Overcoming Drag at the Water-Air Interface Constrains Body Size in Whirligig Beetles
Abstract
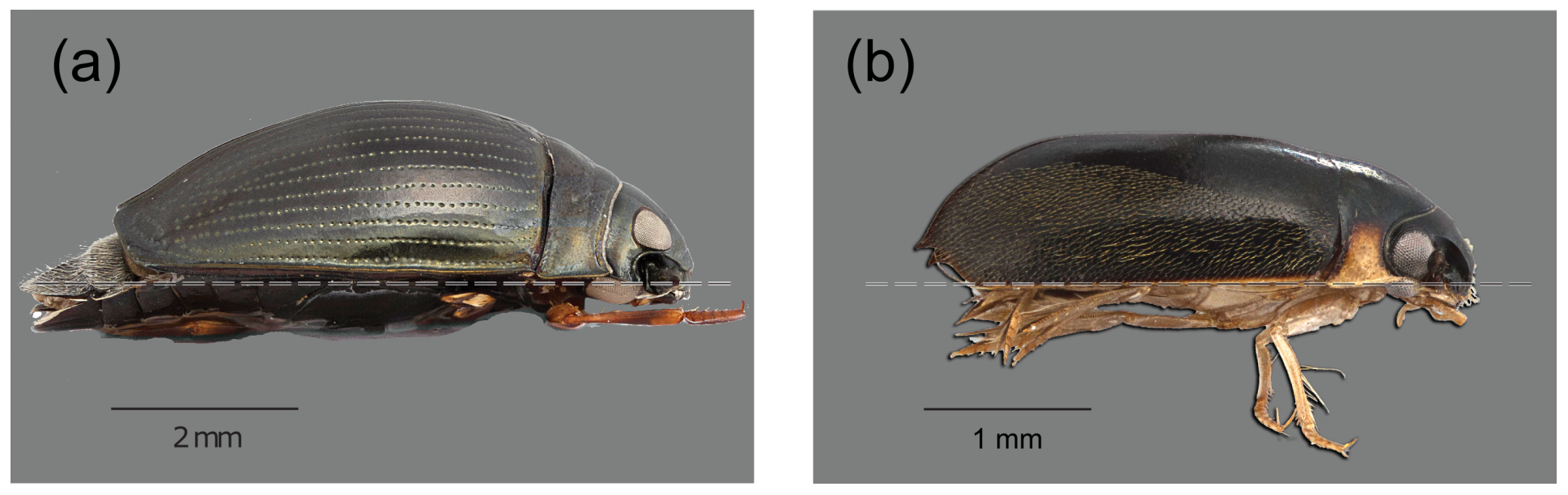
1. Introduction
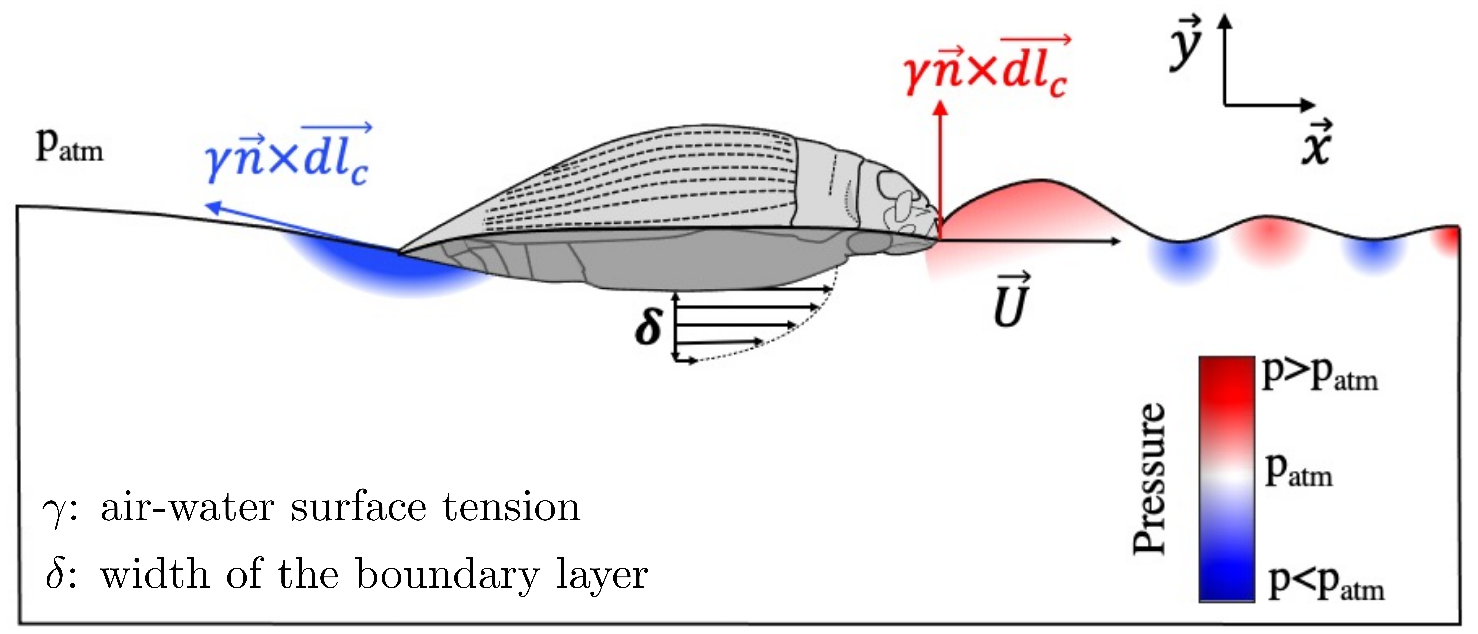
2. Scaling Drag Forces with the Insect Size
2.1. Wave Resistance
2.2. Viscous Drag
2.3. Total Drag
3. Scaling of the Insect Thrust Capacity and Comparing It with Drag
3.1. Scaling of Maximal Thrust Force
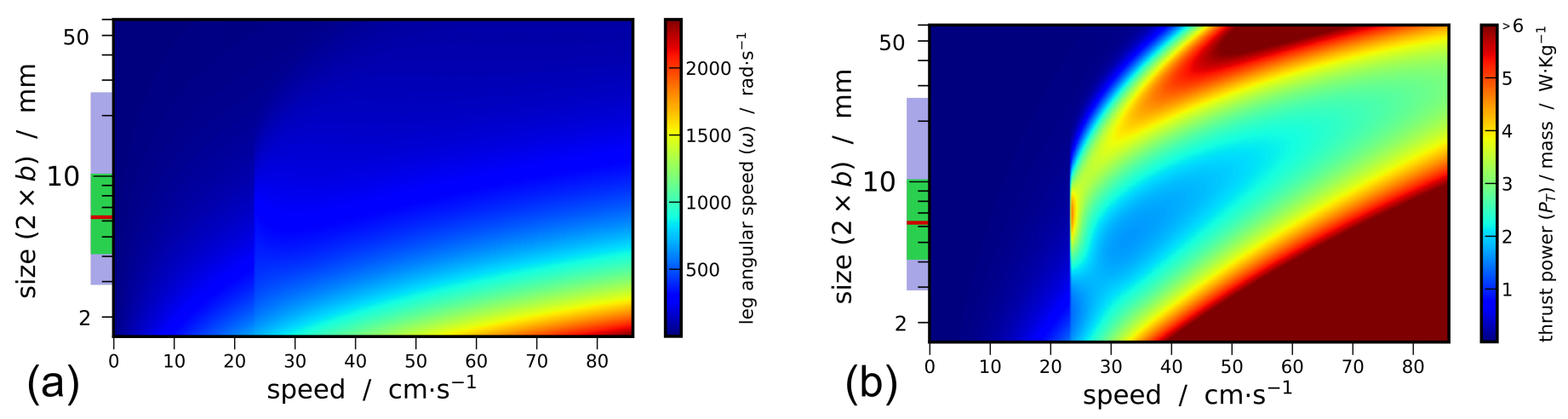
3.2. Thrust Power
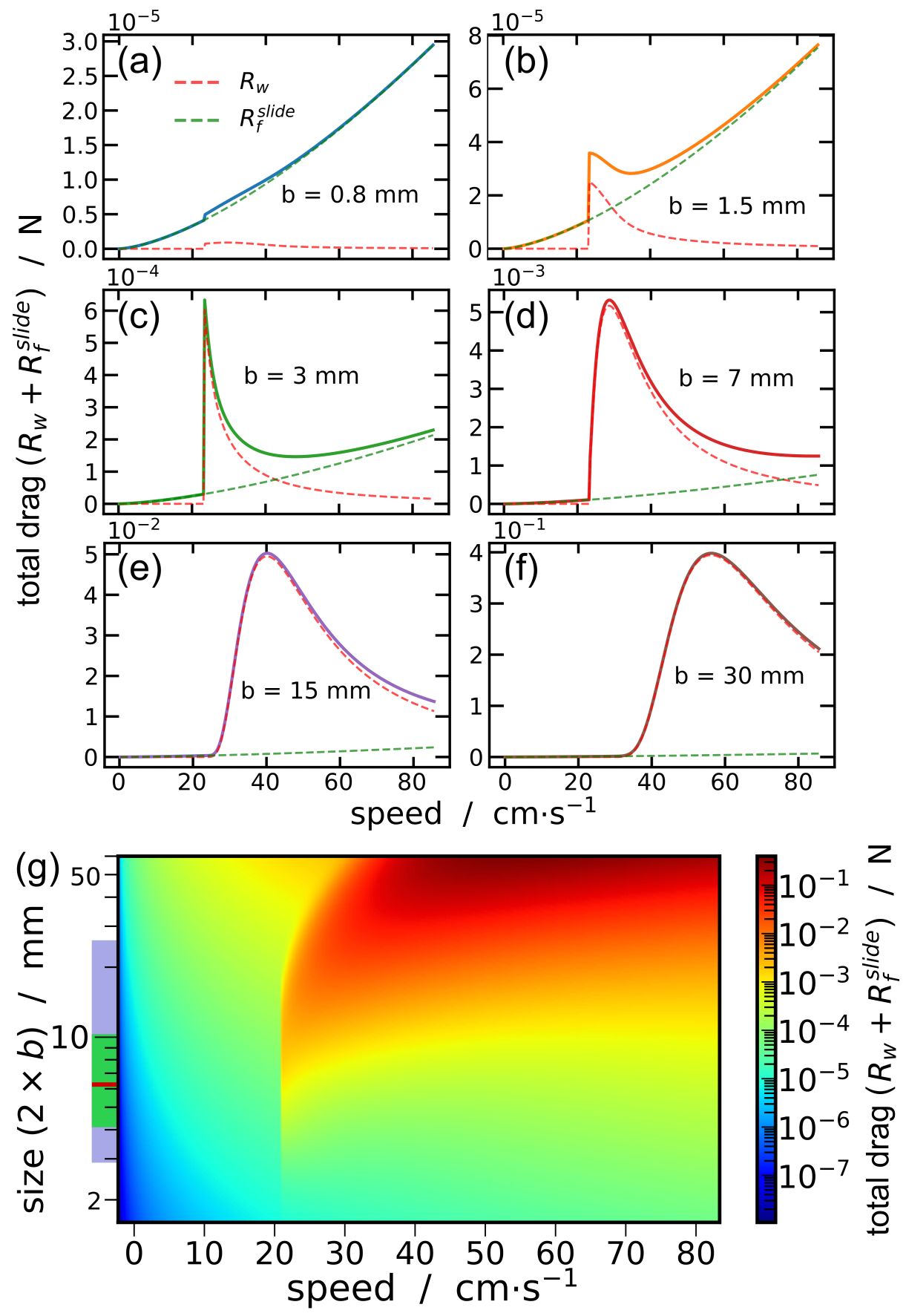
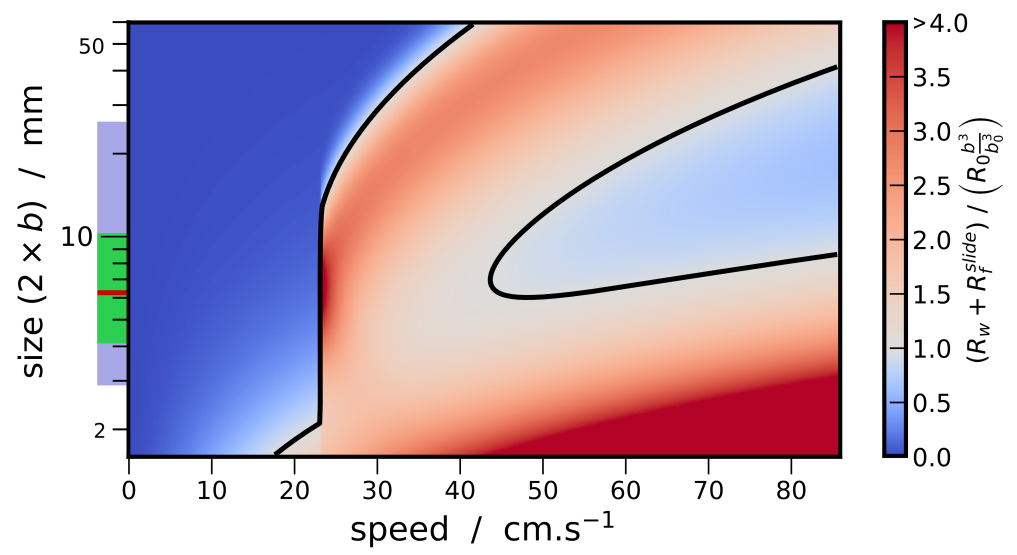
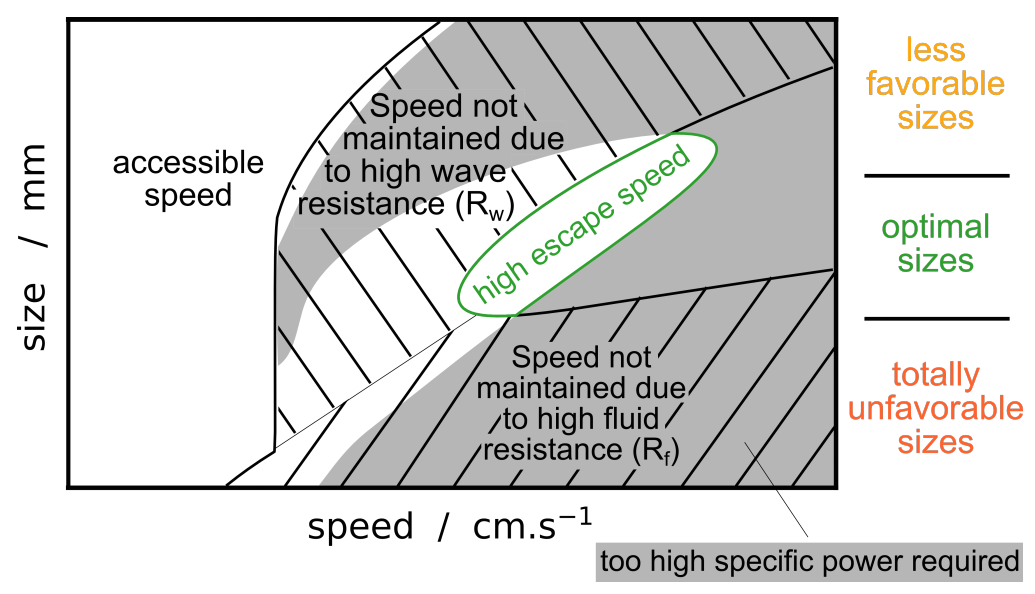
3.3. Synthesis
- (i)
- Beetles of a size length smaller than mm cannot achieve a high speed regime (>23 cm/s). Indeed for these small sizes, the viscous resistance exceeds the wave resistance. The ratio comparing drag and thrust then reads, and diverges as size decreases and speed increases. With an upper estimation of for the muscle mass ratio of whirligig beetles, reduce to mm.
- (ii)
- Larger-sized insects, between approximately 6 and mm, can achieve high speed regimes but cannot maintain themselves in a middle-range speed where the wave resistance reaches its maximum. As explained previously, the avoidance of intermediate speeds has been observed and partially explained by Voise and Casas [22].
- (iii)
- This size range insects, when reaching speed beyond wave resistance peak, have a maximal speed limited by the specific thrust power required increasing with speed.
- (iv)
