Modulates Glycolysis and Proliferation of Endometrial Carcinoma through MiR-490-3p/HK2 Axis. Am. J. Transl. Res. 2019, 11, 7137–7145. [Google Scholar]
- Zhu, L.; Wang, X.; Wang, T.; Zhu, W.; Zhou, X. MiR-494-3p Promotes the Progression of Endometrial Cancer by Regulating the PTEN/PI3K/AKT Pathway. Mol. Med. Rep. 2018, 19, 581–588. [Google Scholar] [CrossRef]
- Tan, A.; Luo, R.; Ruan, P. MiR-495 Promotes Apoptosis and Inhibits Proliferation in Endometrial Cells via Targeting PIK3R1. Pathol. Res. Pract. 2019, 215, 594–599. [Google Scholar] [CrossRef]
- He, Y.; Xu, S.; Qi, Y.; Tian, J.; Xu, F. Long Noncoding RNA SNHG25 Promotes the Malignancy of Endometrial Cancer by Sponging MicroRNA-497-5p and Increasing FASN Expression. J. Ovarian Res. 2021, 14, 163. [Google Scholar] [CrossRef]
- Xing, Y.; Sun, X.; Li, F.; Jiang, X.; Jiang, A.; Li, X.; Lv, R.; Shao, L. Long Non-Coding RNA (LncRNA) HOXB-AS3 Promotes Cell Proliferation and Inhibits Apoptosis by Regulating ADAM9 Expression through Targeting MiR-498-5p in Endometrial Carcinoma. J. Int. Med. Res. 2021, 49, 03000605211013548. [Google Scholar] [CrossRef]
- Chen, S.; Sun, K.-X.; Liu, B.-L.; Zong, Z.-H.; Zhao, Y. MicroRNA-505 Functions as a Tumor Suppressor in Endometrial Cancer by Targeting TGF-α. Mol. Cancer 2016, 15, 11. [Google Scholar] [CrossRef]
- Yuan, S.; Zheng, P.; Sun, X.; Zeng, J.; Cao, W.; Gao, W.; Wang, Y.; Wang, L. Hsa_Circ_0001860 Promotes Smad7 to Enhance MPA Resistance in Endometrial Cancer via MiR-520h. Front. Cell. Dev. Biol. 2021, 9, 738189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Han, Y.; Zhang, X.; Xiao, N.; Jiang, T.; Zhu, S.; Wang, E.; Chen, C. MiR-522 Facilitates the Prosperities of Endometrial Carcinoma Cells by Directly Binding to Monoamine Oxidase, B. Kaohsiung J. Med. Sci. 2019, 35, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, M.; Lv, X.; Zhou, J.; Gao, L. CircCCL22 Regulates CDC25A via Sponging MiR-543 and Promotes Proliferation and Metastasis in Endometrial Cancer. Mol. Biotechnol. 2024, 66, 2522–2531. [Google Scholar] [CrossRef] [PubMed]
- Bing, L.; Hong, C.; Li-Xin, S.; Wei, G. MicroRNA-543 Suppresses Endometrial Cancer Oncogenicity via Targeting FAK and TWIST1 Expression. Arch. Gynecol. Obstet. 2014, 290, 533–541. [Google Scholar] [CrossRef]
- Zhong, Y.; Wang, Y.; Dang, H.; Wu, X. LncRNA AFAP1-AS1 Contributes to the Progression of Endometrial Carcinoma by Regulating MiR-545-3p/VEGFA Pathway. Mol. Cell. Probes 2020, 53, 101606. [Google Scholar] [CrossRef]
- Wang, N.; Guo, Y.; Song, L.; Tong, T.; Fan, X. Circular RNA Intraflagellar Transport 80 Facilitates Endometrial Cancer Progression through Modulating MiR-545-3p/FAM98A Signaling. J. Gynecol. Oncol. 2022, 33, e2. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, L.; Liu, Y. Targeting Thyroid Receptor Interacting Protein 6 by MicroRNA-589-5p Inhibits Cell Proliferation, Migration, and Invasion in Endometrial Carcinoma. Cancer Biother. Radiopharm. 2019, 34, 529–536. [Google Scholar] [CrossRef]
- Shi, Y.; Zha, J.; Zuo, M.; Yan, Q.; Song, H. Long Noncoding RNA CHL1-AS1 Promotes Cell Proliferation and Migration by Sponging MiR-6076 to Regulate CHL1 Expression in Endometrial Cancer. J. Cell. Biochem. 2020, 121, 2655–2663. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, D.-L.; Yu, P. LncRNA H19 Regulates the Expression of Its Target Gene HOXA10 in Endometrial Carcinoma through Competing with MiR-612. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 4820–4827. [Google Scholar] [CrossRef]
