Non-Coding RNA as a Biomarker in Lung Cancer
Abstract
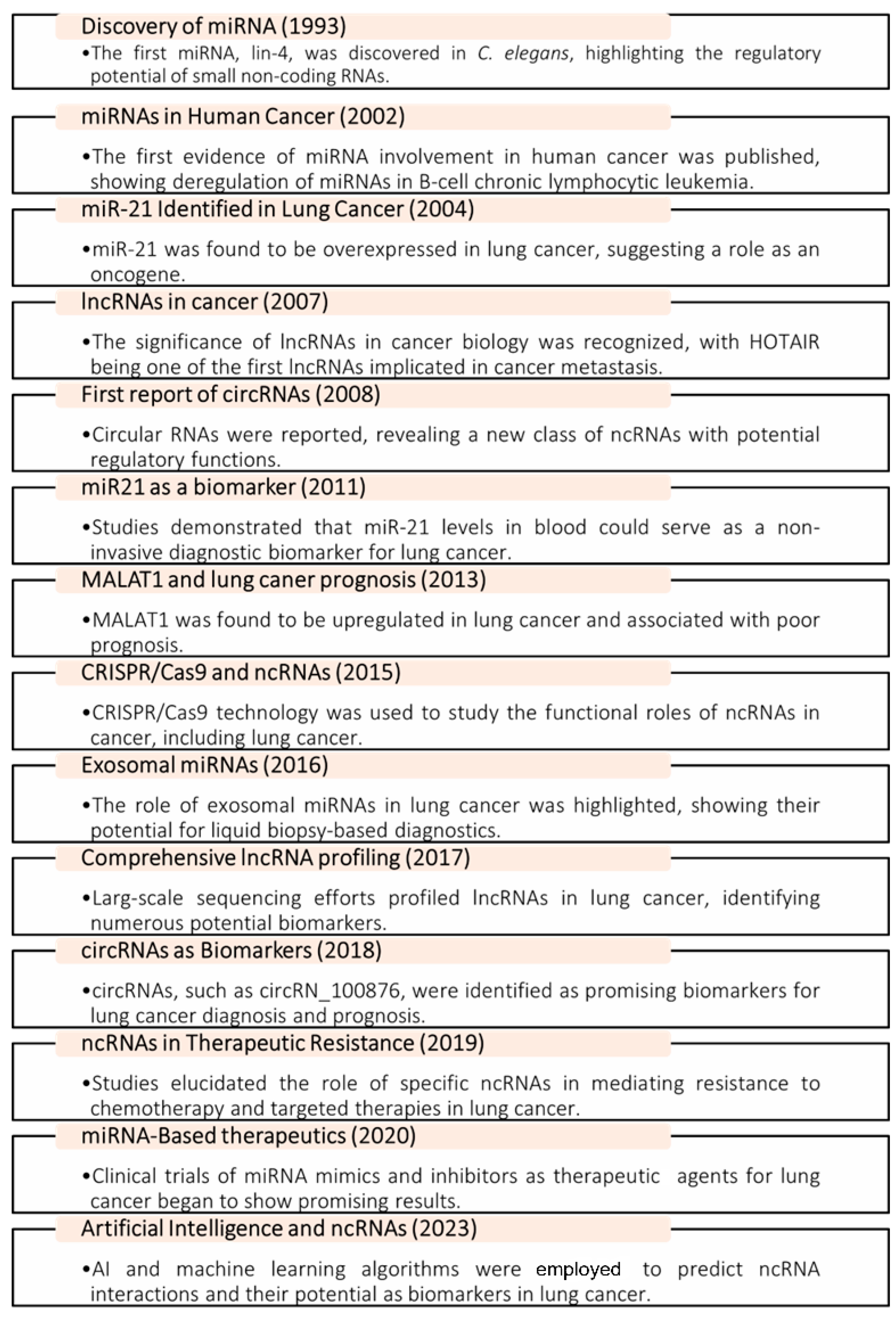
1. Introduction
2. Biological Functions of ncRNAs in Lung Cancer
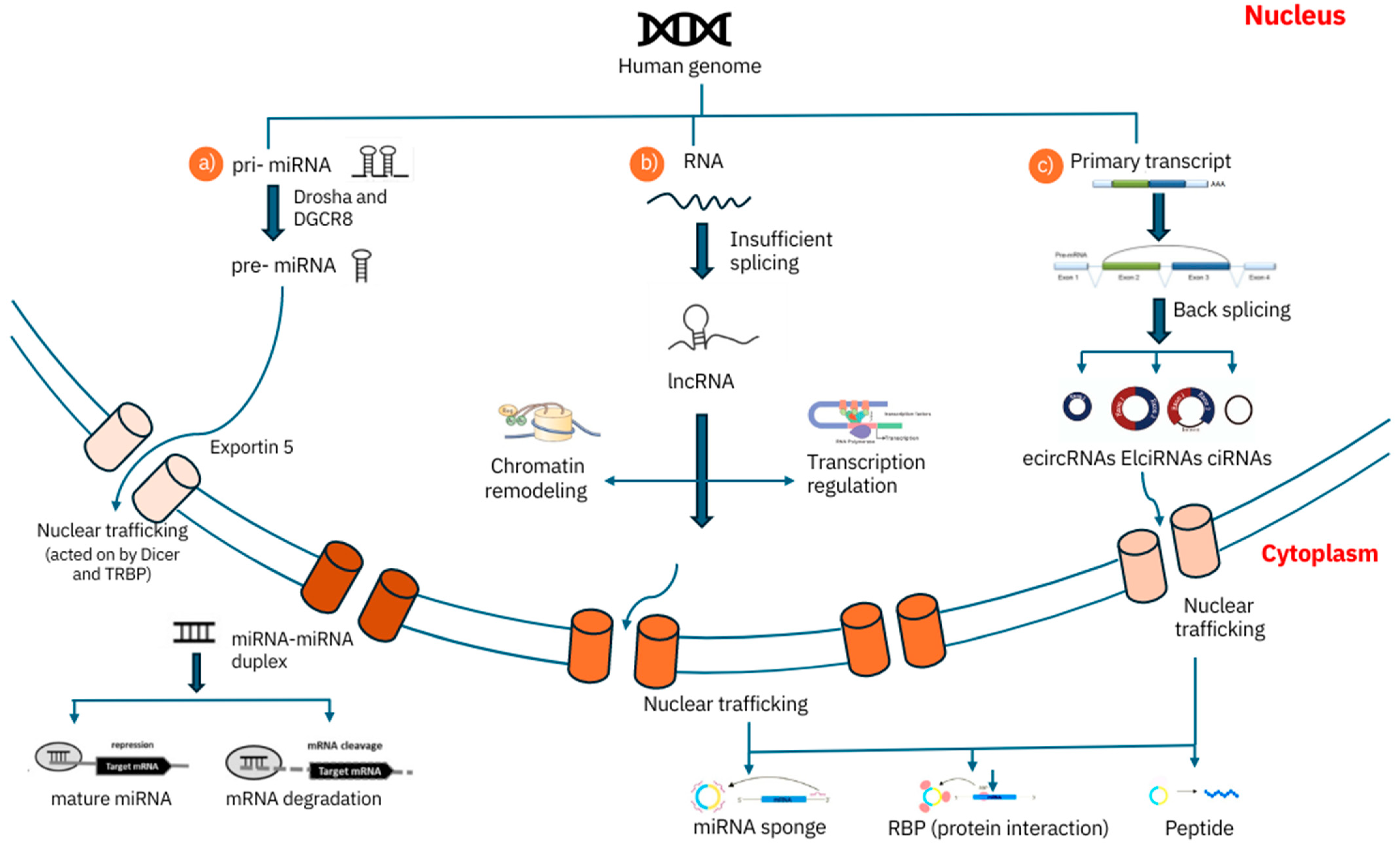
2.1. MicroRNAs (miRNAs)
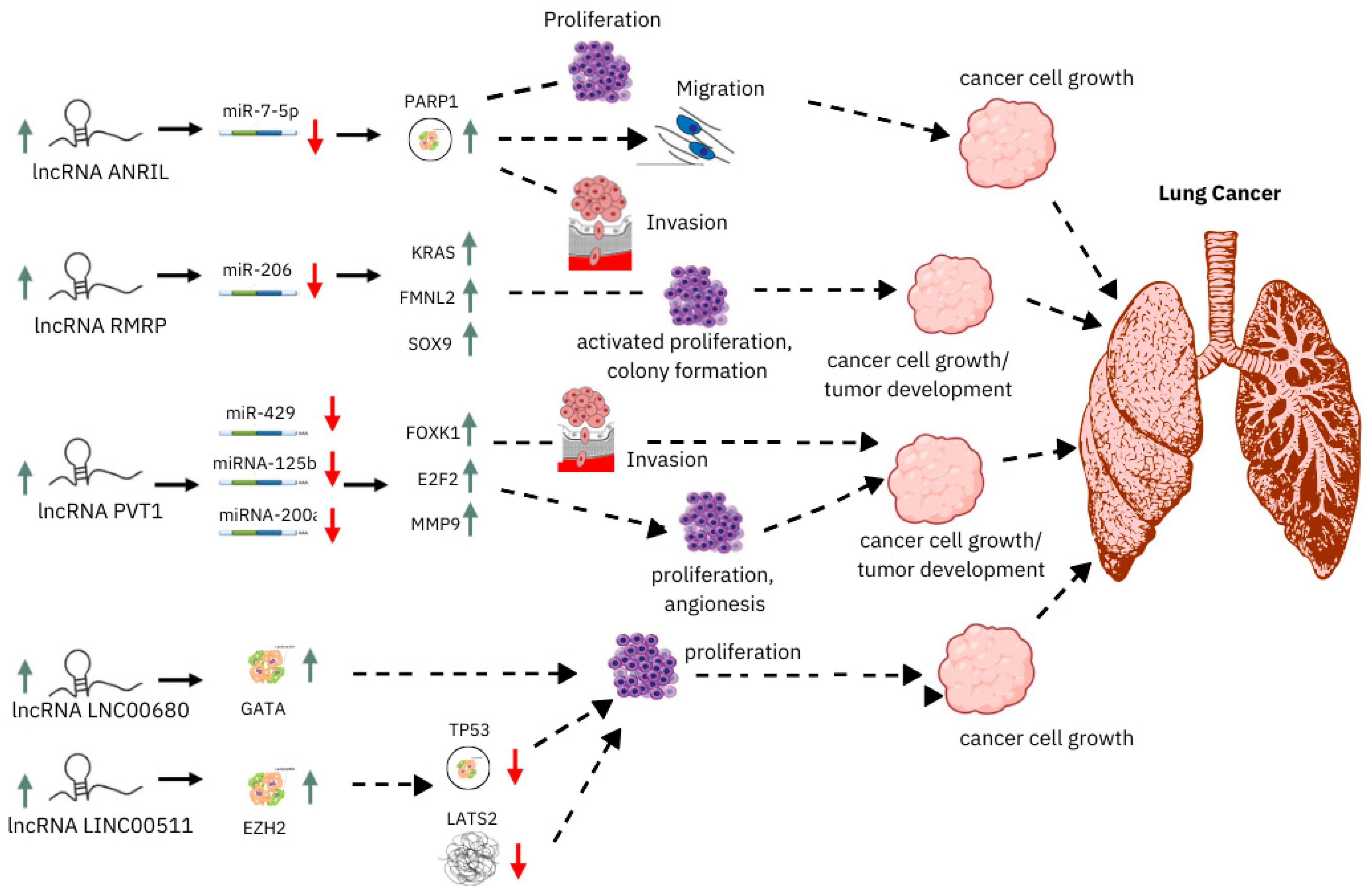
2.2. Long Non-Coding RNAs (lncRNAs)
2.3. Circular RNAs (CircRNAs)
3. ncRNAs as Diagnostic Biomarkers
3.1. miRNAs as Diagnostic Biomarkers
3.2. lncRNAs as Diagnostic Biomarkers
3.3. circRNAs as Diagnostic Biomarkers
4. ncRNAs as Prognostic Biomarkers
miRNAs as Prognostic Biomarkers
5. Therapeutic Response Monitoring
5.1. ncRNAs
5.2. lncRNAs
5.3. miRNAs
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, X.; Yu, H. Global burden of cancer. Yale J. Biol. Med. 2006, 79, 85–94. [Google Scholar] [PubMed]
- Li, C.; Lei, S.; Ding, L.; Xu, Y.; Wu, X.; Wang, H.; Zhang, Z.; Gao, T.; Zhang, Y.; Li, L. Global burden and trends of lung cancer incidence and mortality. Chin. Med. J. 2023, 136, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, P.; Pande, B.; Acharya, R.; Bhaskar, L.V.K.S.; Verma, H.K. Unravelling the Triad of Lung Cancer, Drug Resistance, and Metabolic Pathways. Diseases 2024, 12, 93. [Google Scholar] [CrossRef] [PubMed]
- Lam, D.C.-L.; Liam, C.-K.; Andarini, S.; Park, S.; Tan, D.S.W.; Singh, N.; Jang, S.H.; Vardhanabhuti, V.; Ramos, A.B.; Nakayama, T.; et al. Lung Cancer Screening in Asia: An Expert Consensus Report. J. Thorac. Oncol. 2023, 18, 1303–1322. [Google Scholar] [CrossRef]
- Howlader, N.; Forjaz, G.; Mooradian, M.J.; Meza, R.; Kong, C.Y.; Cronin, K.A.; Mariotto, A.B.; Lowy, D.R.; Feuer, E.J. The Effect of Advances in Lung-Cancer Treatment on Population Mortality. N. Engl. J. Med. 2020, 383, 640–649. [Google Scholar] [CrossRef]
- Hadjicharalambous, M.R.; Lindsay, M.A. Long Non-Coding RNAs and the Innate Immune Response. Noncoding RNA 2019, 5, 34. [Google Scholar] [CrossRef] [PubMed]
- Verma, H.K.; Ratre, Y.K.; Mazzone, P.; Laurino, S.; Bhaskar, L.V.K.S. Micro RNA facilitated chemoresistance in gastric cancer: A novel biomarkers and potential therapeutics. Alex. J. Med. 2020, 56, 81–92. [Google Scholar] [CrossRef]
- Le, P.; Romano, G.; Nana-Sinkam, P.; Acunzo, M. Non-Coding RNAs in Cancer Diagnosis and Therapy: Focus on Lung Cancer. Cancers 2021, 13, 1372. [Google Scholar] [CrossRef]
- Drula, R.; Braicu, C.; Harangus, A.; Nabavi, S.M.; Trif, M.; Slaby, O.; Ionescu, C.; Irimie, A.; Berindan-Neagoe, I. Critical function of circular RNAs in lung cancer. Wiley Interdiscip. Rev. RNA 2020, 11, e1592. [Google Scholar] [CrossRef]
- Statello, L.; Guo, C.J.; Chen, L.L.; Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef] [PubMed]
- Aliperti, V.; Skonieczna, J.; Cerase, A. Long Non-Coding RNA (lncRNA) Roles in Cell Biology, Neurodevelopment and Neurological Disorders. Noncoding RNA 2021, 7, 36. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Cao, J.; Chen, L.; Xi, X.; Wang, S.; Zhu, Y.; Yang, L.; Ma, L.; Wang, D.; Yin, J.; et al. Noncoding RNAs Serve as Diagnosis and Prognosis Biomarkers for Hepatocellular Carcinoma. Clin. Chem. 2019, 65, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Gutschner, T.; Richtig, G.; Haemmerle, M.; Pichler, M. From biomarkers to therapeutic targets-the promises and perils of long non-coding RNAs in cancer. Cancer Metastasis Rev. 2018, 37, 83–105. [Google Scholar] [CrossRef] [PubMed]
- Guglas, K.; Kozłowska-Masłoń, J.; Kolenda, T.; Paszkowska, A.; Teresiak, A.; Bliźniak, R.; Lamperska, K. Midsize noncoding RNAs in cancers: A new division that clarifies the world of noncoding RNA or an unnecessary chaos? Rep. Pract. Oncol. Radiother. 2022, 27, 1077–1093. [Google Scholar] [CrossRef]
- Galamb, O.; Barták, B.K.; Kalmár, A.; Nagy, Z.B.; Szigeti, K.A.; Tulassay, Z.; Igaz, P.; Molnár, B. Diagnostic and prognostic potential of tissue and circulating long non-coding RNAs in colorectal tumors. World J. Gastroenterol. 2019, 25, 5026–5048. [Google Scholar] [CrossRef]
- Xie, S.; Wu, Z.; Qi, Y.; Wu, B.; Zhu, X. The metastasizing mechanisms of lung cancer: Recent advances and therapeutic challenges. Biomed. Pharmacother. 2021, 138, 111450. [Google Scholar] [CrossRef]
- Rapisuwon, S.; Vietsch, E.E.; Wellstein, A. Circulating biomarkers to monitor cancer progression and treatment. Comput. Struct. Biotechnol. J. 2016, 14, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Sonea, L.; Buse, M.; Gulei, D.; Onaciu, A.; Simon, I.; Braicu, C.; Berindan-Neagoe, I. Decoding the Emerging Patterns Exhibited in Non-coding RNAs Characteristic of Lung Cancer with Regard to their Clinical Significance. Curr. Genom. 2018, 19, 258–278. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, L.; Feng, B.; Han, S.; Cui, S.; Chu, X.; Chen, L.; Wang, R. Non-coding RNAs as emerging regulators of epithelial to mesenchymal transition in non-small cell lung cancer. Oncotarget 2017, 8, 36787–36799. [Google Scholar] [CrossRef]
- Kiełbowski, K.; Ptaszyński, K.; Wójcik, J.; Wojtyś, M.E. The role of selected non-coding RNAs in the biology of non-small cell lung cancer. Adv. Med. Sci. 2023, 68, 121–137. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Qin, T.; Qi, T. Precision Medicine in Lung Cancer Theranostics: Paving the Way from Traditional Technology to Advance Era. Cancer Control 2022, 29, 1–8. [Google Scholar] [CrossRef]
- Ricciuti, B.; Mecca, C.; Crinò, L.; Baglivo, S.; Cenci, M.; Metro, G. Non-coding RNAs in lung cancer. Oncoscience 2014, 1, 674–705. [Google Scholar] [CrossRef] [PubMed]
- Walter, N.G. Are non-protein coding RNAs junk or treasure?: An attempt to explain and reconcile opposing viewpoints of whether the human genome is mostly transcribed into non-functional or functional RNAs. Bioessays 2024, 46, e2300201. [Google Scholar] [CrossRef]
- Ortholan, C.; Puissegur, M.P.; Ilie, M.; Barbry, P.; Mari, B.; Hofman, P. MicroRNAs and lung cancer: New oncogenes and tumor suppressors, new prognostic factors and potential therapeutic targets. Curr. Med. Chem. 2009, 16, 1047–1061. [Google Scholar] [CrossRef] [PubMed]
- Tiedt, S.; Dichgans, M. Role of Non-Coding RNAs in Stroke. Stroke 2018, 49, 3098–3106. [Google Scholar] [CrossRef]
- Tüncel, Ö.; Kara, M.; Yaylak, B.; Erdoğan, İ.; Akgül, B. Noncoding RNAs in apoptosis: Identification and function. Turk. J. Biol. 2022, 46, 1. [Google Scholar]
