Abstract
Solving problems of detonation control is associated with obtaining detailed information about the gas dynamics accompanying the detonation process. This paper focuses on the dynamics of real gas flow through a plane detonation wave. The influence of real gas parameters on the Chapman–Jouguet detonation process has been studied. The process is described using the Rankine–Hugoniot system of equations. To model the thermodynamic properties of a real gas, the van der Waals equation of state is used. Equations are obtained to determine the ratio of speeds and pressures during the passage of a wave. The influence of van der Waals parameters on changes in the parameters of the detonation process was elucidated. An increase in parameter A slows down the increase in pressure in the detonation wave, and an increase in parameter B enhances it. Differences in the speed of combustion products for ideal and real gases are shown. For an ideal gas, combustion products flow from the detonation front at a critical (sonic) speed. For a van der Waals gas, the speed of combustion products may be greater than the critical one. Moreover, both factors, additional pressure (A) and additional volume (B), lead to acceleration of combustion products. Effects of heat release on the process parameters were elucidated.
1. Introduction
The study of detonation processes in gaseous media is of interest for the aviation, aerospace and coal mining industries, as well as for hydrogen energy [1,2]. The shock waves generated in physical processes of high energy density are very intense. Achieving compression to ignite nuclear fuel requires a pressure of the order of a gigabar. Under such conditions, the use of the ideal gas approximation in modeling the detonation process is not applicable. It is necessary to consider the equation of state of a real gas, the effects of endothermicity and exothermicity upon impact, and realistic boundary conditions [3]. In [3], the stability of shock waves in a van der Waals gas is analyzed and the effect of heat release on the shock wave stability is shown. The effects of real gas parameters on the critical conditions for detonation initiation in a nonstationary flow are considered in [4]. The effects of a real gas amplify with an increase in the reduced activation energy and/or a decrease in the heat capacity of the gas. A simple but reliable method for evaluating the effect of real gas properties on detonation initiation under high pressure conditions is also given in [4]. Paper [5] presents the results of a study of anomalous waves in the detonation dynamics of a non-ideal gas. Modeling of fluid flow using the non-ideal gas equation of state affects the speed of sound, the speed of the wave, and the speed of the reaction. The effect of van der Waals gas parameters on the reaction rate and wave velocity is shown.
The group invariance method is used to analyze the propagation of a strong shock wave in a rotating non-ideal gas with an azimuthally magnetic field [6,7,8]. The effect of changing the gas non-ideality parameter, the adiabatic index, the Mach number, and the environmental azimuth velocity index on the shock wave strength and flow parameters is shown. It was revealed that these parameters have a damping effect on the shock wave.
In [9], the influence of a real gas on stable planar detonation in a mixture of hydrogen and air at elevated pressure is studied. It is shown that the non-ideal equation of state and the corresponding thermodynamic functions increase the Chapman–Jouguet velocity but decrease the temperature after impact compared to the ideal gas model. The phase transition caused by a shock wave in a real gas is studied in [10,11,12]. The influence of the unperturbed thermodynamic state of a real gas on the shock wave parameters and phase transitions in it is shown. A complete description of the characteristics of shock waves propagating in a van der Waals fluid is given [12]. The strength of these shock waves can vary from weak to strong. The study was performed using the theory of hyperbolic systems. The results are compared with numerical calculations.
The results of studying the propagation of shock waves in a non-ideal gas are presented in [13,14]. The authors demonstrate the effect of the parameters of the van der Waals gas on the intensity of heat and mass transfer processes during the passage of the shock wave front. In [15], exact closed-form solutions for sonic shocks of finite amplitude were obtained for the case of a van der Waals gas with a constant specific heat. Calculation examples are given that include sonic booms embedded in smooth inviscid flows.
Recent theoretical results on the dynamics of gaseous detonations are presented in [16]. An asymptotic analysis is carried out with the preservation of the physical mechanisms governing the modifications of the internal structure of detonation.
The objective of the present study is to perform analytical modeling of the variation in the parameters of the van der Waals gas flow as it passes through a direct shock wave. Of interest is the study of the influence of the degree of compression of real gases and van der Waals constants on the shock adiabat, as well as on the dynamics of velocity jumps.
Advances in investigations of the dynamics of detonation waves contribute to further research of the effects of thermodynamic parameters of real gases on the detonation process. This will enable a better understanding of the physics of the process and help to develop means of controlling the detonation process. The objective of the present work is to theoretically study the detonation process in real gases, determine the influence of the gas equation of state on the detonation parameters, and compare the new results with those of the ideal gas model. The novelty of the work lies in obtaining theoretical results that make it possible to evaluate the influence of van der Waals gas parameters on the characteristics of the detonation process.
