Abstract
The scientific field of microcarrier systems has gained significant advancements, especially in drug delivery and controlled release mechanisms. This manuscript provides a comprehensive overview of the progress in developing pectin-derived microcarriers fabricated using microfluidic technology. Pectin, a naturally occurring polysaccharide, has garnered attention due to its biocompatibility, biodegradability, and ability to form hydrogels, making it an ideal candidate for forming microcarriers. The integration of microfluidic technology in synthesizing these carriers has revolutionized their design and functionality, enabling precise control over size, morphology, and encapsulation efficiency. This review systematically analyzes the methodologies employed in the microfluidic fabrication of pectin-based microparticles, highlighting the significant advantages this technology offers, such as reduced use of solvents, enhanced reproducibility, and scalability.
Keywords:
pectin; microfluidic technology; microparticles; drug delivery; emulsion; hydrogel; Janus particles 1. Introduction
Microparticles, with sizes ranging from 1 to 1000 µm, have been the focus of numerous investigations over the past decades. Consequently, a variety of applications have been developed across different fields, including the medical and pharmaceutical sectors [1,2], materials technology [3,4], food industry [5,6], and environmental science [7,8]. The biomedical sector has been one of the primary beneficiaries of advancements in microparticle technology, with notable applications in drug delivery [9], tissue engineering [10], imaging [11], and the development of biosensors [12,13]. These systems are renowned for their high surface area-to-volume ratio, capacity to transport large quantities of drugs, and ability to deliver them to specific sites within the body [14].
The synthesis of microparticles has been reported through various protocols developed to meet specific requirements regarding size, composition, and morphology tailored to the desired application [15]. Polymerization, spray drying, solvent evaporation, and self-assembly are common methodologies for the preparation of microparticles [12]. Although widely used, these protocols often fail due to producing particles with a broad size distribution, poor reproducibility, limited functionality, less tunable morphology, and difficulty scaling up [16,17]. These challenges hinder their broader applicability.
The advent of microfluidics, coinciding with the need for miniaturization and planarization in bio(chemical) analyses, has played a transformative role in producing particles with well-defined morphology [18,19,20]. The field of microfluidics focuses on manipulating fluids constrained geometrically within environments with micrometer-scale dimensions [20,21]. The ability to control fluids in channels of such small diameters allows for precise control over the size of droplets and bubbles, reduces synthesis time, and enables process automation [22,23]. Microfluidic-based microparticles used as encapsulation systems provide greater stability to active agents and improved controlled release profiles [18].
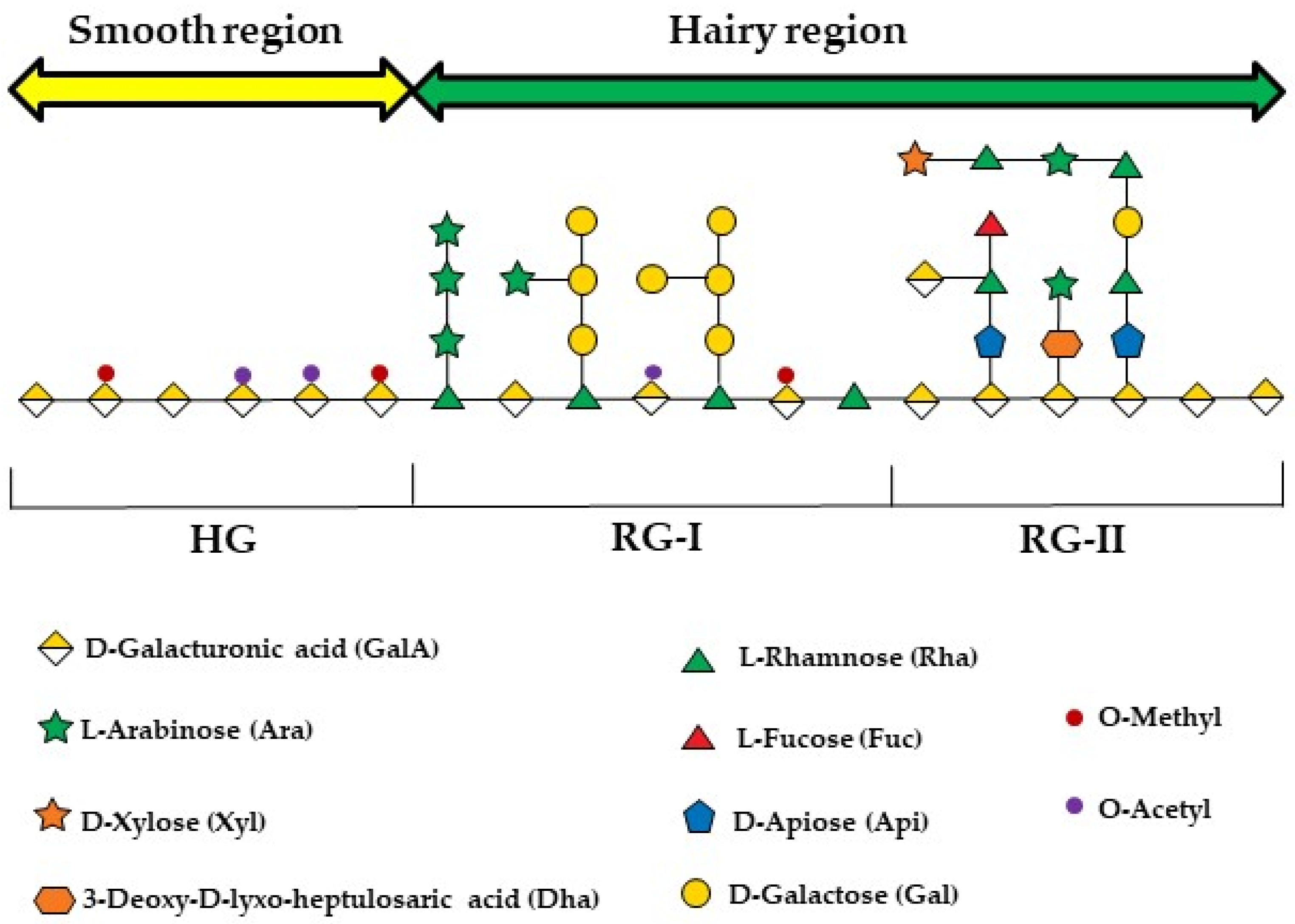
Due to their excellent biocompatibility and biodegradability, biopolymers such as polysaccharides and proteins have gained prominence in the fabrication of microparticles, especially for biomedical applications [14,24]. Pectins are a group of complex polysaccharides naturally present in plant cell walls, providing mechanical strength [25]. Its composition has three main domains: homogalacturonan (HG), rhamnogalacturonan-I (RG-I), and rhamnogalacturonan-II (RG-II). HG is an unbranched long-chain macromolecule comprising α-(1–4) glycosidically linked D-galacturonic acid (GalpA). RG-I is formed by alternating rhamnose residues on the GalpA backbone, with extended side chains that include small amounts of galactan and arabinan. RG-II consists of an HG backbone, which can be substituted with various hetero-oligomeric side chains [26,27]. When extracted, pectins become extremely useful colloids, especially due to their ability to form hydrogels, which can be exploited to fabricate structures with the potential to function as natural carriers.
This review article provides an overview of the use of pectin in the fabrication of microparticles through microfluidic techniques. First, a summary of the basic principles of microfluidics for microparticle preparation is presented. Next, the chemical structure of pectin is discussed in more detail. Finally, the applications of pectin-based microparticles in various fields are examined.
2. Fundamentals of Microfluidic Technology
The study of microfluidics focuses on manipulating fluids on a submillimeter scale (10−9 to 10−18 L) using channels that range in size from tens to hundreds of micrometers [28]. The miniaturization of processes offers several advantages over larger-scale operations, such as reduced waste of samples and reagents required; lower costs and more efficient operations; more efficient mass and energy transfer, which occurs due to the increased specific surface area of the fluids; simultaneous operations that can be performed on the same chip; and the isolation of the sample from the external environment, which reduces the chances of contamination and degradation of the samples [19,29].
In this environment, the relative effects of gravitational force are significantly reduced compared to larger scales, while the forces arising from surface tension and capillarity become dominant [20]. Due to the reduced scale of the channels, the fluid tends to exhibit laminar flow. In laminar flow, the velocity of the fluid particles is not a random function of time, characterized by a smooth and regular flow of the fluids [30]. An immediate consequence of this phenomenon in microfluidics is that, with few exceptions, two or more streams of fluid in contact with each other will not mix, except by diffusion [30]. This fact allows for a didactic division of microfluidic applications for particle production based on the employed mechanism: the first is based on the diffusion–nucleation–growth mechanism, especially through nanoprecipitation and self-assembly processes, and is commonly used for the production of nanoparticles (entities with diameters ranging from 1 to 100 nanometers); the second is based on immiscible liquid/liquid systems, consisting of two or more immiscible phases, commonly employing multiphase flow microfluidic devices, primarily for the generation of microparticles (entities with diameters ranging from 1 to 1000 μm) and/or droplets [23,31]. Subsequently, detailed information on the production of microparticles using microfluidic devices will be provided. For a more comprehensive understanding of nanoparticle fabrication, please consult the recommended literature [23,31].
A system consisting of two immiscible phases, where one phase (the dispersed phase) is dispersed as small droplets in another phase (the continuous phase), is called an emulsion [32]. The two phases often consist of an oily and aqueous phase [33]. The conventional approach to emulsion production is primarily based on the application of shear or impact stresses, such as high-speed mixers and ultrasound, which commonly results in the generation of emulsions with highly polydisperse droplet sizes due to the non-uniformity of the applied stress [17]. In contrast, microfluidic devices enable precise control over droplet generation, resulting in highly monodisperse emulsions with a coefficient of variation (CV) typically lower than 5% [22,34]. The origin of the energy responsible for breaking the dispersed phase into droplets can stem from the hydrodynamic pressure of the flow itself, without external input, as in passive microfluidic devices. On the other hand, when external energy is locally added to the droplet formation process, the devices have active control [35]. Depending on the nature of the external energy input, active techniques can be categorized into electrical, magnetic, centrifugal, optical, thermal, and mechanical controls [35,36]. Adding energy to the system improves mixing performance and reduces the time for specific substances to aggregate or precipitate [37].
Certain dimensionless numbers can be employed to understand the complex phenomenon of droplet breakup in microfluidic systems, which are related to the properties of the fluids, flow conditions, and the geometry/design/material of the channels [17,24]. The dimensionless numbers derive from the forces acting within the medium, namely, inertial force, viscous force, gravitational force, and capillary force. Table 1 represents the considered forces. The first of these, the Reynolds number (Re), describes the relationship between inertial forces and viscous forces. Since inertial forces (generated from fluid momentum) are neglected when compared to viscous forces (forces arising from fluid resistance to flow), the Re exhibits low values (10−6 to 10), and thus, the flow is predominantly laminar [38]. Due to its disregard of interfacial tension, the Re is often overlooked in the study of droplet formation. The Capillary number (Ca), which describes the relationship between viscous forces and capillary pressure, is frequently employed for characterizing microfluidic droplet generation patterns, especially when the effects of inertial forces outweigh those of inertia. Typically, Ca values range from 10−3 to 10 [35]. In situations where inertial forces become significant, such as in cases of high-speed flow, the regime of microfluidic droplet formation can be better described by the Weber number (We), which reflects the balance between inertial forces and surface tension [24,39,40]. Finally, the Bond number (Bo) represents the relationship between gravitational force and surface tension [22]. Bo values increase with rising temperature and the addition of surfactants and are significantly reduced by decreasing the cross-sectional area of the channel from the dominance of interfacial forces over gravity [41,42].

Table 1.
Dimensionless parameters in microfluidic droplet generation.
Droplet breakup can occur through various modes: squeezing, dripping, jetting, tip-streaming, and tip-multi-breaking. While the squeezing mode is driven by channel confinement, capillary instability is responsible for the other modes [35]. In the squeezing mode, the generated droplets are larger than the channel size and highly monodisperse, occurring at low capillary numbers, such as Cac < 0 (10−2) in T-junctions [43]. In this regime, the growing droplet blocks the cross-section of the exit channel, creating a pressure buildup that results in droplet breakup [44,45]. The dripping mode produces droplets smaller than the channel dimensions and highly monodisperse, occurring with a successive increase in the Cac, transitioning from the squeezing to the dripping mode [35,43]. In this mode, droplets are produced right behind the exit of a feeding capillary or ejecting nozzle [46], and it is described when 0 (10−2) < Cac < 0 (1) with Wed < 0 (1) [35,47]. The jetting regime is characterized by the production of polydisperse droplets. Droplet fragmentation in this mode occurs away from the injection source due to Plateau–Rayleigh instability [47]. It generally occurs at high flow rates or low interfacial tension and when Cac + Wed ≥ 0 (1). The tip-streaming and tip-multi-breaking modes produce submicrometer droplets; however, while the former produces monodisperse droplets, the latter produces polydisperse droplets that follow a geometric progression [35,43].