- As size increases, the wave resistance peak widens, thereby a greater thrust effort is likely needed to overcome this peak. Therefore, it should be less probable for the largest whirligig beetles to reach the high speed regime where the wave resistance is greatly reduced. Furthermore, the onset of the wave resistance moves to higher speeds with . Thus the largest species still reach relatively large speeds around cm/s below wave resistance peak.
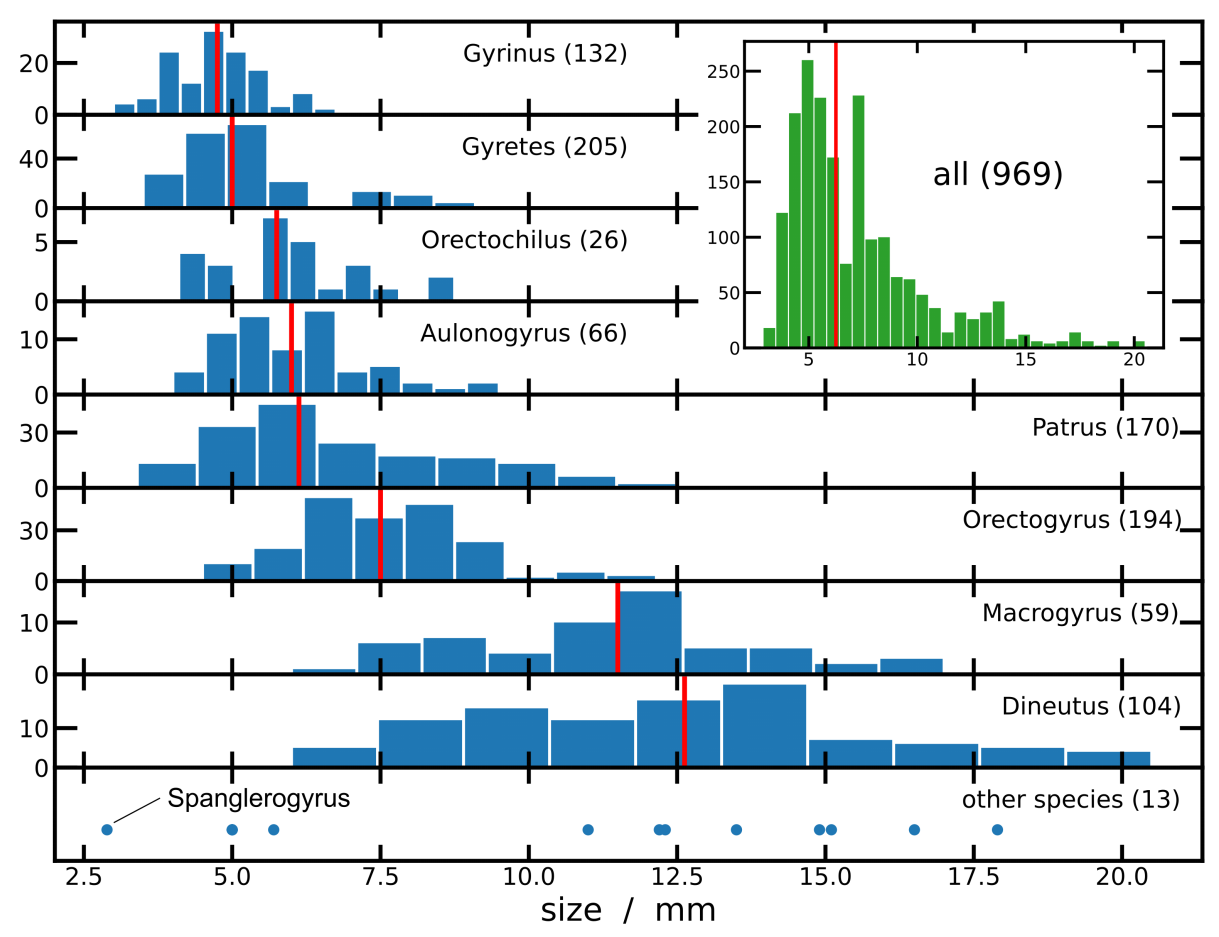
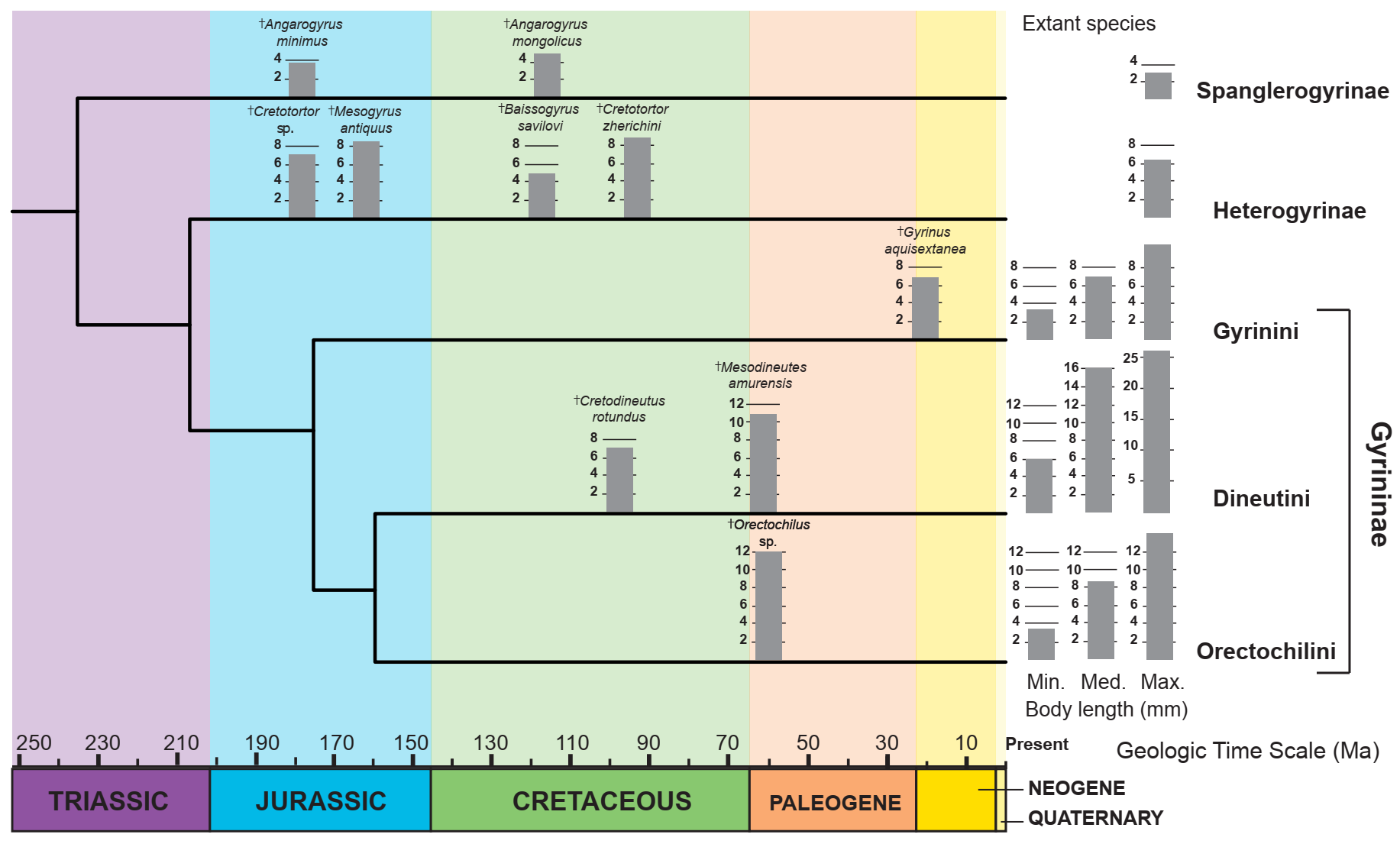
4. Significance for Gyrinidae Ecology and Evolution
5. Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Z.Q. Animal Biodiversity: An Outline of Higher-Level Classification and Survey of Taxonomic Richness; Magnolia Press: Waco, TX, USA, 2011. [Google Scholar]
- Blagodatski, A.; Kryuchkov, M.; Sergeev, A.; Klimov, A.A.; Shcherbakov, M.R.; Enin, G.A.; Katanaev, V.L. Under-and over-water halves of Gyrinidae beetle eyes harbor different corneal nanocoatings providing adaptation to the water and air environments. Sci. Rep. 2014, 4, 1–6. [Google Scholar] [CrossRef]
- Kolmes, S.A. Ecological and sensory aspects of prey capture by the whirligig beetle Dineutes discolor (Coleoptera: Gyrinidae). J. N. Y. Entomol. Soc. 1983, 91, 405–412. [Google Scholar]
- Voise, J.; Casas, J. Echolocation in whirligig beetles using surface waves: An unsubstantiated conjecture. In Studying Vibrational Communication; Springer: Berlin/Heidelberg, Germany, 2014; pp. 303–317. [Google Scholar]
- Nachtigall, W. Funktionelle Morphologie, Kinematik und Hydromechanik des Ruderapparates von Gyrinus. Z. Vgl. Physiol. 1961, 45, 193–226. [Google Scholar] [CrossRef]