- Cong, R.; Kong, F.; Ma, J.; Li, Q.; Yang, H.; Ma, X. The PVT1/MiR-612/CENP-H/CDK1 Axis Promotes Malignant Progression of Advanced Endometrial Cancer. Am. J. Cancer Res. 2021, 11, 1480–1502. [Google Scholar] [PubMed]
- Wu, X.; Cai, D.; Zhang, F.; Li, M.; Wan, Q. Long Noncoding RNA TUSC7 Inhibits Cell Proliferation, Migration and Invasion by Regulating SOCS4 (SOCS5) Expression through Targeting MiR-616 in Endometrial Carcinoma. Life Sci. 2019, 231, 116549. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Han, Y.; Li, S. Oncogenic Circular RNA Circ_0007534 Contributes to Paclitaxel Resistance in Endometrial Cancer by Sponging MiR-625 and Promoting ZEB2 Expression. Front. Oncol. 2022, 12, 985470. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, L.; Sun, X. CircRNA Hsa_circ_0002577 Accelerates Endometrial Cancer Progression through Activating IGF1R/PI3K/Akt Pathway. J. Exp. Clin. Cancer Res. 2020, 39, 169. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y. Hsa_circ_0000437 Inhibits the Development of Endometrial Carcinoma through MiR-626/CDKN1B Axis. Protein Pept. Lett. 2022, 29, 611–620. [Google Scholar] [CrossRef]
- Li, Y.; Huo, J.; He, J.; Ma, X. LncRNA MONC Suppresses the Malignant Phenotype of Endometrial Cancer Stem Cells and Endometrial Carcinoma Cells by Regulating the MiR-636/GLCE Axis. Cancer Cell. Int. 2021, 21, 331. [Google Scholar] [CrossRef]
- Ni, J.; Liang, S.; Shan, B.; Tian, W.; Wang, H.; Ren, Y. Methylation-associated Silencing of MiR-638 Promotes Endometrial Carcinoma Progression by Targeting MEF2C. Int. J. Mol. Med. 2020, 45, 1753–1770. [Google Scholar] [CrossRef]
- Wang, B.; Lu, Y.; Feng, E. Hsa_circ_0001610 Knockdown Modulates MiR-646-STAT3 Axis to Suppress Endometrial Carcinoma Progression. J. Gene Med. 2021, 23, e3337. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, S.; Zong, Z.H.; Guan, X.; Zhao, Y. CircRNA WHSC1 Targets the MiR-646/NPM1 Pathway to Promote the Development of Endometrial Cancer. J. Cell. Mol. Med. 2020, 24, 6898–6907. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.X.; Wang, C.; Mao, L.W.; Wang, Y.L.; Xia, L.Q.; Zhao, W.; Shen, J.; Chen, J. Long Noncoding RNA HOTAIR Mediates the Estrogen-Induced Metastasis of Endometrial Cancer Cells via the MiR-646/NPM1 Axis. Am. J. Physiol. Cell. Physiol. 2018, 314, C690–C701. [Google Scholar] [CrossRef]
- Sun, X.; Dongol, S.; Qiu, C.; Xu, Y.; Sun, C.; Zhang, Z.; Yang, X.; Zhang, Q.; Kong, B. MiR-652 Promotes Tumor Proliferation and Metastasis by Targeting RORA in Endometrial Cancer. Mol. Cancer Res. 2018, 16, 1927–1939. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-F.; Wen, L.-N. LncRNA SNHG14 Promotes Proliferation of Endometrial Cancer through Regulating MicroRNA-655-3p. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 10410–10418. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.; Qiu, H.; Si, L.; Zhen, Y.; Chu, D.; Guo, R. Long Noncoding RNA BMPR1B-AS1 Facilitates Endometrial Cancer Cell Proliferation and Metastasis by Sponging MiR-7-2-3p to Modulate the DCLK1/Akt/NF-ΚB Pathway. Cell. Cycle 2022, 21, 1599–1618. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Zhang, J. N6-Methyladenosine Modified Circ-NAB1 Modulates Cell Cycle and Epithelialmesenchymal Transition via CDKN3 in Endometrial Cancer. Cell. Mol. Biol. 2024, 70, 161–169. [Google Scholar] [CrossRef]
- Ke, J.; Shen, Z.; Hu, W.; Li, M.; Shi, Y.; Xie, Z.; Wu, D. LncRNA DCST1-AS1 Was Upregulated in Endometrial Carcinoma and May Sponge MiR-92a-3p to Upregulate Notch1. Cancer Manag. Res. 2020, 12, 1221–1227. [Google Scholar] [CrossRef]