The traditional droplet breakup modes (squeezing, dripping, and jetting) can be applied to different channel geometries [35]. Co-flow (coaxial junction) utilizes sets of coaxial microchannels. In this configuration, the dispersed fluid is introduced into an inner channel, while the continuous phase flows into an outer concentric channel in the same direction [19]. The resulting droplets are generally larger than the inner microchannel dimension when the breakup modes are dripping or jetting [47]. The flow-focusing geometry features a constriction region where dispersed and continuous phases flowing coaxially meet, generating a filament that elongates and eventually breaks, forming droplets [19]. The sudden constriction of the dispersed phase induces high viscous shear forces, thereby enabling the generation of droplets with sizes down to a few hundred nanometers [14,17]. In a cross-flow geometry, the dispersed phase undergoes shear at a cross junction characterized by an angle θ (0° < θ ≤ 180°). The T-junction is the most common case, where the junction is orthogonal to the dispersed phase. The simplicity and ability to produce monodisperse droplets (commonly with a CV of less than 2%) make the cross-flow geometry widely used [35].
The fabrication of microfluidic devices constitutes a pivotal stage in generating droplets with the desired shape and functionality. The design of such devices must be meticulously engineered to allow for the desired flow pattern, which is achieved through specific geometric structures and physicochemical characteristics, including surface properties [19]. The advancement in silicon-based microelectronics and microelectromechanical systems (MEMSs) has propelled the development of initial microfluidic devices, typically constructed from silicon and glass, with production techniques adapted from those used in semiconductor and plastic industries [28,48], such as micromachining, photolithography, replica molding, embossing, and injection molding [49]. A typical glass-based microfluidic fabrication process via micromachining begins with lithography. A photoresist is deposited on the surface to be microstructured. Light exposes specific areas by using a mask with predefined patterns, transferring the patterns to the substrate. In the next step, etching selectively removes material from the substrate where the photoresist has been cleared, creating the desired microchannels. Finally, bonding joins the micromachined substrates to form enclosed channels. This bonding can be achieved through heat or special adhesives [19,50]. Nevertheless, traditional manufacturing methods exhibit several drawbacks, including high costs, lengthy processes, the requirement for clean-room facilities, and limited finishing [19,23,49]. Recent manufacturing methods, such as 3D printing, wherein 3D printers are employed to create devices through successive layers of material, have facilitated the production of devices at lower costs and with less complex processes [49].
The selection of materials for constructing microfluidic devices significantly influences particle synthesis, as scale reduction enhances the properties of these materials [51]. Various materials have been explored, including glass, silicon, paper, ceramics, metals, and polymers [50,51,52]. Microfluidic devices based on inorganic materials, such as glass, silicon, and ceramics, commonly exhibit high solvent compatibility, stable electroosmotic mobility, and mechanical rigidity, along with optical clarity for glass-based devices and semiconductor properties for silicon-based devices [23,53]. However, microfluidic devices based on inorganic materials are challenging and expensive to manufacture, often impeding scale-up efforts [53]. Additionally, these materials are impermeable to gases, limiting applications involving cell culture [23]. Due to these factors, polymers have been preferred as the material of choice for chip fabrication. Various polymers have been utilized, including elastomers such as poly(dimethylsiloxane) (PDMS), and thermoplastics such as polymethylmethacrylate (PMMA), polycarbonate (PC), polystyrene (PS), polyvinylchloride (PVC), and cyclic olefin copolymer (COC) [53]. The primary advantages of employing polymers for microfluidic device fabrication lie in their considerably lower costs than glass and silicon and their diverse properties, which vary among available polymers, enabling them to cater to a wide range of applications [49].
3. Polymeric Microparticles
Polymer-based microparticles are renowned for their versatility. These materials find utility across diverse domains, serving industrial applications to impart protection, functionality, and finishing to products such as paper, metals, and wood, as well as advanced applications in biomedical and analytical sectors. Examples include drug delivery for chemotherapy, the fabrication of supports for chromatographic columns, spheres employed in flow cytometry, and the recovery of DNA and proteins [54]. A range of polymers is available for use in microparticle fabrication, depending on the desired properties and purposes, which can be categorized as follows: synthetic polymers, including poly(ethylene glycol), polyglycerol, poly(acrylic acid), and poly(acrylamide), and natural polymers (also referred to as biopolymers), encompassing polysaccharides (such as alginate, chitosan, agarose, and pectin), proteins (including gelatin, collagen, and albumin), and bacterial-derived biopolymers (such as bacterial cellulose and dextran) [17].
Despite their technical advantages, using synthetic polymers in microparticle fabrication, particularly those intended for biomedical applications, has been subject to debate. Many of these polymers have fossil origins, either directly or indirectly, and more sustainable alternatives must be sought with the rational use of petroleum sources [55].
Due to their highly organized nature and alignment with properties such as biocompatibility, biodegradability, low antigenicity, and high bioactivity, biopolymers have garnered attention from researchers in the field of microparticle fabrication via microfluidics [14,56]. In this context, abundant polysaccharides in nature present unique opportunities for cost-effective microparticle production [14]. Alginate [57], chitosan [58], and cellulose [59] stand among the polysaccharide examples already explored in microfluidic applications.
4. The Case of Pectin
Pectin, a complex biomacromolecule and abundant heteropolysaccharide in the primary cell wall and middle lamella of plants, plays diverse roles, ranging from maintaining cell integrity, which influences the firmness and texture of fruits and vegetables, to functions in immunity against phytopathogens [60,61,62,63]. Due to its significant presence in plant matrices, unused and discarded residues from agriculture, the agro-industry, and the food industry are its primary sources, including apple pomace, citrus peel, and sugar beet pomace [64,65]. Commercially, due to its ability to interact with water, pectin is commonly used as a solution thickener and gelling agent in food and pharmaceutical products [65,66].
Structurally, pectin is a complex and heterogeneous polysaccharide, primarily composed of homogalacturonan (HG), rhamnogalacturonan I (RG-I), and rhamnogalacturonan II (RG-II) domains (Figure 1) [26]. The HG, which is the main region, comprises the “smooth” regions of pectin, consisting of a linear chain of α-1,4-D-galactopyranuronic acid (GalpA) residues. When branched by side chains formed by different sugars, some of which are rare, such as D-apiose ((3R,4R)-4-(Hydroxymethyl)tetrahydro-2,3,4-furantriol), L-aceric acid (3-C-Carboxy-5-deoxy-L-xylofuranose), and Kdo (2-Keto-3-Deoxy-D-Mannooctanoic Acid), it forms RG-II [67,68]. The RG-I, in contrast, has a main chain formed by alternating α-L-rhamnose (Rhap) and GalpA residues, which becomes branched when Rhap residues are substituted by side chains composed of either a single sugar residue or combined chains of arabinans, galactans, or arabinogalactans [60].
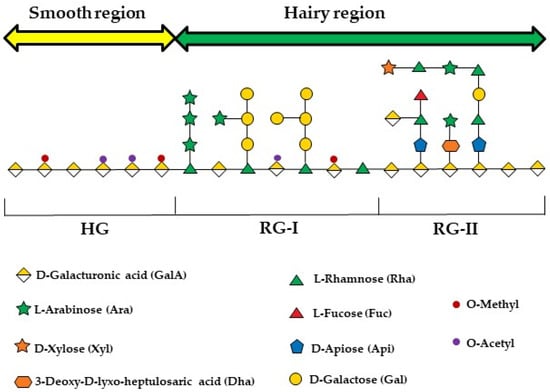
Figure 1.
An illustration of the three polysaccharide segments commonly found in all pectin species: homogalacturonan (HGA), rhamnogalacturonan-I (RG-I), and rhamnogalacturonan-II (RG-II). The figure was created following the symbol nomenclature for glycans guidelines [69,70].
The extraction method used can significantly affect the structure of pectins, influencing their potential applications [66]. The most traditional method for extracting pectins involves heating plant matrices rich in this polysaccharide in acidified solutions, inducing the hydrolysis of protopectin into pectin and its solubilization in the aqueous medium used [71]. The corrosive nature, lengthy extraction steps, high temperatures, low yields, and potential environmental impact have spurred the adoption of green approaches and emerging technologies, such as enzymatic extraction [72], microwave-assisted extraction [73], ultrasound-assisted extraction [74], ohmic heating [75], subcritical extraction [76], and deep eutectic solvents [77].
Gelation with divalent cations, particularly calcium, is one of pectin’s most significant functional properties, serving as a key factor in numerous biological functions and technological applications. Gelation proceeds via a classic “egg-box” mechanism, involving specific and strong interactions between calcium ions and blocks of galacturonic acid residues [78,79]. The degree of esterification (DE), based on the percentage of esterified carboxyl groups in the HG region, is commonly used to classify pectins. Pectins are thereby divided into two types: high-methoxyl pectins (DE > 50%) and low-methoxyl pectins (DE < 50%). The primary characteristic resulting from this classification is the ability to form gels. High-DE pectins form gels when heated in acidic solutions with a low pH (2–3.5) and in the presence of high sugar concentrations (55–75%), while low-DE pectins can form gels across a broader pH range in the presence of divalent cations such as calcium (Ca2+) or zinc (Zn2+) [80]. Due to these properties, high-DE pectins are generally used in the preparation of jams, jellies, or marmalades, while low-DE pectins have a broader range of applications due to their less demanding gelling conditions and the ability to produce gels with different characteristics, making them extensively utilized in the preparation of reduced-calorie products [66]. The DE, along with molecular weight (MW), monosaccharide composition (MC), the RG-I/HG ratio, and spatial conformation, significantly influences pectins’ physicochemical and biological properties. These factors depend on various parameters, including the raw material, vegetative period, harvest time, storage duration, and extraction method [26,81].
Pectin has been recognized for its positive effects on human health. Similar to other dietary fibers, the human body does not produce enzymes capable of degrading pectin, which remains intact upon reaching the intestine and serves as a fermentation substrate for the intestinal microbiota [82]. Two important aspects linked to its beneficial effects are observed in this process: the regulation of the intestinal microbiota and the generation of short-chain fatty acids (SCFAs) [83]. Thus, positive effects of pectin consumption on various human ailments have been reported, such as beneficial effects against hyperglycemia [84], and colorectal cancer [85]. Additionally, due to its resistance to upper gastrointestinal enzymes, pectin has been used as a carrier for the specific delivery of drugs to the colon [25,86].
5. Pectin-Derived Microparticles
The structural attributes of pectin confer emulsifying properties, stabilizing effects, and the ability to alter the viscosity of the medium in various systems where this hydrocolloid is added. In conjunction with its soft and flexible nature, as well as its inherent high biocompatibility, pectin as a building material for fabricating particles/microparticles/microgels has been widely adopted for various purposes [87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102]. Many methodologies have been developed for producing pectin-based microparticles based on electrostatic interactions or emulsions [25,103,104,105].
The most traditional method for producing pectin-based microparticles involves molding pectin with an oppositely charged compound by adjusting the pH of the medium. Unesterified galacturonic acid molecules in low-DE pectins, under certain pH conditions, can have negatively charged deprotonated carboxyl groups that serve as binding sites for electrostatic interactions [103]. Cross-linking is commonly induced with divalent cations such as Ca2+ and Zn2+ through the ionotropic gelation mechanism. Due to its simplicity, this system has been explored as a delivery system for various purposes, such as medications [106,107], bioactives [108,109], and probiotics [110,111]. To enhance the performance of these systems, control swelling, and increase stability [112], pectin hydrogels are additionally cross-linked with proteins like whey protein [113] and gelatin [6], as well as polysaccharides like chitosan [114] and alginate [115].
Due to the hydrophilic functional groups (hydroxyl and carboxyl) and hydrophobic functional groups (carboxylic ester and amide), pectin can be used as an emulsifier [103]. The stability of the formed emulsions is achieved through steric stabilization, primarily attributed to the neutral sugar side chains in RG-I, and electrostatic stabilization, due to the ionization of carboxyl groups in HG [116]. Different gelling mechanisms (acidification with ethanol, ionotropic cross-linking with Fe3+, and ionotropic cross-linking with Ca2+) were explored in the formation of pectin-based emulsion gels for the delivery of bioactives in the gastrointestinal tract [117]. The gelling methods significantly influenced the properties of the emulsion gels, thereby altering the release mechanism of the microparticles. While gels formed by acidification with ethanol and iron exhibited a sustained release profile in the small intestine, calcium gels released their content only during passage through the colon.