- Xu, Z.; Lenaghan, S.C.; Reese, B.E.; Jia, X.; Zhang, M. Experimental studies and dynamics modeling analysis of the swimming and diving of whirligig beetles (Coleoptera: Gyrinidae). PLoS Comput. Biol. 2012, 8, e1002792. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Cho, S.K. Bio-inspired micro/mini propulsion at air-water interface: A review. J. Mech. Sci. Technol. 2012, 26, 3761–3768. [Google Scholar] [CrossRef]
- Tian, L.; Li, Z.; Jin, E.; Ke, Q.; Dong, S.; Ma, Y. Improved flow performance of a centrifugal compressor based on pit formation on the notum of the whirligig beetle (Gyrinidae Latreille). Adv. Mech. Eng. 2015, 7, 1687814015591736. [Google Scholar] [CrossRef]
- Jia, X.; Chen, Z.; Riedel, A.; Si, T.; Hamel, W.R.; Zhang, M. Energy-efficient surface propulsion inspired by whirligig beetles. IEEE Trans. Robot. 2015, 31, 1432–1443. [Google Scholar] [CrossRef]
- Tucker, V.A. Wave-making by whirligig beetles (Gyrinidae). Science 1969, 166, 897–899. [Google Scholar] [CrossRef] [PubMed]
- Härlin, C.; Henrikson, B.I.; Stenson, J.A.; Svensson, J.E. Species-specific predation on gyrinid beetles by the backswimmer, Notonecta glauca. Int. Ver. Theor. Angew. Limnol. Verhandlungen 2005, 29, 717–721. [Google Scholar] [CrossRef]
- Romey, W.L.; Galbraith, E. Optimal group positioning after a predator attack: The influence of speed, sex, and satiation within mobile whirligig swarms. Behav. Ecol. 2008, 19, 338–343. [Google Scholar] [CrossRef]
- Vulinec, K.; Miller, M. Aggregation and predator avoidance in whirligig beetles (Coleoptera: Gyrinidae). J. N. Y. Entomol. Soc. 1989, 97, 438–447. [Google Scholar]