- Chen, S.; Chen, X.; Sun, K.-X.; Xiu, Y.-L.; Liu, B.-L.; Feng, M.-X.; Sang, X.-B.; Zhao, Y. MicroRNA-93 Promotes Epithelial–Mesenchymal Transition of Endometrial Carcinoma Cells. PLoS ONE 2016, 11, e0165776. [Google Scholar] [CrossRef]
- Zhang, K.; Cai, Y.; Zhou, Q.; Sun, H.; Wei, J. Long Non-Coding RNA SNHG14 Impedes Viability, Migration and Invasion of Endometrial Carcinoma Cells Through Modulating MiR-93-5p/ZBTB7A Axis. Cancer Manag. Res. 2020, 12, 9515–9525. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Xu, Y.-P.; Wang, L.-J.; Kong, Y. MiR-940 Potentially Promotes Proliferation and Metastasis of Endometrial Carcinoma through Regulation of MRVI1. Biosci. Rep. 2019, 39, BSR20190077. [Google Scholar] [CrossRef]
- He, Z.; Xu, H.; Meng, Y.; Kuang, Y. MiR-944 Acts as a Prognostic Marker and Promotes the Tumor Progression in Endometrial Cancer. Biomed. Pharmacother. 2017, 88, 902–910. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; Zhang, X.; Lin, Y.; Luo, T.; Xiao, Z.; Zhou, Q. A Dual PI3K/AKT/MTOR Signaling Inhibitor MiR-99a Suppresses Endometrial Carcinoma. Am. J. Transl. Res. 2016, 8, 719–731. [Google Scholar]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Piergentili, R.; Marinelli, E.; Cucinella, G.; Lopez, A.; Napoletano, G.; Gullo, G.; Zaami, S. MiR-125 in Breast Cancer Etiopathogenesis: An Emerging Role as a Biomarker in Differential Diagnosis, Regenerative Medicine, and the Challenges of Personalized Medicine. Noncoding RNA 2024, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Katsushima, K.; Natsume, A.; Ohka, F.; Shinjo, K.; Hatanaka, A.; Ichimura, N.; Sato, S.; Takahashi, S.; Kimura, H.; Totoki, Y.; et al. Targeting the Notch-Regulated Non-Coding RNA TUG1 for Glioma Treatment. Nat. Commun. 2016, 7, 13616. [Google Scholar] [CrossRef]
- Wu, R.; Liu, W.; Yang, Q.; Zhang, J.; Hou, P.; Xiong, J.; Wu, L.; Li, E. LncTUG1 Promotes Hepatocellular Carcinoma Immune Evasion via Upregulating PD-L1 Expression. Sci. Rep. 2023, 13, 16998. [Google Scholar] [CrossRef] [PubMed]
- Beadle, G.W.; Tatum, E.L. Genetic Control of Biochemical Reactions in Neurospora. Proc. Natl. Acad. Sci. USA 1941, 27, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.J. Biochemical Institute Studies IV—Individual Metabolic Patterns and Human Disease: An Exploratory Study Utilizing Predominantly Paper Chromatographic Methods; U. Texas Publication No. 5109; University of Texas: Austin, TX, USA, 1951; 205p. [Google Scholar]
- Wishart, D.S.; Tzur, D.; Knox, C.; Eisner, R.; Guo, A.C.; Young, N.; Cheng, D.; Jewell, K.; Arndt, D.; Sawhney, S.; et al. HMDB: The Human Metabolome Database. Nucleic Acids Res. 2007, 35, D521–D526. [Google Scholar] [CrossRef]
- Pandey, S. Metabolomics for the identification of biomarkers in endometriosis. Arch. Gynecol. Obstet. 2024, 310, 2823–2827. [Google Scholar] [CrossRef] [PubMed]
- Raffone, A.; Troisi, J.; Boccia, D.; Travaglino, A.; Capuano, G.; Insabato, L.; Mollo, A.; Guida, M.; Zullo, F. Metabolomics in endometrial cancer diagnosis: A systematic review. Acta Obstet. Gynecol. Scand. 2020, 99, 1135–1146. [Google Scholar] [CrossRef]
- Albertí-Valls, M.; Megino-Luque, C.; Macià, A.; Gatius, S.; Matias-Guiu, X.; Eritja, N. Metabolomic-Based Approaches for Endometrial Cancer Diagnosis and Prognosis: A Review. Cancers 2023, 16, 185. [Google Scholar] [CrossRef]
- Li, Z.; Munim, M.B.; Sharygin, D.A.; Bevis, B.J.; Vander Heiden, M.G. Understanding the Warburg Effect in Cancer. Cold Spring Harb. Perspect. Med. 2024, a041532. [Google Scholar] [CrossRef]