6. Applications of Pectin in Microparticle Production via Microfluidics
Despite their simplicity, a common challenge with conventional processes is the lack of control over microparticles’ size, shape, and homogeneity [24]. In this regard, methodologies based on microfluidic principles enable the precise and reproducible production of pectin-based particles (Table 2).

Table 2.
The utilization of pectin in the production of microparticles through microfluidic technology and its applications.
In a precursor approach to microfluidic studies, monodisperse pectin microparticles were produced using a microchannel emulsification system [128]. Initially, a W/O emulsion was made using tetraglycerol polyricinoleate and oleic acid as the continuous phase and a 0.5 wt% pectin solution as the dispersed phase. This emulsion was then homogenized in the microchannel with an external aqueous phase containing Tween 20 and calcium ions to create a double W/O/W emulsion. The pectin-loaded internal aqueous phase was then gelled, resulting in the formation of monodisperse pectin microparticles. Although the microchannel technique produces fewer polydisperse particles (commonly less than 5%), it lacks the precision of microfluidic devices in controlling droplet size, shape, anisotropy, uniformity, and internal structure [129].
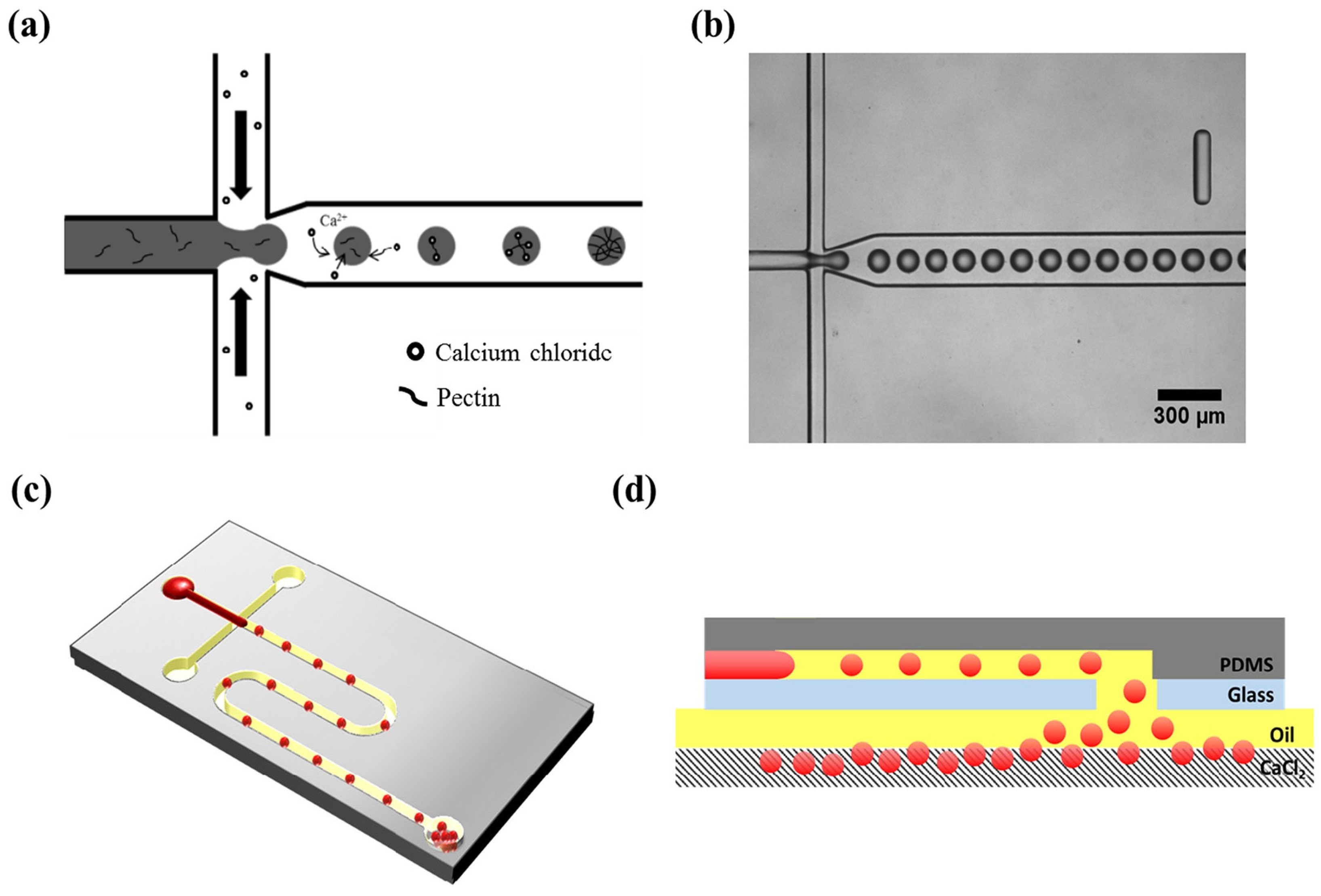
Gelation with divalent cations, especially calcium ions, has been the most widely used approach for creating pectin-based microparticles in microfluidic methods [14]. In an initial exploration of microfluidic concepts, microbeads were successfully produced using pectin and rapeseed oil [118]. A flow-focusing microfluidic device made of polycarbonate, fabricated by micromilling, enabled the formation of monodisperse droplets. Notably, cross-linking time significantly influenced nanoparticle release, with observations suggesting a two-stage cross-linking process: rapid gelation of the outer layer followed by prolonged cross-linking dependent on ion concentration homogenization within the gelling droplet. The importance of calcium ion diffusion during the formation of pectin microparticles was also verified by Fang and Cathala [122]. They demonstrated that the addition of winding channels after droplet formation enhances the homogeneity of the microparticles by improving the mixing between pectin and the cross-linking agent. Finally, the microfluidics method of collecting the newly produced microparticles has been shown to affect their microstructure [123]. The settling collection method, which entails consecutively dropping hydrogels from the outlet hole, effectively preserves the shape of the soft pectin hydrogels compared to the collection via tubing connected to the outlet port of the microfluidic device (Figure 2).
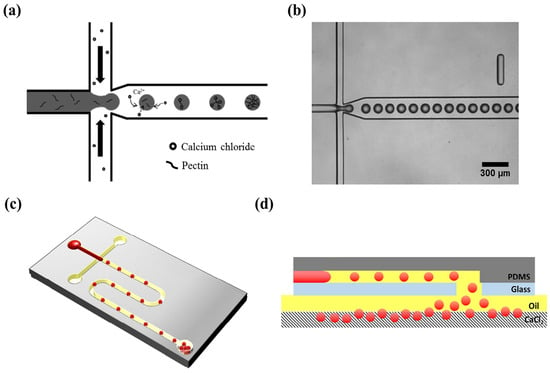
Figure 2.
Microfluidic device for generating pectin hydrogel microspheres: (a) A schematic illustration of external cross-linking within the microchannel. (b) An optical microscope image of pectin droplets within the microchannel. (c) An overall schematic of the microfluidic device containing pectin hydrogel microspheres and the outlet port aligned with the hole in the slide glass. (d) A cross-sectional view of the microfluidic device [123].
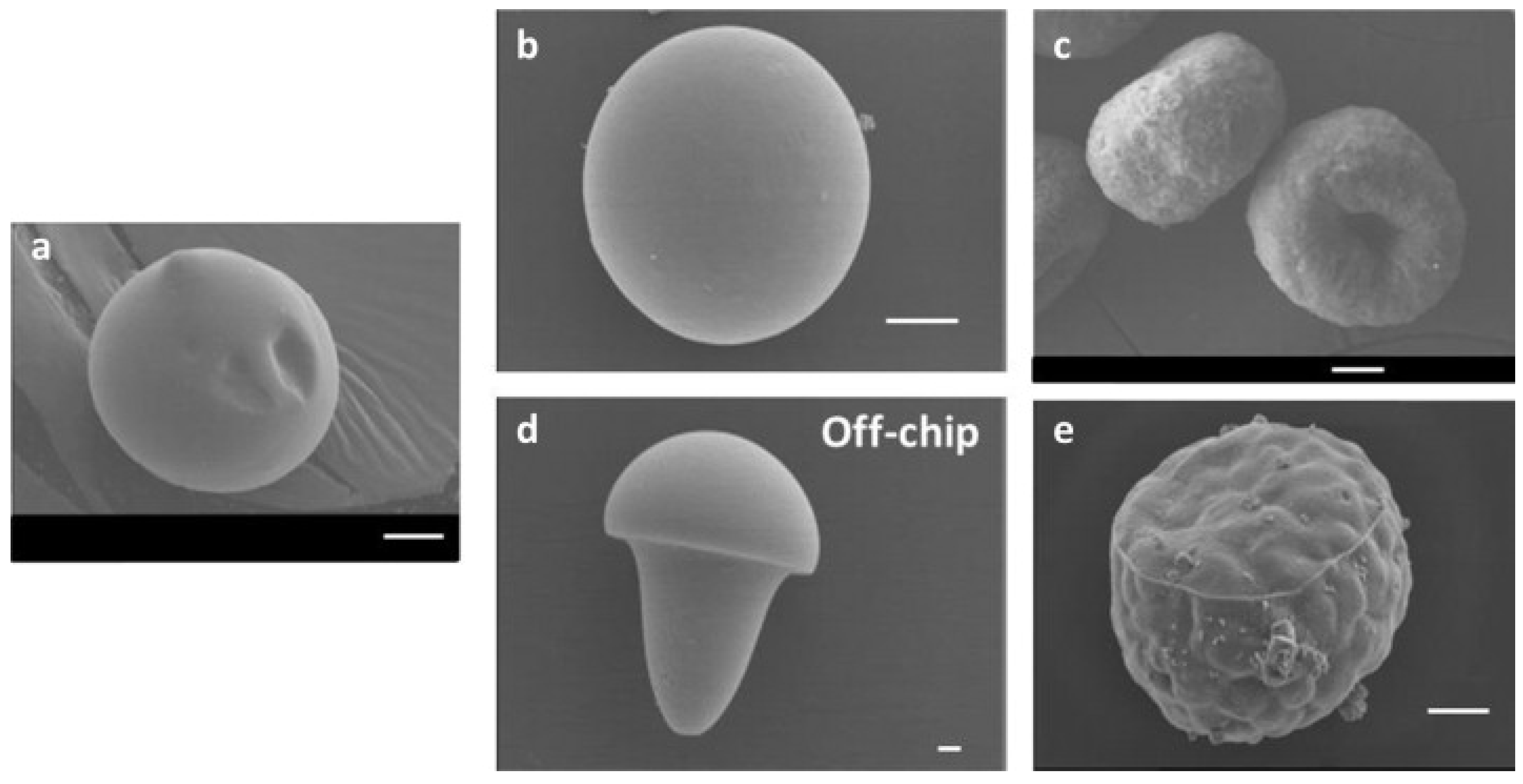
Pectin-based microparticles with diverse morphologies, such as spherical, doughnut-like, oblate ellipsoid, and mushroom-like shapes (Figure 3), were produced using a microfluidic platform. This platform exploited different gelation methods (internal and external gelation) and water diffusion in microfluidic channels (on-chip), as well as the deformation of pre-formed droplets outside the channels (off-chip) [120]. Studies of the swelling behavior of hydrated and dried–rehydrated pectin microparticles indicated that drying did not significantly affect their shapes and swelling capacities, except for the mushroom-like microparticles [121]. The precise control of microparticle morphology opens opportunities for applications in various fields, such as drug delivery, optical devices, and the formation of advanced materials.
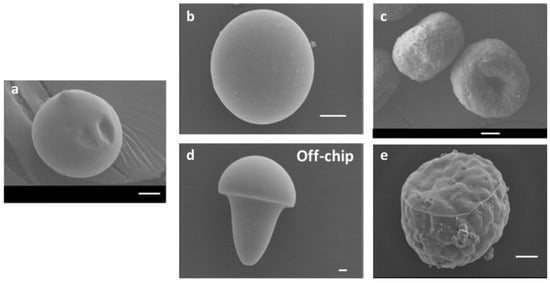
Figure 3.
Scanning electron microscopy images of pectin hydrogel microparticles obtained through various methods: (a) Solvent diffusion using anhydrous DMC without a cross-linking step. (b) Solvent diffusion and external gelation of the dispersed phase using CaCl2 as the cross-linking agent. (c) Solvent diffusion and internal gelation of the dispersed phase using CaCO3 as the cross-linking agent. (d) Without solvent diffusion, utilizing external gelation. (e) Without solvent diffusion, utilizing internal gelation. Scale bars: 10 μm [120].