2. Mathematical Model
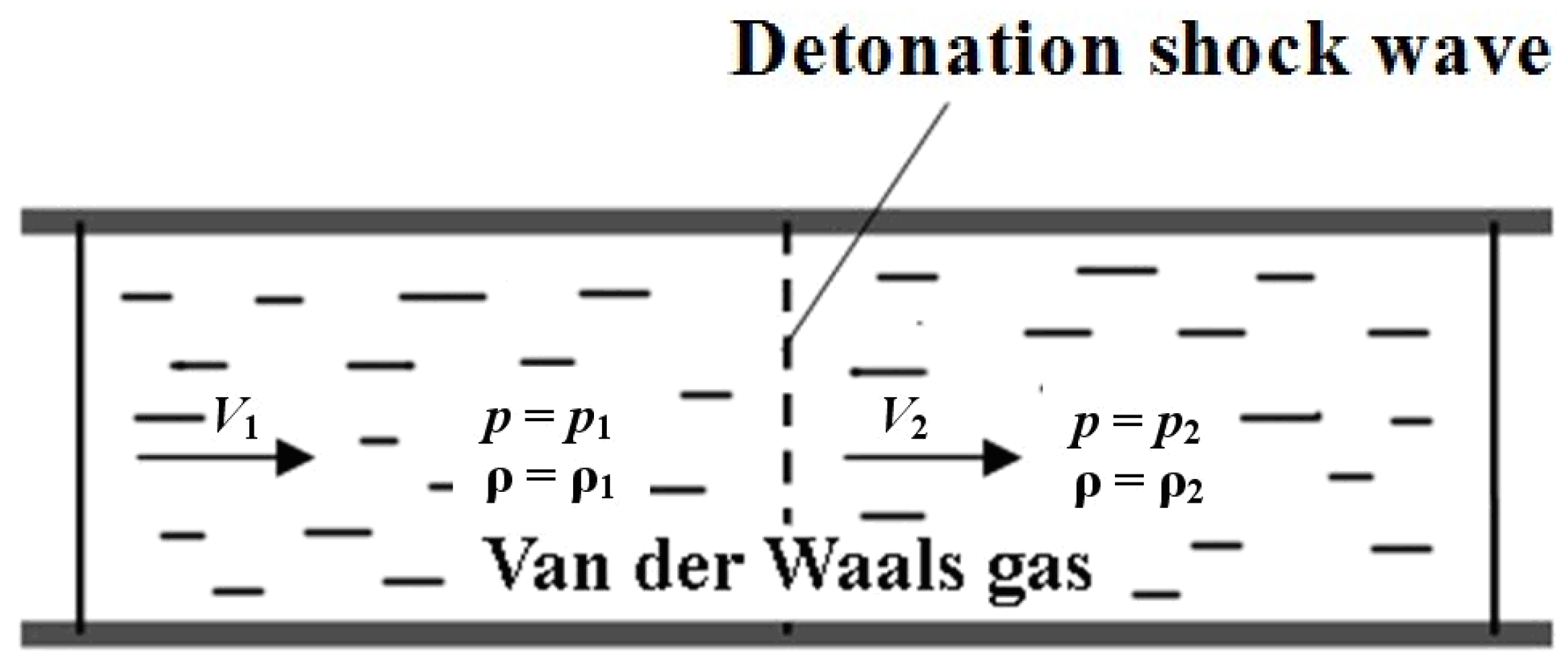
The problem statement is schematically depicted in Figure 1. It was noted above that due to the passage of the flow through the shock wave, its velocity decreases from supersonic to sonic values. When the velocity drops, the main physical characteristics of the flow (density, pressure) also vary. Such a sharp variation in flow parameters (the so-called shock wave) was first considered in [17,18,19,20] as a discontinuity of these parameters (before and after the shock wave). This process can be considered as adiabatic (but not isentropic). In this case, the enthalpy of the flow before and after the discontinuity of its parameters does not change, while the effects of surface friction can be neglected (Figure 1).
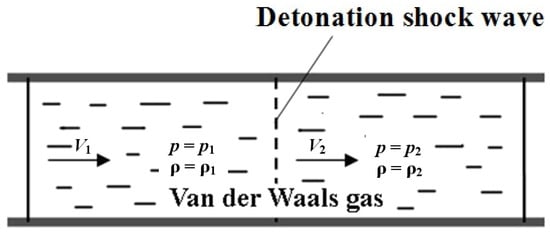
Figure 1.
Schematic representation of the shock wave problem.
The functional dependence of the flow parameters before the shock or combustion wave (deflagration or detonation) in a one-dimensional fluid flow or one-dimensional deformation in a solid is called the Rankine–Hugoniot condition, or the Rankine–Hugoniot jump condition, as well as the Rankine–Hugoniot ratio. They are named after the Scottish engineer and physicist William John McCorn Rankin [17] and the French engineer Pierre Henri Hugoniot [18,19], who made the main contribution to the study of this problem.