- Hishinuma, E.; Shimada, M.; Matsukawa, N.; Shima, Y.; Li, B.; Motoike, I.N.; Shibuya, Y.; Hagihara, T.; Shigeta, S.; Tokunaga, H.; et al. Identification of predictive biomarkers for endometrial cancer diagnosis and treatment response monitoring using plasma metabolome profiling. Cancer Metab. 2023, 11, 16. [Google Scholar] [CrossRef]
- Chang, L.; Xia, J. MicroRNA Regulatory Network Analysis Using miRNet 2.0. Methods Mol. Biol. 2023, 2594, 185–204. [Google Scholar] [CrossRef]
- Androutsopoulos, G.; Styliara, I.; Zarogianni, E.; Lazurko, N.; Valasoulis, G.; Michail, G.; Adonakis, G. The ErbB Signaling Network and Its Potential Role in Endometrial Cancer. Epigenomes 2023, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Fatima, I.; Barman, S.; Rai, R.; Thiel, K.W.W.; Chandra, V. Targeting Wnt Signaling in Endometrial Cancer. Cancers 2021, 13, 2351. [Google Scholar] [CrossRef]
- Gao, S.; Wang, J.; Wang, T.; Wang, J. Is Insulin Resistance a High-Risk Factor for Postmenopausal Endometrial Cancer: Insights from the Triglyceride Glucose (TyG) Index and the Metabolic Score for Insulin Resistance (METS-IR). Int. J. Womens. Health 2024, 16, 2355–2363. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, Q.; Niu, X.; Wang, G.; Zheng, S.; Fu, G.; Wang, Z. MiR-143 Inhibits Bladder Cancer Cell Proliferation and Enhances Their Sensitivity to Gemcitabine by Repressing IGF-1R Signaling. Oncol. Lett. 2017, 13, 435–440. [Google Scholar] [CrossRef]
- Li, D.; Zhong, S.; Zhu, Z.; Jiang, X.; Zhang, J.; Gu, J.; Chen, F. LncRNA MAFG-AS1 Promotes the Progression of Bladder Cancer by Targeting the MiR-143-3p/COX-2 Axis. Pathobiology 2020, 87, 345–355. [Google Scholar] [CrossRef]
- Xiang, W.; Lyu, L.; Huang, T.; Zheng, F.; Yuan, J.; Zhang, C.; Jiang, G. The Long Non-coding RNA SNHG1 Promotes Bladder Cancer Progression by Interacting with MiR-143-3p and EZH2. J. Cell. Mol. Med. 2020, 24, 11858–11873. [Google Scholar] [CrossRef]
- Luo, J.; Chen, J.; Li, H.; Yang, Y.; Yun, H.; Yang, S.; Mao, X. LncRNA UCA1 Promotes the Invasion and EMT of Bladder Cancer Cells by Regulating the MiR-143/HMGB1 Pathway. Oncol. Lett. 2017, 14, 5556–5562. [Google Scholar] [CrossRef]
- Bell, D.W.; Ellenson, L.H. Molecular Genetics of Endometrial Carcinoma. Annu. Rev. Pathol. Mech. Dis. 2019, 14, 339–367. [Google Scholar] [CrossRef]
- Żyła, M.M.; Kostrzewa, M.; Litwińska, E.; Szpakowski, A.; Radosław Wilczyński, J.; Stetkiewicz, T. The Role of Angiogenic Factors in Endometrial Cancer. Menopausal Rev. 2014, 2, 122–126. [Google Scholar] [CrossRef]
- Hong, D.S.; Kang, Y.-K.; Borad, M.; Sachdev, J.; Ejadi, S.; Lim, H.Y.; Brenner, A.J.; Park, K.; Lee, J.-L.; Kim, T.-Y.; et al. Phase 1 Study of MRX34, a Liposomal MiR-34a Mimic, in Patients with Advanced Solid Tumours. Br. J. Cancer 2020, 122, 1630–1637. [Google Scholar] [CrossRef]
- Beg, M.S.; Brenner, A.J.; Sachdev, J.; Borad, M.; Kang, Y.-K.; Stoudemire, J.; Smith, S.; Bader, A.G.; Kim, S.; Hong, D.S. Phase I Study of MRX34, a Liposomal MiR-34a Mimic, Administered Twice Weekly in Patients with Advanced Solid Tumors. Invest. New Drugs 2017, 35, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Peltier, H.J.; Kelnar, K.; Bader, A.G. Effects of MRX34, a Liposomal MiR-34 Mimic, on Target Gene Expression in Human White Blood Cells (HWBCs): QRT-PCR Results from a First-in-Human Trial of MicroRNA Cancer Therapy. J. Clin. Oncol. 2016, 34, e14090. [Google Scholar] [CrossRef]