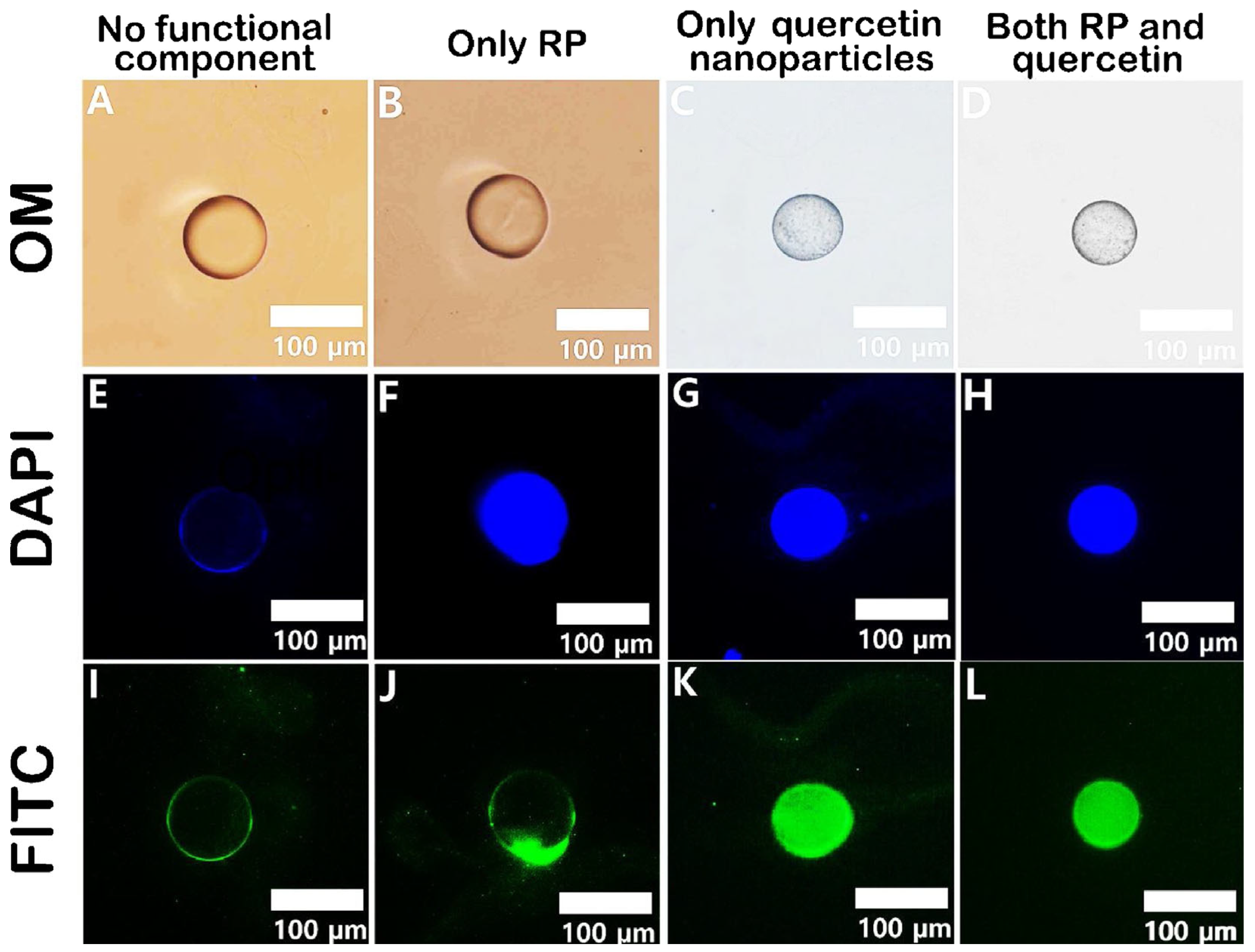
One of the most common applications of microfluidics is the encapsulation and controlled release of active ingredients, such as drugs, nutrients, fragrances, or cosmetics [19]. For this purpose, transport systems must exhibit excellent biocompatibility, biodegradability, and high drug-loading efficiency [27]. To ensure consistency in the drug release profile, effective control over the size, shape, and composition of the microparticles is necessary [22]. Furthermore, multiple active ingredients can be co-encapsulated within a single microparticle, enabling the investigation of synergistic effects [19]. This concept was demonstrated with pectin-based microparticles produced via microfluidics, which were used to encapsulate both hydrophobic and hydrophilic agents within the same system [124]. Using a flow-focusing microfluidic device, monodisperse oil droplets containing retinyl palmitate (RP) and quercetin nanoparticles were continuously generated within an aqueous pectin solution. In a second inlet, this pectin solution flowed through a mixture of water, ethanol, and calcium ions. The pectin around the oil droplets gelled through ionic cross-linking with calcium and ethanol precipitation. The successful encapsulation of the ingredients was demonstrated through a series of optical microscopy images (Figure 4).
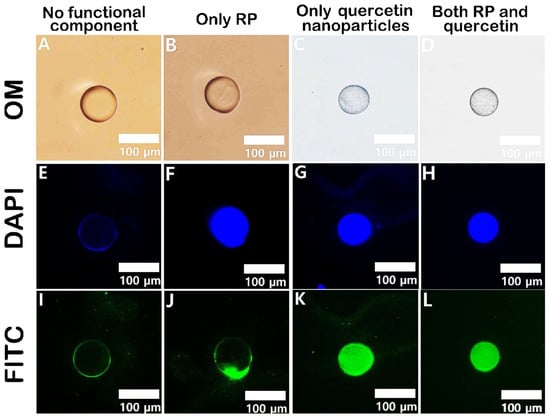
Figure 4.
Optical microscopy images, along with 4′,6-diamidino-2-phenylindole (DAPI)- and fluorescein isothiocyanate (FITC)-filtered fluorescence images, of various pectin microcapsules: (A,E,I) empty pectin microcapsule, (B,F,J) RP-containing pectin capsule, (C,G,K) quercetin-containing pectin capsule, and (D,H,L) pectin capsule containing both RP and quercetin [124].
Pectin-based microgels have emerged as a promising alternative for aquatic protein delivery [127]. Certain aquatic proteins are packed with essential amino acids, and some have health benefits, such as antioxidant and immunomodulatory properties [130,131,132]. Despite this, harsh conditions of temperature and pH can lead to the loss of functional activity of these proteins. Wang et al. [127] fabricated electrostatically crosslinked pectin-based microgels with zinc cations and a layer of cationic chitosan molecules to encapsulate soluble proteins from Fenneropenaeus chinensis and Lateolabrax japonicus. These microgels had uniform size and spherical shape, demonstrating distinct release profiles in the gastrointestinal tract, with an enhanced release under colonic conditions. Thermal analyses indicated interactions between the proteins and the microgels, showing improved thermal stability of the proteins after encapsulation. Additionally, the microgels did not affect the antioxidant and immunostimulatory activities of the proteins.
Advances in tissue engineering and regenerative medicine have employed microfluidic technologies to create a suitable microenvironment for repairing and regenerating lost or damaged tissues, including pectin-based microparticles [133,134]. The protein Bone Morphogenetic Protein-2 (BMP-2), which functions as an osteogenic growth factor and has been applied in bone repair therapies [135,136], was conjugated with carbon dots (CDs), which possess intrinsic fluorescence properties, to create a therapeutic platform where both osteogenesis and bioimaging are needed. Monodisperse BMP-2-CD-loaded pectin droplets, with a size of 35 μm and a CV < 5%, were produced using ionic gelation between pectin and zinc ions, facilitated by an MF flow-focusing droplet generator [125]. To optimize the controlled release of BMP-2-CDs, the pectin microparticles were incorporated into composite scaffolds made of gelatin (G), elastin (E), and hyaluronic acid (HA). These polymers (GE-HA) combined resulted in scaffolds with sustained BMP-2-CD release for 21 days, appropriate degradation, water absorption capacity, and mechanical strength, ideal for enhanced cell adhesion, as demonstrated with MG-63 cells. This approach shows potential for biocompatible structures in therapeutic and bone tissue engineering.
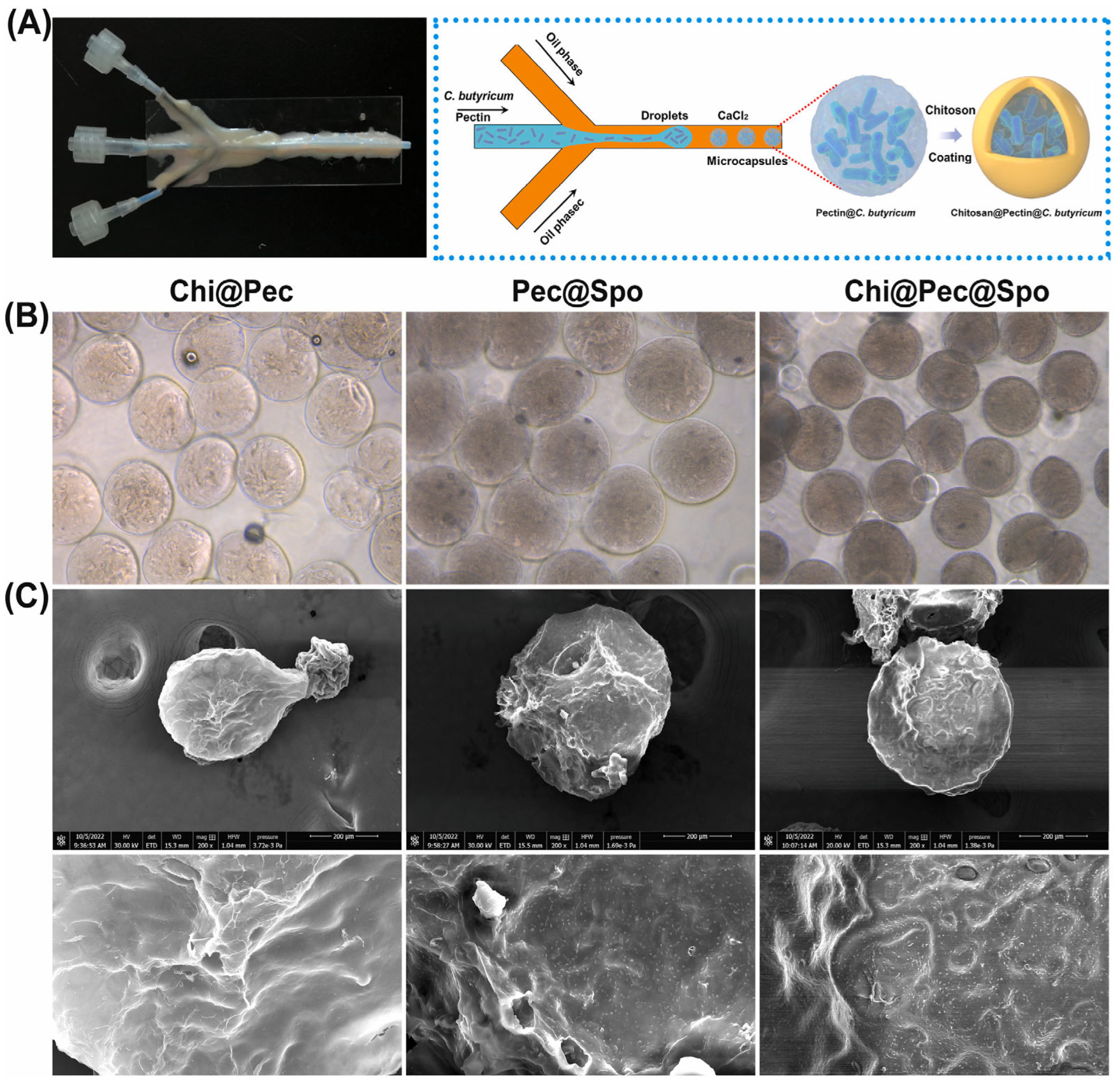
The resistance of pectin to gastrointestinal enzymes can be used for the targeted delivery of drugs, bioactives, and biotics to the colon, aiming to treat or alleviate specific conditions [86]. Inflammatory bowel disease (IBD) is a chronic inflammation of the gastrointestinal tract and is a multifactorial disease triggered by intestinal microbiota dysbiosis, immunological abnormalities, genetic predisposition, and environmental factors. IBD can manifest as either Crohn’s disease (CD) or ulcerative colitis (UC). While CD can affect the entire gastrointestinal tract, UC is generally confined to the colon [137]. A therapy for ulcerative colitis (UC) was developed by regulating microbiota homeostasis by delivering Clostridium butyricum spores, utilizing polysaccharide microparticles, including pectin, via microfluidic technology [126]. Vegetative cells or spores of C. butyricum at a concentration of 8 × 1010 CFU/mL were mixed with a low-DE pectin solution (DE = 34%). This mixture was emulsified in an oil phase using a microfluidic device and subsequently cross-linked with CaCl₂ and chitosan, as illustrated in Figure 5. The encapsulation process effectively increased the thermal stability of both vegetative cells and spores of C. butyricum against heat processing and lyophilization. It also extended their viability during prolonged storage and enhanced their tolerance to gastrointestinal fluids. The chitosan-coated particles demonstrated remarkable stability, likely due to the dense gel network structure formed between pectin and chitosan, which effectively reduced the porosity and permeability of the particles. In a murine model, the microparticles reduced symptoms of acute dextran sulfate sodium (DSS)-induced colitis, such as weight loss and colon shortening. Additionally, histological observations of colon tissue showed a significant reduction in colitis-induced damage, and levels of pro-inflammatory cytokines, including mouse interleukin 6 (IL-6) and tumor necrosis factor-alpha (TNF-α), decreased after treatment. Overall, the results indicated that dietary polysaccharide microcapsules containing C. butyricum are a promising alternative for restoring intestinal damage caused by colitis.