- Takaaki, T.; Yusuke, Y.; Shinichi, S.; Tomohiko, I.; Takahiro, O. Extracellular vesicles as a promising biomarker resource in liquid biopsy for cancer. Extracell. Vesicles Circ. Nucleic Acids 2021, 2, 148–174. [Google Scholar]
- Chakraborty, S.; Hosen, M.I.; Ahmed, M.; Shekhar, H.U. Onco-Multi-OMICS Approach: A New Frontier in Cancer Research. BioMed Res. Int. 2018, 2018, 9836256. [Google Scholar] [CrossRef]
- Tatischeff, I. Current Search through Liquid Biopsy of Effective Biomarkers for Early Cancer Diagnosis into the Rich Cargoes of Extracellular Vesicles. Int. J. Mol. Sci. 2021, 22, 5674. [Google Scholar] [CrossRef]
- Ishola, A.A.; La’ah, A.S.; Le, H.D.; Nguyen, V.Q.; Yang, Y.P.; Chou, S.J.; Tai, H.Y.; Chien, C.S.; Wang, M.L. Non-coding RNA and lung cancer progression. J. Chin. Med. Assoc. 2020, 83, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Gil, N.; Ulitsky, I. Regulation of gene expression by cis-acting long non-coding RNAs. Nat. Rev. Genet. 2020, 21, 102–117. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, J.C.; Oliveira, L.C.; Mathias, C.; Pedroso, G.A.; Lemos, D.S.; Salviano-Silva, A.; Jucoski, T.S.; Lobo-Alves, S.C.; Zambalde, E.P.; Cipolla, G.A.; et al. Long non-coding RNAs in cancer: Another layer of complexity. J. Gene Med. 2019, 21, e3065. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, J.; Wasson, M.D.; Brown, J.M.; Fernando, W.; Marcato, P. LncRNA-miRNA axes in breast cancer: Novel points of interaction for strategic attack. Cancer Lett. 2021, 509, 81–88. [Google Scholar] [CrossRef]
- Yang, J.; Qiu, Q.; Qian, X.; Yi, J.; Jiao, Y.; Yu, M.; Li, X.; Li, J.; Mi, C.; Zhang, J.; et al. Long noncoding RNA LCAT1 functions as a ceRNA to regulate RAC1 function by sponging miR-4715-5p in lung cancer. Mol. Cancer 2019, 18, 171. [Google Scholar] [CrossRef]
- Mondal, P.; Meeran, S.M. Long non-coding RNAs in breast cancer metastasis. Noncoding RNA Res. 2020, 5, 208–218. [Google Scholar] [CrossRef]
- Loaeza-Loaeza, J.; Beltran, A.S.; Hernández-Sotelo, D. DNMTs and Impact of CpG Content, Transcription Factors, Consensus Motifs, lncRNAs, and Histone Marks on DNA Methylation. Genes 2020, 11, 1336. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.J.D.; Man, J.H.S.; Schatz, J.H.; Marsden, P.A. Translational remodeling by RNA-binding proteins and noncoding RNAs. Wiley Interdiscip. Rev. RNA 2021, 12, e1647. [Google Scholar] [CrossRef]
- Buccitelli, C.; Selbach, M. mRNAs, proteins and the emerging principles of gene expression control. Nat. Rev. Genet. 2020, 21, 630–644. [Google Scholar] [CrossRef]
- Zhang, X.Z.; Liu, H.; Chen, S.R. Mechanisms of Long Non-Coding RNAs in Cancers and Their Dynamic Regulations. Cancers 2020, 12, 1245. [Google Scholar] [CrossRef]
- Taniue, K.; Akimitsu, N. The Functions and Unique Features of LncRNAs in Cancer Development and Tumorigenesis. Int. J. Mol. Sci. 2021, 22, 632. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Baker, D.; Ten Dijke, P. TGF-β-Mediated Epithelial-Mesenchymal Transition and Cancer Metastasis. Int. J. Mol. Sci. 2019, 20, 2767. [Google Scholar] [CrossRef] [PubMed]
- Hirose, T.; Yamazaki, T.; Nakagawa, S. Molecular anatomy of the architectural NEAT1 noncoding RNA: The domains, interactors, and biogenesis pathway required to build phase-separated nuclear paraspeckles. Wiley Interdiscip. Rev. RNA 2019, 10, e1545. [Google Scholar] [CrossRef] [PubMed]
- Rinn, J.L.; Chang, H.Y. Long Noncoding RNAs: Molecular Modalities to Organismal Functions. Annu. Rev. Biochem. 2020, 89, 283–308. [Google Scholar] [CrossRef]
- Zhao, Y.; Teng, H.; Yao, F.; Yap, S.; Sun, Y.; Ma, L. Challenges and Strategies in Ascribing Functions to Long Noncoding RNAs. Cancers 2020, 12, 1458. [Google Scholar] [CrossRef]
- Juneja, T.; Shah, S. MicroRNAs and Noncoding RNAs as Gene Regulators and Potential Therapeutic Agents. In Breast Cancer: From Bench to Personalized Medicine; Shakil Malik, S., Masood, N., Eds.; Springer Nature: Singapore, 2022; pp. 213–234. [Google Scholar]
- Miruna, G.; Robert, K.; Franziska, A.; Summer, W.; Holly, C.; Martin, B.; Vitaly, V.; Jiling, Z.; Siegfried, H.; Alexander, K. Multiple Genetic Polymorphisms within microRNA Targets and Homologous microRNA-Binding Sites: Two More Factors Influencing microRNA-Mediated Regulation of Gene Expression. In Advances in Genetic Polymorphisms; Nouha Bouayed, A., Balkiss, A., Eds.; IntechOpen: Rijeka, Croatia, 2023; Chapter 6. [Google Scholar]
- Roos, D.; de Boer, M. Mutations in cis that affect mRNA synthesis, processing and translation. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166166. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.A.; Arora, S.; Prakasam, G.; Calin, G.A.; Syed, M.A. MicroRNA in lung cancer: Role, mechanisms, pathways and therapeutic relevance. Mol. Asp. Med. 2019, 70, 3–20. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, H.; Jiang, M.; Zhang, S.; Chen, J.; Fan, X. Targeting MicroRNA-21 Suppresses Gastric Cancer Cell Proliferation and Migration via PTEN/Akt Signaling Axis. Cell Transplant. 2019, 28, 306–317. [Google Scholar] [CrossRef]
- Matsuhashi, S.; Manirujjaman, M.; Hamajima, H.; Ozaki, I. Control Mechanisms of the Tumor Suppressor PDCD4: Expression and Functions. Int. J. Mol. Sci. 2019, 20, 2304. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.L.; Tsai, Y.M.; Lien, C.T.; Kuo, P.L.; Hung, A.J. The Roles of MicroRNA in Lung Cancer. Int. J. Mol. Sci. 2019, 20, 1611. [Google Scholar] [CrossRef]
- Fu, J.; Imani, S.; Wu, M.Y.; Wu, R.C. MicroRNA-34 Family in Cancers: Role, Mechanism, and Therapeutic Potential. Cancers 2023, 15, 4723. [Google Scholar] [CrossRef] [PubMed]
- Mehrzad, N.; Zamani, M.S.; Rahimi, A.; Shamaei, M.; Karimipoor, M. Methylation Status of miR-34a and miR-126 in Non-Small Cell Lung Cancer (NSCLC) Tumor Tissues. Iran. Biomed. J. 2024, 28, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Rokavec, M.; Li, H.; Jiang, L.; Hermeking, H. The p53/miR-34 axis in development and disease. J. Mol. Cell Biol. 2014, 6, 214–230. [Google Scholar] [CrossRef] [PubMed]