- Mechahougui, H.; Gutmans, J.; Colarusso, G.; Gouasmi, R.; Friedlaender, A. Advances in Personalized Oncology. Cancers 2024, 16, 2862. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Ma, J.; Zhang, Q.; Li, W.; Wang, C. Predicting Gene Mutation Status via Artificial Intelligence Technologies Based on Multimodal Integration (MMI) to Advance Precision Oncology. Semin. Cancer Biol. 2023, 91, 1–15. [Google Scholar] [CrossRef]
- The ESMO Personalised Medicine Task Force. What Is Personalised Medicine? Let Us Help You Understand. Available online: https://www.esmo.org/content/download/20122/337223/1/ESMO-Patient-Guide-Personalised-Cancer-Medicine.pdf (accessed on 10 February 2025).
- Salari, P.; Larijani, B. Ethical Issues Surrounding Personalized Medicine: A Literature Review. Acta Med. Iran. 2017, 55, 209–217. Fiore, R.N.; Goodman, K.W. Precision Medicine Ethics: Selected Issues and Developments in next-Generation Sequencing, Clinical Oncology, and Ethics. Curr. Opin. Oncol. 2016, 28, 83–87. [Google Scholar] [CrossRef]
- Krzyszczyk, P.; Acevedo, A.; Davidoff, E.J.; Timmins, L.M.; Marrero-Berrios, I.; Patel, M.; White, C.; Lowe, C.; Sherba, J.J.; Hartmanshenn, C.; et al. The Growing Role of Precision and Personalized Medicine for Cancer Treatment. Technology 2018, 6, 79–100. [Google Scholar] [CrossRef]
- Gazola, A.A.; Lautert-Dutra, W.; Archangelo, L.F.; Reis, R.B.D.; Squire, J.A. Precision Oncology Platforms: Practical Strategies for Genomic Database Utilization in Cancer Treatment. Mol. Cytogenet. 2024, 17, 28. [Google Scholar] [CrossRef]
- Zeng, J.; Shufean, M.A. Molecular-Based Precision Oncology Clinical Decision Making Augmented by Artificial Intelligence. Emerg. Top Life Sci. 2021, 5, 757–764. [Google Scholar] [CrossRef]
- European Commission. Europe’s Beating Cancer Plan Communication from the Commission to the European Parliament and the Council. Issued in February 2022. Available online: https://health.ec.europa.eu/system/files/2022-02/eu_cancer-plan_en_0.pdf (accessed on 20 August 2023).
- European Commission. Directorate General for Research and Innovation. Conquering Cancer: Mission Possible; Publications Office: Luxembourg, 2020. Available online: https://op.europa.eu/en/publication-detail/-/publication/b389aad3-fd56-11ea-b44f-01aa75ed71a1/https://op.europa.eu/en/publication-detail/-/publication/b389aad3-fd56-11ea-b44f-01aa75ed71a1 (accessed on 29 August 2023).
- Hickman, J.A.; Tannock, I.F.; Meheus, L.; Hutchinson, L. The European Union and personalised cancer medicine. Eur. J. Cancer 2021, 150, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Gambardella, V.; Tarazona, N.; Cejalvo, J.M.; Lombardi, P.; Huerta, M.; Roselló, S.; Fleitas, T.; Roda, D.; Cervantes, A. Personalized Medicine: Recent Progress in Cancer Therapy. Cancers 2020, 12, 1009. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Rajendran, B.K.; Cui, T.; Sun, J.; Zhao, Y.; Palaniyandi, T.; Selvam, M. Advances in Predicting Breast Cancer Driver Mutations: Tools for Precision Oncology (Review). Int. J. Mol. Med. 2025, 55, 6. [Google Scholar] [CrossRef] [PubMed]
- Piergentili, R.; Basile, G.; Nocella, C.; Carnevale, R.; Marinelli, E.; Patrone, R.; Zaami, S. Using ncRNAs as Tools in Cancer Diagnosis and Treatment—The Way towards Personalized Medicine to Improve Patients’ Health. IJMS 2022, 23, 9353. [Google Scholar] [CrossRef]
- PCM4EU—Personalised cancer medicine for all EU citizens. Project set to run from 1st January 2023 to 30th June 2025. Available online: https://health.ec.europa.eu/document/download/67f62913-43d0-4092-bfed-2347caadcfe1_en?filename=fs_cancer_pcm4eu.pdf (accessed on 10 February 2025).