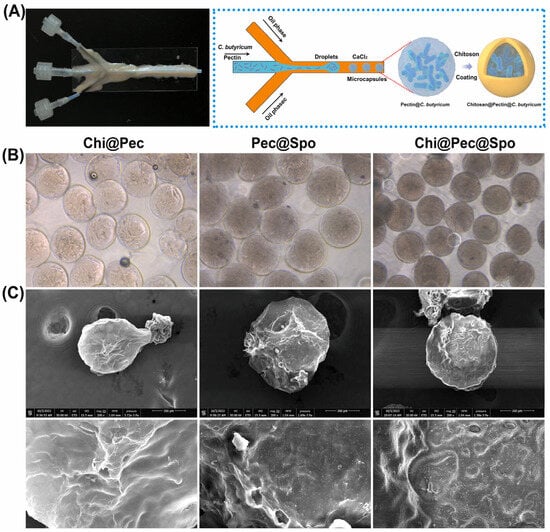
Figure 5.
(A) The microfluidic device (left) and a schematic of the microcapsule preparation process (right), and (B) microscope images and (C) SEM images of chitosan–pectin, pectin–spore, and chitosan–pectin–spore microcapsules [126].
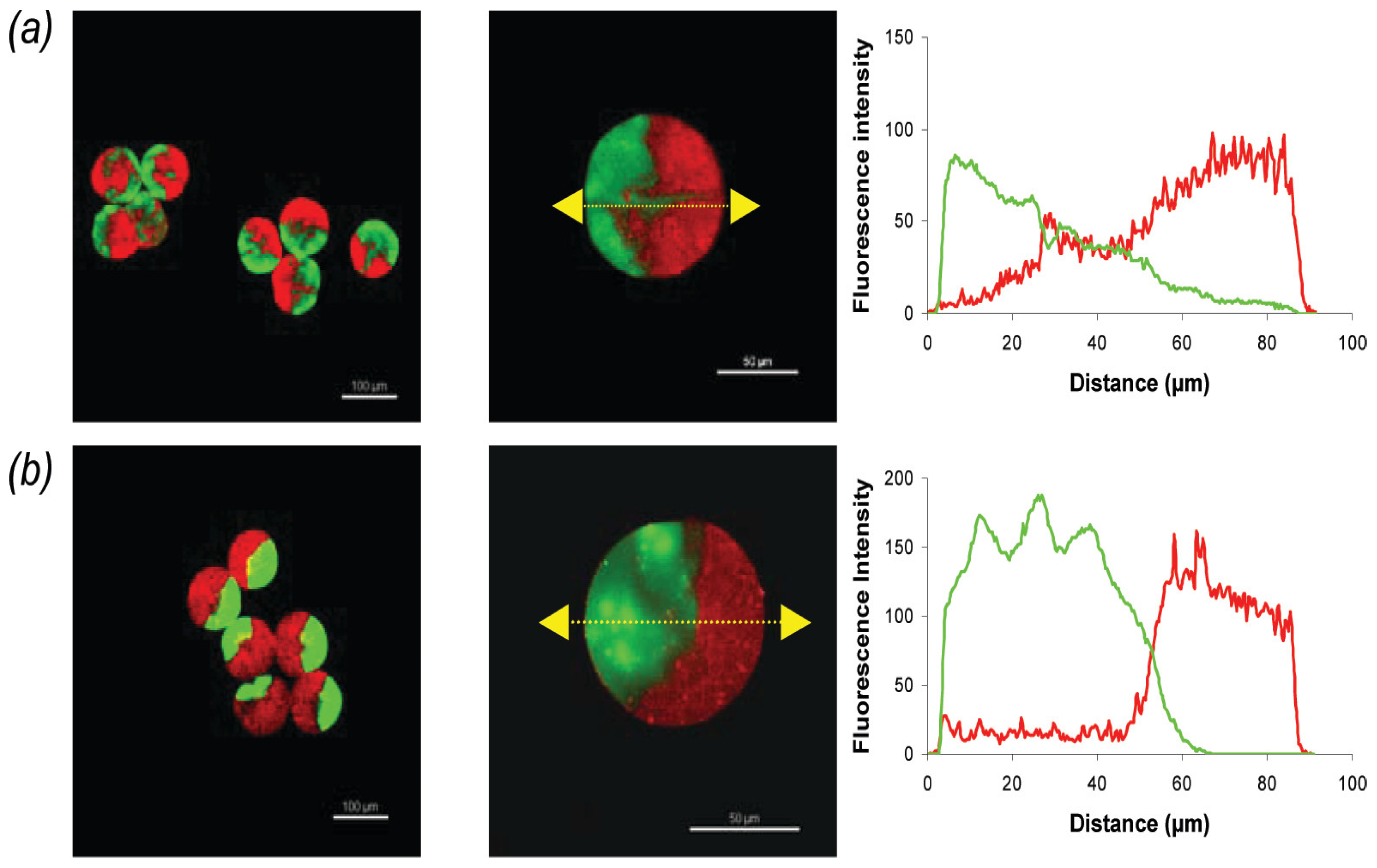
Named after a two-faced Roman god of the same name, Janus particles were initially proposed by Pierre-Gilles de Gennes [138]. Simply put, Janus particles consist of particles with surfaces or regions exhibiting distinct physical or chemical properties, within the same particle, often of different natures [139,140]. This asymmetry provides distinct optical, electrical, or magnetic properties that can be exploited for various purposes, such as controlling molecular recognition and self-assembly processes [139,141]. As demonstrated by Marquis et al. [119], pectin is a suitable hydrogel for the synthesis of homo (pectin–pectin) or hetero (pectin–alginate) Janus particles (Figure 6). In a microfluidic system with a flow-focusing device, droplets of pectin or alginate with CaCO3 were generated in sunflower seed oil. Subsequently, sunflower seed oil with surfactant and acetic acid was added to induce gelation. Homo Janus particles were successfully produced, although their interface was not as clearly defined as that of hetero particles. Conformational constraints from esterified and non-esterified galacturonic acids, coupled with convective and interdiffusive mixing phenomena, were responsible for the invaginations at the homo Janus interface. In hetero Janus particles loaded with bovine serum albumin (BSA), microscopic analysis showed BSA transferring to the charged alginate pole. This transfer was ascribed to diminished attractive electrostatic interactions between BSA amino groups and pectin carboxylic groups, owing to the presence of methoxy groups along the pectin chain [120].
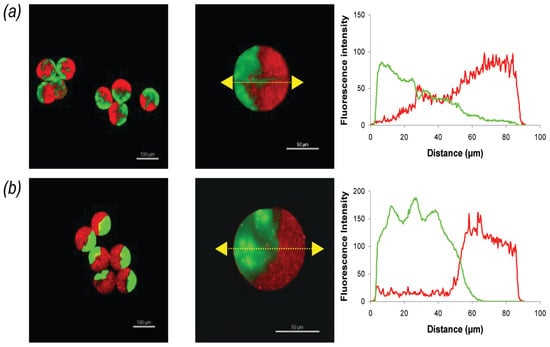
Figure 6.
Fluorescence confocal microscopy images and fluorescence intensity profiles for (a) FA–pectin/Bodipy–pectin homo Janus microbeads and (b) FA–alginate/Bodipy–pectin hetero Janus microbeads. Excitation wavelengths for FA and Bodipy were set at 488 nm and 561 nm, respectively, with emission fluorescence recorded between 500 and 530 nm (green) and 570 and 620 nm (red). Flow rates for FA–pectin (or FA–alginate), Bodipy–pectin, and oil in each channel were 1 μL/min, 1 μL/min, and 18 μL/min, respectively. Scale bars: 100 μm (left) and 50 μm (middle) [119].
7. Conclusions
The introduction of microfluidic technology has revolutionized the field of microcarriers, driven by the need for precise control over particle characteristics such as size, shape, and composition to meet the demands of diverse applications. Pectin’s complex composition offers remarkable versatility for microparticle fabrication, enabling its utilization in biomolecule delivery, bioimaging technologies, and regenerative medicine. Combining pectin with other biopolymers holds immense potential for modulating its properties. Novel particle concepts, such as Janus particles, present exciting possibilities for pectin, paving the way for exploration in new fields like molecular recognition.
Despite the progress achieved, this technology remains underutilized. Many applications realized with pectin microparticles produced by conventional methods have not yet been explored with monodisperse microparticles. Additionally, there is a lack of research on delivering compounds or drugs to the colon, an area with clear untapped potential. Furthermore, no studies have been found on the application of pectin and microfluidic technology for developing nanoparticles or nanocarriers, which are crucial for addressing needs that microparticles cannot fulfill.
Author Contributions
P.B.V.d.S.: conceptualization, writing—original draft preparation, and writing—review and editing. J.P.F.: conceptualization, supervision, writing—review and editing, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge The National Council for Scientific and Technological Development (CNPq) for J.P.F.’s productivity scholarship (CNPq Proc. #307842/2022-3) and the São Paulo Research Foundation (FAPESP) for P.B.V.d.S.’s scholarship (#2022/12270-1). The study was financially supported by grants #2013/07914-8 and #2022/12834-2 from the São Paulo Research Foundation (FAPESP).