- Siemens, H.; Jackstadt, R.; Hünten, S.; Kaller, M.; Menssen, A.; Götz, U.; Hermeking, H. miR-34 and SNAIL form a double-negative feedback loop to regulate epithelial-mesenchymal transitions. Cell Cycle 2011, 10, 4256–4271. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Mazumdar, M.; Mukherjee, S.; Bhattacharjee, P.; Adhikary, A.; Manna, A.; Chakraborty, S.; Khan, P.; Sen, A.; Das, T. Restoration of p53/miR-34a regulatory axis decreases survival advantage and ensures Bax-dependent apoptosis of non-small cell lung carcinoma cells. FEBS Lett. 2014, 588, 549–559. [Google Scholar] [CrossRef]
- Feng, J.; Hu, S.; Liu, K.; Sun, G.; Zhang, Y. The Role of MicroRNA in the Regulation of Tumor Epithelial-Mesenchymal Transition. Cells 2022, 11, 1981. [Google Scholar] [CrossRef]
- Lai, X.N.; Li, J.; Tang, L.B.; Chen, W.T.; Zhang, L.; Xiong, L.X. MiRNAs and LncRNAs: Dual Roles in TGF-β Signaling-Regulated Metastasis in Lung Cancer. Int. J. Mol. Sci. 2020, 21, 1193. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, J.; Deng, X.; Xiong, F.; Zhang, S.; Gong, Z.; Li, X.; Cao, K.; Deng, H.; He, Y.; et al. The role of microenvironment in tumor angiogenesis. J. Exp. Clin. Cancer Res. 2020, 39, 204. [Google Scholar] [CrossRef]
- Zaravinos, A. The Regulatory Role of MicroRNAs in EMT and Cancer. J. Oncol. 2015, 2015, 865816. [Google Scholar] [CrossRef]
- Rupaimoole, R.; Slack, F.J. MicroRNA therapeutics: Towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discov. 2017, 16, 203–222. [Google Scholar] [CrossRef]
- Ma, K.X.; Wang, H.J.; Li, X.R.; Li, T.; Su, G.; Yang, P.; Wu, J.W. Long noncoding RNA MALAT1 associates with the malignant status and poor prognosis in glioma. Tumour Biol. 2015, 36, 3355–3359. [Google Scholar] [CrossRef] [PubMed]
- Sufianov, A.; Begliarzade, S.; Beilerli, A.; Liang, Y.; Ilyasova, T.; Beylerli, O. Circular RNAs as biomarkers for lung cancer. Non-Coding RNA Res. 2023, 8, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Rong, Y.; Tang, X.; Yi, K.; Wu, J.; Wang, F. Circular RNAs Are Promising Biomarkers in Liquid Biopsy for the Diagnosis of Non-small Cell Lung Cancer. Front. Mol. Biosci. 2021, 8, 625722. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Li, Y.; Zhao, F.; Zhou, L.; Jia, R. Circular RNA Foxo3: A Promising Cancer-Associated Biomarker. Front. Genet. 2021, 12, 652995. [Google Scholar] [CrossRef]
- Rao, D.; Yu, C.; Sheng, J.; Lv, E.; Huang, W. The Emerging Roles of circFOXO3 in Cancer. Front. Cell Dev. Biol. 2021, 9, 659417. [Google Scholar] [CrossRef] [PubMed]
- Verduci, L.; Tarcitano, E.; Strano, S.; Yarden, Y.; Blandino, G. CircRNAs: Role in human diseases and potential use as biomarkers. Cell Death Dis. 2021, 12, 468. [Google Scholar] [CrossRef]
- Qu, S.; Yang, X.; Li, X.; Wang, J.; Gao, Y.; Shang, R.; Sun, W.; Dou, K.; Li, H. Circular RNA: A new star of noncoding RNAs. Cancer Lett. 2015, 365, 141–148. [Google Scholar] [CrossRef]
- Korać, P.; Antica, M.; Matulić, M. MiR-7 in Cancer Development. Biomedicines 2021, 9, 325. [Google Scholar] [CrossRef]
- Böhmdorfer, G.; Wierzbicki, A.T. Control of Chromatin Structure by Long Noncoding RNA. Trends Cell Biol. 2015, 25, 623–632. [Google Scholar] [CrossRef]
- Long, Y.; Wang, X.; Youmans, D.T.; Cech, T.R. How do lncRNAs regulate transcription? Sci. Adv. 2017, 3, eaao2110. [Google Scholar] [CrossRef]
- Liu, M.; Sun, W.; Liu, Y.; Dong, X. The role of lncRNA MALAT1 in bone metastasis in patients with non-small cell lung cancer. Oncol. Rep. 2016, 36, 1679–1685. [Google Scholar] [CrossRef] [PubMed]
- Kulcheski, F.R.; Christoff, A.P.; Margis, R. Circular RNAs are miRNA sponges and can be used as a new class of biomarker. J. Biotechnol. 2016, 238, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y. Circular RNAs as novel biomarkers with regulatory potency in human diseases. Future Sci. OA 2018, 4, Fso314. [Google Scholar] [CrossRef] [PubMed]
- Kyei, B.; Li, L.; Yang, L.; Zhan, S.; Zhang, H. CDR1as/miRNAs-related regulatory mechanisms in muscle development and diseases. Gene 2020, 730, 144315. [Google Scholar] [CrossRef]
- Yarmishyn, A.A.; Ishola, A.A.; Chen, C.Y.; Verusingam, N.D.; Rengganaten, V.; Mustapha, H.A.; Chuang, H.K.; Teng, Y.C.; Phung, V.L.; Hsu, P.K.; et al. Circular RNAs Modulate Cancer Hallmark and Molecular Pathways to Support Cancer Progression and Metastasis. Cancers 2022, 14, 862. [Google Scholar] [CrossRef]
- Ginn, L.; Shi, L.; Montagna, M.; Garofalo, M. LncRNAs in Non-Small-Cell Lung Cancer. Noncoding RNA 2020, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Pardini, B.; Sabo, A.A.; Birolo, G.; Calin, G.A. Noncoding RNAs in Extracellular Fluids as Cancer Biomarkers: The New Frontier of Liquid Biopsies. Cancers 2019, 11, 1170. [Google Scholar] [CrossRef] [PubMed]
- Malakoti, F.; Targhazeh, N.; Karimzadeh, H.; Mohammadi, E.; Asadi, M.; Asemi, Z.; Alemi, F. Multiple function of lncRNA MALAT1 in cancer occurrence and progression. Chem. Biol. Drug Des. 2023, 101, 1113–1137. [Google Scholar] [CrossRef]
- Wang, W.; Li, X.; Liu, C.; Zhang, X.; Wu, Y.; Diao, M.; Tan, S.; Huang, S.; Cheng, Y.; You, T. MicroRNA-21 as a diagnostic and prognostic biomarker of lung cancer: A systematic review and meta-analysis. Biosci. Rep. 2022, 42, BSR20211653. [Google Scholar] [CrossRef]
- Ren, M.M.; Xu, S.; Wei, Y.B.; Yang, J.J.; Yang, Y.N.; Sun, S.S.; Li, Y.J.; Wang, P.Y.; Xie, S.Y. Roles of HOTAIR in lung cancer susceptibility and prognosis. Mol. Genet. Genom. Med. 2020, 8, e1299. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.T.; Zhao, S.H.; Liu, Q.P.; Lv, M.Q.; Zhou, D.X.; Liao, Z.J.; Nan, K.J. Over-expression of CircRNA_100876 in non-small cell lung cancer and its prognostic value. Pathol. Res. Pract. 2017, 213, 453–456. [Google Scholar] [CrossRef] [PubMed]
- Beylerli, O.; Gareev, I.; Sufianov, A.; Ilyasova, T.; Guang, Y. Long noncoding RNAs as promising biomarkers in cancer. Noncoding RNA Res. 2022, 7, 66–70. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, L.; Li, Y.; Wu, Y.; Li, X.; Wu, X. Four long noncoding RNAs act as biomarkers in lung adenocarcinoma. Open Med. 2021, 16, 660–671. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Yu, L.; Yan, H.; Tang, S.; Wang, Z.; Dai, T.; Chen, H.; Zhang, S.; Hu, H.; Liu, T.; et al. LncRNAs in non-small cell lung cancer: Novel diagnostic and prognostic biomarkers. Front. Mol. Biosci. 2023, 10, 1297198. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Meng, X.; Mi, H.; Chi, Y.; Li, S.; Jin, Z.; Tian, H.; He, J.; Shen, W.; Tian, H.; et al. Circulating lncRNA XLOC_009167 serves as a diagnostic biomarker to predict lung cancer. Clin. Chim. Acta 2018, 486, 26–33. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Lyu, C.; Buchner, A.; Pohla, H. Diagnostic and Prognostic Role of miR-192 in Different Cancers: A Systematic Review and Meta-Analysis. BioMed Res. Int. 2021, 2021, 8851035. [Google Scholar] [CrossRef]