Thus, the dynamics of gas flow through a direct shock wave is described using the Rankine–Hugoniot system of equations [17,18,19], which includes the mass conservation law
and the momentum conservation law
as well as the law of conservation of total enthalpy
Here, V is velocity, is density of a pure gas, p is pressure, h is enthalpy, and q measures the energy removed (q < 0) or released (q > 0) per unit mass by the corresponding process undergoing across the shock; subscript “1” denotes the parameters before the shock wave, subscript “2” denotes the parameters after the shock wave.
System (1)–(3) is closed with the equation of state of the van der Waals gas
where is individual (specific) gas constant, α and β are van der Waals constants, v = 1/ is specific volume, and T is temperature.
Parameter α characterizes the additional pressure, and parameter β characterizes the additional volume of space not filled with molecules.
Using Equation (4), one can obtain a relation for enthalpy
where cv is heat capacity at constant volume (isochoric heat capacity).
Now the law of conservation of total enthalpy (3) can be re-cast as
3. Hugoniot Equation
Next, we transform Equation (2) with allowance for Equation (1) to the following form
and multiply this equation by
As a result, we get
It follows further from Equation (6) that
Comparing Equations (9) and (10), one can obtain
where
is isentropic expansion exponent, cp is specific heat capacity at constant pressure and is the specific isochoric heat capacity, a1 is the speed of sound.
Solving Equation (11) with respect to the value of p2/p1, one can obtain
In dimensionless form, this equation is
where
Equations (15) and (16) describe the modified Hugoniot equation for detonation. For ideal gas (A = B = 0) and flow without energy removed or released, Equation (16) reduces to
which is the well-established Hugoniot adiabatic for pure ordinary gases [21]. Here .
Equation (16) exhibits an asymptote for / expressed as
For the condition expressed as Equation (19), the pressure jump (16) becomes infinite.
For an ideal gas (A = B = 0), Equation (19) for the density jump is reduced to
Hence, air under conditions when it can be considered as an ideal gas (k = 1.4), when passing through a shock wave, cannot increase its density more than six times. Equation (19) shows that the asymptote of the limiting increase in density shifts to the region of smaller values / with an increase in the parameter value . As is known, the parameter β describes an additional volume of space that is not filled with molecules. Consequently, an increase in this volume leads to the fact that the distance between the molecules increases, and the concentration of molecules decreases. Therefore, with increasing pressure, the growth of density slows down. For example, if there are thousands of molecules in the volume, then with an increase in pressure, their concentration will increase by some amount and, consequently, the density will also increase. If there are only two molecules in the volume, then the increase in pressure will in no way affect the concentration. Therefore, the density will not increase. In addition, the value of the adiabatic exponent affects the position of the density asymptote. With an increase in the value of this parameter, the position of the asymptote also shifts towards smaller values of /. It also follows from Equation (19) that the parameter A does not affect the position of the asymptote in any way. Recall that this parameter characterizes the additional pressure arising in the near-wall layer. In the problem under consideration, there is no interaction with a solid surface. Evidently, therefore, this factor does not affect the position of the density asymptote.
In [13], the equation for ultimate compression was obtained under assumption that
This equation has the following form:
As can be seen, the use of approximation (21) somewhat weakens the influence of parameter B on the compression limit. For monatomic gases, this weakening is k = 5/3 times, and for diatomic gases, k = 7/5.
Limits given by Equations (19), (20) and (22) exist during detonation only in unsteady, strongly pinched waves. With a detonation that propagates spontaneously at a constant speed, the limiting density, is equal to a different value at a speed tending to infinity. This value will be determined below.