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge The National Council for Scientific and Technological Development (CNPq) and the São Paulo Research Foundation (FAPESP) for scholarships.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pei, Y.; Wang, J.; Khaliq, N.U.; Meng, F.; Oucherif, K.A.; Xue, J.; Horava, S.D.; Cox, A.L.; Richard, C.A.; Swinney, M.R.; et al. Development of Poly(Lactide-Co-Glycolide) Microparticles for Sustained Delivery of Meloxicam. J. Control. Release 2023, 353, 823–831. [Google Scholar] [CrossRef]
- Birk, S.E.; Boisen, A.; Nielsen, L.H. Polymeric Nano- and Microparticulate Drug Delivery Systems for Treatment of Biofilms. Adv. Drug Deliv. Rev. 2021, 174, 30–52. [Google Scholar] [CrossRef]
- Lee, S.; Koo, J.; Kang, S.-K.; Park, G.; Lee, Y.J.; Chen, Y.-Y.; Lim, S.A.; Lee, K.-M.; Rogers, J.A. Metal Microparticle—Polymer Composites as Printable, Bio/Ecoresorbable Conductive Inks. Mater. Today 2018, 21, 207–215. [Google Scholar] [CrossRef]
- Scholtz, L. Correlating Semiconductor Nanoparticle Architecture and Applicability for the Controlled Encoding of Luminescent Polymer Microparticles. Sci. Rep. 2024, 14, 11904. [Google Scholar] [CrossRef]
- Peñalva, R.; Martínez-López, A.L.; Gamazo, C.; Gonzalez-Navarro, C.J.; González-Ferrero, C.; Virto-Resano, R.; Brotons-Canto, A.; Vitas, A.I.; Collantes, M.; Peñuelas, I.; et al. Encapsulation of Lactobacillus Plantarum in Casein-Chitosan Microparticles Facilitates the Arrival to the Colon and Develops an Immunomodulatory Effect. Food Hydrocoll. 2023, 136, 108213. [Google Scholar] [CrossRef]
- Günter, E.A.; Melekhin, A.K.; Belozerov, V.S.; Martinson, E.A.; Litvinets, S.G. Preparation, Physicochemical Characterization and Swelling Properties of Composite Hydrogel Microparticles Based on Gelatin and Pectins with Different Structure. Int. J. Biol. Macromol. 2024, 258, 128935. [Google Scholar] [CrossRef]
- Dong, Z.; Xu, H.; Bai, Z.; Wang, H.; Zhang, L.; Luo, X.; Tang, Z.; Luque, R.; Xuan, J. Microfluidic Synthesis of High-Performance Monodispersed Chitosan Microparticles for Methyl Orange Adsorption. RSC Adv. 2015, 5, 78352–78360. [Google Scholar] [CrossRef]
- Caruso, M.R.; D’Agostino, G.; Wasserbauer, J.; Šiler, P.; Cavallaro, G.; Milioto, S.; Lazzara, G. Filling of Chitosan Film with Wax/Halloysite Microparticles for Absorption of Hydrocarbon Vapors. Adv. Sustain. Syst. 2024, 8, 2400026. [Google Scholar] [CrossRef]
- Polat, H.K.; Aytekin, E.; Karakuyu, N.F.; Çaylı, Y.A.; Çalamak, S.; Demirci, N.; Ünal, S.; Kurt, N.; Çırak, R.; Erkan, E.; et al. Harnessing Silk Fibroin Microparticles for Metformin Delivery: A Novel Approach to Treating Corneal Neovascularization. J. Drug Deliv. Sci. Technol. 2024, 96, 105625. [Google Scholar] [CrossRef]
- Shim, H.-E.; Kim, Y.-J.; Park, K.H.; Park, H.; Huh, K.M.; Kang, S.-W. Enhancing Cartilage Regeneration through Spheroid Culture and Hyaluronic Acid Microparticles: A Promising Approach for Tissue Engineering. Carbohydr. Polym. 2024, 328, 121734. [Google Scholar] [CrossRef]
- Zheng, C.; Teng, C.P.; Yang, D.-P.; Lin, M.; Win, K.Y.; Li, Z.; Ye, E. Fabrication of Luminescent TiO2:Eu3+ and ZrO2:Tb3+ Encapsulated PLGA Microparticles for Bioimaging Application with Enhanced Biocompatibility. Mater. Sci. Eng. C 2018, 92, 1117–1123. [Google Scholar] [CrossRef]
- Galogahi, F.M.; Zhu, Y.; An, H.; Nguyen, N.-T. Core-Shell Microparticles: Generation Approaches and Applications. J. Sci. Adv. Mater. Devices 2020, 5, 417–435. [Google Scholar] [CrossRef]
- Khanthaphixay, B.; Wu, L.; Yoon, J.-Y. Microparticle-Based Detection of Viruses. Biosensors 2023, 13, 820. [Google Scholar] [CrossRef]
- Jo, Y.K.; Lee, D. Biopolymer Microparticles Prepared by Microfluidics for Biomedical Applications. Small 2020, 16, 1903736. [Google Scholar] [CrossRef]
- Zhang, X.; Qu, Q.; Zhou, A.; Wang, Y.; Zhang, J.; Xiong, R.; Lenders, V.; Manshian, B.B.; Hua, D.; Soenen, S.J.; et al. Core-Shell Microparticles: From Rational Engineering to Diverse Applications. Adv. Colloid Interface Sci. 2022, 299, 102568. [Google Scholar] [CrossRef]
- Galogahi, F.M.; Ansari, A.; Teo, A.J.T.; Cha, H.; An, H.; Nguyen, N.-T. Fabrication and Characterization of Core–Shell Microparticles Containing an Aqueous Core. Biomed. Microdevices 2022, 24, 40. [Google Scholar] [CrossRef]
- Li, W.; Zhang, L.; Ge, X.; Xu, B.; Zhang, W.; Qu, L.; Choi, C.-H.; Xu, J.; Zhang, A.; Lee, H.; et al. Microfluidic Fabrication of Microparticles for Biomedical Applications. Chem. Soc. Rev. 2018, 47, 5646–5683. [Google Scholar] [CrossRef]
- Zhang, Q.; Kuang, G.; Wang, L.; Fan, L.; Zhao, Y. Tailoring Drug Delivery Systems by Microfluidics for Tumor Therapy. Mater. Today 2024, 73, 151–178. [Google Scholar] [CrossRef]
- Shang, L.; Cheng, Y.; Zhao, Y. Emerging Droplet Microfluidics. Chem. Rev. 2017, 117, 7964–8040. [Google Scholar] [CrossRef]
- Sackmann, E.K.; Fulton, A.L.; Beebe, D.J. The Present and Future Role of Microfluidics in Biomedical Research. Nature 2014, 507, 181–189. [Google Scholar] [CrossRef]
- Elvira, K.S.; i Solvas, X.C.; Wootton, R.C.R.; deMello, A.J. The Past, Present and Potential for Microfluidic Reactor Technology in Chemical Synthesis. Nature Chem. 2013, 5, 905–915. [Google Scholar] [CrossRef]
- Sattari, A.; Hanafizadeh, P.; Hoorfar, M. Multiphase Flow in Microfluidics: From Droplets and Bubbles to the Encapsulated Structures. Adv. Colloid Interface Sci. 2020, 282, 102208. [Google Scholar] [CrossRef]
- Gimondi, S.; Ferreira, H.; Reis, R.L.; Neves, N.M. Microfluidic Devices: A Tool for Nanoparticle Synthesis and Performance Evaluation. ACS Nano 2023, 17, 14205–14228. [Google Scholar] [CrossRef]
- Moreira, A.; Carneiro, J.; Campos, J.B.L.M.; Miranda, J.M. Production of Hydrogel Microparticles in Microfluidic Devices: A Review. Microfluid. Nanofluid 2021, 25, 10. [Google Scholar] [CrossRef]
- Khotimchenko, M. Pectin Polymers for Colon-Targeted Antitumor Drug Delivery. Int. J. Biol. Macromol. 2020, 158, 1110–1124. [Google Scholar] [CrossRef]
- Cui, J.; Zhao, C.; Feng, L.; Han, Y.; Du, H.; Xiao, H.; Zheng, J. Pectins from Fruits: Relationships between Extraction Methods, Structural Characteristics, and Functional Properties. Trends Food Sci. Technol. 2021, 110, 39–54. [Google Scholar] [CrossRef]
- Sun, R.; Niu, Y.; Li, M.; Liu, Y.; Wang, K.; Gao, Z.; Wang, Z.; Yue, T.; Yuan, Y. Emerging Trends in Pectin Functional Processing and Its Fortification for Synbiotics: A Review. Trends Food Sci. Technol. 2023, 134, 80–97. [Google Scholar] [CrossRef]
- Whitesides, G.M. The Origins and the Future of Microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef]
- Fuciños, C.; Rodríguez-Sanz, A.; García-Caamaño, E.; Gerbino, E.; Torrado, A.; Gómez-Zavaglia, A.; Rúa, M.L. Microfluidics Potential for Developing Food-Grade Microstructures through Emulsification Processes and Their Application. Food Res. Int. 2023, 172, 113086. [Google Scholar] [CrossRef] [PubMed]
- Beebe, D.J.; Mensing, G.A.; Walker, G.M. Physics and Applications of Microfluidics in Biology. Annu. Rev. Biomed. Eng. 2002, 4, 261–286. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Fontana, F.; Python, A.; Hirvonen, J.T.; Santos, H.A. Microfluidics for Production of Particles: Mechanism, Methodology, and Applications. Small 2020, 16, 1904673. [Google Scholar] [CrossRef] [PubMed]
- MacClements, D.J.; McClements, D.J. Food Emulsions: Principles, Practices, and Techniques, 2nd ed.; CRC Series in Contemporary Food Science; CRC Press: Boca Raton, FL, USA, 2005; ISBN 978-0-8493-2023-1. [Google Scholar]
- Lu, Y.; Zhang, Y.; Zhang, R.; Gao, Y.; Miao, S.; Mao, L. Different Interfaces for Stabilizing Liquid–Liquid, Liquid–Gel and Gel–Gel Emulsions: Design, Comparison, and Challenges. Food Res. Int. 2024, 187, 114435. [Google Scholar] [CrossRef]
- Shah, R.K.; Shum, H.C.; Rowat, A.C.; Lee, D.; Agresti, J.J.; Utada, A.S.; Chu, L.-Y.; Kim, J.-W.; Fernandez-Nieves, A.; Martinez, C.J.; et al. Designer Emulsions Using Microfluidics. Mater. Today 2008, 11, 18–27. [Google Scholar] [CrossRef]
- Zhu, P.; Wang, L. Passive and Active Droplet Generation with Microfluidics: A Review. Lab Chip 2017, 17, 34–75. [Google Scholar] [CrossRef] [PubMed]
- Chong, Z.Z.; Tan, S.H.; Gañán-Calvo, A.M.; Tor, S.B.; Loh, N.H.; Nguyen, N.-T. Active Droplet Generation in Microfluidics. Lab Chip 2016, 16, 35–58. [Google Scholar] [CrossRef]
- Liu, J.; Fu, Q.; Li, Q.; Yang, Y.; Zhang, Y.; Yang, K.; Sun, G.; Luo, J.; Lu, W.; He, J. Research Strategies for Precise Manipulation of Micro/Nanoparticle Drug Delivery Systems Using Microfluidic Technology: A Review. Pharm. Front. 2024, 6, e69–e100. [Google Scholar] [CrossRef]
- Alavi, S.E.; Alharthi, S.; Alavi, S.F.; Zeinab Alavi, S.; Zahra, G.E.; Raza, A.; Shahmabadi, H.E. Microfluidics for Personalized Drug Delivery. Drug Discov. Today 2024, 29, 103936. [Google Scholar] [CrossRef]
- Baroud, C.N.; Gallaire, F.; Dangla, R. Dynamics of Microfluidic Droplets. Lab Chip 2010, 10, 2032. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, J.R.D.O.; De La Torre, L.G.; Costa, A.L.R. Droplet-Based Microfluidics as a Platform to Design Food-Grade Delivery Systems Based on the Entrapped Compound Type. Foods 2023, 12, 3385. [Google Scholar] [CrossRef] [PubMed]
- Günther, A.; Jensen, K.F. Multiphase Microfluidics: From Flow Characteristics to Chemical and Materials Synthesis. Lab Chip 2006, 6, 1487–1503. [Google Scholar] [CrossRef]
- Raji, F.; Kahani, A.; Sahabi, M.; Rahbar-kalishami, A.; Padrela, L. Investigating the Effectiveness of the Main Channel in Microfluidic Liquid-Liquid Extraction Process. Int. Commun. Heat Mass Transf. 2023, 147, 106986. [Google Scholar] [CrossRef]
- Jiang, Z.; Shi, H.; Tang, X.; Qin, J. Recent Advances in Droplet Microfluidics for Single-Cell Analysis. TrAC Trends Anal. Chem. 2023, 159, 116932. [Google Scholar] [CrossRef]
- De Menech, M.; Garstecki, P.; Jousse, F.; Stone, H.A. Transition from Squeezing to Dripping in a Microfluidic T-Shaped Junction. J. Fluid Mech. 2008, 595, 141–161. [Google Scholar] [CrossRef]
- Kovalchuk, N.M.; Simmons, M.J.H. Review of the Role of Surfactant Dynamics in Drop Microfluidics. Adv. Colloid Interface Sci. 2023, 312, 102844. [Google Scholar] [CrossRef]
- Montanero, J.M.; Gañán-Calvo, A.M. Dripping, Jetting and Tip Streaming. Rep. Prog. Phys. 2020, 83, 097001. [Google Scholar] [CrossRef]
- Almeida, D.R.S.; Gil, J.F.; Guillot, A.J.; Li, J.; Pinto, R.J.B.; Santos, H.A.; Gonçalves, G. Advances in Microfluidic-based Core@Shell Nanoparticles Fabrication for Cancer Applications. Adv. Healthc. Mater. 2024, 2400946. [Google Scholar] [CrossRef]