- Jia, E.; Ren, N.; Zhang, R.; Zhou, C.; Xue, J. Circulating miR-17 as a promising diagnostic biomarker for lung adenocarcinoma: Evidence from the Gene Expression Omnibus. Transl. Cancer Res. 2020, 9, 5544–5554. [Google Scholar] [CrossRef]
- Li, C.; Sun, L.; Zhou, H.; Yang, Y.; Wang, Y.; She, M.; Chen, J. Diagnostic value of microRNA-25 in patients with non-small cell lung cancer in Chinese population: A systematic review and meta-analysis. Medicine 2020, 99, e23425. [Google Scholar] [CrossRef]
- Hao, Q.; Zhou, X. The emerging role of long noncoding RNA RMRP in cancer development and targeted therapy. Cancer Biol. Med. 2022, 19, 140–146. [Google Scholar] [CrossRef]
- Hussain, M.S.; Afzal, O.; Gupta, G.; Goyal, A.; Almalki, W.H.; Kazmi, I.; Alzarea, S.I.; Alfawaz Altamimi, A.S.; Kukreti, N.; Chakraborty, A.; et al. Unraveling NEAT1’s complex role in lung cancer biology: A comprehensive review. EXCLI J. 2024, 23, 34–52. [Google Scholar]
- Smolarz, M.; Widlak, P. Serum Exosomes and Their miRNA Load-A Potential Biomarker of Lung Cancer. Cancers 2021, 13, 1373. [Google Scholar] [CrossRef]
- Markou, A.; Zavridou, M.; Lianidou, E.S. MicroRNA signatures as clinical biomarkers in lung cancer. Curr. Biomark. Find. 2015, 5, 35–45. [Google Scholar]
- Wozniak, M.B.; Scelo, G.; Muller, D.C.; Mukeria, A.; Zaridze, D.; Brennan, P. Circulating MicroRNAs as Non-Invasive Biomarkers for Early Detection of Non-Small-Cell Lung Cancer. PLoS ONE 2015, 10, e0125026. [Google Scholar] [CrossRef] [PubMed]
- Cheong, J.K.; Tang, Y.C.; Zhou, L.; Cheng, H.; Too, H.P. Advances in quantifying circulatory microRNA for early disease detection. Curr. Opin. Biotechnol. 2022, 74, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Mohandas, S.; Jagannathan, P.; Henrich, T.J.; Sherif, Z.A.; Bime, C.; Quinlan, E.; Portman, M.A.; Gennaro, M.; Rehman, J. Immune mechanisms underlying COVID-19 pathology and post-acute sequelae of SARS-CoV-2 infection (PASC). eLife 2023, 12, e86014. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Huang, G.; Zhang, J.; Zhang, L.; Liang, Z. Prognostic and clinicopathological significance of long noncoding RNA MALAT-1 expression in patients with non-small cell lung cancer: A meta-analysis. PLoS ONE 2020, 15, e0240321. [Google Scholar] [CrossRef]
- Tong, Y.S.; Wang, X.W.; Zhou, X.L.; Liu, Z.H.; Yang, T.X.; Shi, W.H.; Xie, H.W.; Lv, J.; Wu, Q.Q.; Cao, X.F. Identification of the long non-coding RNA POU3F3 in plasma as a novel biomarker for diagnosis of esophageal squamous cell carcinoma. Mol. Cancer 2015, 14, 3. [Google Scholar] [CrossRef]
- Schmidt, L.H.; Spieker, T.; Koschmieder, S.; Schäffers, S.; Humberg, J.; Jungen, D.; Bulk, E.; Hascher, A.; Wittmer, D.; Marra, A.; et al. The long noncoding MALAT-1 RNA indicates a poor prognosis in non-small cell lung cancer and induces migration and tumor growth. J. Thorac. Oncol. 2011, 6, 1984–1992. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, T.; Xiao, J. Circular RNAs: Promising Biomarkers for Human Diseases. EBioMedicine 2018, 34, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lou, C.; Ma, X.; Zhou, C.; Zhao, X.; Li, N.; Tian, H.; Meng, X. Serum exosomal hsa_circ_0069313 has a potential to diagnose more aggressive non-small cell lung cancer. Clin. Biochem. 2022, 102, 56–64. [Google Scholar] [CrossRef]
- Roy, S.; Kanda, M.; Nomura, S.; Zhu, Z.; Toiyama, Y.; Taketomi, A.; Goldenring, J.; Baba, H.; Kodera, Y.; Goel, A. Diagnostic efficacy of circular RNAs as noninvasive, liquid biopsy biomarkers for early detection of gastric cancer. Mol. Cancer 2022, 21, 42. [Google Scholar] [CrossRef]
- de Fraipont, F.; Gazzeri, S.; Cho, W.C.; Eymin, B. Circular RNAs and RNA Splice Variants as Biomarkers for Prognosis and Therapeutic Response in the Liquid Biopsies of Lung Cancer Patients. Front. Genet. 2019, 10, 390. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Tian, W.; Wang, S.; Ji, X.; Zhou, B. CircRNAs as promising biomarker in diagnostic and prognostic of lung cancer: An updated meta-analysis. Genomics 2021, 113, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Townson, L.J.; Naumov, N.G.; Chambers, F.A. The Role of Apoptosis in Tumor Progression and Metastasis. Curr. Mol. Med. 2003, 3, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, X.; Wei, F.; Zhang, X.; Yu, J.; Zhao, H.; Sun, Q.; Yan, F.; Yan, C.; Li, H.; et al. Diagnostic and prognostic value of circulating miR-21 for cancer: A systematic review and meta-analysis. Gene 2014, 533, 389–397. [Google Scholar] [CrossRef]
- Wu, Z.H.; Wang, X.L.; Tang, H.M.; Jiang, T.; Chen, J.; Lu, S.; Qiu, G.Q.; Peng, Z.H.; Yan, D.W. Long non-coding RNA HOTAIR is a powerful predictor of metastasis and poor prognosis and is associated with epithelial-mesenchymal transition in colon cancer. Oncol. Rep. 2014, 32, 395–402. [Google Scholar] [CrossRef]
- Bhat, A.A.; Younes, S.N.; Raza, S.S.; Zarif, L.; Nisar, S.; Ahmed, I.; Mir, R.; Kumar, S.; Sharawat, S.K.; Hashem, S.; et al. Role of non-coding RNA networks in leukemia progression, metastasis and drug resistance. Mol. Cancer 2020, 19, 57. [Google Scholar] [CrossRef]
- Izzotti, A.; Ceccaroli, C.; Geretto, M.; Ruggieri, F.G.; Schenone, S.; Di Maria, E. Predicting Response to Neoadjuvant Therapy in Colorectal Cancer Patients the Role of Messenger-and Micro-RNA Profiling. Cancers 2020, 12, 1652. [Google Scholar] [CrossRef] [PubMed]
- Si, L.; Tian, H.; Yue, W.; Li, L.; Li, S.; Gao, C.; Qi, L. Potential use of microRNA-200c as a prognostic marker in non-small cell lung cancer. Oncol. Lett. 2017, 14, 4325–4330. [Google Scholar] [CrossRef]
- Hakami, M.A.; Hazazi, A.; Khan, F.R.; Abdulaziz, O.; Alshaghdali, K.; Abalkhail, A.; Nassar, S.A.; Omar, B.I.A.; Almarshadi, F.; Gupta, G.; et al. PVT1 lncRNA in lung cancer: A key player in tumorigenesis and therapeutic opportunities. Pathol. Res. Pract. 2024, 253, 155019. [Google Scholar] [CrossRef]