- Romey, W.L. Position preferences within groups: Do whirligigs select positions which balance feeding opportunities with predator avoidance? Behav. Ecol. Sociobiol. 1995, 37, 195–200. [Google Scholar] [CrossRef]
- Romey, W.L.; Wallace, A.C. Sex and the selfish herd: Sexual segregation within nonmating whirligig groups. Behav. Ecol. 2007, 18, 910–915. [Google Scholar] [CrossRef]
- Romey, W.L.; Smith, A.L.; Buhl, J. Flash expansion and the repulsive herd. Anim. Behav. 2015, 110, 171–178. [Google Scholar] [CrossRef]
- Larsén, O. On the Morphology and Function of the Locomotor Organs of the Gyrinidae and Other Coleoptera; Entomologiska Sällskapet i Lund: Lund, Sweden, 1966. [Google Scholar]
- Nachtigall, W. Locomotion: Mechanics and hydrodynamics of swimming in aquatic insects. In The Physiology of Insecta; Elsevier: Amsterdam, The Netherlands, 1974; pp. 381–432. [Google Scholar]
- Blake, R. Hydrodynamics of swimming in the water boatman, Cenocorixa bifida. Can. J. Zool. 1986, 64, 1606–1613. [Google Scholar] [CrossRef]
- Whittlesey, R.W. Wake-based unsteady modeling of the aquatic beetle Dytiscus marginalis. J. Theor. Biol. 2011, 291, 14–21. [Google Scholar] [CrossRef]
- Liu, S.P.; Wipfler, B.; Beutel, R.G. The unique locomotor apparatus of whirligig beetles of the tribe Orectochilini (Gyrinidae, Coleoptera). J. Zool. Syst. Evol. Res. 2018, 56, 196–208. [Google Scholar] [CrossRef]
- Voise, J.; Casas, J. The management of fluid and wave resistances by whirligig beetles. J. R. Soc. Interface 2010, 7, 343–352. [Google Scholar] [CrossRef]