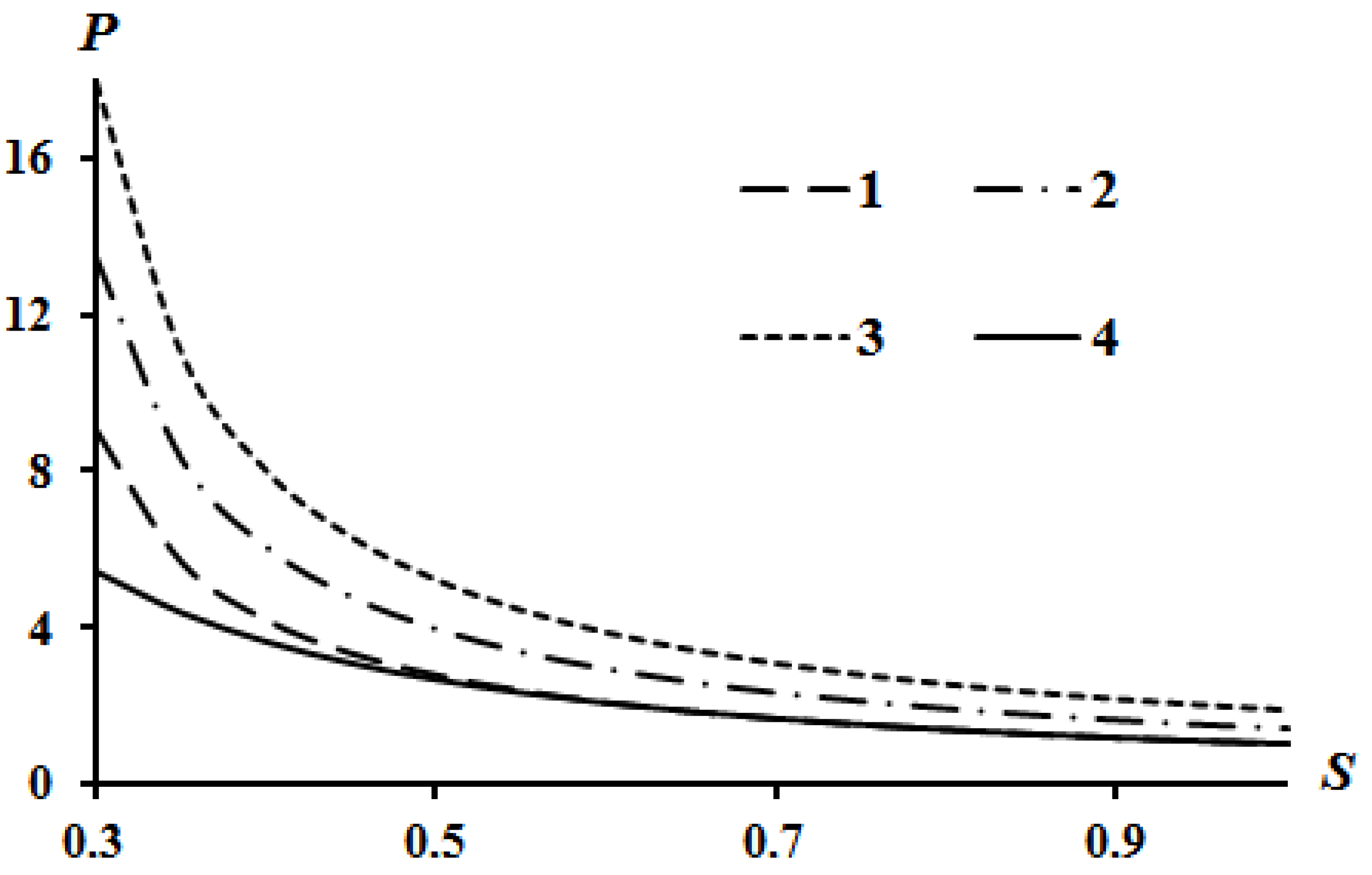
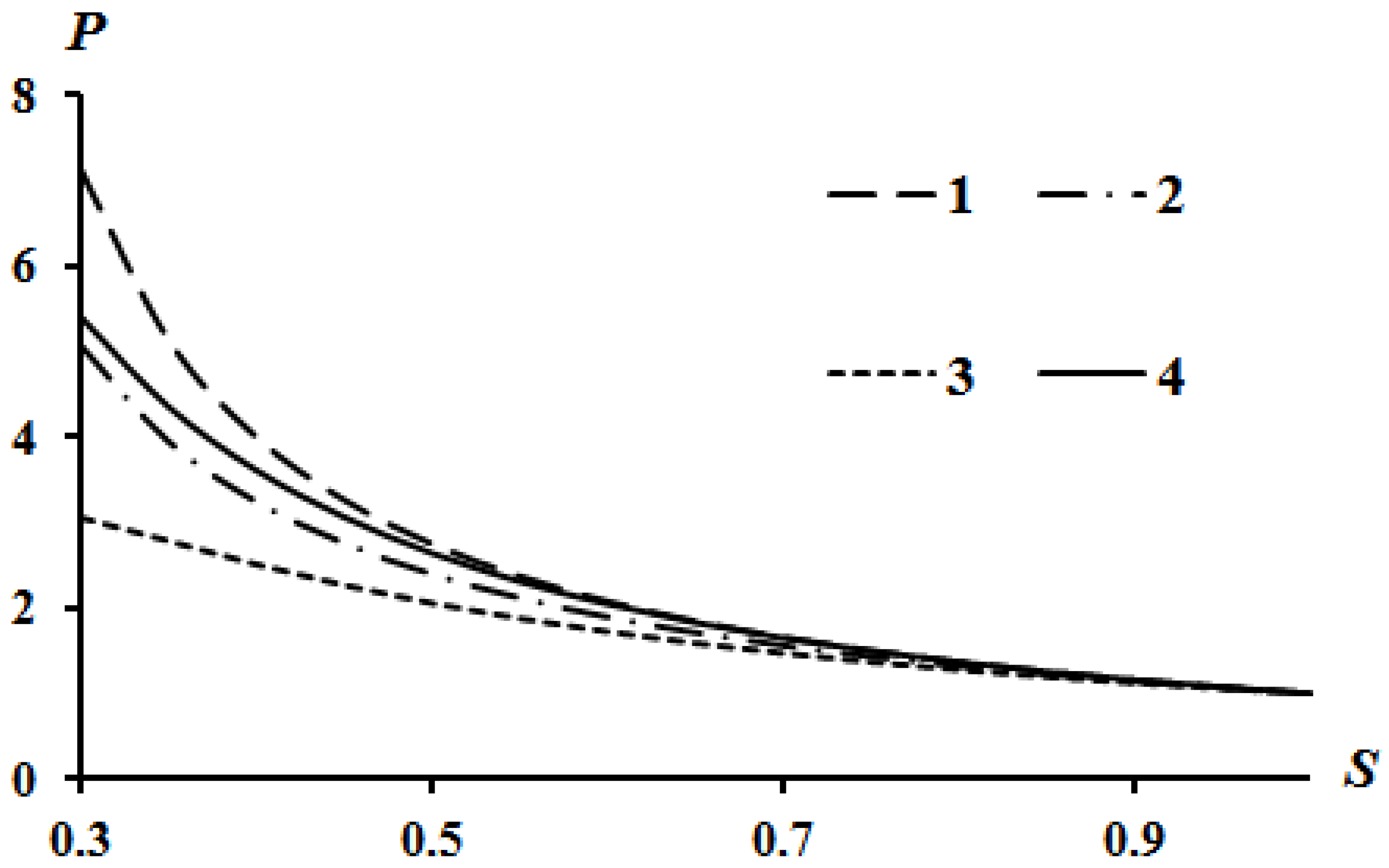
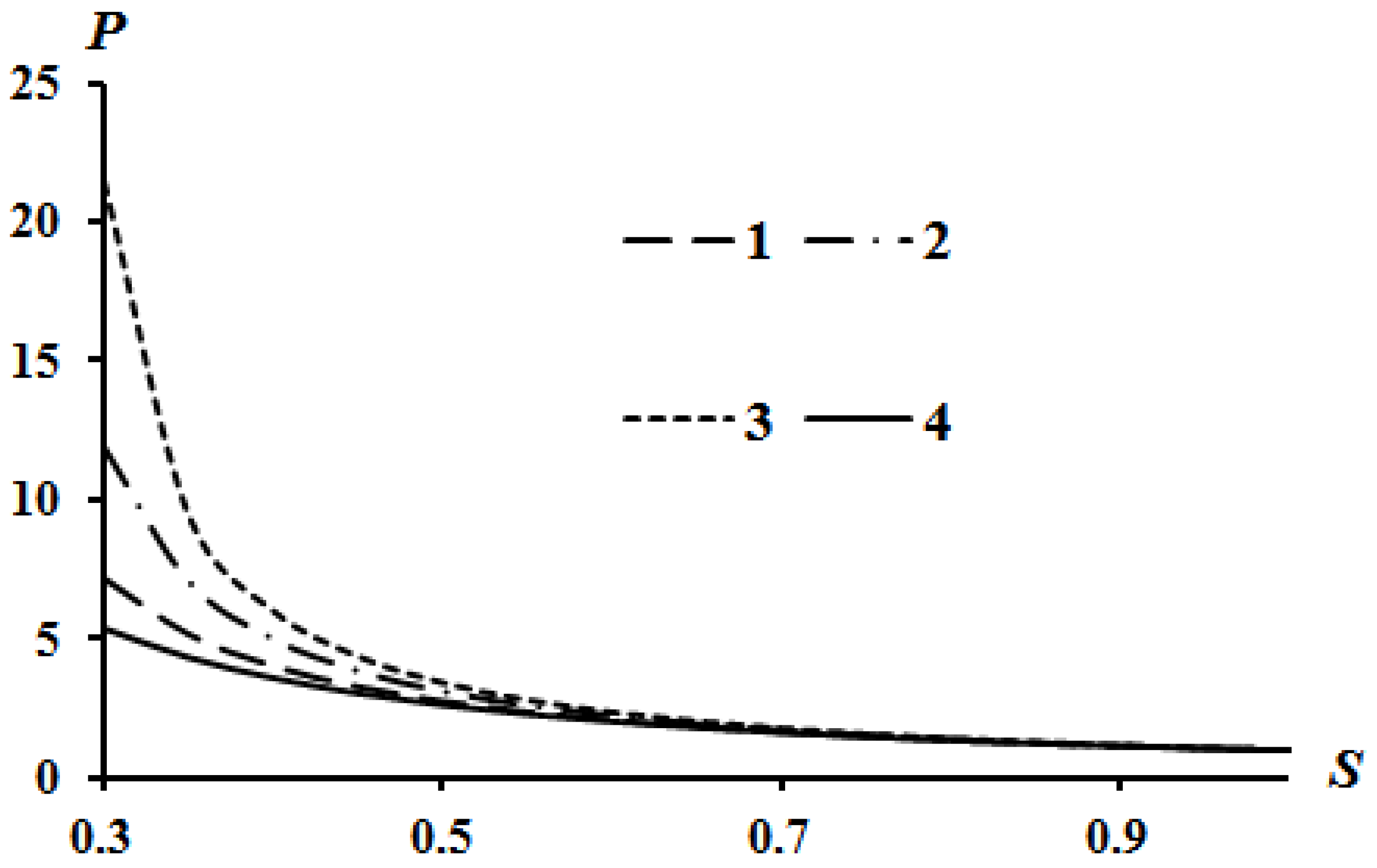
The results of calculations according to Equation (16) are shown in Figure 2, Figure 3 and Figure 4. Curve 4 in Figure 2, Figure 3 and Figure 4 corresponds to the Poisson isentrope P = S−k.