- Fiorini, G.S.; Chiu, D.T. Disposable Microfluidic Devices: Fabrication, Function, and Application. BioTechniques 2005, 38, 429–446. [Google Scholar] [CrossRef]
- Scott, S.; Ali, Z. Fabrication Methods for Microfluidic Devices: An Overview. Micromachines 2021, 12, 319. [Google Scholar] [CrossRef] [PubMed]
- Aralekallu, S.; Boddula, R.; Singh, V. Development of Glass-Based Microfluidic Devices: A Review on Its Fabrication and Biologic Applications. Mater. Des. 2023, 225, 111517. [Google Scholar] [CrossRef]
- Mohammadi, M.; Ahmed Qadir, S.; Mahmood Faraj, A.; Hamid Shareef, O.; Mahmoodi, H.; Mahmoudi, F.; Moradi, S. Navigating the Future: Microfluidics Charting New Routes in Drug Delivery. Int. J. Pharm. 2024, 124142. [Google Scholar] [CrossRef]
- Niculescu, A.-G.; Chircov, C.; Bîrcă, A.C.; Grumezescu, A.M. Fabrication and Applications of Microfluidic Devices: A Review. Int. J. Mol. Sci. 2021, 22, 2011. [Google Scholar] [CrossRef]
- Elvira, K.S.; Gielen, F.; Tsai, S.S.H.; Nightingale, A.M. Materials and Methods for Droplet Microfluidic Device Fabrication. Lab Chip 2022, 22, 859–875. [Google Scholar] [CrossRef]
- Dendukuri, D.; Doyle, P.S. The Synthesis and Assembly of Polymeric Microparticles Using Microfluidics. Adv. Mater. 2009, 21, 4071–4086. [Google Scholar] [CrossRef]
- El Itawi, H.; Fadlallah, S.; Perré, P.; Allais, F. Microfluidics for Polymer Microparticles: Opinion on Sustainability and Scalability. Sustain. Chem. 2023, 4, 171–183. [Google Scholar] [CrossRef]
- Campos, E.; Branquinho, J.; Carreira, A.S.; Carvalho, A.; Coimbra, P.; Ferreira, P.; Gil, M.H. Designing Polymeric Microparticles for Biomedical and Industrial Applications. Eur. Polym. J. 2013, 49, 2005–2021. [Google Scholar] [CrossRef]
- Mok, J.H.; Niu, Y.; Zhao, Y. Continuous-Flow Viscoelastic Profiling of Calcium Alginate Hydrogel Microspheres Using a Microfluidic Lab-on-a-Chip Device. Food Hydrocoll. 2024, 153, 109979. [Google Scholar] [CrossRef]
- Yang, D.; Gao, K.; Bai, Y.; Lei, L.; Jia, T.; Yang, K.; Xue, C. Microfluidic Synthesis of Chitosan-Coated Magnetic Alginate Microparticles for Controlled and Sustained Drug Delivery. Int. J. Biol. Macromol. 2021, 182, 639–647. [Google Scholar] [CrossRef]
- Leontidou, T.; Yu, Z.; Hess, J.; Geisler, K.; Smith, A.G.; Coyne, A.; Abell, C. Microfluidic Preparation of Composite Hydrogel Microparticles for the Staining of Microalgal Cells. Colloids Surf. B Biointerfaces 2023, 221, 113026. [Google Scholar] [CrossRef] [PubMed]
- Zdunek, A.; Pieczywek, P.M.; Cybulska, J. The Primary, Secondary, and Structures of Higher Levels of Pectin Polysaccharides. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1101–1117. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Kanyuka, K.; Papp-Rupar, M. Pectin: A Critical Component in Cell-Wall-Mediated Immunity. Trends Plant Sci. 2023, 28, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Voragen, A.G.J.; Coenen, G.-J.; Verhoef, R.P.; Schols, H.A. Pectin, a Versatile Polysaccharide Present in Plant Cell Walls. Struct. Chem. 2009, 20, 263–275. [Google Scholar] [CrossRef]
- Kaczmarska, A. Effect of Enzymatic Modification on the Structure and Rheological Properties of Diluted Alkali-Soluble Pectin Fraction Rich in RG-I. Sci. Rep. 2024, 14, 11454. [Google Scholar] [CrossRef] [PubMed]
- Sabater, C.; Villamiel, M.; Montilla, A. Integral Use of Pectin-Rich by-Products in a Biorefinery Context: A Holistic Approach. Food Hydrocoll. 2022, 128, 107564. [Google Scholar] [CrossRef]
- Reichembach, L.H.; Petkowicz, C.L.d.O. Pectins from Alternative Sources and Uses beyond Sweets and Jellies: An Overview. Food Hydrocoll. 2021, 118, 106824. [Google Scholar] [CrossRef]
- Toniazzo, T.; Fabi, J.P. Versatile Polysaccharides for Application to Semi-Solid and Fluid Foods: The Pectin Case. Fluids 2023, 8, 243. [Google Scholar] [CrossRef]
- Pedrosa, L.D.F.; Nascimento, K.R.; Soares, C.G.; Oliveira, D.P.D.; De Vos, P.; Fabi, J.P. Unveiling Plant-Based Pectins: Exploring the Interplay of Direct Effects, Fermentation, and Technological Applications in Clinical Research with a Focus on the Chemical Structure. Plants 2023, 12, 2750. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, D.J. Structure and Growth of Plant Cell Walls. Nat. Rev. Mol. Cell Biol. 2024, 25, 340–358. [Google Scholar] [CrossRef]
- Mohnen, D. Pectin Structure and Biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef]
- Neelamegham, S.; Aoki-Kinoshita, K.; Bolton, E.; Frank, M.; Lisacek, F.; Lütteke, T.; O’Boyle, N.; Packer, N.H.; Stanley, P.; Toukach, P.; et al. Updates to the Symbol Nomenclature for Glycans Guidelines. Glycobiology 2019, 29, 620–624. [Google Scholar] [CrossRef]
- Riyamol; Gada Chengaiyan, J.; Rana, S.S.; Ahmad, F.; Haque, S.; Capanoglu, E. Recent Advances in the Extraction of Pectin from Various Sources and Industrial Applications. ACS Omega 2023, 8, 46309–46324. [Google Scholar] [CrossRef]
- Vasco-Correa, J.; Zapata Zapata, A.D. Enzymatic Extraction of Pectin from Passion Fruit Peel (Passiflora Edulis f. Flavicarpa) at Laboratory and Bench Scale. LWT 2017, 80, 280–285. [Google Scholar] [CrossRef]
- Mao, Y.; Robinson, J.P.; Binner, E.R. Current Status of Microwave-Assisted Extraction of Pectin. Chem. Eng. J. 2023, 473, 145261. [Google Scholar] [CrossRef]
- Liu, D.; Xia, W.; Liu, J.; Wang, X.; Xue, J. Ultrasound-Assisted Alkali Extraction of RG-I Enriched Pectin from Thinned Young Apples: Structural Characterization and Gelling Properties. Food Hydrocoll. 2024, 151, 109879. [Google Scholar] [CrossRef]
- Sharifi, A.; Hamidi-Esfahani, Z.; Ahmadi Gavlighi, H.; Saberian, H. Assisted Ohmic Heating Extraction of Pectin from Pomegranate Peel. Chem. Eng. Process.—Process Intensif. 2022, 172, 108760. [Google Scholar] [CrossRef]
- Yilmaz-Turan, S.; Gál, T.; Lopez-Sanchez, P.; Martinez, M.M.; Menzel, C.; Vilaplana, F. Modulating Temperature and pH during Subcritical Water Extraction Tunes the Molecular Properties of Apple Pomace Pectin as Food Gels and Emulsifiers. Food Hydrocoll. 2023, 145, 109148. [Google Scholar] [CrossRef]
- Turan, O.; Isci, A.; Yılmaz, M.S.; Tolun, A.; Sakiyan, O. Microwave-Assisted Extraction of Pectin from Orange Peel Using Deep Eutectic Solvents. Sustain. Chem. Pharm. 2024, 37, 101352. [Google Scholar] [CrossRef]
- Braccini, I.; Pérez, S. Molecular Basis of Ca2+ -Induced Gelation in Alginates and Pectins: The Egg-Box Model Revisited. Biomacromolecules 2001, 2, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Egg-Box Model-Based Gelation of Alginate and Pectin: A Review. Carbohydr. Polym. 2020, 242, 116389. [Google Scholar] [CrossRef] [PubMed]
- Marić, M.; Grassino, A.N.; Zhu, Z.; Barba, F.J.; Brnčić, M.; Rimac Brnčić, S. An Overview of the Traditional and Innovative Approaches for Pectin Extraction from Plant Food Wastes and By-Products: Ultrasound-, Microwaves-, and Enzyme-Assisted Extraction. Trends Food Sci. Technol. 2018, 76, 28–37. [Google Scholar] [CrossRef]
- Tan, H.; Nie, S. Deciphering Diet-Gut Microbiota-Host Interplay: Investigations of Pectin. Trends Food Sci. Technol. 2020, 106, 171–181. [Google Scholar] [CrossRef]
- Tang, X.; De Vos, P. Structure-Function Effects of Different Pectin Chemistries and Its Impact on the Gastrointestinal Immune Barrier System. Crit. Rev. Food Sci. Nutr. 2023, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Guan, S.; Yuan, Y.; Wang, Y.; Mst Nushrat, Y.; Liu, Y.; Tong, Y.; Yu, S.; Hua, X. The Digestive Behavior of Pectin in Human Gastrointestinal Tract: A Review on Fermentation Characteristics and Degradation Mechanism. Crit. Rev. Food Sci. Nutr. 2023, 1–24. [Google Scholar] [CrossRef]
- Brouns, F.; Theuwissen, E.; Adam, A.; Bell, M.; Berger, A.; Mensink, R.P. Cholesterol-Lowering Properties of Different Pectin Types in Mildly Hyper-Cholesterolemic Men and Women. Eur. J. Clin. Nutr. 2012, 66, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, L.D.F.; Fabi, J.P. Dietary Fiber as a Wide Pillar of Colorectal Cancer Prevention and Adjuvant Therapy. Crit. Rev. Food Sci. Nutr. 2023, 64, 6177–6197. [Google Scholar] [CrossRef]
- Das, S. Pectin Based Multi-Particulate Carriers for Colon-Specific Delivery of Therapeutic Agents. Int. J. Pharm. 2021, 605, 120814. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, Z. Citrus Pectin-Derived Carbon Microspheres with Superior Adsorption Ability for Methylene Blue. Nanomaterials 2017, 7, 161. [Google Scholar] [CrossRef]
- Michel, C.R.; Martínez-Preciado, A.H. CO Sensing Properties of Novel Nanostructured La2O3 Microspheres. Sens. Actuators B Chem. 2015, 208, 355–362. [Google Scholar] [CrossRef]
- Chou, W.-M.; Wang, L.-L.; Yu, H.H. Electrophoretic Ink Display Prepared by Jelly Fig Pectin/Gelatin Microspheres. Smart Sci. 2015, 3, 74–79. [Google Scholar] [CrossRef][Green Version]
- Zamri, N.I.I.; Zulmajdi, S.L.N.; Daud, N.Z.A.; Mahadi, A.H.; Kusrini, E.; Usman, A. Insight into the Adsorption Kinetics, Mechanism, and Thermodynamics of Methylene Blue from Aqueous Solution onto Pectin-Alginate-Titania Composite Microparticles. SN Appl. Sci. 2021, 3, 222. [Google Scholar] [CrossRef]
- Munarin, F.; Giuliano, L.; Bozzini, S.; Tanzi, M.C.; Petrini, P. Mineral Phase Deposition on Pectin Microspheres. Mater. Sci. Eng. C 2010, 30, 491–496. [Google Scholar] [CrossRef]
- Dini, C.; Islan, G.A.; De Urraza, P.J.; Castro, G.R. Novel Biopolymer Matrices for Microencapsulation of Phages: Enhanced Protection Against Acidity and Protease Activity. Macromol. Biosci. 2012, 12, 1200–1208. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, Y.; Shuai, X.; Liang, R.; Chen, J.; Liu, C. Pectin/Activated Carbon-Based Porous Microsphere for Pb2+ Adsorption: Characterization and Adsorption Behaviour. Polymers 2021, 13, 2453. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, Y.; Ding, G.; Geng, Q.; Zhu, J.; Guo, M.; Duan, Y.; Wang, B.; Cao, Y. Synthesis, Characterization, and Application of Microbe-Triggered Controlled-Release Kasugamycin–Pectin Conjugate. J. Agric. Food Chem. 2015, 63, 4263–4268. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Huang, X.; Gao, Y.; Huang, X.; Xiao, H.; McClements, D.J. Core–Shell Biopolymer Nanoparticle Delivery Systems: Synthesis and Characterization of Curcumin Fortified Zein–Pectin Nanoparticles. Food Chem. 2015, 182, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.-Z.; Huang, X.-N.; Wu, Z.; Yin, S.-W.; Zhu, J.; Tang, C.-H.; Yang, X.-Q. Fabrication of Zein/Pectin Hybrid Particle-Stabilized Pickering High Internal Phase Emulsions with Robust and Ordered Interface Architecture. J. Agric. Food Chem. 2018, 66, 11113–11123. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Miyauchi, M.; Rahmatika, A.M.; Cao, K.L.A.; Tanabe, E.; Ogi, T. Enhanced Protein Adsorption Capacity of Macroporous Pectin Particles with High Specific Surface Area and an Interconnected Pore Network. ACS Appl. Mater. Interfaces 2022, 14, 14435–14446. [Google Scholar] [CrossRef] [PubMed]