- Fish, F.E.; Nicastro, A.J. Aquatic turning performance by the whirligig beetle: Constraints on maneuverability by a rigid biological system. J. Exp. Biol. 2003, 206, 1649–1656. [Google Scholar] [CrossRef] [PubMed]
- Devereux, H.L.; Twomey, C.R.; Turner, M.S.; Thutupalli, S. Whirligig beetles as corralled active Brownian particles. J. R. Soc. Interface 2021, 18, 20210114. [Google Scholar] [CrossRef]
- Steinmann, T.; Cribellier, A.; Casas, J. Singularity of the water strider propulsion mechanisms. J. Fluid Mech. 2021, 915, A118. [Google Scholar] [CrossRef]
- Steinmann, T.; Arutkin, M.; Cochard, P.; Raphaël, E.; Casas, J.; Benzaquen, M. Unsteady wave pattern generation by water striders. J. Fluid Mech. 2018, 848, 370–387. [Google Scholar] [CrossRef]
- Gao, P.; Feng, J.J. A numerical investigation of the propulsion of water walkers. J. Fluid Mech. 2011, 668, 363. [Google Scholar] [CrossRef]
- Bush, J.W.; Hu, D.L. Walking on water: Biolocomotion at the interface. Annu. Rev. Fluid Mech. 2006, 38, 339–369. [Google Scholar] [CrossRef]
- Miller, K.B.; Bergsten, J. Phylogeny and classification of whirligig beetles (Coleoptera: Gyrinidae): Relaxed-clock model outperforms parsimony and time-free Bayesian analyses. Syst. Entomol. 2012, 37, 706–746. [Google Scholar] [CrossRef]
- Lighthill, M.J.; Lighthill, J. Waves in Fluids; Cambridge University Press: Cambridge, UK, 2001. [Google Scholar]
- Moisy, F.; Rabaud, M. Mach-like capillary-gravity wakes. Phys. Rev. E 2014, 90, 023009. [Google Scholar] [CrossRef]