- Ishola, A.A.; Chien, C.S.; Yang, Y.P.; Chien, Y.; Yarmishyn, A.A.; Tsai, P.H.; Chen, J.C.; Hsu, P.K.; Luo, Y.H.; Chen, Y.M.; et al. Oncogenic circRNA C190 Promotes Non-Small Cell Lung Cancer via Modulation of the EGFR/ERK Pathway. Cancer Res. 2022, 82, 75–89. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, M.; Dong, H.; Wang, M.; Cheng, Y.; Wang, S.; Ma, W.; Xu, H. Comprehensive Analysis of Acetylation-Related lncRNAs and Identified AC099850.3 as Prognostic Biomarker in Non-Small Cell Lung Cancer. J. Oncol. 2021, 2021, 4405697. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Pan, J.; Fang, S.; Zhou, C.; Tian, H.; He, J.; Shen, W.; Meng, X.; Jin, X.; Gong, Z. LncRNA DPP10-AS1 promotes malignant processes through epigenetically activating its cognate gene DPP10 and predicts poor prognosis in lung cancer patients. Cancer Biol. Med. 2021, 18, 675–692. [Google Scholar] [CrossRef]
- Liu, C.; Li, X.; Hao, Y.; Wang, F.; Cheng, Z.; Geng, H.; Geng, D. STAT1-induced upregulation of lncRNA KTN1-AS1 predicts poor prognosis and facilitates non-small cell lung cancer progression via miR-23b/DEPDC1 axis. Aging 2020, 12, 8680–8701. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; He, X.; Yao, Q.; Wang, C.; Lu, X.; Wang, R.; Miao, D. LncRNA PTTG3P promotes tumorigenesis and metastasis of NSCLC by binding with ILF3 to maintain mRNA stability and form a positive feedback loop with E2F1. Int. J. Biol. Sci. 2023, 19, 4291–4310. [Google Scholar] [CrossRef] [PubMed]
- Abd El Fattah, Y.K.; Abulsoud, A.I.; AbdelHamid, S.G.; Hamdy, N.M. Interactome battling of lncRNA CCDC144NL-AS1: Its role in the emergence and ferocity of cancer and beyond. Int. J. Biol. Macromol. 2022, 222, 1676–1687. [Google Scholar] [CrossRef]
- Liu, W.; Wan, Q.; Zhou, E.; He, P.; Tang, L. LncRNA LINC01833 is a Prognostic Biomarker and Correlates with Immune Infiltrates in Patients with Lung Adenocarcinoma by Integrated Bioinformatics Analysis. J. Oncol. 2023, 2023, 3965198. [Google Scholar] [CrossRef]
- Liang, J.; Jin, W.; Xu, H. An efficient five-lncRNA signature for lung adenocarcinoma prognosis, with AL606489.1 showing sexual dimorphism. Front. Genet. 2022, 13, 1052092. [Google Scholar] [CrossRef]
- Zheng, Q.; Min, S.; Zhou, Q. Identification of potential diagnostic and prognostic biomarkers for LUAD based on TCGA and GEO databases. Biosci. Rep. 2021, 41, BSR20204370. [Google Scholar] [CrossRef]
- Wang, C.; Tan, S.; Li, J.; Liu, W.R.; Peng, Y.; Li, W. CircRNAs in lung cancer-Biogenesis, function and clinical implication. Cancer Lett. 2020, 492, 106–115. [Google Scholar] [CrossRef]
- Mutlu, M.; Raza, U.; Saatci, Ö.; Eyüpoğlu, E.; Yurdusev, E.; Şahin, Ö. miR-200c: A versatile watchdog in cancer progression, EMT, and drug resistance. J. Mol. Med. 2016, 94, 629–644. [Google Scholar] [CrossRef] [PubMed]
- Klicka, K.; Grzywa, T.M.; Mielniczuk, A.; Klinke, A.; Włodarski, P.K. The role of miR-200 family in the regulation of hallmarks of cancer. Front. Oncol. 2022, 12, 965231. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Zhang, F.; Guo, Y.; Huang, J.; Xie, Y.; Yue, S.; Chen, M.; Jiang, H.; Li, M. miR-200c enhances sensitivity of drug-resistant non-small cell lung cancer to gefitinib by suppression of PI3K/Akt signaling pathway and inhibites cell migration via targeting ZEB1. Biomed. Pharmacother. 2017, 85, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Slaby, O.; Laga, R.; Sedlacek, O. Therapeutic targeting of non-coding RNAs in cancer. Biochem. J. 2017, 474, 4219–4251. [Google Scholar] [CrossRef]
- He, J.; Wu, F.; Han, Z.; Hu, M.; Lin, W.; Li, Y.; Cao, M. Biomarkers (mRNAs and Non-Coding RNAs) for the Diagnosis and Prognosis of Colorectal Cancer-From the Body Fluid to Tissue Level. Front. Oncol. 2021, 11, 632834. [Google Scholar] [CrossRef] [PubMed]
- Lone, S.N.; Nisar, S.; Masoodi, T.; Singh, M.; Rizwan, A.; Hashem, S.; El-Rifai, W.; Bedognetti, D.; Batra, S.K.; Haris, M.; et al. Liquid biopsy: A step closer to transform diagnosis, prognosis and future of cancer treatments. Mol. Cancer 2022, 21, 79. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Sun, G. MiRNA and Potential Prognostic Value in Non-Smoking Females with Lung Adenocarcinoma by High-Throughput Sequencing. Int. J. Gen. Med. 2023, 16, 683–696. [Google Scholar] [CrossRef] [PubMed]
- Piergentili, R.; Basile, G.; Nocella, C.; Carnevale, R.; Marinelli, E.; Patrone, R.; Zaami, S. Using ncRNAs as Tools in Cancer Diagnosis and Treatment-The Way towards Personalized Medicine to Improve Patients’ Health. Int. J. Mol. Sci. 2022, 23, 9353. [Google Scholar] [CrossRef] [PubMed]
- Grillone, K.; Caridà, G.; Luciano, F.; Cordua, A.; Di Martino, M.T.; Tagliaferri, P.; Tassone, P. A systematic review of non-coding RNA therapeutics in early clinical trials: A new perspective against cancer. J. Transl. Med. 2024, 22, 731. [Google Scholar] [CrossRef]
- Tomar, D.; Yadav, A.S.; Kumar, D.; Bhadauriya, G.; Kundu, G.C. Non-coding RNAs as potential therapeutic targets in breast cancer. Biochim. Biophys. Acta Gene Regul. Mech. 2020, 1863, 194378. [Google Scholar] [CrossRef]
- Rönnau, C.G.; Verhaegh, G.W.; Luna-Velez, M.V.; Schalken, J.A. Noncoding RNAs as novel biomarkers in prostate cancer. BioMed Res. Int. 2014, 2014, 591703. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.P.; Diallo, A.; Cruceanu, C.; Fiori, L.M.; Laboissiere, S.; Guillet, I.; Fontaine, J.; Ragoussis, J.; Benes, V.; Turecki, G.; et al. Biomarker discovery: Quantification of microRNAs and other small non-coding RNAs using next generation sequencing. BMC Med. Genom. 2015, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Guerrero, A.; Kelnar, K.; Peltier, H.J.; Bader, A.G. Synergy between next generation EGFR tyrosine kinase inhibitors and miR-34a in the inhibition of non-small cell lung cancer. Lung Cancer 2017, 108, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhu, X.; Pu, C.; Song, X. Upregulated plasmacytoma variant translocation 1 promotes cell proliferation, invasion and metastasis in colorectal cancer. Mol. Med. Rep. 2018, 17, 6598–6604. [Google Scholar] [CrossRef] [PubMed]
- Onagoruwa, O.T.; Pal, G.; Ochu, C.; Ogunwobi, O.O. Oncogenic Role of PVT1 and Therapeutic Implications. Front. Oncol. 2020, 10, 17. [Google Scholar] [CrossRef]