- Méndez, D.A.; Schroeter, B.; Martínez-Abad, A.; Fabra, M.J.; Gurikov, P.; López-Rubio, A. Pectin-Based Aerogel Particles for Drug Delivery: Effect of Pectin Composition on Aerogel Structure and Release Properties. Carbohydr. Polym. 2023, 306, 120604. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Saipul Bahri, N.S.N.; Rahmatika, A.M.; Cao, K.L.A.; Hirano, T.; Ogi, T. Rapid Indomethacin Release from Porous Pectin Particles as a Colon-Targeted Drug Delivery System. ACS Appl. Bio Mater. 2023, 6, 2725–2737. [Google Scholar] [CrossRef] [PubMed]
- Osvaldt Rosales, T.K.; Pessoa da Silva, M.; Lourenço, F.R.; Aymoto Hassimotto, N.M.; Fabi, J.P. Nanoencapsulation of Anthocyanins from Blackberry (Rubus Spp.) through Pectin and Lysozyme Self-Assembling. Food Hydrocoll. 2021, 114, 106563. [Google Scholar] [CrossRef]
- Da Silva, M.P.; Rosales, T.K.O.; de Pedrosa, L.F.; Fabi, J.P. Creation of a New Proof-of-Concept Pectin/Lysozyme Nanocomplex as Potential β-Lactose Delivery Matrix: Structure and Thermal Stability Analyses. Food Hydrocoll. 2023, 134, 108011. [Google Scholar] [CrossRef]
- Han, S.S.; Ji, S.M.; Park, M.J.; Suneetha, M.; Uthappa, U.T. Pectin Based Hydrogels for Drug Delivery Applications: A Mini Review. Gels 2022, 8, 834. [Google Scholar] [CrossRef]
- Li, D.; Li, J.; Dong, H.; Li, X.; Zhang, J.; Ramaswamy, S.; Xu, F. Pectin in Biomedical and Drug Delivery Applications: A Review. Int. J. Biol. Macromol. 2021, 185, 49–65. [Google Scholar] [CrossRef]
- Gutierrez-Alvarado, K.; Chacón-Cerdas, R.; Starbird-Perez, R. Pectin Microspheres: Synthesis Methods, Properties, and Their Multidisciplinary Applications. Chemistry 2022, 4, 121–136. [Google Scholar] [CrossRef]
- Huang, M.; Sun, Y.; Tan, C. Recent Advances in Emerging Pectin-Derived Nanocarriers for Controlled Delivery of Bioactive Compounds. Food Hydrocoll. 2023, 140, 108682. [Google Scholar] [CrossRef]
- Aydin, Z.; Akbugˇa, J. Preparation and Evaluation of Pectin Beads. Int. J. Pharm. 1996, 137, 133–136. [Google Scholar] [CrossRef]
- Günter, E.A.; Popeyko, O.V. Calcium Pectinate Gel Beads Obtained from Callus Cultures Pectins as Promising Systems for Colon-Targeted Drug Delivery. Carbohydr. Polym. 2016, 147, 490–499. [Google Scholar] [CrossRef]
- de Moura, S.C.S.R.; Berling, C.L.; Germer, S.P.M.; Alvim, I.D.; Hubinger, M.D. Encapsulating Anthocyanins from Hibiscus Sabdariffa L. Calyces by Ionic Gelation: Pigment Stability during Storage of Microparticles. Food Chem. 2018, 241, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, G.L.A.; Pacheco, S.; Ribeiro, A.P.O.; Galdeano, M.C.; Gomes, F.S.; Tonon, R.V. Encapsulation of a Lycopene-Rich Watermelon Concentrate in Alginate and Pectin Beads: Characterization and Stability. LWT 2019, 116, 108589. [Google Scholar] [CrossRef]
- Heumann, A.; Assifaoui, A.; Da Silva Barreira, D.; Thomas, C.; Briandet, R.; Laurent, J.; Beney, L.; Lapaquette, P.; Guzzo, J.; Rieu, A. Intestinal Release of Biofilm-like Microcolonies Encased in Calcium-Pectinate Beads Increases Probiotic Properties of Lacticaseibacillus Paracasei. npj Biofilms Microbiomes 2020, 6, 44. [Google Scholar] [CrossRef]
- Cava, E.L.; Gerbino, E.; Sgroppo, S.C.; Gómez-Zavaglia, A. Pectin Hydrolysates from Different Cultivars of Pink/Red and White Grapefruits (Citrus Paradisi [Macf.]) as Culture and Encapsulating Media for Lactobacillus Plantarum. J. Food Sci. 2019, 84, 1776–1783. [Google Scholar] [CrossRef]
- Raghav, N.; Vashisth, C.; Mor, N.; Arya, P.; Sharma, M.R.; Kaur, R.; Bhatti, S.P.; Kennedy, J.F. Recent Advances in Cellulose, Pectin, Carrageenan and Alginate-Based Oral Drug Delivery Systems. Int. J. Biol. Macromol. 2023, 244, 125357. [Google Scholar] [CrossRef]
- Souza, F.N.; Gebara, C.; Ribeiro, M.C.E.; Chaves, K.S.; Gigante, M.L.; Grosso, C.R.F. Production and Characterization of Microparticles Containing Pectin and Whey Proteins. Food Res. Int. 2012, 49, 560–566. [Google Scholar] [CrossRef]
- Lemos, T.S.A.; De Souza, J.F.; Fajardo, A.R. Magnetic Microspheres Based on Pectin Coated by Chitosan towards Smart Drug Release. Carbohydr. Polym. 2021, 265, 118013. [Google Scholar] [CrossRef]
- Günter, E.A.; Popeyko, O.V.; Belozerov, V.S.; Martinson, E.A.; Litvinets, S.G. Physicochemical and Swelling Properties of Composite Gel Microparticles Based on Alginate and Callus Cultures Pectins with Low and High Degrees of Methylesterification. Int. J. Biol. Macromol. 2020, 164, 863–870. [Google Scholar] [CrossRef]
- Ngouémazong, E.D.; Christiaens, S.; Shpigelman, A.; Van Loey, A.; Hendrickx, M. The Emulsifying and Emulsion-Stabilizing Properties of Pectin: A Review. Comp. Rev. Food Sci. Food Safe 2015, 14, 705–718. [Google Scholar] [CrossRef]
- Feng, L.; Wang, Y.; Liu, T.; Zhao, C.; Chen, Y.; Wang, F.; Bao, Y.; Zheng, J. Pectin-Based Emulsion Gels Prepared by Acidic and Ionotropic Methods for Intestinal Targeted Delivery in Vitro. Food Hydrocoll. 2024, 154, 110118. [Google Scholar] [CrossRef]
- Ogończyk, D.; Siek, M.; Garstecki, P. Microfluidic Formulation of Pectin Microbeads for Encapsulation and Controlled Release of Nanoparticles. Biomicrofluidics 2011, 5, 013405. [Google Scholar] [CrossRef]
- Marquis, M.; Renard, D.; Cathala, B. Microfluidic Generation and Selective Degradation of Biopolymer-Based Janus Microbeads. Biomacromolecules 2012, 13, 1197–1203. [Google Scholar] [CrossRef]
- Marquis, M.; Davy, J.; Cathala, B.; Fang, A.; Renard, D. Microfluidics Assisted Generation of Innovative Polysaccharide Hydrogel Microparticles. Carbohydr. Polym. 2015, 116, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Marquis, M.; Davy, J.; Fang, A.; Renard, D. Microfluidics-Assisted Diffusion Self-Assembly: Toward the Control of the Shape and Size of Pectin Hydrogel Microparticles. Biomacromolecules 2014, 15, 1568–1578. [Google Scholar] [CrossRef] [PubMed]
- Fang, A.; Cathala, B. Smart Swelling Biopolymer Microparticles by a Microfluidic Approach: Synthesis, in Situ Encapsulation and Controlled Release. Colloids Surf. B Biointerfaces 2011, 82, 81–86. [Google Scholar] [CrossRef]
- Kim, C.; Park, K.; Kim, J.; Jeong, S.; Lee, C. Microfluidic Synthesis of Monodisperse Pectin Hydrogel Microspheres Based on in Situ Gelation and Settling Collection. J. Chem. Technol. Biotechnol. 2017, 92, 201–209. [Google Scholar] [CrossRef]
- Noh, J.; Kim, J.; Kim, J.S.; Chung, Y.S.; Chang, S.T.; Park, J. Microencapsulation by Pectin for Multi-Components Carriers Bearing Both Hydrophobic and Hydrophilic Active Agents. Carbohydr. Polym. 2018, 182, 172–179. [Google Scholar] [CrossRef]
- Rajabnejad Keleshteri, A.; Moztarzadeh, F.; Farokhi, M.; Mehrizi, A.A.; Basiri, H.; Mohseni, S.S. Preparation of Microfluidic-Based Pectin Microparticles Loaded Carbon Dots Conjugated with BMP-2 Embedded in Gelatin-Elastin-Hyaluronic Acid Hydrogel Scaffold for Bone Tissue Engineering Application. Int. J. Biol. Macromol. 2021, 184, 29–41. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, A.; Yan, Z.; Shen, B.; Zhu, L.; Jiang, L. Enhanced Tolerance to Environmental Stress of Clostridium Butyricum Spore Encapsulated in Citrus Peel Pectin Polysaccharide for Colitis Therapy. Food Biosci. 2024, 60, 104436. [Google Scholar] [CrossRef]
- Wang, M.; Cheng, Y.; Li, X.; Nian, L.; Yuan, B.; Cheng, S.; Wang, S.; Cao, C. Effects of Microgels Fabricated by Microfluidic on the Stability, Antioxidant, and Immunoenhancing Activities of Aquatic Protein. J. Future Foods 2025, 5, 57–67. [Google Scholar] [CrossRef]
- Kawakatsu, T.; Trägårdh, G.; Trägårdh, C. Production of W/O/W Emulsions and S/O/W Pectin Microcapsules by Microchannel Emulsification. Colloids Surf. A Physicochem. Eng. Asp. 2001, 189, 257–264. [Google Scholar] [CrossRef]
- Vladisavljević, G.T.; Kobayashi, I.; Nakajima, M. Production of Uniform Droplets Using Membrane, Microchannel and Microfluidic Emulsification Devices. Microfluid. Nanofluid. 2012, 13, 151–178. [Google Scholar] [CrossRef]
- Cheung, R.; Ng, T.; Wong, J. Marine Peptides: Bioactivities and Applications. Mar. Drugs 2015, 13, 4006–4043. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Anil, S.; Kim, S.-K.; Shim, M. Marine Fish Proteins and Peptides for Cosmeceuticals: A Review. Mar. Drugs 2017, 15, 143. [Google Scholar] [CrossRef]
- Zhang, W.; Boateng, I.D.; Xu, J. Novel Marine Proteins as a Global Protein Supply and Human Nutrition: Extraction, Bioactivities, Potential Applications, Safety Assessment, and Deodorization Technologies. Trends Food Sci. Technol. 2024, 143, 104283. [Google Scholar] [CrossRef]
- Nadine, S.; Chung, A.; Diltemiz, S.E.; Yasuda, B.; Lee, C.; Hosseini, V.; Karamikamkar, S.; De Barros, N.R.; Mandal, K.; Advani, S.; et al. Advances in Microfabrication Technologies in Tissue Engineering and Regenerative Medicine. Artif. Organs 2022, 46, E211–E243. [Google Scholar] [CrossRef]
- Sherstneva, A.A.; Demina, T.S.; Monteiro, A.P.F.; Akopova, T.A.; Grandfils, C.; Ilangala, A.B. Biodegradable Microparticles for Regenerative Medicine: A State of the Art and Trends to Clinical Application. Polymers 2022, 14, 1314. [Google Scholar] [CrossRef] [PubMed]
- Poon, B.; Kha, T.; Tran, S.; Dass, C.R. Bone Morphogenetic Protein-2 and Bone Therapy: Successes and Pitfalls. J. Pharm. Pharmacol. 2016, 68, 139–147. [Google Scholar] [CrossRef]
- Halloran, D.; Durbano, H.W.; Nohe, A. Bone Morphogenetic Protein-2 in Development and Bone Homeostasis. J. Dev. Biol. 2020, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Kapoor, B.; Gulati, M. Bacterial Consortia-The Latest Arsenal to Inflammatory Bowel Disease Bacteriotherapy. Med. Microecol. 2024, 20, 100107. [Google Scholar] [CrossRef]
- De Gennes, P.G. Soft Matter. Science 1992, 256, 495–497. [Google Scholar] [CrossRef]
- Su, H.; Hurd Price, C.-A.; Jing, L.; Tian, Q.; Liu, J.; Qian, K. Janus Particles: Design, Preparation, and Biomedical Applications. Mater. Today Bio 2019, 4, 100033. [Google Scholar] [CrossRef]
- Walther, A.; Müller, A.H.E. Janus Particles: Synthesis, Self-Assembly, Physical Properties, and Applications. Chem. Rev. 2013, 113, 5194–5261. [Google Scholar] [CrossRef]
- Hu, J.; Zhou, S.; Sun, Y.; Fang, X.; Wu, L. Fabrication, Properties and Applications of Janus Particles. Chem. Soc. Rev. 2012, 41, 4356. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).