- Raphaël, E.; De Gennes, P.G. Capillary gravity waves caused by a moving disturbance: Wave resistance. Phys. Rev. E 1996, 53, 3448. [Google Scholar] [CrossRef]
- Benzaquen, M.; Raphael, E. Capillary-gravity waves on depth-dependent currents: Consequences for the wave resistance. EPL Europhys. Lett. 2012, 97, 14007. [Google Scholar] [CrossRef]
- Chevy, F.; Raphaël, E. Capillary gravity waves: A “fixed-depth” analysis. EPL Europhys. Lett. 2003, 61, 796. [Google Scholar] [CrossRef][Green Version]
- Chepelianskii, A.; Chevy, F.; Raphael, E. Capillary-gravity waves generated by a slow moving object. Phys. Rev. Lett. 2008, 100, 074504. [Google Scholar] [CrossRef]
- Closa, F.; Chepelianskii, A.; Raphael, E. Capillary-gravity waves generated by a sudden object motion. Phys. Fluids 2010, 22, 052107. [Google Scholar] [CrossRef]
- Benzaquen, M.; Chevy, F.; Raphaël, É. Wave resistance for capillary gravity waves: Finite-size effects. EPL Europhys. Lett. 2011, 96, 34003. [Google Scholar] [CrossRef]
- Le Merrer, M.; Clanet, C.; Quéré, D.; Raphaël, É.; Chevy, F. Wave drag on floating bodies. Proc. Natl. Acad. Sci. USA 2011, 108, 15064–15068. [Google Scholar] [CrossRef]
- Burghelea, T.; Steinberg, V. Wave drag due to generation of capillary-gravity surface waves. Phys. Rev. E 2002, 66, 051204. [Google Scholar] [CrossRef]
- Schlichting, H.; Gersten, K. Boundary-Layer Theory; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Pucci, G.; Ho, I.; Harris, D.M. Friction on water sliders. Sci. Rep. 2019, 9, 1–7. [Google Scholar]
- Gazzola, M.; Argentina, M.; Mahadevan, L. Scaling macroscopic aquatic locomotion. Nat. Phys. 2014, 10, 758–761. [Google Scholar] [CrossRef]