- Derderian, C.; Orunmuyi, A.T.; Olapade-Olaopa, E.O.; Ogunwobi, O.O. PVT1 Signaling Is a Mediator of Cancer Progression. Front. Oncol. 2019, 9, 502. [Google Scholar] [CrossRef]
- Bohosova, J.; Kubickova, A.; Slaby, O. lncRNA PVT1 in the Pathogenesis and Clinical Management of Renal Cell Carcinoma. Biomolecules 2021, 11, 664. [Google Scholar] [CrossRef]
- Hu, J.; Gao, W. Long noncoding RNA PVT1 promotes tumour progression via the miR-128/ZEB1 axis and predicts poor prognosis in esophageal cancer. Clin. Res. Hepatol. Gastroenterol. 2021, 45, 101701. [Google Scholar] [CrossRef]
- Ding, C.; Yang, Z.; Lv, Z.; Du, C.; Xiao, H.; Peng, C.; Cheng, S.; Xie, H.; Zhou, L.; Wu, J.; et al. Long non-coding RNA PVT1 is associated with tumor progression and predicts recurrence in hepatocellular carcinoma patients. Oncol. Lett. 2015, 9, 955–963. [Google Scholar] [CrossRef]
- Li, M.Y.; Tang, X.H.; Fu, Y.; Wang, T.J.; Zhu, J.M. Regulatory Mechanisms and Clinical Applications of the Long Non-coding RNA PVT1 in Cancer Treatment. Front. Oncol. 2019, 9, 787. [Google Scholar] [CrossRef]
- Wang, X.; Hui, S.; Tan, C.; Deng, Z.; Wang, X.; Weng, W.; Zhang, M.; Ni, S.; Wang, L.; Huang, D.; et al. Comprehensive analysis of immune subtypes reveals the prognostic value of cytotoxicity and FAP(+) fibroblasts in stomach adenocarcinoma. Cancer Immunol. Immunother. 2023, 72, 1763–1778. [Google Scholar] [CrossRef] [PubMed]
- Houshmand, M.; Yazdi, N.; Kazemi, A.; Atashi, A.; Hamidieh, A.A.; Anjam Najemdini, A.; Mohammadi Pour, M.; Nikougoftar Zarif, M. Long non-coding RNA PVT1 as a novel candidate for targeted therapy in hematologic malignancies. Int. J. Biochem. Cell Biol. 2018, 98, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Zhang, M.; Nie, F.; Huang, Z.; He, J.; Li, W.; Han, L. Transcriptome analysis of EGFR tyrosine kinase inhibitors resistance associated long noncoding RNA in non-small cell lung cancer. Biomed. Pharmacother. 2017, 87, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Minguet, J.; Smith, K.H.; Bramlage, P. Targeted therapies for treatment of non-small cell lung cancer--Recent advances and future perspectives. Int. J. Cancer 2016, 138, 2549–2561. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Franchina, T.; Ricciardi, G.; Battaglia, A.; Picciotto, M.; Adamo, V. Heterogeneous Responses to Epidermal Growth Factor Receptor (EGFR) Tyrosine Kinase Inhibitors (TKIs) in Patients with Uncommon EGFR Mutations: New Insights and Future Perspectives in this Complex Clinical Scenario. Int. J. Mol. Sci. 2019, 20, 1431. [Google Scholar] [CrossRef]
- Cheema, P.K.; Gomes, M.; Banerji, S.; Joubert, P.; Leighl, N.B.; Melosky, B.; Sheffield, B.S.; Stockley, T.; Ionescu, D.N. Consensus recommendations for optimizing biomarker testing to identify and treat advanced EGFR-mutated non-small-cell lung cancer. Curr. Oncol. 2020, 27, 321–329. [Google Scholar] [CrossRef]
- Jänne, P.A. Challenges of detecting EGFR T790M in gefitinib/erlotinib-resistant tumours. Lung Cancer 2008, 60, S3–S9. [Google Scholar] [CrossRef]
- Wu, L.; Ke, L.; Zhang, Z.; Yu, J.; Meng, X. Development of EGFR TKIs and Options to Manage Resistance of Third-Generation EGFR TKI Osimertinib: Conventional Ways and Immune Checkpoint Inhibitors. Front. Oncol. 2020, 10, 602762. [Google Scholar] [CrossRef]
- Heery, R.; Finn, S.P.; Cuffe, S.; Gray, S.G. Long Non-Coding RNAs: Key Regulators of Epithelial-Mesenchymal Transition, Tumour Drug Resistance and Cancer Stem Cells. Cancers 2017, 9, 38. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, M.; Deng, Y. Long Noncoding RNAs in Taxane Resistance of Breast Cancer. Int. J. Mol. Sci. 2023, 24, 12253. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Y.; Yuan, S.; Wen, F.; Liu, J.; Zou, L.; Zhang, J. Regulatory role of long non-coding RNA UCA1 in signaling pathways and its clinical applications. Oncol. Lett. 2021, 21, 404. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Sun, X.; Jiang, X. UCA1 involved in the metformin-regulated bladder cancer cell proliferation and glycolysis. Tumour Biol. 2017, 39, 1010428317710823. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Yan, S.; Wang, M.; Jiang, L.; Ma, P.; Lu, B.; Chen, Q.; Wei, C.; Wang, Z. LncRNA UCA1 Induces Acquired Resistance to Gefitinib by Epigenetically Silencing CDKN1A Expression in Non-small-Cell Lung Cancer. Front. Oncol. 2020, 10, 656. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, Y.; Fan, M.; Ren, H.; Chen, M.; Shi, P. Identification of the ferroptosis-related long non-coding RNAs signature to improve the prognosis prediction and immunotherapy response in patients with NSCLC. BMC Med. Genom. 2021, 14, 286. [Google Scholar] [CrossRef]
- Wang, H.; Guan, Z.; He, K.; Qian, J.; Cao, J.; Teng, L. LncRNA UCA1 in anti-cancer drug resistance. Oncotarget 2017, 8, 64638–64650. [Google Scholar] [CrossRef]
- Florczuk, M.; Szpechcinski, A.; Chorostowska-Wynimko, J. miRNAs as Biomarkers and Therapeutic Targets in Non-Small Cell Lung Cancer: Current Perspectives. Target. Oncol. 2017, 12, 179–200. [Google Scholar] [CrossRef]
- Zhang, X.; Yao, J.; Guo, K.; Huang, H.; Huai, S.; Ye, R.; Niu, B.; Ji, T.; Han, W.; Li, J. The functional mechanism of miR-125b in gastric cancer and its effect on the chemosensitivity of cisplatin. Oncotarget 2018, 9, 2105–2119. [Google Scholar] [CrossRef]
- MacDonagh, L.; Gray, S.G.; Finn, S.P.; Cuffe, S.; O’Byrne, K.J.; Barr, M.P. The emerging role of microRNAs in resistance to lung cancer treatments. Cancer Treat. Rev. 2015, 41, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zheng, J.; Chen, L.; Diao, H.; Liu, Y. Predictive and Prognostic Roles of Abnormal Expression of Tissue miR-125b, miR-221, and miR-222 in Glioma. Mol. Neurobiol. 2016, 53, 577–583. [Google Scholar] [CrossRef]
- Zhao, A.; Zeng, Q.; Xie, X.; Zhou, J.; Yue, W.; Li, Y.; Pei, X. MicroRNA-125b induces cancer cell apoptosis through suppression of Bcl-2 expression. J. Genet. Genom. 2012, 39, 29–35. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, G.; Jiang, Y. The Emerging Roles of miR-125b in Cancers. Cancer Manag. Res. 2020, 12, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Theng, P.Y.; Le, M.T.N. Essential functions of miR-125b in cancer. Cell Prolif. 2021, 54, e12913. [Google Scholar] [CrossRef] [PubMed]