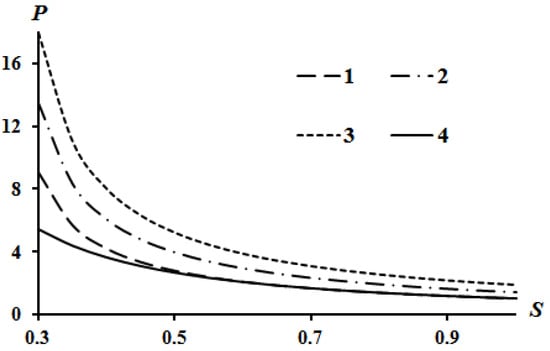
Figure 2.
Effect of heat release Q on the Hugoniot curve at A = 0.1; B = 0.05: 1—Q = 0; 2—Q = 2; 3—Q = 4.
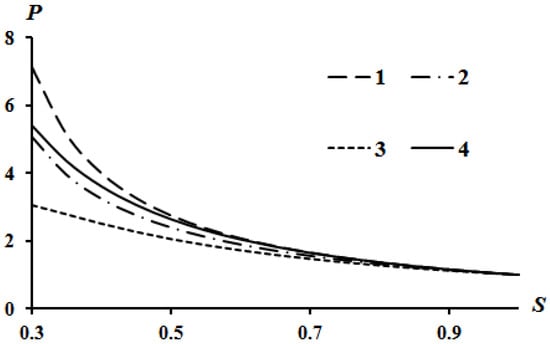
Figure 3.
Effect of the parameter A on the Hugoniot curve at Q = 0; B = 0: 1—A = 0; 2—A = 0.1; 3—A = 0.2.
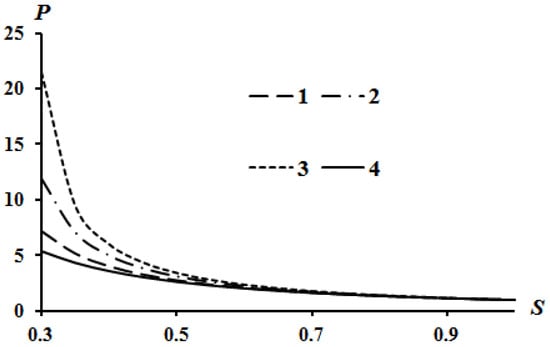
Figure 4.
Effect of the parameter B on the Hugoniot curve at Q = 0; A = 0: 1—B = 0; 2—B = 0.05; 3—B = 0.08.
It can be seen that an increase in heat release (Q) leads to an equidistant rise in the Hugoniot curve compared to the case of Q = 0. An increase in the value of parameter A leads to a lowering of the left side of the Hugoniot curve (Figure 3), and an increase in the value of parameter B leads to an increase in the left side of the Hugoniot curve (Figure 4). These tendencies are described in [13] which used assumption (21). The physical interpretation of these tendencies is also given in [13]. However, all curves converge at a point provided there is no heat release Q = 0.
4. Ultimate Compression and Speed Characteristics
To determine which point on the Hugoniot curve corresponds to stable normal detonation with a minimum velocity, we use the Jouguet selection rule [22,23]. This point corresponds to a point on the Hugoniot curve D through which the tangent passes, which also passes through the point . The equation of the tangent is determined using the following condition [22,23].
From Equation (16) we find
Let us determine the inclination angle of this tangent. To do this, we find from Equations (1) and (2)
From (25) we find the angle of the tangent at point D
where is the Mach number in front of the detonation wave, and PD, SD are the ratios of pressures and densities at point D of the Hugoniot curve.