- Marden, J.H.; Allen, L.R. Molecules, muscles, and machines: Universal performance characteristics of motors. Proc. Natl. Acad. Sci. USA 2002, 99, 4161–4166. [Google Scholar] [CrossRef] [PubMed]
- Marden, J.H. Scaling of maximum net force output by motors used for locomotion. J. Exp. Biol. 2005, 208, 1653–1664. [Google Scholar] [CrossRef] [PubMed]
- Polilov, A.A.; Makarova, A.A. The scaling and allometry of organ size associated with miniaturization in insects: A case study for Coleoptera and Hymenoptera. Sci. Rep. 2017, 7, 1–7. [Google Scholar]
- Gustafson, G.T.; Prokin, A.A.; Bukontaite, R.; Bergsten, J.; Miller, K.B. Tip-dated phylogeny of whirligig beetles reveals ancient lineage surviving on Madagascar. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Nel, A. Les Gyrinidae fossiles de France (Coleoptera). Ann. Soc. Entomol. Fr. 1989, 25, 321–330. [Google Scholar]
- Liang, Z.; Qi, Z.; Chen, J.; Jia, F. Cretodineutus rotundus gen. et sp. nov., the oldest adult whirligig beetle from the Upper Cretaceous of Myanmar (Coleoptera, Gyrinidae, Gyrininae). Cretac. Res. 2020, 106, 104251. [Google Scholar] [CrossRef]
- Beutel, R.G.; Wang, B.; Tan, J.J.; Ge, S.Q.; Ren, D.; Yang, X.K. On the phylogeny and evolution of Mesozoic and extant lineages of Adephaga (Coleoptera, Insecta). Cladistics 2013, 29, 147–165. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, X.; Jarzembowski, E.A.; Chen, L.; Wang, B. First record of adult Coptoclava longipoda Ping (Coleoptera: Coptoclavidae) from the Lower Cretaceous of Laiyang, China. Cretac. Res. 2018, 92, 205–209. [Google Scholar] [CrossRef]