- Sianou, A.; Galyfos, G.; Moragianni, D.; Andromidas, P.; Kaparos, G.; Baka, S.; Kouskouni, E. The role of microRNAs in the pathogenesis of endometrial cancer: A systematic review. Arch. Gynecol. Obstet. 2015, 292, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Shahin, R.K.; Elkady, M.A.; Abulsoud, A.I.; Abdelmaksoud, N.M.; Abdel Mageed, S.S.; El-Dakroury, W.A.; Zewail, M.B.; Elazazy, M.; Sobhy, M.H.; Nomier, Y.; et al. miRNAs orchestration of gallbladder cancer-Particular emphasis on diagnosis, progression and drug resistance. Pathol. Res. Pract. 2023, 248, 154684. [Google Scholar] [CrossRef]
- Sun, Y.M.; Lin, K.Y.; Chen, Y.Q. Diverse functions of miR-125 family in different cell contexts. J. Hematol. Oncol. 2013, 6, 6. [Google Scholar] [CrossRef]
- Sur, D.; Balacescu, L.; Cainap, S.S.; Visan, S.; Pop, L.; Burz, C.; Havasi, A.; Buiga, R.; Cainap, C.; Irimie, A.; et al. Predictive Efficacy of MiR-125b-5p, MiR-17-5p, and MiR-185-5p in Liver Metastasis and Chemotherapy Response Among Advanced Stage Colorectal Cancer Patients. Front. Oncol. 2021, 11, 651380. [Google Scholar] [CrossRef]
- Lv, Y.; Lv, X.; Yang, H.; Qi, X.; Wang, X.; Li, C.; Shang, X.; Guo, H.; Zhang, J.; Zhang, Y. LncRNA SNHG6/miR-125b-5p/BMPR1B Axis: A New Therapeutic Target for Triple-Negative Breast Cancer. Front. Oncol. 2021, 11, 678474. [Google Scholar] [CrossRef]
- Wang, H.; Tan, G.; Dong, L.; Cheng, L.; Li, K.; Wang, Z.; Luo, H. Circulating MiR-125b as a marker predicting chemoresistance in breast cancer. PLoS ONE 2012, 7, e34210. [Google Scholar] [CrossRef]
- Shi, L.; Fei, X.; Wang, Z.; You, Y. PI3K inhibitor combined with miR-125b inhibitor sensitize TMZ-induced anti-glioma stem cancer effects through inactivation of Wnt/β-catenin signaling pathway. In Vitro Cell Dev. Biol. Anim. 2015, 51, 1047–1055. [Google Scholar] [CrossRef]
- Ricciuti, B.; Mencaroni, C.; Paglialunga, L.; Paciullo, F.; Crinò, L.; Chiari, R.; Metro, G. Long noncoding RNAs: New insights into non-small cell lung cancer biology, diagnosis and therapy. Med. Oncol. 2016, 33, 18. [Google Scholar] [CrossRef]
- Pant, J.; Mittal, P.; Singh, L.; Marwah, H. Evolving Strategies in NSCLC Care: Targeted Therapies, Biomarkers, Predictive Models, and Patient Management. Curr. Pharmacogenom. Pers. Med. 2023, 20, 146–164. [Google Scholar] [CrossRef]
- Yang, M.; Shen, H.; Qiu, C.; Ni, Y.; Wang, L.; Dong, W.; Liao, Y.; Du, J. High expression of miR-21 and miR-155 predicts recurrence and unfavourable survival in non-small cell lung cancer. Eur. J. Cancer 2013, 49, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Snyder, M.; Iraola-Guzmán, S.; Saus, E.; Gabaldón, T. Discovery and Validation of Clinically Relevant Long Non-Coding RNAs in Colorectal Cancer. Cancers 2022, 14, 3866. [Google Scholar] [CrossRef] [PubMed]
- Gu, P.; Zhang, L.; Wang, R.; Ding, W.; Wang, W.; Liu, Y.; Wang, W.; Li, Z.; Yan, B.; Sun, X. Development and Validation of a Novel Hypoxia-Related Long Noncoding RNA Model With Regard to Prognosis and Immune Features in Breast Cancer. Front. Cell Dev. Biol. 2021, 9, 796729. [Google Scholar] [CrossRef] [PubMed]
- Ho, D.; Quake, S.R.; McCabe, E.R.B.; Chng, W.J.; Chow, E.K.; Ding, X.; Gelb, B.D.; Ginsburg, G.S.; Hassenstab, J.; Ho, C.M.; et al. Enabling Technologies for Personalized and Precision Medicine. Trends Biotechnol. 2020, 38, 497–518. [Google Scholar] [CrossRef] [PubMed]
- Hulstaert, E.; Morlion, A.; Levanon, K.; Vandesompele, J.; Mestdagh, P. Candidate RNA biomarkers in biofluids for early diagnosis of ovarian cancer: A systematic review. Gynecol. Oncol. 2021, 160, 633–642. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, W.; Wang, J.; Ao, X.; Xue, J. Non-coding RNAs in lung cancer: Molecular mechanisms and clinical applications. Front. Oncol. 2023, 13, 1256537. [Google Scholar] [CrossRef]
- Mordente, A.; Meucci, E.; Martorana, G.E.; Silvestrini, A. Cancer Biomarkers Discovery and Validation: State of the Art, Problems and Future Perspectives. Adv. Exp. Med. Biol. 2015, 867, 9–26. [Google Scholar]
- Di Martino, M.T.; Riillo, C.; Scionti, F.; Grillone, K.; Polerà, N.; Caracciolo, D.; Arbitrio, M.; Tagliaferri, P.; Tassone, P. miRNAs and lncRNAs as Novel Therapeutic Targets to Improve Cancer Immunotherapy. Cancers 2021, 13, 1587. [Google Scholar] [CrossRef]
- Tao, H.; Yang, J.J.; Zhou, X.; Deng, Z.Y.; Shi, K.H.; Li, J. Emerging role of long noncoding RNAs in lung cancer: Current status and future prospects. Respir. Med. 2016, 110, 12–19. [Google Scholar] [CrossRef]
| ncRNA | Type | Expression in Lung Cancer | Detection Method | Diagnostic Accuracy | Source | Sample Size/Method | References |
|---|---|---|---|---|---|---|---|
| miR-21 | miRNA | Upregulated | qRT-PCR | High sensitivity and specificity | Tissue and A549 cells | Meta-analyses use quantitative reverse-transcription PCR (qRT-PCR) | [81] |
| HOTAIR | lncRNA | Upregulated | qRT-PCR | Correlates with disease stage | Tissue | 818 NSCLC patients vs. adjacent normal lung tissue samples; used quantitative real-time PCR (qRT-PCR) | [82] |
| circRNA_100876 | circRNA | Upregulated | qRT-PCR | Differential expression in plasma | Tissue | 101 pairs of NSCLC tissues compared to adjacent normal lung tissues; used quantitative real-time PCR (qRT-PCR) | [83] |
| MALAT1 | lncRNA | Upregulated | qRT-PCR | Area under the ROC curve (AUC) of 0.79 | Tumor tissues, blood/plasma, and urine | Quantitative real-time PCR (qRT-PCR), RNA sequencing, and in situ hybridization | [84] |
| SPRY4-IT1, ANRIL, and NEAT1 | lncRNA | Upregulated | qRT-PCR | AUC of 0.876; high sensitivity and specificity in NSCLC | Plasma | Detected in 50 patients and 50 volunteers using quantitative real-time PCR | [84] |
| GAS5 | lncRNA | Upregulated | qRT-PCR | AUC of 0.832; differential expression in NSCLC tissues and plasma | Tissue samples and liquid biopsies (blood/plasma) | Detected in 40 patients with advanced stage (III and IVB) lung adenocarcinoma using qRT-PCR | [84] |
| LINC00963 and DLX6-AS1 | lncRNA | Upregulated | qRT-PCR | Differential expression in plasma associated with lung adenocarcinoma (LUAD) | Plasma samples | 90 lung cancer patients + 90 healthy controls, using qRT-PCR | [85] |