- European Cancer Imaging Initiative. Launched on 23 January 2023. Available online: https://digital-strategy.ec.europa.eu/en/policies/cancer-imaging (accessed on 10 February 2025).
- European Commission. Smart Specialisation Platform. European Partnership on Personalised Medicine. TOPIC ID: HORIZON-HLTH-2023-CARE-08-01. Last Updated on 9 August 2023. Available online: https://ec.europa.eu/info/funding-tenders/opportunities/portal/screen/opportunities/topic-details/horizon-hlth-2023-care-08-01 (accessed on 30 August 2023).
- Marchant, G.E.; Lindor, R.A. Personalized Medicine and Genetic Malpractice. Genet. Med. 2013, 15, 921–922. [Google Scholar] [CrossRef]
- Brothers, K.B.; Rothstein, M.A. Ethical, Legal and Social Implications of Incorporating Personalized Medicine into Healthcare. Per. Med. 2015, 12, 43–51. [Google Scholar] [CrossRef]
- Ledford, H. US Personalized-Medicine Industry Takes Hit from Supreme Court. Nature 2016, 536, 382. [Google Scholar] [CrossRef]
- Donohue, K.E.; Gooch, C.; Katz, A.; Wakelee, J.; Slavotinek, A.; Korf, B.R. Pitfalls and Challenges in Genetic Test Interpretation: An Exploration of Genetic Professionals Experience with Interpretation of Results. Clin. Genet. 2021, 99, 638–649. [Google Scholar] [CrossRef]
- Berger, S.M.; Appelbaum, P.S.; Siegel, K.; Wynn, J.; Saami, A.M.; Brokamp, E.; O’Connor, B.C.; Hamid, R.; Martin, D.M.; Chung, W.K. Challenges of Variant Reinterpretation: Opinions of Stakeholders and Need for Guidelines. Genet. Med. 2022, 24, 1878–1887. [Google Scholar] [CrossRef]
- Galante, N.; Bedeschi, M.F.; Beltrami, B.; Bailo, P.; Silva Palomino, L.A.; Piccinini, A. Reviewing Hereditary Connective Tissue Disorders: Proposals of Harmonic Medicolegal Assessments. Int. J. Legal Med. 2024, 138, 2507–2522. [Google Scholar] [CrossRef]
- Milunsky, A. Obstetrics, Genetics, and Litigation. Acta Obstet. Gynecol. Scand. 2021, 100, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Statello, L.; Guo, C.-J.; Chen, L.-L.; Huarte, M. Gene Regulation by Long Non-Coding RNAs and Its Biological Functions. Nat. Rev. Mol. Cell. Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef]
- Tsai, M.-C.; Manor, O.; Wan, Y.; Mosammaparast, N.; Wang, J.K.; Lan, F.; Shi, Y.; Segal, E.; Chang, H.Y. Long Noncoding RNA as Modular Scaffold of Histone Modification Complexes. Science 2010, 329, 689–693. [Google Scholar] [CrossRef]
- Gulìa, C.; Signore, F.; Gaffi, M.; Gigli, S.; Votino, R.; Nucciotti, R.; Bertacca, L.; Zaami, S.; Baffa, A.; Santini, E.; et al. Y RNA: An Overview of Their Role as Potential Biomarkers and Molecular Targets in Human Cancers. Cancers 2020, 12, 1238. [Google Scholar] [CrossRef] [PubMed]
- Cao, J. The Functional Role of Long Non-Coding RNAs and Epigenetics. Biol. Proced. Online 2014, 16, 42. [Google Scholar] [CrossRef]
- Qian, Y.; Shi, L.; Luo, Z. Long Non-Coding RNAs in Cancer: Implications for Diagnosis, Prognosis, and Therapy. Front. Med. 2020, 7, 612393. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).