- Wilkinson, S.; Rundle, S.; Brewin, P.; Ormerod, S. A study of the whirligig beetle Dineutus indicus (Aube) (Gyrinidae) in a Nepalese hillstream. Entomologist 1995, 114, 131–137. [Google Scholar]
- Lipp, A.; Wolf, H.; Lehmann, F.O. Walking on inclines: Energetics of locomotion in the ant Camponotus. J. Exp. Biol. 2005, 208, 707–719. [Google Scholar] [CrossRef][Green Version]
- Beutel, R.G.; Yan, E.; Yavorskaya, M.; Büsse, S.; Gorb, S.N.; Wipfler, B. On the thoracic anatomy of the Madagascan Heterogyrus milloti and the phylogeny of Gyrinidae (Coleoptera). Syst. Entomol. 2019, 44, 336–360. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jami, L.; Gustafson, G.T.; Steinmann, T.; Piñeirua, M.; Casas, J. Overcoming Drag at the Water-Air Interface Constrains Body Size in Whirligig Beetles. Fluids 2021, 6, 249. https://doi.org/10.3390/fluids6070249
Jami L, Gustafson GT, Steinmann T, Piñeirua M, Casas J. Overcoming Drag at the Water-Air Interface Constrains Body Size in Whirligig Beetles. Fluids. 2021; 6(7):249. https://doi.org/10.3390/fluids6070249
Chicago/Turabian StyleJami, Ludovic, Grey T. Gustafson, Thomas Steinmann, Miguel Piñeirua, and Jérôme Casas. 2021. "Overcoming Drag at the Water-Air Interface Constrains Body Size in Whirligig Beetles" Fluids 6, no. 7: 249. https://doi.org/10.3390/fluids6070249
APA StyleJami, L., Gustafson, G. T., Steinmann, T., Piñeirua, M., & Casas, J. (2021). Overcoming Drag at the Water-Air Interface Constrains Body Size in Whirligig Beetles. Fluids, 6(7), 249. https://doi.org/10.3390/fluids6070249