| ADAMTS9-AS2 | lncRNA | Upregulated | qRT-PCR | Differential expression in plasma | Blood/plasma | Quantitative real-time PCR (qRT-PCR), RNA sequencing (RNA-seq), and microarray analysis | [86] |
| XLOC_009167 | lncRNA | Upregulated | qRT-PCR | Differentially expressed lncRNAs between tumor and normal tissues were identified | Serum | Using quantitative real-time PCR (qRT-PCR) data from the GEO database | [87] |
| miRNA-192 | miRNA | Upregulated | qRT-PCR | Contributes to the early diagnosis of NSCLC | Tissue samples, serum, and plasma | 1038 cancer patients and 938 healthy controls | [88] |
| miRNA-17 | miRNA | Upregulated | qRT-PCR | Diagnostic biomarker for lung adenocarcinoma screening | Serum | 5 microarray datasets from the Gene Expression Omnibus (GEO) database, comprising a total of 87 LUAD samples and 83 healthy controls, using quantitative real-time PCR (qRT-PCR) data | [89] |
| miR-25 | miRNA | Upregulated | qRT-PCR | Enhanced diagnostic accuracy for NSCLC in liquid biopsy settings | Serum or plasma samples | 6 studies with a total of 480 NSCLC patients and 451 healthy controls, using qRT-PCR | [90] |
| RMRP | lncRNA | Upregulated | qRT-PCR | Upregulated in CRC tissues as compared to adjacent normal tissues | Serum, plasma, and tissue | Quantitative real-time PCR (qRT-PCR), RNA sequencing (RNA-seq), and in situ hybridization | [91] |
| NEAT1 | miRNA sponge | Upregulated | qRT-PCR | Part of a 4-lncRNA panel with high diagnostic value in NSCLC | Tissue | 208 lung cancer samples, 208 non-cancer samples, quantitative real-time PCR (qRT-PCR), RNA sequencing (RNA-seq), in situ hybridization (ISH), and fluorescence in situ hybridization (FISH) | [92] |
| ncRNA | Type | Expression in Lung Cancer | Prognostic Implication | Source | Sample Size/ Detection Method | Reference |
|---|---|---|---|---|---|---|
| miR-200c | miRNA | Downregulated | Poor prognosis and chemo-resistance | Tissue and A549 cells | Meta-analyses use quantitative reverse-transcription PCR (qRT-PCR) | [111] |
| PVT1 | lncRNA | Upregulated | Poor survival rates | Tissue and cell lines | Quantitative real-time PCR (qRT-PCR), RNA sequencing (RNA-seq), and in situ hybridization (ISH) | [112] |
| circRNA_0000190 | circRNA | Upregulated | Tumor progression and poor prognosis | NSCLC tissues and cell lines | Quantitative real-time PCR (qRT-PCR), RNA sequencing (RNA-seq), and fluorescence in situ hybridization (FISH) | [113] |
| AC099850.3 | lncRNA | Upregulated | Overall survival (OS), disease-free survival (DSS), and progress-free survival (PFS) | Tissue and cell lines including COLO 320 and SK-PN-DW | Quantitative real-time PCR (qRT-PCR), RNA sequencing (RNA-seq), and in situ hybridization (ISH) | [114] |
| DPP10-AS1 | lncRNA | Upregulated | Promotes proliferation; associated with poor prognosis | Tissues and cell lines | 94 paired lung cancer tissues and adjacent normal tissues, detected using quantitative real-time PCR (qRT-PCR) and in situ hybridization (ISH) | [115] |
| KTN1-AS1 | lncRNA | Upregulated | Correlated with TNM stage, histological grade, and lymph node metastasis; high expression reduces OS | NSCLC tissues and cell lines | 90 pairs of NSCLC tissues and adjacent normal tissues, using quantitative real-time PCR (qRT-PCR) | [116] |
| PTTG3P | lncRNA | Upregulated | High expression associated with shorter OS and DFS in NSCLC patients | Cell lines including A549, H1299, PC-9, 16HBE, etc. | Using quantitative real-time PCR (qRT-PCR) in these cell lines | [117] |
| ccdc144nl-AS1 | lncRNA | Upregulated | Promotes cellular function by targeting miR-490-3p | NSCLC tissues and cell lines | 128 pairs of NSCLC tissues and paracancerous tissues, using quantitative real-time PCR (qRT-PCR) | [118] |
| AC018629.1 | lncRNA | Upregulated | Part of a four-lncRNA signature associated with overall survival in LUAD patients | Lung adenocarcinoma tissues using data from The Cancer Genome Atlas (TCGA) database | 446 LUAD patients from TCGA database using RNA sequencing data from TCGA database | [85] |
| LINC01833 | lncRNA | Upregulated | Correlates with immune infiltrates in LUAD patients | Lung adenocarcinoma tissues using bioinformatics analysis of public databases | Hundreds of LUAD samples were analyzed using RNA sequencing data from TCGA database | [119] |
| AL138789.1 | lncRNA | Upregulated | Part of a four-lncRNA signature associated with overall survival in LUAD patients | Lung adenocarcinoma tissues using data from The Cancer Genome Atlas (TCGA) database | 535 LUAD samples from TCGA database were analyzed using RNA sequencing data from TCGA database | [120] |
| AC119424.1 | lncRNA | Upregulated | Part of a four-lncRNA signature associated with overall survival in LUAD patients | LUAD and normal tissues | 535 LUAD samples and 59 normal lung samples; RNA sequencing data analysis from TCGA microarray data analysis from GEO datasets | [121] |
| AC122134.1 | lncRNA | Upregulated | Part of a four-lncRNA signature associated with overall survival in LUAD patients | Lung adenocarcinoma tissues using data from The Cancer Genome Atlas (TCGA) database | 535 LUAD samples and 59 normal lung tissue samples from TCGA database, detected using RNA sequencing data from TCGA database | [85] |
| circ_0001946 | circRNA | Upregulated | Promotes tumor progression by sponging miR-135a-5p | Tissues and cell lines | RNA sequencing (RNA-seq), quantitative real-time PCR (qRT-PCR), and microarray analysis | [122] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suri, C.; Swarnkar, S.; Bhaskar, L.; Verma, H.K. Non-Coding RNA as a Biomarker in Lung Cancer. Non-Coding RNA 2024, 10, 50. https://doi.org/10.3390/ncrna10050050
Suri C, Swarnkar S, Bhaskar L, Verma HK. Non-Coding RNA as a Biomarker in Lung Cancer. Non-Coding RNA. 2024; 10(5):50. https://doi.org/10.3390/ncrna10050050
Chicago/Turabian StyleSuri, Chahat, Shashikant Swarnkar, LVKS Bhaskar, and Henu Kumar Verma. 2024. "Non-Coding RNA as a Biomarker in Lung Cancer" Non-Coding RNA 10, no. 5: 50. https://doi.org/10.3390/ncrna10050050
APA StyleSuri, C., Swarnkar, S., Bhaskar, L., & Verma, H. K. (2024). Non-Coding RNA as a Biomarker in Lung Cancer. Non-Coding RNA, 10(5), 50. https://doi.org/10.3390/ncrna10050050