On the other hand, the same tangent is determined using Equation (24)
The solution of the system of Equations (27) and (28) allows us to find the coordinates of point D on the Hugoniot curve. Eliminating PD from Equation (28) based on Equation (27), we obtain a fourth-order polynomial equation
From Equation (29) we find SD, and then from Equation (27), we determine the value of PD. The equations for SD and и PD are very cumbersome, and therefore are not presented here. For an ideal gas (A = B = 0), the equations for SD and и PD go over to the well-known Jouguet relations
The limit of the equation for SD at speed tending to infinity is
This equation determines the ultimate compression in detonation, which corresponds to the Jouguet condition. For an ideal gas (B = 0), Equation (32) transforms into the Jouguet equation [22,23]. As can be seen, the influence of parameter B on the ultimate compression during detonation is qualitatively the same as the asymptote of the ultimate compression for a nonreacting gas (19). Also, the ultimate compression during detonation does not depend on parameter A.
Let us determine the velocity of detonation products. To do this, we transform Equation (24) as follows:
Here we take into account the fact that at point D, the derivatives of the Poisson isentrope (denoted by the letter I in Equation (33)) and the Hugoniot Equation (16) for an ideal gas A = B = 0 (denoted by the letter H in Equation (33)) are equal to each other [22,23]. The binomial series is also used here under the assumption of small values of parameter B.
Let us multiply Equation (33) by . This gives
Now we transform the equations for the velocity of detonation products (26)
Taking this into account, we rewrite Equation (34)
A comparison of Equations (35) and (36) allows us to find the equation for the velocity of detonation products
or in dimensionless form
In Equations (37) and (38), parameter P can be eliminated using the modified Hugoniot Equation (16).
Equations (37) and (38) show that, for an ideal gas, combustion products flow from the detonation front at a critical (sonic) velocity. For the van der Waals gas, the velocity of the combustion products can be greater than the critical one. Moreover, both the additional pressure (A) and the additional volume (B), lead to acceleration of combustion products. The additional pressure transfers its energy to the flow, and thus causes acceleration. The additional volume results in a reduction in the compression ratio as well as in the ultimate compression (32). Hence, the flow velocity increases.
If we use limiting compression (32) in Equation (38), we obtain then
Equation (39) shows that the Mach number after the detonation wave for a van der Waals gas can be greater than unity. This is very important for the design of detonation jet engines.
5. Conclusions
This paper examines the process of the passage of a detonation wave in a real gas, whose equation of state is described using the van der Waals equation. A modified Rankine–Hugoniot equation has been obtained, which makes it possible to analyze the dynamics of a real gas when a detonation wave passes through it. Equations are obtained to determine the ratio of speeds and pressures during the passage of a wave. Also, the influence of van der Waals parameters on the variation of the parameters of the detonation process is shown. Calculations indicated that an increase in parameter A slows down the pressure increase in the detonation wave, and an increase in parameter B enhances it. The difference in the speed of combustion products for ideal and real gas was demonstrated. For an ideal gas, combustion products flow from the detonation front at a critical (sonic) speed. For a van der Waals gas, the speed of combustion products may be greater than the critical one. Moreover, both factors, additional pressure (A) and additional volume (B), lead to acceleration of combustion products. The additional pressure transfers its energy to the fluid flow, and thus causes acceleration. The additional volume leads to a decrease in the compression ratio as well as the ultimate compression (32). Consequently, the flow rate increases. The thermal effect leads to the acceleration of the flow before the shock wave.
Author Contributions
Conceptualization, A.A.A. and I.V.S.; methodology, M.M.K.; formal analysis, validation and investigation, A.A.A., I.V.S. and Y.Y.K.; writing—original draft preparation, A.A.A., I.V.S. and Y.Y.K.; writing—review and editing, A.A.A., I.V.S. and Y.Y.K. All authors have read and agreed to the published version of the manuscript.
Funding
The research contribution of A.A.A., M.M.K. and Y.Y.K. was funded in frames of the program of research projects of the National Academy of Sciences of Ukraine “Support of priority for the state scientific researches and scientific and technical (experimental) developments” 2022–2023. Project: “Development of scientific principles of heat transfer and combustion processes to improve technologies for obtaining and using renewable fuels with the aim of decarbonizing the energy sector of Ukraine”.
Data Availability Statement
Data will be made available on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Urzay, J. Supersonic combustion in air-breathing propulsion systems for hypersonic flight. Annu. Rev. Fluid Mech. 2018, 50, 593–627. [Google Scholar] [CrossRef]
- Hishida, M.; Fujiwara, T.; Wolanski, P. Fundamentals of rotating detonation. Shock Waves 2009, 19, 1–10. [Google Scholar] [CrossRef]
- Calvo-Rivera, A.; Huete, C.; Velikovich, A.L. The stability of expanding reactive shocks in a van der Waals fluid. Phys. Fluids 2022, 34, 046106. [Google Scholar] [CrossRef]
- Weng, Z.; Mével, R.; Law, C.K. On the critical initiation of planar detonation in Noble-Abel and van der Waals Gas. Combust. Flame 2023, 255, 112890. [Google Scholar] [CrossRef]
- Pielemeier, K.R.; Powers, J.M. Anomalous Waves in Non-Ideal Detonation Dynamics; AIAA 2022-0396; AIAA SciTech Forum: San Diego, CA, USA, 2022. [Google Scholar] [CrossRef]
- Chauhan, S.; Singh, D.; Arora, R. Similarity solution for isothermal flow behind the magnetogasdynamic cylindrical shock wave in a rotating non-ideal gas with the effect of gravitational field. Phys. Fluids 2022, 34, 117118. [Google Scholar] [CrossRef]
- Nath, G.; Devi, A. A self-similar solution for unsteady adiabatic and isothermal flows behind the shock wave in a non-ideal gas using Lie group analysis method with azimuthal or axial magnetic field in rotating medium. Eur. Phys. J. Plus 2021, 136, 477. [Google Scholar] [CrossRef]
- Yadav, S.; Singh, D.; Arora, R. Lie group of invariance technique for analyzing propagation of strong shock wave in a rotating non-ideal gas with azimuthal magnetic field. Math. Methods Appl. Sci. 2022, 45, 11889–11904. [Google Scholar] [CrossRef]
- Weng, Z.; Méve, R. Real gas effect on steady planar detonation and uncertainty quantification. Combust. Flame 2022, 245, 112318. [Google Scholar] [CrossRef]
- Taniguchi, S.; Mentrelli, A.; Ruggeri, T.; Sugiyama, M.; Zhao, N. Prediction and simulation of compressive shocks with lower perturbed density for increasing shock strength in real gases. Phys. Rev. E—Stat. Nonlinear Soft Matter Phys. 2010, 82, 036324. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, T.; Mentrelli, A.; Sugiyama, M. Admissible shock waves and shock-induced phase transition in a van der waals fluid (Part II—Rankine-Hugoniot conditions and shock admissibility). Waves Stab. Contin. Media 2010, 279–288. [Google Scholar] [CrossRef]
- Zhao, N.; Mentrelli, A.; Ruggeri, T.; Sugiyama, M. Admissible shock waves and shock-induced phase transition in a van der Waals fluid. Phys. Fluids 2011, 23, 086101. [Google Scholar] [CrossRef]
- Avramenko, A.A.; Shevchuk, I.V.; Dmitrenko, N.P. Shock Wave in van der Waals Gas. J. Non-Equilib. Thermodyn. 2022, 47, 255–267. [Google Scholar] [CrossRef]
- Avramenko, A.A.; Shevchuk, I.V.; Kovetskaya, M.M.; Kovetska, Y.Y. Self-similar analysis of gas dynamics for van der Waals gas in slipping flow after normal shock wave. Phys. Fluids 2023, 35, 026110. [Google Scholar] [CrossRef]
- Cramer, M.S.; Sen, R. Exact solutions for sonic shocks in van der Waals gases. Phys. Fluids 1987, 30, 377–385. [Google Scholar] [CrossRef]
- Clavin, P. Advances in the analytical study of the dynamics of gaseous detonation waves. C. R. Méc. 2019, 347, 273–286. [Google Scholar] [CrossRef]
- Rankine, W.J.M. On the thermodynamic theory of waves of finite longitudinal disturbance. Philos. Trans. R. Soc. 1870, 160, 277–288. [Google Scholar]
- Hugoniot, H. Mémoire sur la propagation des mouvement dans les corps et spécialement dans les gaz parfaits (première partie) [Memoir on the propagation of movements in bodies, especially perfect gases (first part)]. J. Éc. Polytech. 1887, 57, 97. (In French) [Google Scholar]
- Hugoniot, H. Mémoire sur la propagation des mouvements dans les corps et spécialement dans les gaz parfaits (deuxième partie) [Memoir on the propagation of movements in bodies, especially perfect gases (second part)]. J. Éc. Polytech. 1889, 58, 125. (In French) [Google Scholar]
- Anderson, J.D. Modern Compressible Flow: With Historical Perspective; McGraw-Hill: New York, NY, USA, 1990. [Google Scholar]
- Loitsyanskii, L.G. Mechanics of Liquids and Gases (International Series of Monographs in Aeronautics and Astronautics), 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 1966. [Google Scholar]
- Jouguet, E. Sur la propagation des réactions chimiques dans les gaz. J. Math. Pures Appl. 1905, 1, 347–425. Available online: http://www.numdam.org/item/JMPA_1905_6_1__347_0/ (accessed on 26 September 2023).
- Jouguet, E. Sur la propagation des réactions chimiques dans les gaz. J. Math. Pures Appl. 1906, 2, 5–86. Available online: http://www.numdam.org/item/?id=JMPA_1906_6_2__5_0 (accessed on 26 September 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).